Abstract
Neural integration of glutamate- and dopamine-coded signals within the nucleus accumbens (NAc) is a fundamental process governing cellular plasticity underlying reward-related learning. Intra-NAc core blockade of NMDA or D1 receptors in rats impairs instrumental learning (lever-pressing for sugar pellets), but it is not known during which phase of learning (acquisition or consolidation) these receptors are recruited, nor is it known what role AMPA/kainate receptors have in these processes. Here we show that pre-trial intra-NAc core administration of the NMDA, AMPA/KA, and D1 receptor antagonists AP-5 (1 μg/0.5 μL), LY293558 (0.01 or 0.1 μg/0.5 μL), and SCH23390 (1 μg/0.5 μL), respectively, impaired acquisition of a lever-pressing response, whereas post-trial administration left memory consolidation unaffected. An analysis of the microstructure of behavior while rats were under the influence of these drugs revealed that glutamatergic and dopaminergic signals contribute differentially to critical aspects of the initial, randomly emitted behaviors that enable reinforcement learning. Thus, glutamate and dopamine receptors are activated in a time-limited fashion—only being required while the animals are actively engaged in the learning context.
In order to survive in changing environments, animals must be able to acquire, consolidate, and retrieve pertinent information regarding a given stimulus situation. The ability to learn associations between various stimuli and events, including motor actions, is the basis of instrumental learning (Rescorla 1991; Dickinson and Balleine 1994). Appetitive instrumental learning occurs when an animal associates its behavior with a favorable outcome such as food, sex, or the avoidance of pain. For instance, in a common experimental model of instrumental learning, a hungry rat learns to press a lever to obtain a food reward.
The nucleus accumbens (NAc) and its associated circuitry have been linked to the acquisition of adaptive motor responses and the control of behaviors related to natural reinforcers (Setlow 1997; Parkinson et al. 2000; Corbit et al. 2001). Because of the rich glutamatergic and dopaminergic innervation of the NAc from regions associated with motivational, cognitive, and sensory processes, many studies have focused on the role of these neurotransmitter systems with respect to instrumental and incentive learning (Berridge and Robinson 1998; Cardinal et al. 2002; Beninger and Gerdjikov 2004; Kelley 2004). For example, blockade of glutamate (N-methyl-d-aspartate, NMDA) or dopamine D1 receptors within the NAc core potently impairs instrumental learning, and coinfusion of low, individually ineffective doses of AP-5 and SCH23390 also prevents learning, suggesting that convergence of both systems on post-synaptic neurons is required (Smith-Roe and Kelley 2000). The coincident detection of glutamate and dopamine signals has been shown to be required for long-term potentiation (Wickens et al. 1996; Arbuthnott et al. 2000; Floresco et al. 2001; Kerr and Wickens 2001) by regulating the transcription and translation of plasticity-related immediate-early genes through various second messenger systems (Sharp et al. 1995; Sutton and Beninger 1999; Berke and Hyman 2000; Horvitz 2002; Reynolds and Wickens 2002; Steward and Worley 2002; Kelley 2004). Indeed, post-training inhibition of cAMP-dependent protein kinase (PKA) (Baldwin et al. 2002a) or inhibition of de novo protein synthesis within the NAc core (Hernandez et al. 2002) prevents the consolidation, or long-term stabilization, of memory for response-outcome contingencies.
In the aforementioned studies, pre-trial blockade of NMDA and D1 receptors appeared to prevent the encoding (or acquisition) of information; however, it is possible that disruption of the consolidation phase of learning or retrieval could have contributed to the observed impairments. Thus, it remains unclear as to whether glutamate and dopamine are required only to initiate plasticity or whether these neurotransmitters also modulate consolidation. As such, post-trial infusions are often used to temporally dissociate encoding from consolidation (Breen and McGaugh 1961). Therefore, the present study compared the effects of pre- and post-trial infusions of antagonists specific for NMDA or D1 receptors in the NAc core of male Sprague-Dawley rats in the same task. In addition, we investigated the effects of an antagonist specific for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate (AMPA/KA) receptors, since their role in instrumental learning has not yet been described. Lastly, we used a time-stamp behavioral analysis program that records the temporal relationship of task-related events and behaviors during training in order to gain insight into which behaviors are critical for instrumental learning.
Results
Histology
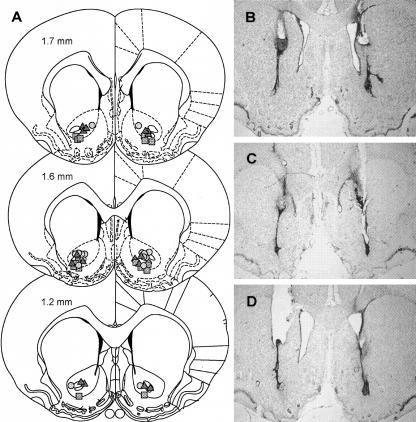
Histological analysis of cannulae and injector placements is shown in Figure 1. Figure 1A depicts schematically representative injector placements for each drug treatment. Only rats that had placements within the NAc core were included in the study. Figure 1, B-D, shows Nissl-stained coronal sections from rats receiving LY293558, AP-5, or SCH23390, respectively, demonstrating that the drugs or injections did not cause observable gross damage to tissue surrounding the injection site.
Figure 1.
Histological analyses of representative nucleus accumbens core injections' sites. (A) Stereotaxic coordinates displayed within the sections are in millimeters from bregma. NAc core injection sites representative of LY293558, AP-5, and SCH23390 infusions are indicated by circles, triangles, and squares, respectively. Adapted with permission from Elsevier © 1998, Paxinos and Watson (1998). (B-D) Nissl stains of coronal sections indicating cannulae and injector tracts terminating in the NAc core for the LY293558, AP-5, and SCH23390 experiments, respectively.
Experiments 1 and 2: Effects of intra-accumbens pre- or post-trial AMPA/KA receptor antagonism on instrumental learning, the microstructure of behavior, and unconditioned behavior
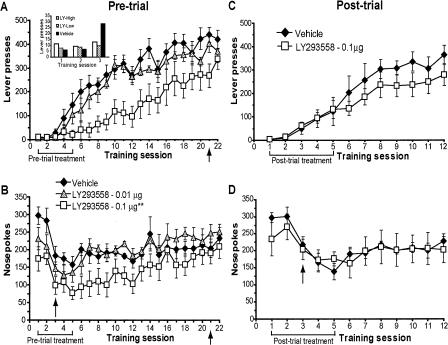
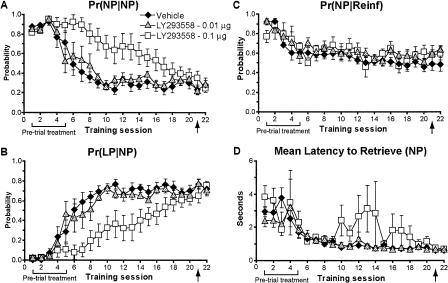
As can be observed from the lever-pressing data shown in Figure 2A, pre-trial infusions of LY293558 into the NAc core produced a significant, dose-dependent impairment of instrumental learning. This impairment was long lasting but not permanent as drug-treated rats eventually learned the task. Repeated-measures analysis of variance (ANOVA) on the lever-pressing data indicated a main effect of treatment (F(2,14) = 6.99, P = 0.008) over sessions 1-20, as well as a significant session × treatment interaction (F(38,266) = 6.99, P = 0.005). A Newman-Keuls post hoc test revealed that the number of responses made by the high-dose group was significantly different from those of both the vehicle and low-dose groups (P = 0.05), whereas responses from the low-dose and control groups were not significantly different from each other (P > 0.05). Corrected F-values were calculated to analyze overall session × treatment interactions (simple main effects) between the high-dose and control groups. This analysis revealed a significant session × treatment interaction for the high-dose group (F(19,266) = 2.69, P = 0.0002), indicating that these rats learned to lever-press at a slower rate over sessions. No session × treatment interaction was found for the low-dose group (P = 0.9). It can also be seen from Figure 2A that pre-trial administration of LY293558 had no effect after the task had been learned (session 21). An ANOVA over sessions 20-21 revealed no significant session × treatment interaction (P = 0.3), indicating that the drug did not affect retrieval or performance of the task.
Figure 2.
