Abstract
The prefrontal cortex (PFC) is known to actively hold information “online” for a period of seconds in working memory for guiding goal-directed behavior. It has been proposed that relevant information is stored in other brain regions, which is retrieved and held in working memory for subsequent assimilation by the PFC in order to guide behavior. It is uncertain whether PFC stores information outside the temporal limits of working memory. Here, we demonstrate that although enhanced cAMP-dependent protein kinase A (PKA) activity in the PFC is detrimental to working memory, it is required for performance in tasks involving conflicting representations when memory storage is needed for minutes. This study indicates that distinct molecular mechanisms within the PFC underlie information storage for seconds (working memory) and for minutes (short-term memory). In addition, our results demonstrate that short-term memory storage within the prefrontal cortex is required for guiding behavior in tasks with conflicts and provides a plausible mechanism by which the prefrontal cortex executes cognitive control.
The prefrontal cortex (PFC) is engaged by tasks that require the selection and flexible use of information for guiding thought and planning action (Luria 1966; Milner 1982). This structure has been shown to actively maintain information for a period of seconds, a process termed working memory, that is critical for many of its higher cognitive functions (Baddeley 1992). Delay match-to-sample tasks in the monkey, and analogous tasks in the rat, have demonstrated that prefrontal neurons exhibit persistent delay period activity for the purposes of guiding a future response (Fuster and Alexander 1971; Kojima and Goldman-Rakic 1982; Baeg et al. 2003). However, this persistent delay activity, a neural correlate of working memory, has only been observed to last for up to 20 sec (Bauer and Fuster 1976; Fuster and Jervey 1982; Diamond et al. 1989; Leung et al. 2002). For longer delay periods, spatial and object recognition memory tasks require the function of the medial temporal lobe, especially the hippocampus. For example, lesion of the hippocampus in rats results in profound deficits in nonmatching-to-sample tasks when the delay is 1-2 min or longer, but not when the delay is reduced to 4 sec (Clark et al. 2001).
In addition to its role in working memory, the PFC plays a critical role in guiding appropriate responses when conflicting representations exist and higher cognitive control is required in order to suppress a habitual behavior (Mesulam 1998; Matsumoto and Tanaka 2004). For example, neuronal activity within the PFC is increased when subjects are asked to perform a color-naming task (Stroop test) if the read word is presented in a different color (e.g., the word “red” presented in a blue color) (Kerns et al. 2004). It is thought that the PFC assimilates relevant information stored in distinct brain regions in order to guide behavior. In paradigms when conflicting representations are not presented, such as in the standard Morris water maze where the location of the hidden platform is fixed, lesion of the medial PFC (mPFC, homolog to the dorsolateral prefrontal cortex in monkeys and humans; Kolb 1984) has no effect on performance. In the present study, we tested whether memory storage within the mPFC lasting minutes is required for performance when the platform location is moved following a pair of training-testing trials in order to provide conflict. We examined the effects of targeted mPFC infusions of Sp-cyclic adenosine monophosphorothioate (Sp-cAMPS) or Rp-cyclic adenosine monophosphorothioate (Rp-cAMPS), diastereomers of cAMP that selectively activate and inhibit cAMP-dependent protein kinase A (PKA), respectively.
The cAMP/PKA pathway is known to be required for both short-term (lasting minutes to hours) and long-term (lasting days) memory storage in the hippocampus and amygdala (Frey et al. 1993; Bernabeu et al. 1997; Pittenger and Kandel 2003). Previous studies using the antagonist Rp-cAMPS show that while PKA activity within the mPFC is not required for working memory, its activation by Sp-cAMPS impairs performance (Taylor et al. 1999). Our present study corroborates these findings on working memory and shows that PKA activity within the mPFC is necessary for memory lasting minutes, specifically in a task that presents conflicting representations. Taken together, these results suggest that distinct molecular mechanisms underlie working and short-term memory.
Results
Analysis of infusion sites
Following the completion of the behavioral studies, cannulae placement was verified by histological examination of the needle tracks. Figure 1A shows the nonredundant sites for the mPFC infusions of the animals used. All of the infusions were within a 0.5-mm region of the prelimbic/infralimbic cortices along the anterior-posterior axis.
