Abstract
Waterpipe tobacco (WPT) smoking is a public health concern, particularly among youth and young adults. The global spread of WPT use has surged because the introduction of pre-packaged flavored and sweetened WPT, which is widely marketed as a safer tobacco alternative. Besides flavorants and sugars, WPT additives include humectants, which enhance the moisture and sweetness of WPT, act as solvents for flavors, and impart smoothness to the smoke, thus increasing appeal to users. In the United States, unlike cigarette tobacco flavoring (with the exception of menthol), there is no FDA product standard or policy in place prohibiting sales of flavored WPT. Research has shown that the numerous fruit, candy, and alcohol flavors added to WPT entice individuals to experience those flavors, putting them at an increased risk of exposure to WPT smoke-related toxicants. Additionally, burning charcoal briquettes—used as a heating source for WPT—contributes to the harmful health effects of WPT smoking. This review presents existing evidence on the potential toxicity resulting from humectants, sugars, and flavorants in WPT, and from the charcoal used to heat WPT. The review discusses relevant studies of inhalation toxicity in animal models and of biomarkers of exposure in humans. Current evidence suggests that more data are needed on toxicant emissions in WPT smoke to inform effective tobacco regulation to mitigate the adverse impact of WPT use on human health.
Keywords: waterpipe, hookah, flavorants, humectants, sugars, charcoal
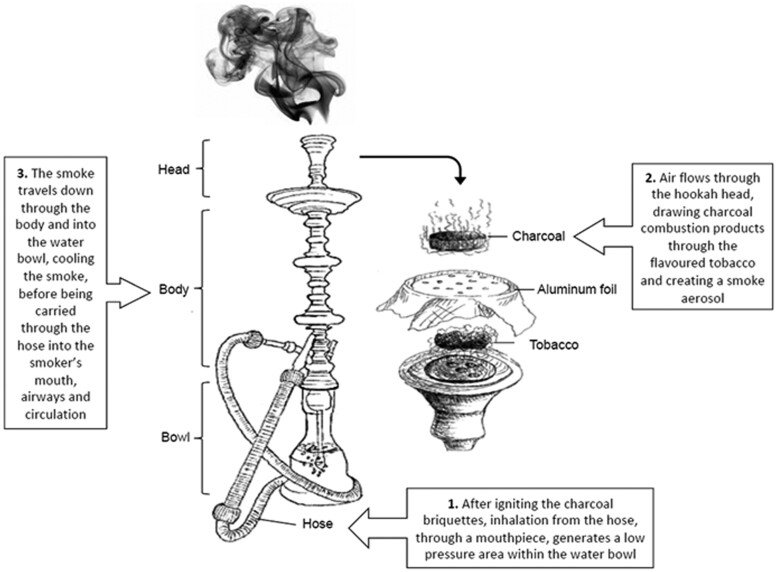
Waterpipe tobacco (WPT) smoking is a centuries-old tobacco use method in which burning charcoal heats tobacco, producing smoke that passes through a water-filled bowl before reaching the user’s mouth, lungs, and circulatory system (Fig. 1, Rezk-Hanna and Benowitz 2019). The use of a waterpipe, also known as hookah, shisha, and narghile, to smoke tobacco has become increasingly popular worldwide, particularly among youth and young adults in several eastern Mediterranean, eastern European, and Western countries, including the United States (Jawad et al. 2018; Zheng et al. 2022). Nationally representative data from the Population Assessment of Tobacco and Health (PATH) Study from 2013 to 2018 indicated that among US adolescents (12–17 yrs) and young adults (18–24 yrs), 4.8% and 18.5% of individuals who never-used WPT initiated WPT use during that period, respectively, and 10.6% and 14.1% of individuals who ever-used WPT increased the frequency of WPT use during the same period, respectively (Gautam et al. 2022).
Fig. 1.
Diagram of waterpipe elements. (Copyright: Mary Rezk-Hanna)
There are many adverse health consequences associated with WPT use, including lung and esophageal cancer, and diminished parameters of cardiopulmonary and cardiovascular function (Raad et al. 2011; Montazeri et al. 2017; Qasim et al. 2019; Al Ali et al. 2020; Hassane et al. 2022; Mahfooz et al. 2023). Nevertheless, there continues to be broad social acceptance of use in the United States and worldwide due in part to misinformation about the associated risks (Cobb et al. 2010). WPT is often perceived as safe or a safer alternative to other combustible tobacco products, and this perception may lead to initiation and continued use of WPT (Kuk et al. 2022). For example, data from Wave 1 of the PATH Study (2013–2014) showed that US adolescents (12–17 yrs) who perceived WPT to be neither harmful nor addictive were 173% more likely to initiate WPT ever use, and 166% more likely to first report past 30-d use, compared with their counterparts who considered WPT to be both harmful and addictive (Kuk et al. 2022). Common misbeliefs about WPT use that may encourage initiation and continued use include: (1) WPT smoking is less addictive than cigarettes (Elton-Marshall et al. 2020), thus misguiding users about their ability to quit WPT use; (2) water through which the smoke passes “filters out” toxicants, resulting in the misperception that WPT is a safer product (Cobb et al. 2010); and (3) WPT use is a social activity not typically occurring on a daily or frequent basis, leading users to assume that intermittent use is safe despite the substantial exposure levels of smoke toxicants (Cobb et al. 2010). This lack of perceived harm has enhanced the social acceptance of WPT use (Cobb et al. 2010).
The growing popularity of WPT use has been attributed to several factors: (1) the introduction of flavored and sweetened WPT providing pleasant, smooth smoke; (2) the availability of WPT in numerous desirable aromatic flavors, including fruit, candy and alcohol flavors; (3) increased accessibility to WPT through sales in convenience stores, tobacco retailers, and online; (4) unregulated advertisements and marketing claims fueling misperceptions of reduced harm compared with cigarette use; (5) flourishing of WPT discussions on social media platforms; and (6) rapid emergence and proliferation of hookah lounges/cafes in close proximity to colleges providing patrons a social setting with food, drinks, and entertainment, or a place to study with friends while smoking and sharing a waterpipe (Maziak et al. 2004b; Maziak 2010, 2011; Kassem et al. 2015, 2019; Ma et al. 2022).
WPT is available in 3 forms: (1) unflavored tobacco (known as Ajami, Isfahani, or Tumbak/Tombak), which consists of dry tobacco leaves; (2) unflavored sweetened tobacco (known as ma’assel), which consists of tobacco leaves infused with honey, molasses, and other sweet syrups; and (3) flavored and sweetened tobacco (also known as flavored ma’assel), which consists of tobacco leaves infused with honey, molasses, and other sweet syrups and a variety of flavoring agents. This review focuses on flavored and sweetened waterpipe tobacco, also referred to hereafter as flavored waterpipe tobacco, waterpipe tobacco, or WPT.
This review examines the following aspects of flavored and sweetened WPT: toxicity of WPT smoke in animal models, nicotine intake and biomarkers of exposure in humans, biomarkers of secondhand smoke exposure, and toxicity resulting from humectants, sugars, flavorants, and charcoal. We conclude with a review of WPT regulations in the United States and provide suggestions for future research that could be leveraged to help mitigate the adverse impacts of WPT use on public health.
Toxicity of WPT smoke
Toxicity of WPT smoke in animal models
Acute and chronic animal exposure to WPT smoke has been shown to induce lung inflammation and injury (Table 1). For example, in mice, WPT smoke exposure elevated oxidative stress and inflammatory responses in the lungs with increased recruitment of leukocytes and respective cytokines (Nemmar et al. 2013; Khabour et al. 2018). WPT smoke exposure resulted in increased expression of matrix metalloproteinases, MMP9 and MMP12, in the lungs of mice, indicating potential chronic lung injury, inflammatory responses, and extracellular matrix (ECM) remodeling (Greenlee et al. 2007; Khabour et al. 2015). WPT smoke exposure can result in a dysregulation of the circadian clock gene profile in the lungs, which has been associated with multiple chronic lung diseases (Khan et al. 2019). Daily exposure to WPT smoke for 2 mo showed severe DNA damage in the lungs, kidneys, bone marrow, and liver of mice (Abi-Gerges et al. 2020). Prenatal exposure to WPT smoke has been shown to increase asthmatic risk in offspring of mice, elevate inflammation and oxidative stress in the lung and hippocampus, and potentially contribute to short- and long-term memory impairment in offspring rats (Al-Sawalha et al. 2017, 2018). To gain a more complete toxicological profile of WPT use in animal models, future research should investigate different WPT products with carefully manipulated additives and smoking durations.
Table 1.
Toxicity of flavored and sweetened WPT smoke in animal studies.
| Species/strain | Exposure type | Duration | Puff profile | WPT flavor (brand) | Toxicity (target organs) |
|---|---|---|---|---|---|
| Balb/c mice | Whole body |
|
a |
|
Airway inflammation (Khabour et al. 2018). |
| Balb/c mice | Whole body |
|
a |
|
Increased inflammation responses and oxidative stress in lungs (Khabour et al. 2012). |
| Balb/C mice | Nose only | 1 mo | b |
|
Increased the risk of thrombogenicity, and heart inflammatory response (Nemmar et al. 2019). |
| Balb/c mice—male | Whole body |
|
a |
|
Increased oxidative stress and levels of MMP1, 3, and 9 in heart (Rababa’h et al. 2019). |
| C57BL/6 mice | Whole body |
|
a |
|
Increased the risk of thrombosis (Alarabi et al. 2020). |
| C57BL/6 mice | Whole body |
|
a |
|
Lung inflammation, DNA damage noticed in lung, kidney, liver, and bone marrow (Abi-Gerges et al. 2020). |
| C57BL/6 mice | Nose only |
|
b |
|
Inflammation and DNA damage were noticed in the lungs after WPT smoke exposure (Nemmar et al. 2019) |
| C57BL/6 mice | Nose only |
|
b |
|
Increased the risk of thrombosis, oxidative stress, and DNA damage in heart (Nemmar et al. 2022). |
| C57BL/6 mice | Nose only |
|
b |
|
Increased lung inflammation, oxidative stress, DNA damage, and asthmatic risk (Nemmar et al. 2020a). |
| C57BL/6 mice | Nose only |
|
b |
|
Increased DNA damage, oxidative stress, and the risk of interstitial fibrosis in heart (Nemmar et al. 2017). |
| Wister rats | Whole body |
|
a |
|
Oxidative stress was elevated in brain; induced short- or long-term memory loss (Alzoubi et al. 2015). |
| Wistar rats | Whole body |
|
a |
|
Blood pressure and fasting glucose level were increased after WPT smoke exposure (Al-Sawalha et al. 2020). |
| Wistar rats—male | Whole body |
|
a |
|
WPT smoke exposure caused memory loss (Alzoubi et al. 2019). |
| Balb/c mice | Nose only |
|
b |
|
Increased inflammation in heart and risk of thrombus (Nemmar et al. 2015b). |
| Balb/c mice | Nose only |
|
b |
|
Lung inflammation and oxidative stress were noticed after WPT smoke exposure (Nemmar et al. 2013). |
| BALB/C mice | Nose only |
|
b |
|
Inflammation, oxidative stress, and DNA damage were noticed in kidney (Nemmar et al. 2020c). |
| Balb/c mice | Nose only |
|
b |
|
WPT smoke-induced inflammation and oxidative stress were noticed in lung (Nemmar et al. 2015a). |
| Balb/c mice | Nose only |
|
b |
|
Induced lower levels of antioxidant, testosterone, and luteinizing hormone in plasma (Ali et al. 2015). |
| C57BL/6 mice—female | Nose only |
|
a |
|
Lymphocyte activity was inhibited by WPT smoke (Reyes-Caballero et al. 2020). |
| Gprc5a or Lcn2 KO mice | Whole body |
|
a |
|
Increased the risk of lung tumor development (Hassane et al. 2022). |
| Wistar rats | Whole body |
|
a |
|
Dysregulated the male hormonal levels and increased oxidative stress in testes (Al-Sawalha et al. 2021). |
| Balb/c mice | Whole body |
|
a |
|
Increased lung inflammation and oxidative stress, and the allergic risk in offspring (Al-Sawalha et al. 2017). |
| Wistar rats | Whole body |
|
a |
|
Either short- or long-term memory were affected. Catalase level in brain was increased in late gestation and whole gestation WPT smoke exposure (Al-Sawalha et al. 2018). |
| Wister rats | Whole body |
|
a |
|
Lower body weight and survival rate in offspring (Al-Sawalha et al. 2018). |
2.6/3s puff duration with 17 s interval.