Memory consolidation is not affected by AMPA/KA receptor blockade. (A,B) Pre-trial infusions of LY293558 (0, 0.01, or 0.1 μg) dose-dependently impaired the acquisition of instrumental learning as measured by decreased (A) lever presses and (B) nose pokes. Infusions after the task was learned had no effect on memory retrieval/performance. (Inset) Lever presses made over the first three sessions. Vehicle group, n = 6; 0.01 μg group, n = 5; 0.1 μg group, n =6. (C,D) Post-trial infusions of LY293558 (0 or 0.1 μg) failed to affect (C) lever-pressing or (D) nose-poking, indicating memory for the task was consolidated normally. Vehicle group, n = 7; 0.1 μg group, n = 5. Arrows next to session 3 in B and D mark the end of noncontingent reinforcement. For all panels, data are shown as the mean number of lever presses or nose pokes ± SEM. Brackets and arrows beneath the x-axis indicate the frequency of injections. (**) Main effect of treatment on lever-pressing, P < 0.01 (see Results for nose-poke statistics).
Figure 2B shows nose-poking behavior. Note that the normal pattern observed in vehicle-infused rats is such that nose-poking is very high in the first two training sessions (see Fig. 2B, and Figs. 4B and 6B below) and then decreases and levels off. This pattern is due to the free, randomly delivered sugar pellets available during sessions 1 and 2. LY293558 lowered the number of nose pokes made by the high-dose group relative to both the vehicle and low-dose groups. Similar to lever-pressing, nose-poking by LY293558-treated rats recovered to levels observed in the other groups once drug treatment ended. ANOVA revealed a significant treatment effect of pre-trial LY293558 on the number of nose pokes made into the food trough over sessions 1-20 (F(2,14) = 3.79, P = 0.05). A Newman-Keuls post hoc test indicated that the number of nose pokes made by the high-dose group was significantly different from those of the low-dose group (P = 0.05). Additionally, the drug did not affect nose-poking when infused prior to session 21 (P = 0.5, sessions 20-21) after the animals had become proficient in the task.
Figure 4.
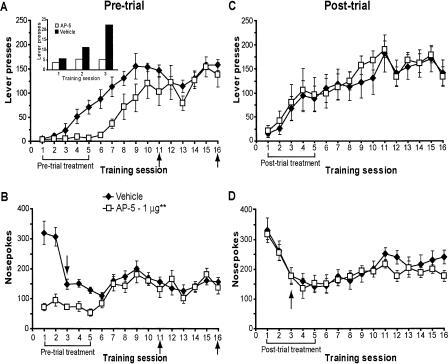
NMDA receptor activity is not necessary for memory consolidation. (A,B) Pre-trial infusions of AP-5 (0 or 1 μg) impaired the acquisition of instrumental learning as measured by decreased (A) lever presses and (B) nose pokes. Infusions after the task was consolidated show no effect on memory retrieval or performance. (Inset) Lever presses made over the first three sessions. Vehicle group, n =7; 1 μg group, n =8. (C,D) Post-trial infusions of AP-5 (0 or 1 μg) failed to affect (C) lever-pressing or (D) nose-poking, indicating memory for the task was consolidated normally. Vehicle group, n = 7; 1 μg group, n = 8. Arrows next to session 3 in B and D mark the end of noncontingent reinforcement. For all panels, data are shown as the mean number of lever presses or nose pokes ± SEM. Brackets and arrows beneath the x-axis indicate the frequency of injections. (**) Main effect of treatment on lever-pressing in the pre-trial condition, P < 0.01 (see Results for nose-poke statistics).
Figure 6.
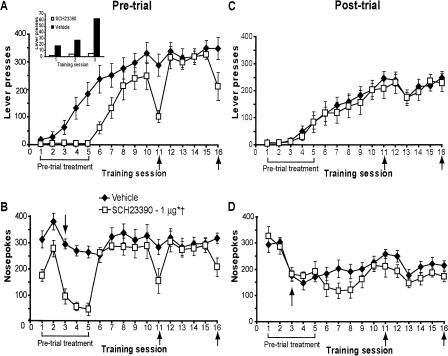
D1 receptor blockade does not affect memory consolidation. Pre-trial infusions of SCH23390 (0 or 1 μg) impaired the acquisition of instrumental learning as measured by decreased (A) lever presses and (B) nose pokes. Only infusions of SCH29330 after the task was consolidated significantly decreased lever-pressing, whereas a nonsignificant decrease in nose-poking was observed. (Inset) Lever presses made over the first three sessions. Vehicle group, n = 9; 1 μg group, n =8. Post-trial infusions of SCH23390 (0 or 1 μg) failed to affect (C) lever-pressing or (D) nose-poking, indicating that memory for the task was consolidated normally. Vehicle group, n = 8; 1 μg group, n = 7. Arrows next to session 3 in B and D mark the end of noncontingent reinforcement. For all panels, data are shown as the mean number of lever presses or nose pokes ± SEM. Brackets and arrows beneath the x-axis indicate the frequency of injections. (*) Main effect of treatment over sessions 1-10 on lever-pressing, P ≤ 0.05. (†) Main effect of treatment over sessions 10-11 on lever-pressing, P ≤ 0.05 during the pre-trial condition (see Results for nose-poke statistics).
In contrast to the effects of pre-trial infusions, post-trial administration of the high-dose of LY293558 produced no obvious learning impairments (Fig. 2C). ANOVA conducted on lever-pressing data over sessions 2-12 indicated that post-trial infusions of LY293558 had no effect on the consolidation of memory for the lever-pressing task (P = 0.4). Nose pokes were similarly not affected by the antagonist over the same period (P = 0.8) (Fig. 2D).
An analysis of the microstructure of behavior was performed only on rats that received pre-trial infusions, as post-trial administration of LY293558 did not affect learning. Briefly, the following conditional probabilities of several task-oriented behaviors were calculated: the probability of a nose poke given that the previous event was also a nose poke [Pr(NP|NP)], the probability of a lever press given that a nose poke had just occurred [Pr(LP|NP)], and the probability of a nose poke given that a reinforcer was delivered [Pr(NP|Reinf)]. The mean latency (in seconds) to retrieve a reinforcer was also calculated. In order to better appreciate drug effects on these measures, it is first useful to observe what happens as vehicle-infused animals learn the task (note Figs. 3 and 5 and 7 below). As vehicle-treated animals learn the response-outcome relationship between lever-pressing and food delivery, successively fewer attempts are made to retrieve food without first pressing the lever [decreased Pr(NP|NP)], the tendency to lever-press immediately after retrieving a reward increases [increased Pr(LP|NP)], and latencies to retrieve rewards decrease. Finally, the probability of a nose poke occurring following the delivery of a reinforcer remains relatively constant across days, or tends to drift down slightly.
Figure 3.
AMPA/KA receptor blockade disrupts specific patterns of behavior during operant training. (A-D) An analysis of the microstructure of behavior of rats given pre-trial infusions of LY293558 (0, 0.01, or 0.1 μg) (see Fig. 2A,B). The 0.1-μg dose of LY293558 prevented (A) the learning-related decrease in the probability to make consecutive nose pokes, Pr(NP|NP), and (B) the increase in the probability to lever-press after retrieving a reward, Pr(LP|NP), as demonstrated by controls and the 0.01-μg group, until the task was well-learned. No reliable changes were observed in (C) the probability to retrieve a reward upon delivery, Pr(NP|Reinf), or (D) the latency to retrieve rewards. Post-learning infusions had no effect. Error bars indicate the SEM. Brackets and arrows beneath the x-axis indicate the frequency of pre-trial injections.
Figure 5.
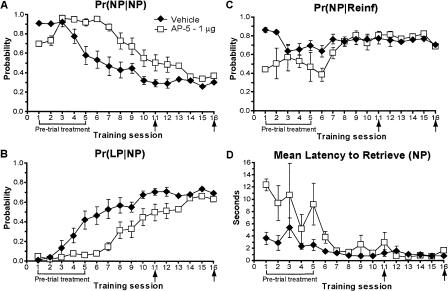
NMDA receptor blockade differentially disrupts specific patterns of behavior during operant training. (A-D) An analysis of the microstructure of behavior of rats given pre-trial infusions of AP-5 (0 or 1 μg) (see Fig. 4A,B). (A) Unlike LY293558, AP-5 decreased the tendency to make consecutive nose pokes, Pr(NP|NP), while free rewards were given (see Materials and Methods), then increased the Pr(NP|NP) relative to controls until the task was well-learned. (B) AP-5 reduced the normal increase in the probability to lever-press after retrieving a reward and, in contrast to LY293558, AP-5 also (C) reduced the probability to retrieve rewards while (D) increasing the latency in which to retrieve them until the task was well-learned. Post-learning infusions had no effect. Error bars indicate the SEM. Brackets and arrows beneath the x-axis indicate the frequency of pre-trial injections.
