Abstract
Endochondral bone formation is characterized by the progressive replacement of a cartilage anlagen by bone at the growth plate with a tight balance between the rates of chondrocyte proliferation, differentiation, and cell death. Deficiency of matrix metalloproteinase-9 (MMP-9) leads to an accumulation of late hypertrophic chondrocytes. We found that galectin-3, an in vitro substrate of MMP-9, accumulates in the late hypertrophic chondrocytes and their surrounding extracellular matrix in the expanded hypertrophic cartilage zone. Treatment of wild-type embryonic metatarsals in culture with full-length galectin-3, but not galectin-3 cleaved by MMP-9, mimicked the embryonic phenotype of Mmp-9 null mice, with an increased hypertrophic zone and decreased osteoclast recruitment. These results indicate that extracellular galectin-3 could be an endogenous substrate of MMP-9 that acts downstream to regulate hypertrophic chondrocyte death and osteoclast recruitment during endochondral bone formation. Thus, the disruption of growth plate homeostasis in Mmp-9 null mice links galectin-3 and MMP-9 in the regulation of the clearance of late chondrocytes through regulation of their terminal differentiation.
INTRODUCTION
Normal skeletal development depends on the tightly regulated differentiation of chondrocytes and strict coordination between synthesis and degradation of the extracellular matrix (ECM). Most bones in the vertebrate skeleton form by the process of endochondral ossification, in which a cartilage template is replaced by bone (for review see Karsenty and Wagner, 2002; Kronenberg, 2003). This process occurs separately at two different sites in long bones, with the primary site arising in the center of the shaft (diaphysis) and the secondary sites in the ends of the bones (epiphyses). Diaphyseal differentiation of chondrocytes leads to the formation of two symmetrical structures, the growth plates, composed of columns of differentiating chondrocytes, which progress gradually from the proliferating to the hypertrophic state. These growth plates maintain their size and overall structure throughout long bone growth and are pushed apart while mineralized bone is formed between them, causing overall bone elongation. This developmental process depends on the integration of cartilage matrix remodeling, vascular invasion of hypertrophic cartilage and trabecular bone formation. Differentiation of chondrocytes is accompanied by changes in expression of ECM macromolecules in the growth plate. Chondrocytes express collagen type II (Col2) during proliferation and maturation, but primarily express collagen type X (Col10) when they differentiate to the hypertrophic stage. The specialized matrix surrounding the hypertrophic chondrocytes (HC) becomes calcified; HC then undergo programmed cell death and the resulting empty lacunae are invaded by capillaries. Concomitantly, the cartilage matrix is partially degraded and osteoblasts colonize this template and synthesize trabecular bone (Karsenty and Wagner, 2002; Kronenberg, 2003).
Multiple signaling molecules regulate proliferation and maturation of chondrocytes. These include Indian hedgehog (Ihh), parathyroid hormone (PTH),and parathyroid hormone–related protein (PTHrP) and their receptors fibroblast growth factor (FGF) FGF18 and its receptor FGFR3, vascular endothelial growth factor (VEGF) and connective tissue growth factor (CTGF), bone morphogenetic proteins (BMPs), and Wnt proteins (reviewed in Karsenty and Wagner, 2002; Kronenberg, 2003). In addition, the transcription factors Sox9 and Runx2/Cbfa1 play major roles in this process.
In contrast, much less is known concerning the late differentiation of HC and their terminal differentiation or programmed cell death. The protease inhibitor cystatin 10 (Koshizuka et al., 2003) and the transcription factor c-maf (MacLean et al., 2003) have been shown to play a role in the last steps of chondrocyte differentiation. Particularly compelling evidence has been obtained from transgenic mice deficient for matrix metalloproteinases (MMPs). In Mmp-9 null mice, HC express Col10 and differentiate relatively normally as matrix mineralization occurs, but their disappearance is delayed and the hypertrophic area expands dramatically during the rapid growth phase of postnatal skeletal development in these animals (Vu et al., 1998). Deficiency in MMP-9 decreases the rate of HC programmed cell death, trabecular bone formation, and initial vascular recruitment (Vu et al., 1998). MMP-13 (also called collagenase-3) also regulates the late differentiation of HC (Stickens et al., 2004; Wu et al., 2002).
Galectin-3 is expressed in skeletal tissues, including notochord, developing cartilage and bone (Fowlis et al., 1995) and osteoblasts (Aubin et al., 1996). It is an excellent substrate of MMP-9 in vitro (Ochieng et al., 1994). Galectin-3, which was first identified as macrophage marker 2 protein (Ho and Springer, 1982), belongs to the protein family of β-galactoside–specific lectins. It lacks a signal sequence and, as such, is present intracellularly, but is also secreted by a nonclassical pathway (Sato et al., 1993). Depending on cell type it can be localized in ECM, on the cell surface, in the cytoplasm or nucleus (Barondes et al., 1994). Multiple studies have underlined the extracellular functions of galectin-3 and assigned it as a new matricellular protein (for review, see Ochieng et al., 2004). Galectin-3 has been implicated in a variety of cellular functions, notably, differentiation, RNA splicing and cell adhesion, depending on its subcellular localization. These functions depend on interaction of galectin-3 with various ligands, including glycosylated ECM molecules such as laminin or fibronectin and hensin advanced glycation end products (AGE), integrins, and intracellular proteins such as Bcl-2 (Yang et al., 1996). Expression of galectin-3 is under the control of the transcription factor Runx2/Cbfa1 (Stock et al., 2003), a key regulator of osteoblast differentiation and chondrocyte maturation. Interestingly, Galectin-3 null mice exhibit a growth plate phenotype with precocious HC apoptosis uncoupled from the other features of endochondral ossification, namely ECM degradation, vascular invasion and trabecular bone formation (Colnot et al., 2001).
In this study we took advantage of Mmp-9 null mice to evaluate the mechanisms regulating terminal differentiation of HC, during the critical phase of rapid bone growth in young mice. We have identified galectin-3 as a physiologically relevant endogenous substrate of MMP-9 and a downstream regulator required during endochondral ossification.
MATERIALS AND METHODS
Animal Studies
The generation of Mmp-9 null mice has been described previously (Vu et al., 1998). All the animals used in this study were maintained under protocols approved by the University of California San Francisco Animal Care and Facilities Committee.
Histological Analysis and Immunohistochemistry
For histological analysis, metatarsals were dissected from 2-wk-old mutant animals and littermate controls; embryonic humeri were dissected from mutant animals and littermate controls at embryonic day (E)15.0 and E16.0. Tissues were fixed in 4% paraformaldehyde at 4°C overnight. The tissues were then washed in phosphate-buffered saline (PBS) and decalcified in 0.5 M EDTA (pH 7.4) for 7 d (2-wk-old metatarsals) or 1 d (E16.0 humeri) at 4°C before processing and embedding in paraffin.
For semithin sections, samples were fixed, decalcified, dehydrated, and embedded in Embed 812 (Electron Microscopy Sciences, Fort Washington, PA). Semithin sections (1 μm) were stained with Toluidine blue (Sigma, St. Louis, MO).
Paraffin sections (5 μm) were stained with Safranin O and Fast Green or Alcian blue and Nuclear fast red (Sigma). For TRAP staining, sections were deparaffinized and rehydrated and stained using a leukocyte acid phosphatase kit and Fast Red Violet as a substrate (Sigma) at 37°C for 1 h. The sections were then washed in distilled water and counterstained with Methyl Green (Sigma). Mononucleated and multinucleated TRAP-positive cells were counted on a minimum of six serial sections chosen among the most median part of six different metatarsals for WT and Mmp-9 null animals at 40× magnification and expressed as total number of TRAP-positive cells along the chondro-osseous junction.