2s puff duration with 58 s interval.
Biomarkers of exposure to WPT smoke
Although WPT is not directly burned, the temperature that WPT reaches during smoking (∼150°C) can result in toxicant generation (Brinkman et al. 2020b). Toxicants stem primarily from the thermal degradation of WPT constituents or from the heating source itself (e.g. charcoal), and include polycyclic aromatic hydrocarbons (PAHs), carbon monoxide (CO), and volatile organic compounds (VOCs) (Olsson and Petersson 2003; Monzer et al. 2008; Jacob et al. 2011; Kassem et al. 2014b). The uptake of these toxicants in the body is assessed by quantifying biomarkers of exposure similar to those measured from cigarette smoking. Biomarkers measured in people who smoke WPT include the metabolites of nicotine, tobacco-specific nitrosamines (TSNA), PAHs, and VOCs (Etemadi et al. 2023).
Levels of NNAL (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol), a metabolite of the carcinogenic nicotine-derived nitrosamine ketone (NNK), were higher in urine samples of participants who smoke WPT exclusively compared with participants who do not use any tobacco (Kassem et al. 2017). Another study found that children ≤5 yrs old living in homes of participants who exclusively smoked WPT daily had 37.3 times significantly higher levels of urinary NNAL than their counterparts living in homes of participants who did not smoke any tobacco (Kassem et al. 2014a). However, TSNA emissions from WPT smoking and resulting NNAL biomarker levels were generally lower than those reported for cigarette smokers (Jacob et al. 2013; Radwan et al. 2013). WPT smoking is associated with high urinary concentrations of hydroxy-PAH metabolites, especially those of high molecular weight PAHs (e.g. hydroxypyrene) (Jacob et al. 2013). Many VOC metabolites are also increased in the urine of people who smoke WPT, especially those of benzene (Kassem et al. 2014b), which stems primarily from the use of charcoal (Olsson and Petersson 2003). Using charcoal as the heating source for WPT increases users’ exposure to benzene, PAHs, and CO (Monzer et al. 2008).
Other toxic compounds found in WPT smoke are the semivolatile furans (Brinkman et al. 2020b), especially 5-(hydroxymethyl)-2-furaldehyde and 2-furaldehyde, which were present in WPT smoke at 3900 and 230 times higher levels, respectively, than in cigarette smoke (Brinkman et al. 2020b). Some urinary furan metabolites were higher in participants who exclusively smoked WPT compared with participants who did not use any tobacco (Kassem et al. 2020).
Several studies have measured acute biomarkers of exposure in controlled experimental settings and natural settings such as homes and hookah lounges/bars. Irrespective of the timing of the most recent WPT use, people who smoke WPT had significantly higher concentrations of all of the biomarkers mentioned above (Etemadi et al. 2019). This indicates that people who smoke WPT are chronically exposed to many toxicants and carcinogens.
Moreover, biomarkers of harm, including inflammation, oxidative stress, immunity, tissue injury, and repair were elevated in people who smoke WPT (Khan et al. 2020). For example, plasma levels of biomarkers of oxidative stress and inflammation, such as IL-1β, IL-6, IL-8, and TNFα, were significantly higher in people who smoke WPT compared with people who do not smoke any tobacco, indicating elevated systemic inflammation response (Khan et al. 2020). Similarly, urinary biomarkers of oxidative stress and inflammation, such as 8-isoprostanes, MPO, RAGE, En-RAGE, and MMP-9, were also elevated in people who smoked WPT (Khan et al. 2020). Analyses of nationally representative data from Wave 1 of the PATH Study (2013–2014) showed that cardiovascular disease-related biomarkers of potential harm, including serum sICAM-1 and urinary F2-isoprostane, were lower among people who smoke WPT exclusively than people who smoke cigarettes exclusively (Rezk-Hanna et al. 2023a). However, these findings represent patterns of WPT smoking predominantly shared among US adults who report non-daily intermittent use of WPT and do not reflect solitary, daily use (Rezk-Hanna et al. 2023a).
Nicotine intake from WPT
Although many people who smoke WPT believe that WPT is not addictive (Maziak et al. 2004a; Primack et al. 2008; Smith-Simone et al. 2008), emerging studies have shown that its use is associated with nicotine dependence (Aboaziza and Eissenberg 2015). When people who smoke cigarettes and are nicotine-dependent smoke low-nicotine-yield cigarettes, they compensate by smoking more intensely (more frequent and larger volume puffing) to attain their accustomed level of nicotine intake (Benowitz 2001). Similarly, compensation occurs among people experienced with smoking WPT when they smoke WPT with lower nicotine emissions (Brinkman et al. 2020a).
WPT typically contains cut-up tobacco leaves and up to ∼70 weight-% of additives. The additives-to-tobacco ratio drives the nicotine content of WPT (e.g. WPTs with higher concentrations of additives have lower nicotine concentrations). The reported nicotine content of WPT ranges from 0.5 to 6.3 mg/g of head-filler (Hadidi and Mohammed 2004; Kulak et al. 2017). Some nicotine is lost to the water when the smoke is pulled through the waterpipe (Edwards et al. 2021). Data obtained from smoking machines show that water in the bowl reduced nicotine content in WPT mainstream smoke between 1.4- and 3.1-fold; the nicotine content of water-filtered WPT mainstream smoke ranged from 13 to 46 µg per puff (Erythropel et al. 2021); and total nicotine inhaled for a typical WPT smoking session can be as high as 9000 µg/session (Shihadeh et al. 2015).
WPT use is associated with significant nicotine intake. For example, a study of 55 participants who smoke WPT found a 4-fold increase in cotinine (a urinary biomarker of nicotine) following smoking WPT at a hookah bar (St Helen et al. 2014). Similarly, a study of 105 participants who exclusively smoke WPT found 8.6- and 8.4-fold increases in urinary cotinine levels following smoking WPT at a hookah lounge (n = 55) and following smoking WPT in a home setting (n = 50), respectively (Kassem et al. 2018a). Another study found a substantial increase in plasma nicotine concentration among 16 participants who smoked WPT in a clinical research ward (Jacob et al. 2011). Overall, a significant uptake of nicotine from WPT smoking underscores its addiction potential.
WPT and secondhand smoke exposure
People who do not smoke any tobacco but live in homes where WPT is used are also exposed to nicotine, toxicants, and carcinogens. For example, a study found that children ≤5 yrs old living in homes of people who smoke WPT daily had significantly higher levels of urinary cotinine, NNAL, and 3-HPMA (a metabolite of acrolein) compared with children of people who do not smoke any tobacco (Kassem et al. 2014a). Another study found that adults who do not smoke WPT but socialize with people who smoke WPT had significantly higher levels of urinary cotinine and 3-HPMA following social gatherings where only WPT was used, and about half (47%) had detectable levels of NNAL in urine (Kassem et al. 2017, 2018a, 2018b). Indeed, more studies are needed to investigate exposure to WPT secondhand and thirdhand smoke, particularly among people who reside in homes where WPT is smoked, such as children, women of reproductive age or pregnant, adolescents, and older adults with pre-existing cardiopulmonary diseases.
Contribution of additives to the toxicity of WPT
Humectants
The most common WPT consumed worldwide, flavored and sweetened WPT, called ma’assel (Maziak 2015), has been shown to contain up to 70 weight-% of the humectants glycerol and propylene glycol (Schubert et al. 2012b). Humectants in WPT enhance WPT’s moisture and sweetness, act as solvents for flavors, and impart smoothness to the smoke, thus increasing the product’s appeal (Adetona et al. 2020; Wagener et al. 2021; Keller-Hamilton et al. 2022). Humectants may replace more expensive ingredients such as molasses or honey to reduce the price of mass-produced hookah tobacco (Brinkman et al. 2020b). Because WPT does not burn self-sustainably and is instead heated indirectly by charcoal, the maximum temperature of WPT is much lower than the combustion zone of a burning cigarette, 150 °C and 950 °C, respectively (Baker and Bishop 2004; Shihadeh and Saleh 2005). As a result, this leads to the intact transfer of most WPT humectants to the smoke, forming up to 23% of the collected total particulate matter (TPM), namely tar (Schubert et al. 2011).
Humectants make limited contributions to aldehyde emissions in cigarettes (e.g. glycerol generally only present at 1–3 weight-%) (Yip et al. 2010), but when present as the main ingredients in e-cigarettes (e.g. glycerol, propylene glycol present in the range of 80–99 weight-%), they do contribute substantially to the emission of aldehydes and other toxicants (Saliba et al. 2018; Ooi et al. 2019; Strongin 2019; El-Hage et al. 2020; AlGemayel et al. 2022). A study indicates that the presence of acrolein in WPT smoke is positively related to the humectant (glycerol) content of the unburned WPT (Almomen et al. 2023). Another study showed that glycerol in WPT notably contributed to VOC in mainstream WPT smoke (Perraud et al. 2019).
The presence of glycerol and propylene glycol in WPT strongly correlates with WPT flavorant levels. For example, 1 flavored WPT brand contains 20 times higher levels of humectants compared with an unflavored WPT brand (Adetona et al. 2020). Humectants also increase smoke production, as they can constitute up to 23% of the tar thereby facilitating nicotine delivery and greater smoking satisfaction (Keller-Hamilton et al. 2022). There is a need to further study the impact of humectants on toxicant generation in WPT smoke.
Sugars
Reducing sugars (e.g. glucose and fructose) can make up 34 weight-% of WPT as seen in Table 1 in Jaccard et al. (2020). Total sugar content levels, or the sum of fructose, glucose, and sucrose, were comparable between a flavored brand and those in an “unflavored” WPT brand by a factor of ∼2 (Adetona et al. 2020). WPT is enriched with ∼15–50 times higher concentrations of simple sugars than other combustible tobacco products such as cigarettes (Maziak and Sharma 2020). The sweet sensory perceptions associated with flavored and sweetened WPT are cited as reinforcing factors for WPT use (Martinasek et al. 2011). Flavorings and other additives, especially sweeteners (e.g. sugars, honey, syrup), contribute to the appeal and uptake of WPT smoking among youth (Martinasek et al. 2011; Hoffman et al. 2016; Ben Taleb et al. 2020; Maziak et al. 2020; Wagener et al. 2021). Indeed, an analysis of WPT-related tweets on the social media platform X (formerly Twitter) found that most flavors mentioned and preferred were associated with sweet sensations: fruit, sweets, and beverage/alcohol (Feliciano et al. 2023).
