Abstract
The nucleus accumbens (NAc) is central to heroin addiction. Activation of opiate receptors in the NAc dissociates Gi/o into α and βγ subunits. Gαi inhibits cAMP production, but βγ regulates several molecular pathways, including protein kinase A (PKA). We show in NAc/striatal neurons that opiates paradoxically activate PKA signaling by means of βγ dimers. Activation requires Gαi3 and an activator of G protein signaling 3 (AGS3). AGS3 competes with βγ for binding to Gαi3-GDP and enhances the action of unbound βγ. AGS3 and Gαi3 knockdown prevents opiate activation of PKA signaling. In rats self-administering heroin, AGS3 antisense in the NAc core, but not shell, eliminates reinstatement of heroin-seeking behavior, a model of human relapse. Thus, Gαi3/βγ/AGS3 appears to mediate μ opiate receptor activation of PKA signaling as well as heroin-seeking behavior.
Keywords: μ opiate receptor, nucleus accumbens core, addiction, βγ subunits, Gαi3
Heroin addiction, a worldwide socioeconomic and public health problem, is difficult for physicians to treat, often because addicts frequently relapse when they try to stop using heroin during abstinence. Heroin activates opiate receptors (1). However, we do not understand the postsynaptic cellular mechanisms produced by μ opiate receptor (MOR) activation that contribute to craving for and relapse to heroin. Sharma et al. (2) provided pioneering evidence in NG108-15 cells that δ opiate receptor (DOR) activation initially decreases cAMP levels, followed by restoration of cAMP to normal levels during continued exposure to morphine; cAMP increases further in response to opiate receptor blockade. Many investigators have confirmed an opiate-induced increase of cAMP/protein kinase A (PKA) signaling that is exaggerated upon opiate withdrawal (3, 4). In vivo, increased cAMP/PKA activity may be associated with opiate-induced tolerance and dependence and is thought to contribute to the reinforcing properties of opiates (5).
Postsynaptic Gi/o-coupled opiate receptors are expressed on GABAergic medium spiny neurons in the nucleus accumbens (NAc), a brain region thought to account, in part, for craving, reward, and reinforcement of addicting drugs (6, 7). MOR and DOR activation dissociates heterotrimeric Gi/o proteins into free α and βγ subunits. Gαi released from Gi/oβγ inhibits several isoforms of adenylyl cyclase (AC) (8) and appears to account for opiate inhibition of cAMP production. By contrast, βγ dimers, which are simultaneously released during receptor activation, can stimulate AC2 and AC4 (9-13). However, the precise molecular mechanisms that facilitate this paradoxical Gi/o-coupled opiate receptor-induced stimulation of cAMP/PKA signaling are not clearly understood.
Recent evidence suggests that an activator of G protein signaling 3 (AGS3) regulates G protein signaling in the NAc (14). By competing with βγ for binding to Gαi3, AGS3 appears to stabilize a specific Gαi3-GDP complex formed before the reassociation of free Gαi3 and βγ subunits (15). The bound form of Gαi3 does not inhibit AC. Both AGS3 and MOR are highly expressed in the NAc (6, 16). This expression suggests that AGS3 could play a role in MOR-induced G protein signaling and heroin addiction. Here, we identify Gαi3 and AGS3 in primary NAc/striatal cultures as molecular components preferentially associated with the MOR. Gαi3 and AGS3 appear to be required to enhance the action of free βγ dimers, which mediate MOR stimulation of PKA signaling. Relapse, or reinstatement of heroin addiction, is a major limitation for effective treatment of human heroin addiction. In rats trained to self-administer heroin, we find that selective expression of AGS3 antisense in the NAc core prevents reinstatement of heroin-seeking behavior, a valid model of human heroin craving and relapse. By contrast, AGS3 antisense in the NAc shell is without effect. These findings suggest that AGS3 may play a critical role in MOR stimulation of PKA signaling in NAc neurons and in the development or maintenance of heroin addiction.
Methods
Preparation and Analysis of Cells. Cells were prepared from NAc/striatum of newborn (postnatal day 0) male Sprague-Dawley rat pups, plated at 5 × 104 cells per slide, and grown in Neurobasal A medium (GIBCO) supplemented with B27 supplement (GIBCO) and glutamate. One-half of the medium was changed 1 day after plating and weekly thereafter. Cells were treated on day 10. Immunostaining and images were obtained as described in ref. 17. For quantification of PKA Cα translocation, random fields on each slide were selected, and cells scored for PKA Cα staining in Golgi, nucleus, cytoplasm, and neurites. For all experiments, at least five fields were scored for each experiment for a total number of at least 40 cells per slide. Data shown were obtained by two independent observers who were blind to the experimental condition. Staining for glutamic acid decarboxylase indicates that >90% of cells are GABAergic neurons (data not shown).