For galectin-3 immunostaining, sections were deparaffinized and washed in PBS; endogenous peroxidase activity was quenched in 0.3% methanol in H2O2 for 30 min and washed in PBS, and then sections were heated in citrate buffer before the initial blocking step done in PBS + 3% bovine serum albumin (BSA) for 30 min. Sections were washed in PBS and blocked in PBS + 5% goat serum for 1 h. The sections were then incubated with a rat monoclonal anti-mouse galectin-3 antibody described previously (Colnot et al., 1999) and diluted in PBS containing 1 mg/ml BSA at 4°C overnight. After primary antibody incubation, the slides were washed in PBS, blocked again in PBS plus 5% goat serum for 30 min, and then incubated with a biotinylated goat anti-rat antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:200 in PBS for 1 h, washed, and then incubated with Vector Elite ABC reagent (Vector Laboratory, Burlingame, CA) for 1 h, washed, developed with DAB substrate, and counterstained with Methyl Green (Sigma). Galectin-3–positive cells were counted on a minimum of six serial sections chosen among the most median part of six different metatarsals for wild-type (WT) and Mmp-9 null animals at 40× magnification and expressed as total number of galectin-3–positive cells along chondro-osseous junction or percentage of galectin-3–positive HC.
For PECAM immunostaining, sections were deparaffinized and washed in PBS; endogenous peroxidase activity was quenched in 0.3% methanol in H2O2 for 30 min and washed in PBS, and then sections were treated with Ficin (Zymed Laboratories, South San Francisco, CA) for 15 min at ambient temperature. Blocking treatment was done as described, and the sections were then incubated with a rat monoclonal anti-mouse CD31(PECAM) antibody (clone MEC 13.3, PharMingen, San Diego, CA) at dilution 1:100 in PBS + 1 mg/ml BSA at 4°C overnight. Secondary antibody incubation and revelation were done as described above.
In Situ Hybridization
Plasmids were linearized with the appropriate restriction enzymes to transcribe either sense or antisense 35S-labeled riboprobes (Col2 and Col10: Albrecht et al., 1997; Mmp9, Mmp13, Mt1-Mmp, and Op: Colnot and Helms, 2001; and Gal3 probes Colnot et al., 2001) were described previously).
Slides were deparaffinized, treated with proteinase K (20 μg/ml) for 5 min at ambient temperature, and hybridized with 35S-labeled antisense riboprobes in hybridization buffer (50% deionized formamide, 300 mM NaCl, 20 mM Tris-HCl, pH 8.0, 5 mM EDTA, 0.5 mg/ml yeast tRNA, 10% dextran sulfate, and 1× Denhardt's) in a humidified chamber at 55°C for embryonic tissues and at 45°C for postnatal tissues, overnight. After hybridization, slides were treated with RNase A, washed to a final stringency of 50% formamide, 2× SSC at 60 or 50°C, dipped in emulsion, exposed for 1–2 wk, developed, and counterstained with DAPI (Colnot et al., 2003; Ferguson et al., 1999).
Protein Extraction and Western Blotting
Hypertrophic cartilage area of growth plates were dissected from Mmp-9 null animals and littermate controls from 2-wk-old mice and frozen immediately in liquid nitrogen. Tissues were reduced to powder under nitrogen using a very small pestle and mortar and then tissues were homogenized using an Ultra Turrax homogenizer. Total proteins were extracted in RIPA buffer (20 mM Tris, pH 7.2, 10 mM EDTA, 0.3 M NaCl, 0.1% Triton X-100, 0.05% Tween-20) with protease inhibitors (5 μg/ml aprotinin, benzamidine, 5 μg/ml leupeptin, 5 μg/ml pepstatin; Sigma) overnight at 4°C. After centrifugation extracts were quantified by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). For protein profile analysis, 5 μg of total protein (corresponding approximately to the equivalent of proteins extracted from 2 growth plates) was loaded per lane on 4–10% gradient gel run in 4-morpholinopropanesulphonic acid (MOPS) running buffer (Novex Invitrogen, Carlsbad, CA) under reducing conditions; the gels were fixed and stained with a Fast Silver kit (Geno Technology, St. Louis, MO).
For Western blotting analysis 40 μg total protein was loaded per lane on 10% Bis-Tris gel run in MOPS running buffer under reducing (for galectin-3, CTGF, transglutaminase or collagen type II) or nonreducing (for VEGF) conditions. After transfer to PVDF membrane (Bio-Rad, Hercules, CA), blotting was performed with a goat polyclonal anti-mouse VEGF antibody that preferentially recognizes VEGF dimers (R&D Systems, Minneapolis, MN), a mouse monoclonal anti–galectin-3 antibody clone A3/A12 (Research Diagnostics, Flanders, NJ), a rabbit polyclonal anti-transglutaminase II antibody or a mouse monoclonal anti–collagen type II antibody (NeoMarkers, Fremont, CA), and a rabbit polyclonal anti-CTGF antibody (Torrey Pines Biolabs, San Diego, CA) diluted according to the manufacturer's recommendations. After incubation with horseradish peroxidase–conjugated secondary antibody, goat polyclonal anti-rabbit, sheep polyclonal anti-mouse (Amersham Biosciences, Buckinghamshire, England), rabbit polyclonal anti-goat (Sigma) diluted 1/10,000, blots were developed with ECL (Amersham Biosciences, Buckinghamshire, England). Equal loading was verified on a gel by Coomassie blue staining.
Embryonic Metatarsal Cultures
Metatarsals from hind limbs of E16.5 mice were dissected and cultured as described previously (Blavier and Delaisse, 1995). In brief, the three middle metatarsals from each hind limb of E16.5 mice were carefully dissected and cultured for 4 d on Millicell culture plate inserts in 700 μl BGJb medium (Life Technologies Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum. Recombinant human galectin-3 was purified as previously described (Massa et al., 1993) by bacterial expression and lactose affinity chromatography followed by dialysis against PBS with 1 mM EDTA. Cleaved galectin-3 was obtained after digestion with active MMP-9 as described previously (Ochieng et al., 1994) and digestion was verified by running samples on 12% SDS polyacrylamide gels and silver-stained. Cleaved or uncleaved proteins were added to a final concentration of 0.1 μM. Medium was changed daily. After 4 d of culture, metatarsals were fixed and processed for paraffin sections. Safranin O and TRAP staining were done as described above. Paraffin sections stained with Safranin O were photographed under microscope at a magnification of 10× and size of the growth plate was measured on a minimum of three serial sections chosen among the most median part of several different metatarsals for WT and mutant animals as indicated.
Statistical Analysis
The quantitative data were compared between the means of two groups using unpaired Student's t tests. Results are expressed as mean plus or minus SEM and significant differences are indicated as P values. GraphPad calculator was used to perform the tests.
Image Acquisition
Photomicrographs were acquired with a microscope Leica DMR (Deerfield, IL) equipped with HC Plan Fluotar 5/0.15; 10/0.30; 20/0.50 or Plan Apo 40/0.75; 100/1.40 coupled to a Leica DC500 digital camera using Leica firecam 1.2.0 software. Images were assembled using Adobe Photoshop software version 6.0.1 (San Jose, CA). In situ hybridization images were taken under dark-field and image analysis was performed as previously described (Ferguson et al., 1999).