People who smoke WPT are exposed to toxicants from the thermal degradation of the sugar additives in WPT, which pyrolyze to form respiratory toxicants (van Nierop et al. 2019; Jaccard et al. 2020). The thermal degradation of sugar additives in WPT leads to the emission of toxicants and carcinogens, including carbonyls, aldehydes, and semivolatile furans (Talhout et al. 2006; Daher et al. 2010; Schubert et al. 2012a; Shihadeh et al. 2015; Soussy et al. 2016; Kassem et al. 2018b; Perraud et al. 2019). Compared with cigarette smoke, WPT smoke contains several orders of magnitude higher concentrations of semivolatile furans, including furfural and 5-hydroxymethylfurfural, both sugar degradation products (Schubert et al. 2012a; Brinkman et al. 2020b). However, the lack of acute and chronic inhalation toxicity data for semivolatile furans is a significant gap in the current understanding of WPT toxicology and is thus a barrier to effective tobacco control (Maziak and Sharma 2020).
Flavorants
WPT smokers have reported higher enjoyment, liking, satisfaction, and calmness when using flavored WPT than when using unflavored varieties (Leavens et al. 2018; Ben Taleb et al. 2019; Maziak et al. 2020). One study reported that out of 237 commercial WPT products (including steam stones and herbal molasses) sold in the European Union (EU) countries, 75% were “fruit” flavored, and authors categorized these into 8 main flavor categories and 48 unique flavor subcategories (Bakker-‘t Hart et al. 2022). The most frequently detected flavoring chemicals (excluding sugars) included vanillin, ethyl vanillin (both typical “dessert” flavorants), dihydrocoumarin (“spice”), ethyl butyrate, ethyl acetate, ethyl-2-methylbutyrate, isoamyl acetate (all “fruity”), maltol (“dessert”), menthol (“minty”), and benzyl alcohol (“fruity/floral”). The popularity of flavored WPT among young people indicates that flavors facilitate nicotine initiation, which is concerning due to nicotine’s well-known effects on the developing brain and other organs and its addictiveness (CDC 2012; Alomari et al. 2018; Colyer-Patel et al. 2023).
Numerous WPT flavors have been reported (Schubert et al. 2013; Javed et al. 2017), with each flavored WPT typically containing a mixture of several flavoring chemicals (Schubert et al. 2013), some of which possess allergenic, irritant, and toxicological properties (Silverman et al. 1946; Gupta et al. 1991; Schubert et al. 2013; Hua et al. 2019). The adverse health effects associated with inhaling flavored WPT smoke are understudied, with very little available clinical and pre-clinical data (Schubert et al. 2013; Nemmar et al. 2020a).
The chemical flavorings in WPT smoke can lead to additive or synergetic toxicological responses compared with unflavored WPT smoke, as seen in animal models (Nemmar et al. 2020a, 2020b). In a mouse model, 1 experimental study evaluated the effects of unflavored, apple-flavored, or strawberry-flavored WPT smoke on pulmonary responses (Nemmar et al. 2020a). Following 1 mo of exposure, authors found that unflavored and flavored WPT smoke-induced significant lung function and structure changes compared with air-exposed control mice (Nemmar et al. 2020a). Although apple and strawberry-flavored WPT smoke altered levels of IL-6 and catalase, nitric oxide and cleaved caspase-3 levels were only significantly changed in the strawberry WPT smoke-exposed group (Nemmar et al. 2020a). Thus, different toxicities between flavored and unflavored WPT were observed, with strawberry-flavored WPT smoke being the most harmful to mice.
Further, another study evaluated the effect of unflavored and apple-flavored WPT smoke on the cardiovascular system of mice over 1 mo (Nemmar et al. 2020b). It found that, compared with air, inhaling WPT smoke increased blood pressure levels and altered markers for thrombosis and blood vessel reactivity (Nemmar et al. 2020b). The addition of the apple flavor led to increased cardiovascular dysfunction with increased oxidative stress and inflammation in the heart (Nemmar et al. 2020b). These 2 studies confirm, at the pre-clinical level, a differential cardiopulmonary toxicity potential of flavored WPT smoke vs. unflavored WPT smoke (Nemmar et al. 2020a, 2020b).
The remainder of this section describes research findings for several different flavorant classes and their related compounds. This is expanded in Table S1, which lists select flavor-related compounds, grouped by their chemical classification and flavor category.
Esters and lactones
Esters were either the most or second most abundant class of flavorants across all flavored WPT products studied (Farag et al. 2018). For example, lactones (cyclic esters) were characteristic of peach-flavored products (Farag et al. 2018). At elevated temperatures, esters may form harmful carboxylic acids (Narimani et al. 2022).
Ketones
Of significant concern is the finding of 2,3-butanedione (diacetyl) (Farag et al. 2018). Diacetyl, the notorious “buttery” flavor identified as the causative agent of Bronchiolitis obliterans (“popcorn lung”) (Harber et al. 2006), is a known respiratory toxicant (Silverman et al. 1946; van Rooy et al. 2007). Carvone, a terpenoid ketone and the principal flavorant in spearmint, possesses insecticidal properties, and, interestingly, has also been described to be present in cinnamon-flavored WPT, likely to add a minty undertone.
Terpenes and terpenoids
Terpenes and terpenoids were the second most common class of flavorants found in apple- and licorice-flavored WPT products (Farag et al. 2018). Terpenes are somewhat prone to thermal degradation, potentially forming toxicants such as formaldehyde and isoprene during heating (Meehan-Atrash et al. 2017). Although some terpenes, such as β-caryophyllene, show anti-inflammatory, antioxidant, and cytoprotective effects, most terpenes, especially monoterpenes, have demonstrated high cytotoxicity in several model organisms, α-terpineol (Table S1) and terpinolene are among the most toxic terpenes, along with humulene and β-linalool. Limonene, found in watermelon WPT products (Farag et al 2018), exhibited cytotoxicity and inflammatory responses in naïve monocytes (Morris et al. 2021).
Nitrogen-containing compounds
Nitrosoazetidine was found at trace levels in apple- and melon-flavored WPT products (Farag et al. 2018). Nitrosoazetidine, when administered by gavage, is a liver carcinogen in animals (Lijinsky et al. 1984); however, its inhalation safety needs to be investigated.
Aldehydes
Aldehydes can cause varying degrees of mucus membrane irritation, eventually resulting in inflammation when inhaled at sufficient concentrations and frequency (Dinu et al. 2020). Cinnamaldehyde, the principal component of cinnamon flavor, is cytotoxic (Behar et al. 2016). Human embryonic stem cells are sensitive to low concentrations of cinnamaldehyde (Behar et al. 2014), a potentially significant concern for pregnant women using WPT. Flavorant molecules can also break down during heating to form toxic levels of formaldehyde, acetaldehyde, acrolein, and glyoxal (Khlystov and Samburova 2016). Ethyl vanillin, an aldehyde commonly found in “dessert” flavors but also in green grape-flavored WPT, was found to be cytotoxic to human bronchial epithelial cells treated with the flavorant (Morris et al. 2021).
Semivolatile furans
Semivolatile furans, such as furfural, can impart sweet, caramel, and almond (The Good Scents Company Information System; Zhang et al. 2010) aromas to WPT smoke and can lead to pulmonary irritation upon inhalation (Gupta et al. 1991). As noted above, semi-volatile furans can be generated from the thermal degradation of sugars.
Aromatic compounds
Aromatic compounds were abundant in mango-flavored WPT (Farag et al. 2018). Diphenyl ether, which has a harsh metallic aroma, irritates the mucus membranes and the upper respiratory tract. Prolonged exposure can damage multiple organs (Stanfill et al. 2006).
Alcohols
Of the alcohols, β-linalool is a non-irritant but auto-oxidizes to an allergenic product (Christensson et al. 2009). Overexposure to 1-hexanol, found mainly in apple and melon-flavored WPT, can lead to eye and respiratory tract irritation as well as central nervous system depression (Cometto-Muñiz et al. 1997; Mckee et al. 2015).
Implications of the findings on flavorant classes
Additional research will expand the inhalation toxicity knowledge base of flavorants and toxicants arising from the thermal breakdown of specific WPT flavorants during smoking. There is a clear need to correlate the presence and concentration of volatile flavoring compounds in flavored WPT smoke with altered pathophysiological cardiopulmonary responses. Despite the scarcity of studies on this topic, a diversity of research efforts provides evidence of the possible inhalation toxicity of 13 flavoring chemicals used in WPT (Table 2).
Table 2.
Selected WPT flavorants, related compounds, odor, and applicable toxicity studies.
| Compound class | WPT flavorants and related compounds | Characteristic odor | Relevant WPT flavors | Toxicity studies |
|---|---|---|---|---|
| Esters | 2-Hexenol acetate | Fruity | Melon, apple, unflavored | Acute inhalation toxicity at dosage of 500 ppm (Silverman 1946). |
| Ethyl cinnamate | Spices/cinnamon | Guava | Cytotoxicity in lung fibroblast and epithelium (Behar et al. 2018). | |
| n-Hexyl acetate | Fruity | Melon | Acute inhalation toxicity at dosage of 500 ppm (Silverman 1946). | |
| Triacetin | Odorless | Green grape | Cytotoxicity in lung fibroblast and epithelium (Behar et al. 2018). | |
| Ketones | 2,3-Butanedione (diacetyl) | Buttery | Melon, unflavored | Peribronchial inflammation, mild nasal and laryngeal injury after exposure of diacetyl 100–400 ppm for at least 4 wks (Morgan et al. 2008). |
| Terpenes and Terpenoids | Limonene | Citrus/fruity | Watermelon | Cytotoxicity and induced inflammatory responses in naïve monocyte (Morris et al. 2021). |
| Aldehydes and Furans | Ethyl vanillin | Vanilla/dessert | Green grape | Induced cytotoxicity in lung epithelium and associated with lung obstructive or restrictive diseases (Hua et al. 2019). |
| p-Anisaldehyde | Spices | Licorice | Cytotoxicity in lung fibroblast and epithelium (Behar et al. 2018). | |
| Furfural | Sweet | Caramel, almond | Irritated when inhaled and induced injury in parenchymal area (Gupta et al. 1991). | |
| Furaneol | Fruity | Strawberry | Cytotoxicity to lung epithelium (Hua et al. 2019). | |
| Aromatic compounds | Phenol | Sweet | Apple, green grape, guava, melon | Phenol exposure at 1.7 mg/ml showed cytotoxicity and mitochondrial activity inhibition in ex vivo human lung slice (Galina et al. 2018). |
| Alcohols | 2-Ethyl-1-hexanol | Odorless | Melon | Acute exposure to 1 mg/m3 caused irritation to nasal, throat, and respiratory track (Ernstgard et al. 2010). |
| Eugenol | Spice/clove | Green grape | Cytotoxicity in lung fibroblast and epithelium (Behar et al. 2018). |
Contribution of the heating source to the toxicity of WPT
WPT is an assisted-combustion tobacco product, and an external source of heating is needed due to the presence of high levels of humectants in WPT that prevent self-sustained combustion (Maziak et al. 2004b). Traditionally, the most widely used external heating source has been charcoal. Charcoal is known to naturally contain a large variety of trace elements and heavy metals (Elsayed et al. 2016). Studies have demonstrated the presence of heavy metals such as lead, arsenic, cadmium, and chromium, as well as VOCs, such as benzene, in WPT charcoal emissions (Schubert et al. 2015; Shihadeh et al. 2015). As a result, WPT users are exposed to these harmful compounds via mainstream smoke, generally at levels higher than from combustible cigarettes (Schubert et al. 2015; Shihadeh et al. 2015).