Figure 1.
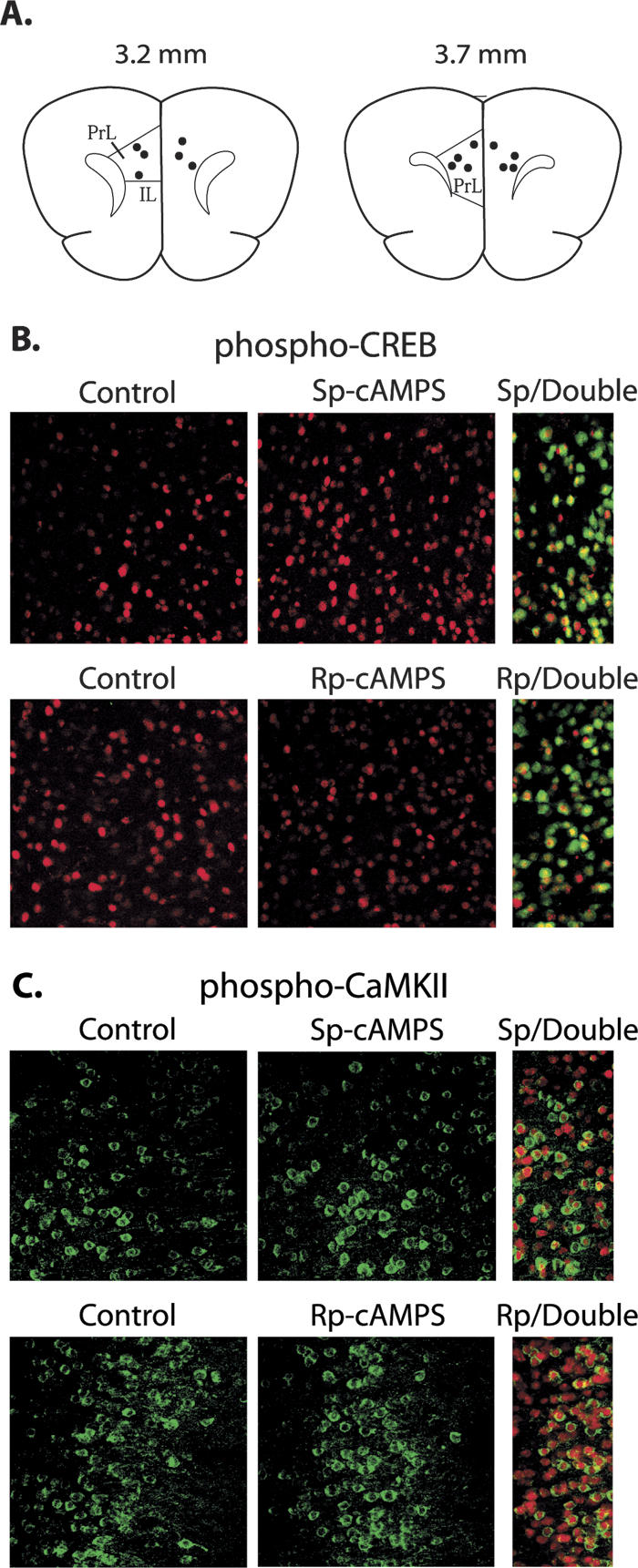
Intra-mPFC infusions of Sp-cAMPS increase, whereas Rp-cAMPS infusions decrease, phospho-CREB immunoreactivity in neurons. (A) Depictions of a coronal section (adapted from Paxinos and Watson 1997) demonstrating nonredundant locations of infusion needle track termini within the mPFC for the animals used in this study (3.2 and 3.7 mm anterior to bregma). (B) Intra-mPFC Sp-cAMPS infusion increased phospho-CREB immunoreactivity (red), whereas Rp-cAMPS infusions decreased phospho-CREB immunoreactivity, compared to vehicle-infused contralateral hemispheres of the same animal. Phospho-CREB immunoreactivity colocalized with the neuron-specific antigen NeuN (green) immunoreactivity. (C) Neither Sp-cAMPS nor Rp-cAMPS had an effect on phospho-CaMKII immunoreactivity (green) compared to the vehicle-infused contralateral side. Phospho-CaMKII immunoreactivity colocalized with the neuron-specific antigen NeuN (green). cc, corpus callosum; IL, infralimbic cortex; PL, prelimbic cortex
Intra-mPFC infusions of Sp-cAMPS and Rp-cAMPS enhance and decrease phospho-CREB immunoreactivity, respectively
To test the efficacy of Sp-cAMPS and Rp-cAMPS in altering substrate protein phosphorylation in mPFC neurons, immunohistochemistry for phospho-CREB on coronal brain sections containing the prelimbic/infralimbic cortices was performed. Numerous studies have shown that Ser133 of CREB is phosphorylated by PKA. Therefore, we used phospho-CREB immunoreactivity as a means of observing the effectiveness of Sp-cAMPS and Rp-cAMPS. Unilateral infusions of 10 μg of Sp-cAMPS or Rp-cAMPS into the mPFC (similar concentrations were shown to influence working memory performance; Taylor et al. 1999) were administered using the contralateral hemisphere of the same animal as a vehicle-infused control. The representative confocal images in Figure 1B show that the PKA activator Sp-cAMPS enhanced, whereas the inhibitor Rp-cAMPS decreased, phospho-CREB immunoreactivity. Neither Sp-cAMPS- nor Rp-cAMPS-infusion altered phospho-CaMKII immunoreactivity, suggesting that these agents did not cause a generalized change in phospho-immunoreactivity (Fig. 1C). Double labeling with an antibody for the neuron-specific protein NeuN (Fig. 1B,C, right panels) revealed that both phospho-CREB and phospho-CaMKII immunoreactivities could be observed in neurons.
PKA activation impairs performance in the delay match-to-place working memory task
It had been reported that activation of PKA by intra-mPFC infusion of Sp-cAMPS impairs working memory in a delay alternation task (Taylor et al. 1999). In the present study, to examine the effect of PKA manipulation using a different working memory task, animals received either intra-mPFC infusions of Sp-cAMPS (n = 7) or vehicle (n = 7), and 15 min later were tested in the delay match-to-place task as outlined in Figure 2A. In this task, animals are required to locate a hidden escape platform during location trials and, following a 5-sec delay period in which they are removed from the training context, allowed to relocate the platform (matching trial). Following a 4-min intertrial interval (ITI), the platform is moved and the paired trials are repeated. The vehicle-infused animals were able to locate the platform significantly faster during the match trials compared to the location trials, indicating normal working memory (vehicle: loc = 45.1±5.4 sec vs. match = 24.3±3.6 sec, P < 0.05; Fig. 2B). However, animals receiving infusions of the PKA activator Sp-cAMPS were unable to locate the platform significantly faster, suggesting a working memory impairment (Sp-cAMPS: loc = 45.3±4.0 sec vs. match = 35.2±5.5 sec, n.s.). No difference in swimming speed was observed between the two groups (Sp-cAMPS = 26.8±1.3 cm/sec; vehicle = 27.7±1.0 cm/sec, n.s.). To test whether PKA activity is necessary for spatial working memory, separate groups of animals received bilateral intra-mPFC infusions of Rp-cAMPS (n = 18) or vehicle (n = 15) before testing in the delay match-to-place task. Both vehicle- and Rp-cAMPS-infused animals had a significantly decreased latency to platform during the matching trials compared with the location trials, demonstrating working memory (vehicle: loc = 42.2±2.7 sec vs. match = 28.1±3.0 sec, P < 0.05; Rp-cAMPS: loc = 32.9±2.4 sec vs. match = 25.2±2.7 sec, P < 0.05; Fig. 2C). Interestingly, a significant decrease in latency to platform was observed during the location trial in the Rp-cAMPS-infused group compared to vehicle-infused controls (Rp-cAMPS: loc = 32.9±2.4 sec; vehicle: loc = 42.2±2.7 sec, P < 0.05; Fig. 2C). These findings are consistent with previous studies demonstrating that stimulation, but not inhibition, of mPFC PKA activity impairs working memory (Taylor et al. 1999; Ramos et al. 2003).
