Abstract
The δ subunit of the GABAA receptor (GABAAR) is highly expressed in the dentate gyrus of the hippocampus. Genetic deletion of this subunit reduces synaptic and extrasynaptic inhibition and decreases sensitivity to neurosteroids. This paper examines the effect of these changes on hippocampus-dependent trace fear conditioning. Compared to controls, δ knockout mice exhibited enhanced acquisition of tone and context fear. Hippocampus-independent delay conditioning was normal in these animals. These results suggest that reduced inhibition in the dentate gyrus facilitates the acquisition of trace fear conditioning. However, the enhancement in trace conditioning was only observed in female knockout mice. The sex-specificity of this effect may be a result of neuroactive steroids. These compounds vary during the estrus cycle, can increase GABAergic inhibition, and have been shown to impair hippocampus-dependent learning. We propose that activation of GABAARs by neuroactive steroids inhibits learning processes in the hippocampus. Knockouts are immune to this effect because of the reduced neurosteroid sensitivity that accompanies deletion of the δ subunit. Relationships between neurosteroids, hippocampal excitability, and memory are discussed.
In the central nervous system, fast inhibitory transmission is mediated primarily by γ-aminobutyric acid type A receptors (GABAARs) (Macdonald and Olsen 1994; Olsen and Homanics 2000). These ligand-gated receptors are pentamers composed of various subunit combinations (α, β, γ, ε, π, ρ, and δ) that exhibit differential expression in the brain (Barnard et al. 1998; Sieghart and Sperk 2002). For example, the δ subunit is highly expressed in the dentate gyrus, but not CA3 or CA1 regions of the hippocampus (Persohn et al. 1992; Peng et al. 2002). The pharmacologic and kinetic properties of GABAARs are highly dependent on the specific configuration of these subunits (Quirk et al. 1994; Gunther et al. 1995; Smith and Olsen 1995; Khan et al. 1996; Benke et al. 1997). Recent studies have shown that genetic deletion of the δ subunit of the GABAAR produces a significant reduction in neurosteroid sensitivity (Mihalek et al. 1999; Vicini et al. 2002; Porcello et al. 2003; Spigelman et al. 2003) and accelerates the decay of spontaneous mIPSCs and evoked IPSPs in hippocampal granule cells and thalamic relay neurons (Mihalek et al. 1999; Porcello et al. 2003; Spigelman et al. 2003). These data suggest that δ-containing GABAARs contribute to synaptic and extrasynaptic inhibition and facilitate modulation by neuroactive steroids.
Pharmacologic or genetic manipulations that reduce GABAergic inhibition often enhance synaptic plasticity and learning (Introini-Collison et al. 1994; Crestani et al. 1999, 2002; Staubli et al. 1999; Shumyatsky et al. 2002). For example, recent experiments on GABAAR mutants (α5 and γ2) found enhanced trace but normal delay conditioning (Crestani et al. 1999, 2002). Trace conditioning is a form of hippocampus-dependent learning in which the conditional stimulus (CS) and unconditional stimulus (US) are separated in time (Solomon et al. 1986; Moyer Jr. et al. 1990; McEchron et al. 1998; Huerta et al. 2000; Quinn et al. 2002). This is distinct from hippocampus-independent delay conditioning in which the CS and US are presented contiguously.
Previous work has demonstrated that trace conditioning engages the CA1 region of the hippocampus (Moyer Jr. et al. 1996, 2000; McEchron and Disterhoft 1999; McEchron et al. 2001, 2003; Leuner et al. 2003) and requires NMDAR-dependent plasticity in this region (Huerta et al. 2000). Relatively little is known about the contribution of the dentate gyrus to trace conditioning, although recent studies suggest this region also plays an important role. For example, exposure to a trace-conditioned cue produces significant increases in immediate early gene expression in the dentate gyrus (Weitemier and Ryabinin 2004). Trace conditioning also increases neurogenesis in the dentate gyrus (Gould et al. 1999) and is impaired by a reduction in the number of newly generated neurons in this region (Shors et al. 2001). The current experiments used δ knockout (KO) mice to examine the effects of reduced inhibition and neurosteroid sensitivity in the dentate gyrus on the acquisition of trace fear conditioning. We also looked for nonspecific effects of δ subunit deletion by examining performance on open-field and RotaRod tests.