As with all incomplete combustion of carbon, the burning of charcoal yields CO, a compound that, when inhaled, preferentially binds to blood hemoglobin over oxygen, thereby reducing oxygen distribution in the body (Bleecker 2015). There is ample evidence that WPT use will result in much higher CO exposure compared with combustible cigarette use (Rezk-Hanna and Benowitz 2019), and studies have concluded that as much as 90% of CO and PAH emissions from WPT use stem from the charcoal briquettes rather than the WPT itself (Monzer et al. 2008). Moreover, different types of charcoal may contribute differently to emissions, with quick-light charcoal emitting significantly higher levels of CO compared with natural charcoal (Medford et al. 2015). Unfortunately, there exist ample medical case studies from across the globe describing cases of CO poisoning due to WPT use (Ashurst et al. 2012; Medford et al. 2015; Retzky 2017; Verweij et al. 2019).
More recently, electric heaters for waterpipes have been introduced, likely due to the known health risks associated with charcoal heating (El Hourani et al. 2019). Replacing charcoal with an electric heater was found to reduce CO and PAH levels by up to 90%, consistent with the evidence laid out above, yet an increase in the emission of acrolein was found, likely resulting from increasing degradation of humectants (Monzer et al. 2008; El Hourani et al. 2019). One clinical study found that using electrical heaters to heat WPT resulted in a reduction of nicotine delivery and in a reduction of exposure to CO and benzene compared with charcoal-based WPT use (Brinkman et al. 2020a). However, the study also reported that participants puffed greater volumes of smoke more aggressively to compensate for lower nicotine emissions, ultimately increasing tobacco-related exposures. A machine-smoking study reported that using electric heaters instead of charcoal reduced mainstream CO and PAH but increased semivolatile furan yields (El Hourani et al. 2019). One concern with electric heating devices is potential metal exposure from the heating element, similar to e-cigarette elements (Williams et al. 2017). Concerning the health effects of combustible charcoal-heated vs. electrically heated WPT, a study found that, similar to cigarette smoking, electrically heated WPT smoking acutely impairs endothelial function, one of the earliest signs of development of atherosclerotic cardiovascular disease (Rezk-Hanna et al. 2019). Furthermore, in traditional charcoal-heated WPT smoking, the acute vascular dysfunction is masked by the effects of high levels of CO, which acts as a vasodilator (Rezk-Hanna et al. 2019).
An emerging concern is the availability of WPT charcoal in various enticing flavors, e.g. apple, pineapple, orange, lemon, mint, peach, strawberry, and watermelon (Starlight Charcoal), which may contribute to the appeal of WPT use and/or increase toxicant exposure. Furthermore, manufacturers of coconut shell charcoal are using descriptors implying reduced harm, such as “environment-friendly” or “chemical-free” (Starlight Charcoal).
WPT package labeling concerns
Without adequate regulations specific to WPT marketing and package labeling, WPT companies advertise their products as comprising mainly molasses and dried fruit, touting them as harmless tobacco alternatives (Rezk-Hanna et al. 2014; Jawad et al. 2015; World Health Organization 2015). However, current scientific evidence does not support these claims (Raad et al. 2011; Montazeri et al. 2017; Al Ali et al. 2020; Hassane et al. 2022).
One concern is the inaccurate labeling of WPT constituents, such as nicotine. Although data on nicotine content and its yields in smoke delivered from WPT are essential to assessing the addictive potential of these products, 1 study that measured plasma nicotine levels in people who smoke WPT found that nicotine labeling on WPT packaging did not necessarily correlate with nicotine delivery (Vansickel et al. 2012). This finding indicates inaccurate labeling of WPT products, which may mislead those who smoke WPT (Vansickel et al. 2012). More research is needed to assess the accuracy of nicotine labeling on WPT packaging, such as comparing measured nicotine levels in neat WPT with levels indicated on the packaging label.
Of particular concern is the marketing and advertisement of WPT flavorings. Table 3 lists WPT package labeling concerns that have been shown to promote widespread WPT use, social acceptance of the behavior, and misperceptions about the addictive potential and adverse health effects of using these products, particularly among youth and young adults (Villanti et al. 2017; Maziak et al. 2020; Soneji et al. 2021). Table 3 provides examples of labeling concerns, such as the use of attractive names of flavorings, lack of disclosure of product ingredients, and use of reduced harm descriptors. Global regulatory bodies are encouraged to consider these WPT package labeling concerns to mitigate misleading messages of safety of use.
Table 3.
Flavored and sweetened WPT package labeling concerns.
| Labeling concerns | Characteristics |
|---|---|
| Use of attractive names of flavorings | Use of fruit, candy, and alcohol flavoring names attracting youth, such as apple martini, sweet passion fruit, peaches n cream, bubble gum, gummy bears, tequila sunrise, Arabian coffee, etc. |
| Lack of disclosure of product ingredients | Inaccurate labeling of tobacco product constituents, including nicotine concentrations (Vansickel et al. 2012); lack of disclosure on specific ingredients, including sugar and sweetener levels (Rezk-Hanna et al. 2023b); and use of misleading label information about product ingredients (e.g. zero tar) (Jawad et al. 2017). |
| Use of reduced harm descriptors | Use of descriptors implying reduced harm (e.g. “healthy,” “clean,” “pure,” “organic,” and “fresh”); use of large size pictures implying “safe and healthy” tobacco products (e.g. fruits, vegetables, and herbs) (Jawad et al. 2017). |
Regulation of flavored and sweetened WPT in the United States
The US Food and Drug Administration (FDA) first gained legal authority to regulate cigarettes, smokeless, and roll-your-own tobacco in 2009 when the US Congress passed the Family Smoking Prevention and Tobacco Control Act (TCA) (U.S. Government Printing Office 2009). In 2016, the FDA’s regulatory authorities were extended to all tobacco products, including WPT and its associated components and parts (FDA 2016). Despite those regulatory efforts, there continues to be an increase in WPT popularity, lack of user awareness of potential harms, and availability of WPT in appealing flavors (Aljarrah et al. 2009; Maziak 2011).
The regulatory context for WPT in the United States is complicated by differing, and often conflicting, federal, state, and local regulations. A 2015 study surveying Clean Indoor Air Acts (CIAA) from each of the 50 states and the District of Columbia found that policies varied greatly between states, and that many state CIAAs contained language that resulted in WPT exclusion from the regulation in question. This was especially significant for waterpipe venues (e.g. hookah lounges, bars), with as many as 24 states allowing waterpipe venues to be exempt from the state CIAA, and a further 14 states having “percentage of sales requirements” for tobacco that could enable exemptions for the venues (Martinasek et al. 2015). In another example of conflicting regulations, a 2017 study evaluating local and statewide WPT-relevant policies in Pennsylvania found that local-level reform attempts were prevented or rolled back by preemptions from the state, and some state regulations were constrained by federal preemptions (Colditz et al. 2017). Ultimately, tobacco control policies at federal, state, and local levels in the United States must be amended to be effective, consistent, and specific in their verbiage around WPT, and to reduce constraints from preemptions.
Conclusion
Despite the known health risks associated with flavored and sweetened WPT use, particularly from additives and heating sources, WPT use remains a global phenomenon. The public, particularly youth and young adults, may be more susceptible to initiate or continue WPT use because of availability of enticing flavors and additives, packaging tactics, and lack of regulation, as well the influence of societal norms. Those factors could intensify toxicant exposure and adverse health outcomes including nicotine addiction. This review summarizes our cumulative knowledge of the association of WPT flavors, additives, and charcoal with the ensuing toxicity as determined by animal models and biomarkers of exposure in clinical and epidemiological studies. We also highlight gaps in the existing literature and regulations of flavored and sweetened WPT toxicity.
Future directions
Based on the findings in this review, Table 4 suggests future research related to the toxicity of WPT additives (e.g. humectants, sweeteners, flavorants), heating sources and other device components, impact of WPT marketing and advertisements, and misleading or inaccurate communications of WPT (e.g. point-of-sale advertising, product packaging inserts, and labeling), as well as health education strategies to increase awareness of the toxicity and associated health risks of WPT use. Effective WPT-related policy and regulatory efforts depend on high-quality independent evidence. Thus, research funding specifically tailored to WPT is critical so that new data can continue to inform federal, state, and local regulation of WPT production, marketing, and sales, to protect public health.
Table 4.
Suggested future research for flavored and sweetened WPT and health education strategies.
|
Supplementary Material
Acknowledgments
The authors appreciate the continuous support of CASEL’s Toxicity Special Interest Group (SIG) who helped coordinate this article.
Contributor Information
Nada O F Kassem, Health Promotion and Behavioral Science, San Diego State University, San Diego, CA 92182, United States; Hookah Tobacco Research Center, San Diego State University Research Foundation, San Diego, CA 92123, United States.
Robert M Strongin, Department of Chemistry, Portland State University, Portland, OR 97207-0751, United States.
Andrea M Stroup, Behavioral Health and Health Policy Practice, Westat, Rockville, MD 20850, United States.
Marielle C Brinkman, College of Public Health, The Ohio State University, Columbus, OH 43210, United States; Center for Tobacco Research, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43214, United States.
Ahmad El-Hellani, Center for Tobacco Research, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43214, United States; Division of Environmental Health Sciences, College of Public Health, The Ohio State University, Columbus, OH 43210, United States.
Hanno C Erythropel, Department of Chemical and Environmental Engineering, Yale University, New Haven, CT 06511, United States; Department of Psychiatry, Yale School of Medicine, Yale Center for the Study of Tobacco Products (YCSTP), New Haven, CT 06511, United States.
Arash Etemadi, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD 20892, United States.
Maciej L Goniewicz, Department of Health Behavior, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, United States.
Eleanore G Hansen, Division of Environmental Health Science, School of Public Health, University of Minnesota, Minneapolis, MN 55455, United States.
Noura O Kassem, Hookah Tobacco Research Center, San Diego State University Research Foundation, San Diego, CA 92123, United States.
Dongmei Li, Department of Clinical and Translational Research, Obstetrics and Gynecology, Public Health Sciences, University of Rochester Medical Center, Rochester, NY 14642, United States.
Sandy Liles, Hookah Tobacco Research Center, San Diego State University Research Foundation, San Diego, CA 92123, United States.
Alexandra Noël, Department of Comparative Biomedical Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA 70803, United States.
Mary Rezk-Hanna, School of Nursing, University of California, Los Angeles, Los Angeles, CA 90095, United States.
Qixin Wang, Ragon Institute of Mass General, MIT, and Harvard, Cambridge, MA 02139, United States.
Irfan Rahman, Department of Environmental Medicine, University of Rochester Medical Center, Rochester, NY 14642, United States.
Author contributions
Nada O.F. Kassem, Robert M. Strongin, Andrea M. Stroup, Marielle C. Brinkman, Ahmad El-Hellani, Hanno C. Erythropel, Arash Etemadi, Maciej L. Goniewicz, Eleanore G. Hansen, Noura O. Kassem, Dongmei Li, Sandy Liles, Alexandra Noël, Mary Rezk-Hanna, Qixin Wang, and Irfan Rahman contributed to writing the initial draft, determining the overall organization of the manuscript, writing specific sections, finding, and inserting references, and proofreading and revising text.
Supplementary material
Supplementary material is available at Toxicological Sciences online.
