Abstract
Pontine neuronal activation during auditory stimuli increases ontogenetically between postnatal days (P) P17 and P24 in rats. Pontine neurons are an essential component of the conditioned stimulus (CS) pathway for eyeblink conditioning, providing mossy fiber input to the cerebellum. Here we examined whether the developmental limitation in pontine responsiveness to a CS in P17 rats could be overcome by direct stimulation of the CS pathway. Eyeblink conditioning was established in infant rats on P17-P18 and P24-P25 using pontine stimulation as a CS. There were no significant age-related differences in the rate or level of conditioning. Eyeblink conditioned responses established with the stimulation CS were abolished by inactivation of the ipsilateral cerebellar nuclei and overlying cortex in both age groups. The findings suggest that developmental changes in the CS pathway play an important role in the ontogeny of eyeblink conditioning.
Eyeblink classical conditioning has been used as a model system for examining developmental changes in the neural mechanisms underlying motor learning (Freeman Jr. and Nicholson 2004). It is well established that eyeblink conditioning in adult mammals depends on cerebellar interactions with its afferent and efferent brainstem nuclei (Mauk and Donegan 1997; Christian and Thompson 2003). The cerebellum receives input stimulation from an auditory conditioned stimulus (CS) through the pontine mossy fiber projection (Steinmetz et al. 1986, 1987, 1989; Steinmetz 1990; Steinmetz and Sengelaub 1992; Tracy et al. 1998). Input stimulation from an air puff or face shock unconditioned stimulus (US) reaches the cerebellum through the climbing fiber projection from the inferior olive (McCormick et al. 1985; Mauk et al. 1986; Steinmetz et al. 1989). The convergence of mossy and climbing fiber activation of cerebellar neurons is thought to result in changes in synaptic efficacy that constitute the substrate of learning and drive the production of the eyeblink conditioned response (CR). Cerebellar output produces inhibitory feedback to the inferior olive and excitatory feedback to the pons (Sears and Steinmetz 1991; Clark et al. 1997; Kim et al. 1998; Bao et al. 2000; Medina et al. 2002). The feedback connections are thought to be important for acquisition and maintenance of CRs (Medina et al. 2002).
Eyeblink conditioning emerges ontogenetically between postnatal days (P) P17 and P24 in rats (Stanton et al. 1992). Neurophysiological, neuropharmacological, and neuroanatomical analyses of the ontogeny of eyeblink conditioning in rats indicate that the developmental emergence of eyeblink conditioning is due to developmental changes in the CS and US pathways (Freeman Jr. and Nicholson 2004). The most substantial developmental change in the US pathway is an increase in the magnitude of cerebellar inhibitory feedback to the inferior olive (Nicholson and Freeman Jr. 2003a,b). The developmental change in inhibitory feedback is due to an increase in inhibitory synapses within the inferior olive (Nicholson and Freeman Jr. 2003a). Developmental addition of inhibitory synapses within the inferior olive results in a decrease in complex spike activity in the cerebellar cortex, which influences the maintenance of synaptic plasticity within the cerebellum. Younger rats with less olivary inhibition have higher baseline rates of climbing fiber activity and as a consequence, they are less capable than adults of maintaining learning-specific plasticity in the cerebellum between training trials and sessions.
Initial analysis of CS pathway development indicated that there is an ontogenetic change that results in weaker input to the cerebellum (Nicholson and Freeman Jr. 2004). A subset of neurons in the pontine nuclei exhibits developmental changes in the latency and magnitude of activation following the onset of a tone CS (Freeman Jr. and Muckler 2003). Younger rats have a weaker pontine response to acoustic stimuli, resulting in less activation of cerebellar neurons during the CS. The developmental change in pontine responsiveness is thought to influence eyeblink conditioning by limiting activity-dependent synaptic plasticity in the cerebellum.