Results
Trace conditioning
Females
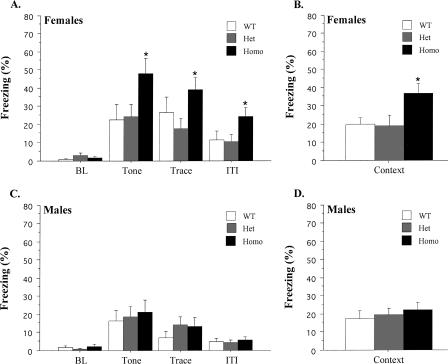
Data from female mice during the tone test are displayed in Figure 1A. The data are presented as percent time spent freezing during four periods: Baseline (BL), Tone, Trace, and Intertrial Interval (ITI). BL freezing levels were low and did not differ among genotypes (F(2,27) = 2.34, p > 0.05). Mice showed a significant increase in freezing when the trace-conditioned tone was presented (F(1,27) = 47.5, p < 0.05), and this effect interacted with genotype (F(2,27) = 3.8, p < 0.05). Post hoc tests (Fisher's PLSD; p < 0.05) revealed that homozygous (Homo) KOs exhibited a larger increase in tone fear than either heterozygous (Het) KO or wild-type (WT) mice. This enhancement was observed during the entire test (F(2,27) = 3.52, p < 0.05) and did not interact with stimulus period (F(4,54) = 1.22, p > 0.05). The day after the tone test, mice were placed in the original training environment for a context test. Figure 1B shows the freezing scores for females averaged across the entire 8-min test. As observed during the tone test, female Homo KOs froze significantly more than Het or wild-type mice (F(2,27) = 4.66, p < 0.05).
Figure 1.
Data from the tone and context tests following trace fear conditioning. (A) Mean (±SEM) percent freezing for female mice during each period of the tone test. (B) Mean (±SEM) percent freezing for female mice during the context test. (C) Mean (±SEM) percent freezing for male mice during each period of the tone test. (D) Mean (±SEM) percent freezing for male mice during the context test. An asterisk (*) indicates a significant group difference (p < 0.05).
Males
Male freezing during the tone test is illustrated in Figure 1C. Freezing levels were low during the BL period and did not differ among genotypes (F(2,24) = 1.1, p > 0.05). Mice exhibited an increase in freezing during the tone presentations (F(1,24) = 30.2, p < 0.05) that did not interact with genotype (F < 1). A repeated-measures ANOVA on the Tone, Trace, and ITI periods found a significant effect of stimulus period (F(2,48) = 19.27, p < 0.05) and post hoc tests (Fisher's PLSD; p < 0.05) revealed that most freezing occurred during the tone. There were no overall freezing differences among genotypes during these periods (F < 1). The day after the tone test, male mice were placed back in the original training environment for a context test. Figure 1D illustrates freezing scores averaged across the entire 8-min test. Homo, Het, and wild-type mice exhibited similar levels of context freezing during this test (F < 1).
An ANOVA on the tone test data revealed a main effect of sex (F(1,51) = 10.64, p < 0.05) as males froze significantly less than females. This effect did not interact with genotype (F < 1), suggesting a general increase in freezing for females across all periods. In contrast, an ANOVA conducted on the context freezing scores from male and female mice did not find an effect of sex (F(1,51) = 2.29, p > 0.05).
Delay conditioning
Females
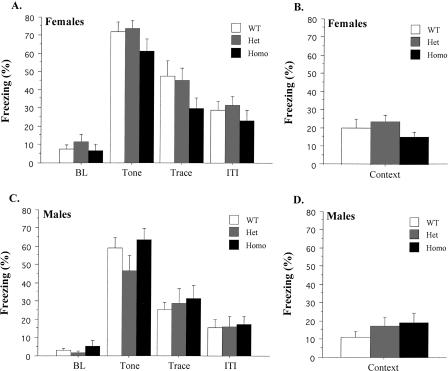
Female data during the tone test are illustrated in Figure 2A. BL freezing levels were low and did not differ among genotypes (F < 1). All groups showed a significant increase in freezing when the delay-conditioned tone was presented (F(1,24) = 477.39, p < 0.05), and this effect did not interact with genotype (F(2,24) = 1.09, p > 0.05). Freezing to the tone following delay conditioning was significantly greater than that observed following trace conditioning (F(1,55) = 37.49, p < 0.05). There was a significant effect of stimulus period during the test (F(2,48) = 103.78, p < 0.05) as most freezing occurred during the tone (Fisher's PLSD; p < 0.05). There were no freezing differences among genotypes during any of the stimulus periods (F(2,24) = 1.76, p > 0.05). The day after the tone test, female mice were placed in the original training environment for a context test. Figure 2B shows the freezing scores for females averaged across the entire 8-min test. Homo, Het, and wild-type mice exhibited similar levels of context freezing during this test (F(2,24) = 1.3, p > 0.05).
Figure 2.
Data from the tone and context tests following delay fear conditioning. (A) Mean (±SEM) percent freezing for female mice during each period of the tone test. (B) Mean (±SEM) percent freezing for female mice during the context test. (C) Mean (±SEM) percent freezing for male mice during each period of the tone test. (D) Mean (±SEM) percent freezing for male mice during the context test.
