Abstract
Some echoviruses (EV) that bind decay-accelerating factor (DAF) also bind cells of human and murine origins in a DAF-independent manner. Pretreatment of cells with heparinase 1 or heparin blocks the binding of radiolabeled virus to the cell surface, and heparin prevents infection of rhabdomyosarcoma cells by certain EV, including several low-passage clinical isolates of EV 6 and some EV that do not bind DAF. These studies suggest that heparan sulfate may be of in vivo relevance as an attachment molecule for EV.
Hemagglutination by picornaviruses of the genus Enterovirus is mediated by binding to decay-accelerating factor (DAF; CD55), a 70-kDa glycosylphosphatidylinositol-linked glycoprotein that is expressed on serum-exposed cells and that is involved in complement regulation and cell signaling (1, 14, 20, 22, 26, 30). The affinity of echovirus (EV) 11 (EV11) for DAF has previously been determined (15), although hemagglutination inhibition assays and the use of soluble DAF (sDAF) to block infection of rhabdomyosarcoma (RD) cells have suggested that DAF-binding enteroviruses exhibit a range of affinities for this receptor (20). Further studies also have suggested that certain enteroviruses require additional cell surface proteins for infection (2, 21, 27, 28).
It has been demonstrated that EV6, derived from the D'Amori type strain by serial passaging in RD cells, is a weakly hemagglutinating, DAF-binding virus (20). Cell infection required cell surface DAF and was blocked by 15 μM sDAF, and a specific interaction with DAF was demonstrated by surface plasmon resonance (22). In contrast to a HeLa cell-passaged D'Amori-derived DAF-binding isolate reported by Bergelson et al. (1), which was found not to bind Chinese hamster ovary (CHO) cells, our EV6 strain bound murine NIH 3T3, CHO, and RD cells in a DAF-independent manner (20; R. M. Powell and D. J. Evans, unpublished results). This result implied that it might bind a widely expressed molecule conserved between humans and rodents. We considered the glycosaminoglycan (GAG) heparan sulfate (HS) a likely candidate, since it is present on the surface of most cells and is known to be bound by a number of viruses (5, 17, 29, 31), including the picornavirus foot-and-mouth disease virus (FMDV) (13).
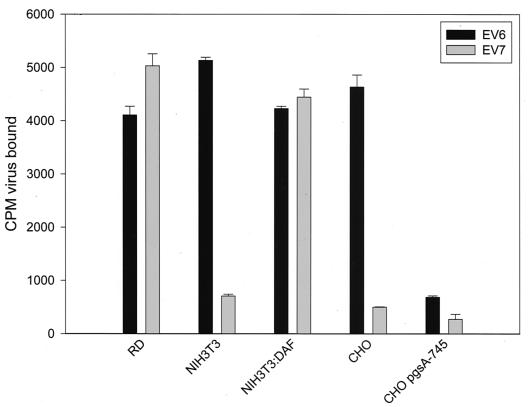
We investigated whether EV6 interacted with HS by quantifying the binding of 35S metabolically labeled EV6 particles (prepared and used as previously described [20]) to CHO pgsA-745 cell lines, which are deficient in GAG synthesis (25) (Fig. 1). EV6 was bound for 2 h at 4°C, and unbound virus was removed by washing with cold serum-free Dulbecco modified Eagle medium (DMEM). Next, cells were subjected to quantification by scintillation counting and comparison with similarly treated cells exposed to radiolabeled EV7, the binding of which is known to be DAF dependent (21, 30). As expected, EV7 bound RD cells but bound neither NIH 3T3 cells in the absence of DAF nor CHO cells (which do not express DAF). EV6 displayed a distinctly different cell interaction profile, binding NIH 3T3 cells in a DAF-independent manner (as previously shown [21]) and GAG-positive CHO cells but displaying only background levels of binding to CHO pgsA-745 cells (Fig. 1). This analysis indicated that EV6 binding to CHO cells and, by inference, to NIH 3T3 cells might involve GAGs.