Funding
This work is a cross-institution collaborative project from the Toxicity Special Interest Group supported, in part, by U54DA046060 from the Center for Coordination of Analytics, Science, Enhancement and Logistics (CASEL) in Tobacco Regulatory Science (National Institute of Drug Abuse [NIDA] and the U.S. Food and Drug Administration’s Center for Tobacco Products [FDA CTP]). Research reported in this publication was supported, in part, by the Tobacco-Related Disease Research Program (TRDRP) (T30IR0894 and T32IR4777 to NOFK), (T30IP1013 to MR-H), the National Institutes of Health/NIDA (R01DA051005 to EGH), and the National Institutes of Health/FDA (R01ES025257 to RMS), (U54DA046060 to AMS), (UC2FD007229 and R01CA255563 to MCB), (UC2FD007229 and R01DA052565 to AEH), (U54DA036151 to HCE), (U54CA228110 to MLG), (U54CA228110 to DL), (1R01HL152435-01A to MR-H), (1R01HL152435-01A and U54 CA228110 to IR).
Conflicts of interest. MLG received a research grant from Pfizer and served as a member of a scientific advisory board to Johnson&Johnson; all other authors declare no conflict of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the authors’ institutions, the National Institutes of Health (NIH), the U.S. Food and Drug Administration (FDA), or the Tobacco-Related Disease Research Program (TRDRP).
References
- Abi-Gerges A, Dagher-Hamalian C, Abou-Khalil P, Chahine JB, Hachem P, Khalil C. 2020. Evaluation of waterpipe smoke toxicity in C57BL/6 mice model. Pulm Pharmacol Ther. 63:101940. 10.1016/j.pupt.2020.101940 [DOI] [PubMed] [Google Scholar]
- Aboaziza E, Eissenberg T. 2015. Waterpipe tobacco smoking: what is the evidence that it supports nicotine/tobacco dependence? Tob Control. 24(Suppl 1):i44–i53. 10.1136/tobaccocontrol-2014-051910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adetona A, Brinkman M, Strozier E, Karam H, Butler J, Kim H, Lim J, McCauley M, Clark P. 2020. Do they differ? Flavored versus unflavored waterpipe tobacco flavor ingredients. Tob Regul Sci. 6(5):336–354. 10.18001/TRS.6.5.4 [DOI] [Google Scholar]
- Al-Sawalha N, Alzoubi K, Khabour O, Alyacoub W, Almahmmod Y, Eissenberg T. 2018. Effect of prenatal exposure to waterpipe tobacco smoke on learning and memory of adult offspring rats. Nicotine Tob Res. 20(4):508–514. 10.1093/ntr/ntx142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sawalha NA, Al-Bo’ul HF, Alzoubi KH, Khabour OF, Thanawala VJ. 2017. Effect of prenatal waterpipe tobacco smoke on airway inflammation in murine model of asthma of adult offspring mice. Inhal Toxicol. 29(8):366–373. 10.1080/08958378.2017.1385113 [DOI] [PubMed] [Google Scholar]
- Al-Sawalha NA, Almahmmod Y, Awawdeh MS, Alzoubi KH, Khabour OF. 2020. Effect of waterpipe tobacco smoke exposure on the development of metabolic syndrome in adult male rats. PLoS One. 15(6):e0234516. 10.1371/journal.pone.0234516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sawalha NA, Pokkunuri ID, Alzoubi KH, Khabour OF, Almomani BN. 2021. Waterpipe tobacco smoke exposure during lactation-susceptibility of reproductive hormones and oxidative stress parameters in male progeny rats. Reprod Sci. 28(1):37–42. 10.1007/s43032-020-00282-8 [DOI] [PubMed] [Google Scholar]
- Alarabi AB, , KarimZA, , RamirezJEM, , HernandezKR, , LozanoPA, , RiveraJO, , AlshboolFZ, , Khasawneh FT. 2020. Short-term exposure to waterpipe/hookah smoke triggers a hyperactive platelet activation state and increases the risk of thrombogenesis. Arterioscler Thromb Vasc Biol. 40(2):335–349. 10.1161/ATVBAHA.119.313435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Ali R, Vukadinović D, Maziak W, Katmeh L, Schwarz V, Mahfoud F, Laufs U, Böhm M. 2020. Cardiovascular effects of waterpipe smoking: a systematic review and meta-analysis. Rev Cardiovasc Med. 21(3):453–468. 10.31083/j.rcm.2020.03.135 [DOI] [PubMed] [Google Scholar]
- AlGemayel C, Honein E, Hellani AE, Salman R, Saliba NA, Shihadeh A, Zeaiter J; Baha and Walid Bassatne Department of Chemical and Petroleum Engineering, Maroun Semaan Faculty of Engineering and Architecture, American University of Beirut. 2022. Kinetic modeling of the pyrolysis of propylene glycol. Eng. Sci. 20:162–179. 10.30919/es8d757 [DOI] [Google Scholar]
- Ali BH, , AdhamSA, , Al BalushiKA, , ShalabyA, , WalyMI, , ManojP, , BeegamS, , YuvarajuP, , Nemmar A. 2015. Reproductive toxicity to male mice of nose only exposure to water-pipe smoke. Cell Physiol Biochem. 35(1):29–37. 10.1159/000369672 [DOI] [PubMed] [Google Scholar]
- Aljarrah K, Ababneh ZQ, Al-Delaimy WK. 2009. Perceptions of hookah smoking harmfulness: predictors and characteristics among current hookah users. Tob Induc Dis. 5(1):16. 10.1186/1617-9625-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almomen S, Aldossari M, Khaleel Y, Altamimi M, Alharbi O, Alsuwaydani A, Almutairi M, Alyousef S, Hafiz R, Alshomer F, et al. 2023. Effect of glycerol concentration on levels of toxicants emissions from water-pipe tobacco smoking (WTS). BMC Public Health. 23(1):1858. 10.1186/s12889-023-16740-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomari MA, Al-Sheyab NA, Khabour OF, Alzoubi KH. 2018. Brain-derived neutrophic factor in adolescents smoking waterpipe: the Irbid TRY. Int J Dev Neurosci. 67:14–18. 10.1016/j.ijdevneu.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Halboup AM, Alomari MA, Khabour OF. 2019. Swimming exercise protective effect on waterpipe tobacco smoking-induced impairment of memory and oxidative stress. Life Sci. 239:117076. 10.1016/j.lfs.2019.117076 [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Khabour OF, Alharahshah EA, Alhashimi FH, Shihadeh A, Eissenberg T. 2015. The effect of waterpipe tobacco smoke exposure on learning and memory functions in the rat model. J Mol Neurosci. 57(2):249–256. 10.1007/s12031-015-0613-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashurst JV, Urquhart M, Cook MD. 2012. Carbon monoxide poisoning secondary to hookah smoking. J Am Osteopath Assoc. 112(10):686–688. https://www.ncbi.nlm.nih.gov/pubmed/23055468 [PubMed] [Google Scholar]
- Baker RR, Bishop LJ. 2004. The pyrolysis of tobacco ingredients. J Anal Appl Pyrolysis. 71(1):223–311. [Google Scholar]
- Bakker-‘t Hart IME, Bakker F, Pennings JLA, Weibolt N, Eising S, Talhout R. 2022. Flavours and flavourings in waterpipe products: a comparison between tobacco, herbal molasses and steam stones. Tob Control. 32(5):627–634. 10.1136/tobaccocontrol-2021-056955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar R, Davis B, Wang Y, Bahl V, Lin S, Talbot P. 2014. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 28(2):198–208. 10.1016/j.tiv.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Behar RZ, Luo W, Lin SC, Wang Y, Valle J, Pankow JF, Talbot P. 2016. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control. 25(Suppl 2):ii94–ii102. 10.1136/tobaccocontrol-2016-053224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar RZ, Luo W, McWhirter KJ, Pankow JF, Talbot P. 2018. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci Rep. 8(1):8288. 10.1038/s41598-018-25575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Taleb Z, Breland A, Bahelah R, Kalan ME, Vargas-Rivera M, Jaber R, Eissenberg T, Maziak W. 2019. Flavored versus nonflavored waterpipe tobacco: a comparison of toxicant exposure, puff topography, subjective experiences, and harm perceptions. Nicotine Tob Res. 21(9):1213–1219. 10.1093/ntr/nty131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Taleb Z, Vargas M, Ebrahimi Kalan M, Breland A, Eissenberg T, Brown D, Maziak W. 2020. The effect of flavoured and non-flavoured tobacco on subjective experience, topography and toxicant exposure among waterpipe smokers. Tob Control. 29(Suppl 2):s72–s79. 10.1136/tobaccocontrol-2019-054972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. 2001. Compensatory smoking of low-yield cigarettes. Smoking and tobacco control monograph. [accessed 2024 July 27]. https://cancercontrol.cancer.gov/sites/default/files/2020-06/m13_3.pdf
- Bleecker ML. 2015. Carbon monoxide intoxication. Handb Clin Neurol. 131:191–203. 10.1016/B978-0-444-62627-1.00024-X [DOI] [PubMed] [Google Scholar]
- Brinkman MC, Kim H, Buehler SS, Adetona AM, Gordon SM, Clark PI. 2020a. Evidence of compensation among waterpipe smokers using harm reduction components. Tob Control. 29(1):15–23. 10.1136/tobaccocontrol-2018-054502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman MC, Teferra AA, Kassem NO, Kassem NO. 2020b. Effect of electric heating and ice added to the bowl on mainstream waterpipe semivolatile furan and other toxicant yields. Tob Control. 29(Suppl 2):s110–s116. 10.1136/tobaccocontrol-2019-054961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2012. Preventing tobacco use among youth and young adults: a report of the surgeon general. Reports of the Surgeon General, Issue. [accessed 2024 July 27]. https://www.cdc.gov/tobacco/sgr/2012/index.htm
- Christensson JB, Forsström P, Wennberg AM, Karlberg AT, Matura M. 2009. Air oxidation increases skin irritation from fragrance terpenes. Contact Dermatitis. 60(1):32–40. [DOI] [PubMed] [Google Scholar]
- Cobb C, Ward KD, Maziak W, Shihadeh AL, Eissenberg T. 2010. Waterpipe tobacco smoking: an emerging health crisis in the United States. Am J Health Behav. 34(3):275–285. 10.5993/ajhb.34.3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz JB, Ton JN, James AE, Primack BA. 2017. Toward effective water pipe tobacco control policy in the United States: synthesis of federal, state, and local policy texts. Am J Health Promot. 31(4):302–309. 10.4278/ajhp.150218-QUAL-736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colyer-Patel K, Kuhns L, Weidema A, Lesscher H, Cousijn J. 2023. Age-dependent effects of tobacco smoke and nicotine on cognition and the brain: a systematic review of the human and animal literature comparing adolescents and adults. Neurosci Biobehav Rev. 146:105038. 10.1016/j.neubiorev.2023.105038 [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Hudnell HK. 1997. Agonistic sensory effects of airborne chemicals in mixtures: odor, nasal pungency, and eye irritation. Percept Psychophys. 59(5):665–674. 10.3758/bf03206014 [DOI] [PubMed] [Google Scholar]
