Abstract
Objective
To establish a practical risk stratification system (RSS) based on ultrasonography (US) and clinical characteristics for predicting soft tissue masses (STMs) malignancy.
Methods
This retrospective multicenter study included patients with STMs who underwent US and pathological examinations between April 2018 and April 2023. Chi-square tests and multivariable logistic regression analyses were performed to assess the association of US and clinical characteristics with the malignancy of STMs in the training set. The RSS was constructed based on the scores of risk factors and validated externally.
Results
The training and validation sets included 1027 STMs (mean age, 50.90 ± 16.64, 442 benign and 585 malignant) and 120 STMs (mean age, 51.93 ± 17.90, 69 benign and 51 malignant), respectively. The RSS was constructed based on three clinical characteristics (age, duration, and history of malignancy) and six US characteristics (size, shape, margin, echogenicity, bone invasion, and vascularity). STMs were assigned to six categories in the RSS, including no abnormal findings, benign, probably benign (fitted probabilities [FP] for malignancy: 0.001–0.008), low suspicion (FP: 0.008–0.365), moderate suspicion (FP: 0.189–0.911), and high suspicion (FP: 0.798–0.999) for malignancy. The RSS displayed good diagnostic performance in the training and validation sets with area under the receiver operating characteristic curve (AUC) values of 0.883 and 0.849, respectively.
Conclusion
The practical RSS based on US and clinical characteristics could be useful for predicting STM malignancy, thereby providing the benefit of timely treatment strategy management to STM patients.
Critical relevance statement
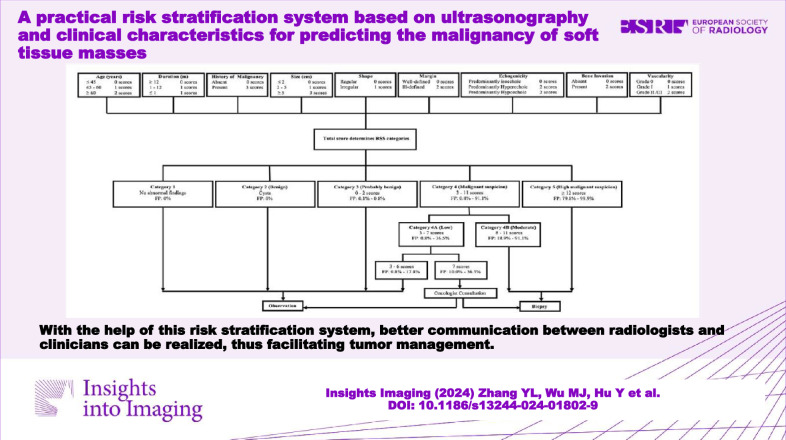
With the help of the RSS, better communication between radiologists and clinicians can be realized, thus facilitating tumor management.
Key Points
There is no recognized grading system for STM management.
A stratification system based on US and clinical features was built.
The system realized great communication between radiologists and clinicians in tumor management.
Graphical Abstract
Keywords: Diagnosis, Soft tissue mass, Ultrasonography
Introduction
Soft tissue masses (STMs), a kind of common clinical disease with a morbidity of approximately 300 cases/100,000 people, can occur in all body parts [1]. Among them, benign STMs far outnumber malignant STMs, with malignant cases accounting for less than 1% [2]. Sadly, a malignant STM has a poor prognosis, increasing the patient’s emotional and financial burdens [3]. Therefore, avoiding over-examination and intervention of benign cases and focusing on malignant cases are crucial for STM management. MRI is the primary modality for STM diagnosis, but its time consumption, cost, and inaccessibility for patients with claustrophobia or metal stents greatly limit its clinical use [4]. As a first-line alternative, ultrasonography (US) is convenient and valuable in diagnosing STMs [5–7].
Currently, the US diagnosis of STMs has been divided into ultrasomics and multimodal US. Although ultrasomics can transform images into high-throughput, extractable features that allow an objective analysis of STMs, the data processing is complex and unsuitable for promotion [8]. Multimodal US combines gray-scale US with novel techniques such as elastography and contrast-enhanced US and has an accuracy range of 77–88% [5, 6, 9–11]. However, its application is limited due to the differences in equipment and observers’ experience. Worse still, no consensus exists on the suspicious image features for malignancy. Therefore, standardization of US image acquisition and interpretation of STMs is necessary for widespread application. The broadly used breast imaging reporting and data system (BI-RADS) and thyroid imaging reporting and data system (TI-RADS) that define malignant features and classify the degree of malignancy can help manage breast or thyroid lesions and can serve as references for establishing a risk stratification system (RSS) for STMs [12, 13]. To our knowledge, no US features-based RSS currently exists for STM management. Due to disease heterogeneity, adopting the TI-RADS or BI-RADS without modification is inappropriate. Specific evaluation and scoring of risk US features for STMs is still needed. Moreover, clinical features such as age and growth speed have been reported to be important in the diagnosis of STMs [14]. The integration of clinical features with US features may contribute to the reliability of the system, as well as clinical utility.
This study aimed to establish a practical RSS for predicting the malignancy of STMs using retrospective data of US and clinical information and validated the system on independent multicenter datasets. This strategy has the potential to achieve precise preoperative prediction of STM malignancy and help guide treatment planning.
Methods
Patients
This retrospective study was approved by the ethics committee of Hospital #1 (The First Affiliated Hospital of Nanjing Medical University, Nanjing, China) with an exemption for written informed consent. Informed consent for biopsy or surgery was obtained from all the patients. The Declaration of Helsinki was followed in this study.
