Abstract
Synapsins are evolutionarily conserved, highly abundant vesicular phosphoproteins in presynaptic terminals. They are thought to regulate the recruitment of synaptic vesicles from the reserve pool to the readily-releasable pool, in particular when vesicle release is to be maintained at high spiking rates. As regulation of transmitter release is a prerequisite for synaptic plasticity, we use the fruit fly Drosophila to ask whether Synapsin has a role in behavioral plasticity as well; in fruit flies, Synapsin is encoded by a single gene (syn). We tackled this question for associative olfactory learning in larval Drosophila by using the deletion mutant syn97CS, which had been backcrossed to the Canton-S wild-type strain (CS) for 13 generations. We provide a molecular account of the genomic status of syn97CS by PCR and show the absence of gene product on Western blots and nerve-muscle preparations. We found that olfactory associative learning in syn97CS larvae is reduced to ∼50% of wild-type CS levels; however, responsiveness to the to-be-associated stimuli and motor performance in untrained animals are normal. In addition, we introduce two novel behavioral control procedures to test stimulus responsiveness and motor performance after “sham training.” Wild-type CS and syn97CS perform indistinguishably also in these tests. Thus, larval Drosophila can be used as a case study for a role of Synapsin in associative learning.
Synapsins are phylogenetically conserved and highly abundant presynaptic phosphoproteins associated with the cytoplasmic side of synaptic vesicles. The working model of Synapsin function in synaptic vesicle housekeeping (review by Hilfiker et al. 1999; for a critical review see Sudhof 2004) proposes that the balance between the readily-releasable and the reserve pool of synaptic vesicles, the latter being tethered to the cytoskeleton, is regulated by the phosphorylation status of Synapsins; thus, phosphorylation of Synapsins regulates the number of vesicles available for release. If Synapsin function is compromised, synaptic output per se remains functional, whereas the ability to maintain synaptic output at high, sustained spiking rates is compromised (Chi et al. 2003; Gitler et al. 2004). Given a role in regulating synaptic output, which is a prerequisite for synaptic plasticity, we ask whether Synapsin might have a role in behavioral plasticity as well. This seems timely, because despite much work on the cellular, molecular, developmental, and physiological levels (Angers et al. 2002; Chin et al. 2002; Ferreira and Rapoport 2002; Chi et al. 2003; Gitler et al. 2004; Hilfiker et al. 2005; for reviews see Hilfiker et al. 1999 and Sudhof 2004), the functional significance of Synapsin for behavior remains less well understood. In humans, Garcia et al. (2004) recently found that a mutation in the synapsin I gene causes severe neurological and behavioral phenotypes, including epilepsy and learning impairments. In the mouse, Silva et al. (1996) found learning impairments in synapsin II, but not synapsin I knockout mice; these results correlated with decreased post-tetanic potentiation in synapsin II, but not synapsin I mutants. In mice lacking all three synapsin genes, Gitler et al. (2004) documented that such triple mutants show delayed responses in a number of tested reflexes and diminished ability to hang from a suspended wire; they also noted that these animals show seizures upon disturbance by opening of the cage, reduced levels of piloerection, and difficulties maintaining balance when the cage is shaken. Importantly for the current context, Gitler et al. (2004) reported that in a test for spatial memory in an eight-arm radial maze, these animals performed poorly; reportedly, this phenotype is not due to deficits in motivation or motor ability.
In the genome of the fruit fly Drosophila melanogaster, only one synapsin gene (syn) is found (Klagges et al. 1996), which makes interpretation of phenotypes relatively straightforward. syn97 was recently described as carrying a 1.4-kb deletion spanning parts of the regulatory sequence of the syn gene and half of its first exon (Fig. 1A). As a consequence, adult syn97 mutants lack detectable Synapsin (Godenschwege et al. 2004) and hence—regarding adult flies—qualify as null mutants. Whether this is also true for larvae is at present unknown. In any event, the availability of a null mutant provides an opportunity to test whether behavioral plasticity might depend on Synapsin function. We tackled this question with regard to olfactory associative learning in larval Drosophila (Scherer et al. 2003; Hendel et al. 2005; Neuser et al. 2005). Such an endeavor seems timely, as the larva is a widely used model system to study synaptic physiology (Koh et al. 2000).
Figure 1.