- Daher N, Saleh R, Jaroudi E, Sheheitli H, Badr T, Sepetdjian E, Al Rashidi M, Saliba N, Shihadeh A. 2010. Comparison of carcinogen, carbon monoxide, and ultrafine particle emissions from narghile waterpipe and cigarette smoking: sidestream smoke measurements and assessment of second-hand smoke emission factors. Atmos Environ (1994). 44(1):8–14. 10.1016/j.atmosenv.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinu V, Kilic A, Wang Q, Ayed C, Fadel A, Harding SE, Yakubov GE, Fisk ID. 2020. Policy, toxicology and physicochemical considerations on the inhalation of high concentrations of food flavour. NPJ Sci Food. 4(1):15. 10.1038/s41538-020-00075-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RL Jr, Venugopal PD, Hsieh JR. 2021. Aquatic toxicity of waterpipe wastewater chemicals. Environ Res. 197:111206. 10.1016/j.envres.2021.111206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage R, El-Hellani A, Salman R, Talih S, Shihadeh A, Saliba NA. 2020. Vaped humectants in E-Cigarettes are a source of phenols. Chem Res Toxicol. 33(9):2374–2380. 10.1021/acs.chemrestox.0c00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hourani M, Talih S, Salman R, Karaoghlanian N, Karam E, El Hage R, Saliba NA, Shihadeh A. 2019. Comparison of CO, PAH, nicotine, and aldehyde emissions in waterpipe tobacco smoke generated using electrical and charcoal heating methods. Chem Res Toxicol. 32(6):1235–1240. 10.1021/acs.chemrestox.9b00045 [DOI] [PubMed] [Google Scholar]
- Elsayed Y, Dalibalta S, Abu-Farha N. 2016. Chemical analysis and potential health risks of hookah charcoal. Sci Total Environ. 569–570:262–268. 10.1016/j.scitotenv.2016.06.108 [DOI] [PubMed] [Google Scholar]
- Elton-Marshall T, Driezen P, Fong GT, Cummings KM, Persoskie A, Wackowski O, Choi K, Kaufman A, Strong D, Gravely S, et al. 2020. Adult perceptions of the relative harm of tobacco products and subsequent tobacco product use: longitudinal findings from waves 1 and 2 of the population assessment of tobacco and health (PATH) study. Addict Behav. 106:106337. 10.1016/j.addbeh.2020.106337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernstgard L, Norback D, Nordquist T, Wieslander G, Walinder R, Johanson G. 2010. Acute effects of exposure to 1 mg/m(3) of vaporized 2-ethyl-1-hexanol in humans. Indoor Air. 20(2):168–175. 10.1111/j.1600-0668.2009.00638.x [DOI] [PubMed] [Google Scholar]
- Erythropel HC, Garcia Torres DS, Woodrow JG, de Winter TM, Falinski MM, Anastas PT, O’Malley SS, Krishnan-Sarin S, Zimmerman JB. 2021. Quantification of flavorants and nicotine in waterpipe tobacco and mainstream smoke and comparison to E-cigarette aerosol. Nicotine Tob Res. 23(3):600–604. 10.1093/ntr/ntaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadi A, Abnet CC, Dawsey SM, Freedman ND. 2023. Biomarkers of tobacco carcinogenesis in diverse populations: challenges and opportunities. Cancer Epidemiol Biomarkers Prev. 32(3):289–291. 10.1158/1055-9965.EPI-22-1289 [DOI] [PubMed] [Google Scholar]
- Etemadi A, Poustchi H, Chang CM, Blount BC, Calafat AM, Wang L, De Jesus VR, Pourshams A, Shakeri R, Shiels MS, et al. 2019. Urinary biomarkers of carcinogenic exposure among cigarette, waterpipe, and smokeless tobacco users and never users of tobacco in the Golestan cohort study. Cancer Epidemiol Biomarkers Prev. 28(2):337–347. 10.1158/1055-9965.Epi-18-0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MA, Elmassry MM, El-Ahmady SH. 2018. The characterization of flavored hookahs aroma profile and in response to heating as analyzed via headspace solid-phase microextraction (SPME) and chemometrics. Sci Rep. 8(1):17028. 10.1038/s41598-018-35368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. 2016. Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. A rule by the Food and Drug Administration on 05/10/2016. (2016-10685). [accessed 2024 July 27]. https://www.federalregister.gov/documents/2016/05/10/2016-10685/deeming-tobacco-products-to-be-subject-to-the-federal-food-drug-and-cosmetic-act-as-amended-by-the [PubMed]
- Feliciano JR, Li D, Xie Z. 2023. Public perceptions of flavored waterpipe smoking on twitter. Int J Environ Res Public Health. 20(7):5264. 10.3390/ijerph20075264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galina B, Krabbe J, Schettgen T, Rieg A, Schröder T, Spillner J, Kalverkamp S, Braunschweig T, Kintsler S, Krüger I, et al. 2018. Low concentration of phenol in medical solutions can induce bronchoconstriction and toxicity in murine, rat and human lungs. Eur Res J. 52(Suppl 62):PA1059. 10.1183/13993003.congress-2018.PA1059 [DOI] [Google Scholar]
- Gautam P, Sharma E, Kalan ME, Li W, Ward KD, Sutherland MT, Cano MA, Li T, Maziak W. 2022. Prevalence and predictors of waterpipe smoking initiation and progression among adolescents and young adults in waves 1-4 (2013-2018) of the population assessment of tobacco and health (PATH) study. Nicotine Tob Res. 24(8):1281–1290. 10.1093/ntr/ntac051 [DOI] [PubMed] [Google Scholar]
- The Good Scents Company Information System. [Retrieved 2024 July 5]. http://www.thegoodscentscompany.com/index.html
- Greenlee KJ, Werb Z, Kheradmand F. 2007. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 87(1):69–98. 10.1152/physrev.00022.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GD, Misra A, Agarwal DK. 1991. Inhalation toxicity of furfural vapours: an assessment of biochemical response in rat lungs. J Appl Toxicol. 11(5):343–347. 10.1002/jat.2550110508 [DOI] [PubMed] [Google Scholar]
- Hadidi KA, Mohammed FI. 2004. Nicotine content in tobacco used in hubble-bubble smoking. Saudi Med J. 25(7):912–917. https://www.ncbi.nlm.nih.gov/pubmed/15235699 [PubMed] [Google Scholar]
- Harber P, Saechao K, Boomus C. 2006. Diacetyl-induced lung disease. Toxicol Rev. 25(4):261–272. 10.2165/00139709-200625040-00006 [DOI] [PubMed] [Google Scholar]
- Hassane M, Rahal Z, Karaoghlanian N, Zhang J, Sinjab A, Wong JW, Lu W, Scheet P, Lee JJ, Raso MG, et al. 2022. Chronic exposure to waterpipe smoke elicits immunomodulatory and carcinogenic effects in the lung. Cancer Prev Res (Phila). 15(7):423–434. 10.1158/1940-6207.CAPR-21-0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Salgado RV, Dresler C, Faller RW, Bartlett C. 2016. Flavour preferences in youth versus adults: a review. Tob Control. 25(Suppl 2):ii32–ii39. 10.1136/tobaccocontrol-2016-053192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Omaiye EE, Luo W, McWhirter KJ, Pankow JF, Talbot P. 2019. Identification of cytotoxic flavor chemicals in top-selling electronic cigarette refill fluids. Sci Rep. 9(1):2782. 10.1038/s41598-019-38978-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard G, Tafin Djoko D, Korneliou A, Belushkin M. 2020. Analysis of waterpipe aerosol constituents in accordance with the ISO standard 22486. Toxicol Rep. 7:1344–1349. 10.1016/j.toxrep.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P 3rd, Abu Raddaha AH, Dempsey D, Havel C, Peng M, Yu L, Benowitz NL. 2011. Nicotine, carbon monoxide, and carcinogen exposure after a single use of a water pipe. Cancer Epidemiol Biomarkers Prev. 20(11):2345–2353. 10.1158/1055-9965.EPI-11-0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Abu Raddaha AH, Dempsey D, Havel C, Peng M, Yu L, Benowitz NL. 2013. Comparison of nicotine and carcinogen exposure with water pipe and cigarette smoking. Cancer Epidemiol Biomarkers Prev. 22(5):765–772. 10.1158/1055-9965.Epi-12-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed F, ALHarthi SS, BinShabaib MS, Gajendra S, Romanos GE, Rahman I. 2017. Toxicological impact of waterpipe smoking and flavorings in the oral cavity and respiratory system. Inhal Toxicol. 29(9):389–396. 10.1080/08958378.2017.1384084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad M, Charide R, Waziry R, Darzi A, Ballout RA, Akl EA. 2018. The prevalence and trends of waterpipe tobacco smoking: a systematic review. PLoS One. 13(2):e0192191. 10.1371/journal.pone.0192191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad M, Darzi A, Lotfi T, Nakkash R, Hawkins B, Akl EA. 2017. Waterpipe product packaging and labelling at the 3rd international hookah fair; does it comply with article 11 of the framework convention on tobacco control? J Public Health Policy. 38(3):303–313. 10.1057/s41271-017-0078-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad M, Nakkash RT, Hawkins B, Akl EA. 2015. Waterpipe industry products and marketing strategies: analysis of an industry trade exhibition. Tob Control. 24(E4):e275–e279. 10.1136/tobaccocontrol-2015-052254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem NO, Daffa RM, Liles S, Jackson SR, Kassem NO, Younis MA, Mehta S, Chen M, Jacob P 3rd, Carmella SG, et al. 2014a. Children’s exposure to secondhand and thirdhand smoke carcinogens and toxicants in homes of hookah smokers. Nicotine Tob Res. 16(7):961–975. 10.1093/ntr/ntu016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem NO, Jackson SR, Boman-Davis M, Kassem NO, Liles S, Daffa RM, Yasmin R, Madanat H, Hovell MF. 2015. Hookah smoking and facilitators/barriers to lounge use among students at a US university. Am J Health Behav. 39(6):832–848. 10.5993/ajhb.39.6.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem NO, Kassem NO, Jackson SR, Liles S, Daffa RM, Zarth AT, Younis MA, Carmella SG, Hofstetter CR, Chatfield DA, et al. 2014b. Benzene uptake in hookah smokers and non-smokers attending hookah social events: regulatory implications. Cancer Epidemiol Biomarkers Prev. 23(12):2793–2809. 10.1158/1055-9965.Epi-14-0576 [DOI] [PubMed] [Google Scholar]
- Kassem NOF, Jackson SR, Kassem NO, Liles S, Posis AIB, Hovell MF. 2019. College student beliefs and behavior regarding sharing when smoking hookahs. Am J Health Behav. 43(1):133–144. 10.5993/ajhb.43.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem NOF, Kassem NO, Liles S, Jackson SR, Chatfield DA, Jacob P, Benowitz NL, Hovell MF. 2017. Urinary NNAL in hookah smokers and non-smokers after attending a hookah social event in a hookah lounge or a private home. Regul Toxicol Pharmacol. 89:74–82. 10.1016/j.yrtph.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem NOF, Kassem NO, Liles S, Jackson SR, Posis AIB, Chatfield DA, Hovell MF. 2018a. Levels of urine cotinine from hookah smoking and exposure to hookah tobacco secondhand smoke in hookah lounges and homes. Int J High Risk Behav Addict. 7(1):e67601. H 10.5812/ijhrba.67601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem NOF, Kassem NO, Liles S, Zarth AT, Jackson SR, Daffa RM, Chatfield DA, Carmella SG, Hecht SS, Hovell MF. 2018b. Acrolein exposure in hookah smokers and non-smokers exposed to hookah tobacco secondhand smoke: implications for regulating hookah tobacco products. Nicotine Tob Res. 20(4):492–501. 10.1093/ntr/ntx133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem NOF, Peterson LA, Liles S, Kassem NO, Zaki FK, Lui KJ, Vevang KR, Dodder NG, Hoh E, Hovell MF. 2020. Urinary metabolites of furan in waterpipe tobacco smokers compared to non-smokers in home settings in the US. Toxicol Lett. 333:202–210. 10.1016/j.toxlet.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Hamilton B, Mehta T, Hale JJ, Leavens ELS, Shihadeh A, Eissenberg T, Brinkman MC, Wagener TL. 2022. Effects of flavourants and humectants on waterpipe tobacco puffing behaviour, biomarkers of exposure and subjective effects among adults with high versus low nicotine dependence. Tob Control. 31(4):527–533. 10.1136/tobaccocontrol-2020-056062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabour OF, , AlzoubiKH, , Bani-AhmadM, , DodinA, , EissenbergT, , Shihadeh A. 2012. Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhal Toxicol. 24(10):667–675. 10.3109/08958378.2012.710918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabour OF, Alzoubi KH, Abu Thiab TM, Al-Husein BA, Eissenberg T, Shihadeh AL. 2015. Changes in the expression and protein level of matrix metalloproteinases after exposure to waterpipe tobacco smoke. Inhal Toxicol. 27(13):689–693. 10.3109/08958378.2015.1085471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabour OF, Alzoubi KH, Al-Sawalha N, Ahmad MB, Shihadeh A, Eissenberg T. 2018. The effect of chronic exposure to waterpipe tobacco smoke on airway inflammation in mice. Life Sci. 200:110–114. 10.1016/j.lfs.2018.03.034 [DOI] [PubMed] [Google Scholar]