Figure 2.
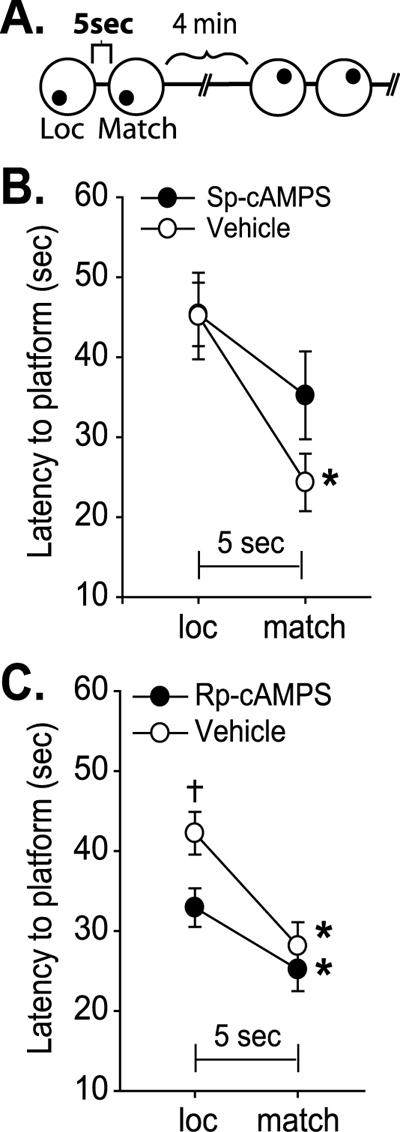
Increased prefrontal PKA activity interferes with working memory. (A) Schematic of the chronological sequence of the delay match-to-place working memory task showing two paired-trials (location and matching trials), with two different platform locations, separated by an intertrial interval (ITI). (B) Average latency to the hidden platform (sec) for location trials (loc) and matching trials (match) during the working memory task for bilateral intra-mPFC infused vehicle and Sp-cAMPS (10μg/side) groups. Vehicle-infused animals had a significantly decreased latency to platform in matching trials, and Sp-cAMPS-infused animals did not, indicating a working memory impairment. (C) Average latency to the hidden platform (sec) for location and match trials during the working memory task for intra-mPFC infused vehicle and Rp-cAMPS (10μg/side) groups. Both groups had a significantly decreased latency to platform in matching trials, displaying normal working memory. However, Rp-cAMPS-infused animals had a significantly lower latency to platform during location trials than vehicle-infused animals. *, significant decrease in latency between match trial and location trial within a group. †, significant difference in location trial latency between groups. P ≤ 0.05.
Intra-mPFC PKA activity is required for short-term memory
The delay match-to-place task utilized in the present study allows for the observation of residual memory for the previous platform location during the subsequent location trial that is given 4 min later. As a result of short-term memory for the prior paired-trial, animals return to the previous platform location before performing a generalized search for the platform (Runyan et al. 2005). Our results (Fig. 2C) revealed that the Rp-cAMPS-infused group had significantly shorter latencies to find the hidden platform during the location trial compared to the vehicle-treated control animals. Examination of the swim traces demonstrated that, unlike vehicle-infused controls, Rp-cAMPS-infused animals did not return to the previous platform location, indicative of a short-term memory impairment (Fig. 3A). When examined on a paired-trial by paired-trial basis, we found that during the location trial of the first paired-trial, no difference between vehicle- and drug-infused animals occurred (Rp-cAMPS: loc = 31.9±4.9 sec; vehicle: loc = 36.7±5.0 sec, n.s.; Fig. 3B). However, on subsequent paired-trials (those preceded by a previous paired-trial [trials 2-4]), vehicle-infused animals demonstrated an increase in latency to platform during location trials compared to Rp-cAMPS-infused animals (Rp-cAMPS: loc = 33.1±2.6 sec; vehicle: loc = 42.8±2.4 sec, P < 0.05; Fig. 3C). Taken together, these results suggest that PKA activity within the PFC is required for short-term memory in this task.
Figure 3.
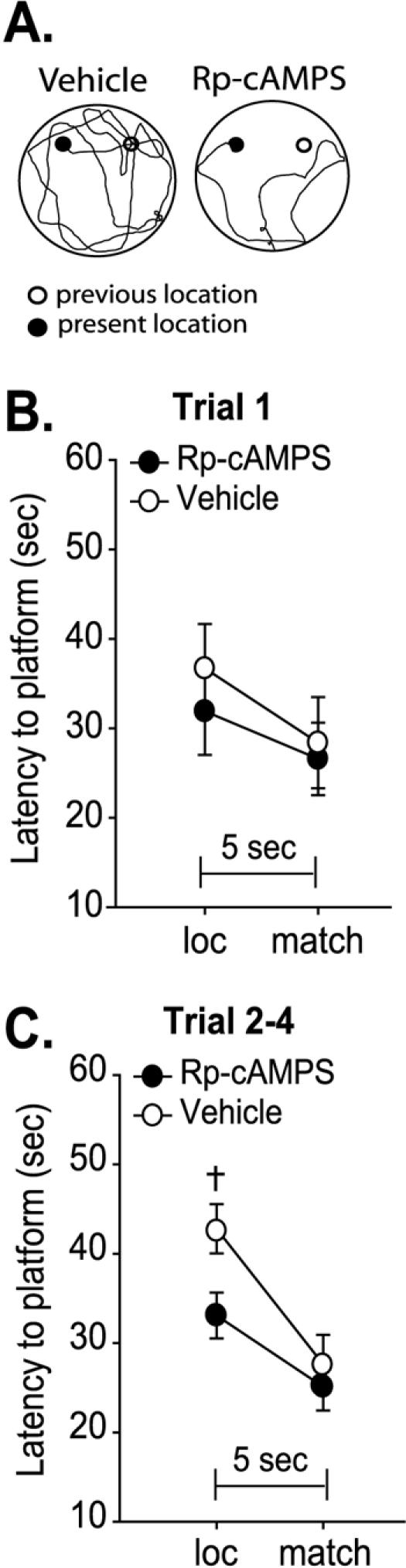
Decreased prefrontal PKA activity does not influence working memory but resulted in impaired short-term memory between trials. (A) Representative location trial swim traces illustrating that although vehicle-infused animals return to the previous trial's platform location, Rp-cAMPS-infused animals did not. Graph shows average latency to the hidden platform (sec) for location trials and matching trials for the first paired-trial (B) and for trials 2-4 (C), demonstrating that the decrease in latency to platform observed in Rp-cAMPS-infused animals occurred only when there was a previous platform location. *, significant decrease in latency between match trial and location trial within a group. †, significant difference in location trial latency between groups. P ≤ 0.05.