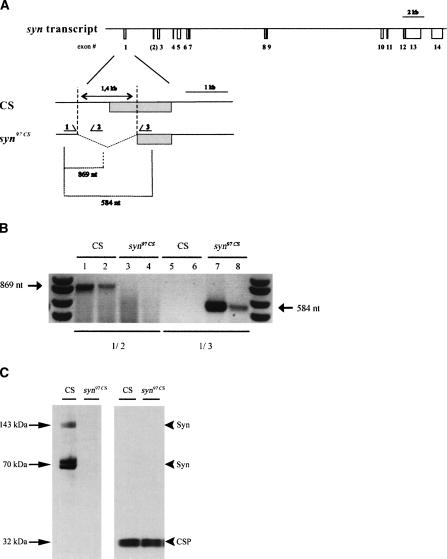
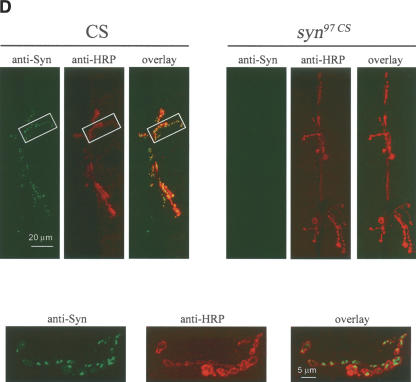
(A) Genomic organization of the Drosophila synapsin locus. syn97CS carries a 1.4-kb deletion spanning parts of the regulatory sequence and half of the first exon of the syn gene. The arrows indicate the binding sites for the PCR primers upstream (primer 1), within (primer 2), and downstream (primer 3) of the deletion. (B) syn97CS is a deletion mutant. In a single-larva PCR approach, primer combination 1/2 yields a 869-nt product in wild-type CS (two independent samples in lanes 1,2) but not in syn97CS (two independent samples in lanes 3,4), whereas primer combination 1/3 yields a 584-nt product in syn97CS (two independent samples in lanes 7,8) but not in wild-type CS (two independent samples in lanes 5,6). (C,D) syn97CS lack Synapsin. (C) Western blot from brains of larval Drosophila. The blot shows separate staining for Synapsin (left panel) and, after stripping the blot from the SYNORF1 antibody, for CSP as loading control (right panel). The left lanes were loaded from wild-type CS, the right lane from syn97CS.The SYNORF1 antibody labels bands at 74 and 143 kDa, where Synapsin is expected (Klagges et al. 1996; Godenschwege et al. 2004). These bands represent fused triple and double bands, respectively, and are absent in syn97CS. (D) Synapsin localizes to synaptic terminals. Immunofluorescence images of synaptic terminals innervating the larval body wall muscle pair 6/7 using double labeling with the SYNORF1 antibody and, for visualization of the motorneuron terminals, an anti-HRP antiserum. The anti-HRP antiserum stains neuronal cell membranes and thus visualizes motorneuron terminals (middle panels for wild-type CS and syn97CS in D). Synapsin immunoreactivity is seen exclusively in boutons of wild-type CS (leftmost panel for wild-type CS in D), where it colocalizes with anti-HRP (right panel for wild-type CS in D). In syn97CS larvae, no Synapsin immunoreactivity can be found (left panel for syn97CS in D). The insets in the lower part of the figure show magnifications of the area boxed in the upper panel; left and middle insets show Synapsin and HRP labeling, respectively; the right inset shows the overlay. Obviously, the membrane of the synaptic boutons is stained by the anti-HRP antiserum; the center of these terminals shows Synapsin immunoreactivity.
We exerted much effort in avoiding confounding effects of “marker” genes and genetic background. We outcrossed syn97 to the wild-type control strain CS for 13 generations such that the resulting syn97CS and wild-type CS essentially share the same genetic background. Such care is warranted given the effects of genetic background (De Belle and Heisenberg 1996) and of “marker” genes (Zhang and Odenwald 1995), which are often used to monitor the presence of transgenic constructs. We are thus confident that phenotypes in syn97CS are indeed attributable to the syn97 mutation and allow conclusions about Synapsin function.
After confirming the genomic status of syn97CS by PCR, we provide a characterization of syn97CS in the larva at the protein level. We show that in syn97CS immunoreactivity for Synapsin is absent on Western blots and from synaptic boutons at the neuromuscular junction. We then investigated whether syn97CS are defective in olfactory associative learning, and found that learning ability is reduced to ∼50% of wild-type CS levels. By introducing two additional, novel “sham training” control procedures, we made a special effort to test whether this learning defect may be secondary to any sensory or motor defects, which we found is not the case.
Results
Larval syn97 is null mutant on the protein level
In a single-larva approach, syn97CS showed a PCR product only for that combination of primers which lie up- and downstream of the deletion (primers 1 and 3; Fig. 1A,B), but not for those primers which lie upstream and within the deletion (primers 1 and 2; Fig. 1A,B). In wild-type CS, however, the 1/2 combination gives a product, but the 1/3 combination does not (Fig. 1B). This confirms the genomic status of syn97CS as carrying the reported 1.4-kb deletion of the syn gene (Godenschwege et al. 2004).
At the protein level, syn97CS clearly is a null mutant. syn97CS lack Synapsin immunoreactivity on the Western blot: Bands for the expected isoforms of Synapsin at 143 and 74 kDa (Klagges et al. 1996; Godenschwege et al. 2004) were detected in homogenates from larval brains of wild-type CS, but not from syn97CS (Fig. 1C). In Figure 1C, the blot was successively probed with the SYNORF1 antibody and, after stripping, with an antibody labeling the Cysteine String Protein (CSP) (band at 32 kDa) as a loading control.
To verify the absence of Synapsin immunoreactivity in situ, we investigated the synaptic terminals innervating the larval body wall musculature. We focused on the much-investigated muscle pair 6/7 and double-labeled the preparation with the SYNORF1 antibody and, in order to visualize the motorneurons, with an anti-HRP antiserum labeling neuronal cell membranes. Synapsin immunoreactivity was clearly seen in wild-type CS but not in syn97CS (top panels in Fig. 1D). Synapsin immunoreactivity colocalized with HRP immunoreactivity (overlay for wild-type CS in Fig. 1D). Specifically, a magnification of the boxed areas in Figure 1D shows that the membrane at the circumference of the synaptic boutons is stained by the anti-HRP antiserum, whereas Synapsin staining is seen cen-trally in these boutons; at these central sites, Synapsin colocalizes with Synaptotagmin immunoreactivity (Godenschwege et al. 2004), confirming its synaptic localization. Thus, the syn97CS strain obviously carries the genomic deletion of the syn gene as reported by Godenschwege et al. (2004) (Fig. 1A,B) and, also at the larval stage, qualifies as a null mutant for Synapsin at the protein level (Fig. 1C,D). In a next step, we therefore asked whether these mutants would be altered in their learning ability.
