Abstract
How mitochondrial DNA (mtDNA) copy number is determined and modulated according to cellular demands is largely unknown. Our previous investigations of the related DNA helicases Pif1p and Rrm3p uncovered a role for these factors and the conserved Mec1/Rad53 nuclear checkpoint pathway in mtDNA mutagenesis and stability in Saccharomyces cerevisiae. Here, we demonstrate another novel function of this pathway in the regulation of mtDNA copy number. Deletion of RRM3 or SML1, or overexpression of RNR1, which recapitulates Mec1/Rad53 pathway activation, resulted in an approximately twofold increase in mtDNA content relative to the corresponding wild-type yeast strains. In addition, deletion of RRM3 or SML1 fully rescued the ∼50% depletion of mtDNA observed in a pif1 null strain. Furthermore, deletion of SML1 was shown to be epistatic to both a rad53 and an rrm3 null mutation, placing these three genes in the same genetic pathway of mtDNA copy number regulation. Finally, increased mtDNA copy number via the Mec1/Rad53 pathway could occur independently of Abf2p, an mtDNA-binding protein that, like its metazoan homologues, is implicated in mtDNA copy number control. Together, these results indicate that signaling through the Mec1/Rad53 pathway increases mtDNA copy number by altering deoxyribonucleoside triphosphate pools through the activity of ribonucleotide reductase. This comprises the first linkage of a conserved signaling pathway to the regulation of mitochondrial genome copy number and suggests that homologous pathways in humans may likewise regulate mtDNA content under physiological conditions.
INTRODUCTION
Since the discovery that the mitochondrial genome is present at multiple copies per cell (100–10,000 in humans) and is subject to dynamic regulation with regard to tissue type, metabolic signals, and environmental stimuli, an understanding of the pathways and mechanisms that regulate cellular mitochondrial DNA (mtDNA) copy number has been sought (Moraes, 2001). Although nuclear gene products that have direct roles in mtDNA replication or stability have been implicated in copy number regulation (Schultz et al., 1998; Zelenaya-Troitskaya et al., 1998; Ekstrand et al., 2004; Matsushima et al., 2004; Tyynismaa et al., 2004), signaling pathways involved in modulating cellular mtDNA content have not been fully elucidated. However, important insight into mtDNA copy number regulation and its clinical significance has been gleaned from the study of human mitochondrial disease patients. For example, several mtDNA-depletion syndromes have been characterized, the hallmark of which is decreased mtDNA copy number and/or integrity in certain tissues (Moraes et al., 1991; Elpeleg et al., 2002). Identification of the nuclear genetic defects underlying several of these diseases has underscored a critical role for cellular deoxyribonucleoside triphosphate (dNTP) pools in mtDNA copy number and stability. For example, mutations in the mitochondrial thymidine kinase gene cause mitochondrial depletion myopathy (Saada et al., 2001), mutations in the thymidine phosphorylase gene result in mitochondrial neurogastrointestinal encephalomyopathy (Nishino et al., 1999), and mutations in the deoxyguanosine kinase gene cause hepatocerebral mtDNA depletion syndrome (Mandel et al., 2001). Although these findings and those of others (Bestwick et al., 1982; Tang et al., 2000) implicate alterations in cellular dNTP pools in the maintenance of mtDNA in humans, how dNTPs are sensed and regulated with regard to mtDNA replication and copy number is currently unknown.
The budding yeast Saccharomyces cerevisiae has proven a valuable model system in which to probe mechanisms of mtDNA replication and stability (Shadel, 1999). In this regard, the abundant high mobility group-box, mtDNA-binding protein Abf2p has been shown to be a key player in these processes (Zelenaya-Troitskaya et al., 1998). Abf2p has multiple functions in mtDNA metabolism, including stabilization of recombination intermediates (MacAlpine et al., 1998), segregation and dynamics of nucleoids (Newman et al., 1996; Okamoto et al., 1998; Zelenaya-Troitskaya et al., 1998), and oxidative DNA damage resistance (O'Rourke et al., 2002). It is also one of the first protein factors proposed to have a direct role in regulating mtDNA copy number. Specifically, in glycerol medium, where mitochondrial respiration is required for survival, deletion of the ABF2 gene results in an ∼50% reduction of mtDNA copy number, whereas moderate overexpression of Abf2p increases copy number ∼1.5- to 2-fold (Zelenaya-Troitskaya et al., 1998). It is worth emphasizing that the ∼78-kb mtDNA genome in yeast is present normally at 20–50 copies/cell comprising 10–20% of the total cellular DNA (Williamson and Fennell, 1979). Therefore, a doubling of mtDNA copy number is a substantial and significant increase in total cellular DNA in this organism.
The human homolog of Abf2p is mitochondrial transcription factor A (h-mtTFA or TFAM) (Parisi and Clayton, 1991; Shadel and Clayton, 1993). Interestingly, h-mtTFA has evolved to provide a critical role in transcription initiation that involves a C-terminal tail that is not present in yeast Abf2p (Dairaghi et al., 1995) and binds to the transcription factors h-mtTFB1 and h-mtTFB2 to facilitate promoter recognition (McCulloch and Shadel, 2003; Shadel, 2004). Because mtDNA replication in mammals is likely primed by RNA derived from transcription, h-mtTFA has a dual role in gene expression and mtDNA replication (Shadel and Clayton, 1997). Recently, the abundance of h-mtTFA has been reported to be greater than previously thought (Alam et al., 2003), and an additional role for vertebrate mtTFA homologues in packaging mtDNA, in much the same way as its yeast counterpart, has been postulated by several groups (Shen and Bogenhagen, 2001; Ekstrand et al., 2004; Kanki et al., 2004; Matsushima et al., 2004). In fact, these reports suggest that h-mtTFA can influence mtDNA copy number primarily, if not exclusively, through its proposed DNA packaging function and therefore independently of its role in transcription. However, if and how the abundance or activity of mtTFA dynamically regulates mtDNA copy number awaits further clarification (Maniura-Weber et al., 2004). Furthermore, whether other pathways exist to dynamically regulate mtDNA copy number has not been addressed adequately.
Pif1p is a conserved DNA helicase (Zhou et al., 2000) that resides both in the nucleus and mitochondria in S. cerevisiae (Foury and Lahaye, 1987; Schulz and Zakian, 1994). Our previous analysis of the petite phenotype of pif1 null strains led us to postulate a role for this unique helicase in the repair or tolerance of oxidative mtDNA damage, perhaps by governing the rate of mtDNA replication or regulating mtDNA copy number (O'Rourke et al., 2002). Consistent with this model, we reported recently (O'Rourke et al., 2005) that the mtDNA instability (petite-induction) phenotype of a pif1 null strain can be partially rescued by deletion of the RRM3 gene, encoding the related DNA helicase Rrm3p (Ivessa et al., 2002), or by overexpression of RNR1, the gene encoding a large subunit of ribonucleotide reductase. Deletion of RRM3 or overexpression of RNR1 activates or partially recapitulates, respectively, signaling through the conserved Mec1/Rad53 intra-S-phase checkpoint pathway (Ivessa et al., 2003; O'Rourke et al., 2005), which results in increased de novo synthesis of dNTPs as a primary endpoint (Zhao et al., 1998). Together, these data led us to postulate that Pif1p, Rrm3p, and dNTP pool regulation by the Mec1/Rad53 pathway are critical for mtDNA stability (O'Rourke et al., 2005). In this report, we demonstrate that mtDNA copy number is dynamically regulated by activation of the Mec1/Rad53 pathway, thereby defining the first conserved signaling pathway that controls cellular mtDNA copy number.