Total RNA was isolated by using TRIzol (Invitrogen) and treated with DNase. First-strand cDNA was synthesized by using 1 μg of RNA in a final volume of 40 μl containing 0.5 μg of oligo(dT), 500 μM each dNTP, and 20 units of Superscript reverse transcriptase (GIBCO). Ten microliters of this reaction was taken for PCR using AGS3-specific primers. PCR was performed at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s for 25 cycles. PCR products were analyzed on a 1.2% agarose gel.
Herpes simplex virus (HSV) vectors expressing antisense RNA were created by cloning antisense oligonucleotides into HSVLacZ vector under control of mouse U6 polymerase III promoter. The sequences for AGS3 antisense correspond to nucleotides 306-330 of AGS3. The sequences of antisense for Gαi, Gβ, or Gγ were designed as described in ref. 18. Ad5AGS3 antisense (AS) under the control of a CMV promoter was created by replacing the E1A gene of the adenovirus. HSV (multiplicity of infection 1) and adenovirus (multiplicity of infection 100) were used to infect primary NAc/striatal neurons.
To assay cAMP-response element (CRE)-luciferase, rat NAc/striatal neurons were plated at 2.5 × 104 cells per 24-well plate and grown for 10 days. Cell transfection, drug treatment, and luciferase assay were carried out as described in ref. 10.
Preparation of Membrane and Solubilized MOR. Confluent primary NAc/striatal cultures in 100-mm dishes were washed with PBS and homogenized with a Brinkmann Polytron homogenizer in 1 ml of buffer (20 mM Tris, pH 7.4/1 mM EGTA/1 mM MgCl2/250 mM sucrose). The homogenate was centrifuged at 120 × g for 30 min. The resulting supernatant was centrifuged at 27,000 × g for 30 min. Crude membrane pellet was resuspended in buffer A, which contains 50 mM Hepes (pH 7.4), 100 mM NaCl, 5 mM MgCl2, 2 mM EDTA, 1 mM EGTA, 2 mM DTT, and a protease inhibitor mixture (Roche Molecular Biochemicals). Membranes were centrifuged at 27,000 × g for 30 min, resuspended in fresh buffer A, incubated with 100 nM [d-Ala2,N-MePhe4,Gly5-ol]enkephalin (DAMGO) for 10 min at 30°C, and then chilled on ice for 10 min. 3-[(3-Cholamidopropyl)dimethyl-ammonio]-1-propanesulfonate was added to the membrane suspension to obtain a final concentration of 10 mM. The preparation was incubated on ice with moderate agitation for 30 min and subsequently centrifuged for 1 h at 50,000 × g. The supernatant fraction was saved for immunoprecipitation.
Immunoprecipitation and Western Blotting. Solubilized membranes, prepared from cells stimulated with 100 nM DAMGO, were immunoprecipitated with anti-MOR antibodies. Protein complexes were captured by adding Protein A/G-Sepharose beads (Santa Cruz Biotechnology) and continuing the incubation for 30 min at 4°C. The mixture was then microcentrifuged at 4°C, and pellets were washed and resuspended in 2× Laemmli loading buffer. Resuspended samples were boiled for 5 min, and supernatant was loaded on 10% SDS/10% PAGE. Membrane transfer and protein detection were carried out as described in ref. 17.
Animal Surgery and Behavioral Assay. Sprague-Dawley male rats were anesthetized, implanted with jugular catheters and bilateral intracranial guide cannulas, and trained as described in refs. 19 and 20. After animals met maintenance criterion (active lever presses do not vary by >10% across three consecutive days), extinction and reinstatement were conducted as described (19, 20), except that a heroin prime was used. Before reinstatement, animals received a microinfusion of Ad5AGS3 AS or Ad5LacZ. Ad5LacZ was used to confirm viral expression and to control for the effect of viral expression on reinstatement. On days 4-6 after infusion, the subjects were returned to extinction conditions. Reinstatement testing was conducted on day 7 after infusion. Each rat received a priming injection of heroin (0.25 mg/kg s.c.) before placement in the self-administration chamber for a 3-h extinction session. After behavioral testing, rats were anesthetized, and brains were fixed and sectioned as described in ref. 19 to verify infusion sites. There was no evidence of toxicity on histological examination of the NAc from experimental animals (data not shown).