- Khan NA, Lawyer G, McDonough S, Wang Q, Kassem NO, Kas-Petrus F, Ye D, Singh KP, Kassem NO, Rahman I. 2020. Systemic biomarkers of inflammation, oxidative stress and tissue injury and repair among waterpipe, cigarette and dual tobacco smokers. Tob Control. 29(Suppl 2):s102–s109. 10.1136/tobaccocontrol-2019-054958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Yogeswaran S, Wang Q, Muthumalage T, Sundar IK, Rahman I. 2019. Waterpipe smoke and e-cigarette vapor differentially affect circadian molecular clock gene expression in mouse lungs. PLoS One. 14(2):e0211645. 10.1371/journal.pone.0211645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlystov A, Samburova V. 2016. Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ Sci Technol. 50(23):13080–13085. 10.1021/acs.est.6b05145 [DOI] [PubMed] [Google Scholar]
- Kuk AE, Bluestein MA, Chen B, Harrell M, Spells CE, Atem F, Perez A. 2022. The effect of perceptions of hookah harmfulness and addictiveness on the age of initiation of hookah use among Population Assessment of Tobacco and Health (PATH) youth. Int J Environ Res Public Health. 19(9):5034. 10.3390/ijerph19095034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak JA, Goniewicz ML, Giovino GA, Travers MJ. 2017. Nicotine and pH in waterpipe tobacco. Tobacco Reg Sci. 3(1):102–107. 10.18001/TRS.3.1.10 [DOI] [Google Scholar]
- Leavens EL, Driskill LM, Molina N, Eissenberg T, Shihadeh A, Brett EI, Floyd E, Wagener TL. 2018. Comparison of a preferred versus non-preferred waterpipe tobacco flavour: subjective experience, smoking behaviour and toxicant exposure. Tob Control. 27(3):319–324. 10.1136/tobaccocontrol-2016-053344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijinsky W, Kovatch R, Knutsen G. 1984. Carcinogenesis by nitrosomorpholines, nitrosooxazolidines and nitrosoazetidine given by gavage to Syrian golden hamsters. Carcinogenesis. 5(7):875–878. 10.1093/carcin/5.7.875 [DOI] [PubMed] [Google Scholar]
- Ma C, Yang H, Zhao M, Magnussen CG, Xi B. 2022. Prevalence of waterpipe smoking and its associated factors among adolescents aged 12-16 years in 73 countries/territories. Front Public Health. 10:1052519. 10.3389/fpubh.2022.1052519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfooz K, Vasavada AM, Joshi A, Pichuthirumalai S, Andani R, Rajotia A, Hans A, Mandalia B, Dayama N, Younas Z, et al. 2023. Waterpipe use and its cardiovascular effects: a systematic review and meta-analysis of case-control, cross-sectional, and non-randomized studies. Cureus. 15(2):e34802. 10.7759/cureus.34802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinasek MP, Gibson-Young LM, Davis JN, McDermott RJ. 2015. Waterpipe tobacco smoking impact on public health: implications for policy. Risk Manag Healthc Policy. 8:121–129. 10.2147/RMHP.S68267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinasek MP, McDermott RJ, Martini L. 2011. Waterpipe (hookah) tobacco smoking among youth. Curr Probl Pediatr Adolesc Health Care. 41(2):34–57. 10.1016/j.cppeds.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Maziak W. 2010. Commentary: the waterpipe—a global epidemic or a passing fad. Int J Epidemiol. 39(3):857–859. 10.1093/ije/dyq054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W. 2011. The global epidemic of waterpipe smoking. Addict Behav. 36(1-2):1–5. 10.1016/j.addbeh.2010.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W. 2015. Rise of waterpipe smoking. BMJ. 350:H1991. 10.1136/bmj.h1991 [DOI] [PubMed] [Google Scholar]
- Maziak W, Ben Taleb Z, Ebrahimi Kalan M, Ward-Peterson M, Bursac Z, Osibogun O, Eissenberg T. 2020. Effect of flavour manipulation on low and high-frequency waterpipe users’ puff topography, toxicant exposures and subjective experiences. Tob Control. 29(Suppl 2):S95–S101. 10.1136/tobaccocontrol-2019-055040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Eissenberg T, Rastam S, Hammal F, Asfar T, Bachir ME, Fouad MF, Ward KD. 2004a. Beliefs and attitudes related to narghile (waterpipe) smoking among university students in Syria. Ann Epidemiol. 14(9):646–654. 10.1016/j.annepidem.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Maziak W, Sharma E. 2020. Building the evidence base for waterpipe regulation and policy. Tob Control. 29(Suppl 2):S59–S61. 10.1136/tobaccocontrol-2019-055391 [DOI] [PubMed] [Google Scholar]
- Maziak W, Ward KD, Afifi Soweid RA, Eissenberg T. 2004b. Tobacco smoking using a waterpipe: a re-emerging strain in a global epidemic. Tob Control. 13(4):327–333. 10.1136/tc.2004.008169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee RH, Adenuga MD, Carrillo J-C. 2015. Characterization of the toxicological hazards of hydrocarbon solvents. Crit Rev Toxicol. 45(4):273–365. 10.3109/10408444.2015.1016216 [DOI] [PubMed] [Google Scholar]
- Medford MA, Gasier HG, Hexdall E, Moffat AD, Freiberger JJ, Moon RE. 2015. Research report: charcoal type used for hookah smoking influences CO production. Undersea Hyperb Med. 42(4):375–380. https://www.ncbi.nlm.nih.gov/pubmed/26403022 [PubMed] [Google Scholar]
- Meehan-Atrash J, Luo W, Strongin RM. 2017. Toxicant formation in dabbing: the terpene story. ACS Omega. 2(9):6112–6117. 10.1021/acsomega.7b01130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri Z, Nyiraneza C, El-Katerji H, Little J. 2017. Waterpipe smoking and cancer: systematic review and meta-analysis. Tob Control. 26(1):92–97. 10.1136/tobaccocontrol-2015-052758 [DOI] [PubMed] [Google Scholar]
- Monzer B, Sepetdjian E, Saliba N, Shihadeh A. 2008. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food Chem Toxicol. 46(9):2991–2995. 10.1016/j.fct.2008.05.031 [DOI] [PubMed] [Google Scholar]
- Morgan DL, Flake GP, Kirby PJ, Palmer SM. 2008. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol Sci. 103(1):169–180. 10.1093/toxsci/kfn016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AM, Leonard SS, Fowles JR, Boots TE, Mnatsakanova A, Attfield KR. 2021. Effects of E-Cigarette flavoring chemicals on human macrophages and bronchial epithelial cells. Int J Environ Res Public Health. 18(21):11107. 10.3390/ijerph182111107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimani M, Adams J, da Silva G. 2022. Toxic chemical formation during vaping of ethyl ester flavor additives: a chemical kinetic modeling study. Chem Res Toxicol. 35(3):522–528. 10.1021/acs.chemrestox.1c00437 [DOI] [PubMed] [Google Scholar]
- Nemmar A, , Al-SalamS, , YuvarajuP, , BeegamS, , YasinJ, , Ali BH. 2017. Chronic exposure to water-pipe smoke induces cardiovascular dysfunction in mice. Am J Physiol Heart Circ Physiol. 312(2):H329–H339. 10.1152/ajpheart.00450.2016 [DOI] [PubMed] [Google Scholar]
- Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH. 2019. Waterpipe smoke exposure triggers lung injury and functional decline in mice: protective effect of gum Arabic. Oxid Med Cell Longev. 2019:8526083. 10.1155/2019/8526083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH. 2020a. Comparative study on pulmonary toxicity in mice induced by exposure to unflavoured and apple- and strawberry-flavoured tobacco waterpipe smoke. Oxid Med Cell Longev. 2020:6450450. 10.1155/2020/6450450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Zaaba NE, Yasin J, Ali BH. 2020b. Waterpipe tobacco smoke inhalation triggers thrombogenicity, cardiac inflammation and oxidative stress in mice: effects of flavouring. Int J Mol Sci. 21(4):1291. 10.3390/ijms21041291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A, Al-Salam S, Beegam S, Zaaba NE, Elzaki O, Yasin J, Ali BH. 2022. Waterpipe smoke-induced hypercoagulability and cardiac injury in mice: influence of cessation of exposure. Biomed Pharmacother. 146:112493. 10.1016/j.biopha.2021.112493 [DOI] [PubMed] [Google Scholar]
- Nemmar A, Al Hemeiri A, Al Hammadi N, Yuvaraju P, Beegam S, Yasin J, Elwasila M, Ali BH, Adeghate E. 2015a. Early pulmonary events of nose-only water pipe (shisha) smoking exposure in mice. Physiol Rep. 3(3):e12258. 10.14814/phy2.12258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A, Beegam S, Yuvaraju P, Yasin J, Ali BH, Adeghate E. 2020c. Nose-only water-pipe smoke exposure in mice elicits renal histopathological alterations, inflammation, oxidative stress, DNA damage, and apoptosis. Front Physiol. 11:46. 10.3389/fphys.2020.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A, Raza H, Yuvaraju P, Beegam S, John A, Yasin J, Hameed RS, Adeghate E, Ali BH. 2013. Nose-only water-pipe smoking effects on airway resistance, inflammation, and oxidative stress in mice. J Appl Physiol (1985). 115(9):1316–1323. 10.1152/japplphysiol.00194.2013 [DOI] [PubMed] [Google Scholar]
- Nemmar A, Yuvaraju P, Beegam S, Ali BH. 2015b. Short-term nose-only water-pipe (shisha) smoking exposure accelerates coagulation and causes cardiac inflammation and oxidative stress in mice. Cell Physiol Biochem. 35(2):829–840. 10.1159/000369741 [DOI] [PubMed] [Google Scholar]
- Olsson M, Petersson G. 2003. Benzene emitted from glowing charcoal. Sci Total Environ. 303(3):215–220. 10.1016/S0048-9697(02)00403-5 [DOI] [PubMed] [Google Scholar]
