Abstract
The crucial role of the medial temporal lobe (MTL) in episodic memory is well established. Although there is little doubt that its anatomical subregions—the hippocampus, peri-, entorhinal and parahippocampal cortex (PHC)—contribute differentially to mnemonic processes, their specific functions in episodic memory are under debate. Data from animal, human lesion, and neuroimaging studies suggest somewhat contradictory perspectives on this functional specialization: a general participation in declarative memory, an exclusive involvement in associative mnemonic processes, and a specific contribution to spatial memory are reported for the hippocampus, adjacent cortices, and the PHC. A functional lateralization in humans dependent on the verbalizability of the material is also discussed herein. To further elucidate the differential contributions of the various MTL subregions to encoding, we employed an object-location association memory paradigm. The memory for each of the studied associations was tested twice: by the object, and by the location serving as retrieval cue. The memory accuracy in response to both cue types was also assessed parametrically. Brain activity during encoding which leads to different degrees of subsequent memory accuracy under the two retrieval conditions was compared. We found the bilateral posterior PHC to participate in encoding of both the object associated with a location and the location associated with an object. In contrast, activity in an area in the left anterior PHC and the right anterior MTL was only correlated with the memory for the location associated with an object.
Events—consisting of items in their spatiotemporal context—are stored in the episodic memory (Tulving 1983). Thus a vital aspect of episodic memory encoding is the formation of associations between an item and the context in which it was encountered. It is known from neuroimaging studies that a subset of brain areas involved in the online processing of a particular stimulus is also active during its encoding (Otten and Rugg 2001). The crucial role of the medial temporal lobe (MTL) in episodic memory is also well established. However, the exact contributions of the various MTL subregions to mnemonic processing are currently a matter of debate (Squire et al. 2004).
Animal, human lesion, and imaging data point in somewhat contradictory directions. Whereas many reports emphasize a specific role in spatial mnemonic processes of the hippocampus, adjacent cortices, and the PHC (Bohbot et al. 2000; Burgess et al. 2002), others claim a more general contribution to mnemonic processes of the same structures (Schacter and Wagner 1999; Eichenbaum 2000). Several studies aimed to dissociate functionally specialized areas within the MTL, for example, a material-dependent lateralization (Reber et al. 2002) or a differential involvement in associative and nonassociative memory processes of MTL subregions (Rugg and Yonelinas 2003). Although previous data clearly suggest a division of labor within the MTL, so far no unequivocal attribution of a cognitive process to a certain subarea was possible (Squire et al. 2004).
The goal of the present study was to investigate how distinct areas within the MTL are involved in encoding different parts of the same episode. In particular we aimed to explore the collaboration and division of labor within the MTL at encoding of object-location associations. For this purpose we employed a variation of a previously published subsequent memory paradigm where brain activity during encoding of object-location associations is correlated with the performance in a succeeding memory test (Sommer et al. 2005). In each session, subjects encoded 16 unique object-location associations. Each of the resulting associative memory traces was probed twice: (1) The object served as retrieval cue, and subjects had to recognize the location associated with that object. (2) The location served as retrieval cue, and subjects had to recognize the object associated with that location. Memory accuracy for the location as well as for the object was assessed parametrically. Thus it was determined to what extent the same associative memory trace was accessible separately cued by its two components. The activity during encoding which led to stepwise decreasing memory accuracy was compared. This parametric design has the advantage that no baseline or control condition is necessary, which facilitates the interpretation of activity patterns (Stark and Squire 2001b).
We hypothesized that activity in areas of the MTL preferentially involved in spatial encoding would predict the subsequent memory performance only for the location associated with an object and vice versa. In addition, activity in areas contributing to the encoding of both objects and locations or to the formation of association between these would correlate with the subsequent memory accuracy for both.
Results
Behavior
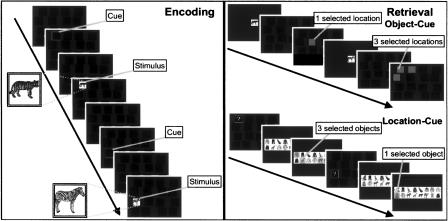
We employed a substantial modification of a previously introduced recognition-memory paradigm with a significantly prolonged recognition test and two different retrieval cue types. We were concerned about three issues: (1) difficulty of the task, (2) differences in both cue types, and (3) better understanding of the cognitive processes followed by both retrieval cues. A description of the task is given in Figure 1.
Figure 1.
Description of the task. In the encoding phase (left panel), each trial consisted of an orienting cue indicating the position of the next object in an array of 16 black boxes, and then (after a jittered ISI) the picture of a common object appeared. Subjects were asked to make an artificial/natural-judgement. In the retrieval phase (right panel), each object-location association was tested twice in randomly intermixed order: (1) by the object and (2) by the location serving as retrieval cue. When retrieval was cued by the object, one of the studied objects appeared in the center of the screen, followed by the same array of black boxes. The task was to indicate, by selecting one or more locations depending on the accuracy of memory, which object was associated during the encoding phase. When retrieval was cued by the location, the 16 locations appeared on the screen, one with a white question mark, followed by the same 16 objects randomly rearranged in two rows in the middle of the screen. The task was to indicate, by selecting one or more objects depending on the accuracy of memory, which object was associated with the location during the encoding phase.
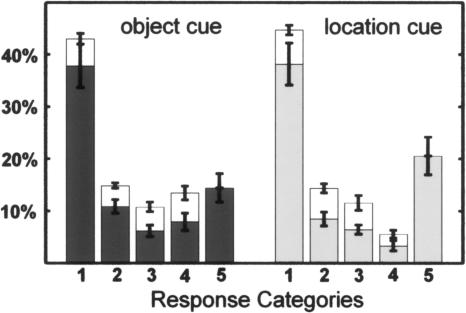
Response accuracy during the encoding task was high (98.8%, SD 1.2), and only objects that were categorized correctly by the subject were analyzed further. We found that 96.8% of all responses in the subsequent memory test in response to both cue types (object as well as a location retrieval cue) fell into the five categories 1 to 4 and 16 selected locations, where the latter one corresponds to the indication of a forgotten location. To simplify further analyses, only these responses' categories (RCs) were included and will be referred to as RC1 to RC4 (one to four selected objects/locations) and RC 5 (indication of a forgotten object/location). The proportions of the various RCs are not equally distributed. A cue type × RC ANOVA revealed a significant main effect of response-category [F(4,56) = 22.2; P < 0.000] and a significant interaction [F(4,56) = 10.1; P < 0.000]. RC1 is more frequent than the remaining responses' categories (post hoc Tukey HSD tests P < 0.000) in response to both cue types. RC4 was more frequent after an object cue and RC5 after a location cue (post hoc Tukey HSD tests P < 0.000, P = 0.01 respectively; Fig. 2).
Figure 2.
