Abstract
After harvesting of cones used for beer production, the remaining hop vegetative biomass requires disposal. The hop plant contains bioactive compounds in all its parts—cones, leaves, and roots—exhibiting interesting antioxidant, antiviral, and antibacterial properties. In this work, extracts obtained from hop leaves, a plant material often neglected in the hop cultivation, have been investigated; the qualitative UHPLC-MS/MS and GC-TOF-MS characterization revealed the presence of bioactive compounds such as polyphenols, α- and β-acids and terpenes are present. The extract retained antioxidant activity, as verified by Folin-Ciocalteu, DPPH, ABTS and FRAP assays, and demonstrated some antimicrobial activity when combined with antibiotics, particularly against Gram-positive bacterial strains. Additionally, the extracts showed an ability to interact with proteins as human insulin, amyloid beta peptide, mucin and bovine serum albumin (BSA), has been detected, indicating their potential to counteract inflammatory processes and protect against Alzheimer's disease. These findings suggest that hop vegetative biomass, typically considered waste, can be potentially transformed into a valuable resource with applications in various fields, from nutraceuticals to pharmaceuticals and cosmetics, aligning with a circular economy perspective.
Keywords: Humulus lupulus, Antioxidant, Anti-amyloid, Synergistic antimicrobic, Biomass recycle
Graphical abstract
Highlights
-
•
Biomass from hop (Humulus lupulus L.) leaves can be a matrix to extract bioactive compounds.
-
•
Leaf extracts maintain antioxidant properties.
-
•
The extracts with antibiotics showed a synergistic to additive effect.
-
•
Leaf extracts exhibit anti-amyloid and anti-denaturation properties.
-
•
Hop leaves can switch from a by-product of beer-making process to a resource.
1. Introduction
Hop (Humulus lupulus L.), belonging to the Cannabaceae family, is well-known throughout the world as one of the raw material used in the brewing industry. Hop cones are widely used to maintain the microbiological stability of the brew and to give its characteristic aroma and flavour. Over the last decades, an increasing part of the scientific community has turned its focus on exploring the biological effects of plants used in traditional medicine, and hop is one of them [1]. Several phytochemical studies have been performed to investigate the composition of hop cones, leading to the isolation and identification of pharmacologically relevant secondary metabolites, such as flavanones, terpenes, bitter acids, flavonol glycosides (i.e. quercetin), catechins (i.e. epicatechin gallate), and prenylflavonoids [2,3], many of which have multiple phenol structure. Because of the presence of these bioactive molecules, hop presents numerous beneficial properties for human health. Among them, bitter acid humulone and lupulone exhibit antibacterial activities against Gram-positive (as Lactobacillus, Streptococcus, Staphylococcus, Listeria, Clostridium and Bacillus species), and Gram-negative bacteria (i.e. Helicobacter pylori and Brucella spp.), and some fungi as Candida, Trichophyton, Fusarium and Mucor species [[4], [5], [6], [7]]. The prenylflavonoids xanthohumol, 8-prenylnaringenin and 6-prenylnaringenin can inhibit the growth of S. aureus and have antibacterial activity against pathogens involved in skin inflammation and Acne vulgaris [[8], [9], [10]].
The presence of numerous polyphenols is responsible for the antioxidant properties of hop cone extracts: they scavenge free radicals involved in many disorders where the oxidative stress plays a crucial role, including cancer, inflammation, neurodegenerative and age-related diseases [4,8,11]. In addition, it has been recently shown the hop's capacity to hinder in vitro aggregation of amyloid beta peptide and the ability to increase autophagy, promoting the clearance of misfolded and aggregated proteins [12].
Several studies have shown that hop cones are richer in bioactive molecules in respect to other hop components, for example the content of phenolic substances in cone extract is 10-times greater than in hop leaves [1,8]. But even so, recently hop leaves have been receiving attention as antioxidants and potential antibacterial agents [13]. Xanthohumol, the most abundant polyphenol which represents 0.1%–1% of the dry weight of hop cones, can also be found in other hop plant parts, as in the trichomes on the underside of hop leaves [2,9]. The concentration of phenolic compounds in hop leaf extract is related to their antioxidant potential, with expected differences related to the genotype and the environmental growing conditions [14,15].
Based on this acquiring, hop vegetative biomass, to date considered a waste and used only for compost production, could represent a secondary noticeable income for hop growers; in fact, this biomass is approximately 10–15 t/ha annually (2.6 kg/plant) [8], and can be used as a matrix to extract bioactive compounds by different types of potential users.
The main aim of this study is to evaluate whether extracts from hop vegetative biomass maintain their antioxidant activity and to investigate their anti-inflammatory, antibacterial, and amyloidogenic beneficial properties. The goal is to potentially transform these extracts into resources for various fields, including nutraceutical, pharmaceutical, and cosmetics. To achieve this, hop leaf extracts were first characterized in terms of polyphenolic content and antioxidant properties, using spectrophotometric assays and by studying their compositional profile with UHPLC-MS/MS and GC-TOF-MS. Subsequently, the antimicrobial activity against Gram-positive and Gram-negative reference bacterial strains was studied by determining the Minimum Inhibitory Concentration (MIC) and evaluating any synergistic effect when hop leaf extracts were combined with different antibiotics. The anti-inflammatory activity of extracts was assessed using a protein denaturation inhibition assay. Furthermore, the extracts exhibited interesting protective effects on protein activity, including protection against thermal unfolding and amyloid aggregation. Specifically, the inhibitory effect on amyloid fibrillation is here presented for two different proteins, human insulin, and amyloid beta peptide, both associated with significant diseases.
Additionally, the interaction of Humulus lupulus extracts with mucin was investigated by UV–Vis absorption spectroscopy as a preliminary study of their potential gastrointestinal protective effect.
2. Material and methods
2.1. Plant material
Leaves were harvested at the same time as the cones, at the end of August, from hop plants, cv. Cascade, grown in a commercial field in the seismic crater of the Marche region. Cascade is a well-known and widespread hop cultivar, cultivated in Italy [16], and mostly used in the brewing process to confer floral and fruity aroma to beer [16,17].
2.2. Extraction of bioactive molecules from hop leaves
Hop leaves were gently cleaned, weighted and lyophilized by a freeze dryer Lyovapor L-200 (Buchi, Milan, Italy) at −54 °C and 1.00 mbar. After pulverization, the grinded freeze-dried leaves were extracted with a solvent mixture ethanol:water (80:20) with a dilution factor of 1:20 (1 g of leaves’ powder in 20 mL of solvent) under agitation (Arex digital shaker, Velp Scientifica srl, Usmate (MB), Italy) at 200 strokes/minute for 2 h. The suspension was centrifuged (2770 rcf, 10 min) and filtered with a filter paper; the concentration of dry matter in the ethanol-water hop leaf extract, determined after lyophilization was 3.5 mg/mL. Part of the extract was lyophilized and stored at −20 °C to be employed in the experiments where a solvent other than ethanol-water was needed.
2.3. Qualitative characterisation of the hop leaf extract
2.3.1. UHPLC-MS/MS
In order to characterize the polyphenolic fraction, both aqueous ethanolic leaf extracts and samples re-suspended after the lyophilisation process were diluted five times with 0.1 % aqueous formic was analysed by a protocol according to Chiancone et al. [18]. A UHPLC Ultimate 3000 separative module (Dionex, Sunnyvale, CA, USA) equipped with a TSQ Vantage triple quadrupole mass spectrometer (Thermo Fisher, San Jose, CA, USA) was used to characterize the samples. In particular, electrospray ionization mode (ESI) was applied for molecule ionization, after a separation on a RP-C18 SunShell column (2.6 m, 100 mm, ChromaNik Technologies, Osaka, Japan) conduced at 40 °C. All the parameters applied for the separation and detection of compounds of interest were the same indicated by Chiancone et al. [18], and, similarly, the detected molecules present in the extracts were recognized by comparing the MS/MS ion spectra with the fragmentation data available in multiple online libraries (Mass Bank Europe, ReSpect, PubChem, and MoNA - Mass Bank of North America) and several previous studies of hop-derived polyphenols. All the solvent used for UHPLC-MS/MS analyses were HPLC grade, and in particular ultra-pure water and acetonitrile were purchased from VWR (Milan, Italy), while formic acid from Sigma-Aldrich (St. Louis, MO, USA).
2.3.2. GC-TOF-MS
A qualitative analysis of the HLE was carried out using a Gas-Chromatography, Time-Of-Flight Mass Spectrometry (GC-TOF-MS) system.