Results
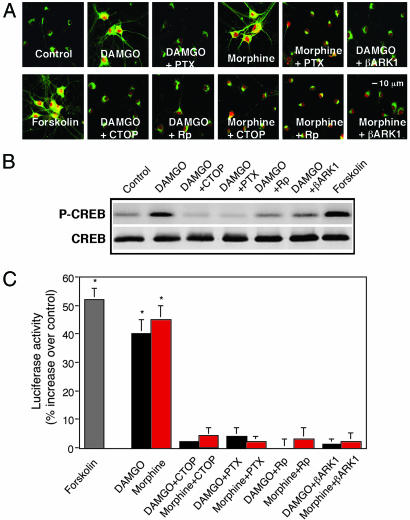
Activation of MOR Induces PKA Signaling. DAMGO (100 nM) or morphine (100 nM) each causes activation and translocation of PKA Cα from Golgi to the nucleus, cytoplasm, and neurites in primary NAc/striatal neurons (Fig. 1A). CTOP, a MOR antagonist, or RP-adenosine 3′,5′-cyclic monophosphorothioate (Rp-cAMPS), a PKA inhibitor, blocks DAMGO and morphine-induced PKA translocation (Fig. 1 A). By contrast, activation of nonaddictive Gi/o-coupled adrenergic receptor α2b or muscarinic receptor M4 by UK 14,304 or carbachol, respectively, has no effect (data not shown). As a positive control, forskolin, a direct and potent activator of AC, causes PKA activation and translocation, like DAMGO and morphine (Fig. 1 A).
Fig. 1.
MOR promotes PKA activation in primary NAc/striatal neurons. (A) Confocal analysis of PKA Cα translocation. Cells were incubated with or without 100 nM DAMGO, 100 nM morphine, or 1 μM forskolin for 10 min. Where indicated, cells were preincubated for 30 min with or without 100 nM d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) or 20 μM RP-adenosine 3′,5′-cyclic monophosphorothioate (Rp), overnight with pertussis toxin (PTX) (50 ng/ml) or Ad5βARK1. (B) Western blot analysis of CREB phosphorylation (P-CREB) at serine-133. (C) Gene activation measured by CRE-luciferase activity. Cells were incubated with DAMGO or morphine as described above. Luciferase was assayed 5 h after drug treatment. Data are the mean ± SEM of at least three experiments. *, P < 0.01, compared with control (one-way ANOVA and Dunnett's test).
PKA Cα translocated into the nucleus phosphorylates CRE-binding protein (CREB) leading to increased CRE-mediated gene expression. DAMGO increases CREB phosphorylation in 10 min (Fig. 1B) and CRE-mediated luciferase activity 5 h later (Fig. 1C); these effects also are blocked by CTOP or Rp-cAMPS. As a positive control, forskolin produced a similar activation (Fig. 1 B and C). These data suggest that increases in cAMP-dependent signaling and CRE-mediated gene expression are MOR- and PKA-dependent.
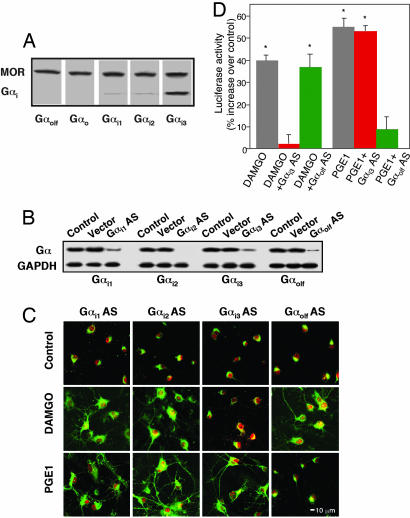
MOR Is Preferentially Coupled with Gαi3. Activation of Gi/o-coupled receptors is thought to inhibit cAMP production by means of Gαi, but MOR activation also can paradoxically stimulate cAMP/PKA signaling (12, 21). We asked whether MOR couples with a specific G protein to facilitate AC stimulation. Solubilized rat NAc/striatal membranes were immunoprecipitated with anti-MOR antibodies and Western-blotted with anti-Gαo, -Gαi1, -Gαi2, -Gαi3, or -Gαolf plus MOR (Fig. 2A). We found that only Gαi3 coimmunoprecipitates predominately with MOR (Fig. 2 A), suggesting that MOR interacts preferentially with Gαi3. This finding was confirmed functionally by using AS directed against Gαi1, Gαi2, Gαi3, or Gαolf to knock down expression of each of these proteins in NAc/striatal neurons (Fig. 2B). Knockdown of Gαi3 specifically blocks DAMGO-induced PKA Cα translocation (Fig. 2C) and CRE-mediated luciferase activity (Fig. 2D). In contrast, AS for Gαi1, Gαi2, or Gαolf was without effect (Fig. 2C). In control studies, AS directed against Gαolf, but not Gαi3, blocks Gαs/Gαolf-coupled prostaglandin E1-induced PKA signaling (Fig. 2 C and D). Antisense for Gαi1 or Gαi2 does not change DAMGO-induced PKA Cα translocation (Fig. 2C) or CRE-mediated gene expression (data not shown), confirming the preferential requirement of Gαi3.
Fig. 2.
