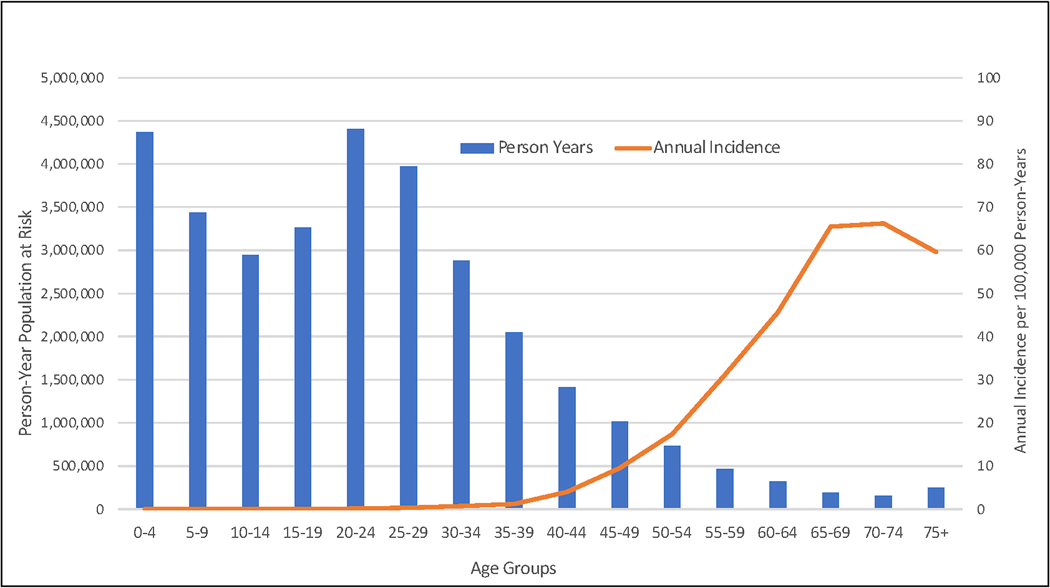
We read Arnold et al’s article[1] with interest and wanted to extend this work to Africa. High incidence of esophageal squamous cell carcinoma (ESCC) has been reported in African countries from Kenya to South Africa[2] where early-onset (age < 45 years) ESCC accounts for 30% of cases in the region. As such, we examined the cumulative lifetime and age-specific incidences of ESCC in Africa. We identified patients with ESCC in 3 major African cancer registries, using the 2013–2017 Cancer Incidence in Five Continents Volume XII (CI5-XII) database and calculated the age-specific, cumulative lifetime, and cumulative early-onset incidence of early-onset ESCC, all of which will allow us to account for the underlying age structure of the population.
We identified 1,022 patients diagnosed with ESCC between 2013 and 2017, of which 47.2% (n=482) were females and 12.3% (n=126) were < 45 years old consistent with early-onset ESCC (Figure 1). Region-level proportions of early-onset ESCC cases varied from 7.6% (26/340) in Eastern Cape, South Africa, to 15.6% (60/384) in Nairobi, Kenya. The cumulative lifetime incidence and cumulative incidence[3] of early-onset ESCC were 1,508 and 33 cases per 100,000 person-year, respectively, and early-onset ESCC only accounted for 2.2% of cumulative lifetime incidence (Table 1 and Supplemental Table 1). We performed similar analyses with data from the United States and the United Kingdom – England; early onset ESCC was 1.5% and 1.1% of all cases, respectively[3]. The associated early onset proportions of cumulative incidences were 0.39% and 0.28%, respectively (Supplemental Table 2). See supplemental materials for further details about methods and limitations.
Figure 1.