The freeze-dried extract was subjected to sequential solid-liquid extractions with solvents of different polarity, in order to solubilize a greater number of compounds of potential interest. First of all, a volume of 5 mL of n-hexane (n-Hexane 99 % RS, Pestipur for pesticide analyses, UN 1208, 2.5 L, Carlo Erba Reagents 447112000) was added to 43.2 mg of the freeze-dried HLE and vigorously shacked for 10 min at room temperature. The lyophilized sample was only partially solubilized, therefore the sample was centrifuged (4000 rpm for 15 min, 10 °C) and the solvent was removed. Aliquots of supernatant were diluted 1:10 using n-hexane and placed in vials for subsequent analyses (N = 5) (Hexane Extracts, HEs). 5 mL of methanol (Methanol RS, for HPLC Plus Gradient Grade, UN 1230, 2.5 L, Carlo Erba Reagents 412383) was then added to the solid residue, shaken vigorously for a further 10 min at room temperature and finally the sample was centrifuged (4000 rpm for 15 min, 10 °C). Aliquots of the methanol supernatant were diluted 1:10 with methanol and placed in vials for analysis (N = 5, Methanol Extracts, MEs). Finally, 5 mL of acetone (Acetone RPE, for analysis ISO, ACS, Reag.Ph.Eur., Reag.USP, UN 1090, 2.5 L, Carlo Erba Reagents 400974) was added to the solid residue and the sample was again vigorously shacked for 10 min at room temperature. The sample was then centrifuged (4000 rpm for 15 min, 10 °C) and aliquots of 100 μL of acetone supernatant were inserted into vials, dried with a gentle flow of nitrogen (pure, compressed, grade 4.6) and 1 mL of methanol was added (N = 5, final dilution 1:10, Acetone Extracts, AEs). At the end of the sequential extraction, the insoluble residue appears as light beige fibrous material.
The GC system was equipped with an Agilent 8990 GC oven, an Agilent G4567A autosampler and a Restek RXI-5MS GC column (30 m, 0.25 mmID, 0.25 μm), connected to a Leco Pegasus BT Time-Of-Flight Mass Spectrometry High Resolution detector (TOF-MS, Resolution 25000 m/Δm). The GC parameters, in brief, were the following: Helium (pure, compressed, grade 5.6) was used as carrier gas at constant flow of 1.5 mL/min, injection was made in split mode with a split ratio of 1:10 (inlet split flow 15 mL/min) at variable temperatures: pursuing the aim of carrying out a qualitative analysis, each sample was repeatedly analysed by injecting the solutions at different temperatures (90 °C, 250 °C or 320 °C) in order to be able to observe both poorly volatile compounds, but also molecules that are more degradable at high temperatures. A linear ramp of temperature from 60 °C to 320 °C was applied to the GC column (oven) for 60 min. The temperature of the transfer line was set up to 250 °C; MS detector acquisition rate was of 10 spectra/sec, with a mass range of 40–1500 mu and an ion extraction frequency of 16 kHz. The emission current was 1.0 mA, the ion source temperature was 250 °C and the electron energy was 70 eV. Finally, the TOF-MS system was tuned up at the beginning of each running using an appropriated perfluorotributylamine (PFTBA) tuning solution focusing the mass at 219 ± 20 mu.
The identification of the unknown compounds was performed by a dedicated deconvolution software (LECO ChromaTOF v5.51.50.0.68774 built 21042021, Data processor v1.2.0.6), comparing the high-resolution mass spectra obtained to those of a mass spectra library owned by the GC system constructor (LIbrary NIST, NIST/EPA/NIH/EI Tandem Mass Spectral Library v2.4 build 25.03.2020.
Mainlib + Replib, total entry = 350643), assessing a minimum of similarity of 500/1000 hits (50 %). The positive scores found were further verified by comparing the Kovats Retention Indexes (RI) available at the NIST library for semi standard non polar DB5 column type, with those determined for the unknown compounds, calculated after a calibration of the retention times using a specific analytical standard of n-alkanes with carbon number between C8 and C40 (C8-C40 Alkane Calibration Standard in dichloromethane, multicomponent solution, 1 mL, suitable for HPLC and gas chromatography analyses, Merk, Supelco 40147-U), applying a maximum tolerance of 30 (RI value). The results of the qualitative analysis therefore represent the most probable hypothesis in terms of similarity of high-resolution mass fragmentation spectra and retention indices, of all the signals returning an acceptable value of the signal to noise ratio (S/N > 20).
As qualitative determinations, the obtained signals (millions of counts) do not consent to directly establish the concentration values, that could be estimated only by the comparison of signals of pure reference standards for confirmation. In addition, information related to the extraction recovery are actually not available for each investigated compounds and solvents, that again could be confirmed only with the use of appropriated test with pure standard solutions. However, in order to hypothesize an order of magnitude of amount of the investigated compounds, a relative concentration of n-alkane was calculated as a comparison. Since the fragmentation intensity is partially related to the retention time, the n-alkane exhibiting the closed retention index (RI) to those of various investigated analytes was used to determine a comparative concentration referred to the millions of counts of the analyte itself. This theoretical value of n-alkanes is not, in any case, to be considered as an estimation of the concentration of the unknown compounds determined by qualitative analysis, but rather a comparative value that can help to understand the order of magnitude associated to the millions of counts observed for each analyte.
2.4. Antioxidant activity and phenolic content
Hop leaf extract in ethanol-water (80:20) was diluted 1:5 with distilled water and subjected to biochemical assays, such as Folin-Ciocalteu, DPPH, ABTS and FRAP, to evaluate its antioxidant activity [18].
2.4.1. Determination of total polyphenol content (TPC)
The total phenolic content (TPC) of the extract was determined using the colorimetric Folin Ciocalteu assay, following the procedure described by Chiancone et al. [18], using a UV–Vis spectrophotometer (BioTek Synergy HT MicroPlate Reader Spectrophotometer). The results were expressed as milligrams of gallic acid equivalents per grams of processed leaves (mg GAE/g). The values were presented as the mean of triplicated assays ± its standard deviation.
2.4.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
DPPH (2,2-Diphenyl-1-picrylhydrazyl, SigmaAldrich Co., Stenheim, Germany) radical was used to test the radical scavenging activity of sample extract as indicated by Chiancone et al. [18], measuring the absorbance at 517 nm wavelength using a UV–Vis spectrophotometer (BioTek Synergy HT MicroPlate Reader Spectrophotometer). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, SigmaAldrich Co., Stenheim, Germany) was used as standard to construct the calibration curve. Radical scavenging activity of the sample was calculated as radical inhibition percentage using the following equation:
where Ac is the absorbance of the control obtained by adding to DPPH ethanol solution an ethanol aliquot equal to the antioxidant solution volume added and As is the absorbance of the reaction solution (hop leaves extract/Trolox standard solution) respectively at 30 min. Results were then expressed as milligrams of TEAC (Trolox Equivalent Antioxidant Capacity) per gram of processed leaves (mg TEAC/g). All solutions were used on the day of preparation and the values were presented as the mean of triplicated assays ± its standard deviation.
2.4.3. ABTS assay
ABTS (2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, SigmaAldrich Co., Stenheim, Germany) tests were performed according to Chiancone et al. [18], registering the absorbance at 734 nm using a UV–Vis spectrophotometer (BioTek Synergy HT MicroPlate Reader Spectrophotometer). The radical scavenging activity of the sample was calculated using the equation previously described for DPPH test. Also in this case, Trolox was the reference compound, and results were expressed as mg TEAC/g. All solutions were used on the day of preparation and the values were presented as the mean of triplicated assays.
2.4.4. FRAP assay
FRAP (Ferric Reducing Antioxidant Power) colorimetric assay was conducted following the protocol of Chiancone et al. [18], measuring absorbance at 593 nm (BioTek Synergy HT MicroPlate Reader Spectrophotometer). Trolox (SigmaAldrich Co., Stenheim, Germany) solutions, between 0.1 and 1 mM, were used as standards to construct the calibration curve. The ferric reducing power was given by the difference between sample and blank absorbance and expressed as mg TEAC/g. All solutions were used on the day of preparation and the values were presented as the mean of triplicated assays ± its standard deviation.
2.5. Antimicrobial activity
2.5.1. Bacterial strains
Antibacterial activity of the HLE was tested against four Gram-positive and four Gram-negative bacterial strains. Gram-positive were represented by two reference staphylococcal isolates and two environmental enterococcal isolates: the Staphylococcus aureus K1902 resistant to erythromycin, S. aureus 1199B resistant to ciprofloxacin, S. aureus Mu50 (ATCC 700699) resistant to methicillin, the Enterococcus faecium LSEF2 resistant to linezolid and the E. faecium KSEG3 resistant to vancomycin [19]. The four Gram-negative strains were: Klebsiella michiganensis 23999A2 [20] resistant to meropenem, Escherichia coli ISZ220 [21] resistant to tetracycline, Acinetobacter baumannii 16 [22] resistant to ciprofloxacin, and the Pseudomonas aeruginosa PA7 resistant to tobramycin.