Larval syn97 are impaired in learning
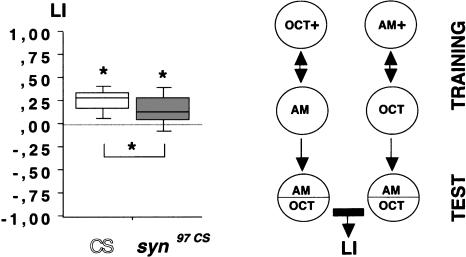
We tested wild-type CS and syn97CS larvae for their ability to associate odors with a fructose reward in an en masse assay (Neuser et al. 2005); we found that both wild-type CS (Fig. 2; one-sample sign test: P < 0.05; n = 27), and syn97CS (Fig. 2; one-sample sign test: P < 0.05; n = 27) learn this association; however, wild-type CS learn significantly better than syn97CS (Fig. 2; P < 0.05, U = 233, sample sizes as above), which show only 50% of the median wild-type CS learning score.
Figure 2.
syn97CS larvae are impaired in learning: en masse assay. In an en masse assay for olfactory associative learning, syn97CS show ∼50% of the learning index (LI) of wild-type CS. The inset figure illustrates the behavioral procedure; please note that in half of the cases we started training with OCT+ or AM+ as indicated; for the other half of the cases, we started training with AM or OCT. *: P < 0.05. Box plots represent the median as the middle line, 25% and 75% quantiles as box boundaries, as well as 10% and 90% quantiles as whiskers, respectively.
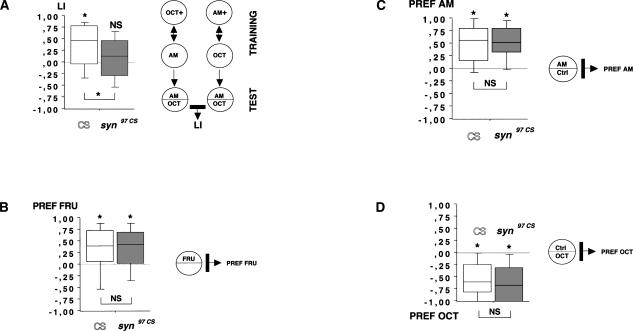
We confirmed this effect in the individual-animal version of this learning paradigm (Scherer et al. 2003; Hendel et al. 2005; Neuser et al. 2005). We found that wild-type CS learn well also in this paradigm (Fig. 3A; one-sample sign test: P < 0.05; n = 39), whereas syn97CS do not show significant learning (Fig. 3A; one-sample sign test: P > 0.05; n = 45). In a direct comparison, wild-type CS learn significantly better than syn97CS (Fig. 3A; P < 0.05, U = 621.5; sample sizes as above), which show a >50% reduction in learning ability.
Figure 3.
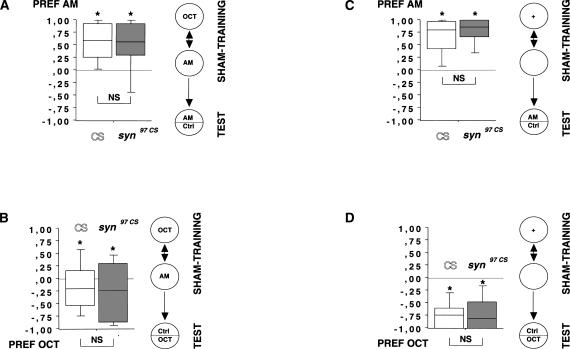
(A) Confirming the learning deficit in syn97CS larvae in an individual-animal version of the learning assay. Learning in syn97CS is reduced to <50% of wild-type CS levels. (B,C,D) Behavioral controls in naive larvae. Responses to the positive reinforcer (B: FRU) and detection of the used odors (C,D: AM and OCT, respectively) are not different between genotypes; thus, the learning impairment in syn97CS is not due to deficits in detecting the to-be-learned stimuli. All experiments used individually assayed larvae. Insets in each figure illustrate the behavioral procedure. *: P < 0.05. For explanation of the box plots, see Figure 2 legend.
Behavioral controls: No defect of syn97 in canonical, naive animal tests
Low learning scores may, apart from “genuine” defects in learning, result from more general defects in the ability to taste or smell or to behaviorally respond to tastants and odors. The canonical approach to these problems is to compare experimentally naive, untrained animals in terms of their responses to the to-be-associated stimuli. Both wild-type CS and syn97CS larvae show a significant preference for fructose over pure agarose (Fig. 3B; wild-type CS: one-sample sign test: P < 0.05; n = 32; syn97CS: one-sample sign test: P < 0.05; n = 32). Importantly, there is no difference between the genotypes with respect to fructose preference (Fig. 3B; P > 0.05; U = 509.0; sample sizes as above).