RESULTS
Absence of MMP-9 Causes an Expanded Zone of Late Differentiated HC
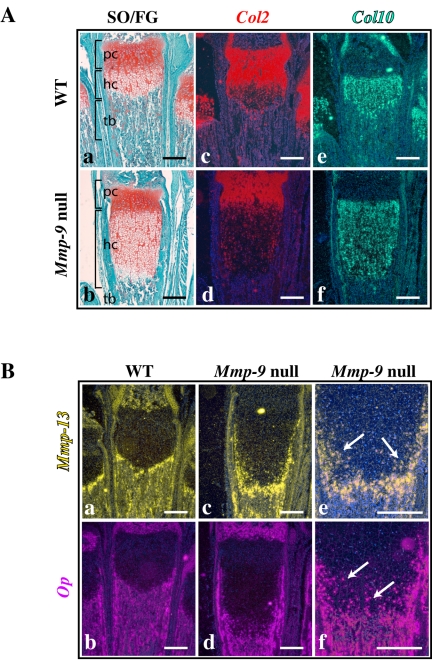
The program of differentiation from proliferating and maturing chondrocytes to HC is characterized by expression of Col10 mRNA (Chan and Jacenko, 1998). As HC further differentiate into late HC, they express Mmp-13 and Osteopontin (Op; Inada et al., 1999; D'Angelo et al., 2000), and their surrounding matrix undergoes mineralization. To investigate the molecular defects leading to the expansion of the HC zone in Mmp-9 null mice, we characterized the differentiation state of the HC in the expanded hypertrophic cartilage zone by analyzing the expression of HC markers by in situ hybridization on sections from metatarsals of 2-wk-old mice. The zone of Col10 expression dramatically expanded in Mmp-9 null compared with WT mice, whereas Col2 expression was unchanged (Figure 1A), indicating that differentiated HC accumulate in the growth plates of Mmp-9 null mice. Moreover, the expanded zones of expression of Mmp-13 and Op showed that late HC persist. In Mmp-9 null mice several rows of HC just above the chondro-osseous junction expressed Mmp-13 and Op (Figure 1B, c–f); in WT mice these late markers were detected in very few cells in the last rows because of a rapid exit of these chondrocytes from the growth plate (Figure 1B, a and b). These observations indicate that, although chondrocytes can undergo late hypertrophy in Mmp-9 null mice, late HC accumulate, due to a defect in their programmed cell death and their rate of exit from the growth plate.
Figure 1.
Accumulation of late terminally differentiated hypertrophic chondrocytes in Mmp-9 null mice. Serial sections of growth plates from 2-wk-old MMP-9 null and WT mice. (A) Safranin O/Fast Green (SO/FG) staining (a and b) and in situ hybridization with antisense probes for collagen type II (Col2; c and d) and collagen type X (Col10; e and f) show an increase in the hypertrophic chondrocyte zone (hc) but not the proliferating chondrocyte zone (pc) in MMP-9 null mice compared with WT. (B) In situ hybridization with antisense probes for late hypertrophic markers, Mmp-13 (a and c) and Osteopontin (Op; b and d) show the accumulation of late hypertrophic chondrocytes in MMP-9 null mice. (e and f) Higher magnification of Mmp-13 and Op expression pattern in MMP-9 null mice. Note that the accumulation of hypertrophic chondrocytes expressing these markers (arrows) in several rows at the chondro-osseous junction. pc, proliferating chondrocytes; hc, hypertrophic chondrocytes; tb, trabecular bone. Scale bars, 250 μm.
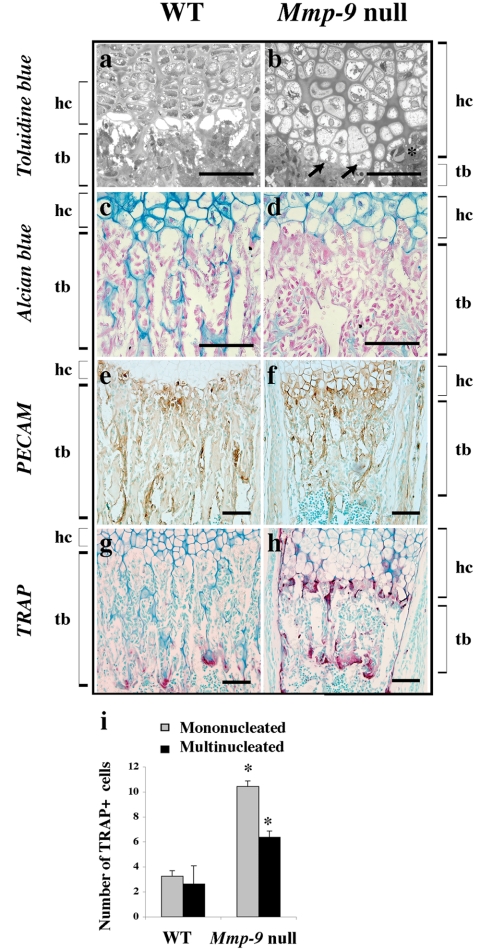
To obtain a more detailed view of the organization of the cells at the chondro-osseous junction, we stained semithin tissue sections from growth plates embedded in plastic with Toluidine blue. In WT mice the chondro-osseous junction was characterized by a row of empty lacunae, whereas in Mmp-9 null mice very few lacunae were empty and the HC appeared very enlarged and fully expanded in the lacunae (Figure 2, A and B). Moreover, mononuclear cells and capillaries were distant from the last intact transverse septa in WT, but were stacked directly under the last row of HC in Mmp-9 null mice (Figure 2B, asterisk). Alcian blue staining indicated a decrease in proteoglycan content at the chondro-osseous junction concomitant with a decrease in trabecular bone in Mmp-9 null mice (Figure 2, C and D). Capillaries exhibited relatively normal morphology and platelet endothelial cell adhesion molecule (PECAM) staining showed no major differences in vascular recruitment at the chondro-osseous junction between Mmp-9 null and WT mice (Figure 2, E and F).
Figure 2.
Altered organization of the chondro-osseous junction in Mmp-9 null mice. Histological analysis of the chondro-osseous junction. (a and b) Representative pictures of semithin sections stained with Toluidine blue showing the abnormal survival of the last row of hypertrophic chondrocyte (arrows) in Mmp-9 null mice compared with WT. A capillary in close contact with last transverse septa is marked by an asterisk (*). (c–h) Longitudinal sections through the central part of growth plates. (c and d) Alcian blue staining; note the decrease in staining in Mmp-9 null mice in the last row of hypertrophic chondrocytes and trabeculae. (e and f) PECAM staining showing no major differences in growth plate vascularization between Mmp-9 null and WT mice. (g and h) TRAP staining, note the increase in TRAP-positive cells recruited at the chondro-osseous junction in Mmp-9 null mice. hc, hypertrophic chondrocytes; tb, trabecular bone. Scale bars, 125 μm. (i) Quantification of mononuclear and multinucleated TRAP-positive cells recruited at the chondro-osseous junction, showing an increase in Mmp-9 null mice (n = 6 different metatarsals; *p ≤ 0.005).
In contrast, the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts increased from 3.3 ± 0.5 cells/section in WT mice to 10.5 ± 1.5 cells/section at the chondro-osseous junction of Mmp-9 null mice (p ≤ 0.005; n = 6; Figure 2, G–I). Multinucleated cells in this region also showed a modest difference, with an average of 6.4 ± 0.5 cells/section in Mmp-9 null mice versus 2.6 ± 0.4 cells/section in WT mice (p ≤ 0.005; n = 6). These results suggest increased recruitment of mononucleated osteoclast precursors and multinucleated osteoclasts in Mmp-9 mice.
Taken together, these data indicate that the extended hypertrophic zone in Mmp-9 null mice at this specific developmental stage results from the accumulation of both HC and late HC. Capillaries are present at the ossification front, but their progression into the hypertrophic cartilage could be delayed. Moreover, the defective disappearance of HC, likely due to a defect in their programmed cell death, is coupled with an increase in osteoclast precursors recruited at the chondro-osseous junction.
Protein Profile Analyses of Hypertrophic Cartilage Reveals Galectin-3 as an In Vivo Target of MMP-9
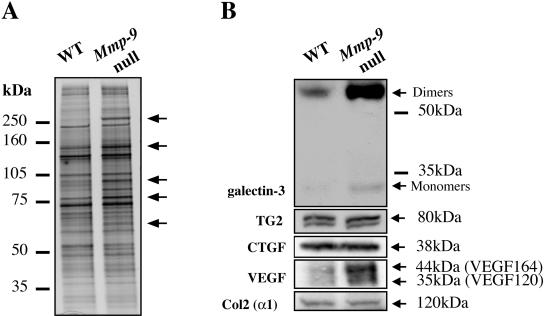
To gain an understanding of the molecular basis for the dysregulated hypertrophic differentiation in Mmp-9 null mice, we compared profiles of proteins extracted from HC zones (including the primary ossification front) of WT and Mmp-9 null HC by SDS-PAGE. We obtained reproducible patterns showing striking differences between genotypes. Several proteins were present at higher levels in Mmp-9 null mice (Figure 3A). Although some proteins were down-regulated with deficiency of MMP-9, we focused on the up-regulated ones, because we were most particularly interested in elucidating the in vivo substrates of MMP-9. To identify these proteins we screened several specific candidates involved in HC differentiation and endochondral bone formation. Western blotting for tissue transglutaminase II (TG2) detected two isoforms, but showed no difference between WT and Mmp-9 null samples. Similarly, we observed no differences for either connective tissue growth factor (CTGF) or collagen type II α1 chain (Col2α1; Figure 3B).