E. coli ATCC 25922, K. quasipneumoniae ATCC 700603, P. aeruginosa ATCC 27853, S. aureus ATCC 29213 and E. faecalis ATCC 29212 were used as control strains.
2.5.2. Antimicrobial susceptibility and checkerboard assays
The dry HLE, dissolved in DMSO, was used as antimicrobial solution and its antimicrobial activity was assessed by the determination of Minimum Inhibitory Concentration (MIC) according to CLSI guidelines [23]. The highest concentration tested was 1 mg/mL, in order not to exceed 2 % DMSO, used as solvent [24]. The results were evaluated after 18–24 h at 37 °C to allow visible growth of all tested strains.
Checkerboard assays were performed as previously described by Isenberg [25] to evaluate any synergistic effect of HLE in combination with different antibiotics. In the microtiter the final antibiotic concentrations varied according to bacterial strain and antibiotic tested (in a range between 1/16 XMIC and 4XMIC) whereas HLE concentrations were in the range 1–1000 μg/mL.
If hop extract showed antibacterial activity, its effect in combination with antibiotic was determined by calculating the Fractional Inhibition Concentration (FIC) index [25] and synergism was defined by a FIC index ≤0.5. On the contrary if HLE showed no antimicrobial activity with MIC greater than 1 mg/mL, the synergism between the HLE and antibiotics was evaluated by the ability of natural compounds to cause a 2-fold decrease of the antibiotic MIC.
2.6. Interaction of hop leaf extract with proteins
2.6.1. In vitro anti-inflammatory activity: bovine serum albumin (BSA) denaturation inhibition
The in vitro anti-inflammatory activity of the HLE was studied by bovine serum albumin (BSA, SigmaAldrich Co., Stenheim, Germany) denaturation inhibition. The reaction mixture consisted of 3 mL of 3 % bovine serum albumin in phosphate buffered saline (PBS, pH 7.4), and 500 μL of DMSO solutions prepared from hop leaves dry extract (or acetylsalicylic acid) at different concentrations (0.06–0.25 mg/mL) [9]. The mixtures were incubated in a water bath at 80 °C for 15 min. The samples were cooled down and turbidity was measured at 660 nm. The percentage of protein denaturation inhibition was calculated using the following equation:
where Ac and As were the absorbance of the control and HLE (or standard) solution, respectively.
2.6.2. Interaction between hop leaf extract and mucin: turbidimetric measurement
The interaction between hop leaf extract and mucin was studied by monitoring the increase of turbidity of the reaction mixture, read at 700 nm using a spectrophotometer (BioTek Synergy HT MicroPlate Reader Spectrophotometer). The dry hop extract and gallic acid solutions were prepared in 1 % ethanol at three different concentrations: 0.125 mg/mL, 0.250 mg/mL and 0.500 mg/mL. Mucin from porcine stomach type III solution (Sigma Aldrich, Co., St. Louis, MO, USA) was prepared at 0.2 % (m/v) using a hydrochloric acid buffer, pH 3.2. Extract and gallic acid solutions (4 mL) were mixed with mucin solution (1 mL). Three reference samples were used: hop leaf extract control (4 mL of extract solution mixed with 1 mL of hydrochloric acid buffer), gallic acid control (4 mL of phenolic compound solution mixed with 1 mL of hydrochloric acid buffer) and mucin control (4 mL of 1 % ethanol mixed with 1 mL of mucin solution). Reaction mixtures were measured after 15 and 120 min at 37 °C [26,27].
2.6.3. Interaction of hop leaf extract with human insulin and amyloid-β peptide
2.6.3.1. Thioflavin T spectrofluorometric measurements
Aβ peptide amyloid fibrillogenesis was monitored in the presence and absence of hop extracts by following in time the fluorescence emission of 12 μM Thioflavin T (ThT, Sigma-Aldrich Co., St. Louis, MO, USA) at 485 nm upon excitation at 450 nm. In order to do this, a 96-well Thermo Scientific Fluoroskan Ascent F2 Microplate was thermostatically controlled at 37 °C. Results are expressed as averages of three experiments. Human insulin (Sigma-Aldrich Co., St. Louis, MO, USA) and amyloid-β peptide 1–40 (Aβ1-40) (Anaspec, Freemont, CA, USA), prepared following the protocol described by Fezoui et al. [28]) were dissolved in a 50 mM phosphate buffer with 20 mM NaCl at pH 7.4 at the concentration of 0.5 mg/mL (∼88 μM), and 0.06 mg/mL (15 μM) respectively. Both the protein solutions were studied in the presence and in the absence of 1 mg/mL hop extracts. The protein samples without the hop compounds were prepared adding to the solution a volume of ethanol:water (80:20) mixture in the same proportion of hop extracts in the corresponding samples, to obtain a coherent comparison, because ethanol is known to affect protein stability, albeit at higher contents [29]. The insulin amyloid aggregation kinetics was studied at 37 °C under strong stirring in the presence of Teflon polyballs, 1/8″ diameter (Polysciences, Inc., Warrington, PA, USA) [30]. Aβ1-40 aggregation kinetics in the presence and in the absence of hop compounds was followed in time by incubating the sample with ThT under agitation at 37 °C.
2.6.3.2. Congo red assay
UV–Visible spectrophotometry was performed to investigate human insulin fibrillogenesis. Absorbance data were obtained in presence of Congo Red (CR), a dye which bounds specifically to crossed β-structures of amyloid aggregates [31]. Hence, a bathochromic shift in the maximal absorbance wavelength when CR is bound to amyloids can be observed from 490 nm to 540 nm. UV–Visible spectrophotometry experiments were performed either in the absence or in the presence of hop leaf extracts.
CR solution was prepared by initially setting up a solution of ethanol (80 % w/w), brought to saturation with NaCl. This solution was filtered and saturated with the dye powder. Once again, to avoid the possibility of aggregates, the solution was filtered with Millipore filters of pore size 0.2 μm.
Each sample was prepared by adding 50 μL of the selected protein sample with 50 μL of the Congo Red solution, and 400 μL of buffer at pH 7.4. The amount of CR used for investigation was selected in previous works [31], and the human insulin aggregation was induced according to literature protocols at pH 7.4 [30]. The ratio between absorption intensity at λ = 538 nm and at λ = 505 nm was calculated for each sample condition and reported as a function of time.
All measurements were performed in triplicate; human insulin (Sigma-Aldrich Co., St. Louis, MO, USA) was dissolved in a a 50 mM phosphate buffer with 20 mM NaCl at pH 7.4, at concentration equal to 0.5 mg/mL (∼88 μM). Protein solutions were studied in the presence and in the absence of HLE at the concentration of 1 mg/mL. HLE concentration was chosen on the basis of the maximal ethanol content that could be added to protein sample, without significantly interfere with its stability in solution and with the aggregation pattern.
3. Results and discussion
3.1. Characterization of the polyphenols profile by UHPLC-MS/MS
The aqueous-ethanolic extracts obtained directly from in field-grown hop (cv. Cascade) leaves (“Direct Extract”, DE), as from the lyophilization step (“Lyophilized sample Extract”, LE) underwent UHPLC-MS/MS analysis and the results as listed in Table 1. More in detail, 15 polyphenolic compounds belonging to different chemical classes were identified; among these, worth to be highlighted is the presence of prenylated phloroglucinol derivates, like α-acids and β-acids. This funding further supports the initial idea that in hop leaves, even at different concentrations, it is possible to individuate the same compounds present in the cones; molecules such as α-acids (co-humulone) and β-acids (lupulone and colupulone) are those that can contribute to the antioxidant and antimicrobial potential of the extracts produced in this study [32,33]. In fact, as inhibitors of diacylglycerol acetyltransferase, lupulone and humulone are known for their antibacterial and antifungal action by affecting the structure of microbial membranes and cell walls [34]. Our study reported a slightly lower number of phloroglucinolic derivatives than those found by Chiancone et al. [35] for vitro-derived hop (Cascade) leaves, suggesting how the different growing conditions (in vitro or open field) could influence the final polyphenolic profile.
Table 1.