Gαi3 AS blocks PKA activation. (A) Coimmunoprecipitation of MOR with Gαi3 subunits. MOR was immunoprecipitated with anti-MOR antibodies and probed with anti-Gαolf, -Gαo, -Gαi1, -Gαi2, or -Gαi3 antibodies. (B) Gαi expression was determined by Western blot analysis in cells transfected with or without HSVGαi1 AS, Gαi2 AS, Gαi3 AS, Gαolf AS, or vector control overnight. GAPDH was assayed as control for protein loading. (C and D) Gαi3 AS inhibition of Cα translocation (C) and CRE-luciferase expression (D). PGE1, prostaglandin E1. Data are the mean ± SEM of at least three experiments. *, P < 0.01, compared with control.
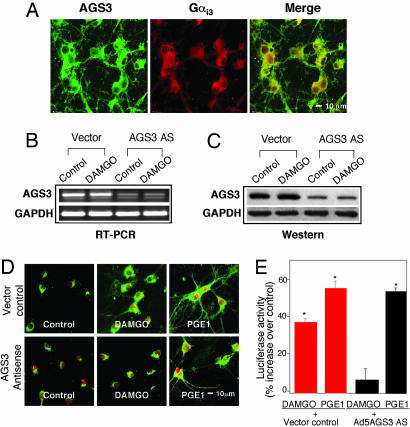
AGS3 Enhances MOR-Induced PKA Signaling. AGS3 also appears to interact preferentially with Gαi3, which is released upon MOR activation. When AGS3 binds to Gαi3, a stable AGS3-Gαi3-GDP complex is formed that maintains inactivation of Gαi3, thereby limiting Gαi3 recycling and its potential inhibition of AC (15). AGS3 and MOR are expressed in the NAc (6, 16). Therefore, AGS3 could modulate MOR signaling through Gαi3. By using immunocytochemistry, we determined that AGS3 and Gαi3 are expressed and colocalized in membranes, cytoplasm, and Golgi in primary NAc/striatal neurons (Fig. 3A). AS directed against AGS3 knocks down AGS3 mRNA and protein by >80% (Fig. 3 B and C) and prevents DAMGO-induced PKA Cα translocation (Fig. 3D) and CRE-mediated luciferase activity (Fig. 3E). By contrast, AGS3 AS is without effect on prostaglandin E1-induced PKA translocation and gene expression, because this activation depends on Gαolf or Gαs, not Gαi3 (Fig. 3 D and E). These findings are consistent with the possibility that, after MOR-induced release of Gαi3 and βγ subunits, AGS3 competes with βγ binding to a Gαi3-GDP complex (22), thereby enhancing MOR-dependent stimulation of AC by βγ. This possibility was confirmed in NAc/striatal neurons preincubated with PTX to inhibit Gαi/o dissociation into αi3 and βγ, or the Ad5βARK1 dominant-negative minigene to inhibit the action of βγ (23). We find that PTX or Ad5βARK1 each blocks DAMGO- or morphine-induced PKA Cα translocation (Fig. 1 A), CREB phosphorylation (Fig. 1B), and CRE-mediated luciferase expression (Fig. 1C).
Fig. 3.
AGS3 AS blocks PKA signaling. (A) Confocal analysis of AGS3 expression and colocalization with Gαi3 subunits. Shown are AGS3 (green), Gαi3 (red), merge (yellow). (B and C) AGS3 expression detected by RT-PCR (B) and Western blot analysis (C). Cells were transfected with or without Ad5AGS3 AS or vector control overnight, treated with or without DAMGO for 10 min, and then lysed for RT-PCR or Western blot analysis. (D and E) AGS3 AS inhibition of Cα translocation (D) and CRE-luciferase expression (E). Cells were transfected with Ad5AGS3 AS or vector control (D) and HSVCRE-luciferase (E) overnight. Data are the mean ± SEM of at least three experiments. *, P < 0.01, compared with control.
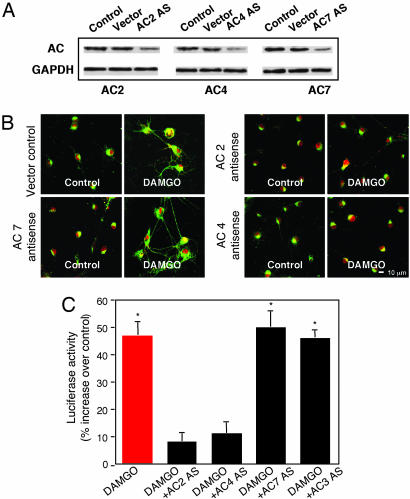
MOR Activation Stimulates AC2 and AC4. Several lines of evidence indicate that βγ dimers stimulate AC2, AC4, and perhaps AC7 (9-13). Western blot analysis shows that AC2, AC4, and AC7 are expressed in primary NAc/striatal neurons; AS against AC2, AC4, or AC7 knocks down the expression of each isoform, respectively (Fig. 4A). DAMGO-induced PKA Cα translocation (Fig. 4B) and CRE-luciferase expression (Fig. 4C) are inhibited by AC2 and AC4 antisense. Antisense for AC3 and AC7 have no effect on DAMGO-induced PKA signaling (Fig. 4 B and C and data not shown). These findings suggest that AC2 and AC4, which are known to be expressed in the NAc (24, 25), appear to be required for βγ-mediated stimulation of cAMP/PKA signaling upon MOR activation.