Figure 3.
Accumulation of uncleaved galectin-3 in the growth plate of Mmp-9 null mice. (A) Protein extracts from WT and Mmp-9 null hypertrophic chondrocyte zones were analyzed by SDS-PAGE and silver staining. Differences in protein profiles were observed as increases in the intensity of several bands in Mmp-9 null samples, as indicated by arrows. (B) Protein extracts from WT and Mmp-9 null hypertrophic chondrocyte zones were analyzed for expression of galectin-3, tissue transglutaminase (TG2), CTGF, VEGF, and α1 chain of collagen type II (Col2; α1) by Western blotting. Note the increase in intact galectin-3 dimers and VEGF in Mmp-9 null mice compared with WT samples.
Interestingly, VEGF and galectin-3 increased in Mmp-9 null samples (Figure 3B). For VEGF, both major isoforms, VEGF164 and VEGF120, increased. We were unable to cleave these VEGF forms with MMP-9, suggesting that they are not direct in vivo substrates. In contrast, galectin-3, a known substrate of MMP-9 in vitro (Ochieng et al., 1994), was particularly enriched in our samples. Moreover, we observed more uncleaved monomeric and dimeric forms of galectin-3 in Mmp-9 null samples compared with WT. The dimeric forms, which were stable to reducing conditions, are likely to be extracellular galectin-3 cross-linked by TG2. Because the antibody used recognizes an epitope in NH2 domain of galectin-3, which contains a cleavage site for MMP-9, we were able to detect only uncleaved galectin-3. These results show that uncleaved galectin-3 accumulates in the HC zone and primary ossification front of mice deficient for MMP-9 and strongly suggest that galectin-3 is a downstream regulator of MMP-9 action in vivo during endochondral ossification.
Galectin-3 Expression Is Disturbed during the Initial Stage of Endochondral Bone Formation in Mmp-9 Null Mice
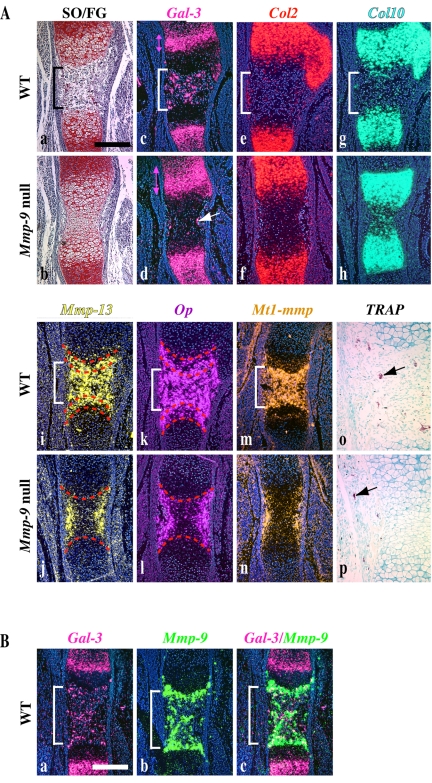
The increase in galectin-3 observed in Mmp-9 null hypertrophic cartilage could be due to an increase in galectin-3 mRNA expression resulting in increased synthesis or an accumulation in galectin-3 protein due to a decrease in its degradation. We first analyzed the galectin-3 mRNA expression pattern in embryonic bones of WT and Mmp-9 null mice relative to other markers of chondrocyte differentiation by in situ hybridization. At embryonic day 15.0 (E15.0), the majority of WT humeri already had primary ossification centers, whereas no ossification centers were observed in Mmp-9 null humeri (Figure 4A, a and b). The expression of Col2 was similar in WT and Mmp-9 null mice (Figure 4A, e and f); however, the zone of Col10 expression expanded in Mmp-9 null embryonic bones, reflecting the expanded HC zone in these animals (Figure 4A, g and h).
Figure 4.
Galectin-3 expression is disturbed during the initial stage of endochondral bone formation in Mmp-9 null mice. (A) Serial longitudinal sections of humeri from E15.0 WT and Mmp-9 null mice were stained with Safranin O/fast green (SO/FG; a and b) and for TRAP activity (o and p, TRAP-positive cells are indicated by black arrows). Adjacent sections were hybridized with antisense RNA probes for Galectin-3 (Gal-3, fuchsia), Collagen type 2 (Col2, red), Collagen type 10 (Col10, green), Mmp-13 (yellow), Osteopontin (Op, purple), or Mt1-mmp (orange). Note the increase in Galectin-3 expression in Mmp-9 null mice as indicated by double pink arrows. Rare Galectin-3–expressing cells are found in the perichondrium of Mmp-9 null samples (white arrow). Red dotted lines indicate the limits of Mmp-13 and Op expression domains in late HC. (B) Serial longitudinal sections of femurs from E16.0 WT mice were hybridized with antisense RNA probes for Galectin-3 and Mmp-9 as indicated. Merging of both signals shows partial overlap between Galectin-3 and Mmp-9 expression. Brackets indicate the primary ossification center in wild-type sections. Scale bars, (A) 250 μm; (B) 500 μm.
Mmp-13 and Op are expressed by hypertrophic chondrocytes and osteoblasts (Tuckermann et al., 2000; McLean et al., 2003) and Mt1-Mmp is expressed by osteoclasts (Sato et al., 1997). Mmp-13 and Op mRNAs appeared in the center of WT humeri in the most terminal rows of HC and in the primary ossification center where osteoclasts and osteoblasts are recruited (Figure 4A, i and k). In Mmp-9 null mice, although the Mmp-13 and Op expression domains appeared to be diminished overall because of a delay in the establishment of the ossification center, their expression domains within the hypertrophic cartilage (delimited by a dotted line) was expanded and revealed that the accumulation of late HC begins at an early embryonic stage of endochondral ossification (Figure 4A, j and l).
Galectin-3 mRNA expression was observed in two distinct zones of the developing bone. It was localized primarily in proliferating and prehypertrophic chondrocytes, and, to a lesser extent, in late HC (Figure 4Ac). A second domain of strong galectin-3 mRNA expression in the ossification center overlapped with the expression domain of Mmp-13, OP, and Mt1-Mmp (Figure 4A, i, k, and m), most likely in osteoclasts and osteoblasts (Niida et al., 1994; Aubin et al., 1996; Colnot et al., 1999). The expression domain of galectin-3 was slightly expanded in early HC in Mmp-9 null embryos, where it overlapped with the Col10 expression domain (Figure 4Ad). As in WT mice, the signal decreased markedly in late HC. Because formation of the primary ossification center was delayed, preosteoclast recruitment and vascular invasion had not yet occurred in Mmp-9 null humeri. Accordingly, no cells expressing galectin-3 had migrated into the ossification center, but instead a few galectin-3–positive cells were present in the perichondrium (Figure 4Ad). Osteoblasts expressing Mmp-13 and Op were detected only in the perichondrium, as were osteoclasts expressing Mt1-Mmp and TRAP (Figure 4A, j, l, n, and p). These results show a delay in the recruitment of osteoclasts and osteoblasts from the perichondrium to the primary ossification center. This delay in recruitment paralleled the delay in the initial removal of late hypertrophic chondrocytes in Mmp-9 null mice at E15.0.
