Abstract
Acetylcholine is the main neurotransmitter at the mammalian neuromuscular junction (NMJ) where nicotinic acetylcholine receptors mediate the signaling between nerve terminals and muscle fibers. We show that under glutamatergic transmission, rat NMJ switches from cholinergic type synapse to glutamatergic synapse. Connecting skeletal muscle to the lateral white matter of the spinal cord by grafting the distal stump of the transected motor nerve produced functional muscle reinnervation. The restored neuromuscular activity became resistant to common curare blockers but sensitive to the glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist. Analysis of the regenerated nerve disclosed new glutamatergic axons and the disappearance of cholinergic fibers. Many axons belonged to the supraspinal neurons located in the red nucleus and the brainstem nuclei. Finally, the innervated muscle displayed high expression and clustering of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunits glutamate receptors 1 and 2. Our data suggest that supraspinal neurons can target skeletal muscle, which retains the plasticity to generate functional glutamatergic NMJ.
Keywords: glutamate, neuromuscular junction, red nucleus
Traumatic paraplegia caused by spinal cord injury is still an irreversible condition. So far, there is no medical or surgical treatment capable of curing paraplegia. The CNS is “nonpermissive” for the advancement of injured axons because of an abundance of growth-inhibitory molecules in the myelin and the glial scar (1-3). In addition, there are no growth-promoting factors at the neuronal growth cone or at the somata. However, when peripheral nerves (PN) are directly grafted into the CNS, central axons can progress throughout the peripheral endoneural tubes, suggesting they can regenerate in an appropriate environment (4-7). The central fibers that are diverted into a nerve graft implanted within a healthy structure derive from neurons axotomized during the grafting procedure and not from uninjured neurons spared by nerve graft implantation (8). Regrowth ceases as soon as axons contact the CNS milieu again. Various studies have demonstrated that central axons can elongate within autologous PN grafted into the spinal cord and form functional synapses with skeletal muscles, leading to motor and sensory recovery (7, 9-11). Spinal cord neurons, as well as the midbrain and brainstem neurons that originate the rubrospinal, vestibulospinal, and reticulospinal tracts, are endowed with a high capability of axonal regeneration into PN transplants (12-15). Thus, in an attempt to bypass a spinal cord lesion by connecting descending motor fibers with skeletal muscles, muscular nerve branches were inserted into the severed lateral bundle of monkey spinal cord (11). The new connection produced muscle reinnervation and restored motor function. This result raised the possibility that the regrowth of axons descending from central noncholinergic neurons and cut during the grafting procedure could be responsible for functional muscle reinnervation. Here we tested this hypothesis by connecting the distal stump of a transected rat motor nerve with lateral white matter. An autologous sural nerve graft was implanted into the acutely severed lateral white matter of the spinal cord and connected to the transected nerve of the internal obliquus abdominis muscle. We found the grafting of the motor nerve into the lateral bundle of the spinal cord led to a new glutamatergic innervation of skeletal muscle, replacing the original cholinergic one (16). By electrophysiological, molecular, and immunohistochemical analysis, we show that reinnervated muscles were reprogrammed by supraspinal neurons to organize functional glutamatergic neuromuscular junctions (NMJ).
Materials and Methods
Surgical Procedure. Experiments were performed on 30 adult male Sprague-Dawley rats (350-400 g). Rats received carprofen (8 mg/kg) and, 10 min later, they were anesthetized with tiletamine (16 mg/kg) and zolazepam (16 mg/kg). All experimental and surgical procedures conformed to the National Research Guide for the Care and Use of Laboratory Animals and were approved by the Animal Research Committees of the University of Brescia. All efforts were made to minimize both the suffering of the animals and the number of animals used. A laminectomy was performed to expose the T10-L1 cord segment, and a 2-mm-deep incision was made in the lateral funiculus of the right T11-T12 spinal cord. A stump of a PN, 50 mm long, taken from the sural nerve of the same animal, was inserted ≈1.5 mm into the cord incision. The graft was secured by tying a 9-0 suture through the epineurium of the PN and the dura mater. The motor nerve innervating the right internal obliquus abdominis muscle was transected, and the distal stump (20 mm long) was anastomosed to the free end of the grafted nerve by an 11-0 nylon suture. Postoperative treatment with enrofloxacine (10 mg/kg per day) was carried out for 6 consecutive days.
A schematic representation of the surgical procedure is shown in Fig. 5, which is published as supporting information on the PNAS web site.
Electrophysiological Recordings. Two months after graft implantation into the right lateral funiculus of the spinal cord, rats were monitored for muscle reinnervation and function by an electro-myographic device (Nicolet Biomedical, Madison, WI). Rats were deeply anesthetized and mechanically ventilated. Control and reinnervated internal obliquus abdominis muscles were exposed, and compound muscle action potentials (CMAP) were measured in response to direct nerve stimulation. Rats were injected with vecuronium (800 μg/kg, i.v.) or with GYKI 52466 (10 mg/kg, i.p.). The nerve stimulation and CMAP recording in reinnervated and control muscle were performed 30 min after drug injection. In the animal group that received both antagonists, muscle responsiveness to nerve stimulation was recorded without drugs, after vecuronium administration, and finally after GYKI 52466 administration. See Supporting Text, which is published as supporting information on the PNAS web site for details.