UHPLC-ESI-MS/MS identification of polyphenol compounds detected in ethanolic extract from hop leaves (cv. Cascade) (DE), as in the extract after lyophilisation (LE). The characteristic mass fragments are reported, as the identification method.
| Peak number | Compound | Retetion time (min) | [M − H]- | MS2 ion fragments (m/z) | DE | LE | References |
|---|---|---|---|---|---|---|---|
| 1 | Dihydrobenzoic acid-O-hexoside | 4 | 315 | 153-152-109-108 | x | x | Chiancone et al. (2022), Martelli et al. (2020) |
| 2 | Galloyl-O-hexoside | 4,46 | 169–125 | x | x | Chiancone et al. (2022), Martelli et al. (2020) | |
| 3 | Hulupone | 4,89 | 331 | 152-168-153 | x | Mass Bank Europe, Mongelli et al. (2015), | |
| 4 | 3-Caffaoylquinic acid | 5,35 | 353 | 191-179-135 | x | x | Shibamoto et al. (2009) |
| 5 | Dihydroxybenzoyl-hexose-pentose | 5,54 | 447 | 152-163-108 | x | x | Varga et al. (2020), |
| 6 | 5-Caffaoylquinic acid | 5,87 | 353 | 191-173-135 | x | x | Shibamoto et al. (2009) |
| 7 | Sinapic acid-O-hexoside I | 5,94 | 385 | 223-208-179-164-149-121 | x | x | Murashige et al. (1962), Chiancone et al. (2022) |
| 8 | Luteolin dihexoside | 6,11 | 609 | 285–447 | x | x | Lin and Harnly (2010) |
| 9 | Benzyl alcohol-hexose-pentose | 6,17 | 407 | 401-269-161 | x | x | Varga et al. (2020), |
| 10 | Sinapic acid-O-hexoside II | 6,25 | 385 | 223-208-179-164-149-121 | x | x | Murashige et al. (1962), Chiancone et al. (2022) |
| 11 | Coumaroylquinic acid III | 6,43 | 337 | 173-191-163-119 | x | x | Mongelli et al. (2015) |
| 12 | Catechine | 6,63 | 289 | 245 | x | Choi et al. (2018), Mass Bank Europe, Mongelli et al. (2015) | |
| 13 | Rutin (Quercetin-3-O-rutinoside) | 6,72 | 609 | 300-301-255 | x | x | Standard compound |
| 14 | Kampferol-O-rutinoside I | 7 | 593 | 447-389-285-284-255 | x | x | Chiancone et al. (2022), Nionelli et al. (2018), |
| 15 | Xanthoumol | 11,32 | 353 | 295-189-133-119 | x | Standard compound | |
| 16 | Cohumulone | 13,08 | 347 | 278-235-223-209-207-195-194-193 | x | x | Chiancone et al. (2022), MoNA – Mass Bank of North America, Martelli et al. (2020) |
| 17 | Humulone/Adhumulone | 13,57 | 361 | 292–249 | x | Principe et al. (2014), Pub Chem, Helmja et al. (2007) | |
| 18 | Post-lupulone | 14,32 | 385 | 273 | x | Chiancone et al. (2022), Principe et al. (2014), Helmja et al. (2007) | |
| 19 | Colupulone | 15,45 | 399 | 287-275-219-207 | x | x | Chiancone et al. (2022), Mass Bank Europe, Principe et al. (2014) |
| 20 | Lupulone | 16,06 | 413 | 301-289-369 | x | x | Standard compound |
Other chemical classes such as glycosylated flavonols (rutin, kampferol-O-rutinoside), quinic acid and derivatives (3-Caffaoylquinic, 5-Caffaoylquinic, Coumaroylquinic acid III), di-hexose and hexose-pentose derivatives (dihydroxybenzoyl-hexose-pentose, benzyl alcohol-hexose-pentose) were found. These last two compounds were identified in Humulus lupulus (L.), cv. Citra, tissues [36] but they were not detected in vitro-derived hop leaf extracts [35]. Previous studies have reported the presence of compounds such as rutin and kaempferol-O-rutinoside in hop flowers and leaves [37,38].
Overall, it is important to consider that polyphenol composition varies significantly depending on several factors including, cultivar, planting site, maturity, environmental conditions throughout its growth [39], extraction solvent type like ethanolic or ethyl acetate solutions and plant material, in in-vitro rather than open field grown plants [40]. The characterization was carried out on both directly obtained leaf extracts (DE) and extracts undergoing the freeze-drying and re-drying process in the same solvent (LE), to assess the influence of the freeze-drying and re-suspension phases. Slight differences were indeed noticed among the two considered samples (Table 1).
In particular, five components as hulupone, cathechin, xanthoumol, humulone/adhumulone, and post-lupulone were found only in the leaf direct extract, while they were lost in the sample re-suspended after the lyophilisation process. Despite this, all the other polyphenolic compounds detected in leaf extracts are present in the lyophilized and re-suspended sample. So, they are supposed to be available to exert their characteristic bioactivities.
3.2. Qualitative analysis by GC-TOF-MS
Table 2 shows the results of the qualitative analysis obtained by GC-TOF-MS techniques. As previously described in detail, the water-alcohol freeze-dried hop leaf extract was subjected to sequential solid-liquid solvation in order to obtain three series of samples, named HEs (n-hexane based), MEs (methanol based) and AEs (acetone based). All these samples were injected at different temperatures (90 °C, 250 °C and 320 °C) in order to obtain signal from molecules characterized by different volatility and degradability.
Table 2.
Results of the qualitative analyses by GC-TOF-MS of extracts in n-hexane (HEs), methanol (MEs) and acetone (AEs), all injected at 90 °C, 250 °C or 320 °C. Information includes name, formula, CAS, retention time (RT), similarity with NIST library score, Kovats retention index (RI), NIST library RI (mean ± s.d. of available observations from the literature; number of observations in brackets), base mass, signal to noise ratio (S/N), number of detected counts (in million) and equivalent alkane concentration in ppb (number of carbons of relative alkane in brackets).
| Sample | Inj. Temp. | Name | Formula | CAS | RT (min) | Similarity | RI | Lib. RI | Base Mass | S/N | Counts (million) | Eq. Alkane Conc. (ppb)a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HEs | 90 °C | 3-Hexene-2,5-diol | C6H12O2 | 7319-23-5 | 4.52 | 70.7 % | 975.1 | 950 (1) | 42.89 | 100 | 5.5 | 68.5 (C9) |
| Threo-2,5-dimethyl-2-(2-methyl-2-tetrahydrofuryl)tetrahydrofuran | C11H20O2 | n/a | 11.49 | 71.0 % | 1266.9 | 1287 (1) | 42.88 | 37 | 1.1 | 14.8 (C12) | ||
| 2,6-Diisopropylnaphthalene | C16H20 | 24157-81-1 | 22.26 | 82.0 % | 1721.9 | 1728 ± 4 (16) | 197.09 | 586 | 3.8 | 127.8 (C17) | ||
| Phytol | C20H40O | 150-86-7 | 29.92 | 87.9 % | 2114.3 | 2102 ± 5 (47) | 70.89 | 316 | 5.0 | 171.8 (C21) | ||
| 250 °C | Acenaphthylene | C12H8 | 208-96-8 | 16.03 | 83.5 % | 1448.3 | 1426 ± 8 (5) | 152.05 | 147 | 1.7 | 42.2 (C14) | |
| Neophytadiene | C20H38 | 504-96-1 | 24.72 | 89.0 % | 1840.6 | 1837 ± 4 (8) | 67.89 | 196 | 2.5 | 94.2 (C18) | ||
| Phytol | C20H40O | 150-86-7 | 29.93 | 81.4 % | 2114.7 | 2102 ± 5 (47) | 70.91 | 194 | 3.6 | 124.9 (C21) | ||
| Squalene | C30H50 | 111-02-4 | 41.06 | 87.9 % | 2833 | 2808 (1) | 68.91 | 1692 | 36.8 | 1487.4 (C28) | ||
| α-Tocopherol | C29H50O2 | 59-02-9 | 45.04 | 90.2 % | 3140 | 3130 ± 18 (5) | 165.06 | 1141 | 74.4 | 3065 (C31) | ||
| β-Amyrone | C30H48O | 638-97-1 | 47.65 | 68.5 % | 3356.9 | 3328 (1) | 218.14 | 434 | 5.1 | 221.8 (C33) | ||
| Lupeol | C30H50O | 545-47-1 | 48.19 | 73.5 % | 3403.6 | 3399 ± 52 (2) | 206.92 | 23 | 6.0 | 199.4 (C34) | ||
| 320 °C | Neophytadiene | C20H38 | 504-96-1 | 24.70 | 84.7 % | 1839.5 | 1837 ± 4 (8) | 67.89 | 116 | 1.3 | 48.9 (C18) | |
| Squalene | C30H50 | 111-02-4 | 41.04 | 77.5 % | 2831.4 | 2808 (1) | 68.91 | 788 | 13.9 | 561.9 (C28) | ||
| α-Tocopherol | C29H50O2 | 59-02-9 | 45.01 | 82.9 % | 3138.1 | 3130 ± 18 (5) | 165.07 | 684 | 31.1 | 1282.2 (C31) | ||
| β-Amyrone | C30H48O | 638-97-1 | 48.16 | 75.4 % | 3401.3 | 3328 (1) | 218.16 | 205 | 2.1 | 69.4 (C34) | ||
| MEs | 90 °C | 2-Octanone | C8H16O | 111-13-7 | 4.29 | 65.1 % | 964.8 | 970 ± 3 (73) | 57.9 | 369 | 26.0 | 324.0 (C9) |
| 250 °C | D-Ribose | C5H10O5 | 50-69-1 | 15.43 | 65.2 % | 1424 | 1436 (1) | 56.88 | 854 | 40.1 | 1021.2 (C14) | |
| 320 °C | 3-Octanamine | C8H19N | 24552-04-3 | 4.27 | 71.4 % | 964 | 995 (1) | 57.91 | 448 | 28.6 | 356.1 (C9) | |
| Benzene, 1,2,4-trimethyl- | C9H12 | 95-63-6 | 5.02 | 74.6 % | 997.8 | 983 ± 5 (122) | 105.11 | 170 | 4.1 | 50.7 (C9) | ||
| 2,4-Di-tert-butylphenol | C14H22O | 96-76-4 | 17.62 | 82.4 % | 1513.9 | 1502 ± 8 (5) | 191.2 | 126 | 10.5 | 224.9 (C15) | ||
| Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester | C35H62O3 | 2082-79-3 | 50.53 | 57.2 % | 3612.9 | 3603 (2) | 56.93 | 210 | 5.6 | 317.7 (C36) | ||
| AEs | 90 °C | 2,4-Di-tert-butylphenol | C14H22O | 96-76-4 | 17.63 | 81.4 % | 1514.3 | 1502 ± 8 (5) | 191.19 | 126 | 9.0 | 192.5 (C15) |
| 250 °C | 1-Tetradecene | C14H28 | 1120-36-1 | 14.67 | 91.2 % | 1392.8 | 1389 ± 1 (34) | 54.91 | 187 | 4.7 | 120.1 (C14) | |
| Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester | C35H62O3 | 2082-79-3 | 50.55 | 72.3 % | 3614.8 | 3603 (1) | 56.92 | 381 | 12.9 | 739.5 (C36) | ||
Concentration of the n-alkane with RI most similar to that of the analyte and related to its number of counts (million), obtained analysing a dilution (1:10) of extracts of 43.2 mg of sample in 5 mL of solvents.