Figure 7.
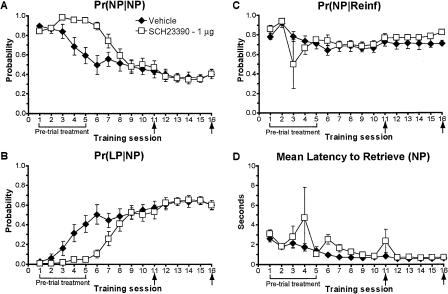
Antagonism of D1 receptors produces disruptions in the microstructure of behavior similar to those caused by LY293558. (A-D) An analysis of the microstructure of behavior of rats given pre-trial infusions of SCH23390 (0 or 1 μg) (see Fig. 6A,B). Until the task was well learned, the 1-μg dose of SCH23390, like LY293558, prevented (A) the learning-related decrease in the probability to make consecutive nose pokes, Pr(NP|NP), and (B) the increase in the probability to lever-press after retrieving a reward, Pr(LP|NP), as demonstrated by controls. No reliable changes were observed in (C) the probability to retrieve a reward upon delivery, Pr(NP|Reinf), or (D) the latency to retrieve rewards. Post-learning infusions had no apparent effect. Error bars indicate the SEM. Brackets and arrows beneath the x-axis indicate the frequency of pre-trial injections.
Figure 3, A and B, shows that intra-accumbens infusion of the high dose of LY293558 prevented the decrease in the Pr(NP|NP) as well as the increase in the Pr(LP|NP), respectively, as is normally observed in the vehicle and low-dose groups during learning. Thus, LY293558-treated rats tended to nose-poke rather than lever-press after a pellet had been retrieved. Neither the Pr(NP|Reinf) (Fig. 3C) nor the latency to retrieve reinforcers (Fig. 3D) was affected by LY293558, however. In no case did LY293558 have an effect on any of these behaviors once the task was learned (session 21).
Experiment 2, designed to assess the unconditioned effects on feeding and locomotor behaviors (Table 1), demonstrated that infusion of the high dose of LY293558 in the NAc core had no significant effect on food intake [although LY293558-treated rats tended to eat slightly more (F(1,6) = 4.95, P = 0.07)] or latency to feed. However, LY293558 significantly decreased the number of feeding bouts (F(1,12) = 6.89, P = 0.04), while simultaneously increasing mean bout length (F(1,12) = 12.01, P = 0.01), and total feeding time (F(1,12) = 10.14, P = 0.02). In terms of locomotor behaviors, rats under the influence of LY293558 made significantly fewer center crossings (F(1,12) = 9.74, P = 0.02), tended to rear less often (F(1,12) = 4.90, P = 0.07), and spent less time rearing relative to the vehicle-infused condition.
Table 1.
Effect of AMPA/KA receptor blockade on feeding and locomotion
| Behavioral measure | Vehicle | LY293558 (0.1 μg) |
|---|---|---|
| Food consumed (g) | 5.0 ± 0.2 | 5.7 ± 0.3 |
| Latency to feed (sec) | 9.5 ± 1.9 | 9.3 ± 2.2 |
| Number of feeding bouts | 24.7 ± 3.2 | 13.7 ± 2.7* |
| Mean bout length (sec) | 30.5 ± 6.4 | 67.7 ± 9.3** |
| Total time feeding (sec) | 637.1 ± 35.4 | 787.1 ± 27.4* |
| Total center crossings | 19.3 ± 4.1 | 6.6 ± 2.2* |
| Total rearing events | 9.6 ± 2.3 | 4.3 ± 1.4 |
| Time spent rearing (sec) | 10.6 ± 2.7 | 5.8 ± 2.1 |
Mean values ± SEM. N = 7; *P < 0.05; **P = 0.01.
Experiment 3: Effects of intra-accumbens pre- or post-trial NMDA receptor antagonism on instrumental learning and the microstructure of behavior
Pre-trial AP-5 infusions into the accumbens also potently impaired instrumental learning relative to controls (Fig. 4A). Again, this effect was reversible as the animals quickly learned the task upon completion of drug treatment. ANOVA revealed a significant treatment effect (F(1,12) = 10.68, P = 0.007) and session × treatment interaction (F(9,108) = 2.13, P = 0.03) on lever-pressing over sessions 1-10. However, no effect of AP-5 was observed when administered after the task had been learned (sessions 10-11, P = 0.2; and sessions 15-16, P = 0.6), indicating the drug did not influence retrieval or task performance.
Nose pokes were also markedly reduced by AP-5 relative to vehicle infusions (Fig. 4B). ANOVA revealed a significant treatment effect (F(1,12) = 27.30, P = 0.0002) and session × treatment interaction (F(9,108) = 10.90, P < 0.0001) on nose-poking behavior over sessions 1-10, whereas no effect of AP-5 was observed after the task had been learned (sessions 10-11, P = 0.5; and sessions 15-16, P = 1.0).
Figure 4, A and B, demonstrates that, in contrast to the effects of pre-trial administration of AP-5, neither lever-pressing (sessions 2-10, P = 0.7) nor nose-poking (sessions 2-10, P = 1.0) was affected by the drug, suggesting that AP-5 did not disrupt consolidation of memory for the task.
As in the first experiment, the Pr(NP|NP) of vehicle-infused animals gradually decreased as learning occurred accompanied by a simultaneous increase in Pr(LP|NP). However, AP-5 disrupted this pattern. During the first two sessions, AP-5 decreased the Pr(NP|NP) relative to controls but then increased the Pr(NP|NP) for much of the remainder of the experiment (Fig. 5A) when reinforcement was contingent only on lever-pressing. Like LY293558, AP-5 also sharply decreased the Pr(LP|NP) until the rats began to learn the task (Fig. 5B). However, in contrast to LY293558, AP-5 decreased the Pr(NP|Reinf) (Fig. 5C) and markedly increased the latency to retrieve rewards (Fig. 5D) until drug treatments ended. After the task was learned, infusions of AP-5 had no effect on any of these measures.
Experiment 4: Effects of pre- or post-trial dopamine D1 receptor antagonism on instrumental learning and the microstructure of behavior
Figure 6A shows that pre-trial blockade of dopamine D1 receptors in the NAc core with SCH23390 markedly impaired instrumental learning. Indeed, ANOVA over sessions 1-10 revealed a significant treatment effect on lever-pressing (F(1,15) = 6.86, P = 0.02) and session × treatment interaction (F(9,135) = 3.06, P = 0.002). Once the task was learned (and in contrast to LY293558 and AP-5), a pre-trial infusion of SCH23390 on session 11 lowered responding significantly (F(1,15) = 7.14, P = 0.02 over sessions 10-11). Interestingly, an additional infusion of SCH23390 5 d later, prior to session 16, failed to affect responding to the same degree (P = 0.1 over sessions 15-16), although there was still a decrement.
SCH23390 also decreased nose-poking behavior as revealed by a significant treatment effect (F(1,15) = 13.26, P = 0.002) and session × treatment interaction (F(9,135) = 6.26, P < 0.0001) over sessions 1-10 (Fig. 6B). Relative to the initial infusions of SCH23390, only moderate, nonsignificant decreases in nose pokes were observed following drug administration after learning had occurred (sessions 10-11, P = 0.2; sessions 15-16, P = 0.1).
Additional rats that received post-trial infusions of SCH23390 into the NAc core demonstrated normal levels of lever-pressing (Fig. 6C; sessions 2-10, P = 0.7) and nose-poking (Fig. 6D; sessions 2-10, P = 0.4) relative to controls, suggesting that blockade of D1 receptors, like AMPA/KA and NMDA receptor blockade, did not affect consolidation of memory for the task.