Immunohistochemical Analysis. Sixty days after nerve grafting, rats were perfused with a 4% paraformaldehyde solution. Six-micrometer muscle sections were processed for immunohistochemistry with antibodies recognizing GluR1 (2 μg/ml, Chemicon) and βIII tubulin (1:1,500, Promega). Sections of control and grafted nerves were reacted with antibodies recognizing choline acetyltransferase (ChAT) (1:1,000, Chemicon), βIII tubulin (1:1,500, Promega), vesicular glutamate transporter 1 (VGluT-1) (1:5,000, Chemicon), and VGluT-2 (1:2,000, Synaptic Systems, Goettingen, Germany). Sections were incubated in PBS containing the CY2-, CY3-, or tetramethylrhodamine B isothiocyanate-conjugated secondary antibodies for 1 hat room temperature. Fluorescence was observed by confocal laser scanning (Bio-Rad 1024) microscopy. See Supporting Text for details.
Western Blot Analysis. Protein extracts were processed for Western blot analysis by using antibodies recognizing ChAT (1:200, Chemicon), vesicular acetylcholine (ACh) transporter (VAChT) (dilution 1:100, Chemicon), VGluT-1 (1:500, Chemicon), VGluT-2 (1: 2,000, Synaptic Systems), GluR1 (1:100, Chemicon), GluR2 (1:200, Chemicon), GluR3 and -4 (1:250, Santa Cruz Biotechnology), NR1 (1:1,500, Chemicon), and β-tubulin (1:1,500 NeoMarkers). See Supporting Text for details.
RT-PCR Analysis. Muscle specimens taken in regions enriched of nerve endings were analyzed by RT-PCR experiments to identify the mRNA transcripts for GluR subunits. Different sets of primer pairs, together with the primer for β-actin, were used as an internal standard. An Agilent Technologies (Palo Alto, CA) 2100 bioanalyzer was used for semiquantitative RT-PCR analysis (17). See Supporting Text for details.
Retrograde Tracing. The retrograde tracing in the regenerated nerve was performed by injecting the cholera toxin β-subunit (CTβ, 1 mg/ml, List Biological Laboratories, Campbell, CA) into the reinnervated obliquus muscle (18). Six days later, the animals were perfused, and CTβ was immunodetected in sections from brain and spinal cord by using the anticholeragenoid antibody (1:4,000; List Biological Laboratories). See Supporting Text for details.
Results
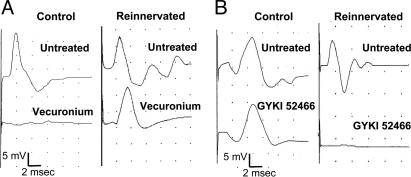
Electrophysiological Recording of Neuromuscular Activity in Reinnervated Rats. Sixty days after nerve implantation into the right lateral funiculus of the spinal cord, rats were monitored for muscle reinnervation and function. Obliquus muscles at both reinnervated and controlateral control sides were exposed, and we recorded the CMAPs in response to direct nerve stimulation (Fig. 1 and Table 1). The reinnervated muscles efficiently responded to nerve electric stimulation, although they showed CMAPs of lower amplitude and longer latency than the control sides. After the first stimulation, a group of rats was injected with the competitive neuromuscular blocking agent vecuronium (800 μg/kg, i.v.), and muscle contractility was again recorded. As expected, the control side was completely blocked by the application of the nicotinic receptor antagonist. Conversely, the reinnervated muscle appeared to be totally insensitive to the curare application (Fig. 1 A). The electrophysiological response of reinnervated muscles resistant to vecuronium application was subsequently blocked by the glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist (AMPA) receptor antagonist GYKI 52466 (19, 20), administered at 10 mg/kg i.p. (Table 1). To confirm the efficacy of AMPA receptor blockade in inhibiting CMAP generation in reinnervated obliquus muscles, the effect of GYKI 52466 was tested in another group of rats just after the first nerve stimulation. Even in these cases, the contractility of reinnervated muscle was efficiently blocked by the AMPA receptor antagonist, whereas the response of the controlateral control side was unaffected (Fig. 1B). A quantitative analysis of data obtained in the two groups of rats is reported in Table 1.
Fig. 1.