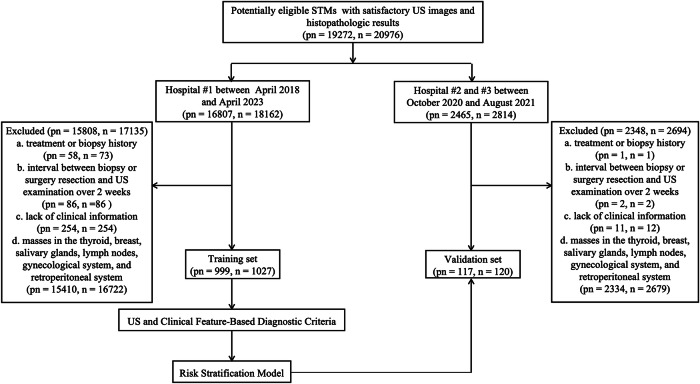
From April 2018 to April 2023, patients with suspicious STMs were reviewed in Hospital #1. Patients were included in this study if they (1) underwent US examination with satisfactory image quality and (2) had a definitive histopathologic result that showed whether the tumor was benign, intermediate, or malignant. Exclusion criteria were that (1) patients had a history of biopsy or treatment of masses before US examination; (2) the intervals between US examination and biopsy or surgery exceeded two weeks; (3) clear records of clinical information were lacking; and (4) masses were located in the thyroid, breast, salivary glands, lymph nodes, gynecological system, and retroperitoneal system. Patients with typical cysts were also excluded because we directly defined Category 1 as having no abnormal findings and Category 2 as benign, showing typical cysts based on BI-RADS and TI-RADS.
Ultimately, 1027 STMs in 999 patients (mean age = 50.90 ± 16.64 years, range 4–92 years, male/female ratio 1:1.08) formed the training set. The validation set included 120 STMs in 117 patients (mean age = 51.93 ± 17.90 years, range 9–84 years, male/female ratio 1:1.14) enrolled in Hospital #2 (The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China) and Hospital #3 (Shanghai Tenth People’s Hospital of Tongji University, Shanghai, China) from October 2020 to August 2021. The inclusion and exclusion criteria were the same as the training set. The participant selection flowchart is shown in Fig. 1.
Fig. 1.
Flowchart of the eligible patients and number of STMs. Note: typical cysts are directly defined as Category 2 in this study so they are not included in the flowchart. n, number of STMs; pn, number of patients; STMs, soft tissue masses; US, ultrasonography
Clinical information collection
All clinical information was obtained from the medical records database. The collected clinical features were determined based on previous literature and the clinical experience of oncologists in Hospital #1 [14]. The following features were collected: sex: male or female; age (years): ≤ 45, 45–60, or ≥ 60; pain: absent or present; duration: months since the mass was discovered, including ≤ 1, 1–12, or ≥ 12; history of malignancy: absent or present; location: head and neck, trunk and hip, or limbs.
Imaging acquisition and interpretation
US examinations were performed using various US instruments, such as LOGIQ E9 (GE Healthcare, Pittsburgh, Pa, USA), Acuson S3000 (Siemens Healthineers, Erlangen, Bavaria, Germany), or EPIQ7 (Philips Medical Systems, Bothell, WA, USA) with linear or convex transducers. The specific scanning protocol criteria were transverse and longitudinal scanning of each STM with full mass exposure. Trapezoidal or panoramic imaging was used when necessary. The maximum transverse- and longitudinal-section grayscale US images of each STM were routinely recorded. Furthermore, the best color Doppler flow imaging (CDFI) images on the same transverse sections were recorded. All examinations were performed by radiologists with over two years of experience in the musculoskeletal US from three separate hospitals, and two radiologists (Y.-L.Z. and M.-J.W.) in Hospital #1 with 2–5 years of experience, who were blind to the clinical information and mass pathology reviewed all grayscale US and CDFI images from all the hospitals. Disagreements were resolved through discussion under the supervision of a senior radiologist (A.L.) with over five years of experience in Hospital #1. Criteria were made based on previous literature and radiologists’ experiences [8, 15–20]. The following features were recorded: layer (relative to the investing fascia): superficial or deep; size: ≤ 2 cm, 2–5 cm, or ≥ 5 cm; shape: regular (ovoid to round) or irregular (not ovoid to round); margin: well-defined (smooth and clear) or ill-defined (jagged, spiculated, blurred, slightly lobulated, or extra-mass extended); echogenicity of the solid and noncalcified component of a mass (relative to adjacent muscle): predominantly isoechoic, predominantly hyperechoic, or predominantly hypoechoic; composition: predominantly solid (cystic portion ≤ 10%), mixed (cystic portion > 10% but ≤ 50%), or predominantly cystic (cystic portion > 50%); calcification: absent or present; bone invasion: absent or present; vascularity: grade 0 (no blood flow in the mass), grade I (only a tiny amount of blood flows with 1–2 punctuate or rod-shaped blood flows), grade II (moderate blood flows with 3–4 punctuate blood flows or a vital blood vessel), or grade III (rich blood flows with more than one important blood vessels).
Reference standard
Histopathologic results of biopsy or surgery served as the reference standards. Malignant STMs included malignant and intermediate STMs defined by the 2020 World Health Organization (WHO) classification of soft tissue tumors [1], metastatic tumors, and hematologic malignancies. Meanwhile, benign STMs referred to benign STMs defined by the 2020 WHO classification.