FIG. 1.
Involvement of GAGs in the DAF-independent binding of EV6 to rodent cells. 35S-Cys-Met metabolically labeled EV6 or EV7 (10,000 counts) was bound to the indicated cell lines for 2 h at 4°C, unbound virus was removed by washing with cold serum-free DMEM, and retained virus was quantified by scintillation counting. The mean and standard deviation for three independent experiments are shown.
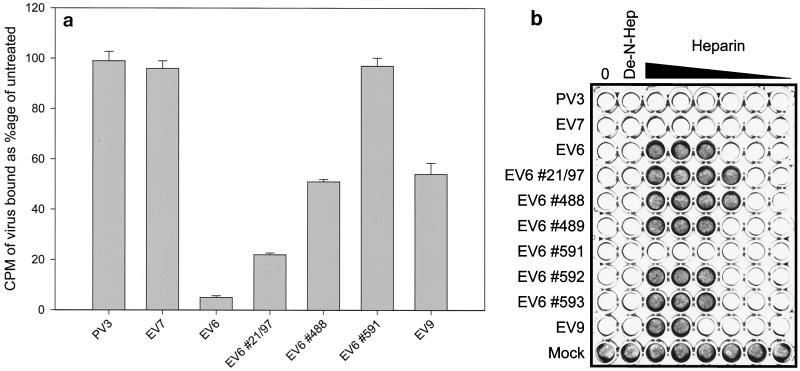
To further investigate a potential role for HS in EV attachment, we determined the effect of heparin—a highly sulfated analogue of cell surface HS—on virus binding to or infection of RD cells (Fig. 2). Heparin (porcine mucosal intestinal; Sigma) or de-N-sulfated heparin (Sigma) was prediluted in serum-free DMEM and mixed with 1,000 50% tissue culture infective doses (TCID50) of virus at room temperature for 15 min. Infection was monitored by adding the virus-heparin mixture to an 80% confluent layer of RD cells in a 96-well plate and incubating the plate for 24 h at 37°C in 5% CO2 prior to staining with crystal violet. The binding of radiolabeled viruses in the presence of heparin was determined as described above. The range of EV6 strains tested was extended to include several clinical isolates (kind gifts from Brian Megson, Public Health Laboratory Service, Colindale, London, England) that had been passaged no more than twice in RD cells since isolation. The Hill strain of EV9, a receptor for which has yet to be identified, and poliovirus type 3 (PV3) were also included in these assays.
FIG. 2.
Heparin inhibits the binding (a) and infection (b) of RD cells by EV6. (a) Binding of radiolabeled EV6, clinical isolates of EV6 (indicated by the number symbol), EV7, EV9, and PV3 to RD cells was monitored in the presence of 1 mg of heparin/ml. Results are presented as the percentage of virus bound in comparison to binding of the untreated virus control (mean and standard deviation). (b) The inhibitory effect of heparin on infection of RD cells was determined by preincubating 1,000 TCID50 of the indicated viruses with dilutions of heparin (doubling dilutions from 2 mg/ml to 62.5 ng/ml) for 15 min before addition to 80% confluent RD cells. Infection was determined by staining with crystal violet after 24 h. The row labeled “Mock” indicates the effect of heparin in the absence of virus; lanes labeled 0 and De-N-Hep contained no heparin or 1 mg of de-N-sulfated heparin/ml, respectively.