Electrophysiological recording of muscle activity in response to direct stimulation of control and grafted nerve. (A) Representative recordings of CMAPs in control and reinnervated muscle. The i.v. injection of vecuronium completely blocked muscle response to control nerve stimulation but not muscle response to stimulation of grafted nerve (Reinnervated). Next, administration of the AMPA receptor antagonist GYKI 52466 totally abolished the muscle response resistant to vecuronium (Table 1). (B) In these experiments, GYKI 52466 was administrated after the first CMAP recording. GYKI 52466 did not modify the control muscle response but totally prevented the response of reinnervated muscle. Traces are from a representative experiment. The stimulus strength was always adjusted to obtain maximal CMAP amplitude. Measurement of mean ± SD of CMAPs amplitude and area are reported in Table 1.
Table 1. CMAP response to nerve stimulation.
| Control
|
Reinnervated
|
|||
|---|---|---|---|---|
| Amplitudes, mV | Areas, mV-ms | Amplitudes, mV | Areas, mV-ms | |
| Expt. 1 | ||||
| No drug | 8.36 ± 5.11 | 10.57 ± 5.84 | 3.87 ± 3.70 | 4.83 ± 3.45 |
| Vecuronium | 0*** | 0**** | 2.96 ± 2.96 | 4.88 ± 4.15 |
| GYKI | — | — | 0* (n = 5) | 0* (n = 5) |
| Expt. 2 | ||||
| No drug | 8.70 ± 5.34 | 12.40 ± 6.03 | 4.60 ± 4.32 | 5.50 ± 5.56 |
| GYKI | 8.52 ± 5.48 | 11.62 ± 5.94 | 0** | 0* |
Measurement of means and SD of CMAP amplitude and area in control and reinnervated muscle. Vecuronium and GYKI 52466 were administered as reported in the Fig. 1 legend. In the first set of experiments, GYKI 52466 abolished muscle response resistant to vecuronium. In the second set of experiments, GYKI 52466 prevented reinnervated muscle response in the absence of vecuronium. The difference of amplitude and area was examined with a paired Student's t test. Expt. 1, n = 9; Expt. 2, n = 8. *, P<0.05; **, P<0.02; ***, P<0.01; ****, P<0.001 vs. no drug.
These results show that the connection of the distal stump of a transected motor nerve with the lateral funiculus of the rat spinal cord produces an effective reinnervation of skeletal muscle. The insensitivity of reinnervated muscle to vecuronium and its acquired sensitivity to a selective blocker of AMPA receptor subtypes suggest that reinnervated muscles have become responsive to glutamatergic transmission.
Evaluation of Cholinergic and Glutamatergic Gene Expression in Grafted Nerves and Reinnervated Muscles. To explore the hall-marks of the muscle-innervating fibers grown into the nerve graft, we investigated the expression of two specific markers for cholinergic neurons, ChAT and VAChT. Western blot analysis was carried out in homogenates of specimens taken from the portion of the obliquus muscle receiving nerve branches. As shown in Fig. 2G, templates from reinnervated muscle revealed a lack of both ChAT and VAChT proteins when compared with the controlateral control tissue. We then investigated the possible expression of VGluT-1/2, which are markers of glutamatergic neurons and axon terminals in CNS. The immunoblot analysis showed a significant expression of both VGluT-1 and -2 in reinnervated muscle but not in the control obliquus muscle. To corroborate these results, we performed an immunofluorescence staining of nerve sections. The staining for βIII tubulin, a selective marker of neuronal microtubules, showed extensive growth of neuronal axons in the grafted nerve. The ChAT antibody was used to double-label the cholinergic fibers, whereas VGluT-1/2 antibodies were used to stain glutamatergic neurites. As shown in Fig. 2 A, ChAT colocalized with βIII tubulin in many of the fibers present in the control nerve, whereas no specific signal for ChAT was found in sections from grafted nerve (Fig. 2B). Conversely, VGluT-1 (Fig. 2 C and D) and VGluT-2 (Fig. 2 E and F) immunostaining was clearly evident in certain localized regions of grafted nerve (Fig. 2 D and F), whereas it was nearly undetectable in sections from control nerve (Fig. 2 C and E).
Fig. 2.
Analysis of cholinergic and glutamatergic markers in control and grafted nerves. (A and B) Confocal images of control (A) and grafted (B) nerve sections double-labeled with antibodies to ChAT (Cy3, red) and βIII tubulin (Cy2, green). (C and D) VGluT-1 (Cy3, red) and βIII tubulin (Cy2, green) were double-labeled in control (C) and grafted (D) nerves. (E and F) VGluT-2 (Cy3, red) and βIII tubulin (Cy2, green) were double-labeled in control (E) and grafted (F) nerves. In Merge, white arrows indicate colocalization of βIII tubulin and ChAT or VGluT. (Bar, 12 μm.) (G) Western blot analysis of proteins from control (C) and reinnervated (R) muscle with antibodies directed to ChAT, VAChT, VGluT-1, and VGluT-2. Membranes reprobed with antibodies to β-tubulin showed equal amounts of loaded proteins.
These data suggest that muscle reinnervation was mainly operated by glutamatergic fibers.