RSS construction and statistical analysis
This study concentrated on creating the RSS Category 3–5 and included six main steps of statistical analysis. (1) All clinical and US features were categorized based on the predetermined criteria. The differences in malignancy frequency and clinical and US features between the training and validation sets were determined via Chi-square tests. (2) The clinical and US features in the training set were assessed by Chi-square tests. (3) The significant features acquired from the univariate analysis were included in the multivariable analysis. The binary logistic regression model determined the risk factors, and their β coefficients and 95% confidence interval (CI) were calculated. A regression equation for fitted probabilities (FP) was gained as well [21]. Ten-fold cross-validation was used to evaluate the multivariable logistic regression model [22]. (4) β coefficients for risk factors from the logistic regression model were used for risk score analyses. The β coefficients were standardized to make the value of the smallest one equal 1. Risk scores for each risk factor were assessed as the closest integer of the standardized β values [23]. An individual’s risk score was then determined by summing the scores of each risk factor. (5) RSS was built based on the risk scores and the FP, and the actual malignancy rates were also calculated. The linear relationships between both the malignancy probabilities and risk scores, as well as the malignancy probabilities and the RSS categories were assessed by Cochran–Armitage trend tests. (6) Finally, the area under the curves (AUCs) with 95% CI, accuracy, sensitivity, and specificity of the RSS in the two sets were calculated.
The R software (version 4.3.1, R Project for Statistical Computing, www.r-project.org) was used for 10-fold cross-validation and the Cochran–Armitage trend tests. Chi-square tests and logistic regression were performed using SPSS 26.0 software (IBM, Ehningen, Germany). Inter-observer agreements of US features in the two sets were assessed by Kappa statistics. p < 0.05 was indicative of a statistically significant difference, and all reported p values were two-sided.
Results
Clinical and US features of patients
Overall, the malignancy frequency distribution, clinical features, and the majority of US features were similar in the two sets, with a difference in vascularity (p = 0.044) (Table 1). The inter-observer agreements of US features in the two sets are shown in Supplementary Table 1. In the training set, there were 442 malignant (233 confirmed surgically and 209 confirmed by biopsies) and 585 benign (469 confirmed surgically and 116 confirmed by biopsies) STMs. In the validation set, there were 51 malignant (21 confirmed surgically and 30 confirmed by biopsies) and 69 benign (32 confirmed surgically and 37 confirmed by biopsies) STMs. The prevalent benign STMs were lipoma (n = 200 vs n = 23, the training set vs the validation set), schwannoma (n = 93 vs n = 12, the training set vs the validation set), and hemangioma (n = 72 vs n = 12, the training set vs the validation set). The malignant STMs in the training set contained 269 malignant soft tissue tumors, with common types being aggressive fibromatosis (n = 35), myxofibrosarcoma (n = 30), and atypical lipomatous tumor (n = 26), 125 metastatic tumors, and 48 hematologic malignancies. In comparison, the malignant STMs in the validation sets contained 31 malignant soft tissue tumors, with common types being myxofibrosarcoma (n = 10), aggressive fibromatosis (n = 4), and atypical lipomatous tumor (n = 4), 18 metastatic tumors, and 2 hematologic malignancies.
Table 1.
Malignancy frequency distributions, clinical and US features of patients in the training and external validation sets
| Features | Training set, (n = 1027) | Validation set, (n = 120) | p value |
|---|---|---|---|
| Malignancy frequency | 442 | 51 | 0.910 |
| Sex | 0.781 | ||
| Male | 493 | 56 | |
| Female | 534 | 64 | |
| Age (year) | 0.592 | ||
| ≤ 45 | 337 | 36 | |
| 45–60 | 344 | 38 | |
| ≥ 60 | 346 | 46 | |
| Pain | 0.731 | ||
| Absent | 709 | 81 | |
| Present | 318 | 39 | |
| Duration (month) | 0.070 | ||
| ≥ 12 | 290 | 46 | |
| 1–12 | 430 | 44 | |
| ≤ 1 | 307 | 30 | |
| History of malignancy | 0.863 | ||
| Absent | 803 | 93 | |
| Present | 224 | 27 | |
| Location | 0.741 | ||
| Head or neck | 146 | 14 | |
| Trunk or hip | 360 | 44 | |
| Limbs | 521 | 62 | |
| Layer | 0.247 | ||
| Superficial | 336 | 33 | |
| Deep | 691 | 87 | |
| Size (cm) | 0.649 | ||
| ≤ 2 | 189 | 24 | |
| 2–5 | 425 | 53 | |
| ≥ 5 | 413 | 43 | |
| Shape | 0.895 | ||
| Regular | 597 | 69 | |
| Irregular | 430 | 51 | |
| Margin | 0.837 | ||
| Well-defined | 675 | 80 | |
| Ill-Defined | 352 | 40 | |
| Echogenicity | 0.090 | ||
| Predominantly isoechoic | 104 | 5 | |
| Predominantly hyperechoic | 141 | 20 | |
| Predominantly hypoechoic | 782 | 95 | |
| Composition | 0.236 | ||
| Predominantly cystic | 6 | 2 | |
| Mixed | 79 | 6 | |
| Predominantly solid | 942 | 112 | |
| Calcification | 0.896 | ||
| Absent | 964 | 113 | |
| Present | 63 | 7 | |
| Bone invasion | 0.708 | ||
| Absent | 967 | 114 | |
| Present | 60 | 6 | |
| Vascularity | 0.044* | ||
| Grade 0 | 338 | 40 | |
| Grade I | 168 | 30 | |
| Grade II | 228 | 27 | |
| Grade III | 293 | 23 |
n number, US ultrasonography
* Indicates a significant difference between the two sets
Construction of RSS
The univariate analysis showed a significant correlation between tumor malignancy and sex, age, pain, duration, history of malignancy, layer, size, shape, margin, echogenicity, calcification, bone invasion, and vascularity (Table 2). The binary logistic regression analysis was performed to assess independent risk factors of malignancy, which included age (45–60 or ≥ 60 years old), duration (1–12 months or ≤ 1 month), history of malignancy, size (2–5 or ≥ 5 cm), irregular shape, ill-defined margin, echogenicity (predominantly hyperechoic or hypoechoic), bone invasion, and vascularity (grade I or II or III) (Table 2). The logistic regression model’s AUC, accuracy, sensitivity, and specificity were 0.917 (95% CI: 0.900–0.933), 0.835, 0.873, and 0.798, respectively. The Hosmer–Lemeshow test indicated the absence of statistical significance (X2 = 6.325, p = 0.611). After 10-fold cross-validation, the model displayed an excellent predictive performance with a mean AUC, accuracy, sensitivity, and specificity of 0.902 (95% CI: 0.883–0.920), 0.815, 0.854, and 0.702, respectively.