Concerning odors, typically one chooses concentrations for the learning experiments such that naive, wild-type animals distribute about equally between them (Scherer et al. 2003). Therefore, if one would compare naive odor choice between wild-type and mutant, one would “ideally” expect both to be indifferent between the two odors. This indifference, however, may come about for different reasons in the two genotypes: the wild-type may be truly indifferent between the two odors, whereas the mutant may be anosmic. This problem of interpretation is typically avoided by testing olfactory detection ability in an odor versus no-odor setup. In the present case, both wild-type CS and syn97CS larvae show significant attraction to both of the odors used (wild-type CS: for amylacetate (AM) in Fig. 3C, one-sample sign test, P < 0.05, n = 72; for 1-octanol (OCT) in Fig. 3D, one-sample sign test, P < 0.05, n = 64) (syn97CS: for AM in Fig. 3C, one-sample sign test, P < 0.05, n = 72; for OCT in Fig. 3D, one-sample sign test, P < 0.05, n = 60). Importantly, there is no difference between the genotypes with respect to preference for either odor (for AM: Fig. 3C, P > 0.05, U = 2400.0; for OCT: Fig. 3D, P > 0.05, U = 1675.5; sample sizes as above). Thus, syn97CS likely are impaired specifically in associating odors with a fructose reward.
Two novel behavioral controls: No defect of syn97 after “sham training”
Clearly, learning can be measured only after training. Therefore, rather than testing experimentally naive animals, one may argue that the olfactory and motor abilities which the animals need during testing must be investigated (as no gustatory abilities are required during testing, this objection does not apply concerning taste). This is because such training by necessity encompasses handling, exposure to reinforcers, and exposure to odors, all of which may alter odor responsiveness on their own behalf (see Discussion). In particular, handling and/or stimulus exposure may render mutants unresponsive to odors, an effect that may feign a “learning” phenotype in such mutants. We therefore tested whether syn97CS are still able to detect and respond to the odors after either of two “sham-training” treatments. These do not involve associative training but the very same handling as during training plus (1) exposure to the odors (but not the reinforcer); (2) exposure to the reinforcer (but not the odors). We found that responses to either odor are equal between wild-type CS and syn97CS after both sham training with odor exposure (for AM responses: Fig. 4A, P > 0.05 U = 786, n = 40, 40; for OCT responses: Fig. 4B, P > 0.05, U = 1053.5, n = 48, 48) and after “sham training” with reward exposure (for AM responses: Fig. 4C, P > 0.05 U = 1014.5, n = 48, 48; for OCT responses: Fig. 4D, P > 0.05, U = 1103.5, n = 48, 48). These results argue that the learning deficit in syn97CS is not secondary to an altered susceptibility to effects of handling, odor exposure, or reward exposure. As the motor abilities that are required for odor detection after sham training are the same as those required to express memory after training, these results also argue that no critical motor abilities are impaired in syn97CS.
Figure 4.
(A-D) No genotype differences after sham training. The two sham training procedures involve the same training procedure as shown in the Fig. 3A inset, except that either the reinforcer (A,B) or the odors (C,D) were omitted. After sham training, animals were tested for their ability to detect AM (A,C) and OCT (B,D), respectively. In neither of the sham training experiments did we uncover any difference between wild-type CS and syn97CS. All experiments used individually assayed larvae. Insets in each figure illustrate the behavioral procedure. *: P < 0.05. For explanation of the box plots, see Figure 2 legend.
After pooling the data in Figure 4 across genotypes, it is obvious that OCT responses are lower after odor-exposure sham training compared to reward-exposure sham training (pooled data from Fig. 4B vs. pooled data from Fig. 4D; P < 0.05, U = 2005, n = 96, 96); the same effect is, albeit less obviously, seen for AM as well (pooled data from Fig. 4A vs. pooled data from 4C; P < 0.05, U = 2817.5, n = 80, 96). Statistical comparisons to naive odor responses (Fig. 3C,D) are not possible, as the data in Figures 3 and 4 were gathered some months apart; therefore in a formal sense it must remain an open question whether this effect represents an increase from naive odor responses due to reward-exposure sham training, or a decrease from naive odor responses due to odor-exposure sham training. Contemplating Figure 3D versus Figure 4B, though, the latter possibility seems the better guess. In any event, whatever the reason(s) for this effect (habituation, adaptation, changes in motivation, changes in the concentration of the odors), three points are important to note: first, there is no reason to question the interpretation of the learning index as a pure measure of associative learning. This is because the learning index reflects the difference between reciprocally trained groups, and the change in odor responses by necessity will happen in both these reciprocally trained groups. In other words, an effect that occurs in both groups cannot cause differences between them. Second, obviously sham training does have effects on odor responses; thus, it is necessary to control for possible between-genotype differences in these effects because they could feign “learning phenotypes.” Third and most important for the present study, wild-type CS and syn97CS are equal in terms of both naive odor responses (Fig. 3C,D), and in terms of odor responses after sham training (Fig. 4A-D). This means that any changes in odor responses that come along with training affect both genotypes in the same way. Thus, the difference between the genotypes in their learning ability (Figs. 2, 3A) cannot be secondary to differences in terms of changed odor responses.