Frequency distribution of the five response categories. The relative proportions of the various response categories (Categories 1-4, number of selected locations/objects; Category 5, the indication of a forgotten location/object) are presented for both retrieval conditions: when retrieval was cued by the object (left) and by the location (right). The dark gray (object cue) and light gray (location cue) bars indicate the frequency of correct responses in a category, the white bars of incorrect responses. The error bars in both panels indicate the standard error of the mean.
A comparison with the behavioral results of a previous study (Sommer et al. 2005) using only object retrieval cues revealed no significant difference in the proportion of the RCs (design-complexity × RC ANOVA, F(4,112) = 0.46).
In addition, the hit rate in RC1 is greater than in the remaining RCs, as a cue type × RC ANOVA revealed [F(3,39) = 14.3; P < 0.000; post hoc Tukey HSD P < 0.01 for RC 1 vs. 2,3,4; Table 1]. Although subjects were instructed to indicate in case of low confidence a forgotten object/location, they selected sometimes false objects/locations, which is defined as “guessing.” The likelihood of a “lucky guess” differs depending on the number of selected locations/objects (see Materials and Methods). Therefore the hit rates must be corrected for “lucky guesses” to test whether they are above chance in all RCs. Assuming that the misses provide a rough estimate of the guessing rate for hits in our paradigm (Snodgrass and Corwin 1988), we adopted the correction for guessing (Rugg et al. 1998) for our recognition task where subjects had to select from 16 alternative objects/locations, as follows: For one selected location, the probability for a lucky guess is 1/16. Therefore the miss rates represent 15/16 of the merely guessed responses, and 1/16 of lucky guesses is hidden in the hit rate. In other words, 1/16 of the miss rate is just by chance correct, and the hit rate must be corrected by this value to get a more valid behavioral measure of the accuracy. Following this rationale, the hit rates in the four categories were corrected for guessing. Importantly, the corrected hit rate in all categories significantly exceeded the particular chance level (P < 0.00 for all RCs).
Table 1.
Behavioral performance in the subsequent memory test
| Categories | 1 | 2 | 3 | 4 | 5 (forgotten) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Object serving as retrieval cue | ||||||||||
| Reaction time in msec (encoding task) | 1278 (222) | 1158 (174) | 1342 (424) | 1214 (227) | 1214 (263) | |||||
| Reaction time in msec (retrieval) | 4920 (340) | 5480 (743) | 6223 (1130) | 5932 (1095) | ||||||
| Hit rate (% of responses in the category) | 87.42 (8.18) | 72.85 (9.24) | 57.80 (14.35) | 62.89 (13.62) | ||||||
| Hit rate, lucky guess corrected | 86.64 (8.18) | 69.45 (9.24) | 49.89 (14.35) | 53.61 (13.62) | ||||||
| Location serving as retrieval cue | ||||||||||
| Reaction time in msec (encoding task) | 1321 (248) | 1298 (263) | 1159 (256) | 1110 (274) | 1204 (347) | |||||
| Reaction time in msec (retrieval) | 6599 (809) | 8469 (2069) | 9810 (3129) | 9302 (2486) | ||||||
| Hit rate (% of responses in the category) | 83.82 (9.00) | 57.90 (16.47) | 61.24 (25.11) | 63.44 (23.69) | ||||||
| Hit rate, lucky guess corrected | 82.81 (9.00) | 52.63 (16.47) | 53.97 (25.11) | 46.54 (25.75) | ||||||
Data are mean ± SD. Category 1—subjects selected the correct location/object; category 2—subjects selected two locations/objects; categories 3 and 4—the same as category 2 but with 3 or 4 selected locations/objects respectively; category 5—subjects indicated that they forgot the location/object. See Materials and Methods section for the rationale of the “lucky guess” correction of the hit rate. The reaction time in the retrieval task is calculated from cue onset until the first selected item.
In the further behavioral analysis, the relative proportion of the five RCs were entered because the relative composition is an indicator for the overall memory performance. The proportions of correct responses were lucky guess-corrected by the misses as outlined above to get a more valid behavioral measurement of memory accuracy.
There was no significant effect of the duration of the preceding or following interstimulus interval (ISI) (0-2 null events, min. 2.5 sec, max. 14.5 sec) for any of the RCs (ISI × cue type × RC ANOVAs F(8,112) = 1.11, respectively F(8,112) = 0.35). The reaction time (RT) of the encoding task also had no influence on the subsequent memory performance (cue type × RT × RC ANOVA F(4,64) = 1.97). The effect of the spatial position in the array of boxes during the encoding phase was analyzed by comparing the influence of the amount of direct neighbors (2, 3, or 4) on the memory performance. RC1 was more frequent for the corner positions (two direct neighbors) during the encoding phase (position × RC × cue type ANOVA, F(8,112) = 10.1; post hoc Tukey HSD P < 0.00). The other RCs were equally distributed over the various spatial positions.
The serial position during retrieval had no significant impact on the memory performance (retrieval position × RC × cue type ANOVA, F(124,1736) = 1.17).
Given that in all 10 sessions the same locations were used in association with different pictures, the locations became more familiar over sessions, whereas the pictures were (despite the familiarization phase) relatively novel. To test the possibilities that (1) this disparity led to differences in the processing of both cue types over time, and (2) subjects became increasingly confused by the multiple former associated pictures, a time × cue type ANOVA for all five RCs was conducted. No significant interaction between cue type and time was observed in any of these analyses.
The frequency of RC1 increased over sessions independent of cue type [main effect session F(9,126) = 4.5, P < 0.00].
Decision latencies were measured as the RT from the occurrence of the retrieval cue until the selection of the first object/location. It took significantly longer to retrieve an object than a location [RT × RC × cue type ANOVA, main effect of cue type F(1,14) = 80.49], and subjects were significantly faster when they selected only one item rather than two, three, or four [main effect of RC F(4,56) = 15.33; post hoc Tukey HSD RC 1 vs. 2, 3, and 4 P < 0.00].
Each association was tested twice, one time with the object and one time with the location as retrieval cue. The correlation between the memory performances in these two tests per item was calculated via a 6 * 6 contingency table (RCs 1,2,3,4, forgotten, and misses). The resulting coefficient of contingency over all subjects and items is C = 0.6124 (df = 25, χ2 = 1345, P < 0.000) at a maximal Cmax = 0.9129. This corresponds to a Cramer's statistic VC = 0.433, which is comparable to the coefficient of a Pearson product moment correlation. The correlation in the individual subjects ranges from VC = 0.36 to VC = 0.48. In response to the second recognition test for each association (independent of the order of retrieval cue types), only the proportion of forgotten responses increased significantly [cue order × RC ANOVA, F(4,56) = 4.66, P < 0.00; post hoc Tukey HSD for RC 5 P < 0.00]. The relative hit rate in each category did not change over the two tests [cue order × RC ANOVA F(3,42) = 0.37].
