Transurethral resection of bladder tumor (TURBT) is a key step in the management of bladder cancer (BCa) for both diagnostic and therapeutic purposes. Diverse strategies have been adopted to improve the quality of resection, such as enhanced tumor visualization, surgical checklists, and en bloc resection of bladder tumor (ERBT) [1], [2], [3]. Despite these improvements, the presence of detrusor muscle (DM) in specimens, which is a surrogate for resection quality, is still suboptimal, even in high-volume centers, so the presence of residual disease may go undetected [4]. This increases the number of second-look TURBT procedures required, significantly impacting the quality of life of patients, escalating health care costs, and prolonging waiting lists. Therefore, we investigated whether confocal microscopy (CFM) could play a role in improving TURBT quality. This technology allows immediate digital imaging of ex vivo fresh tissue, and its application in evaluating surgical margins during prostatectomy showed encouraging preliminary results [5]. Here we describe our first experience with CFM for real-time ex vivo assessment of the presence of DM in TURBT samples.
From January to March 2024, 48 consecutive patients underwent TURBT for suspected BCa and subsequent ex vivo specimen evaluation via CFM (Histolog, SamanTree Medical SA, Lausanne, Switzerland). Figure 1 illustrates the process. All specimens were first analyzed via CFM and then sent for final pathology examination. Two senior urologists (>5 yr of experience) and two young urologists (<5 yr of experience) independently assessed the presence of DM individually for each patient. The whole image acquisition and DM evaluation process takes <5 min.
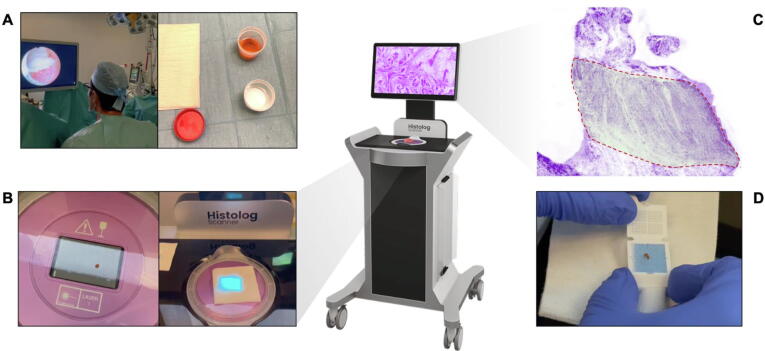
Fig. 1.
Illustration of detrusor muscle evaluation in transurethral resection of bladder tumor specimens via confocal microscopy (Histolog). (A) The specimen is retrieved during conventional or en bloc TURBT, is then processed through immersion in a fluorescent acridine orange stain (Histolog®Dip) for 10 s, then immersed in 0.9% saline solution to eliminate the excess dye and swabbed to remove the excess saline solution. (B) The specimen is moved to the laser scanning surface where a laser diode operating at a wavelength of 488 nm stimulates the fluorescence and fluorescence emission is captured in the wavelength above 500 nm. The live view mode provided by the Histolog® Scanner enables the confirmation of proper positioning and stability of the specimen in real-time. The image acquisition process takes up to 50 s depending on the dimension of the specimen, but for a single sample, it takes ∼30 s. The resolution of the scanning process is 2 µm. The Histolog® provides a comprehensive view of the entire surface without sectioning the specimen. It eliminates background noise at magnifications ranging from optical 0.5× to digital 40×. Images are acquired at a single depth of 20 µm below the surface. (C) The images are displayed with an artificial coloring, which mimics a hematoxylin and eosin stain, tailored to meet the requirements of clinical users. The touch screen enables fast and direct examination of the scanned images with a high-power digital magnification of the areas of interest form 0.5× to 40×. In the area highlighted in green and enclosed in a red dashed line, the detrusor muscle is clearly visible. (D) The specimen is immersed in a solution of formalin 5% and sent for final pathology examination.
