Abstract
Adhesive receptors of the integrin family are primarily involved in cell–extracellular matrix adhesion. Additionally, integrins trigger multiple signaling pathways that are involved in cell migration, proliferation, survival, and differentiation. We previously demonstrated that the activation of integrins containing the β1 subunit leads to a selective increase in potassium currents carried by the human ether-a-go-go-related gene (hERG) channels in neuroblastoma and leukemia cells; this current activation modulates adhesion-dependent differentiation in these cells. We hypothesized that the cross-talk between integrins and hERG channels could be traced back to the assembly of a macromolecular signaling complex comprising the two proteins. We tested this hypothesis in both SH-SY5Y neuroblastoma cells and in human embryonic kidney 293 cells stably transfected with hERG1 and, therefore, expressing only the full-length hERG1 protein on the plasma membrane. The β1 integrin and hERG1 coprecipitate in these cells and colocalize in both intracellular and surface membrane compartments. The two proteins also coprecipitate with caveolin-1, suggesting the localization of the complex in lipid rafts/caveolae. hERG1-transfected cells undergo an activation of hERG currents after β1 integrin-mediated adhesion to fibronectin; concomitant with this activation, the focal adhesion kinase associates with the hERG1 protein and becomes tyrosine phosphorylated. Using hERG1-specific inhibitors, we show that the tyrosine phosphorylation of focal adhesion kinase is strictly dependent on hERG channel activity. Similarly, the activity of the small GTPase Rac1 turned out to be dependent on hERG currents. On the whole, these data indicate that the hERG1 protein associates with β1 integrins and modulates adhesion receptor signaling.
INTRODUCTION
Adhesive receptors of the integrin family are primarily involved in cell–extracellular matrix (ECM) adhesion. In addition to anchoring the ECM to the actin cytoskeleton, integrins trigger multiple signaling pathways that are involved in cell migration, proliferation, differentiation, and antiapoptotic functions (Howe et al., 1998; Giancotti and Ruoslahti, 1999; Schwartz and Baron, 1999). One pathway is centered on activation of the focal adhesion kinase (FAK), which in turn recruits a number of structural and signaling components and leads to the activation of mitogen-activated protein kinases (reviewed in Juliano, 2002). Another interesting model is that of integrin-mediated activation of the extracellular signal-regulated kinase cascade involving the transmembrane protein caveolin-1, the Src family kinase Fyn, and the adaptor protein Shc, but not FAK (Juliano, 2002). Recent studies also have indicated a link between signaling through integrins and activation of small GTPases, particularly RhoA, Rac1, and Cdc42. In addition, several Ras superfamily members influence the activation state of integrins, creating an intricate bidirectional pattern of communication between integrins, small GTPases, and the actin cytoskeleton (Parise et al., 2000). Integrin-mediated activation of Rac1 and other Rho family proteins may involve not only guanine nucleotide exchange factors but also targeting of the GTP-bound protein to sites of adhesion (del Pozo et al., 2004).
Integrins associate laterally to form spatially segregated regions of cell attachment to the matrix (Burridge and Chrzanowska-Wodnicka, 1996); recent data suggest that a fraction of some integrins can be recovered from lipid rafts or caveolae (Baron et al., 2003). In particular β1 and αv integrin subunits associate with caveolin-1 (Wary et al., 1996). Together, these data suggest that membrane compartmentalization and association with membrane proteins play a relevant role in integrin function (Wei et al., 1999). Finally, evidence suggests that integrins form physical complexes at the cell membrane with cell surface receptors, giving rise to signaling platforms at the adhesive sites (Brown, 2002).
Strong evidence is emerging regarding the functional association of ion channels with integrins, and the role of such interaction in the regulation of cellular activity. For example, integrin-dependent adhesion initiates Ca2+ influx in multiple cell types (Davis et al., 2001, 2002). Ion channels can be included among the intracellular targets of integrin-activated phosphotyrosine (p-Tyr) cascades (Davis et al., 2002); conversely, ion channel activity, induced by integrin activation, can itself regulate the phosphorylated state of intracellular proteins, such as FAK (Bianchi et al., 1995). Another important aspect of integrin/ion channel interactions is that integrins may play a role in directing the membrane localization of ion channels (Davis et al., 2002). Furthermore, Kv 1.3 channels are necessary for activation of β1 integrins and subsequent integrin-dependent adhesion and migration in T-lymphocytes. Such functional activation relies on the physical association between the two classes of molecules (Levite et al., 2000).
We have previously reported that a long-lasting activation of human ether-a-go-go-related gene (hERG) K+ channels occurs in neuronal and hemopoietic tumor cells after integrin-mediated adhesion and that such activation is associated with the induction of neurite extensions as well as differentiation in these cells (Arcangeli et al., 1993, 1996; Hofmann et al., 2001). This activation of hERG currents turned out to be specific, because no other K+ currents expressed endogenously in these cells were increased after cell adhesion (Arcangeli et al., 1993, 1996); moreover β1-containing integrins elicited this activity effect (Hofmann et al., 2001). The hERG channel is a member of the EAG family of voltage-gated K+ channels that are involved in controlling multiple cellular activities (Bauer and Schwarz, 2001; Schwarz and Bauer, 2004). The hERG channel constitutes the molecular basis of the cardiac repolarizing current known as Ikr (Sanguinetti et al., 1995). hERG currents also can regulate cell firing in excitable cells (Chiesa et al., 1997; Rosati et al., 2000; Lecchi et al., 2002; Gullo et al., 2003) and contribute to the regulation of cell proliferation and invasiveness in tumor cells (Pillozzi et al., 2002; Lastraioli et al., 2004). Functional hERG channels are tetramers and each subunit consists of a six transmembrane protein, with both N and C termini located intracellularly. The neuroblastoma and leukemia tumor cells, express heterotetrameric hERG channels on the plasma membrane, composed of both full-length hERG1 and the splice variant hERG1B proteins (Crociani et al., 2003).
One potential mechanism explaining the above-described cross-talk between integrins and K+ channels could be the physical interaction between the two surface molecules. Indeed, we recently reported that β1 integrin subunit coprecipitates with hERG proteins in neuroblastoma cells (Cherubini et al., 2002).
To further examine these interactions, we studied a cellular model consisting of human embryonic kidney (HEK) cells stably transfected with the hERG1 or KCNH2. In these cells, we demonstrate that the hERG1 protein is physically linked to β1 integrin and modulates integrin downstream signaling.
MATERIALS AND METHODS
Antibodies
The following antibodies were used for immunoprecipitation (IP)/Western blot (WB) or immunofluorescence (IF) experiments at the concentrations reported in parentheses. Primary antibodies were anti-β1 monoclonal antibody (mAb) (TS2/16) (American Type Culture Collection, Manassas, VA) (IP: 1 and 5 μg antibody/mg protein; IF: 1:50); anti-β1A integrin subunit polyclonal antibody (RM-12) (Immunological Science, Rome, Italy) (WB: 1:1000); anti-hERG1 C terminus polyclonal antibody (C54) (Lastraioli et al., 2004) (WB: 1:1000; IF: 1:200); anti-hERG1 N terminus polyclonal antibody (N135) (Cherubini et al., 2000) (IP: 5 μl antiserum/1.5 mg protein); anti-hERG1B polyclonal antibody (Guasti, Cilia, Crociani, Hofmann, Polvani, Becchetti, Wanke, Tempia, and Arcangeli, unpublished data) (WB: 1:1000); anti-rat ether-a-go-go (rEAG) polyclonal antibody (Alomone Labs, Jerusalem, Israel) (WB: 1:200); anti-caveolin-1 mAb (BD Biosciences Transduction Laboratories, Lexington, KY) (WB: 1:1000); anti-FAK polyclonal antibody (C20; sc-558, Santa Cruz Biotechnology, Santa Cruz, CA) (IP: 2 μg/mg protein; WB: 1:1000); anti-p-Tyr mAb (4G10, Upstate Biotechnology, Lake Placid, NY) (WB: 1:500); anti-p-Tyr397FAK polyclonal antibody (BioSource International, Camarillo, CA) (WB: 1:1000); anti-p-Tyr925FAK polyclonal antibody (Cell Signaling Technology, Beverly, MA) (WB: 1:1000); and anti-Rac mAb (23A8, Upstate Biotechnology) (IP: 2 μg/mg protein, WB: 1:1000).
Secondary antibodies for IF included anti-mouse IgG fluorescein isothiocyanate (FITC)-conjugate (Sigma, 1:200), anti-rabbit IgG Rhodamine B isothiocyanate (RITC)-conjugate antibody (Sigma-Aldrich, St. Louis, MO) (1:200). Secondary antibodies for WB included anti-rabbit peroxidase-conjugate (Sigma-Aldrich) (1:10000) and anti-mouse peroxidase conjugate (Sigma-Aldrich) (1:5000). Secondary antibodies for flow cytometry included anti-mouse IgG FITC-conjugate (Chemicon International, Temecula, CA) (1 μg/106cells).
Production of Anti-hERG1B Antibodies
A specific, ovalbumin coupled, synthetic ERG1B peptide (CRPRAQKGRVRRAVR, a cysteine residue was added to the amino-terminal for conjugating the peptide to ovalbumin) was used for anti-ERG1B antibody production. Antiserum was affinity purified. The specificity of the antibody was tested in Western blot experiments by using hERG1b-HEK 293 and Chinese hamster ovary-transfected cells (Guasti, Cilia, Crociani, Hofmann, Polvani, Becchetti, Wanke, Tempia, and Arcangeli, unpublished data).