Discussion
We report that syn97CS is a protein-null mutant at the larval stage (Fig. 1C,D), and that associative learning in syn97CS larvae is reduced to ∼50% of wild-type CS levels (Figs. 2, 3A). Concerning the behavioral specificity of this learning defect, we tested experimentally naive, untrained animals in terms of their responses to the to-be-associated stimuli and found no difference between wild-type CS and syn97CS (Fig. 3B,C,D). This shows that at the beginning of training, genotypes are equal with respect to their olfactory ability and thus have the same ability to establish odor memories. These kinds of behavior-specificity controls have been state of the art until to date. We took an extra effort and compared olfactory behavior in wild-type CS and in syn97CS after “sham training,” i.e. after (1) handling and exposure to the odors; (2) handling and exposure to the reinforcer. These procedures seem critical to evaluate whether in syn97CS handling or stimulus exposure may deteriorate olfactory or motor abilities, as they are required to express memory during test. That is, handling may deteriorate motivation, lead to fatigue, and/or change the value of odors; repeated odor exposure may reduce olfactory responses by sensory adaptation (Cobb and Domain 2000) or habituation (concerning adult flies: Cho et al. 2004), and sugar exposure may entail motivational changes which distort olfactory behavior (for an analogous effect of electric shock in adult flies, Preat 1998). However, wild-type CS and syn97CS did not differ in odor responses after either sham training regime (Fig. 4A-D); thus, the low learning scores in syn97CS reflect a genuine learning defect.
Gross brain anatomy (data not shown), as well as basic synaptic function (measured by excitatory junction potentials at the neuromuscular junction), the number of synaptic boutons on muscles 6/7 and 12/13, and the number of synaptic vesicles around the active zone of type Ib synapses on these muscles are unaltered in syn97 (Godenschwege et al. 2004). Together, these data suggest a specific contribution of Synapsin for behavioral associative plasticity in Drosophila larva.
In adult Drosophila, a phenotype of syn97CS in odor-shock learning is more moderate than in larvae, i.e., adult syn97CS retain ∼80% of wild-type CS learning levels (Godenschwege et al. 2004). Together with our data, this supports the notion that relatively low levels of learning can be achieved without Synapsin; beyond that level, however, Synapsin is needed. In any event, the common, yet unequally strong, associative learning phenotypes of syn97CS across different learning paradigms and across the stages of metamorphosis suggest a rather general contribution of Synapsin to associative plasticity.
With respect to synaptic plasticity, there is at present no way to directly and in vivo observe synaptic plasticity in central brain neurons in Drosophila—one is limited to observing synaptic plasticity at the larval neuromuscular junction (Koh et al. 2000). Inferences from plasticity phenomena at the larval motorneuron-to-muscle synapse to central brain synapses, however, are of debatable value. Still, the robust larval learning phenotype of syn97CS reported here lays the foundation for three lines of doable further research: (1) When and in which parts of the brain would transgenic expression of the wild-type protein be sufficient to restore learning in the mutant background, and where is Synapsin function necessary in the normal brain? (2) Which functional domains of Synapsin are playing a role (Hilfiker et al. 2005), in particular with respect to the putative phosphorylation sites of the protein? (3) Which role does the editing of the syn mRNA (Diegelmann et al. 2003) play in this respect? The latter two questions may be relevant for Synapsin function in general, and in particular may contribute to our understanding of Synapsin-dependent forms of epilepsy and learning impairments in mice (Silva et al. 1996; Gitler et al. 2004) and humans (Garcia et al. 2004). Along these lines, research on Drosophila, including the larva, should be helpful.
Materials and Methods
We used third-instar feeding stage larvae aged 5 d after egg laying. Flies were kept in mass culture and maintained at 25°C, 60%-70% relative humidity, and a 14/10-h light/dark cycle. Experimenters were blind with respect to genotype and treatment condition in all cases; these were decoded only after the experiments.
Fly strains
We compared wild-type CS larvae to the deletion mutant syn97CS. This strain was generated by 13 outcrossing steps from syn97, which had been obtained from a jump-out mutagenesis of SynP1+P2 (Godenschwege et al. 2004). This jump-out line is characterized by a 1.4-kb deletion spanning parts of the regulatory sequence and half of the first exon of the syn gene (Fig. 1A). The outcrossing regime ensured that the residual phenotypic markers for the presence of the P-element in syn97 were removed and that the genetic background of syn97 and wild-type CS is essentially the same. Outcrossing steps always involved several single couples of heterozygous syn97/CS crossed with CS/CS flies. This resulted in a first filial generation where the genotype of each individual is unknown, but which consists again of syn97/CS and CS/CS flies. These were mated in single pairs to wild-type CS, and the genotype of the questionable part among the parents was determined via single-fly PCR (Gloor et al. 1993). Finally, single crossings among the heterozygous progeny resulted in the newly established strain syn97CS. Heterozygous flies could be identified as they show two PCR products, in contrast to the homozygous wild-type CS and syn97CS.
Single-animal PCR
PCRs were carried out according to Gloor et al. (1993), using material from individual larvae. The primer binding sites were upstream (primer 1: 5′-AGAAAATTTGGCTTGCATGG-3′), within (primer 2: 5′-CGGGGTCTCAGTTTTGTTG-3′), or downstream (primer 3: 5′-CCTCTACTTTTGGCTGCCTG-3′) of the deletion (Fig. 1A). The primer pair 1/2 gives an 869-nucleotide product in only wild-type CS, whereas primer pair 1/3 results in a 584-nucleotide product in only syn97CS flies (because in wild-type CS the template is too long for amplification).