Males
Male data during the tone test are illustrated in Figure 2C. Freezing levels were low during the BL period and did not differ among genotypes (F(2,24) = 1.03, p > 0.05). All groups exhibited a subsequent increase in freezing during the tone presentation (F(1,24) = 218.45, p < 0.05), which did not interact with genotype (F(2,24) = 1.33, p > 0.05). The tone acquired more fear in the delay procedure compared to the trace procedure used in the previous experiment (F(1,52) = 51.45, p < 0.05). Freezing levels during the tone were the same across genotypes (F(2,24) = 1.82, p > 0.05). Mice also exhibited elevated freezing during the Trace and ITI periods. A repeated-measures ANOVA on the Tone, Trace, and ITI periods found a significant effect of stimulus period (F(2,48) = 142.02, p < 0.05), and post hoc tests (Fisher's PLSD; p < 0.05) revealed that most freezing occurred during the tone. There were no freezing differences among genotypes during any of these periods (Fs < 1). The day after the tone test, the same mice were placed back in the original training environment for a context test. Figure 2D illustrates freezing scores averaged across the entire 8-min test. All genotypes exhibited similar levels of context freezing during this test (F(2,24) = 1.06, p > 0.05).
An ANOVA comparing the tone test data from male and female mice revealed a main effect of sex (F(1,48) = 7.47, p < 0.05) as females froze significantly more than males. This effect did not interact with stimulus period (F < 1), suggesting a general increase in freezing across all periods. In contrast to the tone data, an ANOVA on context freezing scores from male and female mice did not reveal an effect of sex (F < 1).
Openfield and RotaRod performance
The δ subunit is expressed in granule cells in the cerebellum (Peng et al. 2002), an area intricately involved in motor function. Therefore, we determined if δ KOs had any changes in motor activity or coordination. Motor impairments could produce performance deficits on learning tasks and confound evaluations of memory function.
Openfield
Females
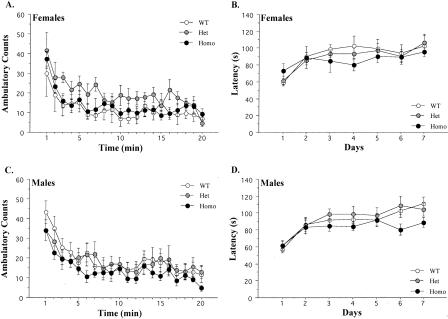
To assess general activity levels, mice were placed on an openfield for 20 min. Overall, Het and Homo KOs exhibited normal activity levels relative to controls (Fig. 3A) (F(2,16) = 1.31, p > 0.05). Across the session, there was a significant decrease in activity (F(19,304) = 9.21, p < 0.05) that did not differ among genotypes (F < 1).
Figure 3.
Data from the openfield and RotaRod tests. (A) Mean (±SEM) activity scores for female mice across the 20-min openfield test. (B) Mean (±SEM) latency to fall from the RotaRod for female mice across 5 d. (C) Mean (±SEM) activity scores for male mice across the 20-min openfield test. (D) Mean (±SEM) latency to fall from the RotaRod for male mice across 7 d.
Males
Both Het and Homo KOs exhibited activity levels that were similar to wild-type controls (Fig. 3C) (F < 1). Across the session, there was a general decrease in activity (F(19,437) = 18.49, p < 0.05) that was observed in all genotypes. The magnitude of this decrease did not differ among genotypes (F < 1). Overall, there was no effect of sex on motor activity in the openfield (F < 1). Males and females also displayed similar decreases in activity across the 20-min session (F < 1).
RotaRod
Females
After the openfield, motor coordination was analyzed on the RotaRod. Female mice displayed an increase in latency to fall across days (Fig. 3B) (F(6,102) = 9.71, p < 0.05) that did not differ among genotypes (F < 1). Overall, there was no effect of genotype (F < 1).
Males
There was an increase in latency to fall across days (Fig. 3D) (F(6,144) = 20.79, p < 0.05) that did not differ among genotypes (F(12,144) = 1.6, p > 0.05). Overall, there was no effect of genotype (F(2,24) = 1.3, p > 0.05). There was also no difference between the performance of males and females overall (F < 1) or across days (F < 1)
Discussion
Our results demonstrate that deletion of the δ subunit of the GABAAR enhances the acquisition of hippocampus-dependent trace fear conditioning in female mice. Delay fear conditioning was normal in δ KOs, suggesting the augmentation was not due to a general increase in motivation or performance. Normal motor activity and coordination were also observed on the RotaRod and openfield tests. Genetic deletion of the δ subunit decreases neurosteroid modulation and reduces inhibition in the dentate gyrus (Spigelman et al. 2003). Therefore, our results suggest that GABAergic inhibition in this region plays an important and selective role in the acquisition of trace fear conditioning. It should be noted that the δ subunit is conspicuously absent from several brain regions known to be essential for fear conditioning (e.g., amygdala, periaqueductal gray) (Fendt and Fanselow 1999; Peng et al. 2002). However, this subunit is highly expressed in the cerebellum, a structure recently implicated in the consolidation of fear learning (Peng et al. 2002; Sacchetti et al. 2002, 2004). Despite this fact, there are several reasons that make it unlikely that the enhanced conditioning we observed resulted from δ-subunit deletion in this region. First, motor learning was completely normal in δ KO mice. Second, manipulations of the cerebellum do not selectively affect hippocampus-dependent learning. In fact, hippocampus-independent delay conditioning is most sensitive to changes in cerebellar activity or placticity (Sacchetti et al. 2002, 2004). This contrasts with the selective enhancement of hippocampus-dependent learning that we observed in the δ KO mice.