Fig. 4.
AC2 and AC4 AS block PKA activation. (A) AC2, AC4, and AC7 expression. Cells were transfected with or without HSVAC2 AS, AC4 AS, or AC7 AS overnight and then lysed. Lysates (20 μg) were Western-blotted with antibodies specific for AC2, AC4, or AC7, respectively. (B and C) AC2 AS and AC4 AS inhibition of Cα translocation (B) and CRE-luciferase expression (C). Cells were transfected with HSVAC2 AS, AC4 AS, AC7 AS, or AC3 AS overnight. Data are the mean ± SEM of at least three experiments. *, P < 0.01, compared with control.
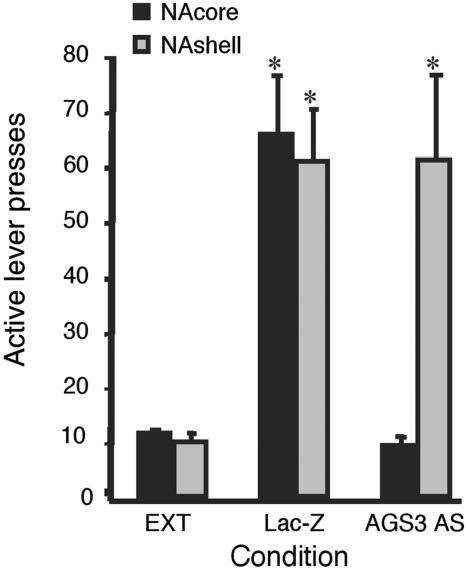
AGS3 Is Required for Heroin-Seeking Behavior. MOR activation of PKA signaling in NAc/striatal neurons requires AGS3. The NAc core has been implicated in heroin-induced reinstatement of drug-seeking behavior (25). Heroin self-administration was established in rats (26). We asked whether heroin-induced reinstatement would be regulated by AGS3 in the NAc. Expression of AGS3 antisense in the core of the NAc abolishes heroin-seeking behavior but is without effect when expressed in the NAc shell (Fig. 5). Moreover, AGS3 AS in the NAc did not affect heroin reinforcement as measured by heroin self-administration (data not shown). Therefore, AGS3 appears to be required in the NAc core primarily for the reinstatement of heroin-seeking behavior, a model of human relapse.
Fig. 5.
Ad5AGS3 AS eliminates heroin-seeking behavior in rats. AS for AGS3 (Ad5AGS3 AS, 2 μl per side, 1012 plaque-forming units/ml) was infused into the core or the shell of the NAc. AGS3 AS in the core of the NAc prevented reinstatement of lever pressing elicited by a priming injection of heroin. Data represent mean active lever presses ± SEM, n = 8 in all groups. *, P < 0.01, compared with extinction responding (EXT) (two-way ANOVA, Tukey's posttest).
Discussion
In this paper, we identify an important MOR-activated pathway in NAc/striatal neurons that stimulates cAMP/PKA signal transduction, followed by activation of CRE-mediated gene expression. AGS3 appears to be a key component of this pathway, because it is required for MOR-induced PKA signaling in primary NAc/striatal neurons. AGS3 in the core of the NAc is also required for reinstatement of heroin-seeking behavior. Our major findings are that: (i) Gαi3 and AGS3 associate preferentially with the MOR in NAc/striatal neurons, which appears to provide specificity for MOR-induced release of βγ dimers from Gi/o; (ii) Gαi3, AGS3, and βγ dimers are required for MOR stimulation of cAMP/PKA signaling and CRE-mediated gene expression in NAc/striatal neurons; and (iii) AGS3 in the core of the NAc is required for heroin-seeking behavior during opiate withdrawal. MOR agonists paradoxically induce PKA Cα activation and translocation to the nucleus and CREB phosphorylation within 10 min, followed by CRE-mediated gene expression hours later (Fig. 1). These events appear to be determined first by the predominant association of the MOR with Gαi3 (Fig. 2) and subsequently, after Gαi3 and βγ subunits are dissociated upon MOR activation, by the binding interaction of Gαi3 with AGS3 (Figs. 2 and 3). AGS3 is thought to bind transiently to the GDP-bound conformation of Gαi3, maintaining attenuation of Gαi3 inhibition of AC (15). Simultaneously, by competing with βγ for binding to Gαi3 (22), AGS3 delays the reassociation of free βγ dimers with Gαi3 and would be expected to prolong βγ stimulation of cAMP production unencumbered by Gαi inhibition (27). Thus, interventions to prevent AGS3 from binding to the Gαi3-GDP complex would be expected to hasten reassociation of free βγ dimers with Gαi3, thereby reducing βγ stimulation of AC. Consistent with this possibility, AS knockdown of AGS3 selectively prevents MOR-dependent increases in cAMP/PKA signaling (Fig. 3); whereas AS knockdown of AGS1 and AGS2 is without effect (data not shown).