Western blot
For each lane in the Western blots, 10 larval brains were homogenized in 10 μL 2 × SDS gel loading buffer; whole-larva homogenates do not yield a signal in Western blots because of insufficient protein concentration and/or degradation by proteases. The sample was heated to 70°C for 5 min and centrifuged for 2 min before electrophoresis. Proteins were separated by 8.5% SDS-PAGE in a Multigel chamber (150 V, 3 h; Biometra) and transferred to nitrocellulose membranes (Khyse-Andersson 1984). Immunoreactions were successively performed with two monoclonal antibodies: SYNORF1 for Synapsin detection (Klagges et al. 1996) (dilution 1:100), and ab49 (Zinsmaier et al. 1990, 1994) (dilution 1:400) for detection of the Cysteine String Protein (CSP; Arnold et al. 2004) as loading control. Visualization was achieved with the “ECL” Western blot detection system (Amersham) according to the manufacturer's specifications. We stripped and reprobed the blot: The membrane was first stained for Synapsin, then incubated for 30 min in stripping buffer to remove the SYNORF1 antibody (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris HCL pH 6.8; 58°C), and only then probed for CSP as loading control. To reduce the background staining of the membrane, in both staining steps the antibodies were dissolved in blocking buffer (5% milk powder in 1 × TBST).
Immunohistochemistry
For double immunofluorescence analyses, larval body wall muscles were dissected in Ca2+-free saline (Stewart et al. 1996) and fixed in ice-cold 4% paraformaldehyde for 30 min. The preparations were washed in PBS/0.1% Triton (PBST) followed by a 1-h incubation with blocking solution, and then incubated overnight at 4°C with the monoclonal anti-Synapsin mouse antibody SYNORF1 (diluted 1:10). The primary antibody was detected after 1 h incubation with Alexa 488 goat antimouse Ig (diluted 1:250) (green); during that step, the preparation was co-incubated with a Texas Red-coupled rabbit anti-HRP antibody (diluted 1:200) (Jackson labs) (red). All incubation steps were followed by multiple PBST washes; the detector incubation step was performed under light protection. Finally, preparations of the larvae were examined under a confocal microscope, aiming at muscle pair 6/7 of the body wall and its innervation by motor neurons.
Learning experiments
Methods for learning experiments follow previous work (Scherer et al. 2003; Hendel et al. 2005; Neuser et al. 2005) (see insets of Figs. 2A and 3A for sketches of the learning paradigm). In brief, we trained groups of 30 larvae and compared olfactory choice performance after either of two reciprocal training regimes: For one regime, animals received amylacetate (AM) with a positive reinforcer and 1-octanol (OCT) without such reinforcer (AM+/OCT); for the second regime, animals were trained reciprocally (AM/OCT+). Then, animals were tested for their choice between AM versus OCT. Associative learning is indicated by systematic differences in test performance between the reciprocal treatment conditions. This conclusion is compelling, as during training animals from both training regimes had identical exposure to both odorants and the reward; what differs between them is solely the contingency between these stimuli. The reciprocally trained groups were run alternately, which allows stringent pairing of data for the calculation of a learning index (LI; see below).
Petri dishes (85-mm inner diameter; Sarstedt) were filled with 1% agarose (electrophoresis grade; Roth), allowed to solidify, covered with their lids, and then left untreated until the following day. As positive reinforcer we used 2 mol fructose (FRU, purity: 99%) added to 1 L of agarose 10 min after boiling.
Experiments were performed in red light under a fume hood at 21°-24°C. Before experiments, we replaced the regular lids of the petri dishes with lids perforated in the center by 15 1-mm holes to improve aeration.
A spoonful of food medium containing larvae was taken from the food bottle and transferred to a glass vial. Thirty animals were collected, briefly washed in tap water, and as a group transferred to the assay plates for the start of training. Each training trial lasted 1 min. Immediately before a trial, two containers loaded with the same odorant (for details see below) were placed on the assay plate on opposite sides of the plate, 7 mm from the edges. Within each reciprocal training condition, for half of the cases we started with AM, for the other with OCT. Thus, for half of the cases we started with an agarose plate that had FRU added to the substrate, and for the other we started with a plate without FRU. Then, the lid was closed and the larvae were allowed to move for 1 min. The larvae were then transferred to a plate with the alternative odorant and the respective other substrate for 1 min. This cycle was repeated three times. Fresh assay plates were used for each trial.
After this training, animals were tested for their odor choice. The larvae were placed in the middle of a fresh, pure agarose assay plate with a container of AM on one side and one of OCT on the other side to create a choice situation. After 3 min, the number of animals on the “AM” or “OCT” side was counted. After this test was completed, the next group of animals was run and trained reciprocally. For both groups, we then calculated an odor preference ranging from -1 to 1. We determined the number of animals observed on the AM side (#AM) minus the number of animals observed on the OCT side (#OCT), divided by the total number of larvae (#TOTAL):
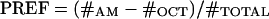 |
(1) |
To determine whether these preferences are different depending on training regime, we took the paired data from the alternately run, reciprocally trained groups and calculated a learning index ranging from -1 to 1 as:
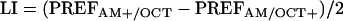 |
(2) |
After the data for one such LI value in one genotype had been collected, the corresponding data for an LI value of the other genotype were gathered, i.e., data from both genotypes were obtained alternately. In a conservative approach, we used nonparametric analyses throughout; comparisons of LIs against zero, i.e., random level, were made with one-sample sign tests, and comparisons of LIs between two genotypes were done with Mann-Whitney U-tests.
Regarding olfactory stimuli, we followed previous work (Scherer et al. 2003; Hendel et al. 2005; Neuser et al. 2005) and used OCT (purity: 99.5%) and AM (purity: 99%, diluted 1:50 in paraffin oil). Odorant was applied by adding 10 μL of odor substance into Teflon containers (5-mm inner diameter) which could be closed by a perforated lid (seven holes, 0.5-mm diameter).