We then analyzed the expression of different glutamate receptor (GluR) subtypes in parallel templates taken from muscle regions enriched with nerve endings. The RT-PCR analysis revealed a lack of NMDA receptor subunit NR1 in both control and reinnervated obliquus muscles but a constitutive expression of AMPA receptor subunits (Fig. 3A). In particular, the mRNA levels for GluR1/2 subunits displayed a trend to increase in reinnervated tissue, whereas no increase was found in GluR3/4 expression levels. Immunoblot analysis of GluRs in homogenates from control and reinnervated muscles confirmed the lack of NR1 subunit in both templates and the significant increase of GluR1/2 proteins in reinnervated muscles (Fig. 3B). Conversely, GluR3/4 were hardly detectable in either specimens (Fig. 3B). The confocal immunofluorescence analysis of GluR1 showed spread punctuate signals in control tissue but large aggregates of immunofluorescence in reinnervated muscle that only partially colocalized with βIII tubulin staining (Fig. 3C). The images were indicative of clusters of postsynaptic AMPA receptors innervated by βIII tubulin-positive presynaptic axons. This physiological and molecular evidence suggests that skeletal muscle can be reinnervated by glutamatergic neurons and may respond to the glutamatergic signal by generating functional glutamatergic NMJ.
Fig. 3.
Expression analysis of GluRs in control and reinnervated muscle. (A) RT-PCR analyses of GluR1, GluR2, GluR3, GluR4, and NR1 transcripts in motor end plate of control (C, open bar) and reinnervated (R, black bar) obliquus muscles. The gel-like images produced by Agilent Bioanalyzer 2001 are shown for the coamplification of β-actin and the different GluR transcripts. Histograms show the means of six individual experiments and standard error. For NR1 analysis, the amplification product from rat cerebral cortex (Cx) was used as a positive control. Statistical analysis was performed by using two-tail Student's t test. (*, P < 0.05). An example of electropherogram traces of β-actin/GluR1 coamplification is reported (Bottom Right). Upper and lower markers are used as internal standards to eliminate sample to sample variation, and calculate size and concentration of each PCR product. (B) Western blot analysis of GluR subunits NR1, GluR1, GluR2, GluR3, GluR4, and of β-tubulin in proteins extracts from control (C) and reinnervated muscles (R). (C) Confocal images of control and reinnervated muscle double labeled with antibodies to GluR1 (Cy2, green) and βIII tubulin (Cy3, red). A punctuate GluR1 staining was evident in control sections. A clustering of GluR1 immunofluorescence appeared in reinnervated muscle at the end of a βIII tubulin-positive axon.
Injection of CTβ into Reinnervated Muscle Produced a Retrograde Labeling of Brainstem and Red Nucleus Neurons. To obtain a labeling of neurons extending their axons into the PN graft, the retrograde tracer CTβ was microinjected into the obliquus muscle. Neuroanatomical tracing confirmed that axons elongated within the PN graft belonged to supraspinal neurons. We analyzed the spinal cord segments either rostral or caudal to the graft implantation, the motor cortex, and those supraspinal regions of the midbrain and brainstem that mainly contribute to the descending pathways in the lateral spinal cord. No labeled neurons were found in the spinal cord or the cortex. Most retrograde labeling was found in red nucleus neurons or in neurons belonging to the medullary reticular formation and vestibular complex. Labeled neurons were found in the nucleus ambiguus, the dorsal and ventral medullary reticular nuclei, and the nucleus gigantocellularis (Fig. 4 A and B). In the red nucleus, most neurons were labeled at the controlateral side to the nerve graft, although a few neurons were also labeled at the ipsilateral side (Fig. 4 C and D).
Fig. 4.
Transverse sections through medullary reticular formations and red nucleus showing retrograde labeling of supraspinal neurons extending axons into the grafted nerve. Two months after PN grafting, the retrograde tracer CTβ was microinjected in the reinnervated muscle. (A) A section from the medullary reticular formation showing the staining of neurons in the nucleus gigantocellularis. Higher-magnification micrograph (Inset) is shown in B. A section from midbrain (C) showing a number of neurons labeled in the red nucleus controlateral to the grafted side and fewer cells stained at the ipsilateral side. Higher-magnification micrograph of controlateral red nucleus (Inset) is shown in D. (Bar, 650 μmin A and C; 200 μmin B and D.)