Here we examined whether the developmental limitation in pontine responsiveness in P17 rats could be overcome by stimulation of the CS pathway. In the first experiment, eyeblink conditioning was established in freely moving infant rats on P17-P18 or P24-P25 using electrical stimulation of the basilar pontine nuclei as a CS. Rat pups were given electrical stimulation through a bipolar electrode implanted in the pontine nuclei paired with a peripheral unconditioned stimulus (US) or unpaired stimulation. The second experiment examined whether learning established with the stimulation CS was cerebellum-dependent. Pups were given paired conditioning using pontine stimulation as the CS on P17-P18 or P24-P25 followed by an infusion of muscimol into the interpositus nucleus, which was expected to inactivate the cerebellar nuclei and overlying cortex (Freeman Jr. et al. 2005).
Results
Associative learning
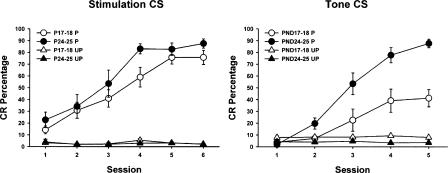
Pontine stimulation induced robust conditioning in both paired groups. Rat pups receiving paired presentations of pontine stimulation and the US showed a significantly higher level of conditioning compared to pups receiving unpaired training during conditioning sessions 2-6 (Fig. 1). Stimulation did not elicit eyeblink responses in pups that received unpaired training in either age group (Fig. 1). A repeated measures ANOVA revealed a significant main effect of the condition factor (F(1,31) = 135.11, P < 0.001), which was due to a higher percentage of CRs in the paired groups relative to the unpaired groups. The difference between paired and unpaired group performance indicates that the CRs produced by paired training were established through associative learning and were not due to pseudoconditioning or sensitization. No statistically significant differences were found between age groups. The current intensity used in the age groups did not differ significantly (P17-P18 paired mean = 73.3 μA, range = 50-100 μA; P17-P18 unpaired mean = 70.8 μA, range = 50-85 μA; P24-P25 paired mean = 67.5 μA, range = 25-100 μA; P24-P25 unpaired mean = 71.7 μA, range = 50-100 μA), indicating that the levels of conditioning seen in P17-P18 and P24-P25 rats were comparable because the stimulation bypassed a developmental limitation in the eyeblink conditioning circuitry and were not due to the use of more intense stimulation in the P17-P18 group. The mean current intensity used in the pups with ineffective electrode placements was 70.7 μA (range 20-100 μA).
Figure 1.
Mean (± standard error of the mean, s.e.m.) conditioned response (CR) percentage for rat pups trained with pontine stimulation (left) or a 2-kHz tone (Nicholson and Freeman Jr. 2004) (right) as the conditioned stimulus (CS) on postnatal days (P) P17-P18 (white symbols) or P24-P25 (black symbols). The pups were given either paired (circles) or unpaired (triangles) presentations of the CS and a periorbital shock unconditioned stimulus (US). The amount of associative learning in each paired group was determined by the increase in responding across training sessions and by the difference in CR percentage between the paired and unpaired conditions in both age groups.
Cerebellar inactivation
A second experiment examined whether the associative learning established using pontine stimulation as a CS depends on the cerebellum. Muscimol was infused into the cerebellar interpositus nucleus to inactivate the cerebellar nuclei and overlying cortex (Freeman Jr. and Rabinak 2005; Freeman Jr. et al. 2005). The infusion was not expected to selectively inactivate the interpositus nucleus (Freeman Jr. et al. 2005). Rat pups were given paired presentations of the pontine stimulation CS and the US for five sessions, followed by a test session in which muscimol (5 nmol in 0.5 μL) was infused prior to an additional paired training session. The infusion session was followed by a test session with additional paired training to assess reversibility of the inactivation effect. As in the first experiment, pups in both age groups acquired eyeblink CRs with paired training (Fig. 2). Both age groups also showed loss of CRs following muscimol inactivation of the cerebellar hemisphere and complete recovery of CRs during the last session (Fig. 2). Figure 3 displays the development of the eyelid CR across training and the effects of muscimol infusion on the CR in a rat trained on P17-P18. An ANOVA revealed a significant effect of the sessions factor (F(6,54) = 32.44, P < 0.001) but no effects related to age. The session effect was due to an increase in CR percentage from session 1 to sessions 3-5, a decrease in CR percentage from session 5 to the inactivation session, and a subsequent increase in CR percentage from the inactivation session to the recovery session (all comparisons, P < 0.05).