Pre-trial SCH23390 infusions produced patterns of disruptions in the microstructure of behavior highly reminiscent of those caused by LY293558 treatment. As such, SCH23390 prevented the normal decrease in the Pr(NP|NP) and increase in the Pr(LP|NP) observed in controls (Fig. 7A,B, respectively). Moreover, SCH23390, like LY293558, had no effect on the Pr(NP|Reif) or the latency to retrieve reinforcers relative to controls (Fig. 7C,D, respectively).
Discussion
The purpose of the present study was to assess the effects of AMPA/KA, NMDA, or D1 receptor antagonism (using LY293558, AP-5, and SCH23390, respectively) within the nucleus accumbens (NAc) core on the acquisition and consolidation of memory for an instrumental lever-pressing task and to assess any behavioral impairments caused by receptor blockade. We report that pre-trial infusions of all three antagonists potently impaired learning in rats whereas post-trial infusions had no effect, suggesting these receptor systems are not required for consolidation. Deficits specific to each receptor antagonist were observed during instrumental training and in the microstructure of task-related behaviors. These data are novel in that no previous studies have examined the role of AMPA/KA receptors during instrumental learning, nor has it been determined whether glutamate and dopamine participate in the acquisition or consolidation phase of this form of learning. Additionally, receptor-specific behavioral deficits have not been previously identified. Thus, under these circumstances, glutamate and dopamine receptors appear to be activated in a context-limited fashion—only being required while the animals are actively engaged in the learning context—and contribute differentially to behaviors emitted during the early learning period.
A context-limited role for AMPA receptor activation within the nucleus accumbens during instrumental learning
Similar to studies examining other forms of learning and memory (De Leonibus et al. 2003; Stefani et al. 2003; Harris et al. 2004), we report that the AMPA/KA receptor antagonist LY293558 administered pre-trial within the NAc core dose-dependently impaired the acquisition of an instrumental lever-pressing task. Although permanent effects of the drug were not observed, the high-dose LY293558 group required more sessions to learn the task after the end of the first five drug treatments relative to typical experiments conducted in our laboratory using AP-5 or SCH23390. It may be that AMPA/KA receptor blockade resulted in a larger learning impairment because of a concurrent decrease in the population of functionally active NMDA receptors, which are both glutamate- and voltage-dependent (Nowak et al. 1984). However, given the strong involvement of AMPA/KA receptors in driving NAc projection neurons, the larger learning impairment probably resulted from the complete inactivation of the affected portion of the nucleus, whereas NMDA (or D1) receptor blockade would have affected neuronal firing and plasticity to a lesser, yet significant, degree (Pennartz et al. 1991; Hu and White 1996; Wolf et al. 2004). Alternatively, it is possible that LY293558 administration has longer-lasting effects on neural function relative to AP-5 or SCH23990, but such effects may be negligible since rats that received the drug post-trial were able to learn normally on the following training sessions.
It is unlikely that pre-trial infusions of LY293558 resulted in motivational or gross motor impairments that could account for the failure to acquire the lever-pressing task. First, an infusion of LY239558 after the task was learned did not reduce lever-pressing. Second, LY293558- and vehicle-treated groups demonstrated the same probability of nose-poking following reward delivery (a nondiscriminative Pavlovian approach behavior learned during autoshaping) and did so with comparable latencies. Finally, it can be seen that the experimental rats sampled the lever as much if not more than controls during the first two formative training sessions (see Fig. 2 inset) and were therefore exposed to the response-outcome contingency yet still failed to learn the task. Taken together, these results strongly suggest that pre-trial infusions impaired some aspect of cellular plasticity or behavior necessary for associative learning. These findings seem to be at odds with several studies reporting antagonist-induced performance deficits (Di Ciano et al. 2001; Micheau et al. 2004; Yun et al. 2004). For example, although not directly comparable to the present study, Di Ciano et al. (2001) reported that LY293558 infused in the NAc core of rats impaired performance of a discriminative Pavlovian approach response. Interestingly, approach responses made to a lever previously associated with food remained unaffected, whereas approaches to a second lever never paired with food increased. Thus, performance deficits do not necessarily signify decreases in responding caused by motor or retrieval impairments. This raises the possibility that the effects of AMPA/KA receptor blockade in the NAc may only become apparent when behavior must be constrained to one of several possible responses or when higher levels of attention are needed (e.g., during discriminative choice).
An analysis of the microstructure of behavior revealed that rats pretreated with the high-dose of LY293558 failed to inhibit their tendency to make consecutive nose pokes into the food magazine or increase their tendency to lever-press after nose-poking as is normally observed in rats learning the response-outcome contingency. These results suggest that the rats tended to nose-poke perseveratively, unable to inhibit that behavior or redirect their attention to other stimuli in their environment (e.g., the newly introduced lever). Thus, glutamate acting on AMPA/KA receptors within the NAc may contribute to proper behavioral set switching, which is critical for the expression of exploratory behaviors needed to first learn the lever-pressing task. This idea is in agreement with several lesion studies that suggest NAc is involved in behavioral flexibility and impulse control (Reading et al. 1991; Cardinal et al. 2001).
AMPA/KA receptors also play an important role in other accumbens-related learned behaviors. For example, Di Ciano et al. report that AMPA/KA receptor blockade in the NAc core together with D1 receptor blockade in the basolateral amygdala blocks cocaine-seeking behavior (Di Ciano and Everitt 2004). Additionally, glutamate release and/or AMPA/KA receptor activation in the NAC core is involved in the expression of behaviors related to drug-paired cues (Hotsenpiller et al. 2001; Park et al. 2002; McFarland et al. 2003), responding for conditioned reinforcers (Burns et al. 1994), and learning spatial strategies during food retrieval (Maldonado-Irizarry and Kelley 1995).
Notably, no effect of LY293558 was observed when administered post-trial, whereas other post-trial treatments, including inhibition of PKA or de novo protein synthesis (Baldwin et al. 2002a; Hernandez et al. 2002), have been shown to impair consolidation when administered at the same time point. Thus, it appears that AMPA/KA receptors may only be required during the encoding or acquisition phase of instrumental learning and are not obviously involved in consolidation (i.e., ongoing gene expression and protein synthesis) once the animal is removed from the stimulus situation.
NMDA receptor activation within the nucleus accumbens core also plays a key role in instrumental learning
Confirming previous results, pre-trial infusions of AP-5 impaired instrumental learning but had no effect on retrieval/performance of the task once learned (Kelley et al. 1997; Smith-Roe and Kelley 2000; Baldwin et al. 2002b). In contrast, post-trial infusions of AP-5 had no effect, suggesting that the role of NMDA receptors within the NAc during instrumental learning also seems to be limited to the initiation of downstream consolidation processes. However, the effects of glutamate receptor (both AMPA/KA and NMDA) blockade on acquisition, consolidation, and retrieval/performance vary widely depending on the site of infusion, timing of post-training infusions, dose, and the learning paradigm being studied (Bonini et al. 2003; De Leonibus et al. 2003).
In this regard, it is important to consider several studies addressing similar questions that report results that are discrepant with the present data. First, Roullet et al. (2001) found that AP-5 injections administered immediately after training but not after a 2-h delay impaired the ability of mice to detect the spatial novelty when tested at 24 h. In contrast, DNQX (an AMPA receptor antagonist) did not affect consolidation when administered immediately or 2 h after training (Roullet et al. 2001). In a second study, Sargolini et al. (2003) found that immediate posttrial injections of AP-5 or DNQX differentially affect consolidation of spatial memory needed to navigate in a water maze. Their results demonstrated that AP-5 prevented consolidation in the place version (where distal visual cues could be used to locate the escape platform) but not in the cued version of the water-maze task (where only one proximal cue could be used to locate the platform). DNQX had no effect on memory in either version of the task (Sargolini et al. 2003). In a very similar study, D2 receptor blockade was found to impair consolidation of memory for a spatial water maze task but not a cued version of the task only when infused immediately post-trial (2-h delay had no effect) (Setlow and McGaugh 1998). In another spatial learning paradigm, Mele et al. (2004) demonstrated that both D1 and D2 receptor blockade impaired spatial memory when antagonists for each receptor were infused immediately post-trial but not after a 2-h delay (Mele et al. 2004). On first consideration, it appears that all of these studies stand in direct contrast to our results. However, it is intriguing to speculate that tasks that have a heavy spatial component (involving hippocampal-dependent processing) require more extended, receptor-mediated “neural rehearsal” mechanisms to be consolidated, whereas receptor-based mechanisms underlying instrumental or procedural learning are temporally constrained by performing appropriate motor responses and experiencing feedback on those actions. This distinction could perhaps explain some of the discrepancies in the efficacy of post-trial manipulations. However, more work is needed to completely rule out the involvement of these receptors in the consolidation of memory for the present task.