All the scores are characterized by a high similarity of the spectra with those of the reference NIST library (at least 50 %), a high correspondence of the Kovats Retention Indices (RI), and an acceptable Signal to Noise ratio (S/N > 20). Overall, these collected results allow us to assess the identification of unknown compounds with a high probability.
In general, different solvents have shown the ability to bring different compounds into solution and n-hexane was found to be capable of solubilizing the greatest number of unknown compounds (11). As an exception, 2,4-di-tert-butylphenol and octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, both show a signal in methanol (MEs) and acetone (AEs) extracts, but an unusual aspect is the fact that these substances show signals at different injection temperatures: considering that the extracts in acetone (AEs) were dried and resuspended up in methanol, it may be possible that the different composition of the extracts could determine different interactions between the compounds, such as to confer different volatility to the same molecules, however further tests in this sense should be carried out to confirm the hypothesis. Among the samples in n-hexane (HEs), several compounds, such as neophytadiene, phytol, squalene, α-tocopherol and β-amyrone, show recognizable signals to different injection temperature, all characterized by a high correspondence of the spectra, of the RIs and with a high S/N, aspect which most corroborates the hypothesis of their identification. It is noteworthy to consider that some terpenes identified by GC-TOF-MS are well known for their antioxidant, antibacterial and antinflammatory properties, in particular α-tocopherol, phytol [41]lupeol [42,43], and squalene [44], these molecules, together with the bioactive compounds highlighted by the UHPLC-MS/MS analysis can confer interesting beneficial properties to hop leaf extracts. As already specified in the Materials and Methods section, this qualitative analysis does not allow to directly estimate the concentration of the various analytes, which could be obtained only by comparing the signals with those of appropriate pure reference standards solutions. In any case, Table 2 includes the millions of counts obtained for each analyte at the various sample extraction and injection conditions. These values, first of all, allow to observe different extraction and analysis recovery for those analytes that showed appreciable signals in various analytical conditions and allow to macroscopically appreciate the different abundance of the various molecules found and identified. In this view, for example, is possible to appreciate that α-tocopherol is among the compounds that exhibit the highest number of million counts, in the various analytical conditions, suggesting that this important vitamin and antioxidant may be one of the most abundant compounds among those identified with qualitative determination.
The n-alkane exhibiting the closed retention index (RI) to those of various investigated analytes was used to determine a comparative concentration referred to the millions of counts of the analyte itself. This theoretical value of n-alkanes can help to understand the order of magnitude associated with the millions of counts observed for each analyte, considering that the results were obtained with a 1:10 dilution of the extracts obtained from 43.2 mg in 5 mL of the various solvents (see Materials and Methods). Future investigations could include the confirmation of some selected analytes by calibration with pure reference standards which would allow obtaining an accurate estimation of the extraction recovery and concentration of these analytes.
3.3. Total polyphenols content in hop leaf extract
The content of phenolic compounds in hop leaf extracts, determined through the Folin-Ciocalteu assay, was 5.4 ± 0.2 mg GAE/g. Our data resulted in line with those reported by Keskin and collaborators [45] who registered a concentration in polyphenols of 6.86 ± 0.05 mg GAE/g for methanolic hop leaf extracts obtained from Pazaryeri, Bilecik, Turkey [45]. The total polyphenol content (TPC) may vary on the basis of the considered hop variety [14], as on the extraction solvent and method applied. Muzykiewicz et al. [46], indeed, reported that different solvent mixtures lead to obtain different polyphenol quantities in hop leaf extracts; in addition, the concentration of polyphenols that can be extracted from hop leaves may be influenced by the drying method; actually, Macchioni et al. [13] describes a lower TPC content in extracts obtained from leaves of several hop cultivars, including Cascade, if they were dried resorting to oven rather than freeze-drying. Comparing our results to those obtained in this latter research, it is possible to note that our TPC value is higher than data shown for Cascade hop leaves dried by oven, but at the same time remained lower than those found for the same variety when leaves were dried by using a freeze-drier [13].
Despite all the slight differences in TPC given by a considerable number of parameters, hop biomass remain a promising source of bioactive compounds with redox properties, which allow them to act as antioxidants. The TPC values found in the present study depended on the components that were identified by the UHPLC-MS/MS technique (flavonols, flavon-3-oils and prenyl flavonoids) and GC-TOF-MS (α-tocopherol, phytol, lupeol and squalene). These compounds, may indeed exert potential antioxidant activity, as described above. Therefore, to deepen study these properties, since the Folin Ciocalteu assay is not specific to phenolic compounds, it could be used as a basis for a rapid screening of the antioxidant activity due to both phenolic and nonphenolic compounds able to reduce the phosphomolybdic/phosphotungstic acid reagent [47].
3.4. Antioxidant activity of hop leaf extract
The antioxidant activity of hop leaf extract has been investigated by three spectrophotometric assays: DPPH, ABTS, and FRAP. It is generally appropriate to use different assays for testing scavenging radical properties, in fact, the extracts can contain substances able to give UV–Vis wavelength absorption similar to that of the compounds of interest, so interfering with the results of a determined test, and altering the result. DPPH radical is widely used in assessing free radical scavenging activity because of the ease of the reaction. ABTS scavenging activity assay acts like the DPPH one and allows to determine antioxidant activity due to both hydrophilic and lipophilic compounds. The antioxidant activity has been assessed by comparison with the hydrogen donation ability of Trolox to scavenge radicals and the relative activities are reported as Trolox equivalent antioxidant capacity (TEAC). Hop leaf extract showed antioxidant activity against both the free radicals investigated; the obtained values of 8.4 ± 0.1 mg TEAC/g and 6.7 ± 1.1 mg TEAC/g for the DPPH and ABTS assays respectively. Regarding results obtained using DPPH assay, data found in the present study seemed to be higher than those descripted by Muzykiewicz et al. [46] who reported values of 3.07 ± 0.14–3.69 ± 0.02 mg TEAC/g for extracts obtained under similar conditions. The comparison of the results obtained in this study with previous published data is of difficult realization, because of some differences in considered hop cultivar, plant organs, achievement of extracts in terms of solvent and/or employed technique, etc. Anyway, our values obtained by the application of DPPH and ABTS assays appear to be higher than those described by Arruda et al. in 2021 [39] for the same hop variety, but these authors considered hop comes, and performed a slightly different extraction process using pure ethanol, and applying a temperature of extraction of 60 °C.