The current results replicate our previous finding that tone and context fear are normal in δ KOs following delay conditioning (Mihalek et al. 1999). We also found that females acquired significantly more tone fear than males following both delay and trace conditioning. Increased tone fear in females is consistent with the rat eyeblink literature, where females acquire tone conditioning at a faster rate and to a higher asymptote than males (Shors et al. 2000). In contrast, previous experiments have found that females tend toward slower acquisition of context fear (Maren et al. 1994). In the current study, context conditioning was not different for male and female mice. This could be explained by the amount of exposure our animals had to the context. A recent study showed that sex differences in the acquisition of context fear depend on the amount of exposure to the training environment (Wiltgen et al. 2001). With longer amounts of context exposure, females acquire the same amount of context fear as males. In the current study, all animals were in the training context for an extended period of time, which likely alleviated any sex difference in context fear.
Using a trace conditioning procedure, we found enhanced tone and context fear in female δ KOs. A performance ceiling did not preclude an effect in male KOs as they showed less trace conditioning than females. Males were also capable of freezing at higher levels, for example, following delay conditioning (Fig. 2C). The sex-specificity of this effect suggests the involvement of neuroactive steroids. There are sex differences in neurosteroid levels and in the response of GABAARs to these compounds (Robel and Baulieu 1995; Wilson and Biscardi 1997; Reddy and Kulkarni 1999). Neurosteroid levels and their efficacy also vary throughout the estrus cycle (Finn and Gee 1993; Palumbo et al. 1995; Reddy and Kulkarni 1999). The general action of these compounds is to increase GABAergic inhibition and impair hippocampus-dependent learning (Finn and Gee 1993; Palumbo et al. 1995). Therefore, the enhanced conditioning observed in female KOs could be a consequence of their reduced responsiveness to neurosteroids. The fact that the enhancement was only observed following trace conditioning, a task that depends on the hippocampus, suggests the dentate gyrus is the site of action.
There is evidence that fluctuations in GABAergic inhibition during the estrus cycle produce changes in learning. In eyeblink conditioning, learning is enhanced in females during proestrus and reduced during estrus and diestrus (Shors et al. 2000). The enhanced learning observed during proestrus results from a rise in estrogen, which increases the formation of dendritic spines. This proliferation is a direct result of reduced GABAergic inhibition (Murphy et al. 1998; Segal and Murphy 2001). In the hippocampus, estrogen receptors are located on glutamic acid decarboxylase (GAD) positive interneurons necessary for the synthesis of GABA. The presence of estradiol significantly reduces the amount of GAD found in these neurons (Murphy et al. 1998). Conversely, GABA agonists increase inhibition and block estrogen-induced increases in dendritic spines (Segal and Murphy 2001). Therefore, enhanced learning during proestrus likely results from a reduction in GABAergic inhibition and the subsequent increase in dendritic spines.