Pre-trial infusions of AP-5 markedly disrupted behavioral patterns normally observed early in learning. In general, noncontingent delivery of sugar during the first two training sessions is highly arousing and can be observed behaviorally by increased levels of nose-poking during the first two sessions relative to the third when sugar delivery is contingent solely upon lever-pressing. We found that superimposing this reinforcement schedule over an FR 1 schedule reduces variability and expedites learning relative to a simple FR 1 schedule (data not shown). However, AP-5-treated rats, unlike LY293558- and SCH23390-treated rats, showed no evidence of any additional arousal induced by noncontingent sugar delivery. Indeed, the same low levels of nose-poking were maintained from sessions 2 to 3, which may reflect an inability to act on the arousing effects of elevated levels of dopamine following food delivery (Hernandez and Hoebel 1988; Schultz et al. 1993, 1997; Wilson et al. 1995; Bassareo and Di Chiara 1999). In fact, AP-5 noticeably increased the latency to retrieve rewards (although the sugar was always eaten when found) and lowered the probability of nose-poking after reinforcer delivery, effects not observed after LY293558 or SCH23390 treatment. This profile might indicate the rats were motorically impaired if it were not for our other studies demonstrating that in nonlearning situations, neither general motor activity nor any aspect of food intake or feeding behavior is impaired by AP-5 (Kelley et al. 1997; Smith-Roe et al. 1999). Thus, the effect of AP-5 on the microstructure of behavior cannot be easily explained by a general deficit in locomotor function. This profile suggests that glutamate signals acting on NMDA receptors in the NAc may be critical for increasing the output and speed of foraging responses under certain motivational and contextual conditions, a concept supported by the recent work of Giertler et al. (2003). When the output of these responses is high over a restricted time window, the probability that random lever presses resulting in reward will occur is also higher. Under the influence of AP-5, rats appear to make fewer attempts at lever-pressing or nose-poking, despite presentation of arousal-inducing food pellets. To this extent, NMDA receptor blockade has also been shown to prevent the reinstatement of cocaine-seeking behaviors controlled by the highly arousing effects of cocaine-associated cues (Bespalov et al. 2000; but see Di Ciano et al. 2001). Although the precise mechanisms are not yet clear, somehow AP-5 prevents the occurrence of associative processes between reward delivery and the animal's actions.
D1 receptor activation within the nucleus accumbens regulates the acquisition and performance of instrumental responses
As demonstrated previously, pre-trial infusions of SCH23390 impaired learning and performance once the animals were allowed to learn the task (Cousins et al. 1994; Smith-Roe and Kelley 2000). Notably, a second post-learning infusion of SCH23390 administered 5 d after the first (the effect of which we have not previously examined) failed to lower responding to the same degree. This diminished effect of SCH23390 suggests that accumbens dopamine is relatively more involved in motor performance early in the learning period while having less involvement once a high degree of proficiency is achieved. Indeed, increases in accumbens DA are attenuated in situations predictive of reward relative to novel learning situations or changes in instrumental contingency (Datla et al. 2002). Therefore, once a response has become “habitual,” brain regions such as the dorsal striatum, which is known to be involved in stimulus-response learning (Packard and McGaugh 1996; Ito et al. 2002; Robbins and Everitt 2002), may be more involved in the expression of a learned instrumental response.
Contrary to findings reported in other learning paradigms (Mele et al. 2004), post-trial infusion of SCH23390 failed to affect instrumental learning, suggesting that D1 receptors, in addition to AMPA/KA and NMDA receptors, are primarily involved in the encoding of task-related information rather than in memory consolidation. That neither glutamate nor D1 receptors seem to be involved in consolidation may be indicative of the unique pattern of convergent inputs from various brain regions into the accumbens. The coincident detection/interaction of glutamatergic and dopaminergic signals might be necessary for the formation of response-outcome contingencies, and this can only occur while the animal is actively engaged within the context of the learning situation, thereby limiting receptor activity to the encoding phase of learning.
Interestingly, AMPA/KA receptor blockade within the NAc core generated a pattern of deficits in both learning and non-learning situations that is remarkably similar to those observed after SCH23990 administration. Recently, in an unconditioned locomotor and feeding experiment, Baldo et al. (2002) reported that SCH23390 (the same dose used in the present study) decreased general motor activity without affecting the latency to eat when infused into the NAc core. The authors also reported that, although SCH23990-treated rats had fewer feeding bouts, the mean duration of each feeding bout increased (Baldo et al. 2002). This is precisely the same pattern of results we observed after administration of LY293558 in the NAc core. Moreover, both SCH23990 and LY293558 decreased lever-pressing and nose-poking (note the patterns of nose-poking over the first three sessions), and produced the same pattern of disruptions on all four measures of the microstructure of behavior. SCH23990 was the only antagonist, however, that impaired performance after the task had been well-learned, indicating at least a dual role for dopamine in the striatum for both associative and motor functions. Indeed, there is much evidence implicating striatal dopamine in both cellular plasticity (Konradi et al. 1993; Cepeda and Levine 1998; Floresco et al. 2001; Thomas and Malenka 2003) and behavioral activation (Fibiger et al. 1976; Salamone 1987; Floresco et al. 1996). Thus, it is possible that under these circumstances both D1 and AMPA/KA receptors could be used to guide proper behavioral set switching early in learning (Koob et al. 1978; Gelissen and Cools 1988; Bakshi and Kelley 1991; van den Bos et al. 1991; Baldo et al. 2002). However, the ability of D1 and AMPA receptors to influence behavioral set switching may be regulated differentially by reward- and sensory-related stimuli, respectively.
Conclusions
The data discussed above demonstrate important and dissociable roles of accumbens AMPA/KA, NMDA, and D1 receptors in appetitive instrumental learning. Receptor stimulation is necessary only when the animals are actively engaged in the learning context, in that post-trial application of all three receptor antagonists has no effect on learning. Moreover, analyses of task-related behavioral profiles suggest that glutamatergic and dopaminergic signals (and perhaps their interaction) contribute to critical aspects of the initial, randomly emitted behaviors that enable reinforcement learning.
During early learning, the output of the NAc core may contribute to the strengthening or weakening of neural representation of a multitude of responses that compete for behavioral expression. Thus, there must be a feedback or gating mechanism for monitoring the input and output of the NAc. For example, the neural coding of responses that result in reward delivery (e.g., jumping, pressing, or biting on the lever), must be able to feed back in some manner, possibly through striato-thalamo-cortico loops (Alexander et al. 1986; Barto 1995; Haber et al. 2000; Guillery and Sherman 2002; McFarland and Haber 2002), in order to facilitate the instantiation of the motor actions resulting in a positive outcome. In addition to encoding information, glutamate and dopamine D1 receptors within the NAc may also be required for the animal to act on important environmental stimuli and monitor the results of their own actions—a mechanism critical for behavioral flexibility of the kind needed to deal with ongoing environmental changes.
Materials and Methods
Animals
A total of 90 male Sprague-Dawley rats (300-325 g) (Harlan Sprague Dawley) were housed in groups of two on a 12-h light/12-h dark cycle. To minimize stress, all rats were handled regularly prior to behavioral procedures. Rats were food-deprived and maintained at 85% of their free-feeding presurgical weight in all experiments. Animal care was in accordance with University of Wisconsin-Madison Animal Care and Use guidelines and the NIH Guide.
Surgery and histology
Rats were anesthetized with ketamine/xylazine (100/10 mg/kg) and implanted bilaterally with 23-gauge cannulae aimed at the NAc core according to standard stereotaxic procedures. Stainless steel stylets were inserted into the cannulae to prevent occlusion. Coordinates for core placements were (in millimeters, based on flat skull system) +1.4 anterior to bregma, ±1.7 from midline, and -5.3 from the skull surface. At least 1 wk was allowed between surgery and the beginning of behavioral testing. After each experiment, rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with 0.9% saline followed by 10% formalin. Brains were removed and stored overnight in 10% formalin, then transferred to 10% sucrose-formalin solution for 24 h before sectioning. Brains were sectioned at 60 μm then stained with cresyl violet. Light microscopy was used to locate the infusion sites (see Fig. 1).