Figure 2.
Mean (± s.e.m.) conditioned response (CR) percentage for rat pups trained with pontine stimulation as the CS on postnatal days (P) P17-P18 (white symbols) or P24-P25 (black symbols). The pups were given paired presentations of pontine stimulation and a shock US. Muscimol was infused into the cerebellar nuclei prior to session 6 to inactivate the cerebellar nuclei and overlying cortex ipsilateral to the conditioned eye. CRs established by paired training of pontine stimulation and the US were abolished by muscimol inactivation. Response recovery was evident in both groups on session 7.
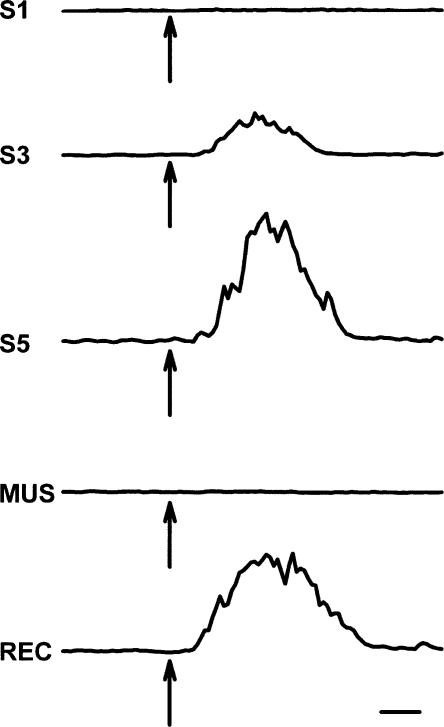
Figure 3.
Traces of eyelid EMG activity recorded from a rat pup trained on P17 and P18 during test trials of pontine stimulation without the US on the first (S1), third (S3), fifth (S5), muscimol inactivation (session 6, MUS), and recovery (session 7, REC) sessions. The arrows indicate the onset time of pontine stimulation. Scale bar, 100 msec.
Electrode and cannula placements
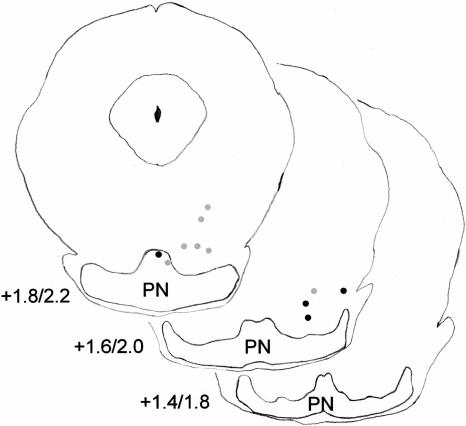
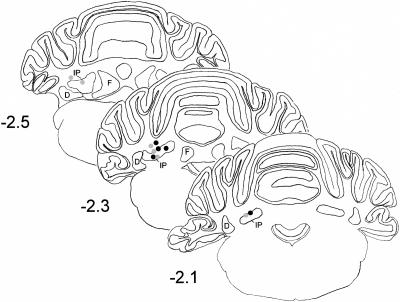
Histological analysis revealed that stimulation sites for rats exhibiting successful conditioning were in or just dorsal (<0.3 mm) to the lateral parts of the basilar pontine nuclei (Fig. 4). Rats that failed to learn had electrode placements in the most medial pontine nucleus or dorsal to the pontine nuclei (Fig. 5). Several of the rats with ineffective electrodes that were placed near the cerebral peduncle exhibited very strong startle-like responses to stimulation but did not exhibit eyeblink conditioning. All the pups given muscimol inactivation had cannula tips placed in or just dorsal to the anterior interpositus nucleus (Fig. 6).