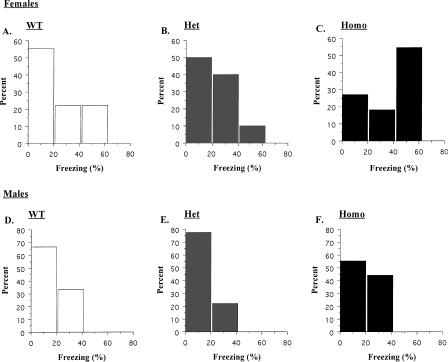
Progesterone, which peaks during estrus, acts to oppose the effects of estrogen. Progesterone increases GABAergic inhibition and reduces the number of dendritic spines in the hippocampus (Woolley and McEwen 1993; Murphy and Segal 2000; Segal and Murphy 2001). It is likely that these changes are mediated by the synthesis of endogenous neurosteroids, natural metabolites of progesterone (Murphy and Segal 2000). Neurosteroid levels and their efficacy vary in the hippocampus during the female cycle (Finn and Gee 1993; Palumbo et al. 1995), and direct administration of these compounds increases inhibition and impairs hippocampus-dependent learning (Paul and Purdy 1992; Johansson et al. 2002). Therefore, it is possible that neurosteroid-mediated inhibition acts to reduce the number of dendritic spines in the hippocampus during estrus and diestrus and impair subsequent learning. δ KOs would be immune to these effects because deletion of this subunit substantially reduces the sensitivity of GABAARs to neurosteroids. A closer analysis of our trace conditioning data supports this idea. Histograms of the trace conditioning scores (averaged across all test periods) are shown in Figure 4. Female wild type (WT) (Fig. 4A) and Hets (Fig. 4B) showed varying amounts of conditioning with most animals freezing at low to moderate levels (0%-40%) and very few mice freezing at high levels (>40%). This distribution was completely reversed in Homo KOs (Fig. 4C). The majority of these animals froze at high levels, and very few exhibited low to moderate conditioning. This suggests that deletion of the δ subunit increased freezing in animals that normally would show low levels of conditioning. The fact that the distribution was completely reversed in KOs (and not simply shifted to the right) suggests that deletion of the δ subunit did not generally enhance learning. Instead, there was a selective benefit for those animals exhibiting the lowest levels of conditioning. These results are exactly what one would predict if neurosteroids impaired hippocampus-dependent learning during the estrus cycle. Male mice did not show a similar profile (Fig. 4D-F). Mice from each genotype, including Homo KOs, showed the same distribution of freezing scores.
Figure 4.
Histograms from the trace fear conditioning test. The data were averaged across all test periods and are expressed as percent animals exhibiting low (0%-20%), moderate (20%-40%), and high (40%-60%) freezing scores.
The ability of neurosteroids to modulate neuronal plasticity has generated substantial interest in their role in learning and memory processes and their potential use as treatments for age-related and cognitive diseases (Schumacher et al. 1997). Recent evidence has also shown that these compounds play a critical role in the mediation of anxiety, particularly in females (Engel and Grant 2001; Toufexis et al. 2004). Consistent with these ideas, we suggest that neurosteroids affect fear learning in females by acting on δ-containing GABAARs in the hippocampus.
Materials and Methods
Subjects
All mice were of a mixed C57Bl/6J × 129Sv/SvJ genetic background. The mice were generated and genotyped as described previously (Mihalek et al. 1999). All experiments were conducted with homozygous (Homo) and heterozygous (Het) KOs and their wild-type littermates. The mice, which ranged from 4 to 6 months of age, were maintained on a 12 h light/12 h dark cycle in the Psychology Department vivarium at UCLA. Animals were group housed with free access to food and tap water. All procedures were performed during the light phase of the light:dark cycle.
Fear conditioning
Fear conditioning took place in four identical observation chambers (28 × 21 × 21 cm; Lafayette Instrument Co.) located in a well-lit room. A video camera was positioned in front of the chambers, which allowed behavior to be observed and recorded by an experimenter in an adjacent room. The floor of each chamber consisted of 33 stainless steel rods (2 mm diameter) spaced 6 mm apart (center to center). The rods were wired to a shock generator and scrambler (Med-Associates Inc.) for the delivery of footshock. A speaker was mounted on the top of each chamber and wired to an audio generator (Med-Associates Inc.) for delivery of the tone CS. Background noise (60 dB) was supplied by a fan positioned underneath the video camera. Prior to conditioning, the chambers were cleaned with a 1% acetic acid solution and pans containing a thin film of the same solution were placed underneath the grid floors. The tone test was conducted in novel chambers (B context) that were structurally different than the conditioning chambers (A context). These chambers had a Plexiglas floor (28 × 21 cm) and two white plastic side walls (24 × 21 cm) placed at 60° to the floor, forming a triangular enclosure. The room was dimly lit by a red lightbulb. Background noise (60 dB) was supplied by a white noise generator. Prior to the tone test, the chambers were cleaned with a 5% sodium hydroxide solution, and pans containing a thin film of the same solution were placed underneath the Plexiglas floors.