MATERIALS AND METHODS
Yeast Strains and Plasmids
The parental strains used in this study were DBY2006 (MATα his3-Δ200 leu2-3, -112 ura3-52 trp1Δ1 ade2) and Y300 (MATa his3-11, 15 leu2-3, -112 ura3-1 trp1-1 ade2-1 can1-100). Specific gene knockouts to produce the pif1, rrm3, rad53, and sml1 null mutations in DBY2006 or Y300 were generated by standard recombination-mediated gene replacement of the desired wild-type locus with a nutritional or drug-selectable marker. The PIF1 gene was replaced by the KANMX4 (G418 resistance) cassette in DBY2006, the RRM3 gene was replaced by the TRP1 gene, the SML1 gene was replaced by the HIS3 gene, and the RAD53 gene was replaced by URA3. The plasmids pRS313-ABF2 (ABF2 CEN HIS3) and pRS316-ABF2 (ABF2 CEN URA3) were used to moderately overexpress Abf2p from its own promoter as described previously (Parisi et al., 1993; Zelenaya-Troitskaya et al., 1998). The plasmid pBAD70 (2μ TRP1 RNR1) used to overexpress the RNR1 gene has been described previously (Huang and Elledge, 1997). The plasmids pRS313, pRS316, and pRS304 (2μ TRP1) were used as a control plasmids with no insert when indicated (Sikorski and Hieter, 1989).
Yeast Growth and Petite-Induction Assays
Yeast strains were grown at 30°C on standard media preparations: rich glucose (YPD), rich glycerol (YPG), or synthetic dextrose (SD) with appropriate nutritional supplements as described previously (O'Rourke et al., 2002). Yeast strains were maintained on YPG (if plasmid selection was not required) or appropriate SD (if plasmid selection was required) solid medium. The petite-induction assays were carried out as described previously (O'Rourke et al., 2002), except that when strains harboring plasmids were involved the petite-induction step was carried out in SD medium rather than YPD. And, in some cases, petite formation was measured on petite-indicator medium (YPG + 0.1% dextrose) rather than by plating identical samples onto YPD and YPG.
mtDNA Copy Number Analysis
The relative amount of mtDNA and nuclear DNA was determined using a quantitative real-time PCR strategy. We routinely use one of two protocols for this measurement, which differ only in the fluorescence method that is used to measure the progress of the PCR reaction. Protocol 1 involves two sets of standard PCR primers and two fluorescently labeled Taqman probes to quantify the amplification signals from the mtDNA target and nuclear target simultaneously in the same reaction, whereas protocol 2 involves separate PCR reactions for the mtDNA and the nuclear DNA assessment and uses SYBR Green to follow the PCR reaction instead of Taqman probes. These two protocols yield virtually identical results (e.g., rrm3Δ in Figure 1 was analyzed using protocol 1 and rrm3Δ in Figures 2b and 3a was analyzed using protocol 2) and are described in detail below.
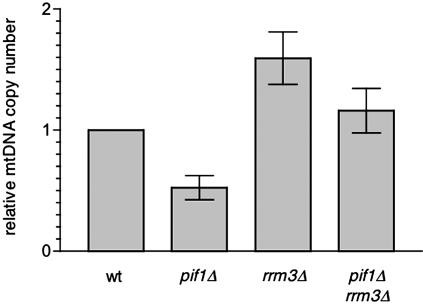
Figure 1.
Pif1p and Rrm3p reciprocally affect mtDNA copy number. The relative mtDNA copy number (plotted on the ordinate) of a pif1 null (pif1Δ), rrm3 null (rrm3Δ), and the corresponding doublemutant (pif1Δ rrm3Δ) strain is shown compared with that of the isogenic wild-type (wt) strain DBY2006. The wt mtDNA copy number was given a value of 1 so that the fold difference in mtDNA copy number [relative to wild type (wt)] is easily compared (see Materials and Methods and Supplemental Table 1 for details on the calculations). The mean of three independent measurements of mtDNA copy number for each strain along with the SD of the measurement observed between the three experiments (brackets) is plotted (Supplemental Table 1).
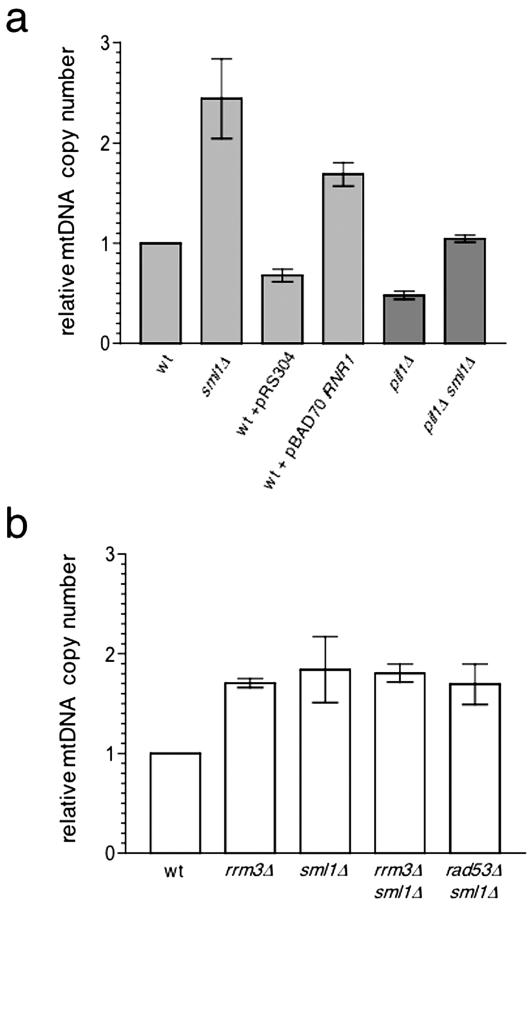
Figure 2.
Activity of the Mec1/Rad53 signaling pathway regulates mtDNA copy number. Plotted in the same manner as in Figure 1 is the relative mtDNA copy number of the strains indicated. (a) Strains analyzed were sml1 null (sml1Δ), pif1 null (pif1Δ), pif1 null and sml1 null (pif1Δ sml1Δ), wild type with the plasmid pBAD70 that overexpresses RNR1 (wt + pBAD70/RNR1), and wild type with empty vector control for pBAD70 comparison (wt + pRS304). (b) Strains analyzed were wild-type DBY2006 (wt), rrm3 null (rrm3Δ), sml1 null (sml1Δ), rrm3 and sml1 null (rrm3Δ sml1Δ), and rad53 and sml1 null (rad53Δ sml1Δ).