Table 2.
Association between STM malignancy and various clinical and US features
| Features | Benign, (n = 585) | Malignant, (n = 442) | p value* | β (95% CI)† | p value† | Score‡ |
|---|---|---|---|---|---|---|
| Sex | 0.034 | |||||
| Male | 264 | 229 | NA | NA | NA | |
| Female | 321 | 213 | NA | NA | NA | |
| Age (year) | < 0.001 | |||||
| ≤ 45 | 253 | 84 | NA | NA | 0 | |
| 45–60 | 205 | 139 | 0.728 (0.271–1.194) | 0.002 | 1 | |
| ≥ 60 | 127 | 219 | 1.534 (1.072–2.011) | < 0.001 | 2 | |
| Pain | < 0.001 | |||||
| Absent | 432 | 277 | NA | NA | NA | |
| Present | 153 | 165 | NA | NA | NA | |
| Duration (month) | < 0.001 | |||||
| ≥ 12 | 229 | 61 | NA | NA | 0 | |
| 1–12 | 207 | 223 | 0.890 (0.429–1.361) | < 0.001 | 1 | |
| ≤ 1 | 149 | 158 | 0.992 (0.502–1.491) | < 0.001 | 1 | |
| History of malignancy | < 0.001 | |||||
| Absent | 523 | 280 | NA | NA | 0 | |
| Present | 62 | 162 | 1.842 (1.368–2.336) | < 0.001 | 3 | |
| Location | 0.684 | |||||
| Head or neck | 86 | 60 | NA | NA | NA | |
| Trunk or hip | 209 | 151 | NA | NA | NA | |
| Limbs | 290 | 231 | NA | NA | NA | |
| Layer | < 0.001 | |||||
| Superficial | 271 | 65 | NA | NA | NA | |
| Deep | 314 | 377 | NA | NA | NA | |
| Size (cm) | < 0.001 | |||||
| ≤ 2 | 160 | 29 | NA | NA | 0 | |
| 2–5 | 267 | 158 | 0.966 (0.402–1.558) | 0.001 | 1 | |
| ≥ 5 | 158 | 255 | 2.119 (1.526–2.744) | < 0.001 | 3 | |
| Shape | < 0.001 | |||||
| Regular | 449 | 148 | NA | NA | 0 | |
| Irregular | 136 | 294 | 0.709 (0.320–1.098) | < 0.001 | 1 | |
| Margin | < 0.001 | |||||
| Well-defined | 505 | 170 | NA | NA | 0 | |
| Ill-Defined | 80 | 272 | 1.729 (1.326–2.144) | < 0.001 | 2 | |
| Echogenicity | < 0.001 | |||||
| Predominantly isoechoic | 95 | 9 | NA | NA | 0 | |
| Predominantly hyperechoic | 109 | 32 | 1.195 (0.273–2.205) | 0.015 | 2 | |
| Predominantly hypoechoic | 381 | 401 | 1.555 (0.714–2.497) | 0.001 | 2 | |
| Composition | 0.263 | |||||
| Predominantly cystic | 5 | 1 | NA | NA | NA | |
| Mixed | 49 | 30 | NA | NA | NA | |
| Predominantly solid | 531 | 411 | NA | NA | NA | |
| Calcification | 0.009 | |||||
| Absent | 559 | 405 | NA | NA | NA | |
| Present | 26 | 37 | NA | NA | NA | |
| Bone invasion | < 0.001 | |||||
| Absent | 581 | 386 | NA | NA | 0 | |
| Present | 4 | 56 | 1.363 (0.266–2.698) | 0.025 | 2 | |
| Vascularity | < 0.001 | |||||
| Grade 0 | 284 | 54 | NA | NA | 0 | |
| Grade I | 101 | 67 | 0.978 (0.423–1.538) | < 0.001 | 1 | |
| Grade II | 105 | 123 | 1.256 (0.739–1.783) | < 0.001 | 2 | |
| Grade III | 95 | 198 | 1.606 (1.082–2.143) | < 0.001 | 2 |
CI confidence interval, NA not applicable, n number, STMs soft tissue masses, US ultrasonography
* Determined with Chi-square tests
† Determined with logistic regression analysis
‡ Scoring criteria for significant risk factors were based on the rounded standardized β coefficients. As the lowest β value was 0.709, its multiplication by 1.41 made it close to 1. To be standardized, all other β values were multiplied by 1.41 and were rounded to the closest integer
In the risk score analysis, the scores for risk factors are shown in Table 2 as well. Table 3 and Fig. 2 show the FP for the total scores. The Cochran–Armitage trend test revealed that the FP increased when the scores increased (Z = 22.350, p < 0.001). Under the cut-off value of 7.5, the risk score model indicated an AUC of 0.912 (95% CI: 0.895–0.930), an accuracy of 0.832, a sensitivity of 0.910, and a specificity of 0.762.
