Abstract
Objectives. To determine whether geographic prioritization of limited COVID-19 vaccine supply was effective for reducing geographic disparities in case rates.
Methods. Rhode Island allocated a portion of the initial COVID-19 vaccine supply to residents of Central Falls, a community already affected by structural policies and inadequate systems that perpetuate health inequities and experiencing disproportionately high COVID-19 morbidity and mortality. The policy was implemented with a culturally and linguistically appropriate community engagement plan and was intended to reduce observed disparities. Using a Bayesian causal analysis with population surveillance data, we evaluated the impact of this prioritization policy on recorded cases over the subsequent 16 weeks.
Results. Early geographic prioritization of Central Falls accelerated vaccine uptake, averting an estimated 520 cases (95% confidence interval = 22, 1418) over 16 weeks and reducing cases by approximately 34% during this period (520 averted vs 1519 expected without early prioritization).
Conclusions. Early geographic prioritization increased vaccine uptake and reduced cases in Central Falls, thereby reducing geographic disparities.
Public Health Implications. Public health institutions should consider geographic prioritization of limited vaccine supply to reduce geographic disparities in case rates. (Am J Public Health. 2024;114(S7):S580–S589. https://doi.org/10.2105/AJPH.2024.307741)
Substantial disparities in COVID-19 morbidity and mortality have been apparent since the onset of the pandemic in the United States, particularly by race/ethnicity, socioeconomic status, and geography.1–4 Black or African American and Hispanic or Latino people have experienced higher rates of COVID-19 diagnosis and mortality than White people,5 as have people of lower socioeconomic status.6 Geographic disparities in COVID-19 case rates often result from structural inequities (e.g., isolation is more difficult in crowded households)7 and financial pressure or essential worker status, increasing hesitancy to get tested or isolate7 and compounding other disproportionate social and economic impacts of the pandemic. These COVID-19 disparities are consistent with those observed for other health conditions in the United States,8 reflecting the strong influence of social and structural factors. Key social determinants of health include economic stability, access to high-quality education and health care, a safe neighborhood and built environment, and community context.9 These social determinants have contributed to COVID-19 disparities directly and through their association with preexisting chronic health conditions that increase risk of severe COVID-19 if infected.2
Early in the pandemic, some state and local agencies implemented programs intended to reduce health disparities, including targeted quarantine and isolation supports, cash assistance, and care navigation.10 As the COVID-19 vaccine became available, new opportunities to improve health equity emerged,11 particularly given the initial limited supply and high demand. The most common approaches to determining early vaccine eligibility were based on age, occupation (e.g., essential workers), and chronic conditions. Although some jurisdictions aimed to incorporate other criteria into their early vaccine prioritization policies, such as race/ethnicity and geography, these policies were often controversial and ultimately abandoned.12–17 However, simulation studies suggest that early COVID-19 vaccination strategies that prioritized people of all ages in geographic areas at high risk for adverse health outcomes may have prevented more deaths than purely age-based strategies.18
The Rhode Island Department of Health (RIDOH) implemented an early COVID-19 geographic vaccine prioritization policy to provide rapid access for residents of communities19–21 with high COVID-19 cases, morbidity, and mortality, high population density, and preexisting structural policies and inadequate systems that perpetuate health inequities. The policy, which was implemented with a robust culturally and linguistically appropriate community engagement plan, was intended to decrease disparities by reducing COVID-19 case rates in prioritized communities toward levels observed in other communities. To increase vaccine uptake, the engagement plan included the dispatch of canvassers who spoke the same languages as community residents to provide information on the vaccine and assist with registration for vaccine appointments, as well as partner with community-based organizations on education and outreach. Adult residents of Central Falls became eligible for vaccination nearly 4 months before all adults statewide,19 and adults in other disproportionately affected communities became eligible between 1 and 4 weeks early.20,21 Consistent with national data, Rhode Island had experienced persistent disparities in COVID-19 case rates, morbidity, and mortality, with people of color and those residing in urban core communities most affected.22–25
Evaluation of this policy is critical for understanding the extent to which it reduced observed disparities. Additionally, understanding the effectiveness of this policy can inform future resource allocation strategies in the context of limited resources. Thus, we aimed to evaluate the impact of Rhode Island’s early COVID-19 geographic vaccine prioritization policy on cases in Central Falls and to consider the potential impact of alternative geographic prioritization scenarios.
METHODS
In this section, we describe the identification of communities disproportionately affected by the COVID-19 pandemic, the specific eligibility strategies within Rhode Island’s geographic vaccine prioritization policy, and the statistical methods of our policy evaluation.
Identification of Communities
Our evaluation focused on Rhode Island residents, accounted for by community of residence, with few exclusions. Hereafter, we use “community” to refer to any of the 57 monitoring regions considered in this analysis, which were generally defined at the zip code tabulation area (ZCTA) level. However, for the 8 municipalities containing at least 1 ZCTA with population less than 1000, ZCTAs were summed to the municipality level (Appendix A.2, available as a supplement to the online version of this article at https://www.ajph.org).
Some aspects of this evaluation considered communities using a 3-tier community risk classification created by RIDOH to help guide COVID-19 surveillance and response efforts. RIDOH assigned each ZCTA to a tier based on community characteristics such as population density, social determinants of health, and COVID-19 burden to date. Generally, tier 1 was considered at highest risk, tier 2 at moderate risk, and tier 3 at lowest risk for SARS-CoV-2 infection and associated COVID-19 morbidity and mortality. RIDOH also used these tier designations to determine geographic prioritization strategies for COVID-19 vaccination. Because of vaccine supply limitations and the community characteristics listed in Methods, 1 tier 1 community, Central Falls, was identified for earliest prioritization, with prioritization of other tier 1 communities following as supply became available.