FRAP test allows to determine the action of electron-donating antioxidants by the reduction, at low pH, of a colourless ferric complex (Fe3+-tripyridyltriazine) to a blue-coloured ferrous complex (Fe2+-tripyridyltriazine). The ethanolic extracts of hop leaves presented a good capacity of inhibit ferric active species (20.7 ± 0.3 mg TEAC/g.), as also reported by previous studies [45]. It is important to consider that the antioxidant capacity may depend on different parameters (hop variety, drying method, extraction procedure), as already stated for polyphenol content; anyway, the results obtained with the extracts from Cascade leaves confirm the potential of this matrix as source of bioactive compounds.
3.5. Antimicrobial susceptibility
The antimicrobial resistance problem is spreading worldwide and there is little prospect of developing new classes of antibiotics in the short term. For this reason, natural extracts have been proposed as alternative antimicrobial agents or adjuvants [5].
Previous studies have shown that compounds extracted from hops have antibacterial activity against different Gram-positive pathogenic strains [5,48]. Boucquet et al. reported that antibacterial activity may be attributed to phenolic compounds that have a greater effect against staphylococci and in particular MRSA (methicillin-resistant S. Aureus) strains [6].
In this context, the potential antibacterial activity of hop leaf extract against both Gram-positive and Gram-negative antibiotic-resistant reference strains was tested.
MICs of hop leaf extracts against the selected strains were determined (Table 3). MIC results showed antibacterial activity only against Gram-positive strains, with a 125–500 μg/mL range; these values, are higher respect to those obtained with cone extracts [49], but similar and in some cases better than those previously obtained in studies on leaves [8]. The observed antimicrobial activity can be attributed to the presence of prenylated flavonoids as xanthohumol and acylphloroglucinol derivatives as lupulone. Gram-negative bacterial growth was not inhibited by hop leaf extracts (MIC ≥1 mg/mL). Therefore, the checkerboard assay was performed only against Gram-positive strains with selected antibiotics.
Table 3.
MIC (μg/mL) values of the hop leaf extracts against Gram-positive strains and the MIC (μg/ml) of the antibiotics tested alone and in presence of the lowest effective concentration of Hop Leaf Extract (HLE).
| Strains | HLE MIC (μg/mL) | Antibiotic | Antibiotic MIC (μg/mL) | Antibiotic MIC (μg/ml) in presence of HLE (μg/mL) | FIC Index | Effect |
|---|---|---|---|---|---|---|
| E. faecium KSEG3 | 500 | Vancomycin | 256 | 128 (62.5) | 0.62 | Additive |
| E. faecium LSEF2 | 500 | Florfenicol | 128 | 64 (1) | 0.5 | Synergistic |
| S. aureus MU50 | 250 | Oxacillin | 256 | 128 (1) | 0.5 | Synergistic |
| S. aureus K1902 | 125 | Erythromycin | 256 | 128 (16) | 0.5 | Synergistic |
3.6. Checkerboard assays
Hop leaf extract was tested in combination with different antibiotics, against which strains were resistant. In both strains of E. faecium a 2-folds decrease of vancomycin and florfenicol MICs was observed at hop extract concentrations of 62.5 and 1 μg/mL respectively. In S. aureus strains a 2-fold reduction in MIC values was observed at all concentrations of extract ranging from 1 to 16 μg/mL.
The combination of hop extract with selected antibiotics showed synergistic to additive effect. The lowest antibiotic and extract concentrations that gave a FIC index corresponding to a synergistic or additive effect are shown in Table 3.
Although the antibiotic MIC values were 2-folds reduced, the susceptible phenotype of strains was not being restored.
As described in literature molecules with antibacterial activity are compounds such as xanthohumol, and α, β-acids (humulone/adhumulone and lulupune/colupulone) [5]. In this work susceptibility assays were performed on testing the antibacterial activity of the overall extract. The low antibacterial activity of the total extract was probably due to the presence of single bioactive compounds at low concentrations unable to effectively inhibit microbial growth.
3.7. Interaction of hop leaf extract with proteins
The ability of polyphenols to interact with proteins and exert a protective effect is well known [9,12,27]; for this purpose, some assays have been conducted on HLE extracts to study the effect of molecules present in them.
3.7.1. Protein denaturation inhibition
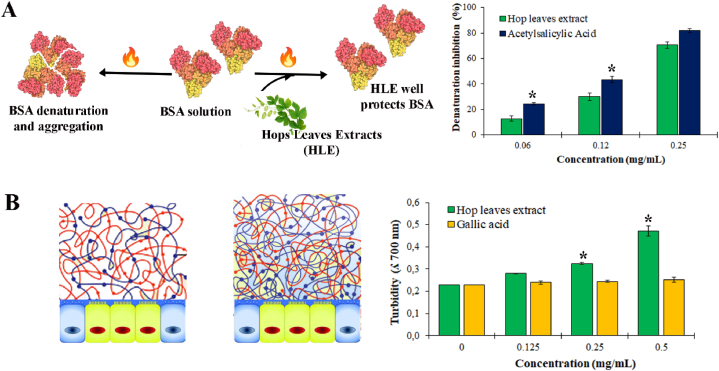
Protein denaturation, which leads to the loss of their quaternary, tertiary and secondary structures of protein, has been documented to be one of the significant causes of inflammation. The denaturation of bovine serum albumin (BSA) leads to the expression of antigens that are associated with a type III hypersensitivity reaction and the initiation of an inflammatory response. Thus, to get an idea of the anti-inflammatory activity of hop leaf extract, its potential to protect from protein denaturation is investigated in this study. The ability of the extract to inhibit heat-induced denaturation, due to the presence of natural polyphenols known for their antioxidant activity can be possibly a contributing factor to its anti-inflammatory activity. The data suggest that hop leaf extract is significantly effective against heat-induced albumin denaturation, and the percent inhibition of denaturation increases with increasing extract concentration. Maximum inhibition of 71 % was found at a concentration of 0.25 mg/mL (Fig. 1A). Acetylsalicylic acid, used as positive control, showed the maximum inhibition of 83 % at the same concentration [50].
Fig. 1.
Effect of HLE extracts on the activity of different proteins. (A) Schematic view of the BSA denaturation assay (left) and histogram showing the BSA denaturation inhibition in the presence of increasing hop leaf extract concentration (right). ∗P < 0.01, significative differences with respect to acetylsalicylic acid. (B) Potential biological effect of the mucin interaction with HLE on mucus barrier integrity (left) and histogram representing the HLE-mucin interaction results (right). ∗P < 0.01, significative differences with respect to control. Statistical analyses were performed by using Student's t-Test.
3.7.2. Interaction between hop leaf extract phenolic compounds and mucin
Gastrointestinal mucus works as selective barrier between lumen and the epithelial cells against potential pathogens and allergens. This represents the first defence line protecting our body from infections and related inflammation. Many dietary molecules, such as polyphenols or their metabolites, act as antibacterial agents and could be therefore used to preserve the barrier functions. In addition, the direct interaction of polyphenols with gastrointestinal mucin could have an important effect on nutrition and gut health. Several studies reported that the interaction of Epigallocatechin-3-gallate (EGCG) and Tannic acid (TA) exhibits strong binding to intestinal mucin and reinforces the mucus barrier [51]. Similarly, quercetin [52], vitamin E, fisetin and other polyphenols promote intestinal structure and immune barrier integrity [27,53,54]. In this context, the HLE ability to exert a potential protective effect on gastrointestinal mucin was evaluated by studying the interaction with porcine intestinal mucus and comparing the results with those obtained with gallic acid, as polyphenol standard (Fig. 1B). At low concentrations, HLE did not interact with mucin protein while a significant interaction was observed at 0.25 and 0.5 mg/mL. It is important to underline the double-side aspect of this result; although the HLE-mucin interaction could lead to a reinforcement of mucus barrier, it could affect the bioavailability of bioactive molecules present in the HLE and so their beneficiary properties. Therefore, the effect of this interaction needs to be deepened, to provide a novel understanding of the health benefits of HLE and evaluate the assess the opportunity to protect the extracts by nanoparticle encapsulation.