Figure 4.
Coronal sections of the rat basilar pontine nuclei (PN) depicting the effective stimulating electrode placements. The electrode placements for groups trained on P17-P18 (left) or P24-P25 (right) in the paired (black dots) or unpaired conditions (gray dots) are shown. The numbers indicate the stereotaxic coordinates in the anterior-posterior dimension relative to lambda.
Figure 5.
Coronal sections of the rat basilar pontine nuclei (PN) depicting the ineffective stimulating electrode placements. The electrode placements for groups trained on P17-P18 (gray dots) or P24-P25 (black dots) are shown. The numbers indicate the stereotaxic coordinates in the anterior-posterior dimension relative to lambda for rats trained on P17-P18 and P24-P25.
Figure 6.
Coronal sections of the rat cerebellum depicting cannula placements. The cannula placements for the groups trained on P17-P18 (gray dots) or P24-P25 (black dots) were in or just dorsal to the anterior interpositus nucleus (IP). D, dentate nucleus; F, fastigial nucleus.
Discussion
Stimulation of the pontine nuclei as a CS was effective for establishing eyeblink conditioning in rat pups on P17-P18 and P24-P25. The conditioning produced in the groups given paired training was the result of associative learning and not due to pseudo-conditioning or sensitization, as indicated by very low response rates in the groups given unpaired training. Conditioning established in both age groups was abolished by muscimol inactivation of the cerebellar nuclei and overlying cortex that are ipsilateral to the conditioned eye.
The conditioning observed in younger rats using pontine stimulation as the CS is remarkable in that no other experimental manipulation has been found that produces conditioning in P17-P18 pups that is equivalent to the conditioning seen in P24-P25 pups (Stanton and Freeman Jr. 2000). A series of experiments by Stanton and colleagues found that systematic alterations in CS salience, US intensity, interstimulus interval, motivational state, amount of training, and CS modality did not reduce the age-related difference in eyeblink conditioning (Stanton and Freeman Jr. 2000). Most of these manipulations influence the rate and magnitude of conditioning in rat pups but do not differentially affect younger rats. Pontine stimulation is, therefore, the only manipulation to date that eliminates the age-related difference in eyeblink conditioning.
The demonstration of robust eyeblink conditioning in rat pups trained on P17-P18 with stimulation of the pontine nuclei as a CS suggests that the developmental emergence of eyeblink conditioning is in part due to developmental changes in the CS pathway. A previous study found a developmental change in the responsiveness of pontine neurons to acoustic stimuli, which might be the primary ontogenetic change in the CS pathway (Freeman Jr. and Muckler 2003). The developmental change in pontine neuronal activity during an acoustic CS is probably not related to maturation of the peripheral auditory system or cochlear nuclei, because behavioral and auditory brainstem response studies indicate that basic auditory function is evident by P15 or P16 in rats (Hyson and Rudy 1984, 1987; Blatchley et al. 1987; Sananes et al. 1988; Stanton et al. 1992). However, we do not know whether the developmental change in pontine responsiveness to acoustic stimuli is due to developmental changes in the projection of the cochlear nuclei to the pons, intrinsic properties of pontine neurons, or feedback projections from the cerebellum and red nucleus (Cartford et al. 1997; Clark et al. 1997). It is also possible that there are developmental changes in the mossy fiber projection to the cerebellum including myelination and increased synaptic efficacy. However, the level of current used to establish conditioning was similar at P17-P18 and P24-P25, suggesting that the important developmental changes in the CS pathway occur in the pons or in a pontine afferent.