Data and Measures
We used RIDOH surveillance data to define weekly counts of administered COVID-19 vaccine doses and recorded cases disaggregated by ZCTA. To obtain community-level sociodemographic characteristics, we downloaded all distinct variables used in the calculation of the US Centers for Disease Control and Prevention’s (CDC’s) Social Vulnerability Index26 at the ZCTA level from the US Census Bureau,27 as well as median household income27 and population density, summing ZCTA-level estimates by community where applicable.
Geographic vaccine eligibility strategies
Using RIDOH’s timeline of vaccine eligibility, shown in Appendix A.1 (available as a supplement to the online version of this article at https://www.ajph.org), we identified 4 distinct strategies within the policy, which were implemented using RIDOH’s ZCTA-based tier designations. The differentiating factor was the timing of communitywide eligibility for all adult residents (aged ≥ 16 years). Specifically, the timing of adult eligibility under the 4 strategies was as follows:
-
1.
December 28, 2020: Central Falls (1 specific tier 1 community, ZCTA 02863);
-
2.
March 22, 2021: remaining tier 1 communities;
-
3.
April 12, 2021: tier 2 communities; and
-
4.
April 19, 2021: tier 3 communities.
Under limited vaccine supply, adult residents of Central Falls became eligible for vaccination nearly 3 months earlier than residents of other tier 1 communities (and nearly 4 months earlier than residents statewide) because of periods with exceptionally high prevaccine case, hospitalization, and mortality rates—even relative to other tier 1 communities—and community characteristics. Such community characteristics included disproportionate disease burden among young, Hispanic/Latino, and Black residents; a high percentage minority and undocumented residents; high population density; preexisting structural policies and inadequate systems that perpetuate health inequities, such as a lack of health infrastructure; and small population size. Thus, we refer to Central Falls as a tier 1* community, and it is the main focus of our policy evaluation. Other tier 1 and tier 2 communities became eligible 1 to 4 weeks earlier than residents statewide.
During the policy implementation period, residents throughout the state also became eligible for vaccination based on age, occupation, chronic conditions, and residence in a congregate care setting. For instance, at the time when all adult residents of Central Falls (tier 1*) became eligible for vaccination, only health care workers and those living or working in congregate care settings were already eligible for vaccination. However, by the time all adult residents of tier 1 and 2 communities became eligible, older age groups and those with chronic conditions statewide had already gained eligibility gradually throughout February and March 2021.
We used the 4 tier-based eligibility strategies to determine the percentage of each community that was eligible for vaccination at any given time, using this percentage as a time-varying continuous intervention that was fully determined by the strategy assignment and the age distribution of the community.
Outcome
Our primary outcome was recorded COVID-19 cases averted at the community level, under different geographic vaccine eligibility strategies. During the study period, all positive SARS-CoV-2 tests were reported to RIDOH. We also model an intermediary outcome, COVID-19 vaccine uptake, measured as the percentage of the community that had received at least 1 vaccine dose.
Sociodemographic covariates
As described briefly in the introduction, RIDOH allocated a portion of the initial supply of COVID-19 vaccines to residents of communities with characteristics identified through sustained monitoring efforts: disproportionately high COVID-19 cases, morbidity, and mortality, including elevated rates of infection and hospitalization among young people; a high percentage of minority and undocumented residents, increasing the importance of culturally relevant outreach; high population density; and preexisting structural policies and inadequate systems that perpetuate health inequities.19–21
Our model relies on a version of the treatment ignorability (no unmeasured confounders) assumption to infer the effect of eligibility assignment: the assignment of eligibility strategy depends only on measured, preassignment covariates and—conditional on those covariates—it is independent of the potential level of vaccine uptake and number of cases that might result from early eligibility assignment. In practical terms, we assumed that assignment of eligibility was dependent on community-level covariates that were known in advance. Thus, it was crucial to understand the motivation for RIDOH’s eligibility decision and adjust accordingly for relevant potential confounders.
We adjusted for measured characteristics to minimize the impact of unmeasured confounding associated with eligibility strategy assignment and recorded COVID-19 cases,28 including all variables used in the calculation of the CDC’s Social Vulnerability Index,26 recorded COVID-19 cases in the prevaccine period, median household income, and population density. These characteristics are summarized in Table 1.