Functional neuroimaging
Object retrieval cue
Brain areas showing a significant positive correlation of brain activity with the five categories of subsequent memory confidence are listed in Table 2. The results of Sommer and colleagues (2005) using an identical design but only an object retrieval cue were replicated. The activity clusters were—probably due to the improved signal to noise ratio using a 3 Tesla-instead of a 1.5 Tesla-MRT—more prominent. In particular, right V1/V2, right dorsal extrastriate area (DE), the bilateral inferior and superior parietal lobes, and the bilateral fusiform and lingual gyri revealed this relationship. In addition, activity in the bilateral PHC and anterior MTL correlated significantly with the accuracy of subsequent memory retrieval. The bilateral frontal eye fields, the superior rostral part of the left premotor cortex, the left anterior prefrontal cortex, left angular gyrus, and the bilateral superior colliculi also showed a significant correlation.
Table 2.
Brain regions showing a significant correlation between activity during encoding and subsequent memory performance when retrieval was cued by the object (upper part) or by the location (lower part)
| Brain region | Hemisphere | Location (MNI coordinates) | Peak Z | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Object serving as retrieval cue | ||||||||||
| Calcarine | Right | 3 | -93 | 21 | 4.45 | |||||
| Dorsal extrastriate cortex | Right | 39 | -72 | 24 | 4.94 | |||||
| Superior parietal cortex | Right | 33 | -63 | 48 | 4.01 | |||||
| Left | -9 | -81 | 48 | 3.82 | ||||||
| Angular gyrus | Left | -30 | -57 | 45 | 4.57 | |||||
| Lingual gyrus | Right | 21 | -63 | -9 | 4.38 | |||||
| Left | -18 | -60 | -6 | 4.76 | ||||||
| Fusiform gyrus | Right | 45 | -45 | -18 | 3.88 | |||||
| Right | 45 | -48 | -18 | 3.53 | ||||||
| Left | -42 | -63 | -24 | 3.54 | ||||||
| Left | -42 | -48 | -27 | 3.39 | ||||||
| Parahippocampal gyrus | Right | 21 | -63 | -9 | 4.57 | |||||
| Left | -36 | -33 | -15 | 3.89 | ||||||
| Frontal eye fields | Left | -27 | 3 | 48 | 4.06 | |||||
| Right | 30 | 3 | 51 | 3.83 | ||||||
| Anterior inferior prefrontal cortex | Left | -45 | 27 | 15 | 4.80 | |||||
| Anterior MTL | Left | -24 | -9 | -27 | 3.37 | |||||
| Right | 30 | -6 | -27 | 4.47 | ||||||
| Colliculi superiori | Right | -9 | 27 | -3 | 3.33 | |||||
| Left | 9 | -24 | -3 | 4.31 | ||||||
| Location serving as retrieval cue | ||||||||||
| Superior parietal cortex | Right | 27 | -69 | 57 | 3.29 | |||||
| Left | -15 | -75 | 57 | 3.36 | ||||||
| Fusiform gyrus | Right | 24 | -51 | -18 | 3.34 | |||||
| Left | -39 | -60 | -24 | 3.25 | ||||||
| Parahippocampal gyrus | Right | 24 | -44 | -6 | 3.57 | |||||
| Left | -27 | -42 | -15 | 3.54 | ||||||
| Inferior prefrontal cortex | Left | -45 | 30 | 18 | 4.80 | |||||
| Lateral occipital complex | Right | 45 | -72 | -21 | 3.34 | |||||
Activity was defined as significant at P < .05 corrected for a spherical volume of interest (10 mm radius, see Materials and Methods).
Location retrieval cue
For the location-retrieval cue, the network of areas where activity during encoding predicted the subsequent memory performance was less extended and also comprised the bilateral superior parietal lobe, bilateral posterior fusiform and parahippocampal gyrus, and the left occipital complex (Table 2). The left anterior inferior prefrontal cortex also showed this correlation.
Conjunction
To identify areas where activity during encoding is correlated with the retrieval success in response to both cue types, we conducted a conjunction analysis. This conjunction revealed a common activity pattern for both cue types consisting of the left fusiform gyrus, the bilateral parahippocampal cortex, and the inferior prefrontal cortex (Table 3). Lowering the statistical threshold by using an uncorrected p-value (p = 0.001) did not reveal more clusters of activity in the MTL. Each object-location association was assessed twice. This successive testing could theoretically exaggerate the size of overlapping effects in the conjunction analysis. Therefore we applied a second model where only the first retrieval occasion of each object-location association was included. The result of this additional analysis confirmed the foci of common activity—with lower Z-values due to the 50% reduction of events—in particular the bilateral parahippocampal cortex, the left fusiform gyrus, and the inferior prefrontal cortex.
Table 3.
Conjunction and interaction analysis
| Brain region | Hemisphere | Location (MNI coordinates) | Peak Z | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conjunction analysis | ||||||||||
| Parahippocampal cortex | Right | 24 | -45 | -6 | 3.38 | |||||
| Left | -24 | -42 | -9 | 3.24 | ||||||
| Fusiform | Left | -33 | -36 | -30 | 3.96 | |||||
| Left | -27 | -51 | -18 | 3.78 | ||||||
| Anterior inferior prefrontal cortex | Left | -42 | 27 | 15 | 4.38 | |||||
| Interaction: object cue > location cue | ||||||||||
| Anterior MTL | Right | 30 | 6 | -27 | 3.52 | |||||
| Parahippocampal cortex | Left | -36 | -33 | -12 | 3.25 | |||||
| Rostral precentral sulcus | Left | -27 | 6 | 45 | 3.28 | |||||
| Angular gyrus | Left | -30 | -57 | 33 | 3.67 | |||||
| Lingual gyrus | Left | -12 | 66 | -6 | 3.23 | |||||
Activity was defined as significant at P < .05 corrected for a spherical volume of interest (10 mm radius, see Materials and Methods).
Interaction
An interaction analysis was conducted to identify areas that are preferentially involved in encoding a location associated with an object, and vice versa. The interaction analyses revealed only areas where activity during encoding predicts the subsequent memory performance in response to an object cue. This analysis exhibited such responses in areas of the right anterior MTL, the left PHC, the left angular gyrus, the left lingual gyrus, and the left rostral precentral sulcus. This is to say activity in these areas correlates with the confidence of remembering the location associated with a particular object. The opposite interaction (location cue over object cue) revealed no suprathreshold voxel. The same pattern of activity was found in the MTL when the statistical threshold was lowered to P = 0.001 uncorrected.
Discussion
In a previous experiment (Sommer et al. 2005) we characterized a network of brain areas where activity during encoding predicts the retrieval success for the former location of an object. In particular, activity in areas of the dorsal and ventral visual stream, the PHC, and the left inferior prefrontal cortex was correlated with the precision of the resulting memory trace.
In the present study we were able to replicate and extend these findings with an improved paradigm that allows a direct comparison between areas involved in encoding two aspects of an object: object-identity and object-location. Statistical conjunction and interaction analyses revealed that activity in the fusiform gyrus, bilateral PHC, and left inferior prefrontal cortex predicts the accuracy of memory for both object-identity and object-location. Activity in the right anterior MTL, left PHC, left angular gyrus, left lingual gyrus, and the rostral precentral sulcus is specifically correlated with the precision of the memory trace for the associated location.