Radiolabeled EV6 binding to RD cells was reduced by 95% in the presence of 1 mg of heparin/ml (Fig. 2a), a result consistent with the observed block in infection observed with heparin concentrations above 0.5 mg/ml (Fig. 2b). In contrast, binding (Fig. 2a) or infection (Fig. 2b) by EV7, PV3, or EV6 clinical isolate #591 was unaffected by the presence of heparin at concentrations of up to 2 mg/ml. EV9 and the other EV6 clinical isolates displayed intermediate phenotypes. Binding by EV6 #21/97 and #488 and by EV9 was reduced by 50 to 75% in the presence of 1 mg of heparin/ml (Fig. 2a). For these clinical isolates, RD cell infection by #21/97 and #488 was more sensitive to inhibition by heparin, whereas the remaining clinical isolates were inhibited by heparin levels comparable to those that blocked wild-type EV6 infection (Fig. 2b). In all cases, de-N-sulfated heparin failed to block infection when added at 2 mg/ml, implying that the sulfation state of the heparin was critical for the observed block, as seen for other HS-binding viruses (3).
The DAF-binding phenotype of the viruses was confirmed by determining whether sDAF blocked infection of RD cells, as previously described (21). RD cell infection by 10,000 TCID50 of wild-type EV6 and the EV6 clinical isolates was blocked by 15 μM sDAF, whereas 3.25 μM sDAF was required to block infection by EV7 (data not shown). EV9 and PV3 do not bind DAF and were unaffected by the presence of sDAF in this assay.
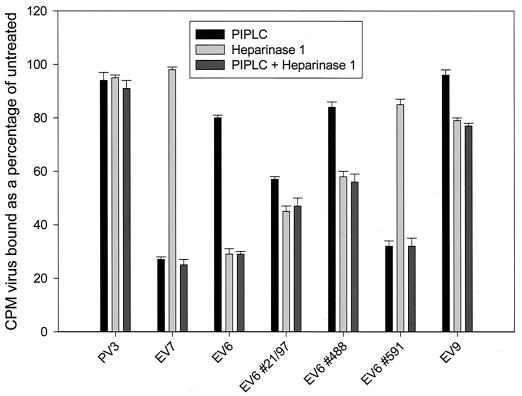
The enzymatic removal of HS by heparinase 1 and glycosylphosphatidylinositol-anchored proteins (including DAF) by phospholipase C (PIPLC) was used to further confirm a role for HS in EV binding and to quantify the relative contributions of HS and DAF to virus binding. RD cells were pretreated with heparinase 1 (Sigma) at 20 U/3.5 × 107 cells for 2 h at 31°C or PIPLC (used as described previously [6]). Cells were then tested for their ability to bind 35S-labeled virus particles as described previously (Fig. 3). Binding of the control PV3, which binds the transmembrane-anchored poliovirus receptor (16), was not reduced by PIPLC treatment, as expected; in addition, consistent with the data presented in Fig. 2, the binding of PV3 was not reduced by heparinase pretreatment. EV7 was similarly unaffected by heparinase treatment, but binding was significantly reduced following pretreatment with PIPLC, as expected for a DAF-binding virus. EV6 binding was reduced by over 70% with heparinase pretreatment but only slightly by PIPLC. These results reflect the weak and transient DAF-binding phenotype of this virus. The EV6 clinical isolate #591 was almost indistinguishable from EV7 in this assay; binding was DAF dependent and was reduced only marginally after the heparinase cleavage of HS, in agreement with the failure of heparin to block infection or binding to RD cells (Fig. 2). The remaining clinical isolates tested were intermediate in phenotype, being affected by both enzymatic treatments. EV9 binding was PIPLC resistant, as would be expected for a virus that does not bind DAF, and was reduced only slightly in the presence of heparinase. Under the conditions used, both heparinase and PIPLC were less efficient at blocking binding than heparin (Fig. 2a) or sDAF (data not shown). We ascribe this result to the incomplete removal of HS or DAF from the cell surface or the recycling of HS or DAF proteins in cells at the nearly physiological temperatures used in these assays.
FIG. 3.
Relative effects of heparinase 1 and PIPLC on enterovirus binding to RD cells. Metabolically labeled, gradient-purified virus particles (10,000 counts) were bound to RD cells following pretreatment of the cell monolayer with heparinase 1 or PIPLC. The results presented are the mean and standard deviation for three independent assays and are expressed as the percentage bound in comparison to binding to untreated RD cells.