3.7.3. Ability of the hop extract to inhibit protein amyloid aggregation
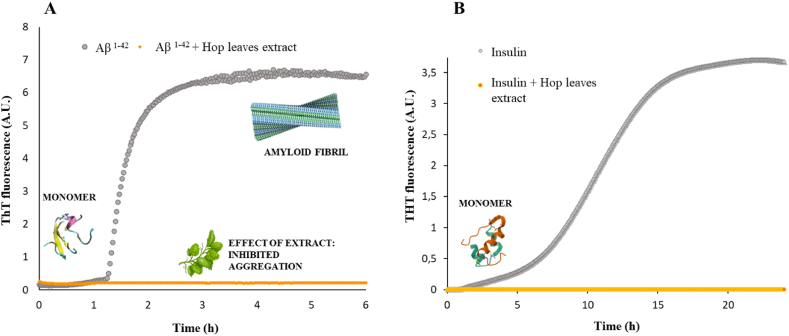
Recent studies have shown how biocompounds present in hop cones exert beneficial effects in counteracting the amyloid aggregation of Aβ1-42 peptide involved in Alzheimer Disease (AD) and its cytotoxic effect [12,55]. To verify whether extracts from leaves of open field grown hop plants result effective in influencing protein amyloid fibrillogenesis, two amylodogenic proteins: Aβ1-40 peptide involved, together with the longer Aβ1-42, in AD disease, and human insulin were considered. Aβ1-40 amyloid aggregation process differs from Aβ1-42 one for the more marked autocatalytic nature and stronger domination from secondary nucleation steps [[56], [57], [58]]. Human insulin is involved in insulin-derived amyloidosis (IDA) and it is able to form fibrils in vitro under conditions more closely resembling physiological pH and temperature (pH 7.0, 37 °C) [30,59]. At this pH, the initial sample is a heterogeneous mixture of monomer/dimers and higher size oligomers and different nucleation mechanisms could coexist. Due the complexity of their aggregation pathways, the two proteins are considered good candidates for evaluating the ability of hop extracts to affect the different structural rearrangements leading to amyloid formation. In order to do this, Thioflavin T (ThT) assay ThT is the most used fluorescent dye to monitor the proteins conversion to amyloid structures, was used. In fact, ThT fluorescence emission dramatically enhance once the dye is bound to the typical cross-beta strands of amyloids [60].
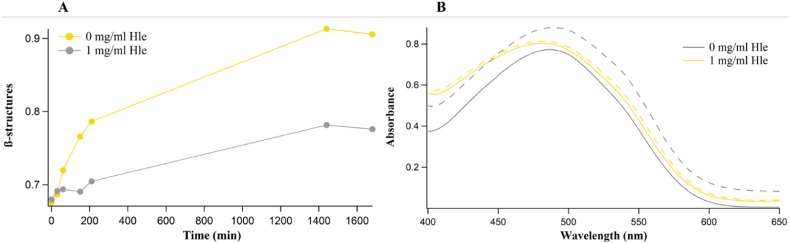
Fig. 2 shows the emission fluorescence at 482 nm of ThT incubated with Aβ and insulin, in the absence and in the presence of hop extracts. In the absence of hop biocompounds, from the aggregation triggering event (T = 37 °C, shearing), ThT fluorescence shows a kinetic profile typical of nucleation-polimerization process [57]. Instead, ThT fluorescence did not significantly increase in time when incubated with the proteins mixed with hop extracts prior triggering the aggregation process, thus suggesting that the hop biocompounds could be affecting in completely inhibiting amyloid formation for both proteins. Moreover, it was verified that the ThT data interpretation of the total inhibition of amyloid type aggregation was not biased by potentially occurring interactions of polyphenols-ThT [61], since we obtained, for human insulin, quite similar results by using an alternative dye for amyloid detection, that is CR (see insect of Fig. 3D). The presence in the Cascade hop leaf extracts of compounds like xanthohumol and caffeoylquinic acid (Table 1), considered at the basis of cone hop beneficial effect in AD, could be responsible for the inhibitory action of Aβ and insulin fibrillogenesis found in this experiments [12,55]. In the left panel of Fig. 3 we reported the ratio between the absorption intensity at 538 nm and at 505 nm, which is proportional to β-structures in solution [32]. On the right panel, two steps of the aggregation kinetic were reported to evidence the shoulder at 538 nm appearing after aggregation. Further studies are needed to understand the contribution of the other compounds so far identified and their possible synergistic action. Also, a dose-dependence of the inhibitory effect of HLE has already been suggested by in progress experiments.
Fig. 2.
In situ real-time ThT fluorescence assay for monitoring the aggregation kinetics. Panel A refers to 15 μM Aβ incubated at 37 °C under stirring in the absence (grey) and in the presence of 1 mg/mL HLE (orange). Panel B: ThT fluorescence assay for monitoring the aggregation kinetics of 88 μM insulin incubated at 37 °C under vigorous agitation in the absence (grey) and in the presence of 1 mg/mL HLE (orange).
Fig. 3.
β-structure estimation obtained by absorption spectroscopy with CR of 88 μM insulin incubated at 37 °C under vigorous agitation in the absence (grey) and in the presence of 1 mg/mL HLE (orange). Panel A: β-structure estimation obtained as ratio between absorption intensity at λ = 538 nm and at λ = 505 nm. Panel B: absorption spectra obtained at time 0 (continuous line) and after 210 min (dashed line).
4. Conclusions
The recovery of valuable compounds from crop waste is a trending objective that meets economic and environmental needs. In this work hop leaves, a waste in the hop cultivation, have been analysed to determine the presence of active substances, interesting for application in nutraceutical, pharmaceutical, and cosmetic fields. Extracts obtained from hop vegetative biomass after harvesting cones have shown to contain characteristic compounds, such as α-acids and β-acids, polyphenols and terpenes, responsible for the antioxidant and antimicrobial properties but also able to confer to the extracts the less known ability to interact with proteins related to relevant biological issues, like Aβ1-40 peptide, human insulin, mucin, and BSA. It must be noted that studies concerning the presence of active ingredients able to hinder in vitro aggregation of amyloid beta peptide and interact with BSA and mucin have never been studied in hop extracts from leaves; consequently, our investigation allows to expand the possible uses of hop leaves obtained as waste material. The limit of this research can be found in the need to improve the extraction method to scale-up the process for an industrial application. These results can encourage the development of new appropriate strategies aimed at enhancing the potential of hop organic waste to be exploited as nutraceuticals as well as ingredients for the development of functional foods.
Funding
This research was partially funded by Regione Marche, POR Marche FSE 2014/2020. This research has received funding from the project Vitality – Project Code ECS00000041, CUP I33C22001330007 - funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.5 - 'Creation and strengthening of innovation ecosystems,' construction of 'territorial leaders in R&D' – Innovation Ecosystems - Project 'Innovation, digitalization and sustainability for the diffused economy in Central Italy – VITALITY' Call for tender No. 3277 of 30/12/2021, and Concession Decree No. 0001057.23-06-2022 of Italian Ministry of University funded by the European Union – NextGenerationEU.; the project has also received funding by University of Parma through the action “Bando di Ateneo 2021 per la ricerca” co-funded by MUR-Italian Ministry of Universities and Research, Italy - D.M. 737/2021 - PNR - PNRR - NextGenerationEU”.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
CRediT authorship contribution statement
Giulia Sabbatini: Writing – review & editing, Writing – original draft, Investigation. Eleonora Mari: Investigation. Maria Grazia Ortore: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Conceptualization. Alessandra Di Gregorio: Investigation. Daniele Fattorini: Writing – review & editing, Writing – original draft, Investigation. Marta Di Carlo: Investigation. Roberta Galeazzi: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Conceptualization. Carla Vignaroli: Writing – review & editing, Writing – original draft, Methodology. Serena Simoni: Investigation. Giorgia Giorgini: Investigation. Valeria Guarrasi: Conceptualization. Benedetta Chiancone: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Conceptualization. Leandra Leto: Investigation. Martina Cirlini: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Conceptualization. Lorenzo Del Vecchio: Investigation. Maria Rosalia Mangione: Investigation. Silvia Vilasi: Writing – review & editing, Writing – original draft, Resources, Investigation, Conceptualization. Cristina Minnelli: Writing – review & editing, Methodology, Investigation, Conceptualization. Giovanna Mobbili: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
M.G.O. thanks Giusanna Di Masi, owner of Luppoleto Malatesta set in Sant'Angelo in Pontano (Macerata, Italy), for providing hop leaves.