Behavioral data
The analysis of subsequent memory performance showed that the challenging retrieval task yielded valid estimates of the precision of the memory traces, and that there are no major performance differences dependent on the cue type. The retrieval of an object associated with a particular location seems to be slightly more difficult, as indicated by the higher forgetting rate. The prolonged decision latencies for the retrieval of objects is probably due to the trial-wise randomly rearranged array of objects, which demanded time to find the remembered pictures in the display.
The employed parametric measurement of memory accuracy shares two important features with classical confidence ratings, on a scale from 1 (low) to 6 (high confidence): (1) a correlation between decision latency and RCs (Murdock and Dufty 1972; Koppell 1977), and (2) a monotonically decreasing hit rate over RCs (Yonelinas 2001). These relationships are explained by signal detection models of memory where decision latency, hit rate, and confidence are reflections of the continuously distributed strength of the memory traces (McNicol and Stewart 1980; Hockley and Murdock 1987). In case of object-location associations, the term “memory trace strength” refers to the strength of the link between the mental representation for the two individual components of an association. Dual-process models hypothesize an additional process, namely recollection, which supports associative recognition (Yonelinas 1999).
Here, the moderate correlation between the performances in the two retrieval conditions indicates that the accessibility for a particular association differs depending on the cue type. Probably due to spontaneous fluctuations of the attentional focus between identity and location, in some associations the location is encoded deeper than the object identity and vice versa. This implies that domain-specific encoding processes exist for objects and locations. This has been shown before in behavioral experiments by explicit manipulations of the encoding tasks (Kohler et al. 2001).
No output encoding (Humphreys and Bowyer 1980) took place, as indicated by the equal performance at the first and second retrievals of each association. Consistent with previous reports, the equal reaction times during encoding for different degrees of subsequent memory indicate that the observed differences in brain activity are not related to differences in task difficulty or time on task (Cansino et al. 2002; Sommer et al. 2005).
Functional results
It is known that a subset of brain areas involved in the online processing of a particular stimulus type is also active during its encoding (Otten and Rugg 2001). The networks of brain areas where activity during encoding correlates with the retrieval success for both cue types show a substantial overlap in our task. This descriptive overlap concerns the fusiform gyrus, the superior parietal lobe, the inferior prefrontal cortex, and the PHC. Such overlapping activity patterns for encoding and retrieval of objects and locations compared to a perceptual baseline have been reported before (Moscovitch et al. 1995; Pihlajamaki et al. 2004).
In addition to areas that contribute to the processing of the item, it is well established that regions of the MTL are involved in episodic memory. The contributions of the various subregions to material-specific and general mnemonic processes are a matter of debate (Squire et al. 2004).
Posterior MTL
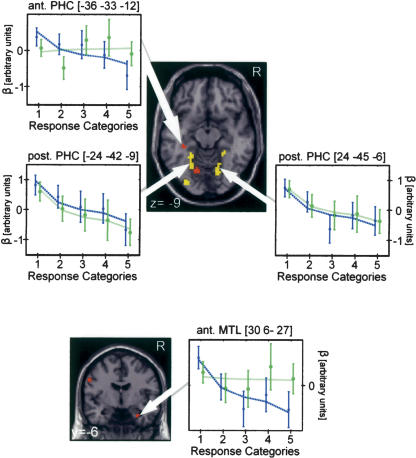
The PHC has been linked with two distinct cognitive processes: spatial cognition (Epstein et al. 2003) and mnemonic processing (Schacter and Wagner 1999). We found evidence of a functional specialization of neighboring areas within the PHC with respect to these cognitive domains (Fig. 3, upper panel).
Figure 3.
Activation in the conjunction and interaction analyses in the posterior and anterior MTL. Statistical map: (yellow clusters) result of the conjunction analysis indicating areas where activity during encoding correlated with the subsequent memory performance in response to both cue types; (red clusters) result of the interaction analysis indicating where activity during encoding correlates only with the subsequent memory performance in response to the object but not to the location cue. For display purposes, the statistical maps are threshold at P < 0.005 uncorrected. Graphs: The blue x's reflect the mean activation across group at the peak voxel during encoding for the different response categories when retrieval was cued by the object, the green circles when retrieval was cued by the location. The error bars represent the 90% confidence interval. The blue (object cue) and green (location cue) lines indicate the fit of the applied contrasts of interests in that voxel. (Categories 1-4: number of selected locations/objects; Category 5: the indication of a forgotten location/object.)
The specific role in spatial coding of areas in the PHC was defined as processing spatial relationships in a visual scene independent of the exact object-identity (Rombouts et al. 1999; Kohler et al. 2002; Epstein et al. 2003), which parallels the association of a particular location to an object in our task.
Human lesion and neuroimaging studies revealed an important role of the posterior MTL in spatial encoding and/or the association of objects and location. Focal lesions in the parahippocampal gyrus lead to topographical amnesia (Aguirre and D'Esposito 1999); lesions in the right MTL result in object-location memory deficits (Smith and Milner 1981, 1989; Pigott and Milner 1993), and neuroimaging studies confirmed the role of the PHC in spatial navigation (Aguirre et al. 1996; Maguire et al. 1998; Janzen and van Turennout 2004).
A more general role in associative mnemonic processes of the PHC is suggested by neuroimaging studies of memory. Areas in that region were found to be active during encoding and retrieval of associative information (Henke et al. 1997, 1999; Rombouts et al. 1997; Krause et al. 1999; Eldridge et al. 2000; Kohler et al. 2002; Davachi et al. 2003; Dobbins et al. 2003; Duzel et al. 2003; Kirwan and Stark 2004; Ranganath et al. 2004). In addition, memory studies using nonassociative stimulus material found the PHC contributing to encoding and retrieval of items (Schacter and Wagner 1999; Kirchhoff et al. 2000; Strange et al. 2002; Morcom et al. 2003), where it is important to note that high-confidence recognition and free recall are mostly accompanied by the retrieval of contextual, associated information. Eichenbaum (2000) concluded from animal experiments that the parahippocampus is involved in the encoding of associations as fused, unitized, or configural representations, as is the case in our paradigm.
Anterior MTL
Although most animal, human lesion, and imaging studies investigating the functional role of the right MTL seem to agree on a contribution of this structure to various aspects of memory, they remain contradictory with respect to its exact nature. In short there exist reports emphasizing (1) a specific role in spatial memory (Maguire et al. 1999), (2) a contribution to all forms of declarative memory (Stark and Squire 2001a), and (3) a selective involvement in associative memory processes (Eichenbaum 2000).
In our paradigm, activity in the right anterior MTL during encoding correlated exclusively with spatial encoding processes: the more active this area was during encoding, the deeper a location is associated with an object, and the more accurately the former location is subsequently retrieved (Fig. 3, lower panel).