The developmental changes in pontine neuronal activity correspond to developmental changes in CS-elicited neuronal activity in the cerebellar cortex and nuclei (Freeman Jr. and Nicholson 2004; Nicholson and Freeman Jr. 2004). As a result of CS pathway development, CS input to the cerebellum in younger rats may not be strong enough to induce synaptic plasticity. Electrical stimulation might increase the number of mossy fibers activated in younger rats relative to a peripheral CS and thereby increase the magnitude of cerebellar neuronal activation, driving activity-dependent synaptic plasticity. Developmental changes in the magnitude of input from the pontine nuclei to the cerebellum might interact with ontogenetic changes in synaptic plasticity mechanisms. However, various forms of synaptic plasticity and changes in excitability have been demonstrated in cultured cerebellar neurons and slices as young as P13 (Aizenman and Linden 2000; Hansel et al. 2001). In the absence of direct evidence for an ontogenetic change in synaptic plasticity mechanisms, our current view is that the developmental change in the magnitude of CS pathway input to the cerebellum accounts for the role of CS pathway maturation in the ontogeny of eyeblink conditioning.
Inactivation of the cerebellar hemisphere ipsilateral to the conditioned eye abolished eyeblink CRs established with pontine stimulation as the CS, as seen in adult rats (Freeman Jr. et al. 2005). The loss of CRs in infant rats following muscimol infusion into the cerebellum suggests that the expression of conditioning established using a pontine stimulation CS requires cerebellar activity. However, muscimol inactivation of the cerebellum does not conclusively establish that the memory underlying conditioning with the stimulation CS is stored in the cerebellum. Studies that use reversible inactivation of the cerebellum and red nucleus are needed to determine whether the memory underlying eyeblink CRs established with a pontine stimulation CS is stored in the cerebellum (Krupa et al. 1993).
Previous studies using pontine stimulation as a CS in adult rats and rabbits found that the acquisition rate with stimulation is faster than the acquisition rate observed with a peripheral CS (Steinmetz et al. 1986; Freeman Jr. and Rabinak 2005). In the present study, the rate of acquisition in infant rats was about the same as that seen with conditioning to a tone CS. Moreover, the rate of acquisition in the infant rats was considerably slower than the rate of acquisition seen in adult rats. These findings suggest that even the older rat pups (P24-P25), which show rapid conditioning, are still undergoing maturational changes in the CS and US pathways.
The present findings and those of previous studies suggest that the ontogeny of eyeblink conditioning is due to developmental changes in the CS and US pathways. The development of the US pathway is characterized by an ontogenetic increase in cerebellar inhibitory regulation of climbing fiber activity (Freeman Jr. and Nicholson 2004). The developmental change in the responsiveness of pontine neurons to a tone CS results in a developmental change in the magnitude of CS-driven input to the cerebellum. The developmental changes in the CS and US pathways are thought to influence the induction and maintenance of synaptic plasticity within the cerebellum and thereby influence the acquisition and retention of eyeblink conditioning.
Materials and Methods
Subjects
The subjects were 46 Long-Evans rat pups. Thirty-five pups had effective electrode placements and were trained on P17-P18 (n = 18) or P24-P25 (n = 17). A subset of the pups with effective electrode placements was implanted with a cannula in the cerebellum and trained on P17-P18 (n = 6) or P24-P25 (n = 5). The remaining 11 pups had ineffective electrode placements. The rats were housed in the animal colony in Spence Laboratories of Psychology at the University of Iowa. The rats were maintained on a 12-h light/12-h dark cycles, with light onset at 7 a.m. Training sessions occurred between 7 a.m. and 7 p.m.
Surgery
The rat pups (P16 and P23) were given i.p. injections of ketamine (100 mg/kg), xylazine (5 mg/kg), and atropine (0.8 mg/kg). The rat's head was positioned and held securely in an infant stereotaxic apparatus and the skull surface was aligned in three planes. Differential electromyographic (EMG) electrodes were implanted in the left upper eyelid, and a ground electrode was connected to one of two skull hooks. The electrode and ground wires were soldered to gold pins in a plastic connector, which was secured to the skull by the skull hook and dental acrylic. The second skull hook was secured to the skull slightly anterior to lambda.