Table 3.
Malignant STMs numbers, total STMs numbers, the corresponding FP, and actual malignancy rates by total scores
| Score | Malignancy, n | Total, n | FP | Malignancy rates |
|---|---|---|---|---|
| 0 | 0 | 6 | 0.001 | 0.000 |
| 1 | 0 | 6 | 0.002–0.003 | 0.000 |
| 2 | 0 | 22 | 0.004–0.008 | 0.000 |
| 3 | 2 | 59 | 0.008–0.017 | 0.034 |
| 4 | 3 | 71 | 0.013–0.039 | 0.042 |
| 5 | 5 | 94 | 0.027–0.099 | 0.053 |
| 6 | 8 | 91 | 0.048–0.178 | 0.088 |
| 7 | 22 | 137 | 0.100–0.365 | 0.161 |
| 8 | 41 | 88 | 0.189–0.539 | 0.466 |
| 9 | 52 | 101 | 0.326–0.805 | 0.515 |
| 10 | 56 | 72 | 0.512–0.845 | 0.778 |
| 11 | 58 | 75 | 0.726–0.911 | 0.773 |
| 12 | 50 | 58 | 0.798–0.946 | 0.862 |
| 13 | 56 | 58 | 0.912–0.976 | 0.966 |
| 14 | 38 | 38 | 0.959–0.985 | 1.000 |
| 15 | 32 | 32 | 0.985–0.992 | 1.000 |
| 16 | 14 | 14 | 0.993–0.996 | 1.000 |
| 17 | 1 | 1 | 0.997 | 1.000 |
| 18 | 4 | 4 | 0.998–0.999 | 1.000 |
FP fitted probabilities, n number of STMs, STMs soft tissue masses
Fig. 2.
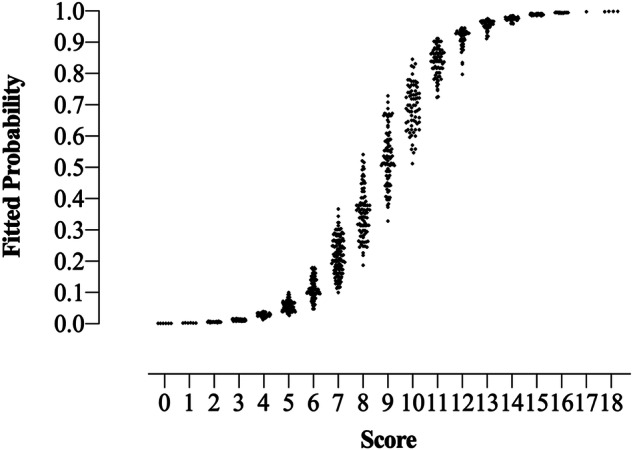
The scatter plot of FP by total scores. Note: the FP tended to increase as total scores increased
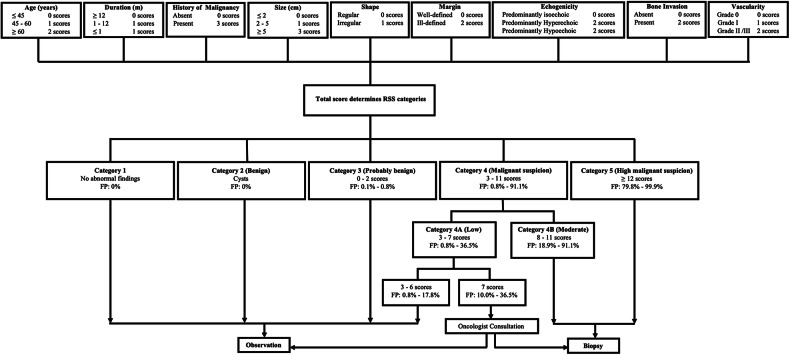
With these findings, we created RSS Category 3 (score: 0–2, FP: 0.001–0.008, actual malignancy rate: 0), 4A (score: 3–7, FP: 0.008–0.365, actual malignancy rate: 0.088), 4B (score 8–11, FP: 0.189–0.911, actual malignancy rate: 0.616), and 5 (score ≥ 12, FP: 0.798–0.999, actual malignancy rate: 0.951) (Fig. 3). The Cochran–Armitage trend test revealed that as the category increased, the FP also increased (Z = 22.239, p < 0.001). The AUC, accuracy, sensitivity, and specificity of the RSS in the training set were 0.883 (95% CI: 0.862–0.904), 0.826, 0.910, and 0.762, respectively. Supplementary Figs. 1–4 show STMs with RSS Categories 3–5. Furthermore, management recommendations were also proposed for each category according to the FP and clinical experience (Fig. 4). The malignancy proportion of Category 4A STMs with a score of 7 was significantly higher than that of Category 4A STMs with a score of ≤ 6 (p < 0.001). Observation was recommended for STMs in Categories 1, 2, 3, and 4A (score 3–6), and biopsy was recommended for STMs in Categories 4B–5. A thorough evaluation for a biopsy or observation was recommended when an STM reached a score of 7.