Histogram graph showing person year population at risk overlapped by annual incidence of ESCC per 100,000 per years
Table 1:
Demographics and Incidence of ESCC in 3 Major African Cancer Registries between 2013 and 2017
| Combined | Nairobi, Kenya | Eastern Cape, South Africa | Kyadondo, Uganda | |
|---|---|---|---|---|
| Number of cases | 1022 | 384 | 340 | 298 |
| Females, n (%) | 482 (47.2%) | 170 (44.3%) | 196 (57.6%) | 116 (38.9%) |
| Age group, n (%) | - | - | - | - |
| 0 – 14 | <5*** | 0 (0%) | <5*** | 0 (0%) |
| 15–29 | 21 (2.1%) | 9 (2.3%) | 9 (2.6%) | <5*** |
| 30–44 | 104 (10.2%) | 51 (13.3%) | 16 (4.7%) | 37 (12.4%) |
| 45–59 | 372 (36.4%) | 132 (34.4%) | 110 (32.4%) | 130 (43.6%) |
| 60–74 | 375 (36.7%) | 142 (37.0%) | 141 (41.5%) | 92 (30.9%) |
| 75=> | 149 (14.6%) | 50 (13.0%) | 63 (18.5%) | 36 (12.1%) |
| Early Onset, n (%) | 126 (12.3%) | 60 (15.6%) | 26 (7.6%) | 40 (13.4%) |
| Cumulative Lifetime Incidence (per 100,000 person-years) * | 1,508 | 1,560 | 1,805 | 1343 |
| Cumulative Early Onset Incidence (per 100,000 person-years) * | 33 | 27 | 82 | 33 |
| Early Onset Proportion of Cumulative Incidence ** | 2.2% | 1.7% | 4.5% | 2.4% |
The cumulative incidence is the sum of the age-specific incidence over each year of age from birth to a defined upper age limit.
The early onset disease proportion of cumulative incidence = cumulative incidence of early onset / cumulative lifetime incidence x 100
Unreported, cells where the number of cases ranged from 1 to 4 were represented as <5 to ensure privacy.
Similar to previous studies[2, 4–6], we report a high proportion of early-onset ESCC in East Africa. However, since early-onset ESCC only accounted for 2.2% of the cumulative lifetime incidence of ESCC, suggesting a reflection of the young age structure of the African population, rather than a higher age-specific incidence. In contrast, early-onset testicular cancer, for example, represents 53.0% of the cumulative lifetime incidence of testicular cancer worldwide[7]. Our results suggest that as life-expectancy increases in Africa, the incidence of ESCC could be expected to rise as individuals who may have avoided other causes of death develop ESCC.
While published studies in Africa report a substantial incidence of early-onset ESCC[8, 9], they did not account for the underlying age structure of the studied population, which can further inform ESCC epidemiology. Further, most published studies of ESCC in Africa report frequency and proportions of ESCC without accounting for the underlying population by age. For example, Dawsey et al. documented a case series where they identified 109 patients who were 30 years of age or younger and diagnosed with esophageal cancer (98% with ESCC) at Tenwek Hospital in Kenya. The youngest individual in the study was 14 years old[9]. Mmbaga et al. reported a case series of 738 patients treated for ESCC in Muhimbili National Hospital in Tanzania, the authors reported that that 13% of cases were ≤ 40 years old at the time of diagnosis[6]. Asombang et al. investigated the age-specific incidence of esophageal cancers, including ESCC and esophageal adenocarcinoma, in Kenya, South Africa, and Zimbabwe, reporting a lower incidence in younger age groups compared to older age groups[10].
Several risk factors for ESCC have also been identified. Buckle et al. identified risk factors uniquely associated with early-onset ESCC, including irregular dental hygiene, exposure to secondhand tobacco smoke, and infestation of grain/nuts by pests. In comparison, lower socioeconomic status, a family history of ESCC, tobacco smoking, consumption of home-brewed alcohol, storage of grain/nuts at home, and the use of firewood for cooking were associated with ESCC, but not with early-onset ESCC[5].
In conclusion, while early-onset ESCC is common in Africa, the cumulative incidence of early-onset ESCC is minimal compared to the total cumulative incidence of ESCC. This is an important epidemiological finding to inform resource allocation, research, treatment and novel approaches to care for cancers[11] including, ESCC in Africa. Future studies are warranted to provide more detailed insights into the complex interplay between demographic factors, risk exposure, and the incidence of early-onset ESCC. This will inform more targeted interventions and enhance resource allocation for the research and treatment of ESCC in Africa.