The NAc core is thought to contribute to addictive behaviors (28). Inactivation of the NAc core, but not the shell, inhibits heroin self-administration (26) and heroin-induced reinstatement of drug-seeking behavior (K.M., unpublished data). Further, self-administration of heroin is associated with up-regulation of gene transcripts involved in neuronal function within the NAc core but down-regulation of these same transcripts within the NAc shell (29). Notably, inactivation of the NAc core (but not shell) (19, 30) or interference with prefrontal glutamatergic projections to the NAc core (20) also prevents cocaine-induced reinstatement of drug-seeking behavior (14). In that study, AGS3 AS in the prefrontal cortex prevented relapse of cocaine-seeking behavior. In this study, AGS3 AS in the NAc core, but not the shell, abolishes reinstatement of heroin-seeking behavior during withdrawal, a valid model of relapse in human heroin addiction (Fig. 5). It is of interest that AGS3 AS in the NAc did not block heroin reinforcement as measured by heroin self-administration (data not shown). This result argues for a specific effect of AGS3 AS on reinstatement and that MOR receptor blockade is not involved in this action. Our findings are consistent with current concepts about the special importance of the core of the NAc in addictive behavior (25, 27) and suggest that AGS3 in the core may regulate craving and relapse in heroin addiction.
The specific requirement for AGS3 in MOR-activated cAMP/PKA signaling appears to depend on the preferential association of Gαi3 with the MOR (Fig. 2 A). Thus, knockdown of Gαi3 eliminates MOR-induced PKA signaling and CRE gene expression; knockdown of other Gαi subunits is without effect (Fig. 2C and data not shown). We propose that in the absence of Gαi3, when βγ dimers would be rapidly degraded (31), a Gαi3βγ complex is not available to dissociate upon MOR activation so that free βγ is not formed and cannot stimulate AC2 or AC4 (12). Thus, Gαi3 as well as AGS3 appears to be required for βγ action. Our finding that the MOR preferentially interacts with Gαi3 (Fig. 2 A) is consistent with other reports that Gαi3 is associated with the MOR and the DOR (32). However, adrenergic receptor α2b and muscarinic receptor M4, which are coupled primarily to Gαi2, only inhibit cAMP production in the same cells (data not shown) and do not activate PKA signaling (10, 33, 34). This result suggests that the preferential association of Gαi3 with the MOR appears to be required for AGS3 regulation of βγ-stimulated cAMP/PKA signaling. However, we have not proven that AGS3/βγ regulation of PKA signaling, which we show in NAc/striatal neurons in vitro, is responsible in the NAc for AGS3/βγ regulation of heroin-seeking behavior in vivo. Other actions of βγ subunits, such as activation of G protein-activated inwardly rectifying potassium channels, could also be involved (35).
A prevailing concept is that short-term activation of MOR and DOR only inhibits AC activity. Several laboratories, however, find that increased cAMP production can be detected within minutes after exposure to opiate agonists (10, 12, 21, 36, 37). Our work suggests that unbound βγ dimers could be responsible for these recent observations. Moreover, our studies also suggest the possibility that βγ dimers may play a more general role in cellular mechanisms involved in addiction to other drugs. Dopamine D2, δ-opioid, and cannabinoid CB1 receptors also appear to be coupled preferentially to Gαi3 (38, 39). We find that βγ dimers are required for dopamine, opiate, cannabinoid, and ethanol stimulation of cAMP/PKA signaling and for voluntary alcohol consumption (10, 17). Thus, Gαi3, βγ, and AGS3 appear to be components of a regulatory mechanism shared in common by those Gi/o-coupled receptors involved in addiction. It is well established that βγ dimers have diverse effects in cells, including stimulation of PKA signaling by means of AC2 and AC4 (11, 40), which could be involved in addiction (3).