Apparatus
Operant chambers (Coulbourn Instruments) equipped with a retractable lever, a house light, and a food trough with a light and photosensors were used in all experiments. Time-stamped stimulus events and data acquisition were controlled by microcomputer (Lablinc, Coulbourn Instruments). The main dependent variables were lever presses and nose pokes into the food trough. Locomotor and feeding assessments were carried out in a novel environment in clear polycarbonate cages similar to the rats' homecages.
Drugs and microinfusion procedure
[(3SR, 4aRS, 6RS, 8aRS)-6-[2-(iH-tetrazol-5-yl) ethyl]-1,2,3,4,4a,5,6,7,8,8a-decahydroiso-quinoline-3-carboxylic acid] (LY293558; a gift from Eli Lilly & Co., Indianapolis, IN) was dissolved in sterile water, whereas D-(-)-2-aminophosphonopentanoic acid (AP-5) and R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390; Sigma) were dissolved in 0.9% isotonic sterile saline. Doses of LY293558 (0.01-0.1 μg/side) were based on those used in the literature (Stefani et al. 2003) as well as on our own pilot experiments, whereas doses of AP-5 (1 μg/side) and SCH23390 (1 μg/side) were based on those used in many previous studies conducted in this laboratory demonstrating their ability to significantly impair learning (Kelley et al. 2003). Microinfusions were administered bilaterally by lowering 30-gauge injector cannulae to 2.5 mm below the end of the guide cannulae. Infusions were conducted via microdrive pump (Harvard Apparatus) at a rate of 0.32 μL/min for 1.33 min (total volume of 0.5 μL/hemisphere). One minute was allowed for diffusion of the drugs before removing injector cannulae and replacing stylets. Before testing, two mock infusions and one 0.9% isotonic sterile saline infusion were given to habituate animals to the drug infusions. Animals were gently held during the infusions. Pre- and post-trial infusions were administered immediately before and after training, respectively (i.e., within 5 min), techniques that have been commonly used in the literature to assess the acquisition and consolidation of memory (Riedel et al. 2003; Di Chiara et al. 2004).
Experimental groups and procedures
Instrumental training
In all experiments, animals were habituated to the operant chambers for 3 d (15 min each day) with sugar pellets (45 mg; BioServe) delivered automatically into the food trough on a random-time (RT) 15-sec schedule for the first day and on an RT 30-sec schedule for the last two habituation days. Sugar pellet delivery during habituation to the operant chamber served to condition (autoshape) the rats to approach the food magazine in which future reinforcers would be delivered. During the first two 15-min instrumental training sessions, sugar was delivered automatically on an RT 30-sec schedule superimposed on a fixed ratio (FR) 1 schedule such that every lever press was rewarded with one sugar pellet. For the remainder of the experiment, the first 50 lever presses in each 15-min session were reinforced on an FR-1 schedule switching to a variable ratio (VR) 2 schedule thereafter, such that on average two lever presses were required to produce a reinforcer. The superimposed RT-30 schedule during the first two sessions was used to maintain approximately equal reinforcement density between experimental and control animals during the early learning period as well as to ensure a high level of motoric arousal to facilitate learning and to provide an opportunity to measure the temporal organization of behavior surrounding reward delivery. Reinforced lever presses and noncontingent delivery of sugar resulted in the simultaneous onset of the food magazine light and sugar delivery (∼0.1 sec in duration). Half of each group was trained in the operant chamber per day with the remaining animals being trained on the following day. The number of training sessions administered depended on the length of time needed for both vehicle- and drug-treated animals to learn the task after treatments ended or until responding on the lever reached a plateau for several sessions.
Experiment 1: Pre- and post-trial infusions of the AMPA receptor antagonist LY293558
In the first part of Experiment 1, rats received pre-trial infusions of 0 μg (n = 6), 0.01 μg (n = 5), or 0.1 μg (n = 6) of LY293558 prior to sessions 1-5 to examine the effect of AMPA/KA receptor blockade on learning and again before session 21 to examine the effect of the drug on memory retrieval and performance. In the second part of the study, different groups of rats received post-trial injections of 0 μg (n = 7) or 0.1 μg (n = 5) of LY293558 immediately after the first five training sessions. The low dose was omitted because no effect was observed with pre-trial infusions with this dose.
Experiment 2: Locomotor and feeding behavior following AMPA receptor blockade
Vehicle-treated rats (n = 7) from Experiment 1 were maintained on the same food deprivation schedule and subsequently used to examine the effect of the 0.1-μg dose of LY293558 on unconditioned feeding and motor behaviors. Using a within-subjects design, the rats were infused as described above with either drug or vehicle (in a randomized order) 5 min before being placed in the locomotor and feeding cages by an experimenter blind to group assignments. On the following day, each rat received the alternate treatment before testing. During each 15-min test session, rats had unlimited access to their normal dietary chow as the following behavioral measures were recorded via a key pad connected to a computer: food consumed (in grams), latency to feed (in seconds), number of feeding bouts, mean bout length (in seconds), total time spent feeding (in seconds), total center crossings, total rearing events, and total time spent rearing (in seconds).
Experiments 3 and 4: Pre- and post-trial infusions of the NMDA antagonist AP-5 and the D1 receptor antagonist SCH23390
In Experiments 3 and 4, rats received pre-trial infusions of AP-5 (0 μg group, n = 7; 1 μg group, n = 8) or SCH23390 (0 μg group, n = 9; 1 μg group, n = 8), respectively, prior to the first five training sessions and again before sessions 11 and 16. In the second part of these studies, different groups of rats received post-trial infusions of AP-5 (0 μg, n = 7; or 1 μg, n = 8) or SCH23390 (0 μg, n = 7; or 1 μg, n = 8) after the first five training sessions. This laboratory has previously characterized the unconditioned (nonlearning-related) effects of AP-5 and SCH23390 on feeding and locomotion at the doses used herein (see Discussion); therefore, no further experiments regarding this issue were carried out (Kelley et al. 1997; Smith-Roe and Kelley 2000; Baldo et al. 2002).
Analysis of the microstructure of behavior
Statistical analyses were supplemented by detailed, microstructural behavior analysis of operant learning experiments in which drugs were administered pre-trial by exporting raw data files from the Colbourn system into Excel. Lever presses, nose pokes, and sugar pellets produced by lever presses or by the RT schedule (free reinforcers) that occurred during each rat's session were time-stamped by Graphic State Notation. The order of events and their temporal relation were analyzed by counting all the dyads of events that occurred. For example, a nose poke (NP) could be followed by a lever press (LP), a reinforcer (Reinf) whether free or earned, or another NP, yielding three types of dyads (NP-LP, NP-Reinf, or NP-NP). These dyads were used to compute conditional probabilities: For example, the probability of a LP given that a NP had just occurred, was the number of NP-LP dyads divided by the total number of NP, or NP-LP + NP-Reinf + NP-NP. Conditional probabilities as well as latencies to retrieve reinforcers were averaged across rats per session and differentiated by group assignment.
Statistical analyses
Data (lever presses and nose pokes) were analyzed by multifactor ANOVA followed by appropriate post hoc comparison (Newman-Keuls test) and analysis of simple main effects for interactions. In the instrumental learning protocol, the between-subjects factor was treatment and the repeated-measures factor was session. Significance was set at P < 0.05. For Experiment 1, ANOVA was conducted on pre-trial data over sessions 1-20 and again on sessions 20-21 to examine the effect of initial treatment on task acquisition and treatment after the task was learned, respectively. Similarly, ANOVA conducted on pre-trial data from Experiments 3 and 4 included sessions 1-10, 10-11, and 15-16. Data from posttrial experiments were analyzed in a similar manner. A one-way repeated measures ANOVA was used to analyze data from the within-subjects locomotion and feeding control experiment (Experiment 2).
Acknowledgments
This work and P.J.H. were supported by National Institute of Drug Abuse Grant DA04788.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.93105.