Cell Culture and Transfection
SH-SY5Y and HEK 293 cells were routinely cultured in DMEM (Hyclone Laboratories, Logan, UT) supplemented with 10% fetal calf serum (FCS) defined (Hyclone Laboratories) and incubated at 37°C in 10% CO2. HEK-hERG1 and HEK-MOCK stably transfected cells, obtained as described previously (Lastraioli et al., 2004), were cultured in the same medium supplemented with geneticin (G418) (Invitrogen, Carlsbad, CA) at a final concentration of 800 μg/ml. HEK 293 cells stably expressing herg1b gene (HEK-hERG1B cells) and HEK 293 cells stably expressing r-eag (HEK-rEAG) were obtained by transfecting the vector pcDNA3.1-herg1b and pcDNA 3.1-r-eag (gift from by Dr. J. Schwarz, University of Hamburg, Hamburg, Germany), respectively, with LipofectAMINE (Invitrogen). The expression of high levels of both proteins and currents was monitored by Western blot experiments and patch-clamp recordings (see below). For some of the experiments, cells were harvested from freshly seeded preparatory cultures by treating with 0.05% trypsin plus 0.02% EDTA and resuspended in DMEM containing 250 μg/ml heat-inactivated bovine serum albumin (BSA) (DMEM + BSA) (Arcangeli et al., 1993). Cells were then plated on the indicated substrates (see below) and incubated for different times at 37°C. In experiments where the cells were kept in suspension, cells were resuspended in DMEM + BSA and inoculated into 25-cm2 tissue culture flasks. Flasks were kept in vertical position, and cell sedimentation was avoided by continuous stirring at very low speed. For the experiment with the activating monoclonal anti-β1 antibody (TS2/16), cells were resuspended in a solution containing 20 μg/ml antibody in DMEM + BSA and kept in suspension through continuous slow speed stirring for 30 min, at 37°C.
Preparation of Substrates and Coating of Culture Dishes
Heat inactivated BSA was prepared as described previously (Arcangeli et al., 1993). Coating of culture dishes with poly-l-lysine (Sigma-Aldrich) and fibronectin (FN) (BD Biosciences, San Jose, CA) was performed as described in Hofmann et al. (2001).
Treatment with hERG Inhibitors
When needed cells were preincubated for 15 min at 37°C with hERG inhibitors (Way 123,398 and E4031 [Alomone Labs], both 40 μM final concentration) and then plated on the appropriate substrate. Way 123,398 was a gift from Dr. W. Spinelli (Wyeth-Ayerst, Princeton, NJ).
Adhesion Assay
Adhesion assay was performed essentially according to Dabizzi et al. (2003).
Immunofluorescence
Cells were seeded onto glass slides previously coated with fibronectin (20 μg/ml), in DMEM + BSA. After 1 h of incubation, cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min at room temperature. After two washes with PBS, cells were permeabilized for 10 min with 0.01% Triton X-100 in PBS (PBS-Tr) and then blocked in PBS, containing 3% BSA, for 1 h at room temperature. Cells were then incubated 2 h at room temperature with a PBS-Tr solution containing the primary antibodies C54 and TS2/16 (see above for details). After three washes (10 min each) with PBS-Tr, cells were incubated with the appropriate secondary antibodies, diluted in PBS-Tr, for 1 h at room temperature. After three final washes with PBS-Tr, coverslips were mounted using a solution containing Mowiol 4-88 reagent (Calbiochem, San Diego, CA), prepared according to manufacturer's instructions. Cells were analyzed with a TCS SPII scanner (Leica, Wetzlar, Germany), connected to an inverted microscope (Leica). The specificity of each staining was verified by setting two distinct, not overlapping emission wavelengths. Images were processed using LCS software package (Leica). Quantification of the immunofluorescence signal was made using the MetaMorph software (integrated morphometry analysis; Universal Imaging, Downingtown, PA).
Patch-Clamp Recording
Patch-clamp recordings and measurements of hERG current density were performed essentially as reported in Hofmann et al. (2001), except that all experiments were performed in physiological extracellular K+ concentration (5 mM).
Cytofluorimetric Assay
The cytofluorimetric assay of β1 integrin expression on HEK-MOCK and HEK-hERG1 cells was performed essentially according to Dabizzi et al. (2003).
Immunoprecipitation, Immunoblotting, and Reprobing of Membranes
All the following steps were performed keeping the cells in ice: cells were first washed with ice-cold PBS and then recovered from the plates with a rubber policeman and collected in PBS. Cells were immediately extracted with 1% NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris-HCl, pH 8, 5 mM EDTA, 10 mM Na4P2O7) supplemented with a tablet of a complete mix of protease inhibitors (Roche Complete Mini; Roche Diagnostics, Mannheim, Germany). Cell lysates were centrifuged at 13,000 × g for 10 min, and supernatants were collected and assayed for protein concentration with the Bradford protein assay method (Bio-Rad, Hercules, CA). Proteins (1.5–2 mg) were subjected to a preclearing step, consisting of an incubation with protein A- or protein G-Sepharose 4B beads (Sigma-Aldrich) for 2 h at 4°C; thereafter, cell lysates were collected and transferred to fresh tubes and immunoprecipitated with the appropriate antibody (see above) overnight at 4°C. A negative control, consisting of a sample where no primary antibody was added to cell lysate (No-IP), also was performed, to detect nonspecific adherence of proteins to the beads. Proteins bound to the beads were washed three times with lysis buffer and then eluted by boiling the samples in Laemmli buffer, analyzed by SDS-PAGE under reducing conditions, and transferred to a nitrocellulose sheet (Hybond P; Amersham Biosciences, Piscataway, NJ). The membrane was incubated 1–4 h at room temperature with 0.1% Tween 20 in PBS (T-phosphate-buffered saline) containing 5% BSA (T-phosphate-BSA) and then incubated overnight at 4°C with the appropriate primary antibodies at the concentration reported above. Membranes were then washed three times with T-phosphate-buffered saline and incubated with the appropriate secondary antibodies for 45 min at room temperature. In some experiments, the antibody was preincubated with an excess of the antigenic peptide used for immunization. After three washes with T-phosphate-buffered saline, the immunoreactivity was determined by an enhanced chemiluminescent reaction (Super Signal; Pierce Chemical, Rockford, IL). For stripping of membranes the ReBlot WB recycling kit (Chemicon International) was routinely used according to manufacturer's instructions.
Rac1 Activity Assay
This assay is based on the interaction of the GTP-bound GTPase Rac1 to the p21-activated kinase (PAK)-Cdc42/Rac interactive binding (CRIB) domain, and it was performed essentially according to Degani et al. (2002). Briefly, to prepare the glutathione S-transferase (GST)-PAK CRIB domain fusion protein, Escherichia coli DH5α cells transformed with the GST-PAK CRIB domain construct (gift from Prof. Tarone, University of Torino, Torino, Italy) were harvested, lysed in lysis buffer (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 100 mM NaCl, 5% glycerol, 0.1% Triton X-100, 1 mM dithiothreitol, 10 μg/ml leupeptin, 0.4 μg/ml pepstatin, 0.1 trypsin inhibitor unit/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride) and sonicated. The amount of fusion protein in the total lysate was estimated after separation by SDS-PAGE and Coomassie Blue staining. For the assay, the cell lysate supernatant was incubated with glutathione-coupled Sepharose 4B beads (Amersham Biosciences) for 1 h at 4°C. The beads were washed three times in PBS and resuspended in an adequate volume of PBS. HEK-MOCK and HEK-hERG1 cells were detached with trypsin-EDTA, centrifuged, and resuspended in DMEM + BSA. When necessary cells were treated with Way 123,398 or E4031 as described above. Cells were incubated in suspension or onto FN-coated dishes for different times. At the end of incubation, cells were washed in ice-cold PBS, incubated 5 min on ice in lysis buffer (50 mM Tris-HCl, pH 7.4, 2 mM MgCl2, 100 mM NaCl, 10% glycerol, 1% NP-40, mix of protease inhibitors [Roche Complete Mini, Roche Diagnostics]), harvested, and centrifuged for 5 min at 4°C at 16,000 × g. Three milligrams of total lysate for each sample was incubated with GST-PAK CRIB domain bound to glutathione-Sepharose beads at 4°C for 1 h. The beads were washed three times with an excess of lysis buffer, bound proteins were eluted in Laemmli buffer and analyzed in a 15% SDS-PAGE gel, together with 40μg of total lysate for each sample, to compare protein amounts. The membrane was then incubated with an anti-Rac1 antibody to reveal the amount of active Rac1 (in pull-down samples) or total Rac1 (in total lysate samples).
Data Acquisition and Analysis
For each condition, at least three experiments were analyzed separately. In hERG1/β1 coimmunoprecipitation (coIP) experiments, the intensity of both the bands relative to hERG1 was normalized on the intensity of the bands corresponding to total β1 protein. The ratio corresponding to results obtained in suspended or untreated cells was set as one (100%). In the other experiments, the intensity of the bands (p-Tyr-FAK or active Rac) was normalized on the intensity of the bands corresponding to total protein (total FAK or total Rac) in the IP or pull-down (PD). The ratio relative to FN treatment was set as 1 (100%) in the experiments where the effect of Way was tested; the ratio obtained from cells kept in suspension was set as 1 (100%) in the experiments on FAK and Rac1 activity in HEK-MOCK and HEK-hERG1 cells. The results of each experiment were then pooled, analyzed, and graphed.