On the conditioning day, mice were brought from the vivarium into a holding room and allowed to sit undisturbed in their homecages for 10 min. Mice then underwent either trace or delay fear conditioning. In each procedure, the mice were placed in the conditioning context and allowed to explore for 3 min before the onset of the tone (20 sec, 80 dB, 2800 Hz). In the trace conditioning groups, tone termination and shock onset were separated by a 20-sec interval (trace interval). In the delay conditioning groups, tone termination was contiguous with shock (2 sec, 0.5 mA) onset. Both the delay and trace conditioning groups received five conditioning trials, each separated by a 200-sec intertrial interval (ITI). The mice were removed from the conditioning chambers 2 min after the last shock presentation and returned to their homecages. Then, 24 h later, the mice were placed in the B context for a tone test. The tone test consisted of a 2-min baseline period (BL) followed by three 20-sec tone presentations. Each tone presentation was separated by a 220-sec ITI. The freezing response (a defensive posture defined as the absence of motion except that necessitated by breathing) was used as a measure of conditional fear (Bolles and Collier 1976). It was measured using a time sampling procedure in which an observer scored the presence or absence of the freezing response for each mouse every 2 sec. Data were transformed into a percent freezing score by dividing the number of freezing observations by the total number of observations and multiplying by 100. For statistical analyses, freezing scores were averaged across the entire test and grouped into four bins: BL, Tone, Trace, and ITI. The Trace bin refers to the 20 sec immediately following tone termination, and the ITI bin is the subsequent 200 sec. This period corresponds to the trace interval used in the trace conditioning experiment. Although a trace interval was not used for the delay conditioning groups during training, freezing was analyzed during this period for comparative purposes. The day after the tone test, the mice were placed back in the original conditioning box (A context) for an 8-min context test. During this test, freezing was scored for each mouse every 8 sec. For trace conditioning, nine wild-type, nine Het, and nine Homo male mice and nine wild-type, 10 Het, and 11 Homo female mice were used. For delay conditioning, nine wild-type, nine Het, and nine Homo male mice and nine wild-type, nine Het, and 10 Homo female mice were used. All mice were naive prior to training.
Openfield
The activity monitors (Med-Associates) were made of polycarbonate plastic (27 × 27 × 20.3 cm.). Activity was tracked by 16 photobeams spaced evenly apart on both x- and y-axes. Mice were placed in the chambers (located in a dimly lit room), and ambulatory counts were calculated over a 20-min period. The chambers were washed thoroughly with Windex between mice. In this experiment, there were eight wild-type, nine Het, and 11 Homo male mice and five wild-type, 10 Het, and five Homo female mice. Data from two males (Hets) and one female (WT) were excluded from statistical analyses because of equipment malfunction.
RotaRod
The RotaRod consisted of a drum made of ribbed plastic and panels that separated the apparatus into five lanes (Technical and Scientific Equipment). Mice were placed on the RotaRod while it was rotating at 5 rpm. As soon as all mice were placed on the apparatus, the acceleration and timer were activated and rotational speed steadily increased from 5 to 60 rpm over the course of 3 min. On each day, every mouse was tested three times sequentially. The average of the three trials was the latency value used for that day. The same mice from the openfield experiment were used on the Rotarod. One male (Het) was excluded from the experiment because of injuries obtained in the homecage.
Acknowledgments
This work was supported by an NIH grant (P01NS35985) to M.S.F. and a UCLA Research Mentorship Fellowship to B.J.W.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.89705.
References
- Barnard, E.A., Skolnick, P., Olsen, R.W., Mohler, H., Sieghart, W., Biggio, G., Braestrup, C., Bateson, A.N., and Langer, S.Z. 1998. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: Classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 50: 291-313. [PubMed] [Google Scholar]
- Benke, D., Michel, C., and Mohler, H. 1997. GABAA receptors containing the α4-subunit: Prevalence, distribution, pharmacology, and subunit architecture in situ. J. Neurochem. 69: 806-814. [DOI] [PubMed] [Google Scholar]
- Bolles, R.C., and Collier, A.C. 1976. The effect of predictive cues on freezing in rats. Anim. Learn. Behav. 4: 6-8. [Google Scholar]