The mPFC is involved in the short-term storage of information in a task with conflicting representations
To further test the involvement of PKA activity in the use of memory lasting minutes, the delay period between the location and the match trials was extended to 1.5 min. As working memory-related delay neuron activity has been reported to last for up to 20 sec, the 1.5-min interval between the location and match trials is beyond the temporal limitations of working memory (Fuster and Jervey 1982) (Fig. 4A). Additionally, the ITI was extended to 10 min in order to reduce the influence of memory for the previous paired-trial. A third group of animals received bilateral intra-mPFC infusions of either Sp-cAMPS (n = 6) or vehicle (n = 7) 15 min before testing. In contrast to the negative influence of PKA activation on working memory, Sp-cAMPS infusion did not affect the ability of the animals to perform the task when the delay period was extended to 1.5 min (vehicle: loc = 39.7±5.2 sec vs. match = 27.4±5.4 sec, P < 0.05; Sp-cAMPS: loc = 42.5±3.6 sec vs. match = 30.5±3.2 sec, P < 0.05; Fig. 4B). Bilateral infusions of Rp-cAMPS (n = 9), however, resulted in the inability to relocate the platform significantly faster (vehicle: loc = 38.4±2.9 sec vs. match = 27.3±3.1 sec, P < 0.05; Rp-cAMPS: loc = 38.6±5.4 sec vs. match = 34.4±2.1 sec, n.s.; Fig. 4C), indicating a requirement for PKA activity in the delayed use of information over minutes. No difference in swimming speed was observed between the two groups (Rp-cAMPS = 23.1±1.2 cm/sec; vehicle = 24.3±0.8 cm/sec, n.s.).
Figure 4.
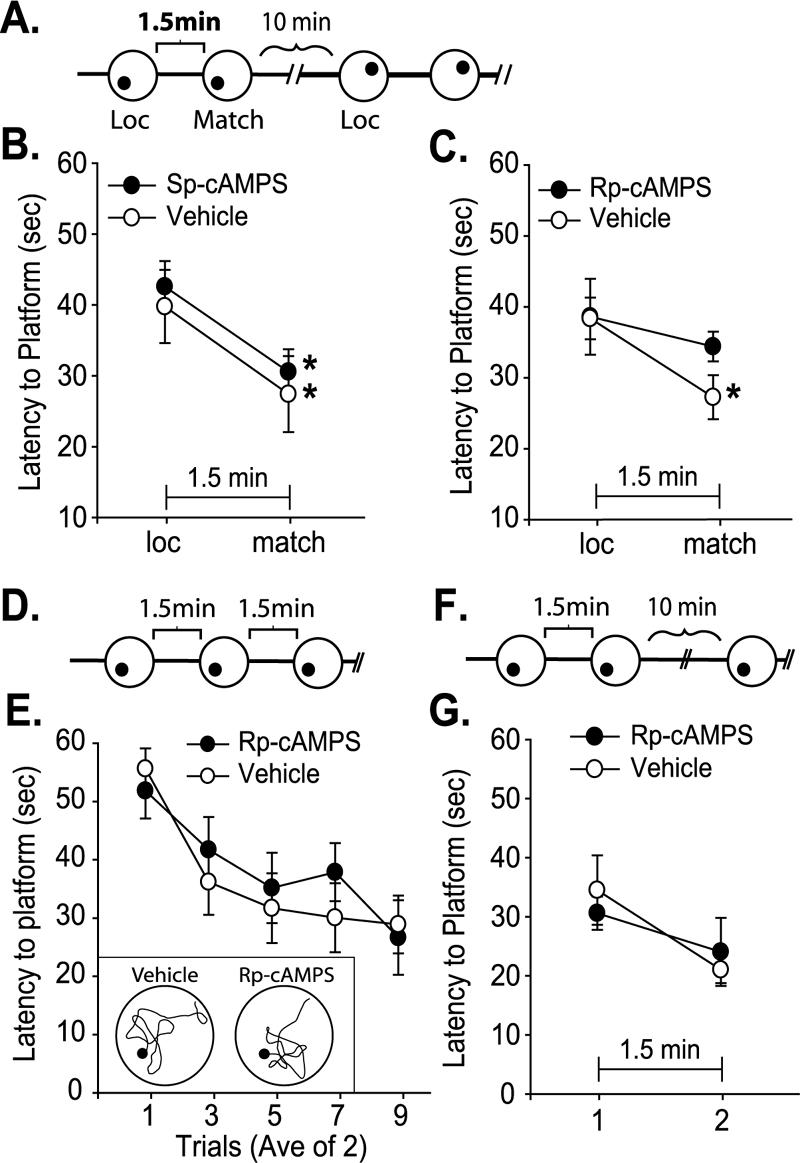
Decreased mPFC PKA activity impairs short-term memory when the platform location is varied. (A) Schematic of the chronological sequence of the delay match-to-place short-term memory task showing two paired-trials, with two different platform locations, separated by an intertrial interval (ITI). (B) Average latency to the hidden platform (min) for location and match trials during the short-term memory task for vehicle and Sp-cAMPS groups. (C) Average latency to the hidden platform (sec) for location and match trials during the short-term memory task for vehicle and Rp-cAMPS groups. (D) Schematic of the chronological sequence of the Morris water maze short-term memory task. (E) Acquisition curves demonstrating that both vehicle- and Rp-cAMPS-infused groups showed comparable learning when the platform was stationary. Inset: Representative swim traces taken from the last trial illustrating that both groups showed localized platform searches. (F) Schematic of the chronological sequence for the stationary delay match-to-place short-term memory task. (G) Average latency to the hidden platform (min) during the stationary delay match-to-place short-term memory task for intra-mPFC infused vehicle and Rp-cAMPS groups. When the platform remains stationary, latency to platform is not affected by intra-mPFC Rp-cAMPS infusion. *, significant decrease in latency between match trial and location trial within a group. P ≤ 0.05.