A bipolar stimulating electrode was then implanted into or just dorsal to the right basilar pontine nuclei. The electrode consisted of two insulated stainless steel wires (50 μm) in a plastic connector. The stereotaxic coordinates for the pontine nuclei were taken from lambda (P16/P23: +1.6/+2.0 anterior, -1.0/-1.0 medial-lateral, -8.3/-8.5 dorsal-ventral). Once the electrode was in place it was cemented with dental acrylic covering the entire length of the electrode above the skull surface, including the plastic connector. The rat pups that were given muscimol infusions into the cerebellum had a 23-gauge guide cannula implanted 0.5 mm dorsal to the left anterior interpositus nucleus. A 30-gauge stylet was inserted into the guide cannula. The stereotaxic coordinates for the cannula were also taken from lambda (P16/P23: -2.3/-2.3 posterior, 2.0/2.0 medial-lateral, and -4.8/-4.9 dorsal-ventral).
A bipolar stimulating electrode used for delivering the US was implanted subdermally, immediately caudal to the left eye. This bipolar electrode was also encased by a plastic connector, which was secured by dental acrylic. Sutures closed the surgical site on both sides of the head stage. Ketofen (5 mg/kg), an analgesic, was administered at the end of surgery, along with a subcutaneous injection of Ringer solution.
Conditioning apparatus
The conditioning apparatus consisted of a small-animal sound attenuation chamber (BRS/LVE) with a small-animal operant chamber (BRS/LVE) contained inside. The rats were kept in the operant chamber during conditioning. Lightweight cables with connectors for the EMG, US, and CS electrodes were attached to a commutator. The back wall of the sound-attenuation chamber was equipped with a small houselight that stayed on during conditioning sessions. The electrode leads from the rat's head stage were connected to peripheral equipment and a desktop computer. Computer software controlled the delivery of stimuli and the recording of eyelid EMG activity (JSA Designs). The US was delivered through a stimulus isolator (model no. 365A; World Precision Instruments). EMG activity was recorded differentially, filtered (500-5000 Hz), amplified (2000×), and integrated (time constant = 20 msec). Pontine stimulation was triggered through a programmable stimulator (Master 8, A.M.P.I.), which controlled signal input to a stimulus isolator (model no. 365A; World Precision Instruments) that delivered the electrical stimulation.
Pontine stimulation
Electrical stimulation of the basilar pontine nuclei functioned as the CS, which was administered in a 200 Hz train of 0.1-msec biphasic pulses for 300 msec. The stimulation threshold for the CS was found before training by setting the stimulating current to 50 μA, and either increasing or decreasing the current in 5-μA increments until a slight movement was detected (Tracy et al. 1998). Observable movements included, but were not limited to, eye blinks, eyeball retractions, and head movements. The level of stimulation during training was set to half the threshold intensity.
Muscimol infusion
Prior to muscimol infusions, the stylet was removed from the guide cannula and replaced with a 30-gauge infusion cannula. The infusion cannula was connected to polyethylene tubing (PE 10, 110-120 cm) that was connected to a 10-μL gas-tight syringe (Hamilton). The syringe was placed in an infusion pump (Harvard Apparatus), and 0.5 μL muscimol (5 nmol in saline, pH 7.4) was infused at a rate of 30 μL/h. The tubing connected to the infusion cannula was cut and sealed with candle wax. The infusion cannula remained in place for the duration of the experimental session.
Paired training
The rat pups in both age groups were given six paired training sessions, three sessions per day. The paired training sessions consisted of 100 trials, each with 90 trials of the stimulation CS paired with the shock US (10 msec, 3.0 mA) and 10 stimulation CS-alone trials, occurring on every tenth trial. The CS-alone trials were included in order to assess behavioral responses (integrated EMG activity) uncontaminated by URs. The interstimulus interval for paired trials was 290 msec. Trials were separated by an intertrial interval that averaged 30 sec. Behavioral data were examined from computer records of EMG responses. CRs were defined as responses that crossed a threshold of 0.4 volts above the baseline activity during the CS period, but at least 80 msec after CS onset, to avoid contamination of the CR measures by the startle (alpha) response. The threshold level is typically less than 10% of the amplitude of the UR. Previous studies have shown that the paired training protocol used in the present study established associative eyeblink CRs in developing rats (Nicholson and Freeman Jr. 2003a,b, 2004).