Discussion
Mammalian NMJ is believed to be a unique cholinergic synapse where ACh is the main neurotransmitter activating postsynaptic nicotinic ACh receptors (16, 21). Conversely, invertebrate NMJ is served by glutamate as major excitatory neurotransmitter (22). The most striking finding of our study is that, under glutamatergic innervation, the mammal NMJ can switch from cholinergic-type to glutamatergic synapse. We found that the connection of the obliquus abdominis muscle nerve with the lateral bundle of rat spinal cord by a PN graft restored a functional muscle innervation. This was revealed by the trophic appearance of reinnervated muscle and the electrophysiological analysis of neuromuscular activity. In line with previous evidence (11), 2 months after nerve grafting, the muscle responded to direct stimulation of the motor nerve. The data obtained from CMAP measurements suggest that the proportion of successfully reinnervated muscle fibers was largely above one-third, but this is an indirect measure. We plan to carry out intracellular recordings to finely document the percentage of muscle fibers that are functionally reinnervated and that generate excitatory postsynaptic potentials. The reinnervated muscles appeared to be insensitive to common curare-mediated blockade when compared with the control one. Muscle resistance to nicotinic blockers strongly suggested that a neurotransmitter other than ACh was responsible for NMJ activation. Indeed, we found that reinnervated muscle was completely paralyzed by selective blockers of glutamate AMPA receptor subtypes. The systemic administration of the AMPA receptor antagonist GYKI 52466 efficiently prevented muscle contraction in response to nerve stimulation on the nerve graft side but not the control side. The replacement of ACh-mediated transmission at the NMJ by a glutamatergic transmission was consistent with data from immunofluorescence analysis of the grafted nerve. Clear ChAT immunoreactivity was found in the control motor nerve, whereas ChAT signals were nearly undetectable in the grafted one. Conversely, the labeling of VGluTs that are markers of glutamatergic innervation (23) disclosed high protein expression in grafted nerves, but this was negligible in the controls.
Taking into consideration the primary role of ACh in activating NMJ, recent research has proposed the participation of glutamate in modulating cholinergic transmission. A glutamate-like immunoreactivity has been found at the level of NMJ and motor nerve terminals, suggesting glutamate as a possible cotransmitter of ACh in motoneurons (24, 25). The application of glutamate to strips of rat diaphragm has been shown to contribute to the maintenance of the resting membrane potential (26) and to inhibit the nonquantal release of ACh from nerve endings (27). Glutamate effects may be mediated by activation of postjunctional NMDA receptors to induce sarcoplasmic synthesis and retrograde diffusion of NO from muscle cells (27). However, because glutamate is a ubiquitous intermediate in cellular metabolism, only the recent identification of VGluTs has allowed reliable investigation on glutamate as neurotransmitter. Three types of VGluTs, VGluT-1, -2, and -3, have been identified in the CNS (23). Although VGluT-1/2 transcripts have been detected in rat spinal motoneurons (28, 29), no VGluT-1/2 proteins have been found to colocalize with VAChT (28, 30) or ChAT (31). Recent studies demonstrating VGluT-2 immunostaining in motoneuron collateral axons projecting to layer VII of the spinal cord (28, 32) confirmed the lack of VGluT-2 and glutamatergic transmission in the cholinergic projections to the NMJ. Furthermore, studies on VGluT-3 expression at the skeletal muscle endplates gave contradictory results (33, 34). The difficulty in demonstrating VGluT expression at the cholinergic synapses indicates that mechanisms responsible for the high concentration of glutamate at the NMJ still need to be clarified or their identification requires more sensitive investigation tools. In line with this evidence, we found no VGluT-1/2 immunoreactivity either in nerve sections or endings from control cases. Conversely, both VGluT-1 and -2 appeared in grafted nerve and its target muscle, giving unambiguous evidence of the growth of glutamatergic axons therein. A wider neurochemical analysis of grafted nerve will possibly disclose the growth of additional nonglutamatergic axons not examined in this study.
VGluT-1/2, but mainly VGluT-2, are largely distributed in the lateral white matter of the spinal cord (31, 35), where they label glutamatergic fibers, including the descending afferents from glutamatergic supraspinal neurons. Interestingly, expression of VGluT-2 has been found in midbrain and brainstem nuclei projecting to the spinal cord, including the red nucleus, reticular formation, and vestibular complex (35-37). In addition, VGluT-1 expression was specifically detected in cells of the gigantocellular reticular nucleus (38). Red nucleus and brainstem neurons extend their axons along the lateral white matter of the spinal cord to form rubrospinal, reticulospinal, and vestibulospinal tracts (18, 39-41). We found that a number of these neurons were retrogradely labeled by CTβ application at the muscular synaptic terminals of the grafted nerve, demonstrating they were primarily involved in the new glutamatergic innervation of obliquus muscle. No labeled neurons were found in the cerebral cortex or in spinal cord segments either rostrally or caudally to the nerve graft site. This finding provides evidence that brain glutamatergic neurons can directly synapse with mammal skeletal muscle to drive motor activity.