Extensive evidence shows that the PFC is involved in organizing behavior specifically when conflicting information exists (Zahrt et al. 1997; Ragozzino et al. 1999; Clarke et al. 2004). To test whether mPFC PKA activity is required when there is no conflict, as when the platform location is stationary, Rp-cAMPS or vehicle was infused into a fourth group of animals before training in a standard Morris water maze task with an ITI of 1.5 min (Fig. 4D). No effect on latency to platform was observed as a result of Rp-cAMPS infusions (n = 7) compared to vehicle controls (n =8) (F(1,6) = 0.0057, P = 0.94), and both groups demonstrated significant acquisition (F(8,48) = 2.76, P < 0.05; Fig. 4E). Additionally, representative swim traces from the final trial show that both vehicle- and Rp-cAMPS-infused animals demonstrate localized platform searches (Fig. 4E, inset). These findings are consistent with previous studies indicating that mPFC lesions do not alter performance in the standard Morris water maze task (de Bruin et al. 1994; Compton et al. 1997).
To specifically examine whether the PKA involvement in short-term memory occurred as a result of varying the platform location between paired-trials, Group 5 was tested in the delay match-to-place paradigm using a fixed platform position (Fig. 4F). Animals were bilaterally infused with either vehicle or 10 μg/side Rp-cAMPS, then tested for short-term memory in four match-to-place trials with a 1.5-min delay period. No significant difference was observed between the latency curves of intra-mPFC Rp-cAMPS infused- (n = 5) and vehicle-infused groups (n =5) (F(1,13) = 0.185, P = 0.67) when the platform position remained static throughout the experiment (Fig. 4G).
Discussion
Several studies have demonstrated that the PFC is involved in the use of information encountered minutes earlier, outside of the upper temporal limits of working memory (Seamans et al. 1998; Floresco and Phillips 2001; Raye et al. 2002). It has been proposed that relevant information is stored in other brain regions, which is retrieved and held in working memory for subsequent integration by the PFC for guiding goal-directed behavior. Here we demonstrate that information storage within the PFC, which is beyond the temporal limits of working memory, is required for guiding behavior in a task that presents conflicting information, but not in a task without such conflicts. Specifically we show that mPFC PKA inhibition impairs short-term memory in a delay match-to-place task only when the platform is moved between trial pairs to provide conflict.
Cognitive control is required for appropriate behavioral responses in situations presenting conflicts in order to suppress a previously learned response and generate a less-familiar response. Imaging studies in human subjects have shown the involvement of the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (dLPFC) in cognitive control (Kerns et al. 2004). It has been proposed that the ACC first detects the conflict and increases the activity in the dLPFC, which guides the behavior (Matsumoto and Tanaka 2004). These studies, however, did not address whether storage of information from previous experience is required for guiding behavior in situations with conflicts. One advantage of the delay match-to-place task used in the present study is that it allows us to assess an animal's working memory and short-term memory during a single session. Since blockade of mPFC PKA activity impaired short-term, but not working, memory in the same animal, these influences of PKA modulation do not appear to be due to a generalized mPFC dysfunction. Although delay period activity within the PFC only occurs for a period of seconds, the present findings suggest that information can be stored via a different molecular mechanism within the PFC beyond the temporal limitations of working memory. This may allow for easy accessibility of information for guiding behavior when pre-existing information may conflict with the present task goals.
Using the phosphorylation of CREB as a marker of PKA activity, we demonstrated that Sp-cAMPS and Rp-cAMPS effectively enhanced and decreased phospho-Ser133 immunoreactivity, respectively. These naturally occurring diastereomers of cAMP have been demonstrated to be useful in the study of PKA due to their specificity, cell permeability, and resistance to cyclic nucleotide phosphodiesterase activity. Evaluation of the extent of detectable increases/decreases in CREB phosphorylation, as well as our histological examination of cannulae placement, did not reveal any influence of the drug outside of the mPFC. However, as changes in CREB phosphorylation were observed in both the prelimbic and infralimbic cortices, we cannot determine which of these two regions (or both) is responsible for the behavioral effects we observed. Furthermore, it has repeatedly been demonstrated that hippocampal function is critical for performance in the Morris water maze task (Morris et al. 1982, 1986), and that short-term memory within this structure is dependent on PKA activity (Vianna et al. 2000). We showed previously that drugs infused into the mPFC do not diffuse to the hippocampus (Runyan et al. 2004). Consistent with this, no effect of mPFC infusion of Rp-cAMPS was observed in the stationary platform version of the Morris water maze task, indicating that the PKA inhibitor did not diffuse into the hippocampus.