Uncontrolled craving and relapse is perhaps the most important clinical problem that limits effective medical treatment of addiction. Current concepts suggest that reinstatement during withdrawal is a valid model of human drug-seeking behavior (craving). One of our most important findings during heroin withdrawal is that AS directed against AGS3 in the core of the NAc, but not the shell, abolishes reinstatement in heroin-addicted rats. This finding is consistent with recent findings that AGS3 appears to be required for cocaine-induced behavioral sensitization and reinstatement (14). We propose that, by competing with βγ for binding to the Gαi3-GDP complex (22), AGS3 enhances the action of βγ dimers, and that Gαi3, AGS3, and βγ dimers appear to be components of a signaling pathway that underlies addictive behavior. Our results suggest that molecular mechanisms that differentially regulate the action of Gαi3, AGS3, and βγ dimers could underlie drug-seeking behavior (craving) and mediate relapse in heroin addicts. These observations open an avenue to develop treatments for heroin addiction directed against relapse.
Acknowledgments
We thank Dr. Susan Taylor (University of California, San Diego) for antibodies to PKA Cα, Dr. R. J. Lefkowitz (Howard Hughes Medical Institute, Duke University Medical Center, Durham, NC) for the βARK1 minigene, Dr. R. L. Neve (Harvard Medical School, Boston) for the HSVPrpUC vector, and Anjlee Mahajan for expert manuscript preparation. This work was supported by National Institutes of Health Grant AA010030 (to I.D.); the University of California, San Francisco (through state funding for ethanol and substance abuse research); and Department of the Army Grant DAMD 17-01-1-0803 (to I.D. and L.Y.).
Abbreviations: AS, antisense; MOR, μ opiate receptor; DOR, δ opiate receptor; PKA, protein kinase A; NAc, nucleus accumbens; AC, adenylyl cyclase; DAMGO, [d-Ala2,N-MePhe4,Gly5-ol]enkephalin; AGS3, activator of G protein signaling 3; HSV, herpes simplex virus; CRE, cAMP-response element; CREB, CRE-binding protein; CTOP, d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2; PTX, pertussis toxin.
References
- 1.Matthes, H. W., Maldonado, R., Simonin, F., Valverde, O., Slowe, S., Kitchen, I., Befort, K., Dierich, A., Le Meur, M., Dolle, P., et al. (1996) Nature 383, 819-823. [DOI] [PubMed] [Google Scholar]
- 2.Sharma, S. K., Klee, W. A. & Nirenberg, M. (1975) Proc. Natl. Acad. Sci. USA 72, 3092-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestler, E. J. & Aghajanian, G. K. (1997) Science 278, 58-63. [DOI] [PubMed] [Google Scholar]
- 4.Avidor-Reiss, T., Bayewitch, M., Levy, R., Matus-Leibovitch, N., Nevo, I. & Vogel, Z. (1995) J. Biol. Chem. 270, 29732-29738. [DOI] [PubMed] [Google Scholar]
- 5.Nestler, E. J. & Malenka, R. C. (2004) Sci. Am. 290 (3), 78-85. [DOI] [PubMed] [Google Scholar]
- 6.Svingos, A. L., Moriwaki, A., Wang, J. B., Uhl, G. R. & Pickel, V. M. (1996) J. Neurosci. 16, 4162-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svingos, A. L., Clarke, C. L. & Pickel, V. M. (1998) J. Neurosci. 18, 1923-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defer, N., Best-Belpomme, M. & Hanoune, J. (2000) Am. J. Physiol. 279, F400-F416. [DOI] [PubMed] [Google Scholar]
- 9.Bayewitch, M. L., Avidor-Reiss, T., Levy, R., Pfeuffer, T., Nevo, I., Simonds, W. F. & Vogel, Z. (1998) J. Biol. Chem. 273, 2273-2276. [DOI] [PubMed] [Google Scholar]
- 10.Yao, L., Fan, P., Jiang, Z., Mailliard, W. S., Gordon, A. S. & Diamond, I. (2003) Proc. Natl. Acad. Sci. USA 100, 14379-14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang, W. J. & Gilman, A. G. (1991) Science 254, 1500-1503. [DOI] [PubMed] [Google Scholar]
- 12.Chan, J. S., Chiu, T. T. & Wong, Y. H. (1995) J. Neurochem. 65, 2682-2689. [DOI] [PubMed] [Google Scholar]