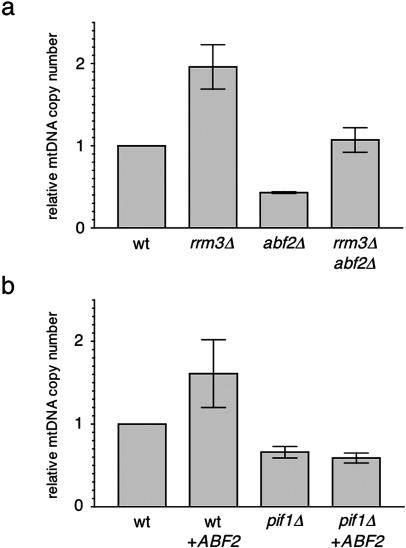
Figure 3.
Increased mtDNA copy number via activation of the Mec1/Rad53 pathway can occur independently of Abf2p. Plotted in the same manner as in Figure 1 is the relative mtDNA copy number of the strains indicated. (a) Strains analyzed were wild-type DBY2006 (wt), rrm3 null (rrm3Δ), abf2 null (abf2Δ), and rrm3 null and abf2 null (rrm3Δ abf2Δ). (b) Strains analyzed were wild-type DBY2006 with a control plasmid lacking the ABF2 gene insert (wt), wild type with a plasmid overexpressing ABF2 from its own promoter (wt + ABF2), pif1 null with a control plasmid lacking the ABF2 gene insert (pif1Δ), and pif1 null with a plasmid overexpressing ABF2 from its own promoter (pif1Δ + ABF2).
In protocol 1 (Figures 1 and 2a), the yeast strains to be analyzed were grown in 10 ml of liquid YPG medium to an optical density (at 600 nm) of 0.6–0.8. The cultures were harvested by centrifugation, and total cellular nucleic acids were isolated using a “smash and grab” protocol essentially as described previously (Hoffman and Winston, 1987). The nucleic acid pellet was dissolved in 100 μl of TE buffer containing DNase-free RNase A (10 μg/ml) and incubated at 37°C for 1.5 h. This DNA was quantified by absorbance at 260 nm and used in a duplex, quantitative, real-time PCR reaction (25-μl total volume) by using the Taqman PCR core reagent kit (Applied Biosystems, Foster City, CA) as follows: 1× buffer A; 200 nM each of dATP, dGTP, and dCTP; 400 nM dUTP; 5.5 mM MgCl2; 125 nM each primer (four total; see below); and 200 nM each probe (two total; see below). The PCR reaction was performed in 96-well format using a Bio-Rad iCycler and analyzed using and accompanying iCycler IQ version 3.1 software.
Each duplex PCR reaction simultaneously measured amplification of the nuclear ACT1 gene and the mtDNA-encoded COX1 gene. For each gene, this involved a set of two PCR primers and an internal probe oligonucleotide that is conjugated to a unique fluorophor at its 5′ end (HEX for ACT1 and 6-FAM for COX1) and a fluorescence quencher moiety (BHQ-1 for ACT1 and TAMRA for COX1) at its 3′ end. Primers and probes were synthesized by Integrated DNA Technologies (Coralville, IA), and their sequences were as follows: ACT1-forward, 5′-GTATGTGTAAAGCCGGTTTTG-3′; ACT1-reverse, 5′-CATGATACCTTGGTGTCTTGG-3′; ACT1 probe, 5′-CGCTCCTCGTGCTGTCTTCCCA-5′; COX1-forward, 5′-CTACAGATACAGCATTTCCAAGA-3′; COX1-reverse, 5′-GTGCCTGAATAGATGATAATGGT-3′; and COX1 probe, 5′-AGTTCACCCTGTACCAGCACCTGA-5′.
The PCR protocol was empirically designed around a primer annealing temperature of 58°C, a probe annealing temperature of ∼68°C, and 45 cycles of real-time PCR amplification and data collection as described in the iCycler manual. For each strain analyzed, the copy number of the mtDNA and the nuclear DNA is calculated using the threshold cycle number (CT), making sure the reactions were in a linear range of detection, by running a variety of dilutions. This is done in triplicate to obtain an average value for CT for COX1 and ACT1, CT (COX1) and CT (ACT1), respectively. The difference (ΔCT) between these averages CT (COX1) - CT (ACT1) is determined and used to arrive at a value of the relative mtDNA copy number (RCN) of each strain, which is equal to 2ΔCT. The fold change in the RCN of each mutant strain is calculated by dividing its RCN by the wild-type RCN value, which yields the fold change compared with wild type, which now has a value of 1. At least three experiments of this type were run for each strain analyzed, and the mean fold change in RCN (labeled in the figures as relative mtDNA copy number) ± 1 SD of these independent trials are plotted in the figures (Supplemental Table 1). In our initial characterization of the pif1Δ, rrm3Δ and pif1Δ rrm3Δ strains, we also measured mtDNA copy number by using a quantitative Southern blotting approach with different ACT1 and COX1 probes. We observed similar results by this method (Supplemental Figure 1).
In protocol 2 (Figures 2b, 3, and 4), yeast growth and nucleic acid isolation were the same as in protocol 1, except 5-ml cultures were used. Various dilutions (≥80-fold) of the template DNA were used in the final PCR reaction to ensure measurements were within the linear range. Nuclear DNA and mtDNA PCR were done in individual wells for each sample dilution in the following standard 50-μl SYBR Green reaction: 25 μl of Bio-Rad iQ SYBR Green Supermix, 10 μl of diluted template, 3 μl of H2O, and 1 μl of each nuclear primer (ACT1 forward and reverse primers, same as protocol 1) or each mtDNA primer (COX3 forward and reverse primers, same as protocol 1). Each primer was stored separately as a working stock of 6.25 μM, and the final concentration in the above-mentioned reaction for each primer was 125 nM. Analysis of the data to generate the relative mtDNA copy number of the strains was performed as described above for protocol 1.
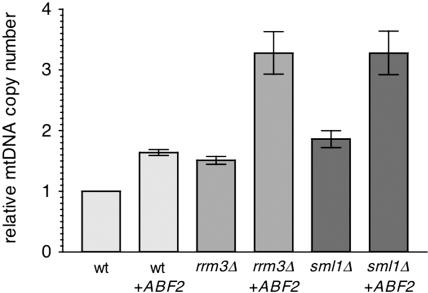
Figure 4.
Abf2p and the Mec1/Rad53 pathways for increased mtDNA copy number are additive. Plotted in the same manner as in Figure 1 is the relative mtDNA copy number of the strains indicated. The strains analyzed were wild-type DBY2006 with a control plasmid lacking the ABF2 gene insert (wt), wild-type with a plasmid overexpressing ABF2 from its own promoter (wt + ABF2), rrm3 null with a control plasmid lacking the ABF2 gene insert (rrm3Δ), rrm3 null with a plasmid overexpressing ABF2 from its own promoter (rrm3Δ + ABF2), sml1 null with a control plasmid lacking the ABF2 gene insert (sml1Δ), and sml1 null with a plasmid overexpressing ABF2 from its own promoter (sml1Δ + ABF2).