- Crestani, F., Lorez, M., Baer, K., Essrich, C., Benke, D., Laurent, J.P., Belzung, C., Fritschy, J.M., Luscher, B., and Mohler, H. 1999. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 2: 833-839. [DOI] [PubMed] [Google Scholar]
- Crestani, F., Keist, R., Fritschy, J.M., Benke, D., Vogt, K., Prut, L., Bluthmann, H., Mohler, H., and Rudolph, U. 2002. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc. Natl. Acad. Sci. 99: 8980-8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, S.R. and Grant, K.A. 2001. Neurosteroids and behavior. Int. Rev. Neurobiol. 46: 321-348. [DOI] [PubMed] [Google Scholar]
- Fendt, M. and Fanselow, M.S. 1999. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 23: 743-760. [DOI] [PubMed] [Google Scholar]
- Finn, D.A. and Gee, K.W. 1993. The influence of estrus cycle on neurosteroid potency at the γ-aminobutyric acidA receptor complex. J. Pharmacol. Exp. Ther. 265: 1374-1379. [PubMed] [Google Scholar]
- Gould, E., Beylin, A., Tanapat, P., Reeves, A., and Shors, T.J. 1999. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2: 260-265. [DOI] [PubMed] [Google Scholar]
- Gunther, U., Benson, J., Benke, D., Fritschy, J.M., Reyes, G., Knoflach, F., Crestani, F., Aguzzi, A., Arigoni, M., Lang, Y., et al. 1995. Benzodiazepine-insensitive mice generated by targeted disruption of the γ 2 subunit gene of γ-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. 92: 7749-7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta, P.T., Sun, L.D., Wilson, M.A., and Tonegawa, S. 2000. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron 25: 473-480. [DOI] [PubMed] [Google Scholar]
- Introini-Collison, I.B., Castellano, C., and McGaugh, J.L. 1994. Interaction of GABAergic and β-noradrenergic drugs in the regulation of memory storage. Behav. Neural Biol. 61: 150-155. [DOI] [PubMed] [Google Scholar]
- Johansson, I.M., Birzniece, V., Lindblad, C., Olsson, T., and Backstrom, T. 2002. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 934: 125-131. [DOI] [PubMed] [Google Scholar]
- Khan, Z.U., Gutierrez, A., and De Blas, A.L. 1996. The α 1 and α 6 subunits can coexist in the same cerebellar GABAA receptor maintaining their individual benzodiazepine-binding specificities. J. Neurochem. 66: 685-691. [DOI] [PubMed] [Google Scholar]
- Leuner, B., Falduto, J., and Shors, T.J. 2003. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 23: 659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, R.L. and Olsen, R.W. 1994. GABAA receptor channels. Annu. Rev. Neurosci. 17: 569-602. [DOI] [PubMed] [Google Scholar]
- Maren, S., De Oca, B., and Fanselow, M.S. 1994. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: Positive correlation between LTP and contextual learning. Brain Res. 661: 25-34. [DOI] [PubMed] [Google Scholar]
- McEchron, M.D. and Disterhoft, J.F. 1999. Hippocampal encoding of non-spatial trace conditioning. Hippocampus 9: 385-396. [DOI] [PubMed] [Google Scholar]
- McEchron, M.D., Bouwmeester, H., Tseng, W., Weiss, C., and Disterhoft, J.F. 1998. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 8: 638-646. [DOI] [PubMed] [Google Scholar]
- McEchron, M.D., Weible, A.P., and Disterhoft, J.F. 2001. Aging and learning-specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning. J. Neurophysiol. 86: 1839-1857. [DOI] [PubMed] [Google Scholar]
- McEchron, M.D., Tseng, W., and Disterhoft, J.F. 2003. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J. Neurosci. 23: 1535-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek, R.M., Banerjee, P.K., Korpi, E.R., Quinlan, J.J., Firestone, L.L., Mi, Z.P., Lagenaur, C., Tretter, V., Sieghart, W., Anagnostaras, S.G., et al. 1999. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc. Natl. Acad. Sci. 96: 12905-12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer Jr., J.R., Deyo, R.A., and Disterhoft, J.F. 1990. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 104: 243-252. [DOI] [PubMed] [Google Scholar]
- Moyer Jr., J.R., Thompson, L.T., and Disterhoft, J.F. 1996. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J. Neurosci. 16: 5536-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer Jr., J.R., Power, J.M., Thompson, L.T., and Disterhoft, J.F. 2000. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J. Neurosci. 20: 5476-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, D.D. and Segal, M. 2000. Progesterone prevents estradiol-induced dendritic spine formation in cultured hippocampal neurons. Neuroendocrinology 72: 133-143. [DOI] [PubMed] [Google Scholar]
- Murphy, D.D., Cole, N.B., Greenberger, V., and Segal, M. 1998. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J. Neurosci. 18: 2550-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, R.W. and Homanics, G.E. 2000. Function of GABAA receptors. In GABA in the nervous system: The view at fifty years (eds. D.L. Martin and R.W. Olsen), pp. 81-96. Lippincott Williams and Wilkins, Philadelphia, PA.