The specification of neurotransmitter and receptor types is a crucial phenomenon for nervous system development and regeneration, enabling communication between the presynaptic neuron and the postsynaptic target cell (42, 43). In embryonic neurons, plasticity of the transmitter phenotype, implying replacement of excitatory transmitters with inhibitory ones, occurs in response to neuronal activity (44). Likewise, it has been shown that sympathetic preganglionic cholinergic fibers can directly reinnervate the smooth muscle of the nictating membrane in adult cats. The muscle activation relies on the switch of the preganglionic fibers from a cholinergic to a noradrenergic phenotype (45). In addition, “normally glutamatergic” mossy fibers projecting to hippocampal CA3 pyramidal cells have been found to generate GABAergic postsynaptic potentials after kindled seizure, as a possible homeostatic reaction to hyperexcitability (46). Open questions arising from transmitter specification plasticity are whether and how changes in presynaptic neurotransmitter phenotype correspond to changes in the expression of postsynaptic transmitter receptors, to achieve functional synapse formation. Another open question is whether transmitter specification can reroute neurons to make synaptic connections with suitable targets, or whether it is itself regulated by specific targets. Our results strongly suggest that glutamatergic postsynaptic specification occurs under glutamatergic innervation of skeletal muscle. The pharmacological analysis of receptors involved in activation of reinnervated NMJ strikingly indicates the presence and function of AMPA-type GluRs. We show that the muscle response to nerve stimulation was totally blocked by the selective AMPA receptor antagonist GYKI 52466 (19, 20). Because AMPA receptors are tetrameric heteromeric complexes of four homologous subunits, GluR1-4, we analyzed the expression of each AMPA receptor gene. In the control muscle all of GluR1-4, transcripts were detected together with very low levels of GluR1/2 translation products. In reinnervated muscle, GluR1/2 subunits displayed an upward mRNA expression trend with a higher significant occurrence at the protein level. Hence, both GluR1 and -2 proteins, hardly detectable in the control muscle, displayed marked expression after reinnervation by glutamatergic axons. Moreover, the immunofluorescence analysis of GluR1 in reinnervated muscle revealed large clusters of postsynaptic AMPA receptors, a key feature of a specialized postsynaptic apparatus (16, 42).
An intriguing subject of future investigation would be to find molecules and mechanisms responsible for synapse assembly between brain neuronal terminals and skeletal muscle. Although the expression of AMPA receptors in mammalian muscle has never been investigated, the NMDA receptors were found to mediate the modulatory effects of glutamate at the NMJ in the rat diaphragm (47). However, studies based on the immunocytochemical detection of NR1 showed that the expression of the NMDA receptor cannot be generalized to all skeletal muscles (48) and may vary with muscle-type fiber composition. We failed to detect any NR1 protein or mRNA in obliquus muscle either before or after glutamatergic innervation. This suggests that activation of glutamate AMPA, but not NMDA receptors, was mainly involved in the signaling between nerve terminals and reinnervated muscle. The presynaptic elimination of cholinergic input did not cause the disappearance of the nicotinic ACh receptor mRNAs (data not shown). Experiments addressed to investigate changes of nicotinic NMJ in muscle subjected to glutamatergic reinnervation would add information about the possible colocalization of GluR with the ACh receptor and the potential role of preexisting pathways in guiding new glutamatergic projections.
It has been established that glutamate has a primary role in neuromuscular transmission of organisms phylogenically distant from mammals, such as invertebrates, including insects and mol-lusks (22, 49, 50). In addition, glutamate acts as cotransmitter of ACh by regulating the development and function of cholinergic synapse in fishes and amphibians (51-53). Our study suggests that mammal skeletal muscles retain the memory of ancestral glutamatergic transmission together with the capability to remodel and reorganize the molecular patterning of NMJ postsynaptic membrane to the glutamatergic input. A future challenge, therefore, would be to define signals and molecular mechanisms responsible for glutamatergic synapse formation and maintenance at mammals NMJ and to understand how these mechanisms interact to achieve differentiation of postsynaptic specialization and restoration of muscle activity. Understanding the enormous potentiality of CNS plasticity in regulating motor function would unravel new strategies for intervention in spinal cord injury.
Supplementary Material
Acknowledgments
We are grateful to Profs. P. Mantegazza and F. Clementi, University of Milan, Milan, Italy, and to Prof. N. Manca, University of Brescia, for critical and constructive discussions. This work was supported by grants from Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR); Programmi di Ricerca di Rilevante Interesse Nazionale (COFIN) 2002, 2003, and 2004; Fondo per gli Investimenti della Ricerca di Base (FIRB) 2002; Centro di Studio e Ricerca sulla Terza Età, Brescia; and Centro di Eccellenza per la Innovazione Diagnostica e Terapeutica (IDET), University of Brescia-MIUR.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NMJ, neuromuscular junction; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; CMAP, compound muscle action potential; ChAT, choline acetyltransferase; ACh, acetylcholine; VAChT, vesicular ACh transporter; VGluT, vesicular glutamate transporter; GluR, glutamate receptor; PN, peripheral nerves; CTβ, cholera toxin β-subunit.