A crucial role in spatial memory of the hippocampus and adjacent areas was first described in the cognitive map theory of hippocampal function (O'Keefe and Nadel 1978) and later confirmed by many findings in rats and monkeys (Suzuki et al. 1997; Broadbent et al. 2004; Burwell et al. 2004; Hampton et al. 2004; Jenkins et al. 2004; Leutgeb et al. 2004; Ludvig et al. 2004).
Human lesions restricted to the right hippocampus result in a loss of spatial memory and imply that its spatial role is lateralized to the right hemisphere (Smith and Milner 1981, 1989; Pigott and Milner 1993; Abrahams et al. 1997; Nunn et al. 1999; Bohbot et al. 2000; Astur et al. 2002; Stepankova et al. 2004). Such a lateralization was supported by neuroimaging studies, which found a preferential processing of words in the left, locations in the right, and pictures in bilateral hippocampus (Schacter and Wagner 1999; Kirchhoff et al. 2000; Reber et al. 2002). A pronounced role in spatial processing of the right hippocampus comes also from navigation experiments (Burgess et al. 2002). Furthermore, right anterior MTL activity during retrieval of previously learned locations of objects was reported (Owen et al. 1996). This cognitive process seems very similar to the retrieval processes in our paradigm when the object served as cue and the location had to be retrieved.
Evidence of a more general role of the right anterior MTL in memory comes from many neuroimaging studies that did not explicitly contrast familiarity-based and associative memory (Schacter and Wagner 1999; Kirchhoff et al. 2000; Stark and Squire 2000; Fletcher and Henson 2001; Strange et al. 2002; Henson et al. 2003; Morcom et al. 2003). Nevertheless, recent studies point toward a selective associative memory function of the anterior MTL (Eldridge et al. 2000; Small et al. 2001; Stark and Squire 2001a; Davachi et al. 2003; Dobbins et al. 2003; Sperling et al. 2003; Zeineh et al. 2003; Jackson III and Schacter 2004; Kirwan and Stark 2004; Preston et al. 2004; Ranganath et al. 2004).
Our finding that an area in the anterior MTL is specifically involved in encoding of spatial associations is not in contradiction to these studies. Animal and human data suggest that subregions of the hippocampus and entorhinal cortex participate in distinct cognitive processes. More specifically there is evidence that only parts of the anterior MTL cortex participate in navigation and spatial encoding in rats (Broadbent et al. 2004; Fyhn et al. 2004). Two neuroimaging studies comparing face and name encoding and object-identity and spatial configuration processing showed a functional specialization within the hippocampus (Small et al. 2001; Pihlajamaki et al. 2004). It was suggested that a functional dissociation within the hippocampus is related to afferents from distinct neocortical areas through the parahippocampal gyrus (Witter et al. 1989). Therefore there might exist subareas in the anterior MTL involved in spatial encoding, and others which are more general involved in mnemonic processing.
Materials and Methods
Subjects
Informed consent was obtained from 21 right-handed healthy subjects. Due to elevated error rates (>50%), six of the subjects were excluded after scanning from the further analysis; the final sample consisted of 15 subjects (seven female; age range 20-28 yrs; mean age 24.4 yrs; recruited by advertisement). Ethics approval was obtained from the local (Hamburg Board of Physicians) ethics committee.
Task
Each of the 10 sessions (+ two practice sessions outside of the scanner) consisted of four phases: “familiarization,” “encoding,” “distraction,” and “retrieval,” where the first three phases were identical to those of a previously published experiment (Sommer et al. 2005). The subjects were kept in the magnetic resonance (MR) scanner during all four phases of each session but were scanned only during the familiarization and encoding phases. In each session, a new subset of 16 pictures of common objects (Snodgrass and Vanderwart 1980) were used including eight natural and eight artificial objects. The order of subsets was randomized over subjects. Because the pictures of each session belong to two categories or semantic fields such as “African animals” and “musical instruments,” or “body parts” and “vehicles,” they are within one category semantically related, like neighbored locations are spatially related. At the beginning of each phase in each session, the instructions appeared on the screen to cue subjects to the task. The background was gray in all four phases, and instructions were presented in white.
Picture stimuli were presented controlled by a PC that ensured synchronization with the MR scanner using the software “Presentations” (http://www.neurobehavioralsystems.com). An LCD projector projected the stimuli on a screen positioned on top of the head coil, and the stimuli were viewed by the subjects through a mirror (10 × 15° field of view). Participants entered the responses by pressing buttons on an MR-compatible response box (familiarization and encoding phase) and computer mouse (retrieval phase).
The familiarization phase was introduced after pilot studies showed that the pictures are not equally familiar and verbalizable for German subjects, which is confirmed by a German standardization study of the Snodgrass and Vanderwart pictures (Genzel et al. 1995). Thus the familiarization phase ensured that all subjects recognized all pictures in a comparable time frame. During this phase the pictures of the particular session were shown in the center of the screen for 2.5 sec in randomized order. Beneath the picture, the common German name for the object was presented. The subjects were instructed to press the response button as soon as they recognized the picture and read the name.
In the encoding phase (Fig. 1), the subjects were shown an irregular array of 16 black boxes with the hidden pictures of the particular subset. Each picture was shown for 2 sec sequentially in a randomized order while the subject had to make a natural/artificial judgment by pressing one of two response buttons. The ISI was jittered between 2.5 and 3.5 sec with 14% null events and a maximum of two consecutive null events. We indicated the location of the next picture in advance (after presenting the last picture) by changing the color of the relevant box to white for 500 msec. This was undertaken to avoid orienting reactions when presenting the images, which may otherwise interfere with spatial attention, Subjects were instructed to fixate on the cued box and keep it in the focus of attention until the picture would appear. All cues were valid.
In the immediately following distraction phase (not scanned), subjects were instructed to count aloud backwards in steps of three from a random number between 80 and 100 displayed on the screen to overwrite working memory and minimize a recency effect.
In the retrieval phase, each of the previously encoded object-location associations were probed twice in randomly intermixed order: (1) by the object (picture) and (2) by the location serving as retrieval cue. In object-retrieval cue trials, one picture was presented in the center of the screen for 3 sec, followed by the empty array of 16 boxes. Subjects were instructed to select the remembered location of the picture in the study phase by moving the mouse cursor to that box and clicking with the mouse button. It was specifically emphasized to the subjects that they should not guess the location but select as many boxes as necessary in case of doubt, or indicate that they forgot the location. In trials with the location as retrieval cue, the empty array of 16 boxes were presented for 3 sec, where one box was marked as retrieval cue by a white question mark. This cue was followed by the 16 slightly downsized pictures of the particular subset randomly arranged in two rows in the center of the screen (Fig. 1). Subjects were instructed to select the object that was presented before at that location by moving the mouse cursor to that picture and clicking with the mouse button. Again it was emphasized to the subjects that they should not guess the picture but select as many as necessary in case of doubt, or indicate that they forgot the picture. At the end of each session a feedback for the overall memory performance in this session was given (total number of correct and “forgotten” responses in location- and object-retrieval cue trials in that session).