RESULTS
The Pif1p and Rrm3p Helicases Reciprocally Influence mtDNA Copy Number
We reported previously that the Pif1p DNA helicase has a critical role in mtDNA damage resistance, mutagenesis, and stability that involves its cooperation with the related DNA helicase Rrm3p (O'Rourke et al., 2002, 2005). Based on these original observations, we speculated that Pif1p and Rrm3p might have a role in regulating mtDNA replication or copy number (O'Rourke et al., 2002, 2005). To test this hypothesis directly, we analyzed mtDNA copy number in pif1 and rrm3 single-mutant and pif1 rrm3 double-mutant strains using a quantitative, real-time PCR assay (Supplemental Table 1). In glycerol medium (YPG), which selects for mitochondrial respiration competence, the mtDNA copy number of a pif1 null mutant strain was reduced to ∼50% of that in the corresponding isogenic wild-type strain DBY2006 (Figure 1). This reduction of copy number in the pif1 strain was completely restored in the pif1 rrm3 double mutant strain (Figure 1), which had wild-type levels of mtDNA. These data are consistent with our previously published results that deletion of rrm3 also rescues the mtDNA instability (petite induction) phenotype of a pif1 null strain (O'Rourke et al., 2005). Of particular additional interest was the striking observation that deletion of RRM3 alone resulted in a near doubling of the mtDNA copy number (Figure 1). To ensure the observed effects on copy number were not strain dependent, we repeated this analysis in a different yeast genetic background (Y300), where virtually identical results were obtained (Zhang and Shadel, unpublished data). Together, these results (Figure 1) are the first to indicate that Pif1p and Rrm3p can influence mtDNA copy number, perhaps in a manner analogous to their cooperative roles in the nucleus.
Signaling through the Mec1/Rad53 Pathway Dynamically Influences mtDNA Copy Number
We showed previously that the kinase Rad53p is phosphorylated in rrm3 and pif1 rrm3 strains and that the mtDNA stability phenotype of a pif1 null mutation is partially rescued by overexpression of RNR1, indicating that signaling through the Mec1 checkpoint pathway is responsible for the increased mtDNA stability in these strains (O'Rourke et al., 2005). Based on these observations and the fact that mtDNA copy number is increased in rrm3 null strains (Figure 1), we tested the hypothesis that mtDNA copy number is dynamically regulated by activity of the Mec1/Rad53 pathway (Figure 5). To do this, we measured mtDNA content in wild-type and pif1 null strains that contain increased activity of ribonucleotide reductase (RNR), the rate-limiting enzyme in dNTP synthesis. Activation of the Mec1 pathway causes transcriptional induction of the RNR genes (Huang and Elledge, 1997) and degradation of Sml1p (Zhao et al., 1998, 2001), an inhibitor of the RNR complex, which together lead to a well-documented increase in dNTP synthesis necessary for S-phase completion or DNA repair. For example, deletion of the SML1 gene alone leads to a ∼2.5-fold increase in all four dNTPs (Zhao et al., 1998). As was the case with rrm3 null strains (Figure 1), we observed an approximately two-fold increase in mtDNA copy number in strains that have SML1 deleted or that overexpress RNR1 from a multicopy plasmid (Figure 2a). In addition, like deletion of RRM3 (Figure 1), deletion of SML1 restored mtDNA copy number to normal levels in a pif1 null strain (Figure 2a). Because overexpression of RNR1 or deletion of SML1 recapitulates a primary known endpoint of Mec1/Rad53 pathway activation (increased dNTP synthesis via elevated cellular RNR activity), these results indicate that increased dNTP synthesis is a significant parameter that influences mtDNA copy number via the Mec1/Rad53 signaling pathway.
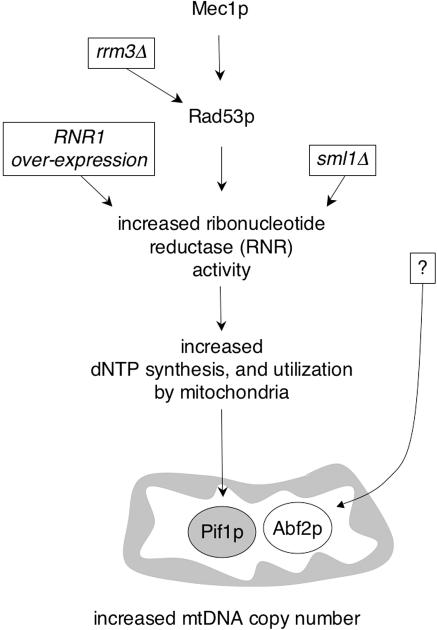
Figure 5.
Proposed model for how signaling via the Mec1/Rad53 pathway influences mtDNA copy number. It is established that the Mec1/Rad53 pathway is responsive to nuclear DNA damage, nuclear replication stress, and S-phase signals that result in increased dNTPs needed to allow replication and repair to proceed. Based on the present study, we propose that signaling through this pathway can also regulate mtDNA copy number, primarily (although not necessarily exclusively; see Discussion) through changes in RNR activity and up-regulation or alteration of cellular dNTP pools. Arrows denote points in the pathway that are activated by the indicated stimulus (for example, deletion of SML1 results in increased activity of RNR). Several conditions tested in this work that resulted in increased mtDNA copy number and how they influence this pathway are shown, including deletion of RRM3 (rrm3Δ) or SML1 (sml1Δ) and overexpression of RNR1. Mitochondrial Pif1p is drawn at the end of this pathway because its petite-induction phenotype is rescued by overexpression of RNR1 (O'Rourke et al., 2005), deletion of RRM3 (O'Rourke et al., 2005), or deletion of SML1 (Table 1), indicating that a process it is involved in is responsive to alterations in dNTP pools. Finally, Abf2p is shown as a separate pathway that can increase mtDNA copy number. That upstream signals which regulate the abundance (or activity) of Abf2p to regulate mtDNA copy number are currently unknown is indicated by a question mark.
To ensure that the effects of deleting SML1 and overexpressing RNR1 were due to similar endpoints in Mec1/Rad53 pathway activation as predicted (Figure 5), we determined whether the petite-induction phenotype of a pif1 null strain is rescued by deletion of SML1. Accordingly, deletion of SML1 partially rescued the petite-induction phenotype of a pif1 null strain (Table 1), in fact, to virtually the same degree as did the overexpression of RNR1 we reported previously (O'Rourke et al., 2005).
Table 1.