TABLE 1—
Summary of Average Sociodemographic Characteristics by COVID-19 Risk Tier Designation: Rhode Island, March 1, 2020–September 18, 2021
| Tier 1*,a (n = 1) | Tier 1 (n = 6) | Tier 2 (n = 9) | Tier 3 (n = 41) | |
| SVI variables, % | ||||
| Below 150% of poverty level | 48.8 | 32.1 | 18.2 | 11.0 |
| Unemployed | 4.6 | 4.7 | 3.6 | 3.4 |
| Housing cost–burdened units | 48.9 | 40.4 | 32.3 | 23.6 |
| No high school diploma | 35.9 | 18.5 | 11.8 | 6.8 |
| Uninsured | 14.7 | 7.1 | 4.5 | 2.6 |
| Aged ≥ 65 y | 7.7 | 12.3 | 16.4 | 20.2 |
| Aged ≤ 17 y | 29.1 | 23.9 | 18.0 | 18.1 |
| Disabled | 17.6 | 14.2 | 14.0 | 12.3 |
| Single-parent households | 11.3 | 13.3 | 6.8 | 4.4 |
| Speak English less than well | 17.0 | 11.8 | 3.0 | 1.0 |
| Minorities | 79.1 | 66.6 | 29.3 | 10.5 |
| Housing with ≥ 10 units | 12.2 | 14.8 | 14.3 | 8.4 |
| Mobile homes | 0.0 | 0.2 | 0.2 | 1.5 |
| Crowded housing (> 1 occupant/room) | 8.8 | 4.0 | 2.0 | 0.8 |
| Households with no vehicle | 22.3 | 15.8 | 9.3 | 5.3 |
| Group quarters | 3.2 | 3.0 | 3.8 | 2.7 |
| Additional variables | ||||
| Population density (per sq mi) | 16 194 | 9 888 | 5 226 | 1 166 |
| Median household income, $ | 34 689 | 46 641 | 64 865 | 88 923 |
| Recorded cases per 100 000 (March–November 2020) | 2 295 | 3 016 | 1 256 | 376 |
| % of population with at least 1 dose as of September 18, 2021 | 69.9 | 61.9 | 65.1 | 68.1 |
| % of population with at least 2 doses as of September 18, 2021 | 60.1 | 55.1 | 60.2 | 63.9 |
Note. The table includes all variables used in the calculation of the Social Vulnerability Index (SVI) and the additional sociodemographic variables used in the models. SVI variables are the percentages of the population with that characteristic. Tiers refer to the 3-tier community risk classification system developed by the Rhode Island Department of Health to help guide COVID-19 surveillance and response efforts. Tier 1 included communities at highest risk for COVID-19 and tier 3 included communities at lowest risk (see Methods).
Under limited vaccine supply, adult residents of Central Falls became eligible for vaccination nearly 3 months earlier than residents of other tier 1 communities (and nearly 4 months earlier than residents statewide) because of periods with exceptionally high prevaccine case, hospitalization, and mortality rates.
Statistical Methods
We used a 2-part model to characterize the overall effect of eligibility strategy on longitudinal trends in case counts. Specifically, we assumed that the mechanism through which eligibility strategy affects case counts is by increasing vaccine uptake and that vaccine uptake is driven both by the percentage of the population eligible for vaccination and community-level covariates (for more detail, see Appendix B, available as a supplement to the online version of this article at https://www.ajph.org). We used the following modeling process:
-
1.
Model observed vaccine uptake at the community level as a function of eligibility, sociodemographic variables, and time.
-
2.
Model recorded case counts at the community level as a function of vaccine uptake, sociodemographic variables, and time.
-
3.
Using the model in step 1, generate predicted vaccine uptake as a function of time for each community under each eligibility strategy.
-
4.
Using the model in step 2, generate predicted number of recorded cases as a function of time, using vaccine uptake predictions generated in step 3. This yields predicted potential outcomes of recorded cases under each of the eligibility strategies for each community.
-
5.
Compute causal effects in terms of the difference in predicted cases under different eligibility strategies.
We used Bayesian machine learning models for the predictive models in steps 1 and 2 to capture potential nonlinearities and interactions without having to specify the precise functional form of the models. Steps 3 through 5 were carried out using posterior predictive sampling from the models, which can be viewed as simulations from fitted models under each strategy.
Specifically, we used soft Bayesian Additive Regression Trees (SoftBart)29,30 to learn observed data models (steps 1 and 2 above). Briefly, SoftBart is a Bayesian nonparametric method for modeling an unknown function as an ensemble of decision trees, aggregating numerous weakly informative decision trees, each explaining a small portion of the unknown function, into a strong learner, while regularizing the impact of each tree.29 Unlike general BART models, SoftBart assigns probabilities to each split rather than hard cutoffs, resulting in smooth predictions with reduced error compared to general BART implementations.29,31 This modeling strategy allowed us to examine every possible interaction among numerous potentially correlated predictors, while prioritizing lower-order interactions, and to estimate causal effects by drawing posterior predictions at the community level. We used default parameter settings. Detailed modeling information is available in online Appendix B.
To evaluate the impact of the eligibility strategy implemented in Central Falls, we compared the predicted number of recorded cases under the tier 1* strategy implemented to that predicted under the tier 3 strategy (i.e., assuming no geographic prioritization) during the period of early prioritization. Similarly, we estimated the potential impact of alternative geographic prioritization scenarios for tier 1 and tier 2 communities, given sufficient vaccine supply, by comparing the predicted number of cases under the tier 1* strategy (i.e., if they had received earliest prioritization) versus the strategy actually implemented for each community.