At E16.0, when vascular invasion was well underway in WT embryonic bones, Mmp-9 mRNA was highly expressed in cells located at the primary ossification front, as is typical of Mmp-9 expression during endochondral bone formation from embryonic stages through adulthood. The Mmp-9 expression pattern partially overlapped with the localization of galectin-3 at the front of ossification and coexpression of these genes was detected in a limited cell population, most likely comprised of (pre-) osteoclasts (Figure 4B). The remaining galectin-3–positive cells, which did not express Mmp-9, may represent another population of ECM resorbing cells expressing Mt1-Mmp and/or osteoblasts expressing Mmp-13 and Op as suggested by the overlapping expression profiles observed at E15.0 (Figure 4A).
Taken together, these results indicate that the expansion of late HC and the delay in their disappearance observed in Mmp-9 null mice begins early in bone development. Concomitant with these defects, we observed an expansion of galectin-3 expression in the upper HC zone and a decrease in the initial recruitment of galectin-3–positive cells in the primary ossification center. These observations suggests that galectin-3 as a downstream regulator of MMP-9 at two steps in endochondral bone formation: HC differentiation, and osteoclast recruitment and differentiation.
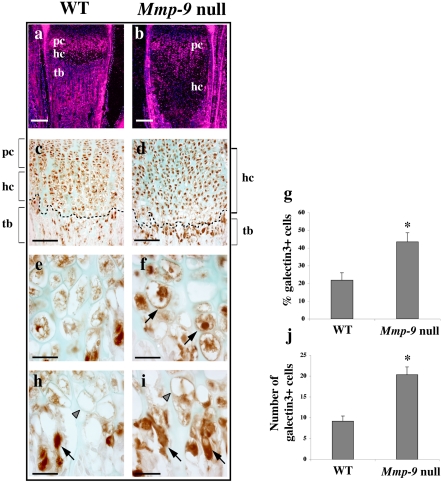
Accumulation of Galectin-3 Protein at the Chondro-osseous Junction of Mmp-9 Null Mice Overlaps with MMP-9 Localization At postnatal 2 wk, galectin-3 mRNA was highly expressed in proliferating and prehypertrophic chondrocytes with an expansion of this expression domain in Mmp-9 null mice. Galectin-3 mRNA was strongly decreased in late HC, although a few sparsely distributed cells exhibited a higher expression (Figure 5, A and B). An antibody to galectin-3–stained proliferating chondrocytes strongly and HC less intensely in WT growth plates (Figure 5C), as previously described (Colnot et al., 1999). At the chondro-osseous junction, galectin-3 was also detected in mononuclear cells, preosteoclasts, some vascular cells and multinucleated osteoclasts (unpublished data). However, in Mmp-9 null samples, the majority of HC stained very strongly positive for galectin-3, and the number of positive cells at the chondro-osseous junction also increased (Figure 5D). In Mmp-9 null mice, galectin-3 protein accumulated in the enlarged late HC in nuclei, along the plasma membrane, extracellularly in the perilacunar space and to a lesser extent weakly in the proximal ECM (Figure 5F). In contrast, in WT mice few late HC exhibited intense nuclear galectin-3 staining, and membrane, perilacunar, and ECM staining were very weak (Figure 5E). Strongly galectin-3–positive HC were only 20.2 ± 4.2% of the total HC population in WT mice, versus 43.5 ± 5.1% (p ≤ 0.005; n = 6) in Mmp-9 null mice (Figure 5G).
Figure 5.
Galectin-3 accumulates at the chondro-osseous junction of Mmp-9 null mice. Paraffin sections of the growth plates of 2-wk-old Mmp-9 null and WT metatarsals. (a and b) In situ hybridization with antisense RNA probe for Galectin-3. (c and d) Immunostaining with monoclonal rat anti–galectin-3. (e and f) Higher magnification of (c and d) showing the increase in galectin-3 protein in hypertrophic chondrocytes (hc) of Mmp-9 null mice. Black arrows indicate galectin-3 staining in perilacunar space. (h and i) Higher magnification of (c and d) showing the increase in recruitment of galectin-3–positive cells at the chondro-osseous junction (indicated by dotted line) in Mmp-9 null mice (black arrows) and the increase in galectin-3 staining in the pericellular matrix of the last row of hypertrophic chondrocytes (gray arrowheads). (g) Quantification of hypertrophic chondrocytes exhibiting high levels of galectin-3 immunostaining. Results are expressed as percentage of total hypertrophic chondrocytes counted. (n = 6 different metatarsals; *p ≤ 0.005). (j) Quantification of galectin-3–positive cells recruited at the chondro-osseous junction expressed as total number of galectin-3–positive cells per section along the ossification front. (n = 6 different metatarsals; *p ≤ 0.005). pc, proliferating chondrocytes; hc, hypertrophic chondrocytes; tb, trabecular bone. Scale bars, (a and b, 250 μm); (c and d, 150 μm); (e, f, h, and i, 30 μm).
The galectin-3 staining in the lacunae and ECM in Mmp-9 null mice increased and persisted in the last rows of HC (Figure 5I). In contrast, the numerous empty lacunae observed at the chondro-osseous junction in WT mice had little galectin-3 staining in the surrounding matrix (Figure 5H). At the chondro-osseous junction, the number of recruited galectin-3–positive cells per section increased to 20.3 ± 1.0 in Mmp-9 null mice compared with 9.2 ± 1.3 (p ≤ 0.005, n = 6) in WT (Figure 5J). Some of these cells were vascular cells, whereas others were preosteoclasts; osteoblasts did not show high expression of galectin-3 (unpublished data).
Thus, the slightly expanded region of galectin-3 mRNA expression in Mmp-9 null mice owed to a modest expansion of the prehypertrophic zone. In the absence of MMP-9, galectin-3 protein accumulated in regions where MMP-9 was located in WT animals, along the last row of HC, their ECM, and the chondro-osseous junction. We did not observe an increase in galectin-3 protein in bone marrow of Mmp-9 null mice, indicating that this accumulation does not result from a global effect of MMP-9 deficiency on galectin-3 translation. The fact that galectin-3 accumulates in a context of decreased ECM remodeling strongly suggests a decrease in galectin-3 degradation. Therefore, galectin-3 appears to be downstream of MMP-9, most likely as a direct substrate, in regulating the final stages of HC differentiation and also in regulating osteoclastic recruitment and/or survival during endochondral bone formation.
Excess Extracellular Galectin-3 Phenocopies Early MMP-9 Deficiency
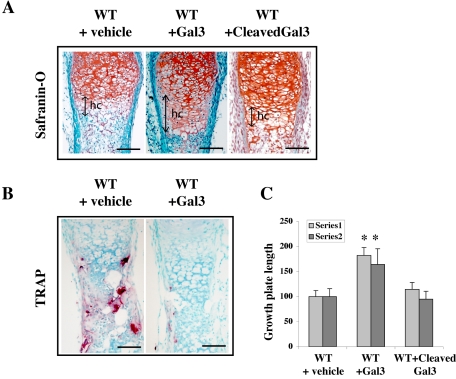
A feature of the Mmp-9 null growth plates was the presence of extracellular galectin-3. This raises the question of whether the growth plate phenotype in Mmp-9 null mice is caused by an overabundance of uncleaved extracellular galectin-3. If this is the case, then addition of excess galectin-3 should delay the initial formation of the growth plate and concomitantly increase the size of the hypertrophic cartilage zone in skeletal elements undergoing endochondral ossification. We tested this hypothesis in organotypic cultures of embryonic metatarsals. We treated metatarsals dissected from E16.5 WT or Mmp-9 null mice for 4 d with recombinant uncleaved (multimeric) or MMP-9–cleaved galectin-3. In three independent experiments, WT embryonic metatarsals treated with intact galectin-3 mimicked the embryonic Mmp-9 null phenotype, exhibiting delayed and decreased recruitment of preosteoclasts to the primary ossification center coupled with an increase in the HC zone (Figure 6). This resembles the early growth plate phenotype of metatarsals from developing embryonic Mmp-9 null mice in vivo and in culture with decreased vascular invasion and recruitment of TRAP-positive cells and an increased HC zone (Engsig et al., 2000). In contrast, we could not detect any significant phenotypic effects in embryonic metatarsals treated with MMP-9–cleaved galectin-3. Mmp-9 null embryonic metatarsals, which already have an endogenous excess of galectin-3, were unaffected by these treatments (unpublished data). Thus, either excess uncleaved multimeric galectin-3, or ablation of Mmp-9 interferes with two pathways, one that directs late differentiation of HC and a second that controls osteoclast differentiation and migration.