Fig. 3.
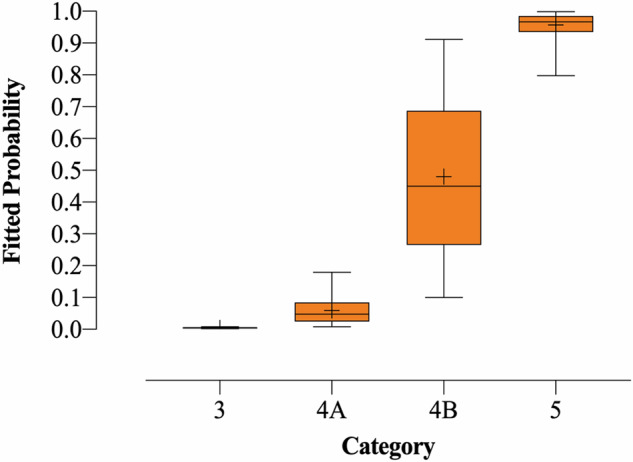
The box plot of FP by detailed categories. Note: the upper edge of the box is the 75th percentile of the FP, and the lower edge represents the 25th percentile. The line in the box represents the medians, and points indicated by + in the box represent the means. The lower and upper ends of vertical lines represent the minimum and maximum values of the FP
Fig. 4.
Workflow shows the detailed categories of RSS and management recommendations. FP, fitted probabilities; RSS, risk stratification system
External validation
For the validation set, the STMs malignancy rates with Categories 3, 4A, 4B, and 5 were 0% (0/5), 13.5% (7/52), 56.1% (23/41), and 95.5% (21/22), respectively. The AUC, accuracy, sensitivity, and specificity of the RSS in the validation set were 0.849 (95% CI: 0.778–0.920), 0.783, 0.863, and 0.725, respectively.
Discussion
US is an effective imaging technique for STM management. Although previous studies have investigated the diagnostic value of US in STM diagnosis and developed diagnostic nomograms, they suffered from issues such as small sample sizes, ambiguous definitions of US features, complex calculations, and ignorance of clinical features. Moreover, previous studies have not yet conducted a US stratification system for STMs, which may confuse clinicians and limit clinical applications [15–17]. In order to effectively manage STMs, we built a practical RSS using the US and clinical features of 1027 STMs.
After standardizing the definitions of different US features, good inter-observer agreements were shown in the training and validation sets. Our findings revealed that age, duration, and history of malignancy in clinical features, and size, shape, margin, echogenicity, bone invasion, and vascularity in US features were indicative of malignancy, mostly aligning with previous studies [15, 16, 24–27]. In terms of clinical features, our study indicated that malignant STMs were independent of sex or location whereas previous studies found that malignant STMs were predominantly male and more likely to be located in the central parts of the trunks [15, 16, 27]. These discrepancies could be attributed to the different study sets in the respective studies. Additionally, patients with histories of malignancy were found to be more likely to suffer from malignant STMs in our study, possibly due to the considerable proportion of metastatic tumors in the training set. In terms of US features, tumor size, shape, margin, bone invasion, and vascularity were probably determined by the biological characteristics of malignant STMs, such as rapid growth, surrounding infiltration, and massive neovascularization [24, 27]. However, the association between echogenicity and malignancy remained controversial [16, 17, 24]. Our study indicated that predominantly hyperechogenicity or hypoechogenicity, rather than isoechogenicity, may indicate malignancy. Wu et al [28] reported that the mass echogenicity in the US could be related to the histopathologic compositions. Notably, hypoechogenicity was likely related to organized tumor cells [28], which is consistent with our study. Though hyperechogenicity was mainly related to benign compositions like adipocytes, cartilage, and osteoid tissues [28], atypical lipomatous tumors, classified as malignant STMs in our study, were histopathologically characterized as adipocytic variants and also appeared hyperechoic on the US [29]. Therefore, our study suggested hyperechogenicity remained a risk factor for malignant STMs.
As we know, our study was the first attempt to create an RSS for the diagnosis of STMs based on clinical and US risk factor weights. After assigning each risk factor a corresponding risk score calculated by standardized β coefficients, we found that as the total scores of STMs increased, both the FP and actual malignancy rates correspondingly increased. This trend was consistent with analogous studies in the thyroid reporting system [21, 30] and indicated the reasonableness of the RSS based on risk scores. In our study, STMs with different FP were classified into 6 categories given the low malignancy rates, the diverse pathological types, and the inherently overlapping US features of STMs [5]. Then, as the category level in the RSS increased, the corresponding FP and actual malignancy rates also increased. Thus, our RSS showed a generalizable diagnostic performance with an AUC value of 0.883 (95% CI: 0.862–0.904) in the training set and an AUC value of 0.849 (95% CI: 0.778–0.920) in the validation set. Management recommendations for different categories of STMs were made based on clinical practice and previous studies [31]. Similar to BI-RADS or TI-RADS, it was recommended in our RSS to perform biopsies on STMs with moderate (Category 4B) or high malignancy suspicion (Category 5) [22]. But for Category 4A in RSS, special attention was needed. Category 4A (score 3–7) was defined as low suspicion for malignancy in our RSS, but its FP range was wider than BI-RADS or TI-RADS. Specifically, in Category 4A, the malignancy rate of STMs with a score of 7 was higher than that of STMs with a score of 3–6, suggesting a need for separate analysis in the management of STMs with a score of 7. In all, clinical observation was recommended for STMs in Categories 1, 2, 3, and 4A (score 3–6), and biopsy was recommended for STMs in Categories 4B–5. For STMs in category 4A with a score of 7, comprehensive evaluation by clinicians was required. We believe that the RSS and corresponding management recommendations could have great value for the diagnosis and treatment of STMs.