Unpaired training
Rat pups in both age groups were given six 200-trial sessions of explicitly unpaired presentations of the CS and US. The same time durations for the CS and US were used as in the paired procedure. The intertrial interval (ITI) was set to average 15 sec to match the total time spent in the conditioning chamber and the temporal distribution of CS and US presentations with the paired groups. The method for defining CRs was the same as that used in the paired procedure.
Histology
After training was completed, rats were euthanized with a lethal injection of sodium pentobarbital (90 mg/kg) and transcardially perfused with 100 mL of physiological saline, followed by 300 mL of 3% formalin. The brains were post-fixed in formalin for 2 d and then put in a solution of 10% sucrose in PBS before sectioning. The brains were sectioned at 50 μm with a sliding microtome. Sections were then stained with cresyl violet. The cannula and stimulating electrode locations were determined by examining serial sections.
Acknowledgments
Support for this research comes from National Institute for Neurological Disorders and Stroke grant NS38890 to J.H.F.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.91105.
References
- Aizenman, C.D. and Linden, D.J. 2000. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat. Neurosci. 3: 109-111. [DOI] [PubMed] [Google Scholar]
- Bao, S., Chen, L., and Thompson, R.F. 2000. Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behav. Neurosci. 114: 254-261. [DOI] [PubMed] [Google Scholar]
- Blatchley, B.J., Cooper, W.A., and Coleman, J.R. 1987. Development of auditory brainstem response to tone pip in the rat. Dev. Brain Res. 32: 75-84. [DOI] [PubMed] [Google Scholar]
- Cartford, M.C., Gohl, E.B., Singson, M., and Lavond, D.G. 1997. The effects of reversible inactivation of the red nucleus on learning-related and auditory-evoked unit activity in the pontine nuclei of classically conditioned rabbits. Learn. Mem. 3: 519-531. [DOI] [PubMed] [Google Scholar]
- Christian, K.M. and Thompson, R.F. 2003. Neural substrates of eyeblink conditioning: Acquisition and retention. Learn. Mem. 10: 427-455. [DOI] [PubMed] [Google Scholar]
- Clark, R.E., Gohl, E.B., and Lavond, D.G. 1997. The learning-related activity that develops in the pontine nuclei during classical eye-blink conditioning is dependent on the interpositus nucleus. Learn. Mem. 3: 532-544. [DOI] [PubMed] [Google Scholar]
- Freeman Jr., J.H. and Muckler, A.S. 2003. Developmental changes in eyeblink conditioning and neuronal activity in the pontine nuclei. Learn. Mem. 10: 337-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman Jr., J.H. and Nicholson, D.A. 2004. Developmental changes in the neural mechanisms of eyeblink conditioning. Behav. Cog. Neurosci. Rev. 3: 3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman Jr., J.H. and Rabinak, C.A. 2005. Eyeblink conditioning in rats using pontine stimulation as a conditioned stimulus. Int. Physiol. Behav. Sci. (in press). [DOI] [PMC free article] [PubMed]
- Freeman Jr., J.H., Halverson, H.E., and Poremba, A. 2005. Differential effects of cerebellar inactivation on eyeblink conditioned excitation and inhibition. J. Neurosci. 25: 889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel, C., Linden, D.J., and D'Angelo, E. 2001. Beyond parallel fiber LTD: The diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 4: 467-475. [DOI] [PubMed] [Google Scholar]
- Hyson, R.L. and Rudy, J.W. 1984. Ontogenesis of learning: II. Variation in the rat's reflexive and learned responses to acoustic stimulation. Dev. Psychobiol. 17: 263-283. [DOI] [PubMed] [Google Scholar]