References
- 1.Schwab, M. E. & Bartholdi, D. (1996) Physiol. Rev. 76, 319-370. [DOI] [PubMed] [Google Scholar]
- 2.Fawcett, J. W. & Asher, R. A. (1999) Brain Res. Bull. 49, 377-391. [DOI] [PubMed] [Google Scholar]
- 3.Silver, J. & Miller, J. H. (2004) Nat. Rev. Neurosci. 5, 146-156. [DOI] [PubMed] [Google Scholar]
- 4.Richardson, P. M., McGuinness, U. M. & Aguayo, A. J. (1980) Nature 284, 264-265. [DOI] [PubMed] [Google Scholar]
- 5.David, S. & Aguayo, A. J. (1981) Science 214, 931-933. [DOI] [PubMed] [Google Scholar]
- 6.Brunelli, G., Milanesi, S., Bartolaminelli, P., De Filippo, G., Brunelli, F. & Bottonelli, P. V. (1983) Traumatology 9, Suppl., 53-56. [PubMed] [Google Scholar]
- 7.Brunelli, G. A. & Brunelli, G. R. (1996) J. Peripher. Nerv. Syst. 1, 111-118. [PubMed] [Google Scholar]
- 8.Friedman, B. & Aguayo, A. J. (1985) J. Neurosci. 5, 1616-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horvat, J. C., Pecot-Dechavassine, M., Mira, J. C. & Davarpanah, Y. (1989) Brain Res. Bull. 22, 103-114. [DOI] [PubMed] [Google Scholar]
- 10.Bertelli, J. A., Orsal, D. & Mira, J. C. (1994) Brain Res. 644, 150-159. [DOI] [PubMed] [Google Scholar]
- 11.Brunelli, G. A. (2001) J. Reconstr. Microsurg. 17, 631-636. [DOI] [PubMed] [Google Scholar]
- 12.Ye, J. H. & Houle, J. D. (1997) Exp. Neurol. 143, 70-81. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi, N. R., Fan, D. P., Giehl, K. M., Bedard, A. M., Wiegand, S. J. & Tetzlaff, W. (1997) J. Neurosci. 17, 9583-9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, M. S. & Gold, B. G. (1999) J. Spinal Cord Med. 22, 287-296. [DOI] [PubMed] [Google Scholar]
- 15.Decherchi, P. & Gauthier, P. (2000) Neuroscience 101, 197-210. [DOI] [PubMed] [Google Scholar]
- 16.Sanes, J. R. & Lichtman, J. W. (2001) Nat. Rev. Neurosci. 2, 791-805. [DOI] [PubMed] [Google Scholar]
- 17.Gottwald, E., Muller, O. & Polten, A. (2001) Electrophoresis 22, 4016-4022. [DOI] [PubMed] [Google Scholar]
- 18.Kuchler, M., Fouad, K., Weinmann, O., Schwab, M. E. & Raineteau, O. (2002) J. Comp. Neurol. 448, 349-359. [DOI] [PubMed] [Google Scholar]
- 19.Bleakman, D., Ballyk, B. A., Schoepp, D. D., Palmer, A. J., Bath, C. P., Sharpe, E. F., Woolley, M. L., Bufton, H. R., Kamboj, R. K., Tarnawa, I., et al. (1996) Neuropharmacology 35, 1689-1702. [DOI] [PubMed] [Google Scholar]
- 20.Szabados, T., Gigler, G., Gacsalyi, I., Gyertyan, I. & Levay, G. (2001) Brain Res. Bull. 55, 387-391. [DOI] [PubMed] [Google Scholar]
- 21.Fatt, P. & Katz, B. (1952) J. Physiol. 117, 109-128. [PMC free article] [PubMed] [Google Scholar]
- 22.Lunt, G. G. & Olsen, R. W. (1988) Comparative Invertebrate Neurochemistry (Cornell University Press, Ithaca, NY).