We wanted to back up our results in the paradigm of Scherer et al. (2003), which used individually assayed animals. This assay differs from the above en masse assay introduced by Neuser et al. (2005) in that (1) a group of eight, rather than 30, was trained; (2) a 1-min break was introduced between training trials; (3) 10, rather than three training trials were given. Most importantly, (4) the test was performed on individual animals. Following Scherer et al. (2003), the position of the individual larva during the test was noted every 20 sec for 5 min as “AM,” “OCT,” or “neutral” (a 7-mm-wide zone in the middle of the assay plate). To calculate the odor preferences for each animal we determined the number of times a given animal was observed on the AM side during the test minus the number of times that animal was observed on the OCT side, divided by the total number of observations. For calculating the LI, we took the pairs of individuals from either of the two training conditions and calculated analogous to equation 2. These data were then statistically compared as detailed above.
Controls for detection of FRU and the odors
In corresponding control assays, we determined the ability of individually assayed animals to detect FRU and the odors. To test the ability to detect FRU, we prepared split petri dishes according to Heimbeck et al. (1999), with one side pure agarose and the other with FRU added to the agarose (for a sketch, see Fig. 3B inset). To test the ability of larvae to detect the odorants used, we took experimentally naive animals and gave them the choice between either paraffin-diluted AM versus paraffin, or between undiluted OCT versus an empty container (for sketches, see Fig. 3C,D insets). For both FRU detection and odor detection, animals were assayed individually; data acquisition, calculation of the PREF values, and data analysis follow the procedure for the odor choice test in individual animals detailed in the preceding paragraph.
Two novel “sham training” controls
Additionally, we introduced two novel sham training controls (see Discussion for a more detailed description of the motivation for these experiments). This seemed warranted to test whether genotype differences in learning may be secondary to differences in the susceptibility to odor or reward exposure. Therefore, we determined the ability of individually assayed animals from both genotypes to detect the odors after either of two sham training treatments. The first tests for genotype-differences with respect to the effects of odor exposure: it consists of the same treatment as in the individual animal learning assay, except that the reinforcer was omitted (for a sketch, see Fig. 4A,B insets). The second tests for differences in terms of reward exposure: it also consists of the same treatment as in the learning assay, but in turn omits the odors (for a sketch, see Fig. 4C,D insets). The tests for odor detection after either kind of sham training involved choices either between paraffin-diluted AM versus paraffin, or between undiluted OCT versus an empty container.
All statistical analyses were performed with StatView on a MacIntosh (significance: P < 0.05).
Acknowledgments
Start-up funds for this research program were provided by the VolkswagenFoundation (I/76 240, to B.G.). Current support comes from the Deutsche Forschungsgemeinschaft (SFB 554-A2 and Graduiertenkolleg GRK 200 Arthropod Behavior to E.B.; SFB 554-B6 to M. Heisenberg in support of B.G.), the International Human Frontiers Science Program Organization (long-term fellowship to H.T.), and the Studienstiftung des Deutschen Volkes (to I.S.). Thanks to K. Gerber for assistance with and B. Poeck for advice concerning the PCR experiments; to M. Porsch for advice to improve Western blotting; to colleagues from the Würzburg lab, in particular D. Dudazek, K. Neuser, X.-b. Mao, and A. Yarali for many helpful contributions. Finally, special thanks go to the referee who pointed to adaptation/habituation as a source of the synapsin phenotype and who thus inspired the sham training controls.
Dedicated to the memory of U. Werner.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.92805.
References
- Angers, A., Fioravante, D., Chin, J., Clearly, L.J., Bean, A.J., and Byrne, J.H. 2002. Serotonin stimulates phosphorylation of Aplysia synapsin and alters its subcellular distribution in sensory neurons. J. Neurosci. 22: 5412-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, C., Reisch, N., Leibold, C., Becker, S., Prüfert, K., Sautter, K., Palm, D., Jatzke, S., Buchner, S., and Buchner, E. 2004. Structure-function analysis of the cysteine string protein in Drosophila: Cysteine string, linker and C terminus. J. Exp. Biol. 207: 1323-1334. [DOI] [PubMed] [Google Scholar]
- Chi, P., Greengard, P., and Ryan, T.A. 2003. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron 38: 69-78. [DOI] [PubMed] [Google Scholar]
- Chin, J., Angers, A., Clearly, L.J., Eskin, A., and Byrne, J.H. 2002. Transforming growth factor β 1 alters synapsin distribution and modulates synaptic depression in Aplysia. J. Neurosci. 22: RC220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, W., Heberlein, U., and Wolf, F.W. 2004. Habituation of an odorant-induced startle response in Drosophila. Genes Brain Behav. 3: 127-137. [DOI] [PubMed] [Google Scholar]
- Cobb, M. and Domain, I. 2000. Olfactory coding in a simple nervous system: Adaptation in Drosophila larvae. Proc. Royal Soc. London (B) 267: 2119-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Belle, J.S. and Heisenberg, M. 1996. Expression of Drosophila mushroom body mutations in alternative genetic backgrounds: A case study of the mushroom body miniature gene (mbm). Proc. Natl. Acad. Sci. 93: 9875-9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann, S., Werner, U., Godenschwege, T.A., Reisch, D., Putz, G., Schwaerzel, M., and Buchner, E. 2003. Molecular and phenotypical characterization of the Drosophila synapsin mutant. In The Neurosciences from research to therapy. Proceedings of the 29th Göttingen Neurobiology Conference. (eds. N. Elsner and H. Zimmermann). Abstract 759. Thieme, Stuttgart, New York.