Results of petite-induction assays on sml1Δ and pif1Δ single-mutant and sml1Δ pif1Δ double-mutant strains
| Relevant strain genotypea | % Respiration competentb |
|---|---|
| Wild-type | 98.3 ± 0.6 |
| sml1Δ | 98.8 ± 0.4 |
| pif1Δ | 23.3 ± 4.6 |
| sml1Δ pif1Δ | 64.0 ± 4.0 |
Strains are derivatives of the wild-type strain DBY2006
Mean percentages of respiration-competent cells (±1 SD from the mean) remaining after 24 h of growth (approximately six generations) in YPD liquid media
To further address the role of signaling through the Mec1/Rad53 pathway on mtDNA copy number, we next determined whether RRM3, RAD53, and SML1 are all in the same genetic pathway with regard to mtDNA copy number. To do this, we compared mtDNA copy number in sml1Δ rad53Δ and rrm3Δ sml1Δ double-mutant strains to that in the corresponding sml1Δ and rrm3Δ single mutant strains. Deletion of the RAD53 gene is lethal, which precludes the analysis of rad53Δ single mutant. However, its lethality is suppressed by simultaneous deletion of SML1, allowing a rad53 null background to be analyzed (Zhao et al., 1998). We reasoned that if the increase in mtDNA copy number observed in an rrm3Δ strain (Figure 1) is mediated through activation of the Mec1/Rad53 pathway, then sml1Δ should be epistatic to rrm3Δ and rad53Δ because SML1 is the most downstream gene in the proposed pathway (Figure 5). As shown in Figure 2b, each double-mutant strain had the same approximate twofold amount of mtDNA (relative to wild type) as the corresponding single mutant strains, indicating that this indeed is the case. We also attempted to analyze mtDNA copy number in a rad53Δ sml1Δ rrm3Δ triple mutant strain, however, because it exhibited a severe growth defect in YPG medium (Lebedeva and Shadel, unpublished observations), we were unable to assess this with any confidence.
mtDNA Copy Number Regulation by the Mec1/Rad53 Pathway Can Occur Independently of Abf2p
Yeast Abf2p is an abundant mitochondrial DNA-binding protein that, among its many proposed functions, influences mtDNA copy number (Zelenaya-Troitskaya et al., 1998). In fact, as discussed in the Introduction, modulation of Abf2p levels (or its mammalian homologues mtTFA) has been postulated by several investigators to be the major pathway for mtDNA copy number regulation in yeast and in mammalian cells via its DNA packaging function. It was therefore of interest to determine whether the Mec1/Rad53 pathway was influencing mtDNA copy number via the same or a different pathway as Abf2p. To address this we measured mtDNA copy number in abf2 null strains that simultaneously have an activated Mec1/Rad53 pathway due to deletion of RRM3. Consistent with the observations of Butow and colleagues (Zelenaya-Troitskaya et al., 1998), deletion of ABF2 resulted in an ∼50% reduction in mtDNA (Figure 3a). As was the case for a pif1 null strain (Figure 1), deletion of RRM3 also restored the mtDNA copy number in the abf2Δ strain to normal levels (Figure 3a), demonstrating that modulation of mtDNA via an activated Mec1/Rad53 pathway can occur in the complete absence of Abf2p. The increase in mtDNA copy number in the rrm3Δ strain is shown in Figure 3A as a positive control for this experiment. However, in contrast to a pif1Δ strain (O'Rourke et al., 2005), deletion of RRM3 did not significantly rescue the petite-induction phenotype of the abf2Δ strain (Table 2), despite the fact that mtDNA copy number was restored to wild-type levels (Figure 3a).
Table 2.
Results of petite-induction assays on abf2Δ single-mutant and abf2Δ rrm3Δ double-mutant strains
| Relevant strain genotypea | % Respiration competentb |
|---|---|
| Wild-type | 96.7 ± 18.1 |
| abf2Δ | 3.8 ± 1.6 |
| abf2Δ rrm3Δ | 6.1 ± 2.5 |
Strains are derivatives of the wild-type strain DBY2006
Mean percentages of respiration-competent cells (±1 SD from the mean) remaining after 24 h of growth (approximately six generations) in YPD liquid media
As a second test of the concept that regulation of mtDNA copy number by the Mec1/Rad53 pathway can occur independently of Abf2p, we performed the following experiment. Abf2p was moderately overexpressed from a CEN plasmid in a wild-type and isogenic pif1 null strain that has decreased mtDNA copy number (Figure 1). As reported by Butow and colleagues (Zelenaya-Troitskaya et al., 1998), this resulted in an ∼1.5- to 2-fold increase in mtDNA copy number in a wild-type strain (Figure 3b). However, unlike deletion of RRM3 (Figures 1 and 2b) or SML1 (Figure 2a), overexpression of Abf2p was unable to increase mtDNA copy number in the pif1 null strain (Figure 3b). These data indicate that increased mtDNA copy number imparted by overexpression of Abf2p or activation of the Mec1/Rad53 pathway is not achieved via the same mechanism.
Increased Levels of Abf2p and Activation of the Mec1/Rad53 Pathway Additively Increase mtDNA Copy Number
Based on the data presented in the previous section (Figure 3), we concluded that the increase in mtDNA copy number resulting from Abf2p overexpression or by activation of Mec1/Rad53 signaling is occurring largely, if not exclusively, through separate pathways. One prediction of this model is that the two pathways should not be epistatic. That is, if the two pathways are independent, then their effects most likely will be additive rather than one pathway masking the effect of the other. To examine this, we overexpressed Abf2p in rrm3Δ or sml1Δ strains that recapitulate an activated Mec1/Rad53 pathway. As already shown, overexpression of Abf2p (Figure 3b), deletion of RRM3 (Figures 1, 2a, and 3b), or deletion of SML1 (Figure 2a), each result in ∼1.5-fold increase in mtDNA copy number (Figure 4). However, when Abf2p was overexpressed in the rrm3Δ or sml1Δ strains, mtDNA copy number was increased approximately threefold above that of the corresponding wild-type strain (Figure 4). These data show that the Abf2p and Mec1/Rad53 pathways for mtDNA copy number amplification are additive and therefore most likely represent independent mechanisms of mtDNA copy number control.
DISCUSSION
There are two main conclusions that we reach based on the results of this study. The first is that there is a direct link between the conserved Mec1/Rad53 nuclear checkpoint pathway and mtDNA copy number (Figure 5), which to our knowledge is the first conserved signaling pathway to be implicated in this process. The second is that regulation of mtDNA copy number by the Mec1/Rad53 pathway can occur independently of the DNA-packaging protein Abf2p, which is involved in a previously elucidated mechanism of mtDNA copy number control, and therefore represents a novel pathway for mitochondrial genome regulation.
A primary endpoint of activation of the Mec1/Rad53 pathway is an increase in dNTP synthesis (Figure 5), mediated by transcriptional induction of the RNR genes, encoding the catalytic and regulatory subunits of the two forms of RNR (Huang and Elledge, 1997), and degradation of the RNR inhibitor protein Sml1p (Zhao et al., 1998, 2001; Zhao and Rothstein, 2002). We provide several lines of evidence that it is this increase in size (or utilization) of the cellular dNTP pool via signaling through the Mec1/Rad53 pathway that can dynamically regulate mtDNA copy number. First, we (O'Rourke et al., 2005) and others (Ivessa et al., 2003) have shown that deletion of the RRM3 gene results in a nuclear DNA replication checkpoint response and phosphorylation of the Rad53p kinase. Under these conditions, we observe a reproducible ∼1.5- to 2-fold increase in mtDNA copy number in otherwise wild-type strains (Figures 1, 2b, 3a, and 4). In addition, deletion of RRM3 rescues the mtDNA depletion we observe in pif1 null (Figure 1) and abf2 null (Figure 3a) strains. Second, overexpression of RNR1 or deletion of SML1, both of which recapitulate an endpoint of an activated Mec1/Rad53 pathway (Figure 5), result in an approximately twofold increase in mtDNA copy number (Figure 2a) similar to that observed in rrm3Δ strains. And third, results of epistasis experiments are consistent with the placement of RRM3, RAD53, and SML1 in a linear genetic pathway of mtDNA copy number regulation (Figure 2b).