Microphotographs
Microphotographs of HEK-MOCK and HEK-hERG1 cells seeded on BSA- and FN-coated dishes for 60 min were taken using an inverted microscope (Nikon TS100) equipped with a Nikon D70 camera. Images were acquired through the Nikon Capture 4.1 software.
RESULTS
β1 Integrin and hERG1 Protein Coimmunoprecipitate in Neuroblastoma Cells
As stated in Introduction, we previously demonstrated that β1 integrin subunit engagement by proteins of the ECM leads to an increase in potassium currents specifically carried by the hERG channels (IhERG) in both neuroblastoma (Arcangeli et al., 1993, 1996) and leukemia (Hofmann et al., 2001) cells. This channel activation then switches on differentiation signals in both cell types. We hypothesized that these phenomena could be traced back to the establishment of a macromolecular signaling complex between the β1 integrin subunit and hERG proteins: indeed, we gathered data in neuroblastoma cells, indicating that the β1 integrin subunit coprecipitates with hERG proteins in these cells (Cherubini et al., 2002).
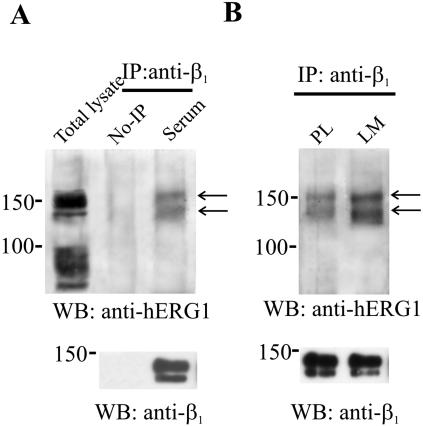
We now show these data relative to the human neuroblastoma cell line (SH-SY5Y cells) (Arcangeli et al., 1996), cultured in standard conditions (i.e., in the presence of FCS (Figure 1A). Cell lysates were subjected to coIP by using an anti-β1 antibody (directed against extracellular epitopes of the protein) to IP and an anti-hERG1 antibody (Lastraioli et al., 2004) for the Western blot. SH-SY5Y cells express heterotetrameric hERG channels composed of both the hERG1 and hERG1B isoforms on the plasma membrane (Crociani et al., 2003). The protein bands visible in Figure 1A (arrows) after coIP show molecular masses of 155 and 135 kDa, suggestive of the full-length hERG1 protein. As reported previously, these two bands indicate the hERG1 protein with various degrees of glycosylation either in the endoplasmic reticulum or on the plasma membrane (Crociani et al., 2003). No visible bands can be detected corresponding to the presumptive molecular mass of the hERG1B protein, i.e., 90 kDa, in the lane corresponding to the total lysate. The same bands also are evident from the coIP obtained from SH-SY5Y cells after adhesion to laminin (LM) shown in Figure 1B. In this experiment, lysates from cells seeded onto polylysine (PL) were taken as controls. In this blot, it also is evident that the amount of both bands of the hERG1 protein that coimmunoprecipitates with β1 is increased after cell adhesion to LM (see Discussion).
Figure 1.
Physical association between the β1 and hERG1 proteins in SH-SY5Y neuroblastoma cells. (A) CoIP between β1 and hERG1 proteins in SH-SY5Y cells cultured in the presence of fetal calf serum (lane serum). CoIP between β1 integrin subunit and hERG1 protein were performed using anti-β1 (TS2/16) antibodies (IP: anti-β1) to IP and anti-hERG1 (C54) (top) or anti-β1 (RM-12) (bottom) antibodies for Western blot. The lane total lysate refers to a Western blot on total lysate of SH-SY5Y cells. The lane no-IP refers to samples where no primary antibody was added to cell lysates, as described in Materials and Methods. Arrows indicate the main immunoprecipitated bands. (B) CoIP between β1 and hERG1 in SH-SY5Y cells seeded onto PL or LM. CoIP conditions are the same as in A.
On the whole, data in Figure 1 suggest that β1 and hERG channels coimmunoprecipitate in neuroblastoma cells and that this coprecipitation is apparently increased after cell adhesion to ECM proteins. Moreover, it is specifically the full-length hERG1 protein that is preferentially associated in the complex with β1.
The Experimental Model: HEK 293 Cells Transfected with the hERG1 Gene
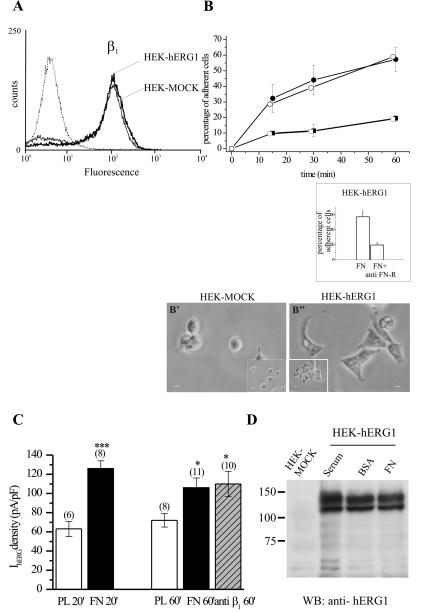
Based on these data, we decided to confirm and define the functional consequences of hERG association with β1 by using a cellular model, consisting of HEK 293 cells stably transfected with the hERG1 gene (HEK-hERG1); HEK cells transfected with the empty vector (HEK-MOCK) were used as a control. HEK-hERG1 cells express hERG channels on the plasma membrane composed of only the full-length hERG1 protein and display large hERG currents. First, we tested whether these cells would be a useful model for mechanistic studies. The results of this analysis are reported in Figure 2. The HEK-hERG1 cells indeed expressed the β1 integrin subunit on their plasma membrane (Figure 2A). No difference in β1 integrin expression was detected between HEK-hERG1 and HEK-MOCK cells. Both cells types adhered to FN to the same extent (Figure 2B); moreover, cell adhesion to FN was impaired by antibodies blocking β1-containing integrins (anti-FN receptor antibodies; Hofmann et al., 2001) (Figure 2B, inset). However, a difference in the extent of cell spreading in the two cell lines could be detected (Figure 2B, microphotographs in inset). In fact, HEK-hERG1 cells seem well spread within 60 min of adhesion onto FN, whereas HEK-MOCK cells remain roughly round or only partially spread.
Figure 2.
Properties of HEK-hERG1 cells. (A) Analysis of β1 surface expression in HEK-hERG1 and HEK-MOCK cells by flow cytometry. Traces relative to cells loaded with anti-β1 antibodies followed by FITC-conjugated secondary antibodies (peaks on the right) and to cells loaded only with the FITC-conjugated secondary antibody (dotted line on the left) are reported. (B) Analysis of HEK-hERG1 (closed symbols) and HEK-MOCK (open symbols) cell adhesion at various times of incubation onto FN (circles) and onto BSA (squares). Inset, effect of anti-β1 integrin antibodies (anti-FN-R) after 60 min of cell adhesion to FN in HEK-hERG1 cells. Microphotographs show the morphology of HEK-MOCK and HEK-hERG1 cells seeded onto FN (B′ and B″, respectively) and on BSA (insets in B′- and B″)-coated dishes. Bars, 10 μm. (C) IhERG density of HEK-hERG1 cells after various times of cell adhesion onto PL, FN, and anti-β1-activating antibodies (TS2/16, 40 μg/ml). IhERG amplitude was measured in whole-cell configuration at –120 mV, after a 15-s preconditioning at 0 mV, and after subtracting traces obtained in the presence of the hERG inhibitor Way 123,398 (Hofmann et al., 2001). Values are means ± SEM of the number of cells analyzed (reported in parentheses). *, significantly different, p < 0.0005; ***, significantly different, p < 0.0001, Student's t test (FN or anti-β1 vs. PL at 60 min and FN vs. PL at 20 min of incubation, respectively). (D) Modulation of hERG1 protein expression by cell adhesion. Proteins were extracted from both HEK-MOCK and HEK-hERG1 cells, and a Western blot was performed on equal amount of protein loaded per lane and visualized by Ponceau staining, as described in Materials and Methods. Serum, cells grown in the presence of fetal calf serum; BSA, cells seeded on BSA-coated dishes for 60 min; FN, cells seeded on FN-coated dishes for 60 min.
Patch-clamp experiments were then performed to test whether hERG channels were activated after integrin-mediated adhesion in the reconstituted system, as found in native systems, endogenously expressing hERG channels (see Introduction). Therefore we measured hERG1 current (IhERG) density in HEK-hERG1 cells seeded on FN at various times of incubation. Cells seeded onto PL were used as controls. PL induces a strong cell adhesion, which is necessary for patch clamping. Results are graphed in Figure 2C. IhERG density increases in HEK-hERG1 cells after 20 min of adhesion to FN, compared with PL-adherent cells. A significant increase in IhERG density also was evident after 60 min of cell adhesion to FN and was elicited by cell adhesion onto anti-β1-activating antibodies (Arcangeli et al., 1996). Finally, the increase in IhERG density turned out to be unrelated to an increased cellular amount of the hERG1 protein, as shown in Figure 2D, where a Western blot of cells seeded on BSA and FN is reported.