Using a delayed alternation task, Taylor et al. (1999) demonstrated that infusion of Sp-cAMPS impairs working memory whereas Rp-cAMPS had no effect, observations consistent with the present report. In contrast, Aujla and Beninger (2001) observed that inhibition of PKA in the mPFC impairs working memory in a delayed win-shift task. However, the duration of the delay used by Aujla and Beninger was 30 min and more fitting of the description of short-term memory used in our study. In this respect, our results are consistent in that PKA inhibition in the mPFC impairs memory lasting minutes. The involvement of PKA in short-term memory has been demonstrated in several species and in multiple mammalian brain structures, suggesting a conservation of this mechanism. For example, in the marine mollusk Aplysia californica, short-term sensitization of the gill-siphon reflex is dependent on presynaptic PKA activity (Castellucci et al. 1982). In rodents, inhibition of PKA by targeted infusion of Rp-cAMPS into the hippocampus, or KT5720 (a PKA-selective inhibitor) into the entorhinal cortex, impairs short-term memory for one-trial step-down inhibitory avoidance (Izquierdo et al. 2000; Vianna et al. 2000). In the present study, impaired short-term memory following PKA inhibition within the PFC was only observed when conflict was present as a result of moving the platform between pairs of training-testing trials. No effect of PKA inhibition was observed when the platform position was stationary. This finding is consistent with studies using lesion of the PFC which show that prefrontal function is not required for performance in the standard version of the water maze (de Bruin et al. 1994; Compton et al. 1997) and studies demonstrating engagement of the PFC in tasks in which cognitive control is necessary in order to guide appropriate behavioral responses (Kerns et al. 2004; Matsumoto and Tanaka 2004). Our results demonstrate that short-term memory storage within the PFC is required for guiding behavior in tasks with conflicts and provides a plausible mechanism by which the PFC executes cognitive control. However, one caveat of this interpretation is that we cannot rule out the possibility that PKA-mediated short-term memory is stored in the PFC as a result of training in tasks without conflict, but that its loss as a result of PKA inhibition simply does not interfere with behavior. Although the exact downstream processes which are activated by enhanced PKA activity in the mPFC are not known at present, it is likely that these processes are similar in nature to those previously attributed to PKA in short-term memory such as increased phosphorylation of S-type K+ channels and increased transmitter release (Shuster et al. 1985; Byrne and Kandel 1996).
It was recently observed that calcineurin, especially PFC calcineurin activity, is involved in maintaining working memory (Runyan et al. 2005). Calcineurin is a calcium/calmodulin-dependent protein phosphatase that can dephosphorylate substrates that are phosphorylated by PKA and other protein kinases. Therefore, although PKA-mediated phosphorylation may be involved in short-term memory, increased prefrontal PKA activity may interfere with intracellular events required for the maintenance of working memory. At present, however, it is not known whether the same cells within the PFC are responsible for both working and short-term memory. Using an in vitro phosphorylation assay, we measured PKA activity following delay periods lasting seconds and minutes but failed to detect any significant changes (data not shown). This inability to detect PKA activation could be due to either a modest activation of PKA or activation of PKA within a limited number of cells. As the downstream substrates that are phosphorylated in response to PKA activity following behavioral training are delineated, these substrates may serve as a means of determining the cell populations involved in working and short-term memory storage. In addition to PKA, other kinases such as CaMKII have been implicated in short-term memory formation. In our recently published work investigating the role of calcium-mediated signaling in prefrontal working memory (Runyan et al. 2005), inhibition of CaMKII did not appear to interfere with short-term memory in this structure. However, definitive experiments to address the role of this and other kinases in short-term memory formation within the mPFC have not been performed.
Taken together, our findings provide experimental evidence for two distinct intracellular mechanisms for information storage within the PFC depending upon the length of time between the acquisition and the use of information. The impairing effect of PKA inhibition on short-term memory was observed to occur selectively when the flexible use of competing information was required. This ability of the PFC could subserve its role in selecting, comparing, and inhibiting conflicting representations in order to organize appropriate behavior within specific contexts.
Materials and Methods
Surgery
Animal protocols were approved by the Institutional Animal Welfare Committee and were in compliance with National Institutes of Health Guide for Care and Use of Laboratory Animals. A total of 89 male Sprague-Dawley rats were implanted bilaterally (under isofurane anesthesia) with sterile stainless-steel guide cannulae aimed at the dorsal border of the prelimbic area using a stereotaxic device (bregma 3.2 mm, lateral ± 0.75, and depth -2.5 mm). All infusion needle tracks examined terminated within the mPFC cortex (prelimbic/infralimbic cortices). Cannula placement was assessed in a representative group of animals.
Behavioral protocol
All behavior tests were performed by an investigator blind to the treatment groups. Twenty-four hours before testing, animals were given five training trials in a delay match-to-place version of the Morris water maze task (Hamm et al. 1996; Steele and Morris 1999; Dash et al. 2004) in order to familiarize them with the task. Each testing session consisted of a location trial, a 5-sec delay, and a matching trial. Each location trial was started from a unique position in the tank, and the hidden platform was in a novel location. Once the platform was found, the animal was allowed to rest on the platform for 10 sec. The animal was then removed from the hidden platform during a delay period (5 sec for working memory test, 1.5 min for short-term memory), and placed in the holding area. The animal was then placed back into the water maze at the same starting position as in the location trial and allowed to search for the hidden escape platform (the matching trial). If the animal failed to locate the platform within 60 sec on any given trial, it was led there by the investigator. After each matched trial, rats were given an intertrial interval (ITI) (4 min for working memory and 10 min for short-term memory) before the next pair of trials. The influence of each drug on working memory performance was assessed in four pairs of trials. All other parameters were kept constant. In the short-term spatial memory test using the standard Morris water maze, animals were given nine location trials with a 1.5-min ITI, and the platform was kept stationary.
Intra-mPFC infusions
Drugs were dissolved in saline at a concentration of 10μg/μL, and infusions were performed bilaterally at a rate of 0.25μL/min for 4 min. Following infusion, the needles were left in place for 2 min to allow for diffusion of the drug. Infusions were carried out 15 min prior to working memory testing. Dosages of Sp-cAMPS and Rp-cAMPS used here were based on previous in vivo studies (Taylor et al. 1999), and their specificities have been extensively characterized (Botelho et al. 1988; Rothermel and Parker Botelho 1988; Sandberg et al. 1991; Schaap et al. 1993).