Together, our data strongly indicate that the relative activity of the Mec1/Rad53 pathway is an important mechanism through which cells can regulate mtDNA copy number. In particular, our data point to fluctuations of cellular dNTP pools in general as an important regulatory parameter for mtDNA copy number (Figure 5). This conclusion is supported further by early work with cell division control (CDC) mutants that also revealed a connection between regulation of cellular dNTP pools (via CDC8 and CDC21) and mtDNA replication rates (Newlon and Fangman, 1975). However, given that there is some recent preliminary evidence that Rrm3p is localized in mitochondria (Prokisch et al., 2004) as well as in the nucleus, we note here, as we discussed previously (O'Rourke et al., 2005), that our data do not discount the possibility that Rrm3p has a direct role in mtDNA metabolism. In addition, our data do not address directly whether signaling through the Mec1/Rad53 pathway can affect mtDNA copy number through mechanisms in addition to increasing dNTP pools via RNR activity or that other pathways might also be involved.
Our assertion that mtDNA copy number and stability are governed by the activity of the Mec1/Rad53 pathway is consistent with several previously published observations. First, mutations in several genes in this pathway, including RNR genes (Huang and Elledge, 1997), DUN1 (Zhao and Rothstein, 2002), and MEC1 and RAD53 (Zhao et al., 2001), result in increased petite mutant formation due to mtDNA instability in yeast. Second, we have shown previously that increased expression of RNR1 or deletion of RRM3 partially rescues the petite-induction phenotype of a pif1 null mutation (O'Rourke et al., 2005). Third, in this study, we have shown that deletion of PIF1 results in reduced mtDNA copy number (Figures 1, 2a, and 3b) that is restored in an sml1 null background (Figure 2a). Finally, the petite-induction phenotype of a pif1 null mutation is partially rescued by deletion of SML1 (Table 1). Together, these results demonstrate that alterations in mtDNA copy number are a significant component of the mechanism causing the mtDNA instability phenotypes of Mec1/Rad53 pathway mutant strains reported by others and support our main conclusion that this pathway is involved in regulating mtDNA content and stability through modulation of RNR activity and dNTP pools. It is also relevant in this regard that overexpression of RNR1 rescues the petite-induction phenotype caused by a point mutation in (or haplo-insufficiency of) MIP1, encoding mitochondrial DNA polymerase γ (Lecrenier and Foury, 1995), indicating that the mtDNA replication machinery is responsive to alterations in the cellular dNTP pools.
Yeast Abf2p has previously been implicated in mtDNA copy number regulation (Zelenaya-Troitskaya et al., 1998). Abf2p is an abundant mtDNA-binding protein that results in increased mtDNA copy number when moderately overexpressed, reduced mtDNA levels in glycerol medium when absent (Figure 3a; Zelenaya-Troitskaya et al., 1998), and low mtDNA copy number and increased mtDNA instability when expressed at higher levels (Zelenaya-Troitskaya et al., 1998). It is generally thought that Abf2p can stabilize mtDNA through a DNA-packaging or nucleoid organization function and thereby increase mtDNA copy number when overexpressed moderately. Given that Abf2p is a previously assigned regulator of mtDNA copy number, it was important to determine whether the regulation of mtDNA copy number by the Mec1/Rad53/dNTP pathway elucidated here (Figure 5) can occur independently of Abf2p. We provide several lines of evidence that suggest that it can. First, as is the case in an otherwise wild-type background, deletion of RRM3 results in an approximately twofold increase in mtDNA copy number in an abf2 null background (Figure 3a), clearly demonstrating that activation of the Mec1/Rad53 pathway by deletion of RRM3 results in increased mtDNA copy number in the absence of Abf2p. Second, the increase in mtDNA copy number observed by overexpression of Abf2p is additive to that observed in RRM3- or SML1-deleted strains (Figure 4), indicating independent contributions of Abf2p and an activated Mec1/Rad53 pathway to increased mtDNA copy number. And third, unlike deletion of RRM3 and SML1 (Figures 1 and 2a), increased expression of Abf2p cannot rescue the copy number defect of a pif1 null strain (Figure 3b), indicating again that an activated Mec1/Rad53 pathway and overexpression of Abf2p are not operating through the same mechanism. However, we acknowledge that the fact that the Mec1/Rad53 pathway can influence mtDNA copy number independently of Abf2p does not necessarily mean that these two pathways are entirely independent. Deciphering if and how these pathways cooperate to establish mtDNA levels in response to changing cellular demands represents an important area for future investigation.
Although there is often a correlation between mtDNA copy number and mtDNA stability (as measured by petite-induction), it is important to emphasize that this is not always the case. For example, deletion of RRM3 (O'Rourke et al., 2005), deletion of SML1 (Table 1), or overexpression of RNR1 (O'Rourke et al., 2005) each partially rescues the petite-induction phenotype of a pif1 null mutation. All of these conditions likewise lead to increased mtDNA copy number in pif1 null and/or wild-type strains (Figures 1 and 2a). However, as reported previously for deletion of RRM3 and overexpression of RNR1 (O'Rourke et al., 2005), deletion of SML1 only partially rescues the petite-induction phenotype of a pif1 null strain (Table 1). Thus, only partial rescue of the pif1 null phenotype is achieved, despite the fact that mtDNA copy number is restored to normal levels under these conditions (Figure 2a). Clearly, mtDNA instability in a pif1 null strain is not entirely due to its reduced mtDNA copy number. The same is true of an abf2 null strain. Although deletion of RRM3 clearly rescues the mtDNA copy number depletion of an abf2 null strain (Figure 3a), it has little or no effect on its petite-induction rate (Table 2). This is most likely explained by the fact that Abf2p (and very likely also Pif1p) has multiple functions that impact mtDNA stability and expression, only a subset of which can be overcome by increasing dNTP pools or mtDNA copy number through the Mec1/Rad53 pathway.
The discovery of a conserved pathway (the Mec1/Rad53 pathway; Figure 5) for mtDNA copy number regulation in yeast that can operate independently of Abf2p is likely relevant to the mechanism of mtDNA copy number regulation in metazoans. For example, it has been known for some time that the mammalian homologues of Abf2p, mtTFA/Tfam, can influence mtDNA copy number. Reduced levels of h-mtTFA are observed in patients with mtDNA depletion (Larsson et al., 1994) and in cultured cells that are artificially depleted of mtDNA (Seidel-Rogol and Shadel, 2002). In addition, moderate overexpression of mtTFA in certain cultured cells results in increased mtDNA copy number (Ekstrand et al., 2004; Kanki et al., 2004; Matsushima et al., 2004) and decreased mtDNA copy number is observed in tissues of homozygous and heterozygous mtTFA knockout mice (Larsson et al., 1998). These and other observations have led many investigators to conclude that mtTFA is the key player in mtDNA copy number regulation (Shen and Bogenhagen, 2001; Ekstrand et al., 2004; Kanki et al., 2004; Matsushima et al., 2004). However, based on the results presented herein, we propose that the activity of mtTFA is likely not the only mechanism, and may not even be the primary mechanism, through which mtDNA copy number is regulated in mammalian cells.