- 13.Baker, L. P., Nielsen, M. D., Impey, S., Hacker, B. M., Poser, S. W., Chan, M. Y. & Storm, D. R. (1999) J. Neurosci. 19, 180-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowers, M. S., McFarland, K., Lake, R. W., Peterson, Y. K., Lapish, C. C., Gregory, M. L., Lanier, S. M. & Kalivas, P. W. (2004) Neuron 42, 269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vries, L., Fischer, T., Tronchere, H., Brothers, G. M., Strockbine, B., Siderovski, D. P. & Farquhar, M. G. (2000) Proc. Natl. Acad. Sci. USA 97, 14364-14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumer, J. B., Chandler, L. J. & Lanier, S. M. (2002) J. Biol. Chem. 277, 15897-15903. [DOI] [PubMed] [Google Scholar]
- 17.Yao, L., Arolfo, M. P., Dohrman, D. P., Jiang, Z., Fan, P., Fuchs, S., Janak, P. H., Gordon, A. S. & Diamond, I. (2002) Cell 109, 733-743. [DOI] [PubMed] [Google Scholar]
- 18.Macrez-Lepretre, N., Kalkbrenner, F., Morel, J. L., Schultz, G. & Mironneau, J. (1997) J. Biol. Chem. 272, 10095-10102. [DOI] [PubMed] [Google Scholar]
- 19.McFarland, K. & Kalivas, P. W. (2001) J. Neurosci. 21, 8655-8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFarland, K., Lapish, C. C. & Kalivas, P. W. (2003) J. Neurosci. 23, 3531-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubovitch, V., Gafni, M. & Sarne, Y. (2003) Brain Res. Mol. Brain Res. 110, 261-266. [DOI] [PubMed] [Google Scholar]
- 22.Lanier, S. M. (2004) Biol. Cell. 96, 369-372. [DOI] [PubMed] [Google Scholar]
- 23.Koch, W. J., Hawes, B. E., Inglese, J., Luttrell, L. M. & Lefkowitz, R. J. (1994) J. Biol. Chem. 269, 6193-6197. [PubMed] [Google Scholar]
- 24.Furuyama, T., Inagaki, S. & Takagi, H. (1993) Brain Res. Mol. Brain Res. 19, 165-170. [DOI] [PubMed] [Google Scholar]
- 25.Mons, N., Harry, A., Dubourg, P., Premont, R. T., Iyengar, R. & Cooper, D. M. (1995) Proc. Natl. Acad. Sci. USA 92, 8473-8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alderson, H. L., Parkinson, J. A., Robbins, T. W. & Everitt, B. J. (2001) Psychopharmacology 153, 455-463. [DOI] [PubMed] [Google Scholar]
- 27.Bernard, M. L., Peterson, Y. K., Chung, P., Jourdan, J. & Lanier, S. M. (2001) J. Biol. Chem. 276, 1585-1593. [DOI] [PubMed] [Google Scholar]
- 28.Kalivas, P. W., McFarland, K., Bowers, S., Szumlinski, K., Xi, Z. X. & Baker, D. (2003) Ann. N.Y. Acad. Sci. 1003, 169-175. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs, E. H., de Vries, T. J., Smit, A. B. & Schoffelmeer, A. N. (2004) FASEB J. 18, 200-202. [DOI] [PubMed] [Google Scholar]
- 30.Ito, R., Robbins, T. W. & Everitt, B. J. (2004) Nat. Neurosci. 7, 389-397. [DOI] [PubMed] [Google Scholar]
- 31.Lee, Y. I., Kim, S. Y., Cho, C. H., Seo, M., Cho, D. H., Kwak, S. J. & Juhnn, Y. S. (2003) FEBS Lett. 555, 329-334. [DOI] [PubMed] [Google Scholar]
- 32.Chalecka-Franaszek, E., Weems, H. B., Crowder, A. T., Cox, B. M. & Cote, T. E. (2000) J. Neurochem. 74, 1068-1078. [DOI] [PubMed] [Google Scholar]
- 33.McClue, S. J. & Milligan, G. (1990) FEBS Lett. 269, 430-434. [DOI] [PubMed] [Google Scholar]
- 34.Migeon, J. C. & Nathanson, N. M. (1994) J. Biol. Chem. 269, 9767-9773. [PubMed] [Google Scholar]
- 35.Peng, L., Mirshahi, T., Zhang, H., Hirsch, J. P. & Logothetis, D. E. (2003) J. Biol. Chem. 278, 50203-50211. [DOI] [PubMed] [Google Scholar]
- 36.Bilecki, W., Wawrzczak-Bargiela, A. & Przewlocki, R. (2004) J. Neurochem. 90, 874-882. [DOI] [PubMed] [Google Scholar]
- 37.Bilecki, W., Hollt, V. & Przewlocki, R. (2000) Eur. J. Pharmacol. 390, 1-6. [DOI] [PubMed] [Google Scholar]
- 38.Senogles, S. E. (1994) J. Biol. Chem. 269, 23120-23127. [PubMed] [Google Scholar]
- 39.Mukhopadhyay, S. & Howlett, A. C. (2001) Eur. J. Biochem. 268, 499-505. [DOI] [PubMed] [Google Scholar]
- 40.Ford, C. E., Skiba, N. P., Bae, H., Daaka, Y., Reuveny, E., Shekter, L. R., Rosal, R., Weng, G., Yang, C. S., Iyengar, R., et al. (1998) Science 280, 1271-1274. [DOI] [PubMed] [Google Scholar]