References
- Alexander, G.E., DeLong, M.R., and Strick, P.L. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann. Rev. Neurosci. 9: 357-381. [DOI] [PubMed] [Google Scholar]
- Arbuthnott, G.W., Ingham, C.A., and Wickens, J.R. 2000. Dopamine and synaptic plasticity in the neostriatum. J. Anat. 196: 587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi, V.P. and Kelley, A.E. 1991. Dopaminergic regulation of feeding behavior: Differential effects of haloperidol infusion into three striatal subregions. Psychobiology 19: 223-232. [Google Scholar]
- Baldo, B.A., Sadeghian, K., Basso, A.M., and Kelley, A.E. 2002. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav. Brain Res. 137: 165-177. [DOI] [PubMed] [Google Scholar]
- Baldwin, A.E., Sadeghian, K., Holahan, M.R., and Kelley, A.E. 2002a. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol. Learn. Mem. 77: 44-62. [DOI] [PubMed] [Google Scholar]
- Baldwin, A.E., Sadeghian, K., and Kelley, A.E. 2002b. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J. Neurosci. 22: 1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barto, A.G. 1995. Adaptive critics and the basal ganglia. In Information processing in the basal ganglia (eds. J.C. Houk et al.), pp. 215-232. MIT Press, Cambridge, MA.
- Bassareo, V. and Di Chiara, G. 1999. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur. J. Neurosci. 11: 4389-4397. [DOI] [PubMed] [Google Scholar]
- Beninger, R.J. and Gerdjikov, T. 2004. The role of signaling molecules in reward-related incentive learning. Neurotox. Res. 6: 91-104. [DOI] [PubMed] [Google Scholar]
- Berke, J.D. and Hyman, S.E. 2000. Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515-532. [DOI] [PubMed] [Google Scholar]
- Berridge, K.C. and Robinson, T.E. 1998. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 28: 309-369. [DOI] [PubMed] [Google Scholar]
- Bespalov, A.Y., Zvartau, E.E., Balster, R.L., and Beardsley, P.M. 2000. Effects of N-methyl-d-aspartate receptor antagonists on reinstatement of cocaine-seeking behavior by priming injections of cocaine or exposures to cocaine-associated cues in rats. Behav. Pharmacol. 11: 37-44. [DOI] [PubMed] [Google Scholar]
- Bonini, J.S., Rodrigues, L., Kerr, D.S., Bevilaqua, L.R., Cammarota, M., and Izquierdo, I. 2003. AMPA/kainate and group-I metabotropic receptor antagonists infused into different brain areas impair memory formation of inhibitory avoidance in rats. Behav. Pharmacol. 14: 161-166. [DOI] [PubMed] [Google Scholar]
- Breen, R.A. and McGaugh, J.L. 1961. Facilitation of maze learning with posttrial injections of picrotoxin. J. Fr. Med. Chir. Thorac. 54: 498-501. [DOI] [PubMed] [Google Scholar]
- Burns, L.H., Everitt, B.J., Kelley, A.E., and Robbins, T.W. 1994. Glutamate-dopamine interactions in the ventral striatum: Role in locomotor activity and responding with conditioned reinforcement. Psychopharmacology 115: 516-528. [DOI] [PubMed] [Google Scholar]
- Cardinal, R.N., Pennicott, D.R., Sugathapala, C.L., Robbins, T.W., and Everitt, B.J. 2001. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292: 2499-2501. [DOI] [PubMed] [Google Scholar]
- Cardinal, R.N., Parkinson, J.A., Hall, J., and Everitt, B.J. 2002. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 26: 321-352. [DOI] [PubMed] [Google Scholar]
- Cepeda, C. and Levine, M.S. 1998. Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev. Neurosci. 20: 1-18. [DOI] [PubMed] [Google Scholar]
- Corbit, L.H., Muir, J.L., and Balleine, B.W. 2001. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J. Neurosci. 21: 3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins, M.S., Wei, W., and Salamone, J.D. 1994. Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: Effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology (Berl) 116: 529-537. [DOI] [PubMed] [Google Scholar]
- Datla, K.P., Ahier, R.G., Young, A.M., Gray, J.A., and Joseph, M.H. 2002. Conditioned appetitive stimulus increases extracellular dopamine in the nucleus accumbens of the rat. Eur. J. Neurosci. 16: 1987-1993. [DOI] [PubMed] [Google Scholar]
- De Leonibus, E., Costantini, V.J., Castellano, C., Ferretti, V., Oliverio, A., and Mele, A. 2003. Distinct roles of the different ionotropic glutamate receptors within the nucleus accumbens in passive-avoidance learning and memory in mice. Eur. J. Neurosci. 18: 2365-2373. [DOI] [PubMed] [Google Scholar]
- Di Chiara, G., Bassareo, V., Fenu, S., De Luca, M.A., Spina, L., Cadoni, C., Acquas, E., Carboni, E., Valentini, V., and Lecca, D. 2004. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology 47 Suppl 1: 227-241. [DOI] [PubMed] [Google Scholar]
- Di Ciano, P. and Everitt, B.J. 2004. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J. Neurosci. 24: 7167-7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano, P., Cardinal, R.N., Cowell, R.A., Little, S.J., and Everitt, B.J. 2001. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J. Neurosci. 21: 9471-9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, A. and Balleine, B. 1994. Motivational control of goal-directed action. Anim. Learn. Behav. 22: 1-18. [Google Scholar]
- Fibiger, H.C., Carter, D.A., and Phillips, A.G. 1976. Decreased intracranial self-stimulation after neuroleptics or 6-hydroxydopamine: Evidence for mediation by motor deficits rather than by reduced reward. Psychopharmacology 47: 21-27. [DOI] [PubMed] [Google Scholar]
- Floresco, S.B., Seamans, J.K., and Phillips, A.G. 1996. A selective role for dopamine in the nucleus accumbens of the rat in random foraging but not delayed spatial win-shift-based foraging. Behav. Brain Res. 80: 161-168. [DOI] [PubMed] [Google Scholar]
- Floresco, S.B., Blaha, C.D., Yang, C.R., and Phillips, A.G. 2001. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J. Neurosci. 21: 6370-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelissen, M. and Cools, A. 1988. Effect of intracaudate haloperidol and apomorphine on switching motor patterns upon current behaviour of cats. Behav. Brain Res. 29: 17-26. [DOI] [PubMed] [Google Scholar]
- Giertler, C., Bohn, I., and Hauber, W. 2003. The rat nucleus accumbens is involved in guiding instrumental responses by stimuli predicting reward magnitude. Eur. J. Neurosci. 18: 1993-1996. [DOI] [PubMed] [Google Scholar]
- Guillery, R.W. and Sherman, S.M. 2002. Thalamic relay functions and their role in corticocortical communication: Generalizations from the visual system. Neuron 33: 163-175. [DOI] [PubMed] [Google Scholar]
- Haber, S.N., Fudge, J.L., and McFarland, N.R. 2000. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 20: 2369-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, G.C., Wimmer, M., Byrne, R., and Aston-Jones, G. 2004. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience 129: 841-847. [DOI] [PubMed] [Google Scholar]
- Hernandez, L. and Hoebel, B.G. 1988. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 42: 1705-1712. [DOI] [PubMed] [Google Scholar]
- Hernandez, P.J., Sadeghian, K., and Kelley, A.E. 2002. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nat. Neurosci. 5: 1327-1331. [DOI] [PubMed] [Google Scholar]
- Horvitz, J.C. 2002. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav. Brain Res. 137: 65-74. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller, G., Giorgetti, M., and Wolf, M.E. 2001. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur. J. Neurosci. 14: 1843-1855. [DOI] [PubMed] [Google Scholar]
- Hu, X.T. and White, F.J. 1996. Glutamate receptor regulation of rat nucleus accumbens neurons in vivo. Synapse 23: 208-218. [DOI] [PubMed] [Google Scholar]
- Ito, R., Dalley, J.W., Robbins, T.W., and Everitt, B.J. 2002. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J. Neurosci. 22: 6247-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, A.E. 2004. Memory and addiction; shared neural circuitry and molecular mechanisms. Neuron 44: 161-179. [DOI] [PubMed] [Google Scholar]