Of the study cohort, 21 patients (44%) had a history of BCa. Ten (21%) underwent EBRT. At final pathology, two patients (4.1%) had T2, three (6.2%) had high-grade T1, and seven (15%) had high-grade carcinoma in situ/Ta disease. The rate of DM sampling was 81% (39/48). The overall sensitivity for assessing the presence of DM via CFM was 88% (95% confidence interval [CI] 82–93%), with specificity of 53% (95% CI 36–69%), positive predictive value (PPV) of 89% (95% CI 85–92%), negative predictive value (NPV) of 50% (95% CI 38–63%), and accuracy of 81% (95% CI 75–87%). Subgroup analysis for evaluations by the young urologists revealed sensitivity of 87% (95% CI 78–94%), specificity of 44% (95% CI 22–69%), PPV of 87% (95% CI 82–91%), NPV of 44% (95% CI 27–63%), and accuracy of 79% (95% CI 70–87). A subgroup analysis of ERBT procedures revealed overall sensitivity of 97% (95% CI 85–99%), specificity of 75% (95% CI 19–99%), PPV of 97% (95% CI 86–99%), NPV of 75% (95% CI 29–96%), and accuracy of 95% (95% CI 83–99%). With an overall agreement rate of 82% (95% CI 76–89), the Gwet coefficient showed substantial agreement between raters and final pathology (0.75, 95% CI 0.62–0.88).
Our initial experience demonstrates the feasibility of CFM for evaluating DM presence in TURBT specimens. The primary advantages of CFM include the speed of processing and acquisition and retention of the sample integrity for subsequent analyses. Our preliminary results suggest that CFM can be adequately interpreted by both senior and young surgeons. Overall, the approach we described might help in reducing DM undersampling through real-time specimen assessment, thereby enhancing the resection and quality subsequently improving patient outcomes.
Our results also demonstrated that interpretation of DM presence is more accurate when an EBRT approach is used. It is well known that ERBT is associated with a high rate of DM presence and provides higher-quality samples than traditional TURBT [6]. Therefore, implementation of ERBT with CFM could lead to greater surgical standardization and ensure high-quality resection regardless of the surgeon’s experience.
We are currently developing a feasibility study to evaluate the impact of CFM implementation during TURBT in clinical practice.
Conflicts of interest: The authors have nothing to disclose.
Data sharing statement: Data are available on reasonable request from the corresponding author.
Acknowledgement
Article processing charges were supported by SamanTree Medical. The sponsor have not influenced the results
Associate Editor: M. Carmen Mir
References
- 1.Mostafid H., Kamat A.M., Daneshmand S., et al. Best practices to optimise quality and outcomes of transurethral resection of bladder tumours. Eur Urol Oncol. 2021;4:12–19. doi: 10.1016/j.euo.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Yanagisawa T., Mori K., Motlagh R.S., et al. En bloc resection for bladder tumors: an updated systematic review and meta-analysis of its differential effect on safety, recurrence and histopathology. J Urol. 2022;207:754–768. doi: 10.1097/JU.0000000000002444. [DOI] [PubMed] [Google Scholar]
- 3.Sari Motlagh R., Mori K., Laukhtina E., et al. Impact of enhanced optical techniques at time of transurethral resection of bladder tumour, with or without single immediate intravesical chemotherapy, on recurrence rate of non-muscle-invasive bladder cancer: a systematic review and network meta-analysis of randomized trials. BJU Int. 2021;128:280–289. doi: 10.1111/bju.15383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariappan P, Johnston A, Trail M, et al. Achieving benchmarks for national quality indicators reduces recurrence and progression in non–muscle-invasive bladder cancer. Eur Urol Oncol. In press. 10.1016/j.euo.2024.01.012. [DOI] [PubMed]
- 5.Baas D.J.H., Vreuls W., Sedelaar J.P.M., et al. Confocal laser microscopy for assessment of surgical margins during radical prostatectomy. BJU Int. 2023;132:40–46. doi: 10.1111/bju.15938. [DOI] [PubMed] [Google Scholar]
- 6.Gallioli A., Diana P., Fontana M., et al. En bloc versus conventional transurethral resection of bladder tumors: a single-center prospective randomized noninferiority trial. Eur Urol Oncol. 2022;5:440–448. doi: 10.1016/j.euo.2022.05.001. [DOI] [PubMed] [Google Scholar]