We implemented all analyses using R version 4.0.2, fitting models using the SoftBart package.30
RESULTS
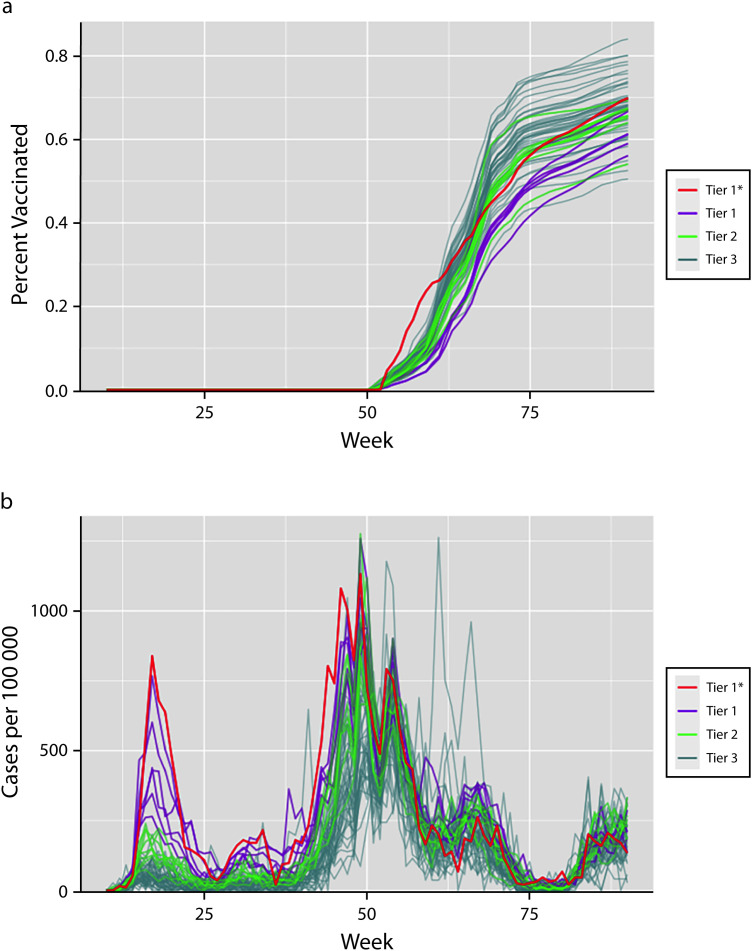
Our analysis included 57 communities in Rhode Island, ranging in population from 2194 to 47 174. Of the 1 046 263 people residing in those 57 communities, 19 437 (1.9%) resided in the tier 1* community, 212 431 (20.3%) in tier 1 communities, 249 017 (23.8%) in tier 2 communities, and 565 378 (54.0%) in tier 3 communities. From March to November of 2020, there were 20 333 COVID-19 cases recorded in tier 1* and tier 1 communities combined, more than half of the state’s 40 179 total recorded cases during this period, despite only 22.2% of the state’s population residing in these communities. Case rates were consistently higher in tier 1* and tier 1 communities than in tier 2 and tier 3 communities during this prevaccine period (Figure 1).
FIGURE 1—
Community Statistics for (a) Observed COVID-19 Vaccine Uptake and (b) Recorded Cases of COVID-19 per 100 000 Population: Rhode Island, March 1, 2020–September 18, 2021
Note. The figure shows the observed data for the 57 communities that we used to fit our model in each stage of the analysis. The horizontal axes spans March 1, 2020 (week 10), to September 18, 2021 (week 90). Tiers refer to the 3-tier community risk classification system developed by the Rhode Island Department of Health to help guide COVID-19 surveillance and response efforts. Tier 1 included communities at highest risk for COVID-19 and tier 3 included communities at lowest risk (see Methods). In each panel, lines representing community-level metrics are colored by tier assignment. Vaccine uptake is measured as the proportion of the population with at least 1 dose of an approved vaccine, while cases are measured as recorded case counts per 100 000 population.
aUnder limited vaccine supply, adult residents of Central Falls became eligible for vaccination nearly 3 months earlier than residents of other tier 1 communities (and nearly 4 months earlier than residents statewide) because of periods with exceptionally high prevaccine case, hospitalization, and mortality rates.
Disparities in social determinants of health were also apparent across the tiers (Table 1). For example, the percentage of the population living below 150% of the federal poverty level ranged from 48.8% in the tier 1* community to 32.1% in tier 1, 18.2% in tier 2, and 11.0% in tier 3 communities. Similarly, the percentage of the population with no high school diploma ranged from 35.9% in the tier 1* community to 6.8% in tier 3 communities, whereas living in crowded housing ranged from 8.8% in the tier 1* community to 0.8% in tier 3 communities.
Impact of Rhode Island’s Policy
The observed vaccine uptake (Figure 1a) and recorded case rate (Figure 1) differed substantially by community over time. Of note, the initial and end-of-study (September 18, 2021) observed vaccine uptake in Central Falls (tier 1*) was higher than in any tier 1 community.
Our models for vaccine uptake and recorded cases by community exhibited good fit to the observed data over time. Example curves of model fit for a community in each tier are available in Figure A1 (available as a supplement to the online version of this article at https://www.ajph.org).
As previously noted, our evaluation focused on the policy impact in Central Falls because of the community characteristics and the wider timeline distinction between the strategy implemented in Central Falls and any other strategy. Compared with a scenario where Central Falls followed the tier 3 strategy (i.e., no geographic prioritization), the tier 1* strategy implemented in Central Falls is estimated to have averted 520 (95% confidence interval [CI] = 22, 1418) recorded cases over 16 weeks, corresponding to 167 (95% CI = 7, 456) cases averted per 100 000 residents per week during this 16-week period. For context, Central Falls has an estimated population of 19 437 and observed 999 cases over this 17-week period. Thus, early prioritization is estimated to have reduced cases by approximately 34% during this period (i.e., 520 averted vs 1519 expected without early prioritization).
Potential Alternative Scenarios
Using modeled vaccine uptake and recorded cases under each strategy by community, we also considered potential alternative prioritization scenarios, given sufficient vaccine supply. Predicted vaccine uptake and recorded case curves under each eligibility strategy for 1 selected community in each tier are available in online Figure A2. In each community, compared with any other eligibility strategy, the tier 1* eligibility strategy resulted in more rapid vaccine uptake and a more rapid decrease in recorded cases. Differences between the other 3 strategies were small.