The multicopy nature of the mitochondrial genome contributes significantly to the modulation of mitochondrial function in different tissue types (Moraes et al., 1991). In addition, mitochondrial function is dynamic, responding to internal and external stimuli such as developmental signals (Shen and Bogenhagen, 2001), metabolic state (Wu et al., 1999; Zong et al., 2002), and exercise (Wu et al., 2002), indicating that signaling pathways exist to alter mtDNA content in response to cellular and environmental cues. Finally, depleted mtDNA levels lead to human diseases (Elpeleg et al., 2002), many of which presumably involve tissue-specific alterations in the cellular dNTP pool for nuclear and mtDNA replication and repair (Marti et al., 2003). Our finding that the conserved Mec1/Rad53 signaling pathway, which regulates cellular dNTP pools, controls mtDNA copy number in yeast (Figure 5) may shed new light onto why dNTP pool perturbations cause mtDNA-depletion syndromes in humans and strongly suggests that signaling pathways that involve the analogous protein kinases in human cells (i.e., ATM, ATR, Chk1, or Chk2) will likewise influence mtDNA copy number. Differential activity of these pathways in specific cell types may provide a mechanism underlying the natural variation in mtDNA copy number observed in various tissues or allow dynamic responses of mtDNA to environmental and metabolic cues, such as those observed during development or exposure to genotoxic stress. Our findings open new avenues of investigation toward understanding mtDNA dynamics and the role of mitochondrial dysfunction and altered mtDNA copy number in human disease and aging.
Supplementary Material
Acknowledgments
We thank Dr. Steve Elledge for the pBAD70 plasmid. G.S.S. thanks Drs. Susan Kaech and David Stern and the Shadel laboratory group for critical insight, interpretation, and helpful comments on the manuscript. This work was supported by Program Project Grant P01 ES011163. Portions of this work also were supported by grant DAAD19-00-1-0560 from the Army Research Office awarded to G.S.S.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-01-0053) on April 13, 2005.
Abbreviations used: dNTP, deoxyribonucleoside triphosphate; mtDNA, mitochondrial DNA; RNR, ribonucleotide reductase.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Alam, T. I., Kanki, T., Muta, T., Ukaji, K., Abe, Y., Nakayama, H., Takio, K., Hamasaki, N., and Kang, D. (2003). Mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 31, 1640-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick, R. K., Moffett, G. L., and Mathews, C. K. (1982). Selective expansion of mitochondrial nucleoside triphosphate pools in antimetabolite-treated HeLa cells. J. Biol. Chem. 257, 9300-9304. [PubMed] [Google Scholar]
- Dairaghi, D. J., Shadel, G. S., and Clayton, D. A. (1995). Addition of a 29 residue carboxyl-terminal tail converts a simple HMG box-containing protein into a transcriptional activator. J. Mol. Biol. 249, 11-28. [DOI] [PubMed] [Google Scholar]
- Elpeleg, O., Mandel, H., and Saada, A. (2002). Depletion of the other genome-mitochondrial DNA depletion syndromes in humans. J. Mol. Med. 80, 389-396. [DOI] [PubMed] [Google Scholar]
- Ekstrand, M. I., Falkenberg, M., Rantanen, A., Park, C. B., Gaspari, M., Hultenby, K., Rustin, P., Gustafsson, C. M., and Larsson, N. G. (2004). Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 13, 935-944. [DOI] [PubMed] [Google Scholar]
- Foury, F., and Lahaye, A. (1987). Cloning and sequencing of the PIF gene involved in repair and recombination of yeast mitochondrial DNA. EMBO J. 6, 1441-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, C. S., and Winston, F. (1987). A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57, 267-272. [DOI] [PubMed] [Google Scholar]
- Huang, M., and Elledge, S. J. (1997). Identification of RNR4, encoding a second essential small subunit of ribonucleotide reductase in Saccharomyces cerevisiae. Mol. Cell. Biol. 17, 6105-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivessa, A. S., Lenzmeier, B. A., Bessler, J. B., Goudsouzian, L. K., Schnakenberg, S. L., and Zakian, V. A. (2003). The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein-DNA complexes. Mol. Cell 12, 1525-1536. [DOI] [PubMed] [Google Scholar]
- Ivessa, A. S., Zhou, J. Q., Schulz, V. P., Monson, E. K., and Zakian, V. A. (2002). Saccharomyces Rrm3p, a 5′ to 3′ DNA helicase that promotes replication fork progression through telomeric and subtelomeric DNA. Genes Dev. 16, 1383-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki, T., Ohgaki, K., Gaspari, M., Gustafsson, C. M., Fukuoh, A., Sasaki, N., Hamasaki, N., and Kang, D. (2004). Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol. Cell. Biol. 24, 9823-9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, N. G., Oldfors, A., Holme, E., and Clayton, D. A. (1994). Low levels of mitochondrial transcription factor A in mitochondrial DNA depletion. Biochem. Biophys. Res. Commun. 200, 1374-1381. [DOI] [PubMed] [Google Scholar]
- Larsson, N. G., Wang, J., Wilhelmsson, H., Oldfors, A., Rustin, P., Lewandoski, M., Barsh, G. S., and Clayton, D. A. (1998). Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 18, 231-236. [DOI] [PubMed] [Google Scholar]
- Lecrenier, N., and Foury, F. (1995). Overexpression of the RNR1 gene rescues Saccharomyces cerevisiae mutants in the mitochondrial DNA polymerase-encoding MIP1 gene. Mol. Gen. Genet. 249, 1-7. [DOI] [PubMed] [Google Scholar]
- MacAlpine, D. M., Perlman, P. S., and Butow, R. A. (1998). The high mobility group protein Abf2p influences the level of yeast mitochondrial DNA recombination intermediates in vivo. Proc. Natl. Acad. Sci. USA 95, 6739-6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel, H., et al. (2001). The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat. Genet. 29, 337-341. [DOI] [PubMed] [Google Scholar]
- Maniura-Weber, K., Goffart, S., Garstka, H. L., Montoya, J., and Wiesner, R. J. (2004). Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic Acids Res. 32, 6015-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti, R., Nishigaki, Y., Vila, M. R., and Hirano, M. (2003). Alteration of nucleotide metabolism: a new mechanism for mitochondrial disorders. Clin. Chem. Lab. Med. 41, 845-851. [DOI] [PubMed] [Google Scholar]
- Matsushima, Y., Garesse, R., and Kaguni, L. S. (2004). Drosophila mitochondrial transcription factor B2 regulates mitochondrial DNA copy number and transcription in Schneider cells. J. Biol. Chem. 279, 26900-26905. [DOI] [PubMed] [Google Scholar]