On the whole, results reported in Figure 2 allowed us to conclude that HEK-hERG1 cells are a good experimental model, because a β1 integrin-dependent activation of hERG channels occurs in these cells similar to the observations in neuroblastoma and leukemia cells. We also decided that the time window wherein to perform further functional experiments was between 20 and 60 min of cell adhesion.
A β1/hERG1 Complex Occurs in HEK-hERG1 Cells
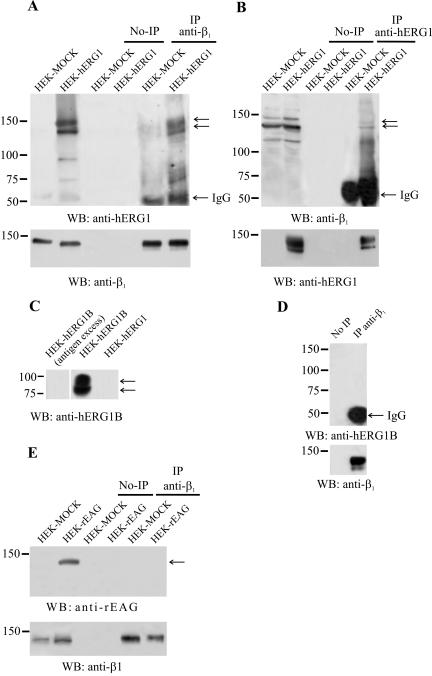
We next analyzed whether hERG1 and β1 proteins were physically associated in HEK-hERG1 cells to form a macromolecular complex. HEK cells transfected with the empty vector (HEK-MOCK) were used as a control in these experiments. CoIP experiments were performed on cells cultured in DMEM supplemented with 10% FCS (Figure 3), by using the same antibodies described in Figure 1A, and an anti-hERG N-terminal antibody to immunoprecipitate (Crociani et al., 2003), along with an anti-β1 antibody (directed against the cytoplasmic tail of the protein) on the Western blot (Figure 3B). Reprobing of the membrane with the antibody used to immunoprecipitate also was performed and is reported in Figure 3, A and B, bottom. Both experiments indicated that the two proteins indeed coassociate in HEK-hERG1 cells in standard culture conditions. The same results were obtained performing IPs on total protein lysates as well as on membrane extracts (our unpublished data). It also is evident from Figure 3A that two bands of the hERG1 protein, at 155 and 135 kDa (arrows) coimmunoprecipitate with β1. Also, the anti-hERG1 IP contained both the precursor and mature form of the β1 (see arrows). Therefore, these data suggest that the coassociation of hERG1 protein with β1 occurs also in the endoplasmic reticulum and hence, the two proteins interact throughout trafficking of the protein toward the plasma membrane.
Figure 3.
Physical association between hERG1 and β1 integrin proteins in HEK-hERG1 cells. HEK-hERG1 and HEK-MOCK cells were cultured in the presence of fetal calf serum: IP and WB were performed as described in Materials and Methods. No-IPs refers to samples where no primary antibody was added to cell lysates, as described in Materials and Methods. (A and B) CoIP between β1 integrin subunit and hERG1 protein. (A) Proteins were extracted from HEK-hERG1 and HEK-MOCK cells and immunoprecipitated using anti-β1 (TS2/16) antibodies (IP anti-β1), followed by Western blotting with anti-hERG1 antibodies (C54) (top) or anti-β1 antibodies (RM-12) (bottom). (B) Proteins extracted from HEK-hERG1 and HEK-MOCK cells were immunoprecipitated using anti-hERG1 antibodies (N135) (IP anti-hERG1), followed by Western blotting with either anti-β1 antibodies (RM-12) (top) or anti-hERG1 antibodies (C54) (bottom). The first two lanes in A and B refer to Western blots on total lysates of both HEK-MOCK and HEK-hERG1 cells. (C) Immunoreactivity of the anti-hERG1B antibody. Proteins were extracted from HEK-hERG1B and HEK-hERG1 cells. The anti-hERG1B antibody reveals two bands in HEK-hERG1B cells, with a predicted molecular mass of 90 and 80 KDa. A negative control was performed, by preincubating the antibody with an excess of the antigen, on HEK-hERG1B cell lysate. (D) CoIP between β1 integrins and hERG1B proteins performed on HEK-hERG1B cells. Cell lysates were immunoprecipitated using anti-β1 (TS2/16) antibodies (IP anti-β1), followed by Western blotting with either anti-hERG1B antibodies (top) or anti-β1 antibodies (RM-12) (bottom). (E) CoIP between β1 integrins and rEAG protein. Proteins extracted from HEK-MOCK and HEK-rEAG cells were immunoprecipitated using anti-β1 (TS2/16) antibodies (IP anti-β1), followed by Western blotting with either anti-rEAG antibodies (top) or anti-β1 antibodies (RM-12) (bottom). No-IP, no immunoprecipitation; IgG, IgG bands represent the primary antibody from the initial immunoprecipitation reacting with the antibody used to visualize the Western blot.
Next, we tested whether β1 integrins associate with other channel proteins, belonging to the same K+ channel family. Specifically, we tested whether the splice variant hERG1B (Crociani et al., 2003) as well as the rEAG protein (Warmke and Ganetzky, 1994) coIP with the β1 integrin subunit. For this purpose, HEK cells stably transfected with hERG1B (HEK-hERG1B) or rEAG (HEK-rEAG) were used. As shown in Figure 3, C–E, neither hERG1B nor rEAG channels coIP with β1.
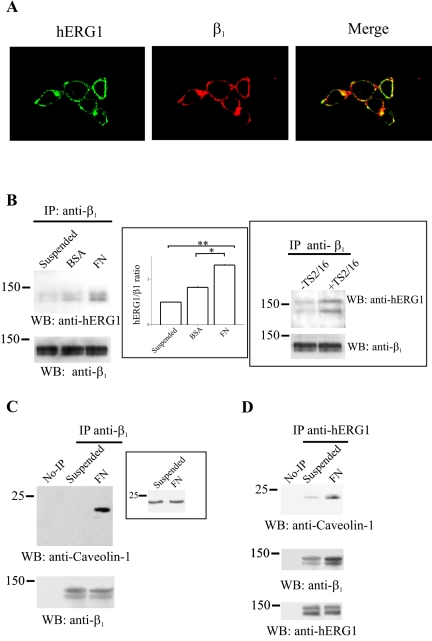
The localization of the β1/hERG1 complex was then studied by immunofluorescence experiments (Figure 4A). Cells were plated onto FN for 1 h, fixed, and labeled with both anti-hERG1 and anti-β1 antibodies; photographs were then taken with a confocal microscope and merged. HEK-MOCK cells were used as a control (our unpublished data). It seems that the localization of the two proteins partially overlaps in HEK-hERG1 cells, confirming results obtained in the coIP experiments reported in Figure 3, A and B (see above). Colocalization occurs only in the membrane surface compartment, where the two proteins are mainly expressed. Interestingly, the colocalization is restricted to specific compartments, suggesting that adhesion to FN might regulate membrane composition and association of β1 integrin to hERG1. A quantitative analysis of confocal images, using MetaMorph software (integrated morphometry analysis; Universal Imaging), showed that 18% (±2, n = 12) of the hERG1 protein and of β1 integrin colocalize in HEK-hERG1 cells.
Figure 4.
Characterization of the β1/hERG1 complex in HEK-hERG1 cells. (A) Colocalization of hERG1 and β1 proteins. Double immunofluorescence was performed on HEK-hERG1 cells seeded onto glass slides coated with 20 μg/ml FN in DMEM + BSA, by using anti-hERG1 antibodies (C54) and a FITC-conjugated secondary antibody (hERG1) and anti-β1 antibodies (TS2/16) and a RITC-conjugated secondary antibody (β1). Photographs were taken with a confocal microscope and merged (merge). A quantitative analysis of confocal images, using the MetaMorph Software (integrated morphometry analysis; Universal Imaging) showed that 18% (±2, n = 12) of the hERG1 protein and of β1 integrin colocalize in HEK-hERG1 cells. (B) CoIP of β1 and hERG1 proteins depends on β1 activity. CoIP experiments were performed on cells kept in suspension (lane suspended) or seeded either onto BSA- (lane BSA)- or FN (lane FN)-coated dishes for 30 min, by using anti-β1 antibodies (TS2/16) to immunoprecipitate and anti-hERG1 antibodies for the Western blot. Reprobing with anti-β1 antibodies also is reported in the bottom panel. Inset (center), densitometric analysis of the amount of hERG1 protein that coimmunoprecipitates with β1 after cell adhesion on different substrates. The analysis was performed as described in Materials and Methods; data are the means ± SEM of three separate experiments. *, significantly different p = 0.00016, Student's t test (FN adherent cells vs. BSA adherent cells); **, significantly different p = 0.0000016, Student's t test (FN adherent cells vs. suspended cells). Inset (right), HEK-hERG1 cells were kept in suspension and treated (lane +TS2/16) or not (lane –TS2/16) for 30 min with activating monoclonal anti-β1 antibodies as described in Materials and Methods. CoIP experiments were performed as described above. (C and D) Colocalization of β1 with caveolin-1 and hERG1 proteins. Proteins were extracted from HEK-hERG1 cells kept in suspension (lane suspended) or seeded onto FN (lane FN)-coated dishes for 30 min and immunoprecipitated using anti-β1 antibodies (TS2/16); no-IPs refer to samples where no primary antibody was added to cell lysates, as described in Materials and Methods. Bands were revealed using anti-caveolin-1 antibodies. Reprobing with anti-β1 antibodies also is reported at the bottom. Inset, total cell lysate obtained from cells treated as in C probed with anti-caveolin-1 antibodies. (D) CoIP of hERG1, caveolin-1 and β1 proteins. Proteins were extracted from HEK-hERG1 cells kept in suspension (lane suspended) or seeded onto FN-coated dishes for 30 min (lane FN), and immunoprecipitated using anti-hERG1 antibodies (N135); bands were revealed using anti-caveolin-1 antibodies (top), anti-β1 antibodies (middle), and anti-hERG1 antibodies (C54) (bottom). All the panels in this figure are representative of at least three different experiments, except for TS2/16 treatment (inset to B, right), which was a single experiment. No-IP, no immunoprecipitation.