- Ooi BG, Dutta D, Kazipeta K, Chong NS. 2019. Influence of the E-cigarette emission profile by the ratio of glycerol to propylene glycol in E-liquid composition. ACS Omega. 4(8):13338–13348. 10.1021/acsomega.9b01504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud V, Lawler MJ, Malecha KT, Johnson RM, Herman D, Staimer N, Kleinman MT, Nizkorodov SA, Smith JN. 2019. Chemical characterization of nanoparticles and volatiles present in mainstream hookah smoke. Aerosol Sci Technol. 53(9):1023–1039. 10.1080/02786826.2019.1628342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primack BA, Sidani J, Agarwal AA, Shadel WG, Donny EC, Eissenberg TE. 2008. Prevalence of and associations with waterpipe tobacco smoking among U.S. university students. Ann Behav Med. 36(1):81–86. 10.1007/s12160-008-9047-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim H, Alarabi AB, Alzoubi KH, Karim ZA, Alshbool FZ, Khasawneh FT. 2019. The effects of hookah/waterpipe smoking on general health and the cardiovascular system. Environ Health Prev Med. 24(1):58. 10.1186/s12199-019-0811-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad D, Gaddam S, Schunemann HJ, Irani J, Abou Jaoude P, Honeine R, Akl EA. 2011. Effects of water-pipe smoking on lung function: a systematic review and meta-analysis. Chest. 139(4):764–774. 10.1378/chest.10-0991 [DOI] [PubMed] [Google Scholar]
- Rababa’h AM, Bsoul RW, Alkhatatbeh MJ, Alzoubi KH, Khabour OF. 2019. Waterpipe tobacco smoke distresses cardiovascular biomarkers in mice: alterations in protein expression of metalloproteinases, endothelin and myeloperoxidase. Inhal Toxicol. 31(3):99–106. 10.1080/08958378.2019.1606366 [DOI] [PubMed] [Google Scholar]
- Radwan G, Hecht SS, Carmella SG, Loffredo CA. 2013. Tobacco-specific nitrosamine exposures in smokers and nonsmokers exposed to cigarette or waterpipe tobacco smoke. Nicotine Tob Res. 15(1):130–138. 10.1093/ntr/nts099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzky SS. 2017. Carbon monoxide poisoning from hookah smoking: an emerging public health problem. J Med Toxicol. 13(2):193–194. 10.1007/s13181-017-0617-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Caballero H, Park B, Loube J, Sanchez I, Vinayachandran V, Choi Y, Woo J, Edwards J, Brinkman MC, Sussan T, et al. 2020. Immune modulation by chronic exposure to waterpipe smoke and immediate-early gene regulation in murine lungs. Tob Control. 29(Suppl 2):S80–S89. 10.1136/tobaccocontrol-2019-054965 [DOI] [PubMed] [Google Scholar]
- Rezk-Hanna M, Adolfo A, Warda US, Brecht ML, Benowitz NL. 2023a. Association of non-daily hookah tobacco smoking and cardiovascular disease-related exposure biomarkers among U.S. users: the population assessment of tobacco and health study. Prev Med Rep. 36:102417. 10.1016/j.pmedr.2023.102417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezk-Hanna M, Benowitz NL. 2019. Cardiovascular effects of hookah smoking: potential implications for cardiovascular risk. Nicotine Tob Res. 21(9):1151–1161. 10.1093/ntr/nty065 [DOI] [PubMed] [Google Scholar]
- Rezk-Hanna M, Macabasco-O’Connell A, Woo M. 2014. Hookah smoking among young adults in Southern California. Nurs Res. 63(4):300–306. 10.1097/nnr.0000000000000038 [DOI] [PubMed] [Google Scholar]
- Rezk-Hanna M, Mosenifar Z, Benowitz NL, Rader F, Rashid M, Davoren K, Moy NB, Doering L, Robbins W, Sarna L, et al. 2019. High carbon monoxide levels from charcoal combustion mask acute endothelial dysfunction induced by hookah (waterpipe) smoking in young adults. Circulation. 139(19):2215–2224. 10.1161/CIRCULATIONAHA.118.037375 [DOI] [PubMed] [Google Scholar]
- Rezk-Hanna M, Talhout R, Jordt SE. 2023b. Sugars and sweeteners in tobacco and nicotine products: food and drug administration's regulatory implications. Nicotine Tob Res. 25(4):838–840. 10.1093/ntr/ntac222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba NA, El Hellani A, Honein E, Salman R, Talih S, Zeaiter J, Shihadeh A. 2018. Surface chemistry of electronic cigarette electrical heating coils: effects of metal type on propylene glycol thermal decomposition. J Anal Appl Pyrolysis. 134:520–525. 10.1016/j.jaap.2018.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert J, Bewersdorff J, Luch A, Schulz TG. 2012a. Waterpipe smoke: a considerable source of human exposure against furanic compounds. Anal Chim Acta. 709:105–112. 10.1016/j.aca.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Schubert J, Hahn J, Dettbarn G, Seidel A, Luch A, Schulz TG. 2011. Mainstream smoke of the waterpipe: does this environmental matrix reveal as significant source of toxic compounds? Toxicol Lett. 205(3):279–284. 10.1016/j.toxlet.2011.06.017 [DOI] [PubMed] [Google Scholar]
- Schubert J, Heinke V, Bewersdorff J, Luch A, Schulz TG. 2012b. Waterpipe smoking: the role of humectants in the release of toxic carbonyls. Arch Toxicol. 86(8):1309–1316. 10.1007/s00204-012-0884-5 [DOI] [PubMed] [Google Scholar]
- Schubert J, Luch A, Schulz TG. 2013. Waterpipe smoking: analysis of the aroma profile of flavored waterpipe tobaccos. Talanta. 115:665–674. 10.1016/j.talanta.2013.06.022 [DOI] [PubMed] [Google Scholar]
- Schubert J, Muller FD, Schmidt R, Luch A, Schulz TG. 2015. Waterpipe smoke: source of toxic and carcinogenic VOCs, phenols and heavy metals? Arch Toxicol. 89(11):2129–2139. 10.1007/s00204-014-1372-x [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Saleh R. 2005. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem Toxicol. 43(5):655–661. 10.1016/j.fct.2004.12.013 [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Schubert J, Klaiany J, El Sabban M, Luch A, Saliba NA. 2015. Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tob Control. 24(Suppl 1):i22–i30. 10.1136/tobaccocontrol-2014-051907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman L, Schulte HF, First MW. 1946. Further studies on sensory response to certain industrial solvent vapors. J Ind Hyg Toxicol. 28(6):262–266. https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/63013 [PubMed] [Google Scholar]
- Smith-Simone S, Maziak W, Ward KD, Eissenberg T. 2008. Waterpipe tobacco smoking: knowledge, attitudes, beliefs, and behavior in two U.S. samples. Nicotine Tob Res. 10(2):393–398. 10.1080/14622200701825023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji S, Knutzen KE, Gravely S, Elton-Marshall T, Sargent J, Lambert E, Hilmi N, Sharma E, Jackson KJ, Wang B, et al. 2021. Transitions in frequency of hookah smoking among youth and adults: findings from waves 1 and 2 of the population assessment of tobacco and health (PATH) study, 2013-15. Addiction. 116(4):936–948. 10.1111/add.15250 [DOI] [PubMed] [Google Scholar]
- Soussy S, El-Hellani A, Baalbaki R, Salman R, Shihadeh A, Saliba NA. 2016. Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tob Control. 25(Suppl 2):ii88–ii93. 10.1136/tobaccocontrol-2016-053220 [DOI] [PubMed] [Google Scholar]
- St Helen G, Benowitz NL, Dains KM, Havel C, Peng M, Jacob P. 3rd. 2014. Nicotine and carcinogen exposure after water pipe smoking in hookah bars. Cancer Epidemiol Biomarkers Prev. 23(6):1055–1066. 10.1158/1055-9965.EPI-13-0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfill SB, Brown CR, Yan X, Watson CH, Ashley DL. 2006. Quantification of flavor-related compounds in the unburned contents of bidi and clove cigarettes. J Agric Food Chem. 54(22):8580–8588. 10.1021/jf060733o [DOI] [PubMed] [Google Scholar]
- Starlight Charcoal. [Retrieved 2024 July 5]. https://www.starlightcharcoal.com/
- Strongin RM. 2019. E-Cigarette chemistry and analytical detection. Annu Rev Anal Chem (Palo Alto Calif). 12(1):23–39. 10.1146/annurev-anchem-061318-115329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhout R, Opperhuizen A, van Amsterdam JG. 2006. Sugars as tobacco ingredient: effects on mainstream smoke composition. Food Chem Toxicol. 44(11):1789–1798. 10.1016/j.fct.2006.06.016 [DOI] [PubMed] [Google Scholar]
- U.S. Government Printing Office. 2009. Family Smoking Prevention and Tobacco Control Act—Public Law 111-31, p. 123 STAT. 1776. [Retrieved 2023 July 15]. https://quittrainblog.com/wp-content/uploads/2014/02/plaw-111publ31.pdf
- van Nierop LE, Pennings JLA, Schenk E, Kienhuis AS, Talhout R. 2019. Analysis of manufacturer’s information on tobacco product additive use. Tob Regul Sci. 5(2):182–205. 10.18001/TRS.5.2.9 [DOI] [Google Scholar]
- van Rooy FG, Rooyackers JM, Prokop M, Houba R, Smit LA, Heederik DJ. 2007. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am J Respir Crit Care Med. 176(5):498–504. 10.1164/rccm.200611-1620OC [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Shihadeh A, Eissenberg T. 2012. Waterpipe tobacco products: nicotine labelling versus nicotine delivery. Tob Control. 21(3):377–379. 10.1136/tc.2010.042416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij BGF, Rood PPM, Schuit SCE, Bouwhuis MG. 2019. Waterpipe smoking: not as innocent as it may seem. Neth J Med. 77(4):156–159. https://www.ncbi.nlm.nih.gov/pubmed/31502549 [PubMed] [Google Scholar]
- Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, Feirman SP, Tworek C, Glasser AM, Pearson JL, et al. 2017. Flavored tobacco product use in youth and adults: findings from the first wave of the PATH study (2013-2014). Am J Prev Med. 53(2):139–151. 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener TL, Leavens ELS, Mehta T, Hale J, Shihadeh A, Eissenberg T, Halquist M, Brinkman MC, Johnson AL, Floyd EL, et al. 2021. Impact of flavors and humectants on waterpipe tobacco smoking topography, subjective effects, toxicant exposure and intentions for continued use. Tob Control. 30(4):366–372. 10.1136/tobaccocontrol-2019-055509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Bozhilov K, Ghai S, Talbot P. 2017. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One. 12(4):e0175430. 10.1371/journal.pone.0175430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2015. Advisory note: waterpipe tobacco smoking: health effects, research needs and recommended actions for regulators. [accessed 2024 July 27]. http://apps.who.int/iris/bitstream/10665/161991/1/9789241508469_eng.pdf
- Yip S, Taylor L, Ashraf-Khorassani M, Yu J, Borgerding M, Coleman W, Bodnar J. 2010. HPLC-MS determination of acrolein and acetone generated from C -labeled glycerol added to cigarette tobacco using two machine-smoking regimes. Contribut Tobacco Nicotine Res. 24(2):48–57. 10.2478/cttr-2013-0881 [DOI] [Google Scholar]
- Zhang Y, Li X, Lo CK, Guo ST. 2010. Characterization of the volatile substances and aroma components from traditional soypaste. Molecules. 15(5):3421–3427. 10.3390/molecules15053421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Xie Z, Li D. 2022. Discussion of waterpipe tobacco smoking on reddit. Heliyon. 8(9):e10635. 10.1016/j.heliyon.2022.e10635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.