Image acquisition
Functional MRI was performed on a 3T system (Siemens Trio) with a gradient-echo EPI T2* sensitive sequence in 38 contiguous axial slices (2-mm thickness with 1-mm gap, TR 2.2 sec, TE 40 msec, flip angle 90°, field of view 210 × 210 mm2, matrix 64 × 64).
Image analysis
The imaging series was realigned, slice-time corrected, normalized into standard anatomical space (MNI), and smoothed with a Gaussian kernel of 10 mm full-width half-maximum. A high-pass filter with a cut-off period of 120 sec was applied.
An event-related analysis of the imaging data was conducted using Statistical Parametric Mapping (SPM2) to compare encoding related activity of individual object-location associations during the study phase. Only trials in which subjects made the correct natural/artificial decision during encoding (98.8%) were analyzed.
These encoding trials were post-hoc classified in two separate analyses according to the subsequent memory performance as response to (1) the object retrieval cue and (2) the location retrieval cue. Both analyses revealed that 96.8% of all responses fell into the following five categories: subjects selected one, two, three, or four locations/objects or indicated the absence of any confident memory. Only encoding trials belonging to these categories were functionally analyzed.
The analysis of the behavioral results showed an effect of the serial position during encoding. This effect was only significant for the first item, which was subsequently retrieved very accurately as indicated by a category 1 response [position × response category × cue type ANOVA, F(60,840) = 2.91; P < 0.000; post hoc Tukey HSD P < 0.05 for response category 1 at position 1 vs. position 4, 7, 8, 9, 10, 11, 12, and 15]. This was interpreted as a Primacy effect. For the following two items in each session, there was a statistical trend in the same direction. To avoid confounding of Primacy and the “normal” subsequent memory effect, the first three items of each session were therefore excluded in cases where only one location/object was subsequently selected (Strange et al. 2002).
These considerations have led to similar models for both types of retrieval cues: a total of seven regressors were created (i.e., stick functions convolved with a canonical hemodynamic response function as implemented in SPM) as the basis function of the following categories. Additionally, six subject-specific movement parameters from the rigid body registration were used as covariates. The first regressor accounted for the Primacy effect as described above. Regressor 2 consisted of the encoding events belonging to retrieval category 1 (subjects selected in the retrieval phase the correct location/object), regressor 3 comprised the events of category 2 (only when the correct location/object was among the selected), regressor 4 of category 3 (only when the correct location/object was among the selected), regressor 5 of category 4 (only when the correct location/object was among the selected), regressor 6 contained the forgotten encoding events, that is, when subjects indicated subsequently the absence of any memory, and regressor 7 the remaining events (errors during the encoding task, false responses during retrieval). In the first-level analysis, mean contrasts of each category were estimated over all sessions.
In two separate second-level analyses with subject as a random effect, the mean contrast images of the first-level analyses were used to compare the activation among the following five categories in both retrieval cue types: one, two, three, four selected locations/objects but only if the correct location/object was one of these selected and the indication of a forgotten location/object. These five categories were weighted depending on the chance level to guess the correct location/object (“lucky guess”) selecting 1, 2, 3, 4, and 16 boxes/pictures (equivalent to a forgotten location/object). This weighting was used as a rational quantification of the various response categories. If a subject randomly selects just one location/object, the chance to choose the correct one is 1 to 15. In cases in which subjects select more than one location/object, the situation is slightly more complicated because there are many possibilities to select two, three, or four locations/objects randomly. For two selected locations there are 105 possibilities to randomly select wrong locations/objects and 15 possibilities to randomly select two locations/objects including the correct one. The probability for a lucky guess is thus 1:7. Following this rationale, the chance level for three, four, and 16 selected locations/objects are 1:4.333, 1:3, and 1:1, respectively. The resulting weights were inverted and normalized, and resulted in the following contrast: 1.60 0.20 -0.27 -0.50 -1.03.
To take previous evidence into account, correction for multiple comparisons was based on volumes of interest rather than on the whole brain. The peak coordinates of activations reported previously served as the center of these spherical volumes (radius 10 mm). These regions included the frontal eye fields, areas in the left inferior prefrontal, parietal, occipital, and parahippocampal cortex as well as the fusiform gyrus as found to be activated in our previous study (Sommer et al. 2005), the lateral occipital complex (Epstein et al. 2003), an area involved in object recognition, the lingual gyrus as part of the visual pathway (Menon et al. 2000), and the anterior MTL as reported by a previous neuroimaging study on object-location associations (Owen et al. 1996).
Interaction
To identify domain-specific areas involved only in successful encoding of objects or locations, a combined second-level analysis with the 10 mean contrast images of each subject (five for each retrieval cue type) was performed. Statistical interactions using the above-described contrasts of interests were used [(1.60 0.20 -0.27 -0.50 -1.03 -1.60 -0.20 0.27 0.50 1.03) and (-1.60 -0.20 0.27 0.50 1.03 1.60 0.20 -0.27 -0.50 -1.03)], and the volumes of interests were defined as described above.
Conjunction
To identify general areas involved in successful encoding of objects and locations, a conjunction analysis as described by Nichols et al. (2005) was conducted in the combined second-level analysis. Volumes of interest were defined as described above. Each event of the encoding phase enters the model twice: for the spatial cue and for the object cue retrieval. In the case of the conjunction analysis, this could potentially lead to an exaggeration of the overlapping activity patterns. To validate the results of the conjunction analysis, we therefore conducted additional analyses in which each encoding event was modeled only once, characterized by the memory performance in the first retrieval occasion.
Acknowledgments
This work was supported by the Volkswagen-Stiftung (T.S., C.B., M.R., T.W.), Deutsche Studienstiftung (J.G.), DFG and BMBF. We thank the Physics and Methods group at NeuroImage Nord for help with MR scanning and A. McNamara for helpful comments.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.90405.