- McCulloch, V., and Shadel, G. S. (2003). Human mitochondrial transcription factor B1 interacts with the C-terminal activation region of h-mtTFA and stimulates transcription independently of its RNA methyltransferase activity. Mol. Cell. Biol. 23, 5816-5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes, C. T. (2001). What regulates mitochondrial DNA copy number in animal cells? Trends Genet. 17, 199-205. [DOI] [PubMed] [Google Scholar]
- Moraes, C. T., Shanske, S., Tritschler, H. J., Aprille, J. R., Andreetta, F., Bonilla, E., Schon, E. A., and DiMauro, S. (1991). mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am. J. Hum. Genet. 48, 492-501. [PMC free article] [PubMed] [Google Scholar]
- Newlon, C. S., and Fangman, W. L. (1975). Mitochondrial DNA synthesis in cell cycle mutants of Saccharomyces cerevisiae. Cell 5, 423-428. [DOI] [PubMed] [Google Scholar]
- Newman, S. M., Zelenaya-Troitskaya, O., Perlman, P. S., and Butow, R. A. (1996). Analysis of mitochondrial DNA nucleoids in wild-type and a mutant strain of Saccharomyces cerevisiae that lacks the mitochondrial HMG box protein Abf2p. Nucleic Acids Res. 24, 386-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino, I., Spinazzola, A., and Hirano, M. (1999). Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 283, 689-692. [DOI] [PubMed] [Google Scholar]
- Okamoto, K., Perlman, P. S., and Butow, R. A. (1998). The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: preferential transmission of mitochondrial DNA to the medial bud. J. Cell Biol. 142, 613-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke, T. W., Doudican, N. A., Mackereth, M. D., Doetsch, P. W., and Shadel, G. S. (2002). Mitochondrial dysfunction due to oxidative mitochondrial DNA damage is reduced through cooperative actions of diverse proteins. Mol. Cell. Biol. 22, 4086-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke, T. W., Doudican, N. A., Zhang, H., Eaton, J. S., Doetsch, P. W., and Shadel, G. S. (2005). Differential involvement of the related DNA helicases Pif1p and Rrm3p in mtDNA mutagenesis and stability. Gene (in press). [DOI] [PubMed]
- Parisi, M. A., and Clayton, D. A. (1991). Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science 252, 965-969. [DOI] [PubMed] [Google Scholar]
- Parisi, M. A., Xu, B., and Clayton, D. A. (1993). A human mitochondrial transcriptional activator can functionally replace a yeast mitochondrial HMG-box protein both in vivo and in vitro. Mol. Cell. Biol. 13, 1951-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokisch, H., et al. (2004). Integrative analysis of the mitochondrial proteome in yeast. PLoS Biol. 2, 795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saada, A., Shaag, A., Mandel, H., Nevo, Y., Eriksson, S., and Elpeleg, O. (2001). Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat. Genet. 29, 342-344. [DOI] [PubMed] [Google Scholar]
- Schultz, R. A., Swoap, S. J., McDaniel, L. D., Zhang, B., Koon, E. C., Garry, D. J., Li, K., and Williams, R. S. (1998). Differential expression of mitochondrial DNA replication factors in mammalian tissues. J. Biol. Chem. 273, 3447-3451. [DOI] [PubMed] [Google Scholar]
- Schulz, V. P., and Zakian, V. A. (1994). The saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 76, 145-155. [DOI] [PubMed] [Google Scholar]
- Seidel-Rogol, B. L., and Shadel, G. S. (2002). Modulation of mitochondrial transcription in response to mtDNA depletion and repletion in HeLa cells. Nucleic Acids Res. 30, 1929-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel, G. S. (1999). Yeast as a model for human mtDNA replication. Am. J. Hum. Genet. 65, 1230-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel, G. S. (2004). Coupling the human transcriptional machinery to human disease. Trends Genet. 20, 513-519. [DOI] [PubMed] [Google Scholar]
- Shadel, G. S., and Clayton, D. A. (1993). Mitochondrial transcription initiation: variation and conservation. J. Biol. Chem. 268, 16083-16086. [PubMed] [Google Scholar]
- Shadel, G. S., and Clayton, D. A. (1997). Mitochondrial DNA maintenance in vertebrates. Annu. Rev. Biochem. 66, 409-435. [DOI] [PubMed] [Google Scholar]
- Shen, E. L., and Bogenhagen, D. F. (2001). Developmentally-regulated packaging of mitochondrial DNA by the HMG-box protein mtTFA during Xenopus oogenesis. Nucleic Acids Res. 29, 2822-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y., Schon, E. A., Wilichowski, E., Vazquez-Memije, M. E., Davidson, E., and King, M. P. (2000). Rearrangements of human mitochondrial DNA (mtDNA): new insights into the regulation of mtDNA copy number and gene expression. Mol. Biol. Cell 11, 1471-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyynismaa, H., Sembongi, H., Bokori-Brown, M., Granycome, C., Ashley, N., Poulton, J., Jalanko, A., Spelbrink, J. N., Holt, I. J., and Suomalainen, A. (2004). Twinkle helicase is essential for mtDNA maintenance and regulates mtDNA copy number. Hum. Mol. Genet. 13, 3219-3227. [DOI] [PubMed] [Google Scholar]
- Williamson, D. H., and Fennell, D. J. (1979). Visualization of yeast mitochondrial DNA with the fluorescent stain `DAPI'. Methods Enzymol. 56, 728-733. [DOI] [PubMed] [Google Scholar]
- Wu, H., Kanatous, S. B., Thurmond, F. A., Gallardo, T., Isotani, E., Bassel-Duby, R., and Williams, R. S. (2002). Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296, 349-352. [DOI] [PubMed] [Google Scholar]
- Wu, Z., Puigserver, P., Andersson, U., Zhang, C., Adelmant, G., Mootha, V., Troy, A., Cinti, S., Lowell, B., Scarpulla, R. C., and Spiegelman, B. M. (1999). Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115-124. [DOI] [PubMed] [Google Scholar]
- Zelenaya-Troitskaya, O., Newman, S. M., Okamoto, K., Perlman, P. S., and Butow, R. A. (1998). Functions of the high mobility group protein, Abf2p, in mitochondrial DNA segregation, recombination and copy number in Saccharomyces cerevisiae. Genetics 148, 1763-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Chabes, A., Domkin, V., Thelander, L., and Rothstein, R. (2001). The ribonucleotide reductase inhibitor Sml1 is a new target of the Mec1/Rad53 kinase cascade during growth and in response to DNA damage. EMBO J. 20, 3544-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Muller, E. G., and Rothstein, R. (1998). A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2, 329-340. [DOI] [PubMed] [Google Scholar]
- Zhao, X., and Rothstein, R. (2002). The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc. Natl. Acad. Sci. USA 99, 3746-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J., Monson, E. K., Teng, S., Schulz, V. P., and Zakian, V. A. (2000). Pif1p helicase, a catalytic inhibitor of telomerase in yeast. Science 289, 771-774. [DOI] [PubMed] [Google Scholar]
- Zong, H., Ren, J. M., Young, L. H., Pypaert, M., Mu, J., Birnbaum, M. J., and Shulman, G. I. (2002). AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA 99, 15983-15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.