- Kelley, A.E., Smith-Roe, S.L., and Holahan, M.R. 1997. Response-reinforcement learning is dependent on N-methyl-d-aspartate receptor activation in the nucleus accumbens core. Proc. Natl. Acad. Sci. 94: 12174-12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, A.E., Andrzejewski, M.E., Baldwin, A.E., Hernandez, P.J., and Pratt, W.E. 2003. Glutamate-mediated plasticity in corticostriatal networks: Role in adaptive motor learning. Ann. NY Acad. Sci. 1003: 159-168. [DOI] [PubMed] [Google Scholar]
- Kerr, J.N. and Wickens, J.R. 2001. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J. Neurophysiol. 85: 117-124. [DOI] [PubMed] [Google Scholar]
- Konradi, C., Kobierski, L.A., Nguyen, T.V., Heckers, S., and Hyman, S.E. 1993. The cAMP-response-element-binding protein interacts, but Fos protein does not interact, with the proenkephalin enhancer in rat striatum. Proc. Natl. Acad. Sci. 90: 7005-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob, G.F., Riley, S.J., Smith, S.C., and Robbins, T.W. 1978. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on feeding, locomotor activity and amphetamine anorexia in the rat. J. Comp. Physiol. Psychol. 92: 917-927. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry, C.S. and Kelley, A.E. 1995. Excitatory amino acid receptors within nucleus accumbens subregions differentially mediate spatial learning in the rat. Behav. Pharmacol. 6: 527-539. [PubMed] [Google Scholar]
- McFarland, N.R. and Haber, S.N. 2002. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J. Neurosci. 22: 8117-8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland, K., Lapish, C.C., and Kalivas, P.W. 2003. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 23: 3531-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele, A., Avena, M., Roullet, P., De Leonibus, E., Mandillo, S., Sargolini, F., Coccurello, R., and Oliverio, A. 2004. Nucleus accumbens dopamine receptors in the consolidation of spatial memory. Behav. Pharmacol. 15: 423-431. [DOI] [PubMed] [Google Scholar]
- Micheau, J., Riedel, G., Roloff, E.L., Inglis, J., and Morris, R.G. 2004. Reversible hippocampal inactivation partially dissociates how and where to search in the water maze. Behav. Neurosci. 118: 1022-1032. [DOI] [PubMed] [Google Scholar]
- Nowak, L., Bregestovski, P., Ascher, P., Herbet, A., and Prochiantz, A. 1984. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307: 462-465. [DOI] [PubMed] [Google Scholar]
- Packard, M.G. and McGaugh, J.L. 1996. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem. 65: 65-72. [DOI] [PubMed] [Google Scholar]
- Park, W.K., Bari, A.A., Jey, A.R., Anderson, S.M., Spealman, R.D., Rowlett, J.K., and Pierce, R.C. 2002. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J. Neurosci. 22: 2916-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, J.A., Cardinal, R.N., and Everitt, B.J. 2000. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Prog. Brain Res. 126: 263-285. [DOI] [PubMed] [Google Scholar]
- Paxinos, G. and Watson, C. 1998. The rat brain in stereotaxic coordinates. Academic Press, San Diego, CA.
- Pennartz, C.M., Boeijinga, P.H., Kitai, S.T., and Lopes da Silva, F.H. 1991. Contribution of NMDA receptors to postsynaptic potentials and paired-pulse facilitation in identified neurons of the rat nucleus accumbens in vitro. Exp. Brain Res. 86: 190-198. [DOI] [PubMed] [Google Scholar]
- Reading, P.J., Dunnett, S.B., and Robbins, T.W. 1991. Dissociable roles of the ventral, medial and lateral striatum on the acquisition and performance of a complex visual stimulus-response habit. Behav. Brain Res. 45: 147-161. [DOI] [PubMed] [Google Scholar]
- Rescorla, R.A. 1991. Associative relations in instrumental learning: The eighteenth Bartlett memorial lecture. Q. J. Exp. Psychol. B 43: 1-23. [Google Scholar]
- Reynolds, J.N. and Wickens, J.R. 2002. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 15: 507-521. [DOI] [PubMed] [Google Scholar]
- Riedel, G., Platt, B., and Micheau, J. 2003. Glutamate receptor function in learning and memory. Behav. Brain Res. 140: 1-47. [DOI] [PubMed] [Google Scholar]
- Robbins, T.W. and Everitt, B.J. 2002. Limbic-striatal memory systems and drug addiction. Neurobiol. Learn. Mem. 78: 625-636. [DOI] [PubMed] [Google Scholar]
- Roullet, P., Sargolini, F., Oliverio, A., and Mele, A. 2001. NMDA and AMPA antagonist infusions into the ventral striatum impair different steps of spatial information processing in a nonassociative task in mice. J. Neurosci. 21: 2143-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone, J. 1987. The actions of neuroleptic drugs on appetitive instrumental behaviors. In Handbook of psychopharmacology (eds. L.L. Iversen and S.H. Snyder), pp. 575-608. Plenum, New York.
- Sargolini, F., Florian, C., Oliverio, A., Mele, A., and Roullet, P. 2003. Differential involvement of NMDA and AMPA receptors within the nucleus accumbens in consolidation of information necessary for place navigation and guidance strategy of mice. Learn. Mem. 10: 285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, W., Apicella, P., and Ljungberg, T. 1993. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 13: 900-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, W., Dayan, P., and Montague, P.R. 1997. A neural substrate of prediction and reward. Science 275: 1593-1598. [DOI] [PubMed] [Google Scholar]
- Setlow, B. 1997. The nucleus accumbens and learning. J. Neurosci. Res. 49: 515-521. [DOI] [PubMed] [Google Scholar]
- Setlow, B. and McGaugh, J.L. 1998. Sulpiride infused into the nucleus accumbens posttraining impairs memory of spatial water maze training. Behav. Neurosci. 112: 603-610. [DOI] [PubMed] [Google Scholar]
- Sharp, F.R., Liu, J., Nickolenko, J., and Bontempi, B. 1995. NMDA and D1 receptors mediate induction of c-fos and junB genes in striatum following morphine administration: Implications for the study of memory. Behav. Brain Res. 66: 225-230. [DOI] [PubMed] [Google Scholar]
- Smith-Roe, S.L. and Kelley, A.E. 2000. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J. Neurosci. 20: 7737-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Roe, S., Sadeghian, K., and Kelley, A.E. 1999. Spatial learning and performance in the radial arm maze is impaired after N-methyl-d-aspartate (NMDA) receptor blockade in striatal subregions. Behav. Neurosci. 113: 703-717. [DOI] [PubMed] [Google Scholar]
- Stefani, M.R., Groth, K., and Moghaddam, B. 2003. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav. Neurosci. 117: 728-737. [DOI] [PubMed] [Google Scholar]
- Steward, O. and Worley, P. 2002. Local synthesis of proteins at synaptic sites on dendrites: Role in synaptic plasticity and memory consolidation? Neurobiol. Learn. Mem. 78: 508-527. [DOI] [PubMed] [Google Scholar]
- Sutton, M.A. and Beninger, R.J. 1999. Psychopharmacology of conditioned reward: Evidence for a rewarding signal at D1-like dopamine receptors. Psychopharmacology (Berl) 144: 95-110. [DOI] [PubMed] [Google Scholar]
- Thomas, M.J. and Malenka, R.C. 2003. Synaptic plasticity in the mesolimbic dopamine system. Philos. Trans. R Soc. Lond. B Biol. Sci. 358: 815-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos, R., Charria Ortiz, G.A., Bergmans, A.C., and Cools, A.R. 1991. Evidence that dopamine in the nucleus accumbens is involved in the ability of rats to switch to cue-directed behaviours. Behav. Brain Res. 42: 107-114. [DOI] [PubMed] [Google Scholar]
- Wickens, J.R., Begg, A.J., and Arbuthnott, G.W. 1996. Dopamine reverses the depression of rat corticostriatal synapses which normally follows high-frequency stimulation of cortex in vitro. Neuroscience 70: 1-5. [DOI] [PubMed] [Google Scholar]
- Wilson, C., Nomikos, G.G., Collu, M., and Fibiger, H.C. 1995. Dopaminergic correlates of motivated behavior: Importance of drive. J. Neurosci. 15: 5169-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, M.E., Sun, X., Mangiavacchi, S., and Chao, S.Z. 2004. Psychomotor stimulants and neuronal plasticity. Neuropharmacology 47 Suppl 1: 61-79. [DOI] [PubMed] [Google Scholar]
- Yun, I.A., Nicola, S.M., and Fields, H.L. 2004. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur. J. Neurosci. 20: 249-263. [DOI] [PubMed] [Google Scholar]