References
- Abrahams, S., Pickering, A., Polkey, C.E., and Morris, R.G. 1997. Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia 35: 11-24. [DOI] [PubMed] [Google Scholar]
- Aguirre, G.K. and D'Esposito, M. 1999. Topographical disorientation: A synthesis and taxonomy. Brain 122: 1613-1628. [DOI] [PubMed] [Google Scholar]
- Aguirre, G.K., Detre, J.A., Alsop, D.C., and D'Esposito, M. 1996. The parahippocampus subserves topographical learning in man. Cereb. Cortex 6: 823-829. [DOI] [PubMed] [Google Scholar]
- Astur, R.S., Taylor, L.B., Mamelak, A.N., Philpott, L., and Sutherland, R.J. 2002. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav. Brain Res. 132: 77-84. [DOI] [PubMed] [Google Scholar]
- Bohbot, V.D., Allen, J.J., and Nadel, L. 2000. Memory deficits characterized by patterns of lesions to the hippocampus and parahippocampal cortex. Ann. NY Acad. Sci. 911: 355-368. [DOI] [PubMed] [Google Scholar]
- Broadbent, N.J., Squire, L.R., and Clark, R.E. 2004. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. 101: 14515-14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, N., Maguire, E.A., and O'Keefe, J. 2002. The human hippocampus and spatial and episodic memory. Neuron 35: 625-641. [DOI] [PubMed] [Google Scholar]
- Burwell, R.D., Saddoris, M.P., Bucci, D.J., and Wiig, K.A. 2004. Corticohippocampal contributions to spatial and contextual learning. J. Neurosci. 24: 3826-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino, S., Maquet, P., Dolan, R.J., and Rugg, M.D. 2002. Brain activity underlying encoding and retrieval of source memory. Cereb. Cortex 12: 1048-1056. [DOI] [PubMed] [Google Scholar]
- Davachi, L., Mitchell, J.P., and Wagner, A.D. 2003. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. 100: 2157-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins, I.G., Rice, H.J., Wagner, A.D., and Schacter, D.L. 2003. Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia 41: 318-333. [DOI] [PubMed] [Google Scholar]
- Duzel, E., Habib, R., Rotte, M., Guderian, S., Tulving, E., and Heinze, H.J. 2003. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J. Neurosci. 23: 9439-9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum, H. 2000. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 1: 41-50. [DOI] [PubMed] [Google Scholar]
- Eldridge, L.L., Knowlton, B.J., Furmanski, C.S., Bookheimer, S.Y., and Engel, S.A. 2000. Remembering episodes: A selective role for the hippocampus during retrieval. Nat. Neurosci. 3: 1149-1152. [DOI] [PubMed] [Google Scholar]
- Epstein, R., Graham, K.S., and Downing, P.E. 2003. Viewpoint-specific scene representations in human parahippocampal cortex. Neuron 37: 865-876. [DOI] [PubMed] [Google Scholar]
- Fletcher, P.C. and Henson, R.N. 2001. Frontal lobes and human memory: Insights from functional neuroimaging. Brain 124: 849-881. [DOI] [PubMed] [Google Scholar]
- Fyhn, M., Molden, S., Witter, M.P., Moser, E.I., and Moser, M.B. 2004. Spatial representation in the entorhinal cortex. Science 305: 1258-1264. [DOI] [PubMed] [Google Scholar]
- Genzel, S., Kerkhoff, G., and Scheffter, S. 1995. PC-gestützte Standardisierung des Bildmaterials von Snodgrass und Vanderwart (1980). Neurolinguistik 9: 41-53. [Google Scholar]
- Hampton, R.R., Hampstead, B.M., and Murray, E.A. 2004. Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus 14: 808. [DOI] [PubMed] [Google Scholar]
- Henke, K., Buck, A., Weber, B., and Wieser, H.G. 1997. Human hippocampus establishes associations in memory. Hippocampus 7: 249-256. [DOI] [PubMed] [Google Scholar]
- Henke, K., Weber, B., Kneifel, S., Wieser, H.G., and Buck, A. 1999. Human hippocampus associates information in memory. Proc. Natl. Acad. Sci. 96: 5884-5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson, R.N., Cansino, S., Herron, J.E., Robb, W.G., and Rugg, M.D. 2003. A familiarity signal in human anterior medial temporal cortex? Hippocampus 13: 301-304. [DOI] [PubMed] [Google Scholar]
- Hockley, W.E. and Murdock, B.B. 1987. A decision model for accuracy and response latency in recognition memory. Psychol. Rev. 94: 341-358. [Google Scholar]
- Humphreys, M.S. and Bowyer, P.A. 1980. Sequential testing effects and the relationship between recognition and recognition failure. Mem. Cognit. 8: 271-277. [DOI] [PubMed] [Google Scholar]
- Jackson III, O. and Schacter, D.L. 2004. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage 21: 456-462. [DOI] [PubMed] [Google Scholar]
- Janzen, G. and van Turennout, M. 2004. Selective neural representation of objects relevant for navigation. Nat. Neurosci. 7: 673-677. [DOI] [PubMed] [Google Scholar]
- Jenkins, T.A., Amin, E., Pearce, J.M., Brown, M.W., and Aggleton, J.P. 2004. Novel spatial arrangements of familiar visual stimuli promote activity in the rat hippocampal formation but not the parahippocampal cortices: A c-fos expression study. Neuroscience 124: 43-52. [DOI] [PubMed] [Google Scholar]
- Kirchhoff, B.A., Wagner, A.D., Maril, A., and Stern, C.E. 2000. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J. Neurosci. 20: 6173-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan, C.B. and Stark, C.E. 2004. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus 14: 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler, S., Moscovitch, M., and Melo, B. 2001. Episodic memory for object location versus episodic memory for object identity: Do they rely on distinct encoding processes? Mem. Cognit. 29: 948-959. [DOI] [PubMed] [Google Scholar]
- Kohler, S., Crane, J., and Milner, B. 2002. Differential contributions of the parahippocampal place area and the anterior hippocampus to human memory for scenes. Hippocampus 12: 718-723. [DOI] [PubMed] [Google Scholar]
- Koppell, S. 1977. Decision latencies in recognition memory: A signal detection theory analysis. J. Exp. Psychol. Hum. Learn. Mem. 3: 445-457. [Google Scholar]
- Krause, B.J., Horwitz, B., Taylor, J.G., Schmidt, D., Mottaghy, F.M., Herzog, H., Halsband, U., and Muller-Gartner, H. 1999. Network analysis in episodic encoding and retrieval of word-pair associates: A PET study. Eur. J. Neurosci. 11: 3293-3301. [DOI] [PubMed] [Google Scholar]
- Leutgeb, S., Leutgeb, J.K., Treves, A., Moser, M.B., and Moser, E.I. 2004. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science 305: 1295-1298. [DOI] [PubMed] [Google Scholar]
- Ludvig, N., Tang, H.M., Gohil, B.C., and Botero, J.M. 2004. Detecting location-specific neuronal firing rate increases in the hippocampus of freely-moving monkeys. Brain Res. 1014: 97-109. [DOI] [PubMed] [Google Scholar]
- Maguire, E.A., Frith, C.D., Burgess, N., Donnett, J.G., and O'Keefe, J. 1998. Knowing where things are: Parahippocampal involvement in encoding object locations in virtual large-scale space. J. Cogn. Neurosci. 10: 61-76. [DOI] [PubMed] [Google Scholar]
- Maguire, E.A., Burgess, N., and O'Keefe, J. 1999. Human spatial navigation: Cognitive maps, sexual dimorphism, and neural substrates. Curr. Opin. Neurobiol. 9: 171-177. [DOI] [PubMed] [Google Scholar]
- McNicol, D. and Stewart, G.W. 1980. Reaction times and the study of memory. In Reaction Times (ed. A.T. Welford), pp. 253-308. Academic Press, London.