Figure 6.
Ectopic addition of multimeric uncleaved galectin-3 partially mimics MMP-9 deficiency. Sections from E16.5 WT metatarsals cultured for 4 d in presence of vehicle alone, full-length mouse recombinant galectin-3–or MMP-9–cleaved galectin-3 stained with (A) Safranin O or (B) for TRAP activity. (C) Quantification of the increase in growth plate length following galectin-3 treatment. Results are expressed as the percentage of increase compared with untreated WT and the results from two representative independent experiments are shown (series 1, n = 5 for vehicle alone, n = 4 for full length galectin-3 and n = 4 for MMP-9 cleaved galectin-3), *p ≤ 0.005); (series 2, n = 5 for vehicle alone, n = 5 for full length galectin-3 and n = 3 for MMP-9 cleaved galectin-3, *p ≤ 0.005).
DISCUSSION
Mmp-9 deficiency leads to abnormal endochondral bone formation, with decreased rates of HC apoptosis and trabecular bone formation (Vu et al., 1998). These defects are initially derived from a delay in vascular invasion and osteoclast recruitment at an early stage in development (Engsig et al., 2000). The growth plate of Mmp-9 null mice is characterized by an extension of the lifespan of late HC, as well as the accumulation of full-length galectin-3, in and around late HC. Ectopic extracellular galectin-3 produces delayed osteoclast recruitment as well as expansion of the hypertrophic cartilage zone due to the delayed removal of HC at the chrondro-osseous junction. Our results point to galectin-3 as a downstream regulator of MMP-9 function at two distinct points during endochondral bone formation, differentiation of late HC and control of the recruitment and/or differentiation of TRAP-positive osteoclasts or osteoclast progenitors to the front of ossification.
Mmp-9 Null Mice Show Altered Expression and Extracellular Activities of Galectin-3 during Endochondral Bone Formation
Galectin-3 is synthesized in proliferating and prehypertrophic chondrocytes, with a clear nuclear and cytoplasmic localization in WT mice. During cartilage differentiation, galectin-3 synthesis decreases gradually until the late hypertrophic stage (Colnot et al., 1999). We report here that the Mmp-9 null phenotype is characterized by an abnormal accumulation of galectin-3 in and around late HC, even though there is no increase in mRNA in this particular zone of the growth plate. Galectin-3 also accumulated in and around (pre)osteoclasts
In culture, exogenous intact galectin-3 disrupted normal embryonic bone development, increasing the HC zone and decreasing recruitment and/or differentiation of preosteoclasts. These effects mimic the phenotype previously observed in embryonic bones of Mmp-9 null mice (Engsig et al., 2000). Galectin-3 cleaved by MMP-9 (monomeric) had no effect in our in vitro metatarsal culture experiments. Thus, we hypothesize that the functions of intact galectin-3 may be regulated through inactivation of the protein by proteolytic cleavage during endochondral bone formation on both sides of the chondro-osseous junction, in late HC and preosteoclasts or osteoclasts.
The slight expansion in the region of galectin-3 expression in proliferating and prehypertrophic zones of the growth plate suggests that MMP-9 may also regulate factors involved in the transition from chondrocyte prehypertrophy to hypertrophy. The expanded galectin-3 mRNA expression early in development was conserved in 2-wk-old mice and could have contributed to the increased HC zone in Mmp-9 null mice during the phase of rapid bone growth through its antiapoptotic effects. However, the expansion of the HC zone in Mmp-9 null mice does not result from an increase in chondrocyte proliferation or in expression of major differentiation markers such as Col10 (Vu et al., 1998).
Galectin-3 Is a Downstream Regulator of MMP-9 in Hypertrophic Chondrocyte Terminal Differentiation
Numerous studies have established intracellular galectin-3 as an antiapoptotic factor. Indeed, galectin-3 contains the anti-death domain characteristic of the Bcl-2 protein family (NWGR; Akahani et al., 1997), and cells that over express galectin-3 display increased resistance to apoptotic stimuli (for review see Krzeslak and Lipinsku, 2004; Wang et al., 2004). Galectin-3 is the only member of the galectin family that promotes cell survival (Akahani et al., 1997; Matarrese et al., 2000). It is now becoming clear that galectin-3 is an extracellular modulator of cell/ECM interactions that exhibits pro- and antiadhesive properties in different processes such as kidney development, angiogenesis, and metastasis (Nangia-Makker et al., 2000; Bullock et al., 2001; Fukushi et al., 2004; Takenaka et al., 2004). However, the previous studies do not differentiate between intracellular and extracellular functions of galectin-3.
We propose that extracellular galectin-3 negatively regulates terminal differentiation of HC, possibly by acting as an antiapoptotic matricellular protein that maintains ECM anchorage. In support of this hypothesis, exogenous galectin-3 expands the population of HC in cultured embryonic metatarsals. This is further supported by the galectin-3 null-mutant phenotype, where increased programmed cell death is observed at the chondro-vascular junction in the absence of galectin-3 (Colnot et al., 2001). It is possible that ECM remodeling by MMPs, particularly MMP-9 present along the last transverse septa, degrades galectin-3, thereby derepressing terminal differentiation or programmed cell death of HC and allowing endochondral ossification to proceed.
The HC of Mmp-9 null mice reached late differentiation as evidenced by their expression of Mmp-13 and Op. However, very few HC expressing Mmp-13 and Op were detectable in WT mice because of the rapid exit of these chondrocytes from the growth plate during the first 3 wk of postnatal life, when growth of long bones is maximal. We conclude that the Mmp-9 null phenotype results from a delay in the disappearance of late HC, characterized by galectin-3 accumulation and is probably due to a defect in the switch to terminal differentiation or programmed cell death.
Because there was no difference in galectin-3 expression in late HC between WT and Mmp-9 null mice, we argue that persistence of galectin-3 in late HC results from a defect in its degradation. We cannot exclude that MMP-9 deficiency induces an increase in galectin-3 because of an indirect role in controlling other proteases involved in galectin-3 degradation, but intriguingly, at this locale, galectin-3 and MMP-9 colocalize and the galectin-3 detected is present in Mmp-9 null mice as intact dimers. Moreover, our immunostaining data support that hypothesis, as strong staining for galectin-3 was seen all around the lacuna in hypertrophic cartilage of Mmp-9 null mice, where late HC survived and accumulated abnormally. The presence of galectin-3 dimers may promote HC survival through maintenance of a strong adhesion to the ECM. Indeed, galectin-3 overexpression can protect cells from apoptosis by improving cell adhesion (Matarrese et al., 2000), which is dependent on oligomerization of galectin-3, particularly through TG2 activity (Mehul et al., 1995; Mahoney et al., 2000). Because TG2 is expressed at identical levels in WT and Mmp-9 null chondrocytes, we hypothesize that the observed accumulation of galectin-3 dimers does not result from an increase in TG2 activity but more likely from an increase in the half-life of the dimers.
In vitro, galectin-3 is a direct substrate for MMP-9, MMP-2, and MMP-13, which cleave the amino terminal domain of the molecule at a common site (Ochieng et al., 1994; Guevremont et al., 2004). Because MMP-13 is expressed in Mmp-9 null HC, and Mmp-13 null mice exhibit only a modest expansion of the HC zone (Stickens et al., 2004), we suggest that MMP-9 is the dominant MMP for cleaving the N-terminal domain of galectin-3 in and around late HC. As this domain allows oligomerization of galectin-3, its integrity may be required for adhesion of HC to the ECM and consequent antiapoptotic effects. Cleavage of this domain by MMP-9 would therefore modify the affinity of galectin-3 for its ligands and promote HC terminal differentiation or programmed cell death. As such, we propose that galectin-3 is a downstream regulator of MMP-9 controlling terminal differentiation of HC at the chondro-osseous junction.