Supplementary Material
Acknowledgments
Funding:
Mohamed Noureldin is supported by NIDDK T32 DK062708
Research reported in this publication was supported by the Office Of The Director, National Institutes Of Health (OD), the National Institute Of Biomedical Imaging And Bioengineering (NIBIB), the National Institute Of Mental Health (NIMH), and the Fogarty International Center (FIC) of the National Institutes of Health under award number U54TW012089 (Abubakar A and Waljee AK)
Footnotes
Disclosures:
AKW is a member of the Gut Editorial Board. All other authors report no disclosures.
Data Availability:
The data for this study is publicly available at: https://ci5.iarc.fr/ci5-xii/download
References
- [1].Arnold M. et al. , “International variation in oesophageal and gastric cancer survival 2012–2014: differences by histological subtype and stage at diagnosis (an ICBP SURVMARK-2 population-based study),” Gut, vol. 71, no. 8, pp. 1532–1543, Aug 2022, doi: 10.1136/gutjnl-2021-325266. [DOI] [PubMed] [Google Scholar]
- [2].Wakhisi J, Patel K, Buziba N, and Rotich J, “Esophageal cancer in north rift valley of Western Kenya,” (in eng), Afr Health Sci, vol. 5, no. 2, pp. 157–63, Jun 2005. [PMC free article] [PubMed] [Google Scholar]
- [3].Bray F FJ, “Cancer Incidence in Five Continents. Chapter 7: Age standardization,” Lyon: International Agency for Research on Cancer, 2017, vol. Vol. XI. Accessed: 9/26/2023. [Online]. Available: https://ci5.iarc.fr/CI5-XI/PDF/Chapter%207.pdf [Google Scholar]
- [4].Cheng ML et al. , “The incidence of oesophageal cancer in Eastern Africa: identification of a new geographic hot spot?,” (in eng), Cancer Epidemiol, vol. 39, no. 2, pp. 143–9, Apr 2015, doi: 10.1016/j.canep.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buckle GC et al. , “Risk Factors Associated With Early-Onset Esophageal Cancer in Tanzania,” (in eng), JCO Glob Oncol, vol. 8, pp. 1–16, Feb 2022, doi: 10.1200/go.21.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mmbaga EJ et al. , “Characteristics of Esophageal Cancer Cases in Tanzania,” Journal of Global Oncology, no. 4, pp. 1–10, 2018, doi: 10.1200/jgo.2016.006619. [DOI] [PMC free article] [PubMed]
- [7].Ferlay J EM, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F, “Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer,” pp. 1–2, 2020. [Online]. Available: https://gco.iarc.fr/today. [Google Scholar]
- [8].White RE, Abnet CC, Mungatana CK, and Dawsey SM, “Oesophageal cancer: a common malignancy in young people of Bomet District, Kenya,” The Lancet, vol. 360, no. 9331, pp. 462–463, 2002/August/10/ 2002, doi: 10.1016/S0140-6736(02)09639-3. [DOI] [PubMed] [Google Scholar]
- [9].Dawsey SP et al. , “Esophageal cancer in young people: a case series of 109 cases and review of the literature,” (in eng), PLoS One, vol. 5, no. 11, p. e14080, Nov 22 2010, doi: 10.1371/journal.pone.0014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Asombang AW et al. , “Systematic review and meta-analysis of esophageal cancer in Africa: Epidemiology, risk factors, management and outcomes,” (in eng), World J Gastroenterol, vol. 25, no. 31, pp. 4512–4533, Aug 21 2019, doi: 10.3748/wjg.v25.i31.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Waljee AK et al. , “Artificial intelligence and machine learning for early detection and diagnosis of colorectal cancer in sub-Saharan Africa,” Gut, vol. 71, no. 7, pp. 1259–1265, Jul 2022, doi: 10.1136/gutjnl-2022-327211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this study is publicly available at: https://ci5.iarc.fr/ci5-xii/download