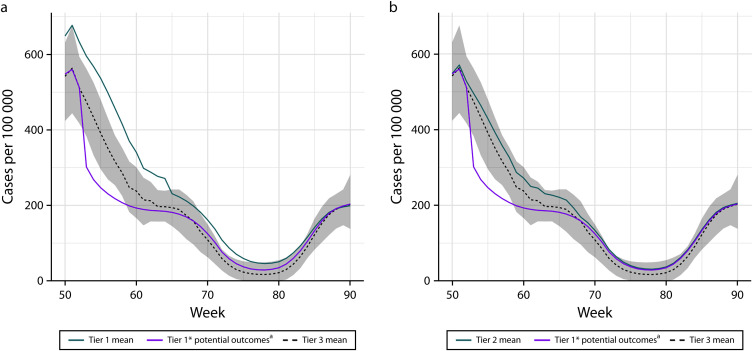
Compared with the actual strategy used, implementing the tier 1* strategy in tier 1 communities would have rapidly reduced recorded case rates to a more equitable level (Figure 2). We are defining “equitable” here as case rates in the range of what tier 3 communities were experiencing. Overall, implementation of the tier 1* strategy in tier 1 communities would have averted an estimated 3363 (95% CI = 433, 7455) recorded cases over 12 weeks, corresponding to 132 (95% CI = 36, 621) cases averted per 100 000 residents per week during this 12-week period and a 35% reduction in cases during this period (i.e., 3363 averted vs 9620 observed; Table 2).
FIGURE 2—
Estimated Case Counts Under Differing Strategies for COVID-19 Risk (a) Tier 1 and (b) Tier 2 Communities: Rhode Island, March 1, 2020–September 18, 2021
Note. One realistic alternative to prioritizing Central Falls in December 2020 would have been to prioritize additional or all tier 1 or even tier 2 communities. This figure suggests that the tier 1* strategy would have rapidly reduced case rates to a more equitable level (i.e., in the range of what tier 3 communities were experiencing) in tier 1 or tier 2 communities when considered in aggregate. It also highlights that case rate disparities were less severe in tier 2 communities. The purple line in each panel shows the potential recorded case counts per 100 000 population, if the indicated set of communities had been prioritized as early as Central Falls (tier 1* strategy). The turquoise line shows the fitted recorded case counts per 100 000 population under the tier-specific implemented strategy. The black dashed line shows the posterior mean and the shaded region the 95% density interval for the tier 3 communities in aggregate. Tiers refer to the 3-tier community risk classification system developed by the Rhode Island Department of Health to help guide COVID-19 surveillance and response efforts. Tier 1 included communities at highest risk for COVID-19 and tier 3 included communities at lowest risk (see Methods). Figure A3 (available as a supplement to the online version of this article at https://www.ajph.org) displays the same results for each tier 1 community individually. aUnder limited vaccine supply, adult residents of Central Falls became eligible for vaccination nearly 3 months earlier than residents of other tier 1 communities (and nearly 4 months earlier than residents statewide) because of periods with exceptionally high prevaccine case, hospitalization, and mortality rates.
TABLE 2—
Average Number of Cases of COVID-19 Averted by the Indicated Strategy Comparison: Rhode Island, March 1, 2020–September 18, 2021
| Community | Population | No. of Observed Cases | Realized Strategy | Avg No./Week | Avg No./Week/100 000 | Total No. Averted (95% CI) | Total No. per 100 000 (95% CI) | % Reduction |
| 02860 | 47 175 | 2 030 | Tier 1 | 53 | 113 | 640 (72, 1 377) | 1357 (153, 2919) | 31 |
| 02904 | 29 881 | 1 360 | Tier 1 | 37 | 123 | 442 (64, 942) | 1480 (216, 3153) | 33 |
| 02905 | 26 020 | 1 019 | Tier 1 | 37 | 143 | 447 (53, 943) | 1719 (204, 3625) | 44 |
| 02907 | 31 277 | 1 262 | Tier 1 | 47 | 151 | 567 (59, 1 315) | 1812 (190, 4203) | 45 |
| 02908 | 37 792 | 2 027 | Tier 1 | 49 | 130 | 588 (67, 1 391) | 1557 (178, 3680) | 29 |
| 02909 | 40 286 | 1 922 | Tier 1 | 57 | 140 | 678 (82, 1 571) | 1683 (203, 3900) | 35 |
| Tier 1 (total) | 212 431 | 9 620 | Tier 1 | 280 | 132 | 3 363 (433, 7 455) | 1583 (204, 3509) | 35 |
| 02861 | 24 764 | 1 273 | Tier 2 | 28 | 113 | 420 (32, 971) | 1696 (128, 3922) | 33 |
| 02893 | 29 283 | 1 344 | Tier 2 | 28 | 97 | 424 (22, 954) | 1448 (76, 3258) | 32 |
| 02895 | 41 616 | 2 262 | Tier 2 | 34 | 82 | 511 (40, 1 113) | 1227 (96, 2676) | 23 |
| 02906 | 28 254 | 1 131 | Tier 2 | 24 | 85 | 362 (−51, 966) | 1280 (−182, 3418) | 32 |
| 02910 | 21 746 | 1 129 | Tier 2 | 22 | 103 | 336 (5, 780) | 1545 (22, 3586) | 30 |
| 02911 | 15 627 | 762 | Tier 2 | 24 | 153 | 360 (2, 813) | 2301 (10, 5203) | 47 |
| 02914 | 21 720 | 1 052 | Tier 2 | 27 | 126 | 410 (31, 913) | 1889 (141, 4203) | 39 |
| 02919 | 29 312 | 1 598 | Tier 2 | 26 | 87 | 384 (−23, 958) | 1309 (−78, 3268) | 24 |
| 02920 | 36 695 | 1 781 | Tier 2 | 30 | 82 | 451 (30, 1 048) | 1229 (83, 2857) | 25 |
| Tier 2 (total) | 249 017 | 12 332 | Tier 2 | 244 | 98 | 3 657 (170, 8 131) | 1468 (68, 3265) | 30 |
Note. CI = confidence interval. Regarding interpretation of the table: in the first row, for example, if community 02860 had received the tier 1* strategy instead of the realized strategy (tier 1), we estimate that they would have seen 53 fewer cases per week or 113 fewer cases per 100 000 residents per week. Community groupings with “(total)” indicate sums of communities; for instance, tier 1 indicates the sum over all tier 1 communities. The “No. of Observed Cases” column displays the number of cases recorded in that community over the time period between full eligibility in Central Falls and full eligibility in that community. Tiers refer to the 3-tier community risk classification system developed by the Rhode Island Department of Health to help guide COVID-19 surveillance and response efforts. Tier 1 included communities at highest risk for COVID-19 and tier 3 included communities at lowest risk (see Methods). The “Community” column contains the zip code tabulation area. Table cells indicating averted cases are the posterior means of all draws from the model. These results, as with results from other Bayesian models, are averages from a distribution of effect sizes, and were similar to results estimated under different model parameters. In Appendix B (available as a supplement to the online version of this article at https://www.ajph.org), we provide additional information on the modeling process and parameter selection.