Immunohistochemistry
Animals received unilateral intra-mPFC infusions of Rp-cAMPS or Sp-cAMPS. The contralateral hemisphere was infused with vehicle and served as an intra-animal control. Twenty min following infusion, animals were decapitated and brains were quickly removed while submerged in ice-cold artificial cerebrospinal fluid. Tissue slabs containing the mPFC were isolated and fixed in ice-cold 4% paraformaldehyde/15% picric acid. Immunohistochemistry was performed on free-floating 40-μ-thick sections using an antibody that specifically recognizes CaMKII when phosphorylated on Thr286 (2 μg/mL), or an antibody recognizing cAMP-response element binding protein (CREB) when phosphorylated on Ser133 (0.2 μg/mL) (Cell Signaling). Immunoreactivity for both phosphorylated CREB and CaMKII was visualized using a species-specific secondary antibody conjugated to Alexa 488 fluorochrome (Molecular Probes). Double-label immunohistochemistry was performed using an antibody against the neuronal marker NeuN (1 μg/mL). NeuN immunoreactivity was visualized using a species-specific secondary antibody conjugated to Alexa 568 fluorochrome. During confocal microscopy, the laser intensities and settings were kept constant for all images.
Data analysis
A two-way repeated measures ANOVA was used for statistical evaluation of differences in latency to the platform between experimental and control groups in location and matching trials as well as for the Morris water maze short-term memory test. Post hoc examination was performed using the Tukey or Fisher LSD tests. A P-value ≤ 0.05 was used as the criterion for significance.
Acknowledgments
We thank Anthony Moore for intellectual and scientific input and for his insightful comments, and Melanie Moody for her surgical expertise. The present research was supported by grants from the NIH (NS35457) and TIRR/Mission Connect Consortium.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.92405.
References
- Aujla, H. and Beninger, R.J. 2001. Hippocampal-prefrontocortical circuits: PKA inhibition in the prefrontal cortex impairs delayed nonmatching in the radial maze in rats. Behav. Neurosci. 115: 1204-1211. [PubMed] [Google Scholar]
- Baddeley, A. 1992. Working memory. Science 255: 556-559. [DOI] [PubMed] [Google Scholar]
- Baeg, E.H., Kim, Y.B., Huh, K., Mook-Jung, I., Kim, H.T., and Jung, M.W. 2003. Dynamics of population code for working memory in the prefrontal cortex. Neuron 40: 177-188. [DOI] [PubMed] [Google Scholar]
- Bauer, R.H. and Fuster, J.M. 1976. Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. J. Comp. Physiol. Psychol. 90: 293-302. [DOI] [PubMed] [Google Scholar]
- Bernabeu, R., Bevilaqua, L., Ardenghi, P., Bromberg, E., Schmitz, P., Bianchin, M., Izquierdo, I., and Medina, J.H. 1997. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. 94: 7041-7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho, L.H., Webster, L.C., Rothermel, J.D., Baraniak, J., and Stec, W.J. 1988. Inhibition of cAMP-dependent protein kinase by adenosine cyclic 3′-, 5′-phosphorodithioate, a second cAMP antagonist. J. Biol. Chem. 263: 5301-5305. [PubMed] [Google Scholar]
- Byrne, J.H. and Kandel, E.R. 1996. Presynaptic facilitation revisited: State and time dependence. J. Neurosci. 16: 425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci, V.F., Nairn, A., Greengard, P., Schwartz, J.H., and Kandel, E.R. 1982. Inhibitor of adenosine 3′:5′-monophosphate-dependent protein kinase blocks presynaptic facilitation in Aplysia. J. Neurosci. 2: 1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, R.E., West, A.N., Zola, S.M., and Squire, L.R. 2001. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus 11: 176-186. [DOI] [PubMed] [Google Scholar]
- Clarke, H.F., Dalley, J.W., Crofts, H.S., Robbins, T.W., and Roberts, A.C. 2004. Cognitive inflexibility after prefrontal serotonin depletion. Science 304: 878-880. [DOI] [PubMed] [Google Scholar]
- Compton, D.M., Griffith, H.R., McDaniel, W.F., Foster, R.A., and Davis, B.K. 1997. The flexible use of multiple cue relationships in spatial navigation: A comparison of water maze performance following hippocampal, medial septal, prefrontal cortex, or posterior parietal cortex lesions. Neurobiol. Learn. Mem. 68: 117-132. [DOI] [PubMed] [Google Scholar]
- Dash, P.K., Moore, A.N., Moody, M.R., Treadwell, R., Felix, J.L., and Clifton, G.L. 2004. Post-trauma administration of caffeine plus ethanol reduces contusion volume and improves working memory in rats. J. Neurotrauma 21: 1573-1583. [DOI] [PubMed] [Google Scholar]
- de Bruin, J.P., Sanchez-Santed, F., Heinsbroek, R.P., Donker, A., and Postmes, P. 1994. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: Evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Res. 652: 323-333. [DOI] [PubMed] [Google Scholar]
- Diamond, A., Zola-Morgan, S., and Squire, L.R. 1989. Successful performance by monkeys with lesions of the hippocampal formation on AB and object retrieval, two tasks that mark developmental changes in human infants. Behav. Neurosci. 103: 526-537. [DOI] [PubMed] [Google Scholar]
- Floresco, S.B. and Phillips, A.G. 2001. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav. Neurosci. 115: 934-939. [PubMed] [Google Scholar]
- Frey, U., Huang, Y.Y., and Kandel, E.R. 1993. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260: 1661-1664. [DOI] [PubMed] [Google Scholar]
- Fuster, J.M. and Alexander, G.E. 1971. Neuron activity related to short-term memory. Science 173: 652-654. [DOI] [PubMed] [Google Scholar]
- Fuster, J.M. and Jervey, J.P. 1982. Neuronal firing in the inferotemporal cortex of the monkey in a visual memory task. J. Neurosci. 2: 361-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm, R.J., Temple, M.D., Pike, B.R., O'Dell, D.M., Buck, D.L., and Lyeth, B.G. 1996. Working memory deficits following traumatic brain injury in the rat. J. Neurotrauma 13: 317-323. [DOI] [PubMed] [Google Scholar]
- Izquierdo, L.A., Vianna, M., Barros, D.M., Mello e Souza, T., Ardenghi, P., Sant' Anna, M.K., Rodrigues, C., Medinam, J.H., and Izquierdo, I. 2000. Short- and long-term memory are differentially affected by metabolic inhibitors given into hippocampus and entorhinal cortex. Neurobiol. Learn. Mem. 73: 141-149. [DOI] [PubMed] [Google Scholar]
- Kerns, J.G., Cohen, J.D., MacDonald III, A.W., Cho, R.Y., Stenger, V.A., and Carter, C.S. 2004. Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023-1026. [DOI] [PubMed] [Google Scholar]
- Kojima, S. and Goldman-Rakic, P.S. 1982. Delay-related activity of prefrontal neurons in rhesus monkeys performing delayed response. Brain Res. 248: 43-49. [DOI] [PubMed] [Google Scholar]
- Kolb, B. 1984. Functions of the frontal cortex of the rat: A comparative review. Brain Res. 320: 65-98. [DOI] [PubMed] [Google Scholar]
- Leung, H.C., Gore, J.C., and Goldman-Rakic, P.S. 2002. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J. Cogn. Neurosci. 14: 659-671. [DOI] [PubMed] [Google Scholar]
- Luria, A. 1966. Human brain and psychological processes. Harper and Row, New York.