We next evaluated whether the β1/hERG1 complex formation was dependent on β1 activation. For this purpose, coIP experiments were performed on HEK-hERG1 cells kept in suspension or seeded for 30 min onto either BSA or FN (Figure 4B). The β1/hERG1 complex is observed at low levels when cells are kept in suspension, increases when cells sediment onto BSA, and almost doubles when adhesion occurs on FN (also see densitometric analysis in inset). Moreover, β1/hERG1 complex formation is stimulated when β1 integrins are engaged by an activating mAb (TS2/16). In fact, HEK-hERG1 cells kept in suspension in the presence of the antibody for 30 min show a higher degree of hERG1 protein that coimmunoprecipitates with β1 with respect to untreated cells (Figure 4, inset on right).
We also tested whether hERG1 and β1 proteins were localized in specific membrane domains. Integrins have been reported to colocalize with caveolin-1, a protein that contributes to the formation of those membrane microdomains known as caveolae/rafts. Furthermore, coclustering with caveolin-1 seems to be critical to β1 integrin signaling (see Introduction). Therefore, we tested whether β1 associates with caveolin-1 in HEK-hERG1 cells. In Figure 4C, HEK-hERG1 cells kept in suspension or seeded onto FN were lysed and immunoprecipitated with an anti-β1 antibody and then probed with an anti-caveolin-1 antibody. It is evident that caveolin-1 only coimmunoprecipitates with β1 when HEK-hERG1 cells are seeded onto FN. On the other hand, the total amount of caveolin-1 does not change during cell adhesion (Figure 4C, inset). We next asked whether the hERG1 protein also coimmunoprecipitates with caveolin-1 and β1 in FN seeded cells; therefore, a coIP experiment was performed using anti-hERG1 antibodies to immunoprecipitate and anti-caveolin-1 and anti-β1 antibodies to reveal the blot. It can be seen (Figure 4D) that hERG1 and caveolin-1 coprecipitate in HEK-hERG1 cells, particularly after cell adhesion onto FN, and that also the β1 integrin is present in the IP.
On the whole, data presented in Figures 3 and 4 show that β1 integrin subunit forms a molecular complex with the hERG1 protein in HEK-hERG1 cells and that the two proteins partially colocalize. Moreover, complex formation seems to be dependent on β1 integrin engagement and activation. The complex also comprises caveolin-1, a protein that is a mediator between integrins and cytoplasmic, signaling proteins in several experimental systems.
hERG1 Channels Modulate Adhesion-Dependent Signaling Pathways: FAK
The presence of hERG1 in a complex with β1 and caveolin-1 suggested to us that the activation of hERG currents could have a potential role in integrin-dependent signaling. In other words, we hypothesized that the activation of hERG currents (Figure 2D), after engagement of β1 integrin by cell adhesion, could somehow be involved in the modulation of adhesion-dependent signaling. This hypothesis also was supported by our previous finding that K+ currents can modulate FAK phosphorylation in neuroblastoma cells after cell adhesion to FN (Bianchi et al., 1995). To test our hypothesis we first looked at FAK expression and activation in HEK-hERG1 cells after adhesion to FN. In these experiments, HEK-MOCK cells were used as controls. The role of hERG channels was analyzed using specific inhibitors of hERG currents. In particular, a specific IhERG inhibitor, namely, Way 123,398 (Way) (Lastraioli et al., 2004) was used. Patch-clamp experiments had shown that Way inhibited IhERG in HEK-hERG1 cells in a dose-dependent manner, with the 40 μM concentration giving 99.5 ± 0.3% inhibition. Therefore, we decided to use this Way concentration in our inhibition experiments.
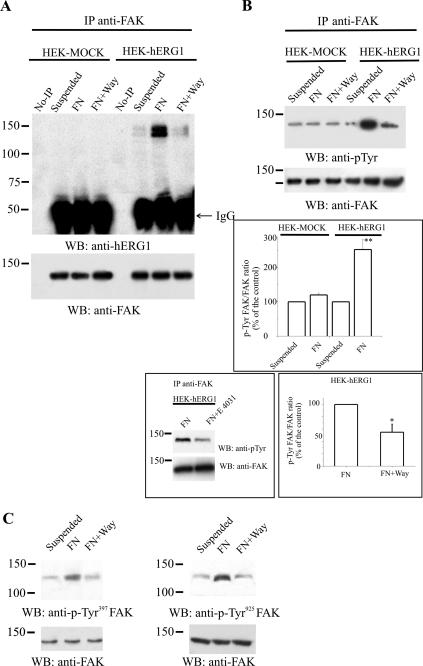
We first tested whether FAK is associated with hERG1 before and after engagement of integrin receptors during cell adhesion. FAK was immunoprecipitated from HEK-hERG1 (as well as HEK-MOCK) cells kept in suspension or after cell adhesion to FN, and the presence of the hERG1 protein in the FAK IP was tested by Western blot analysis. As shown in Figure 5A, hERG1 does coimmunoprecipitate with FAK only in HEK-hERG1 cells (no hERG1 band was detected in the IP from HEK-MOCK cells), especially after cell adhesion to FN. Interestingly, formation of the FAK/hERG1 complex is drastically inhibited by the specific IhERG inhibitor Way. Reprobing with anti-FAK antibodies (lower part of A) ruled out that the Way-dependent effect was due to a different amount of immunoprecipitated FAK.
Figure 5.
Recruitment and modulation of FAK phosphorylation by hERG channel activity in HEK-hERG1 cells. (A) CoIP between FAK and hERG1 protein. Proteins were extracted from HEK-hERG1 and HEK-MOCK cells incubated for 60 min in suspension or seeded onto FN-coated dishes, the latter in the absence or in the presence of Way 123,398 (FN+Way). Proteins were immunoprecipitated using anti-FAK antibodies (C20). No-IPs refers to samples where no primary antibody was added to cell lysates, as described in Materials and Methods. Western blotting was performed and the bands visualized using either anti-hERG1 antibodies (C54) (top) or anti-FAK antibodies (C20) (bottom). (B) Effects of cell adhesion and Way addition on FAK phosphorylation. Proteins were extracted from HEK-MOCK and HEK-hERG1 cells, kept in suspension, or seeded onto FN in the absence or in the presence of Way and then were immunoprecipitated using anti-FAK antibodies (C20) followed by Western blotting with anti-p-Tyr antibodies. Data refer to cells seeded onto FN for 60 min, because the phosphorylation of FAK in HEK-hERG1 cells turned out to be very low at incubation times before 60 min (our unpublished data). Reprobing with anti-FAK antibodies is shown in the lower panels. Inset (top), densitometric analysis of FAK phosphorylation in HEK-MOCK and HEK-hERG1 cells kept in suspension or seeded onto FN. The analysis was performed as described in Materials and Methods; data are means ± SEM of three separate experiments. **, significantly different p = 0.04036, Student's t test (FN adherent HEK-hERG1 cells vs. FN adherent HEK-MOCK cells). Note that results obtained on FN adherent HEK-hERG1 and HEK-MOCK cells were both significantly different compared with the relative controls, i.e., suspended cells (our unpublished data). Inset (left bottom), effect of E4031 on FAK phosphorylation; experimental conditions were the same as in B. Inset (right bottom), densitometric analysis of FAK phosphorylation in HEK-hERG1 cells seeded onto FN in the absence or in the presence of Way. The analysis was performed as described in Materials and Methods; data are means ± SEM of three separate experiments. *, significantly different p = 0.0096, Student's t test (FN adherent HEK-hERG1 cells in the presence of Way vs. FN adherent cells). (C) Effects of cell adhesion and Way addition on FAK phosphorylation of residues Y397 and Y925. Proteins extracted from HEK-hERG1 cells treated as described above were separated by SDS-PAGE, and bands were revealed using anti-pTyr-397 FAK antibodies (left) or anti-pTyr-925 FAK antibodies (right). Reprobing of the membranes with anti-FAK antibodies is reported in the bottom panels. No-IP, no immunoprecipitation; IgG, IgG bands, see legend to Figure 3.