Similarly, implementation of the tier 1* strategy in tier 2 communities would have rapidly reduced case rates to a more equitable level (Figure 2 and online Figure A3). Overall, implementation of the tier 1* strategy in all tier 2 communities would have averted an estimated 3657 (95% CI = 170, 8131) recorded cases over 15 weeks, corresponding to 98 (95% CI = 5, 218) cases averted per 100 000 residents per week during this 15-week period and a 30% reduction in cases during this period (i.e., 3683 averted vs 12 332 observed).
DISCUSSION
In this evaluation of an early COVID-19 geographic vaccine allocation policy implemented in Rhode Island, we found substantial benefits of early eligibility for residents of a community (Central Falls) with disproportionately high COVID-19 morbidity and mortality, high population density, and preexisting social policies and inadequate systems that perpetuate health inequities. All adults in Central Falls became eligible for vaccination nearly 4 months earlier than all adults statewide. This eligibility strategy for Central Falls accelerated vaccine uptake, thereby resulting in approximately 34% lower recorded cases than would have been expected if the community had not received early eligibility. Our study also suggests that, given sufficient vaccine supply, a similar strategy would have benefited other tier 1 and tier 2 communities. Although our analysis focused on recorded cases, these findings are generally consistent with a simulation study suggesting that geographic vaccine prioritization may prevent more deaths than age-based strategies.18
In addition to early geographic prioritization, RIDOH’s community engagement efforts were likely an essential component of the vaccination approach. Vaccine effectiveness is dependent not just on eligibility but also on uptake, and uptake requires both accessibility and interest in getting vaccinated.32 RIDOH implemented the early geographic prioritization policy along with a culturally and linguistically appropriate community engagement plan. Though not directly evaluated in our study, this was likely critical because vaccine access and confidence are decreased by the same structural inequities that predispose communities to adverse health outcomes.32,33 However, even with this robust community engagement plan, many tier 1 and tier 2 communities had lower vaccine uptake than tier 3 communities, suggesting that additional engagement strategies are needed to improve vaccine access and confidence. Such engagement strategies may include identifying vaccine ambassadors and trusted messengers and supporting them in delivering effective messages; offering home-, school-, and workplace-based vaccination to improve access; and trying to combat misinformation, among others.32
Limitations
Our analysis has limitations. First, as with all causal models built from observational data, there may be unmeasured confounding. For example, use of nonpharmaceutical interventions aggregated to the community could be related to increased vaccine uptake and reduced case counts. In this scenario, failing to account for use of nonpharmaceutical interventions could lead to overestimation of policy impact. Second, our counterfactual predictions rely on the accuracy of our models of vaccine uptake and case counts. To maximize flexibility and guard against misspecification, we use Bayesian machine learning models that demonstrate good fit to the observed data but, as with any simulation-based approach, the counterfactual predictions cannot be directly verified. Third, there may be geographic spillover, which is not accounted for in the model (e.g., increased uptake in 1 community could reduce case counts in neighboring communities). If early availability of vaccine in prioritized communities reduces case counts in neighboring communities, not accounting for spillover could lead to underestimation of early prioritization impact. Finally, our analysis relies on counts of recorded cases, which are less than the numbers of infections. Because our analysis was carried out during a period where all positive test results were reported to RIDOH, it is reasonable to assume that reductions in reported cases correspond to reductions in overall infections, but this cannot be directly verified.
It would have been difficult to apply standard techniques for policy evaluation because primary assumptions are not satisfied,34,35 so we applied a causal modeling approach (for more details, see online Appendix B). Despite the limitations, our analysis was strengthened by its use of population surveillance data to estimate the impact of the policy, rather than relying on techniques not well-suited for this application.