- ____. 1987. Ontogenetic change in the analysis of sound frequency in the infant rat. Dev. Psychobiol. 20: 189-207. [DOI] [PubMed] [Google Scholar]
- Kim, J.J., Krupa, D.J., and Thompson, R.F. 1998. Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science 279: 570-573. [DOI] [PubMed] [Google Scholar]
- Krupa, D.J., Thompson, J.K., and Thompson, R.F. 1993. Localization of a memory trace in the mammalian brain. Science 260: 989-991. [DOI] [PubMed] [Google Scholar]
- Mauk, M.D. and Donegan, N.H. 1997. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn. Mem. 3: 130-158. [DOI] [PubMed] [Google Scholar]
- Mauk, M.D., Steinmetz, J.E., and Thompson, R.F. 1986. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proc. Natl. Acad. Sci. 83: 5349-5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, D.A., Steinmetz, J.E., and Thompson, R.F. 1985. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Res. 359: 120-130. [DOI] [PubMed] [Google Scholar]
- Medina, J.F., Nores, W.L., and Mauk, M.D. 2002. Inhibition of climbing fibers is a signal for the extinction of conditioned eyelid responses. Nature 416: 330-333. [DOI] [PubMed] [Google Scholar]
- Nicholson, D.A. and Freeman Jr., J.H. 2003a. Addition of inhibition in the olivocerebellar system and the ontogeny of a motor memory. Nat. Neurosci. 6: 532-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 2003b. Developmental changes in evoked Purkinje cell complex spike responses. J. Neurophysiol. 90: 2349-2357. [DOI] [PubMed] [Google Scholar]
- ____. 2004. Developmental changes in eyeblink conditioning and simple spike activity in the cerebellar cortex. Dev. Psychobiol. 44: 45-57. [DOI] [PubMed] [Google Scholar]
- Sananes, C., Gaddy, J.R., and Campbell, B.A. 1988. Ontogeny of conditioned heart rate to an olfactory stimulus. Dev. Psychobiol. 21: 117-133. [DOI] [PubMed] [Google Scholar]
- Sears, L.L. and Steinmetz, J.E. 1991. Dorsal accessory inferior olive activity diminishes during acquisition of the rabbit classically conditioned eyelid response. Brain Res. 545: 114-122. [DOI] [PubMed] [Google Scholar]
- Stanton, M.E. and Freeman Jr., J.H. 2000. Developmental studies of eyeblink conditioning in a rat model. In Eyeblink classical conditioning: Animal models (eds. D.S. Woodruff-Pak and J.E. Steinmetz), pp. 105-134. Kluwer, Amsterdam.
- Stanton, M.E., Freeman Jr., J.H., and Skelton, R.W. 1992. Eyeblink conditioning in the developing rat. Behav. Neurosci. 106: 657-665. [DOI] [PubMed] [Google Scholar]
- Steinmetz, J.E. 1990. Neuronal activity in the rabbit interpositus nucleus during classical NM-conditioning with a pontine-nucleus-stimulation CS. Psych. Sci. 1: 378-382. [Google Scholar]
- Steinmetz, J.E. and Sengelaub, D.R. 1992. Possible conditioned stimulus pathway for classical eyelid conditioning in rabbits. Behav. Neur. Biol. 57: 103-115. [DOI] [PubMed] [Google Scholar]
- Steinmetz, J.E., Rosen, D.J., Chapman, P.F., Lavond, D.G., and Thompson, R.F. 1986. Classical conditioning of the rabbit eyelid response with a mossy fiber stimulation CS. I. Pontine nuclei and middle cerebellar peduncle stimulation. Behav. Neurosci. 100: 878-887. [DOI] [PubMed] [Google Scholar]
- Steinmetz, J.E., Logan, C.G., Rosen, D.J., Thompson, J.K., Lavond, D.G., and Thompson, R.F. 1987. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proc. Natl. Acad. Sci. 84: 3531-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz, J.E., Lavond, D.G., and Thompson, R.F. 1989. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse 3: 225-233. [DOI] [PubMed] [Google Scholar]
- Tracy, J.A., Thompson, J.K., Krupa, D.J., and Thompson, R.F. 1998. Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behav. Neurosci. 112: 267-285. [DOI] [PubMed] [Google Scholar]