- Ferreira, A. and Rapoport, M. 2002. The synapsins: Beyond the regulation of neurotransmitter release. Cell. Mol. Life Sci. 59: 589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, C.C., Blair, H.J., Seager, M., Coulthard, A., Tennant, S., Buddles, M., Curtis, A., and Goodship, J.A. 2004. Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J. Med. Genet. 41: 183-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler, D., Takagishi, Y., Feng, J., Ren, Y., Rodriguiz, R.M., Wetsel, W.C., Greengard, P. and Augustine, G.J. 2004. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J. Neurosci. 24: 11368-11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor, G.B., Preston, C.R., Johnson-Schlitz, D.M., Nassif, N.A., Phillis, R.W., Benz, W.K., Robertson, H.M., and Engels, W.R. 1993. Type I repressors of P element mobility. Genetics 135: 81-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege, T.A., Reisch, D., Diegelmann, S., Eberle, K., Funk, N., Heisenberg, M., Hoppe, V., Hoppe, J., Klagges, B.R.E., Martin, J.R., et al. 2004. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur. J. Neurosci. 20: 611-622. [DOI] [PubMed] [Google Scholar]
- Hendel, T., Michels, B., Neuser, K., Schipanski, A., Kaun, K., Sokolowski, M.B., Marohn, F., Michel, R., Heisenberg, M., and Gerber, B. 2005. The carrot, not the stick: Appetitive rather than aversive gustatory stimuli support associative olfactory learning in individually assayed Drosophila larvae. J. Comp. Physiol. (A) 191: 265-279. [DOI] [PubMed] [Google Scholar]
- Heimbeck, G., Bugnon, V., Gendre, N., Häberlin, C., and Stocker, R.F. 1999. Smell and taste perception in Drosophila melanogaster larva: Toxin expression studies in chemosensory neurons. J. Neurosci. 19: 6599-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker, S., Benfenati, F., Doussau, F., Nairn, A.C., Czernik, A.J., Augustine, G.J., and Greengard, P. 2005. Structural domains involved in the regulation of transmitter release by synapsins. J. Neurosci. 25: 2658-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker, S., Pieribone, V.A., Czernik, A.J., Kao, H.-T., Augustine, G.J., and Greengard, P. 1999. Synapsins as regulators of neurotransmitter release. Phil. Trans. Royal Soc. (B) 354: 269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khyse-Anderson, J. 1984. Electroblotting of multiple gels: A simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Meth. 10: 203-209. [DOI] [PubMed] [Google Scholar]
- Klagges, B., Heimbeck, G., Godenschwege, T.A., Hofbauer, A., Pflugfelder, G.O., Reifegerste, R., Reisch, D., Schaupp, M., Buchner, S., and Buchner, E. 1996. Invertebrate synapsins: A single gene codes for several isoforms in Drosophila. J. Neurosci. 16: 3154-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, Y.H., Gramates, L.S., and Budnik, V. 2000. Drosophila larval neuromuscular junction: Molecular components underlying synaptic plasticity. Micros. Res. Tech. 49: 14-25. [DOI] [PubMed] [Google Scholar]
- Littleton, J.T., Bellen, H.J., and Perin, M.S. 1993. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development 118: 1077-1088. [DOI] [PubMed] [Google Scholar]
- Neuser, K., Husse, J., Stock, P., and Gerber, B. 2005. Appetitive olfactory learning in Drosophila larvae: Testing for effects of training amount, reinforcer intensity, age, gender, assay type, and memory span. Anim. Behav. 69: 891-898. [Google Scholar]
- Preat, T. 1998. Decreased odor avoidance after electric shock in Drosophila mutants biases learning and memory tests. J. Neurosci. 18: 8534-8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, S., Stocker, R.F., and Gerber, B. 2003. Olfactory learning in individually assayed Drosophila larvae. Learn. Mem. 10: 217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, A.J., Rosahl, T.W., Chapman, P.F., Marowitz, Z., Friedman, E., Frankland, P.W., Cestari, V., Cioffi, D., Sudhof, T.C., and Bourtchuladze, R. 1996. Impaired learning in mice with abnormal short-lived plasticity. Curr. Biol. 6: 1509-1518. [DOI] [PubMed] [Google Scholar]
- Stewart, B.A., Schuster, C.M., Goodman, C.S., and Atwood, H.L. 1996. Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J. Neurosci. 16: 3877-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof, T.C. 2004. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27: 487-507. [DOI] [PubMed] [Google Scholar]
- Zhang, S.D. and Odenwald, W.F. 1995. Misexpression of the white (w) gene triggers male-male courtship in Drosophila. Proc. Natl. Acad. Sci. 92: 5525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsmaier, K.E., Hofbauer, A., Heimbeck, G., Pflugfelder, G.O., Buchner, S., and Buchner, E. 1990. A cysteine-string protein is expressed in retina and brain of Drosophila. J. Neurogenet. 7: 15-29. [DOI] [PubMed] [Google Scholar]
- Zinsmaier, K.E., Eberle, K.K., Buchner, E., Walter, N., and Benzer, S. 1994. Paralysis and early death in cysteine string protein mutants of Drosophila. Science 263: 977-80. [DOI] [PubMed] [Google Scholar]