Public Health Implications
Our analysis suggests that an early geographic COVID-19 vaccine prioritization policy rapidly increased vaccine uptake and reduced recorded cases in Central Falls, thereby reducing geographic disparities. Our findings also suggest that other communities disproportionately affected by the pandemic would have also benefited from this very early prioritization, given sufficient vaccine supply. Reducing rates of COVID-19 cases through early vaccination was critical for improving health equity, as this prevents ongoing transmission and associated morbidity and mortality, and residents are able to maintain daily responsibilities. Public health institutions should consider geographic prioritization of limited vaccine supply within pandemic preparedness and response to improve health equity. Importantly, although our analysis identified benefits of geographic prioritization, we did not aim to determine the optimal vaccine prioritization policy in the context of limited resources. Additional research is needed to estimate the policy’s impact on COVID-19 hospitalization and mortality, which could identify additional benefits and inform endpoints for an optimal-policy decision framework. Finally, future use of our model to identify the marginal effect of specific social determinants of health on vaccine uptake at the community level could be useful for informing vaccination campaigns.
ACKNOWLEDGMENTS
This work was funded by the Rhode Island Department of Health. L. Gargano was supported by the US Centers for Disease Control and Prevention (CDC; grant 6 NH23IP922618-0501). M. Wilson was supported, in part, by the CDC (grant OT21-2103). The analysis was completed in conjunction with CDC OT21- 2103 activities to inform initiative planning efforts, policy development, and allocation of resources. This work was also funded in part by the CDC through the Council of State and Territorial Epidemiologists and Center for Forecasting and Outbreak Analytics (A. Bilinski, cooperative agreement NU38OT000297).
We gratefully acknowledge Ellen Amore, MS, of the Rhode Island Department of Health for her advice on the utilization of vaccination data in this analysis.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
HUMAN PARTICIPANT PROTECTION
This evaluation was classified as exempt by the Rhode Island Department of Health institutional review board (application 2021-02).
REFERENCES
- 1.Lin Q, Susan P, Dylan H, Aresha M-C, Marynia K. Assessment of structural barriers and racial group disparities of COVID-19 mortality with spatial analysis. JAMA Netw Open. 2022;5(3):e220984. 10.1001/jamanetworkopen.2022.0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tai DBG, Aditya S, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):703–706. 10.1093/cid/ciaa815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson SL, Sahar D, Leah B, et al. Spatial disparities of COVID-19 cases and fatalities in United States counties. Int J Environ Res Public Health. 2021;18(16):8259. 10.3390/ijerph18168259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kathe N J, Wani R J. Determinants of COVID-19 case fatality rate in the United States: spatial analysis over one year of the pandemic. J Health Econ Outcomes Res. 2021;8(1):51–62. 10.36469/jheor.2021.22978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueroa JF, Wadhera RK, Mehtsun WT, Kristen R, Jessica P, Jha AK. Association of race, ethnicity, and community-level factors with COVID19 cases and deaths across US counties. Healthc (Amst). 2021;9:100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanijahani A, Iezadi S, Gholipour K, Azami- Aghdash S, Naghibi D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health. 2021;20(1):248. 10.1186/s12939-021-01582-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reitsma MB, Claypool AL, Jason V, et al. Racial/ethnic disparities in COVID-19 exposure risk, testing, and cases at the subcounty level in California. Health Aff (Millwood). 2021;40(6):870–878. 10.1377/hlthaff.2021.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Healthy People 2010 Final Review. Hyattsville, MD: Health Statistics National Center; 2012. [Google Scholar]
- 9. Disease Prevention Office, Promotion Health . Healthy People 2030: Social Determinants of Health. Available at: https://www.cdc.gov/nchs/data/hpdata2010/hp2010_final_review.pdf . Accessed August 1, 2023.
- 10.Bleser WK, Humphrey S, Crook HL, et al. Pandemic-driven health policies to address social needs and health equity. Health Affairs Health Policy Brief. 2022. Available at: https://www.healthaffairs.org/content/briefs/pandemic-driven-health-policies-address-social-needs-and-health-equity. Accessed June 11, 2024. [Google Scholar]
- 11.Jean-Jacques M, Bauchner H. Vaccine distribution—equity left behind? JAMA. 2021;325(9):829–830. 10.1001/jama.2021.1205 [DOI] [PubMed] [Google Scholar]
- 12.Janell R. When a Texas county tried to ensure racial equity in COVID-19 vaccinations, it didn’t go as planned. 2021. Available at: https://time.com/5942884/covid-19-vaccine-racial-inequity-dallas. Accessed June 11, 2024. [Google Scholar]
- 13. Ostrov BF. Sacrificing equity for speed? California’s COVID vaccine rollout stirs concern. 2021. . Available at: https://calmatters.org/health/coronavirus/2021/01/sacrificing-equity-for-speed-californias-covid-vaccine-rollout-stirs-concern . Accessed June 11, 2024.
- 14. Michigan Public Radio . The Social Vulnerability Index, COVID-19 vaccines, and why it makes some Republicans mad. 2021. . Available at: https://www.michiganradio.org/politics-government/2021-02-26/the-social-vulnerability-index-covid-19-vaccines-and-why-it-makes-some-republicans-mad . Accessed June 11, 2024.