- Menon, V., White, C.D., Eliez, S., Glover, G.H., and Reiss, A.L. 2000. Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Hum. Brain Map. 11: 117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom, A.M., Good, C.D., Frackowiak, R.S., and Rugg, M.D. 2003. Age effects on the neural correlates of successful memory encoding. Brain 126: 213-229. [DOI] [PubMed] [Google Scholar]
- Moscovitch, C., Kapur, S., Kohler, S., and Houle, S. 1995. Distinct neural correlates of visual long-term memory for spatial location and object identity: A positron emission tomography study in humans. Proc. Natl. Acad. Sci. 92: 3721-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, B.B. and Dufty, P.O. 1972. Strength theory and recognition memory. J. Exp. Psychol. 94: 284-290. [Google Scholar]
- Nichols, T., Brett, M., Andersson, J., Wager, T., and Poline, J.B. 2005. Valid conjunction inference with the minimum statistic. NeuroImage (in press). [DOI] [PubMed]
- Nunn, J.A., Graydon, F.J., Polkey, C.E., and Morris, R.G. 1999. Differential spatial memory impairment after right temporal lobectomy demonstrated using temporal titration. Brain 122: 47-59. [DOI] [PubMed] [Google Scholar]
- O'Keefe, J. and Nadel, L. 1978. The hippocampus as a cognitive map. Oxford University Press, Oxford, UK.
- Otten, L.J. and Rugg, M.D. 2001. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb. Cortex 11: 1150-1160. [DOI] [PubMed] [Google Scholar]
- Owen, A.M., Milner, B., Petrides, M., and Evans, A.C. 1996. A specific role of the right parahippocampal gyrus in the retrieval of object-location: A positron emission tomography study. J. Cogn. Neurosci. 8: 588-602. [DOI] [PubMed] [Google Scholar]
- Pigott, S. and Milner, B. 1993. Memory for different aspects of complex visual scenes after unilateral temporal- or frontal-lobe resection. Neuropsychologia 31: 1-15. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki, M., Tanila, H., Kononen, M., Hanninen, T., Hamalainen, A., Soininen, H., and Aronen, H.J. 2004. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur. J. Neurosci. 19: 1939-1949. [DOI] [PubMed] [Google Scholar]
- Preston, A.R., Shrager, Y., Dudukovic, N.M., and Gabrieli, J.D. 2004. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus 14: 148-152. [DOI] [PubMed] [Google Scholar]
- Ranganath, C., Yonelinas, A.P., Cohen, M.X., Dy, C.J., Tom, S.M., and D'Esposito, M. 2004. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42: 2-13. [DOI] [PubMed] [Google Scholar]
- Reber, P.J., Wong, E.C., and Buxton, R.B. 2002. Encoding activity in the medial temporal lobe examined with anatomically constrained fMRI analysis. Hippocampus 12: 363-376. [DOI] [PubMed] [Google Scholar]
- Rombouts, S.A., Machielsen, W.C., Witter, M.P., Barkhof, F., Lindeboom, J., and Scheltens, P. 1997. Visual association encoding activates the medial temporal lobe: A functional magnetic resonance imaging study. Hippocampus 7: 594-601. [DOI] [PubMed] [Google Scholar]
- Rombouts, S.A., Scheltens, P., Machielson, W.C., Barkhof, F., Hoogenraad, F.G., Veltman, D.J., Valk, J., and Witter, M.P. 1999. Parametric fMRI analysis of visual encoding in the human medial temporal lobe. Hippocampus 9: 637-643. [DOI] [PubMed] [Google Scholar]
- Rugg, M.D. and Yonelinas, A.P. 2003. Human recognition memory: A cognitive neuroscience perspective. Trends Cogn. Sci. 7: 313-319. [DOI] [PubMed] [Google Scholar]
- Rugg, M.D., Schloerscheidt, A.M., and Mark, R.E. 1998. An electrophysiological comparison of two indices of recollection. J. Mem. Lang. 39: 47-69. [Google Scholar]
- Schacter, D.L. and Wagner, A.D. 1999. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9: 7-24. [DOI] [PubMed] [Google Scholar]
- Small, S.A., Nava, A.S., Perera, G.M., DeLaPaz, R., Mayeux, R., and Stern, Y. 2001. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nat. Neurosci. 4: 442-449. [DOI] [PubMed] [Google Scholar]
- Smith, M.L. and Milner, B. 1981. The role of the right hippocampus in the recall of spatial location. Neuropsychologia 19: 781-793. [DOI] [PubMed] [Google Scholar]
- ____. 1989. Right hippocampal impairment in the recall of spatial location: Encoding deficit or rapid forgetting? Neuropsychologia 27: 71-81. [DOI] [PubMed] [Google Scholar]
- Snodgrass, J.G. and Corwin, J. 1988. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J. Exp. Psychol. Gen. 117: 34-50. [DOI] [PubMed] [Google Scholar]
- Snodgrass, J.G. and Vanderwart, M. 1980. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. Hum. Learn. 6: 174-215. [DOI] [PubMed] [Google Scholar]
- Sommer, T., Rose, M., Weiller, C., and Büchel C. 2005. Contributions of occipital, parietal and parahippocampal cortex to encoding of object-location associations. Neuropsychologia 43: 732-743. [DOI] [PubMed] [Google Scholar]
- Sperling, R., Chua, E., Cocchiarella, A., Rand-Giovannetti, E., Poldrack, R., Schacter, D.L., and Albert, M. 2003. Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage 20: 1400-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire, L.R., Stark, C.E., and Clark, R.E. 2004. The medial temporal lobe. Annu. Rev. Neurosci. 27: 279-306. [DOI] [PubMed] [Google Scholar]
- Stark, C.E. and Squire, L.R. 2000. Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. J. Neurosci. 20: 7776-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 2001a. Simple and associative recognition memory in the hippocampal region. Learn. Mem. 8: 190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 2001b. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proc. Natl. Acad. Sci. 98: 12760-12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepankova, K., Fenton, A.A., Pastalkova, E., Kalina, M., and Bohbot, V.D. 2004. Object-location memory impairment in patients with thermal lesions to the right or left hippocampus. Neuropsychologia 42: 1017-1028. [DOI] [PubMed] [Google Scholar]
- Strange, B.A., Otten, L.J., Josephs, O., Rugg, M.D., and Dolan, R.J. 2002. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J. Neurosci. 22: 523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, W.A., Miller, E.K., and Desimone, R. 1997. Object and place memory in the macaque entorhinal cortex. J. Neurophysiol. 78: 1062-1081. [DOI] [PubMed] [Google Scholar]
- Tulving, E. 1983. Elements of episodic memory. Oxford University Press, New York.
- Witter, M.P., Van Hoesen, G.W., and Amaral, D.G. 1989. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J. Neurosci. 9: 216-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas, A.P. 1999. The contribution of recollection and familiarity to recognition and source-memory judgments: A formal dual-process model and an analysis of receiver operating characteristics. J. Exp. Psychol. Learn. Mem. Cogn. 25: 1415-1434. [DOI] [PubMed] [Google Scholar]
- ____. 2001. Components of episodic memory: The contribution of recollection and familiarity. Philos. Trans. R Soc. Lond. B Biol. Sci. 356: 1363-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh, M.M., Engel, S.A., Thompson, P.M., and Bookheimer, S.Y. 2003. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science 299: 577-580. [DOI] [PubMed] [Google Scholar]