- Palumbo, M.A., Salvestroni, C., Gallo, R., Guo, A.L., Genazzani, A.D., Artini, P.G., Petraglia, F., and Genazzani, A.R. 1995. Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J. Endocrinol. Invest. 18: 853-856. [DOI] [PubMed] [Google Scholar]
- Paul, S.M. and Purdy, R.H. 1992. Neuroactive steroids. FASEB J. 6: 2311-2322. [PubMed] [Google Scholar]
- Peng, Z., Hauer, B., Mihalek, R.M., Homanics, G.E., Sieghart, W., Olsen, R.W., and Houser, C.R. 2002. GABAA receptor changes in δ subunit-deficient mice: Altered expression of α4 and γ2 subunits in the forebrain. J. Comp. Neurol. 446: 179-197. [DOI] [PubMed] [Google Scholar]
- Persohn, E., Malherbe, P., and Richards, J.G. 1992. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J. Comp. Neurol. 326: 193-216. [DOI] [PubMed] [Google Scholar]
- Porcello, D.M., Huntsman, M.M., Mihalek, R.M., Homanics, G.E., and Huguenard, J.R. 2003. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking δ subunit. J. Neurophysiol. 89: 1378-1386. [DOI] [PubMed] [Google Scholar]
- Quinn, J.J., Oommen, S.S., Morrison, G.E., and Fanselow, M.S. 2002. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus 12: 495-504. [DOI] [PubMed] [Google Scholar]
- Quirk, K., Gillard, N.P., Ragan, C.I., Whiting, P.J., and McKernan, R.M. 1994. Model of subunit composition of γ-aminobutyric acid A receptor subtypes expressed in rat cerebellum with respect to their α and γ/δ subunits. J. Biol. Chem. 269: 16020-16028. [PubMed] [Google Scholar]
- Reddy, D.S. and Kulkarni, S.K. 1999. Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behaviors in rats. Pharmacol. Biochem. Behav. 62: 53-60. [DOI] [PubMed] [Google Scholar]
- Robel, P. and Baulieu, E.E. 1995. Neurosteroids: Biosynthesis and function. Crit. Rev. Neurobiol. 9: 383-394. [PubMed] [Google Scholar]
- Sacchetti, B., Baldi, E., Lorenzini, C.A., and Bucherelli, C. 2002. Cerebellar role in fear-conditioning consolidation. Proc. Natl. Acad. Sci. 99: 8406-8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti, B., Scelfo, B., Tempia, F., and Strata, P. 2004. Long-term synaptic changes induced in the cerebellar cortex by fear conditioning. Neuron 42: 973-982. [DOI] [PubMed] [Google Scholar]
- Schumacher, M., Guennoun, R., Robel, P., and Baulieu, E.E. 1997. Neurosteroids in the hippocampus: Neuronal plasticity and memory. Stress 2: 65-78. [DOI] [PubMed] [Google Scholar]
- Segal, M. and Murphy, D. 2001. Estradiol induces formation of dendritic spines in hippocampal neurons: Functional correlates. Horm. Behav. 40: 156-159. [DOI] [PubMed] [Google Scholar]
- Shors, T.J., Beylin, A.V., Wood, G.E., and Gould, E. 2000. The modulation of Pavlovian memory. Behav. Brain Res. 110: 39-52. [DOI] [PubMed] [Google Scholar]
- Shors, T.J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., and Gould, E. 2001. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372-376. [DOI] [PubMed] [Google Scholar]
- Shumyatsky, G.P., Tsvetkov, E., Malleret, G., Vronskaya, S., Hatton, M., Hampton, L., Battey, J.F., Dulac, C., Kandel, E.R., and Bolshakov, V.Y. 2002. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell 111: 905-918. [DOI] [PubMed] [Google Scholar]
- Sieghart, W. and Sperk, G. 2002. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2: 795-816. [DOI] [PubMed] [Google Scholar]
- Smith, G.B. and Olsen, R.W. 1995. Functional domains of GABAA receptors. Trends Pharmacol. Sci. 16: 162-168. [DOI] [PubMed] [Google Scholar]
- Solomon, P.R., Vander Schaaf, E.R., Thompson, R.F., and Weisz, D.J. 1986. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav. Neurosci. 100: 729-744. [DOI] [PubMed] [Google Scholar]
- Spigelman, I., Li, Z., Liang, J., Cagetti, E., Samzadeh, S., Mihalek, R.M., Homanics, G.E., and Olsen, R.W. 2003. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABAA receptor δ subunit. J. Neurophysiol. 90: 903-910. [DOI] [PubMed] [Google Scholar]
- Staubli, U., Scafidi, J., and Chun, D. 1999. GABAB receptor antagonism: Facilitatory effects on memory parallel those on LTP induced by TBS but not HFS. J. Neurosci. 19: 4609-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis, D.J., Davis, C., Hammond, A., and Davis, M. 2004. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J. Neurosci. 24: 10280-10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini, S., Losi, G., and Homanics, G.E. 2002. GABAA receptor δ subunit deletion prevents neurosteroid modulation of inhibitory synaptic currents in cerebellar neurons. Neuropharmacology 43: 646-650. [DOI] [PubMed] [Google Scholar]
- Weitemier, A.Z. and Ryabinin, A.E. 2004. Subregion-specific differences in hippocampal activity between Delay and Trace fear conditioning: An immunohistochemical analysis. Brain Res. 995: 55-65. [DOI] [PubMed] [Google Scholar]
- Wilson, M.A. and Biscardi, R. 1997. Influence of gender and brain region on neurosteroid modulation of GABA responses in rats. Life Sci. 60: 1679-1691. [DOI] [PubMed] [Google Scholar]
- Wiltgen, B.J., Sanders, M.J., Behne, N.S., and Fanselow, M.S. 2001. Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behav. Neurosci. 115: 26-32. [DOI] [PubMed] [Google Scholar]
- Woolley, C.S. and McEwen, B.S. 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 336: 293-306. [DOI] [PubMed] [Google Scholar]