Aberrant Accumulation of Extracellular Galectin-3 Interferes with Recruitment of Osteoclast Progenitors
We observed two distinct effects of galectin-3 during endochondral ossification on cells of the osteoclast lineage. In our embryonic metatarsal culture experiments, an excess of exogenous intact galectin-3 decreased the initial TRAP-positive cells (preosteoclasts and osteoclasts) invading the chondro-osseous junction. Addition of galectin-3 cleaved by MMP-9 had no such effect on recruitment of TRAP-positive cells. In contrast, we observed increased TRAP staining at the front of ossification in 2-wk-old Mmp-9 null mice, in parallel with an increase in the number of galectin-3–positive cells. Osteoclasts and osteoclast precursors, which are monocyte-derived, express galectin-3 (Niida et al., 1994). Interestingly, galectin-3 has been described as a chemoattractant for monocytes and macrophages (Sano et al., 2000). It is likely that full-length galectin mediates this effect since both N- and C-terminal domains are required. Galectin-3 null mice have defective macrophage survival and adhesion (Hsu et al., 2000). However, no defect in osteoclast differentiation was reported in galectin-3 null mice and MMP-9–positive cells seemed to be recruited normally at the chondro-osseous junction (Colnot et al., 2001). It is possible that aberrant persistence of intact galectin-3 induces abnormal osteoclast survival and excess bone turnover.
Mmp-9 Null Mice Have Altered ECM and Disruption of Growth Plate Homeostasis
Homeostasis of the growth plate during long bone growth depends on a delicate balance between the rate of chondrocyte proliferation and the rate of HC disappearance or death. The critical role of metaphyseal vascular supply in this homeostasis has been demonstrated (Trueta and Amato, 1960), and VEGF has been identified as one of the major angiogenic factor involved in this process. Inhibition of VEGF by circulating soluble inhibitors (Gerber et al., 1999) or deficiency in VEGF long isoform expression (VEGF121/121; Maes et al., 2002; Zelzer et al., 2002) results in an enlarged HC zone, disturbed vascularization and altered bone mineralization. Moreover, trabecular bone volume, HC apoptosis, and osteoclast recruitment decrease.
The striking phenotypic similarities between these VEGF mutant mice and the Mmp-9 null mice indicate an important link between these factors. We found that many proteins accumulate aberrantly in extracts from Mmp-9 growth plates. Two mechanisms may contribute to these increases: prolonged synthesis of these proteins by late HC that normally would have exited the growth plate and/or reduced degradation of these proteins in the absence of MMP-9 activity. We identified two of these proteins as VEGF and galectin-3. In contrast to galectin-3, which accumulates most probably because of a decrease in its degradation, VEGF mRNA persists in the expanded hypertrophic cartilage zone of Mmp-9 null mice (Engsig et al., 2000), indicating that late HC synthesize VEGF as long as they survive. Because VEGF is essential for osteoclast recruitment into developing long bones (Engsig et al., 2000) and is a chemoattractant for the monocyte/macrophage lineage (Barleon et al., 1996; Clauss et al., 1996), this increase in VEGF protein may contribute to the increase in TRAP- and galectin-3–positive cells at the chondro-osseous junction of Mmp-9 null mice. It follows then that a decrease in VEGF should result in decreased numbers of TRAP-positive cells, which is indeed the case (Gerber et al., 1999; unpublished observations).
Acknowledgments
We thank Helen Capili, Angelo Kaplan, Ying Yu, and Andrew Tauscher for excellent technical assistance. This work was supported by grants from the National Institutes of Health (AR046238 and DE013058). N.O. was a Michael Geisman research fellow of the Osteogenesis Imperfecta Foundation and D.J.B. is a University of California Regents Fellow.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-12-1119) on March 30, 2005.
Abbreviations used: Col2, collagen type II; Col10, collagen type X; CTGF, connective tissue growth factor; ECM, extracellular matrix; HC, hypertrophic chondrocyte(s); MMP, matrix metalloproteinase; PECAM, platelet endothelial cell adhesion molecule; TRAP, tartrate-resistant acidic phosphatase; TG2, tissue transglutaminase; MT1-MMP, membrane type 1 matrix metalloproteinase; VEGF, vascular endothelial growth factor.
References
- Akahani, S., Nangia-Makker, P., Inohara, H., Kim, H. R., and Raz, A. (1997). Galectin-3, a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 57, 5272-5276. [PubMed] [Google Scholar]
- Albrecht, U., Eichele, G., Helms, J. A., and Lu, H. (1997). Visualization of gene expression patterns by in situ hybridization. In: Molecular and Cellular Methods in Developmental Toxicology, ed. G. Daston, Boca Raton, FL: CRC Press, 23-48.
- Aubin, J. E., Gupta, A. K., Bhargava, U., and Turksen, K. (1996). Expression and regulation of galectin 3 in rat osteoblastic cells. J. Cell Physiol. 169, 468-480. [DOI] [PubMed] [Google Scholar]
- Barleon, B., Sozzani, S., Zhou, D., Weich, H. A., Mantovani, A., and Marme, D. (1996). Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87, 3336-3343. [PubMed] [Google Scholar]
- Barondes, S. H. et al. (1994). Galectins: a family of animal beta-galactoside-binding lectins. Cell 76, 597-598. [DOI] [PubMed] [Google Scholar]
- Blavier, L., and Delaisse, J. M. (1995). Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J. Cell Sci. 108, 3649-3659. [DOI] [PubMed] [Google Scholar]
- Bullock, S. L., Johnson, T. M., Bao, Q., Hughes, R. C., Winyard, P. J., and Woolf, A. S. (2001). Galectin-3 modulates ureteric bud branching in organ culture of the developing mouse kidney. J. Am. Soc. Nephrol. 12, 515-523. [DOI] [PubMed] [Google Scholar]
- Chan, D., and Jacenko, O. (1998). Phenotypic and biochemical consequences of collagen X mutations in mice and humans. Matrix Biol. 17, 1169-1184. [DOI] [PubMed] [Google Scholar]
- Clauss, M., Weich, H., Breier, G., Knies, U., Rockl, W., Waltenberger, J., and Risau, W. (1996). The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 271, 17629-17634. [DOI] [PubMed] [Google Scholar]
- Colnot, C., Sidhu, S. S., Poirier, F., and Balmain, N. (1999). Cellular and subcellular distribution of galectin-3 in the epiphyseal cartilage and bone of fetal and neonatal mice. Cell. Mol. Biol. 45, 1191-1202. [PubMed] [Google Scholar]
- Colnot, C. I., and Helms, J. A. (2001). A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech. Dev. 100, 245-250. [DOI] [PubMed] [Google Scholar]
- Colnot, C., Sidhu, S. S., Balmain, N., and Poirier, F. (2001). Uncoupling of chondrocyte death and vascular invasion in mouse galectin 3 null mutant bones. Dev. Biol. 229, 203-214. [DOI] [PubMed] [Google Scholar]
- Colnot, C., Thompson, Z., Miclau, T., Werb, Z., and Helms, J. A. (2003). Altered fracture repair in the absence of MMP-9. Development 130, 4123-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo, M., Yan, Z., Nooreyazdan, M., Pacifici, M., Sarment, D. S., Billings, P. C., and Leboy, P. S. (2000). MMP-13 is induced during chondrocyte hypertrophy. J. Cell Biochem. 77, 678-693. [PubMed] [Google Scholar]
- Engsig, M. T. et al. (2000). Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J. Cell Biol. 151, 879-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, C., Alpern, E., Miclau, T., and Helms, J. A. (1999). Does adult fracture repair recapitulate embryonic skeletal formation? Mech. Dev. 87, 57-66. [DOI] [PubMed] [Google Scholar]