- 15. M Jagannathan . Should Black and Latino people get priority access to a COVID-19 vaccine? Market Watch. 2020. . Available at: https://www.marketwatch.com/story/should-black-and-latino-people-get-priority-access-to-a-covid-19-vaccine-2020-07-16 . Accessed June 11, 2024.
- 16. S Cline , G Flaccus . Under new program, some Oregon centers can vaccinate anyone. AP. 2021. . Available at: https://apnews.com/article/portland-coronavirus-pandemic-oregon-5ed46270d0da623dd453189d1bc48e2e . Accessed June 11, 2024.
- 17. J Wick . Vaccine access codes for hard-hit Black, Latino communities improperly used in other LA areas. Los Angeles Times. 2021. . Available at: https://www.latimes.com/california/story/2021-02-22/vaccine-access-codes-for-hard-hit-communities-of-color-circulate-widely-in-affluent-l-a . Accessed June 11, 2024.
- 18.Wrigley-Field E, Kiang MV, Riley AR, et al. Geographically targeted COVID-19 vaccination is more equitable and averts more deaths than age-based thresholds alone. Sci Adv. 2021;7(40):eabj2099. 10.1126/sciadv.abj2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. S Doiron , LoSciuto S. Central Falls will be first RI population to begin receiving COVID-19 vaccine. WPRI. 2020. . Available at: https://www.wpri.com/news/local-news/blackstone-valley/central-falls-will-be-first-ri-population-to-begin-receiving-covid-19-vaccine . Accessed June 11, 2024.
- 20. Machado S. Pawtucket, Providence expand vaccine eligibility to all adults in hard-hit ZIP codes. WPRI: . 2021. . Available at: https://www.wpri.com/news/local-news/providence/providence-expands-vaccine-eligibility-to-18-in-hard-hit-zip-codes . Accessed June 11, 2024. [Google Scholar]
- 21. Rhode Island Dept of Health . Press Release: Governor McKee, RIDOH Announce 7,600 COVID-19 Vaccination Appointments to be Posted Tomorrow. 2021. . Available at: https://covid.ri.gov/press-releases/governor-mckee-ridoh-announce-7600-covid-19-vaccination-appointments-be-posted . Accessed June 11, 2024.
- 22.Naganathan S, Paiva M, Soliman L, Amanullah S, Aluisio AR, Genisca AE. Epidemiology and clinical characteristics of emergency department patients with COVID-19 in a Rhode Island healthcare system. R I Med J (2013). 2021;104:24–29. [PubMed] [Google Scholar]
- 23.Barry K, Suskin JA, Testa J, Leonard M, De Groot AS. Expanding access to COVID-19 testing, vaccination and treatment at a free clinic for uninsured Spanish-speaking adults in Providence, RI. Hum Vaccin Immunother. 2022;18(6):2144604. 10.1080/21645515.2022.2144604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhode Island Dept of Health . Rhode Island COVID-19 data: hospital demographic data by year. Available at: https://docs.google.com/spreadsheets/d/1Nq25JKlbQphdBKqZc09mjRKF0HWHO9280p9xCnVvOyY/edit#gid=1113895183 . Accessed October 20, 2023.
- 25. Rhode Island Dept of Health . Rhode Island COVID-19 data: death demographic data by year. Available at: https://docs.google.com/spreadsheets/d/1Nq25JKlbQphdBKqZc09mjRKF0HWHO9280p9xCnVvOyY/edit#gid=939060058 . Accessed October 20, 2023.
- 26. CDC/ATSDR SVI: data and documentation download. 2020. . Available at: https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html . Accessed June 11, 2024.
- 27. US Census Bureau . Demographic and housing estimates. 2021. . Available at: https://data.census.gov . Accessed June 11, 2024. [Google Scholar]
- 28.Karaye IM, Horney JA. The impact of social vulnerability on COVID-19 in the US: an analysis of spatially varying relationships. Am J Prev Med. 2020;59(3):317–325. 10.1016/j.amepre.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linero AR. Soft Bayesian additive regression trees. 2022. . Available at: https://arxiv.org/pdf/2210.16375 . Accessed June 11, 2024.
- 30. Linero AR. SoftBart: implements the SoftBart algorithm 2022. . R package version 1.0.0. Available at: https://cran.r-project.org/web/packages/SoftBart/SoftBart.pdf . Accessed July 8, 2024.
- 31. Chipman HA , George EI , McCulloch RE. BART: Bayesian additive regression trees 2010. . Available at: http://www.jstor.org/stable/27801587 . Accessed June 11, 2024.
- 32.COVID-19 Vaccination Field Guide: 12 Strategies for Your Community. Atlanta, GA: US Centers for Disease Control and Prevention; 2023. [Google Scholar]
- 33.AuYoung M, Rodriguez Espinosa P, Chen W-T, et al. Addressing racial/ethnic inequities in vaccine hesitancy and uptake: lessons learned from the California Alliance Against COVID-19. J Behav Med. 2023;46:153–166. 10.1007/s10865-022-00284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray EJ, Robins JM, Seage GR, Freedberg KA, Hernán MA. A Comparison of agent-based models and the parametric G-formula for causal inference. Am J Epidemiol. 2017;186(2):131–142. 10.1093/aje/kwx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou H, Taber C, Arcona S, Li Y. Difference-in-differences method in comparative effectiveness research: utility with unbalanced groups. Appl Health Econ Health Policy. 2016;14(4):419–429. 10.1007/s40258-016-0249-y [DOI] [PMC free article] [PubMed] [Google Scholar]