Some limitations should be mentioned in our study. First, selection bias is inevitable due to the retrospective design with a limited dataset from three hospitals. The absence of pathological diagnoses for many STMs resulted in a relatively small sample size for benign STMs. Meanwhile, as a referral hospital, the proportion of malignant STMs in this study was far higher than the population-based incidence rate. Thus, a well-designed multicenter study is required to involve more STMs in a prospective setting. Second, the interpretation of images was inevitably subjective, despite the good consistency among observers. Involving more experts in the development and refinement of standard terms, together with applying artificial intelligence techniques to image acquisition and processing, could hopefully address this issue. Third, although our RSS showed good diagnostic performance, there was some overlap of FP between different categories. Fortunately, the potential value of multimodal US in the differential diagnosis of STMs has also been proved [10, 11, 26]. Accordingly, specifications for the acquisition and interpretation of images from the multimodal US should be developed, and a multimodal-US-based RSS could be constructed to improve diagnoses.
In summary, the practical RSS using both clinical and US characteristics may be a valuable tool in predicting STM malignancy, thereby promoting standardized management of STM by clinicians.
Supplementary information
Abbreviations
- AUC
Area under the receiver operating characteristic curve
- BI-RADS
Breast imaging reporting and data system
- CDFI
Color doppler flow imaging
- CI
Confidence interval
- FP
Fitted probabilities
- RSS
Risk stratification system
- STMs
Soft tissue masses
- TI-RADS
Thyroid imaging reporting and data system
- US
Ultrasonography
- WHO
World Health Organization
Authors contributions
Conceptualization: Y.-L.Z., M.-J.W., J.-M.L., and A.L.; methodology: M.-J.W., Y.H., and A.L.; software: A.L.; validation: J.Y., X.-H.Y., and A.L.; formal analysis: J.Y., X.-H.Y., and A.L.; resources: J.Y., X.-H.Y., J.-M.L., and A.L.; data curation: Y.-L.Z., M.-J.W., Y.H., X.-J.P., Q.M., C.-L.M., Y.D., Z.-K.W., Y.-Q.G., Q.-Y.Y., J.Y., X.-H.Y., and A.L.; writing (original draft preparation): Y.-L.Z.; writing (review and editing): A.L.; and supervision: Y.-L.Z., M.-J.W., Y.H., X.-J.P., Q.M., C.-L.M., Y.D., Z.-K.W., Y.-Q.G., Q.-Y.Y., J.Y., X.-H.Y., J.-M.L., and A.L. All authors read and approved the final manuscript.
Funding
This work was supported by the National Nature Science Foundation of China (grant number, 82371979) and the National Key Research and Development Program of China (grant number, 2023YFC2410805).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (2019-SR-295) with an exemption for written informed consent. Informed consent for biopsy or surgery was acquired from all the patients.
Consent for publication
This manuscript is approved by all participants for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ju-Ming Li and Ao Li contributed equally to this work.
Contributor Information
Ju-Ming Li, Email: Lijuming7905@163.com.
Ao Li, Email: cqh2liao@163.com.
Supplementary information
The online version contains supplementary material available at 10.1186/s13244-024-01802-9.
References
- 1.Anderson WJ, Doyle LA (2020) Updates from the 2020 World Health Organization classification of soft tissue and bone tumours. Histopathology 78:644–657 [DOI] [PubMed] [Google Scholar]
- 2.Andritsch E, Beishon M, Bielack S et al (2017) ECCO essential requirements for quality cancer care: soft tissue sarcoma in adults and bone sarcoma. a critical review. Crit Rev Oncol Hematol 110:94–105 [DOI] [PubMed] [Google Scholar]
- 3.Callegaro D, Miceli R, Bonvalot S et al (2016) Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol 17:671–680 [DOI] [PubMed] [Google Scholar]
- 4.Gowda P, Bajaj G, Silva FD, Ashikyan O, Xi Y, Chhabra A (2023) Does the apparent diffusion coefficient from diffusion-weighted MRI imaging aid in the characterization of malignant soft tissue tumors and sarcomas. Skeletal Radiol 52:1475–1484 [DOI] [PubMed] [Google Scholar]
- 5.Griffith JF, Yip SWY, Hung EHY et al (2020) Accuracy of ultrasound in the characterisation of deep soft tissue masses: a prospective study. Eur Radiol 30:5894–5903 [DOI] [PubMed] [Google Scholar]
- 6.Hung EHY, Griffith JF, Yip SWY et al (2020) Accuracy of ultrasound in the characterization of superficial soft tissue tumors: a prospective study. Skeletal Radiol 49:883–892 [DOI] [PubMed] [Google Scholar]
- 7.Burke CJ, Fritz J, Samim M (2023) Musculoskeletal soft-tissue masses: MR imaging-ultrasonography correlation, with an emphasis on the 2020 World Health Organization classification. Magn Reson Imaging Clin N Am 31:285–308 [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, Li A, Zhao CK et al (2023) A multiparametric clinic-ultrasomics nomogram for predicting extremity soft-tissue tumor malignancy: a combined retrospective and prospective bicentric study. Radiol Med 128:784–797 [DOI] [PubMed] [Google Scholar]