We then analyzed whether the adhesion-dependent activation of hERG currents was determinant for FAK phosphorylation and therefore FAK activation. The amount of phosphorylated FAK in HEK-MOCK and HEK-hERG1 cells kept in suspension or after cell adhesion onto FN was tested by immunoprecipitating cellular proteins with an anti-FAK antibody and probing with an anti-p-Tyr antibody. As shown in Figure 5B, the phosphorylation of FAK was higher in HEK-hERG1 cells adherent onto FN compared with suspended cells (see also the inset to B, top). Moreover, such increase in FAK phosphorylation was significantly inhibited by Way addition. When the same experiment was performed on HEK-MOCK cells, it was evident that FAK phosphorylation was lower in the cells adherent onto FN compared with HEK-hERG1 cells and was not inhibited by Way addition (B, lanes on the left). Another inhibitor of hERG currents, E4031, of different chemical structure compared with Way, displayed the same inhibitory effect on FAK phosphorylation in HEK-hERG1 cells (inset to B, left bottom). The average of three separate experiments indicated that FAK phosphorylation in HEK-hERG1 cells was 50% inhibited by Way (inset to B, right bottom). On the whole, it can be concluded that FAK phosphorylation is substantially dependent on hERG channel expression and activity in HEK-hERG1 cells.
The FAK tyrosine residues whose phosphorylation was dependent on hERG activity were therefore studied in HEK-hERG1 cells. Both anti-pTyr-397FAK and anti-pTyr-925FAK antibodies were used and tested on cells kept in suspension or adherent to FN, the latter in the absence or in the presence of hERG blockers. As in Figure 5C, FAK was phosphorylated in residue 397 after 60 min of adhesion to FN, and the phosphorylation on this tyrosine residue was impaired by hERG current inhibition with Way. E4031 exerted the same inhibitory effect (our unpublished data). Also FAK phosphorylation on tyrosine residue 925 was inhibited by the hERG channel blocker Way (panel C). Reprobing of the membranes with anti-FAK antibodies shows that no change occurred in the total amount of FAK protein by the various cell treatments.
Together, these data indicate that hERG1 channels, once activated by β1 integrin-mediated adhesion, recruit FAK into a macromolecular complex. Hence, activated hERG channels modulate both the adhesion-dependent autophosphorylation of FAK in residue 397 and the Src-dependent FAK phosphorylation in residue 925.
hERG1 Channels Modulate Adhesion-dependent Signaling Pathways: Rac 1 GTPase
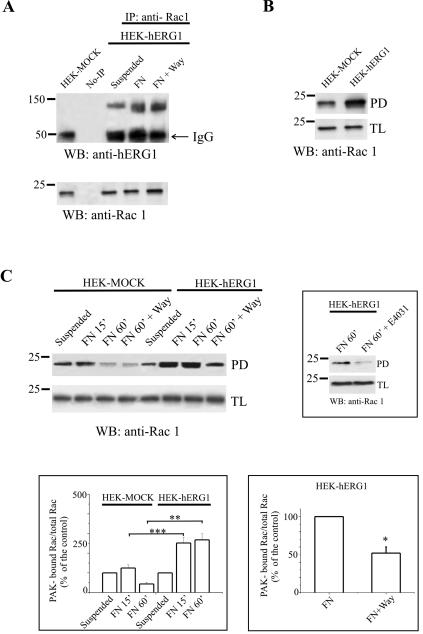
Another relevant pathway downstream to integrin activation is that leading to modulation of small GTPases of the Rho family and thus to the control of cell motility. We therefore tested whether small GTPases also were complexed with the hERG1 protein in HEK-hERG1 cells. Proteins from HEK-hERG1 cells kept in suspension or seeded onto FN were immunoprecipitated with anti-Rho or anti-Rac1 antibodies, respectively, and the IP was probed with anti-hERG1 antibodies. HEK-MOCK were used as a control. Although hERG1 did not coprecipitate with RhoA (our unpublished data), a significant amount of hERG1 coIP with Rac1 in HEK-hERG1 cells (Figure 6A). In contrast to FAK, the Rac1/hERG1 association also took place in suspended cells, although to a lower extent compared with FN-adherent cells and was not affected by inhibition of hERG channels by Way. This result suggests a partially constitutive association of the two proteins (see Discussion). No variation in the total amount of the Rac1 protein in the total lysate was detected in suspended versus FN-adherent cells.
Figure 6.
Physical and functional interaction between hERG1 channels and Rac1 GTPase in HEK-hERG1 cells. (A) Physical interaction between hERG1 channels and Rac1 GTPase in HEK-hERG1 cells. Proteins extracted from either HEK-hERG1 cells kept in suspension or incubated for 60 min onto FN-coated dishes in the absence or in the presence of Way, or from HEK-MOCK cells seeded onto FN, were immunoprecipitated using anti-Rac1 antibodies, and bands were visualized using anti-hERG1 antibodies (C54). No-IPs refers to samples where no primary antibody was added to cell lysates, as described in Materials and Methods. Reprobing with anti-Rac1 antibodies is reported in the panel on the bottom. (B) Basal level of Rac1 activity in HEK-MOCK and HEK-hERG1 cells: samples derive from a pull-down of HEK-MOCK and HEK-hERG1 total lysates by using a GST-PAK-CRIB Domain fusion protein; the membrane was then incubated with an anti-Rac1 antibody; the amount of Rac1 protein in the total lysate is shown as a control (bottom). Data reported in this panel are representative of three different experiments performed. (C) Modulation of Rac1 activity in HEK-MOCK and HEK-hERG1 cells in various experimental conditions: suspended (lane suspended), incubated for 15 or 60 min on FN-coated dishes (lanes FN 15′ and FN 60′, respectively) or on FN-coated dishes in the presence of the hERG inhibitor Way (lane FN 60′+Way). The amount of Rac1 protein in the total lysate is reported on the bottom. Inset (top), effect of E4031 on Rac1 activity of HEK-hERG1 cells seeded onto FN for 60 min; protein extraction and measurement of Rac1 activity was performed as described above. Inset (left bottom), densitometric analysis of Rac-1 activity in HEK-MOCK and HEK-hERG1 cells treated as reported above. The analysis was performed as described in Materials and Methods; data are means ± SEM of three different experiments. Inset (right bottom), densitometric analysis of Rac1 activity in HEK-hERG1 cells seeded onto FN in the absence (bar on the left) or in the presence of Way (bar on the right). The analysis was performed as described in Materials and Methods; data are means ± SEM of three different experiments. *, significantly different, p = 0.0229, Student's t test (FN adherent HEK-hERG1 cells in the presence of Way vs. FN adherent cells). **, significantly different, p = 0.0019, Student's t test (60 min FN adherent HEK-hERG1 cells vs. 60 min FN adherent HEK-MOCK cells); ***, significantly different, p = 0.0117 Student's t test (15 min FN adherent HEK-hERG1 cells vs. 15 min FN adherent HEK-MOCK cells). No-IP, no immunoprecipitation; TL, total lysate.
We subsequently focused our attention to the Rac1 GTPase and on the modulation of its activity. Rac1 activity was tested by measuring the amount of Rac1 bound to a fusion protein containing the PAK/CRIB domain, as described in Degani et al. (2002) (see Materials and Methods). Again, the putative modulation of Rac1 activity by hERG1 channels was tested by using hERG-specific inhibitors in HEK-hERG1 and HEK-MOCK cells. Rac1 turned out to be substantially more active in HEK-hERG1 cells compared with HEK-MOCK cells under basal conditions (Figure 6B). Furthermore, we determined the modulation of Rac1 after cell adhesion to FN. As shown in Figure 6C, Rac1 activity was switched on early in cells seeded onto FN compared with cells kept in suspension. After 60 min of cell adhesion to FN, Rac1 activity further increased only in HEK-hERG1 cells, while falling to below basal levels in HEK-MOCK cells (also see inset to C, left bottom). Interestingly, Rac1 activity was significantly reduced in HEK-hERG1 cells treated with hERG blockers Way and E4031 (inset to C, top). This inhibitory effect occurred only in HEK cells expressing hERG channels; it was absent in HEK-MOCK (Figure 6C). The average of the densitometric analysis performed on three separate experiments (inset to Figure 6C, right bottom) indicates that Rac1 activity is inhibited by 50% after blocking hERG currents. Together, these data indicate that the activity of the small GTPase Rac1 also is modulated by the expression and the activity of the hERG1 channels.
DISCUSSION
Integrin receptors are key regulators of cell adhesion to the ECM as well as to counterreceptors on heterotypic cells (Hynes, 1992). In addition to modulating the actin cytoskeleton, integrins mediate numerous signaling pathways that in turn regulate cell growth, survival, differentiation, and motility (Giancotti and Ruoslahti, 1999). The activation of ion channels can be included in the integrin-dependent signaling pathways (Davis et al., 2002). The β1-containing integrins can specifically activate potassium channels of the hERG family, resulting in the control of neurite extension in neuroblastoma cells (Arcangeli et al., 1993, 1996) and osteoclastic differentiation in leukemia cells (Hofmann et al., 2001). These observations suggested that hERG channels may represent a key step in integrin-regulated downstream signaling. Using a reconstituted cellular model (HEK-hERG1 cells), we show here that β1 integrin subunits and hERG channels can form a complex and that the adhesion dependent-, integrin-mediated hERG current activation is capable of modulating integrin signaling, i.e., FAK phosphorylation and Rac1 activation.