These studies demonstrated that the genus Enterovirus of the family Picornaviridae contains representatives that bind the cell surface GAG HS. We extended these studies to determine the concentrations of heparin required to block RD cell infection by 1,000 TCID50 of a range of enteroviruses (Table 1). This experiment demonstrated that the phenotype is widespread within the human enterovirus B species and that there is no correlation with the ability of the virus to bind DAF. HS binding by FMDV is likely to be an adaptation to cell cultures (12, 13, 19). This is probably not the case for HS-binding EV; the majority of the isolates tested in the present study have been serially passaged in RD cells, and low-passage clinical isolates of EV6 can also exhibit this phenotype (Fig. 2a).
TABLE 1.
Heparin concentrations required to block infection
| Virusa | Heparin (μg/ml) required to block 1,000 TCID50 |
|---|---|
| 9, 25 | 125 |
| 2, 3, 5, 6, 15 | 500 |
| 30 | 1,000 |
| 19, 26 | 2,000 |
| 1, 4, 7, 12, 13, 14, 16, 20, 24, 27, 29, 31, 32, 33, PV3, CBV2, CBV3 | —b |
EV, unless otherwise indicated. CBV2 and CBV3, coxsackie B2 and B3 viruses, respectively. Boldfacing indicates DAF-binding viruses.
—, Insensitive to heparin at 2 mg/ml.
These observations suggest that HS binding by EV may be of in vivo relevance. It is possible that the ability to bind HS provides an additional means of cell association (as outlined in reference 10) that facilitates an interaction with the cell surface molecules that mediate virus entry. This situation resembles that seen for herpes simplex virus type 1, human immunodeficiency virus type 1, dengue virus, and adeno-associated virus (11, 17, 18, 23, 24). Characterized HS-binding motifs contain a small number of predominantly basic residues, and structural analysis of the FMDV-HS interface indicates that a limited number of electrostatic interactions are critical for binding (4, 7–9). This information suggests that the genotypic plasticity of picornaviruses could lead to the acquisition or loss of an HS-binding phenotype relatively rapidly. The in vivo relevance of HS binding remains to be determined and will require the further analysis of clinical virus isolates. Studies are under way to investigate the adaptation to HS binding of EV6 isolates such as #591 to provide a better molecular understanding of this phenotype.
Acknowledgments
These studies were supported by Medical Research Council grants G9006199 and G9901250. A.B.S. was funded by the Ministry of Culture and Higher Education, Tabriz, Iran.
REFERENCES
- 1.Bergelson J M, Chan M, Solomon K R, St. John N F, Lin H, Finberg R W. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci USA. 1994;91:6245–6249. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeois C, Bour J B, Lidholt K, Gauthray C, Pothier P. Heparin-like structures on respiratory syncytial virus are involved in its infectivity in vitro. J Virol. 1998;72:7221–7227. doi: 10.1128/jvi.72.9.7221-7227.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardin A D, Weintraub H J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y P, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 6.Davitz A M, Low M G, Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell-membrane by phosphatidylinositol-specific phospholipase-c (PIPLC)-selective modification of a complement regulatory protein. J Exp Med. 1986;163:1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman S A, Audet S, Beeler J A. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J Virol. 2000;74:6442–6447. doi: 10.1128/jvi.74.14.6442-6447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman S A, Hendry R M, Beeler J A. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virol. 1999;73:6610–6617. doi: 10.1128/jvi.73.8.6610-6617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fry E E, Lea S M, Jackson T, Newman J W, Ellard F M, Blakemore W E, Abu-Ghazaleh R, Samuel A, King A M, Stuart D I. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. EMBO J. 1999;18:543–554. doi: 10.1093/emboj/18.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung S L, Lee P L, Chen H W, Chen L K, Kao C L, King C C. Analysis of the steps involved in dengue virus entry into host cells. Virology. 1999;257:156–167. doi: 10.1006/viro.1999.9633. [DOI] [PubMed] [Google Scholar]