- Fowlis, D., Colnot, C., Ripoche, M. A., and Poirier, F. (1995). Galectin-3 is expressed in the notochord, developing bones, and skin of the postimplantation mouse embryo. Dev. Dyn. 203, 241-251. [DOI] [PubMed] [Google Scholar]
- Fukushi, J., Makagiansar, I. T., and Stallcup, W. B. (2004). NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol. Biol. Cell 15, 3580-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber, H. P., Vu, T. V., Ryan, A. M., Kowalski, J., Werb, Z., and Ferrara, N. (1999). VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5, 623-628. [DOI] [PubMed] [Google Scholar]
- Guevremont, M., Martel-Pelletier, J., Boileau, C., Liu, F. T., Richard, M., Fernandes, J. C., Pelletier, J. P., and Reboul, P. (2004). Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann. Rheum. Dis. 63, 636-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, M. K., and Springer, T. A. (1982). Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J. Immunol. 128, 1221-1228. [PubMed] [Google Scholar]
- Hsu, D. K., Yang, R-Y., Pan, Z., Yu, L., Salomon D. R., Fung-Leung, W.-P., and Liu, F.-T. (2000). Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am. J. Pathol. 156, 1073-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada, M. et al. (1999). Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 214, 279-290. [DOI] [PubMed] [Google Scholar]
- Karsenty, G., and Wagner, E. F. (2002). Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389-406. [DOI] [PubMed] [Google Scholar]
- Koshizuka, Y., Yamada, T., Hoshi, K., Ogasawara, T., Chung, U.-I., Kawano, H., Nakamura, Y., Nakamura, K., Ikegawa, S., and Kawaguchi, H. (2003). Cystatin 10, a novel chondrocyte-specific protein, may promote the last steps of the chondrocyte differentiation pathway. J. Biol. Chem. 278, 48259-48266. [DOI] [PubMed] [Google Scholar]
- Kronenberg, H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332-336. [DOI] [PubMed] [Google Scholar]
- Krzeslak, A., and Lipinska, A. (2004). Galectin-3 as a multifunctional protein. Cell Mol. Biol. Lett. 9, 305-328. [PubMed] [Google Scholar]
- MacLean, H. E., Kim, J. I., Glimcher, M. J., Wang, J., Kronenberg, H. M., and Glimcher, L. H. (2003). Absence of transcription factor c-maf causes abnormal terminal differentiation of hypertrophic chondrocytes during endochondral bone development. Dev. Biol. 262, 51-63. [DOI] [PubMed] [Google Scholar]
- Maes, C., Carmeliet, P., Moermans, K., Stockmans, I., Smets, N., Collen, D., Bouillon, R., and Carmeliet, G. (2002). Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech. Dev. 111, 61-73. [DOI] [PubMed] [Google Scholar]
- Mahoney, S. A., Wilkinson, M., Smith, S., and Haynes, L. W. (2000). Stabilization of neurites in cerebellar granule cells by transglutaminase activity: identification of midkine and galectin-3 as substrates. Neuroscience 101, 141-155. [DOI] [PubMed] [Google Scholar]
- Massa, S. M., Cooper, D. N., Leffler, H., and Barondes, S. H. (1993). L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry 32, 260-267. [DOI] [PubMed] [Google Scholar]
- Matarrese, P., Fusco, O., Tinari, N., Natoli, C., Liu, F. T., Semeraro, M. L., Malorni, W., and Iacobelli, S. (2000). Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int. J. Cancer 85, 545-554. [PubMed] [Google Scholar]
- Mehul, B., Bawumia, S., and Hughes, R. C. (1995). Cross-linking of galectin 3, a galactose-binding protein of mammalian cells, by tissue-type transglutaminase. FEBS Lett. 360, 160-164. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker, P., Honjo, Y., Sarvis, R., Akahani, S., Hogan, V., Pienta, K. J., and Raz, A. (2000). Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 156, 899-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niida, S., Amizuka, N., Hara, F., Ozawa, H., and Kodama, H. (1994). Expression of Mac-2 antigen in the preosteoclast and osteoclast identified in the op/op mouse injected with macrophage colony-stimulating factor. J. Bone Miner. Res. 9, 873-881. [DOI] [PubMed] [Google Scholar]
- Ochieng, J., Fridman, R., Nangia-Makker, P., Kleiner, D. E., Liotta, L. A., Stetler-Stevenson, W. G., and Raz, A. (1994). Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry 33, 14109-14114. [DOI] [PubMed] [Google Scholar]
- Ochieng, J., Furtak, V., and Lukyanov, P. (2004). Extracellular functions of galectin-3. Glycoconjugate J. 19, 527-535. [DOI] [PubMed] [Google Scholar]
- Sano, H., Hsu, D. K., Yu, L., Apgar, J. R., Kuwabara, I., Yamanaka, T., Hirashima, M., and Liu, F. T. (2000). Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J. Immunol. 165, 2156-2164. [DOI] [PubMed] [Google Scholar]
- Sato, S., Burdett, I., and Hughes, R. C. (1993). Secretion of the baby hamster kidney 30-kDa galactoside-binding lectin by from polarized and nonpolarized cell: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp. Cell Res. 207, 8-18. [DOI] [PubMed] [Google Scholar]
- Sato, T., del Carmen Ovejero, M., Hou, P., Heegaard, A. M., Kumegawa, M., Foged, N. T., and Delaisse, J. M. (1997). Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J. Cell Sci. 110, 589-596. [DOI] [PubMed] [Google Scholar]
- Stickens, D., Behonick, D. J., Ortega, N., Heyer, B., Hartenstein, B., Yu, Y., Schorpp-Kistner, M., Fosang, A., Angel, P., and Werb, Z. (2004). Altered endochondral bone formation in matrix metalloproteinase-13 deficient mice. Development 131, 5883-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock, M., Schaffer, H., Stricker, S., Gross, G., Mundlos, S., and Otto, F. (2003). Expression of galectin-3 in skeletal tissues is controlled by Runx2. J. Biol. Chem. 278, 17360-17367. [DOI] [PubMed] [Google Scholar]
- Takenaka, Y., Fukumori, T., and Raz, A. (2004). Galectin-3 and metastasis. Glycoconjugate J. 19, 543-549. [DOI] [PubMed] [Google Scholar]
- Trueta, J., and Amato, V. P. (1960). The vascular contribution to osteogenesis. III. Changes in the growth cartilage caused by experimentally induced ischemia. J. Bone Joint Surg. 42B, 571-587. [DOI] [PubMed] [Google Scholar]
- Tuckermann, J. P., Pittois, K., Partridge, N. C., Merregaert, J., and Angel, P. (2000). Collagenase-3 (MMP-13) and integral membrane protein 2a (Itm2a) are marker genes of chondrogenic/osteoblastic cells in bone formation: sequential temporal, and spatial expression of Itm2a, alkaline phosphatase, MMP-13, and osteocalcin in the mouse. J. Bone Miner. Res. 15, 1257-1265. [DOI] [PubMed] [Google Scholar]
- Vu, T. V., Shipley, J. M., Bergers, G., Berger, J. E., Helms, J. A., Hanahan, D., Shapiro, S. D., Senior, R. M., and Werb, Z. (1998). MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93, 411-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. L., Gray, R. M., Haudek, K. C., and Patterson, R. J. (2004). Nucleocytoplasmic lectins. Biochim. Biophys. Acta 1673, 75-93. [DOI] [PubMed] [Google Scholar]
- Wu, C. W., Tchetina, E. V., Mwale, F., Hasty, K., Pidoux, I., Reiner, A., Chen, J., Van Wart, H. E., and Poole, A. R. (2002). Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J. Bone Miner. Res. 17, 639-651. [DOI] [PubMed] [Google Scholar]
- Yang, R. Y., Hsu, D. K., and Liu, F. T. (1996). Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. USA 93, 6737-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelzer, E. R., McLean, W., Ng, Y. S., Fukai, N., Reginato, A. M., Lovejoy, S., D'Amore, P. A., and Olsen, B. R. (2002). Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 129, 1893-1904. [DOI] [PubMed] [Google Scholar]