A complex between the β1 integrin subunit and hERG proteins occurs in cells endogenously expressing hERG channels (neuroblastoma cells) and in reconstituted systems (HEK 293 stably transfected with herg1 gene) and is modulated upon cell adhesion. Two hERG1 bands, with an apparent molecular mass of 155 and 135 kDa and corresponding to various degree of glycosylation of the protein, coimmunoprecipitate with β1, in both cell types. The coimmunoprecipitation of both bands is increased after adhesion to LM and FN, respectively. This observation cannot be traced back to a nonspecific adherence of the channel to the beads (see the control lane “no-IP” in Figures 1, 3, and 4). We can, therefore, conclude that even a partially glycosylated form of the channel protein of lower molecular mass can reach the plasma membrane, giving rise to functional hERG channels (as recently reported by Gong et al., 2002) and coassemble with β1. The same conclusion can be applied to the coIP of the hERG1 protein with FAK (Figure 5) and Rac1 (Figure 6) (see below).
Using HEK-hERG1 cells, we show that a complex forms between β1 and the full-length hERG1 protein. A physical association between β1 integrin and G protein-gated inwardly rectifying K+ (GIRK) channels was reported previously (McPhee et al., 1998); the integrin-channel binding can occur directly through the conserved amino acid sequence Arg-Gly-Asp (RGD) present on the GIRK protein. A physical and functional association between β1 integrins and Kv 1.3 channels was described in T-lymphocytes (Levite et al., 2000) and melanoma cells (Artym and Petty, 2002). In this case, it was hypothesized that other membrane components could link the two molecules. In our experiments the molecular determinant for linking the hERG protein to integrins is likely the N terminal of the hERG1 protein. Evidence for this is our observation that the β1 integrin does not coprecipitate with the hERG1B protein, which differs from the hERG1 full-length protein only in the N terminus as well as with rEAG protein (Figure 3, C–E).
In our experimental model, the β1/hERG1 complex also includes caveolin-1, suggesting its localization in specific membrane microdomains, i.e., caveolae/lipid rafts. Caveolae are specific detergent-resistant microdomains that contribute to an alternative endocytic pathway and also seem to act as organized transducing centers that concentrate key signaling molecules (Nabi and Le, 2003; Carver and Schnitzer, 2003; Lai, 2003). Integrins are often recovered with caveolin-1 and therefore can be recruited into membrane lipid rafts (Wary et al., 1996; Baron et al., 2003). The functional relevance of integrin association with caveolin-1 in lipid rafts is not known, although it is clear that such recruitment could regulate/modulate integrin signaling pathways (Lai, 2003). Our data suggest that integrins could also contribute to localize hERG channels into caveolae (Figure 4D).
The colocalization of hERG1 with integrins and caveolin-1 suggests a possible explanation to the still unsolved question of how integrins activate hERG channels; the targeting of the channel protein into caveolin-rich membrane domains, where many signaling proteins are concentrated, could bring the hERG1 protein close to those molecules known to regulate hERG channel activity (reviewed in Thomas et al., 2003).
Furthermore, the colocalization of hERG channels with caveolin-1 further reinforces the main message emerging from data reported here: that hERG channels play a modulating role in integrin signaling. This conclusion is supported by data showing that the hERG1 protein coprecipitates with FAK after cell adhesion to FN and that both FAK recruitment and FAK phosphorylation are dependent on hERG current activity. These results confirm and reinforce what has been suggested to occur in neuroblastoma cells (Bianchi et al., 1995). FAK phosphorylation on both Tyr397 and Tyr925 residues also was dependent on hERG current activity suggesting that Src-dependent FAK phosphorylation relies on hERG current activation.
We also show here that hERG channel activity is a modulating factor for Rac1 activation. Not only is Rac1 more active in HEK-hERG1 compared with HEK-MOCK cells but also the FN induced increase in Rac1 activity was inhibited by hERG blockers. This effect could rely on the link between Rac1 and the hERG1 protein (Figure 6). Indeed, the targeting of Rac1 to the plasma membrane, in particular to caveolae/lipid rafts, is an important prerequisite for Rac1 activation (del Pozo et al., 2004).
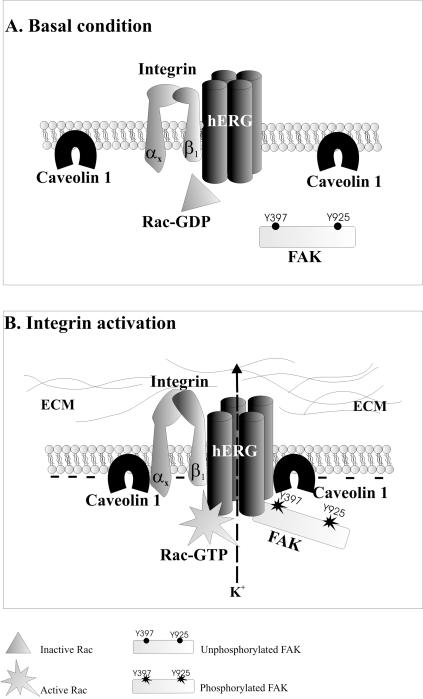
How do hERG channels regulate integrin signaling? Although our understanding is far from being complete, we favor a model depicted in Figure 7. A complex containing β1 integrins and hERG1 channels is physically linked on the plasma membrane; when integrins are activated by cell adhesion to the ECM, they coassemble with caveolin-1, along with hERG channels that are then activated. Meanwhile, signaling proteins (FAK) are complexed with the channel protein in a channel activity-dependent manner. In addition, the signaling molecule Rac1, likely constitutively linked to the channel protein, starts to activate its downstream effectors. Signaling molecules are switched on as the conducting activity of hERG channels increases. The trigger for the signaling pathway might be either the conformational change of the channel protein (dependent upon its activation) or the current flow itself (Olivotto et al., 1996). Currently, it is not possible to distinguish between these two possibilities. As far as we are aware, all the known hERG inhibitors block the ion conduction pathway. Therefore, this problem must be approached by different techniques. Finally, we have focused on hERG channels, Because only this type of channel was activated by integrins in neuroblastoma and leukemia cells (Arcangeli et al., 1996; Hofmann et al., 2001), but, in principle, our model can be applied to any other potassium channel able to associate with integrin receptors.
Figure 7.
Model for regulation of β1 integrin signaling by hERG channel activity. After integrin engagement by the ECM, the β1 subunit, linked to the hERG1 protein, coassembles with caveolin-1. hERG channels are thereby activated, changing their conformation and leading to a hyperpolarization of the membrane. This channel activity induces the recruitment of FAK to hERG1 protein and their subsequent tyrosine phosphorylation of FAK and activation of Rac1.
Last, the modulation of integrin-dependent signaling by hERG channels likely has important implications in the regulation of neurite extension in neuronal cells that express hERG channels on their membrane (Arcangeli et al., 1993, 1996; Bianchi et al., 1995; Chiesa et al., 1997; Gullo et al., 2003; Sacco et al., 2003). This process is apparently regulated by various tyrosine kinases, including FAK (Ivankovic-Dikic et al., 2000), as well as by small GTPases, like Rac1 (reviewed in Luo, 2000). The hERG-dependent Rac1 activation also may represent a necessary step for the activation of a motility and invasive program in hERG-expressing tumor cells, giving a molecular dimension to our previous demonstration that hERG overexpression in cancer cells is responsible for the acquisition of a more invasive, and thus more malignant, phenotype (Lastraioli et al., 2004).
Acknowledgments
The revision of the manuscript by Dr. M. Sanguinetti (University of Utah, Salt Lake City, UT) is acknowledged. We also thank Dr. G. Mugnai, for suggestions on experiments and comments on the manuscript and Dr. S. Polvani for technical assistance in double immunofluorescence analysis. This work was supported by grants from the Telethon Fondazione Onlus (project no. GGP02208 to A. A.), Ministero dell'Università e Ricerca Scientifica e Tecnologica (Cofin '01 and Cofin '03, and FAR 04 to A. A.), Associazione Italiana per la Ricerca sul Cancro (to A. A.), FAR04 (to A. B.), and the Oklahoma Center for the Advancement of Science and Technology (HR01-151 to R.W. A. C. and L. G. are Ph.D. students in experimental and clinical oncology, G. H. is a fellow of the Fondazione Italiana per la Ricerca sul Cancro, S. P. is a fellow of the Associazione Italiana contro le Leucemie (Firenze), and E. C. is a fellow of Telethon Fondazione Onlus.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-10-0940) on March 30, 2005.
Abbreviations used: ECM, extracellular matrix; FAK, focal adhesion kinase; FN, fibronectin; GIRK, G protein-gated inwardly rectifying K+; hERG1, human ether-a-go-go-related gene 1; IF, immunofluorescence; IP, immunoprecipitation(ed); p-Tyr, phosphotyrosine; WB, Western blot.