- 12.Jackson T, Blakemore W, Newman J W, Knowles N J, Mould A P, Humphries M J, King A M. Foot-and-mouth disease virus is a ligand for the high-affinity binding conformation of integrin alpha5beta1: influence of the leucine residue within the RGDL motif on selectivity of integrin binding. J Gen Virol. 2000;81:1383–1391. doi: 10.1099/0022-1317-81-5-1383. [DOI] [PubMed] [Google Scholar]
- 13.Jackson T, Ellard F M, Ghazaleh R A, Brookes S M, Blakemore W E, Corteyn A H, Stuart D I, Newman J W, King A M Q. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J Virol. 1996;70:5282–5287. doi: 10.1128/jvi.70.8.5282-5287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnauchow T M, Tolson D L, Harrison B A, Altman E, Lublin D M, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55) J Virol. 1996;70:5143–5152. doi: 10.1128/jvi.70.8.5143-5152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lea S, Powell R, McKee T, Evans D, Brown D, Stuart D, van der Merwe P. Determination of the affinity and kinetic constants for the interaction between the human echovirus 11 and its cellular receptor CD55. J Biol Chem. 1998;273:30443–30447. doi: 10.1074/jbc.273.46.30443. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 17.Mondor I, Ugolini S, Sattentau Q J. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J Virol. 1998;72:3623–3634. doi: 10.1128/jvi.72.5.3623-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 19.Neff S, Sa-Carvalho D, Rieder E, Mason P W, Blystone S D, Brown E J, Baxt B. Foot-and-mouth disease virus virulent for cattle utilizes the integrin alpha(v)beta3 as its receptor. J Virol. 1998;72:3587–3594. doi: 10.1128/jvi.72.5.3587-3594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell R M, Schmitt V, Ward T, Goodfellow I, Evans D J, Almond J W. Characterization of echoviruses that bind decay accelerating factor (CD55): evidence that some haemagglutinating strains use more than one cellular receptor. J Gen Virol. 1998;79:1707–1713. doi: 10.1099/0022-1317-79-7-1707. [DOI] [PubMed] [Google Scholar]
- 21.Powell R M, Ward T, Evans D J, Almond J W. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J Virol. 1997;71:9306–9312. doi: 10.1128/jvi.71.12.9306-9312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell R M, Ward T, Goodfellow I, Almond J W, Evans D J. Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enteroviruses: implications for the evolution of a DAF-binding phenotype. J Gen Virol. 1999;80:3145–3152. doi: 10.1099/0022-1317-80-12-3145. [DOI] [PubMed] [Google Scholar]
- 23.Putnak J R, Kanesa-Thasan N, Innis B L. A putative cellular receptor for dengue viruses. Nat Med. 1997;3:828–829. doi: 10.1038/nm0897-828. [DOI] [PubMed] [Google Scholar]
- 24.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 25.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafren D R, Bates R C, Agrez M V, Herd R L, Burns G F, Barry R D. Coxsackieviruses B1, B3, and B5 use decay-accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3877. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafren D R, Dorahy D J, Ingham R A, Burns G F, Barry R D. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J Virol. 1997;71:4736–4743. doi: 10.1128/jvi.71.6.4736-4743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shafren D R, Williams D T, Barry R D. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J Virol. 1997;71:9844–9848. doi: 10.1128/jvi.71.12.9844-9848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward T, Pipkin P A, Clarkson N A, Stone D M, Minor P D, Almond J W. Decay accelerating factor (CD55) identified as the receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method. EMBO J. 1994;13:5070–5074. doi: 10.1002/j.1460-2075.1994.tb06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wudunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]