- 23.Fremeau, R. T., Jr., Voglmaier, S., Seal, R. P. & Edwards, R. H. (2004) Trends Neurosci. 27, 98-103. [DOI] [PubMed] [Google Scholar]
- 24.Waerhaug, O. & Ottersen, O. P. (1993) Anat. Embryol. 188, 501-513. [DOI] [PubMed] [Google Scholar]
- 25.Meister, B., Arvidsson, U., Zhang, X., Jacobsson, G., Villar, M. J. & Hokfelt, T. (1993) NeuroReport 5, 337-340. [DOI] [PubMed] [Google Scholar]
- 26.Urazaev, A. K., Naumenko, N. V., Nikolsky, E. E. & Vyskocil, F. (1999) Neurosci. Res. 33, 81-86. [DOI] [PubMed] [Google Scholar]
- 27.Malomouzh, A. I., Mukhtarov, M. R., Nikolsky, E. E., Vyskocil, F., Lieberman, E. M. & Urazaev, A. K. (2003) J. Neurochem. 85, 206-213. [DOI] [PubMed] [Google Scholar]
- 28.Herzog, E., Landry, M., Buhler, E., Bouali-Benazzouz, R., Legay, C., Henderson, C. E., Nagy, F., Dreyfus, P., Giros, B., et al. (2004) Eur. J. Neurosci. 20, 1752-1760. [DOI] [PubMed] [Google Scholar]
- 29.Landry, M., Bouali-Benazzouz, R., El Mestikawy, S., Ravassard, P. & Nagy, F. (2004) J. Comp. Neurol. 468, 380-394. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira, A. L., Hydling, F., Olsson, E., Shi, T., Edwards, R. H., Fujiyama, F., Kaneko, T., Hokfelt, T., Cullheim, S. & Meister, B. (2003) Synapse 50, 117-129. [DOI] [PubMed] [Google Scholar]
- 31.Todd, A. J., Hughes, D. I., Polgar, E., Nagy, G. G., Mackie, M., Ottersen, O. P. & Maxwell, D. J. (2003) Eur. J. Neurosci. 17, 13-27. [DOI] [PubMed] [Google Scholar]
- 32.Nishimaru, H., Restrepo, C. E., Ryge, J., Yanagawa, Y. & Kiehn, O. (2005) Proc. Natl. Acad. Sci. USA 102, 5245-5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraus, T., Neuhuber, W. L. & Raab, M. (2004) Neurosci. Lett. 360, 53-56. [DOI] [PubMed] [Google Scholar]
- 34.Boulland, J. L., Qureshi, T., Seal, R. P., Rafiki, A., Gundersen, V., Bergersen, L. H., Fremeau, R. T., Jr., Edwards, R. H., Storm-Mathisen, J. & Chaudhry, F. A. (2004) J. Comp. Neurol. 480, 264-280. [DOI] [PubMed] [Google Scholar]
- 35.Varoqui, H., Schafer, M. K., Zhu, H., Weihe, E. & Erickson, J. D. (2002) J. Neurosci. 22, 142-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fremeau, R. T., Jr., Troyer, M. D., Pahner, I., Nygaard, G. O., Tran, C. H., Reimer, R. J., Bellocchio, E. E., Fortin, D., Storm-Mathisen, J. & Edwards, R. H. (2001) Neuron 31, 247-260. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko, T., Fujiyama, F. & Hioki, H. (2002) J. Comp. Neurol. 444, 39-62. [DOI] [PubMed] [Google Scholar]
- 38.Ni, B., Wu, X., Yan, G. M., Wang, J. & Paul, S. M. (1995) J. Neurosci. 15, 5789-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shieh, J. Y., Leong, S. K. & Wong, W. C. (1983) J. Comp. Neurol. 214, 79-86. [DOI] [PubMed] [Google Scholar]
- 40.Newman, D. B. (1995) Adv. Neurol. 67, 219-244. [PubMed] [Google Scholar]
- 41.Tracey, D. (2004) in The Rat Nervous System, ed. Paxinos, G. (Academic, New York), 3rd Ed., pp. 149-164.
- 42.Goda, Y. & Davis, G. W. (2003) Neuron 40, 243-264. [DOI] [PubMed] [Google Scholar]
- 43.Spitzer, N. C., Root, C. M. & Borodinsky, L. N. (2004) Trends Neurosci. 27, 415-421. [DOI] [PubMed] [Google Scholar]
- 44.Borodinsky, L. N., Root, C. M., Cronin, J. A., Sann, S. B., Gu, X. & Spitzer, N. C. (2004) Nature 429, 523-530. [DOI] [PubMed] [Google Scholar]
- 45.Ceccarelli, B., Clementi, F. & Mantegazza, P. (1972) J. Physiol. 220, 211-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutierrez, R., Romo-Parra, H., Maqueda, J., Vivar, C., Ramirez, M., Morales, M. A. & Lamas, M. (2003) J. Neurosci. 23, 5594-5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger, U. V., Carter, R. E. & Coyle, J. T. (1995) Neuroscience 64, 847-850. [DOI] [PubMed] [Google Scholar]
- 48.Grozdanovic, Z. & Gossrau, R. (1998) Cell Tissue Res. 291, 57-63. [DOI] [PubMed] [Google Scholar]
- 49.Petersen, S. A., Fetter, R. D., Noordermeer, J. N., Goodman, C. S. & Di Antonio, A. (1997) Neuron 19, 1237-1248. [DOI] [PubMed] [Google Scholar]
- 50.Fox, L. E. & Lloyd, P. E. (1999) J. Neurophysiol. 82, 1477-1488. [DOI] [PubMed] [Google Scholar]
- 51.Fu, W. M., Liou, J. C., Lee, Y. H. & Liou, H. C. (1995) J. Physiol. 489, 813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinard, A., Levesque, S., Vallee, J. & Robitaille, R. (2003) Eur. J. Neurosci. 18, 3241-3250. [DOI] [PubMed] [Google Scholar]
- 53.Todd, K. J., Slatter, C. A. & Ali, D. W. (2004) J. Neurophysiol. 91, 828-840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.