References
- Arcangeli, A., Becchetti, A., Mannini, A., Mugnai, G., De Filippi, P., Tarone, G., Del Bene, M. R., Barletta, E., Wanke, E., and Olivotto, M. (1993). Integrin-mediated neurite outgrowth in neuroblastoma cells depends on the activation of potassium channels. J. Cell Biol. 122, 1131-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli, A., Faravelli, L., Bianchi, L., Rosati, B., Gritti, A., Vescovi, A., Wanke, E., and Olivotto, M. (1996). Soluble or bound laminin elicit in human neuroblastoma cells short- or long-term potentiation of a K+ inwardly rectifying current: relevance to neuritogenesis. Cell Adhes. Commun. 4, 369-385. [DOI] [PubMed] [Google Scholar]
- Artym, V. V., and Petty, H. R. (2002). Molecular proximity of Kv1.3 voltage-gated potassium channels and beta(1)-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. J. Gen. Physiol. 120, 29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, W., Decker, L., Colognato, H., ffrench-Constant, C. (2003). Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr. Biol. 13, 151-155. [DOI] [PubMed] [Google Scholar]
- Bauer, C. K., and Schwarz, J. R. (2001). Physiology of EAG K+ channels. J. Membr. Biol. 182, 1-15. [DOI] [PubMed] [Google Scholar]
- Bianchi, L., Arcangeli, A., Bartolini, P., Mugnai, G., Wanke, E., and Olivotto, M. (1995). An inward rectifier K+ current modulates in neuroblastoma cells the tyrosine phosphorylation of the pp125FAK and associated proteins: role in neuritogenesis. Biochem. Biophys. Res. Commun. 210, 823-829. [DOI] [PubMed] [Google Scholar]
- Brown, E. J. (2002). Integrin-associated proteins. Curr. Opin. Cell Biol. 14, 603-607. [DOI] [PubMed] [Google Scholar]
- Burridge, K., and Chrzanowska-Wodnicka, M. (1996). Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463-518. [DOI] [PubMed] [Google Scholar]
- Carver, L. A., and Schnitzer, J. E. (2003). Caveolae: mining little caves for new cancer targets. Nat. Rev. Cancer 3, 571-581. [DOI] [PubMed] [Google Scholar]
- Cherubini, A., et al. (2002). HERG K+ channels and beta1 integrins interact through the assembly of a macromolecular complex. Ann. N.Y. Acad. Sci. 973, 559-561. [DOI] [PubMed] [Google Scholar]
- Cherubini, A., et al. (2000). HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. Br. J. Cancer 83, 1722-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa, N., Rosati, B., Arcangeli, A., Olivotto, M., and Wanke, E. (1997). A novel role for HERG K+ channels: spike-frequency adaptation. J. Physiol. 501, 313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crociani, O., Guasti, L., Balzi, M., Becchetti, A., Wanke, E., Olivotto, M., Wymore, R. S., and Arcangeli, A. (2003). Cell cycle-dependent expression of HERG1 and HERG1B isoforms in tumor cells. J. Biol. Chem. 278, 2947-2955. [DOI] [PubMed] [Google Scholar]
- Dabizzi, S., Noci, I., Borri, P., Borrani, E., Giachi, M., Balzi, M., Taddei, G. L., Marchionni, M., Scarselli, G. F., and Arcangeli, A. (2003). Luteinizing hormone increases human endometrial cancer cells invasiveness through activation of protein kinase A. Cancer Res. 63, 4281-4286. [PubMed] [Google Scholar]
- Davis, M. J., Wu, X., Nurkiewicz, T. R., Kawasaki, J., Gui, P., Hill, M. A., and Wilson, E. (2001). Regulation of ion channels by protein tyrosine phosphorylation. Am. J. Physiol. 281, H1835-H1862. [DOI] [PubMed] [Google Scholar]
- Davis, M. J., Wu, X., Nurkiewicz, T. R., Kawasaki, J., Gui, P., Hill, M. A., and Wilson, E. (2002). Regulation of ion channels by integrins. Cell Biochem. Biophys. 36, 41-66. [DOI] [PubMed] [Google Scholar]
- Degani, S., Balzac, F., Brancaccio, M., Guazzone, S., Retta, S. F., Silengo, L., Eva, A., and Tarone, G. (2002). The integrin cytoplasmic domain-associated protein ICAP-1 binds and regulates Rho family GTPases during cell spreading. J. Cell Biol. 156, 377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo, M. A., Alderson, N. B., Kiosses, W. B., Chiang, H. H., Anderson, R. G., and Schwartz, M. A. (2004). Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839-842. [DOI] [PubMed] [Google Scholar]
- Giancotti, F. G., and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028-1032. [DOI] [PubMed] [Google Scholar]
- Gong, Q., Anderson, C. L., January, C. T., and Zhou, Z. (2002). Role of glycosylation in cell surface expression and stability of HERG potassium channels. Am. J. Physiol. 283, H77-H84. [DOI] [PubMed] [Google Scholar]
- Gullo, F., Ales, E., Rosati, B., Lecchi, M., Masi, A., Guasti, L., Cano-Abad, M. F., Arcangeli, A., Lopez, M. G., and Wanke, E. (2003). ERG K+ channel blockade enhances firing and epinephrine secretion in rat chromaffin cells: the missing link to LQT2-related sudden death? FASEB J. 17, 330-332. [DOI] [PubMed] [Google Scholar]
- Hofmann, G., et al. (2001). HERG K+ channels activation during beta(1) integrin-mediated adhesion to fibronectin induces an up-regulation of alpha(v)beta(3) integrin in the preosteoclastic leukemia cell line FLG 29.1. J. Biol. Chem. 276, 4923-4931. [DOI] [PubMed] [Google Scholar]
- Howe, A., Aplin, A. E., Alahari, S. K., and Juliano, R. L. (1998). Integrin signaling and cell growth control. Curr. Opin. Cell Biol. 10, 220-231. [DOI] [PubMed] [Google Scholar]
- Hynes, R. O. (1992). Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11-25. [DOI] [PubMed] [Google Scholar]
- Ivankovic-Dikic, I., Gronroos, E., Blaukat, A., Barth, B. U., and Dikic, I. (2000). Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat. Cell Biol. 2, 574-581. [DOI] [PubMed] [Google Scholar]
- Juliano, R. L. (2002). Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 42, 283-323. [DOI] [PubMed] [Google Scholar]
- Lai, E. C. (2003). Lipid rafts make for slippery platforms. J. Cell Biol. 162, 365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastraioli, E., et al. (2004). herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 64, 606-611. [DOI] [PubMed] [Google Scholar]
- Lecchi, M., et al. (2002). Isolation of a long-lasting eag-related gene-type K+ current in MMQ lactotrophs and its accommodating role during slow firing and prolactin release. J. Neurosci. 22, 3414-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levite, M., Cahalon, L., Peretz, A., Hershkoviz, R., Sobko, A., Ariel, A., Desai, R., Attali, B., and Lider, O. (2000). Extracellular K(+) and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and beta1 integrins. J. Exp. Med. 191, 1167-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, L. (2000). Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1, 173-180. [DOI] [PubMed] [Google Scholar]
- McPhee, J. C., Dang, Y. L., Davidson, N., and Lester, H. A. (1998). Evidence for a functional interaction between integrins and G protein-activated inward rectifier K+ channels. J. Biol. Chem. 273, 34696-34702. [DOI] [PubMed] [Google Scholar]
- Nabi, I. R., and Le, P. U. (2003). Caveolae/raft-dependent endocytosis. J. Cell Biol. 161, 673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivotto, M., Arcangeli, A., Carla, M., and Wanke, E. (1996). Electric fields at the plasma membrane level: a neglected element in the mechanisms of cell signaling. Bioessays 18, 495-504. [DOI] [PubMed] [Google Scholar]
- Parise, L. V., Lee, J., and Juliano, R. L. (2000). New aspects of integrin signaling in cancer. Semin. Cancer Biol. 10, 407-414. [DOI] [PubMed] [Google Scholar]
- Pillozzi, S., et al. (2002). HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia 16, 1791-1798. [DOI] [PubMed] [Google Scholar]
- Rosati, B., Marchetti, P., Crociani, O., Lecchi, M., Lupi, R., Arcangeli, A., Olivotto, M., and Wanke, E. (2000). Glucose- and arginine-induced insulin secretion by human pancreatic beta-cells: the role of HERG K(+) channels in firing and release. FASEB J. 14, 2601-2610. [DOI] [PubMed] [Google Scholar]
- Sacco, T., Bruno, A., Wanke, E., and Tempia, F. (2003). Functional roles of an ERG current isolated in cerebellar Purkinje neurons. J. Neurophysiol. 90, 1817-1828. [DOI] [PubMed] [Google Scholar]
- Sanguinetti, M. C., Jiang, C., Curran, M. E., and Keating, M. T. (1995). A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81, 299-307. [DOI] [PubMed] [Google Scholar]
- Schwartz, M. A., and Baron, V. (1999). Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol. 11, 197-202. [DOI] [PubMed] [Google Scholar]
- Schwarz, J. R., and Bauer, C. K. (2004). Functions of erg K+ channels in excitable cells. J. Cell Mol. Med. 8, 22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D., et al. (2003). Regulation of HERG potassium channel activation by protein kinase C independent of direct phosphorylation of the channel protein. Cardiovasc. Res. 59, 14-26. [DOI] [PubMed] [Google Scholar]
- Warmke, J. W., and Ganetzky, B. (1994). A family of potassium channel genes related to eag in Drosophila and mammals. Proc. Natl. Acad. Sci. USA 91, 3438-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary, K. K., Mainiero, F., Isakoff, S. J., Marcantonio, E. E., and Giancotti, F. G. (1996). The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87, 733-743. [DOI] [PubMed] [Google Scholar]
- Wei, Y., Yang, X., Liu, Q., Wilkins, J. A., and Chapman, H. A. (1999). A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144, 1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]