- 9.Hung EH, Griffith JF, Ng AW, Lee RK, Lau DT, Leung JC (2014) Ultrasound of musculoskeletal soft-tissue tumors superficial to the investing fascia. AJR Am J Roentgenol 202:W532–W540 [DOI] [PubMed] [Google Scholar]
- 10.Wu M, Ren A, Xu D, Peng X, Ye X, Li A (2021) Diagnostic performance of elastography in malignant soft tissue tumors: a systematic review and meta-analysis. Ultrasound Med Biol 47:855–868 [DOI] [PubMed] [Google Scholar]
- 11.Wu M, Hu Y, Hang J et al (2020) Qualitative and quantitative contrast-enhanced ultrasound combined with conventional ultrasound for predicting the malignancy of soft tissue tumors. Ultrasound Med Biol 48:237–247 [DOI] [PubMed] [Google Scholar]
- 12.Sedgwick E (2011) The breast ultrasound lexicon: breast imaging reporting and data system (BI-RADS). Semin Roentgenol 46:245–251 [DOI] [PubMed] [Google Scholar]
- 13.Kwak JY, Han KH, Yoon JH et al (2011) Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 260:892–899 [DOI] [PubMed] [Google Scholar]
- 14.Okada K (2016) Points to notice during the diagnosis of soft tissue tumors according to the “Clinical Practice Guideline on the Diagnosis and Treatment of Soft Tissue Tumors”. J Orthop Sci 21:705–712 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhao C, Lv H et al (2023) Benefit of using both ultrasound imaging and clinical information for predicting malignant soft tissue tumors. Ultrasound Med Biol 49:2459–2468 [DOI] [PubMed] [Google Scholar]
- 16.Wu M, Hu Y, Ren A et al (2021) Nomogram based on ultrasonography and clinical features for predicting malignancy in soft tissue tumors. Cancer Manag Res 13:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu H, Ma Q, Li A et al (2022) Diagnostic performance of US and MRI in predicting malignancy of soft tissue masses: using a scoring system. Front Oncol 12:853232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon MA, Chung HW, Chee CG et al (2020) Risk factors for diagnostic failure of ultrasound-guided core needle biopsy of soft-tissue tumors based on World Health Organization classification category and biologic potential. AJR Am J Roentgenol 214:413–421 [DOI] [PubMed] [Google Scholar]
- 19.Araki Y, Yamamoto N, Maeda T et al (2022) Management of soft-tissue tumors with a size of 2–5 cm, including malignancy. Anticancer Res 42:1555–1562 [DOI] [PubMed] [Google Scholar]
- 20.Wu JS, Goldsmith JD, Horwich PJ et al (2008) Bone and soft-tissue lesions: What factors affect diagnostic yield of image-guided core-needle biopsy? Radiology 248:962–970 [DOI] [PubMed] [Google Scholar]
- 21.Kwak JY, Jung I, Baek JH (2013) Image reporting and characterization system for ultrasound features of thyroid nodules: multicentric Korean retrospective study. Korean J Radiol 14:110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan J, Xu X, Cai Y et al (2022) A practical CEUS thyroid reporting system for thyroid nodules. Radiology 305:149–159 [DOI] [PubMed] [Google Scholar]
- 23.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J (2006) Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol 5:735–741 [DOI] [PubMed] [Google Scholar]
- 24.Morii T, Kishino T, Shimamori N et al (2018) Differential diagnosis between benign and malignant soft tissue tumors utilizing ultrasound parameters. J Med Ultrason 45:113–119 [DOI] [PubMed] [Google Scholar]
- 25.Chiou HJ, Chou YH, Chiu SY et al (2009) Differentiation of benign and malignant superficial soft-tissue masses using grayscale and color doppler ultrasonography. J Chin Med Assoc 72:307–315 [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Li A, Wu MJ et al (2023) Added value of contrast-enhanced ultrasound to conventional ultrasound for characterization of indeterminate soft-tissue tumors. Br J Radiol 96:20220404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozturk M, Selcuk MB, Polat AV, Ozbalci AB, Baris YS (2020) The diagnostic value of ultrasound and shear wave elastography in the differentiation of benign and malignant soft tissue tumors. Skeletal Radiol 49:1795–1805 [DOI] [PubMed] [Google Scholar]
- 28.Wu CL, Lai YC, Wang HK, Chen PC, Chiou HJ (2017) Correlation between histological and ultrasonographic findings of soft tissue tumors: To verify the possibility of cell-like resolution in ultrasonography. J Chin Med Assoc 80:721–728 [DOI] [PubMed] [Google Scholar]
- 29.Wei S, Henderson-Jackson E, Qian X, Bui MM (2017) Soft tissue tumor immunohistochemistry update: illustrative examples of diagnostic pearls to avoid pitfalls. Arch Pathol Lab Med 141:1072–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu SY, Zhan WW, Wang WH (2015) Evaluation of thyroid nodules by a scoring and categorizing method based on sonographic features. J Ultrasound Med 34:2179–2185 [DOI] [PubMed] [Google Scholar]
- 31.de Juan Ferré A, Álvarez Álvarez R, Casado Herráez A et al (2021) SEOM clinical guideline of management of soft-tissue sarcoma (2020). Clin Transl Oncol 23:922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.