- Matsumoto, K. and Tanaka, K. 2004. Neuroscience. Conflict and cognitive control. Science 303: 969-970. [DOI] [PubMed] [Google Scholar]
- Mesulam, M.M. 1998. From sensation to cognition. Brain 121: 1013-1052. [DOI] [PubMed] [Google Scholar]
- Milner, B. 1982. Some cognitive effects of frontal-lobe lesions in man. Philos. Trans. R Soc. Lond. B Biol. Sci. 298: 211-226. [DOI] [PubMed] [Google Scholar]
- Morris, R.G., Garrud, P., Rawlins, J.N., and O'Keefe, J. 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297: 681-683. [DOI] [PubMed] [Google Scholar]
- Morris, R.G., Anderson, E., Lynch, G.S., and Baudry, M. 1986. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature 319: 774-776. [DOI] [PubMed] [Google Scholar]
- Paxinos, G. and Watson, C. 1997. The rat brain in stereotaxic coordinates. Academic Press, Inc., San Diego, CA.
- Pittenger, C. and Kandel, E.R. 2003. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos. Trans. R Soc. Lond. B Biol. Sci. 358: 757-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino, M.E., Detrick, S., and Kesner, R.P. 1999. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J. Neurosci. 19: 4585-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, B.P., Birnbaum, S.G., Lindenmayer, I., Newton, S.S., Duman, R.S., and Arnsten, A.F. 2003. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron 40: 835-845. [DOI] [PubMed] [Google Scholar]
- Raye, C.L., Johnson, M.K., Mitchell, K.J., Reeder, J.A., and Greene, E.J. 2002. Neuroimaging a single thought: Dorsolateral PFC activity associated with refreshing just-activated information. Neuroimage 15: 447-453. [DOI] [PubMed] [Google Scholar]
- Rothermel, J.D. and Parker Botelho, L.H. 1988. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3′,5′-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem. J. 251: 757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan, J.D., Moore, A.N., and Dash, P.K. 2004. A role for prefrontal cortex in memory storage for trace fear conditioning. J. Neurosci. 24: 1288-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan, J.D., Moore, A.N., and Dash, P.K. 2005. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn. Mem. 12: 103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg, M., Butt, E., Nolte, C., Fischer, L., Halbrugge, M., Beltman, J., Jahnsen, T., Genieser, H.G., Jastorff, B., and Walter, U. 1991. Characterization of Sp-5,6-dichloro-1-β-D-ribofuranosyl-benzimidazole-3′,5′-monophosphorothioate (Sp-5,6-DCl-cBiMPS) as a potent and specific activator of cyclic-AMP-dependent protein kinase in cell extracts and intact cells. Biochem. J. 279: 521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap, P., Ments-Cohen, M., Soede, R.D., Brandt, R., Firtel, R.A., Dostmann, W., Genieser, H.G., Jastorff, B., and van Haastert, P.J. 1993. Cell-permeable non-hydrolyzable cAMP derivatives as tools for analysis of signaling pathways controlling gene regulation in Dictyostelium. J. Biol. Chem. 268: 6323-6331. [PubMed] [Google Scholar]
- Seamans, J.K., Floresco, S.B., and Phillips, A.G. 1998. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J. Neurosci. 18: 1613-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster, M.J., Camardo, J.S., Siegelbaum, S.A., and Kandel, E.R. 1985. Cyclic AMP-dependent protein kinase closes the serotonin-sensitive K+ channels of Aplysia sensory neurones in cell-free membrane patches. Nature 313: 392-395. [DOI] [PubMed] [Google Scholar]
- Steele, R.J. and Morris, R.G. 1999. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus 9: 118-136. [DOI] [PubMed] [Google Scholar]
- Taylor, J.R., Birnbaum, S., Ubriani, R., and Arnsten, A.F. 1999. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. J. Neurosci. 19: RC23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna, M.R., Izquierdo, L.A., Barros, D.M., Ardenghi, P., Pereira, P., Rodrigues, C., Moletta, B., Medina, J.H., and Izquierdo, I. 2000. Differential role of hippocampal cAMP-dependent protein kinase in short- and long-term memory. Neurochem. Res. 25: 621-626. [DOI] [PubMed] [Google Scholar]
- Zahrt, J., Taylor, J.R., Mathew, R.G., and Arnsten, A.F. 1997. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J. Neurosci. 17: 8528-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]