References
- 1.Hrnčič M.K., Španinger E., Košir I.J., Knez Ž., Bren U. Hop compounds: extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients. 2019;11 doi: 10.3390/nu11020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astray G., Gullón P., Gullón B., Munekata P.E.S., Lorenzo J.M., Humulus lupulus L. As a natural source of functional biomolecules. Appl. Sci. 2020;10 doi: 10.3390/app10155074. [DOI] [Google Scholar]
- 3.Nionelli L., Pontonio E., Gobbetti M., Rizzello C.G. Use of hop extract as antifungal ingredient for bread making and selection of autochthonous resistant starters for sourdough fermentation. Int. J. Food Microbiol. 2018;266:173–182. doi: 10.1016/j.ijfoodmicro.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Lin M., Xiang D., Chen X., Huo H. Role of characteristic components of humulus lupulus in promoting human health. J. Agric. Food Chem. 2019;67:8291–8302. doi: 10.1021/acs.jafc.9b03780. [DOI] [PubMed] [Google Scholar]
- 5.Weber N., Biehler K., Schwabe K., Haarhaus B., Quirin K.W., Frank U., Schempp C.M., Wölfle U. Hop extract acts as an antioxidant with antimicrobial effects against Propionibacterium acnes and Staphylococcus aureus. Molecules. 2019;24 doi: 10.3390/molecules24020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bocquet L., Sahpaz S., Bonneau N., Beaufay C., Mahieux S., Samaillie J., Roumy V., Jacquin J., Bordage S., Hennebelle T., Chai F., Quetin-Leclercq J., Neut C., Rivière C. Phenolic compounds from humulus lupulus as natural antimicrobial products: new weapons in the fight against methicillin resistant staphylococcus aureus, leishmania mexicana and trypanosoma brucei strains. Molecules. 2019;24 doi: 10.3390/molecules24061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer B., Thielmann J., Hickisch A., Muranyi P., Wunderlich J., Hauser C. Antimicrobial activity of hop extracts against foodborne pathogens for meat applications. J. Appl. Microbiol. 2015;118:648–657. doi: 10.1111/jam.12717. [DOI] [PubMed] [Google Scholar]
- 8.Abram V., Čeh B., Vidmar M., Hercezi M., Lazić N., Bucik V., Možina S.S., Košir I.J., Kač M., Demšar L., Poklar Ulrih N. A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crops Prod. 2015;64:124–134. doi: 10.1016/j.indcrop.2014.11.008. [DOI] [Google Scholar]
- 9.Liu M., Hansen P.E., Wang G., Qiu L., Dong J., Yin H., Qian Z., Yang M., Miao J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus) Molecules. 2015;20:754–779. doi: 10.3390/molecules20010754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi N., Satoh-Yamaguchi K., Ono M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine. 2009;16:369–376. doi: 10.1016/j.phymed.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Dudonné S., Vitrac X., Coutiére P., Woillez M., Mérillon J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 12.Palmioli A., Mazzoni V., De Luigi A., Bruzzone C., Sala G., Colombo L., Bazzini C., Zoia C.P., Inserra M., Salmona M., De Noni I., Ferrarese C., Diomede L., Airoldi C. Alzheimer's disease prevention through natural compounds: cell-free, in vitro, and in vivo dissection of hop (humulus lupulus L.) multitarget activity. ACS Chem. Neurosci. 2022;13:3152–3167. doi: 10.1021/acschemneuro.2c00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhäuser C. Broad spectrum antiinfective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 2005;49:827–831. doi: 10.1002/mnfr.200500091. [DOI] [PubMed] [Google Scholar]
- 14.Čeh B., Kač M., Košir I.J., Abram V. Relationships between xanthohumol and polyphenol content in hop leaves and hop cones with regard to water supply and cultivar. Int. J. Mol. Sci. 2006;8:989–1000. http://www.mdpi.org/ijms [Google Scholar]
- 15.Macchioni V., Picchi V., Carbone K. Hop leaves as an alternative source of health-active compounds: effect of genotype and drying conditions. Plants. 2022;11 doi: 10.3390/plants11010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santagostini L., Caporali E., Giuliani C., Bottoni M., Ascrizzi R., Araneo S.R., Papini A., Flamini G., Fico G. Humulus lupulus L. cv. Cascade grown in Northern Italy: morphological and phytochemical characterization. Plant Biosyst. 2020;154:316–325. doi: 10.1080/11263504.2019.1610111. [DOI] [Google Scholar]
- 17.Rodolfi M., Chiancone B., Liberatore C.M., Fabbri A., Cirlini M., Ganino T. Changes in chemical profile of Cascade hop cones according to the growing area. J. Sci. Food Agric. 2019;99:6011–6019. doi: 10.1002/jsfa.9876. [DOI] [PubMed] [Google Scholar]
- 18.Iglesias A., Gimenez Martinez P., Ramirez C., Mitton G., Meroi Arcerito F.R., Fangio M.F., Churio M.S., Fuselli S., Fanovich A., Eguaras M., Maggi M. Valorization of hop leaves for development of eco-friendly bee pesticides. Apidologie. 2021;52:186–198. doi: 10.1007/s13592-020-00808-8. [DOI] [Google Scholar]
- 19.Fioriti S., Coccitto S.N., Cedraro N., Simoni S., Morroni G., Brenciani A., Mangiaterra G., Vignaroli C., Vezzulli L., Biavasco F., Giovanetti E. Linezolid resistance genes in enterococci isolated from sediment and zooplankton in two Italian coastal areas. Appl. Environ. Microbiol. 2021;87:1–10. doi: 10.1128/AEM.02958-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoni S., Leoni F., Veschetti L., Malerba G., Carelli M., Lleò M.M., Brenciani A., Morroni G., Giovanetti E., Rocchegiani E., Barchiesi F., Vignaroli C. The emerging nosocomial pathogen Klebsiella michiganensis : genetic analysis of a KPC-3 producing strain isolated from venus clam. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.04235-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Citterio B., Andreoni F., Simoni S., Carloni E., Magnani M., Mangiaterra G., Cedraro N., Biavasco F., Vignaroli C. Plasmid replicon typing of antibiotic-resistant Escherichia coli from clams and marine sediments. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Atanasio N., de Joannon A.C., Di Sante L., Mangano G., Ombrato R., Vitiello M., Bartella C., Magarò G., Prati F., Milanese C., Vignaroli C., Di Giorgio F.P., Tongiani S. Antibacterial activity of novel dual bacterial DNA type II topoisomerase inhibitors. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute (CLSI) CLSI; Wayne, PA: 2022. Performance Standards for Antimicrobial Susceptibility Testing. CLSI M100. [Google Scholar]
- 24.Laudadio E., Cedraro N., Mangiaterra G., Citterio B., Mobbili G., Minnelli C., Bizzaro D., Biavasco F., Galeazzi R. Natural alkaloid berberine activity against Pseudomonas aeruginosa MexXY-mediated aminoglycoside resistance: in silico and in vitro studies. J Nat Prod. 2019;82:1935–1944. doi: 10.1021/acs.jnatprod.9b00317. [DOI] [PubMed] [Google Scholar]
- 25.Moody J. In: Clinical Microbiology Procedures Handbook. second ed. Isenberg H.D., editor. American Society for Microbiology Press; Washington, DC., USA: 2007. Synergism testing: broth microdilution checkerboard and broth macrodilution methods. update, Vol. 2, section 5. [Google Scholar]
- 26.Quintero-Flórez A., Sánchez-Ortiz A., Gaforio Martínez J.J., Jiménez Márquez A., Beltrán Maza G. Interaction between extra virgin olive oil phenolic compounds and mucin. Eur. J. Lipid Sci. Technol. 2015;117:1569–1577. doi: 10.1002/ejlt.201400613. [DOI] [Google Scholar]
- 27.Yilmaz H., Gultekin Subasi B., Celebioglu H.U., Ozdal T., Capanoglu E. Chemistry of protein-phenolic interactions toward the microbiota and microbial infections. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.914118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fezoui Y., Hartley D.M., Harper J.D., Khurana R., Walsh D.M., Condron M.M., Selkoe D.J., Lansbury Jk P.T., Fink A.L., Teplow D.B. 2000. An Improved Method of Preparing the Amyloid P-Protein for Fibrillogenesis and Neurotoxicity Experiments. [DOI] [PubMed] [Google Scholar]
- 29.Ortore M.G., Mariani P., Carsughi F., Cinelli S., Onori G., Teixeira J., Spinozzi F. Preferential solvation of lysozyme in water/ethanol mixtures. J. Chem. Phys. 2011;135 doi: 10.1063/1.3670419. [DOI] [PubMed] [Google Scholar]
- 30.Sirangelo I., Borriello M., Vilasi S., Iannuzzi C. Hydroxytyrosol inhibits protein oligomerization and amyloid aggregation in human insulin. Int. J. Mol. Sci. 2020;21:1–16. doi: 10.3390/ijms21134636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mari E., Ricci C., Pieraccini S., Spinozzi F., Mariani P., Ortore M.G. Trehalose effect on the aggregation of model proteins into amyloid fibrils. Life. 2020;10 doi: 10.3390/life10050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmja K., Vaher M., Püssa T., Kamsol K., Orav A., Kaljurand M. Bioactive components of the hop strobilus: comparison of different extraction methods by capillary electrophoretic and chromatographic methods. J. Chromatogr. A. 2007;1155:222–229. doi: 10.1016/j.chroma.2006.12.067. [DOI] [PubMed] [Google Scholar]
- 33.Sun S., Wang X., Yuan A., Liu J., Li Z., Xie D., Zhang H., Luo W., Xu H., Liu J., Nie C., Zhang H. Chemical constituents and bioactivities of hops (Humulus lupulus L.) and their effects on beer-related microorganisms. Food Energy Secur. 2022;11 doi: 10.1002/fes3.367. [DOI] [Google Scholar]
- 34.Bocquet L., Rivière C., Dermont C., Samaillie J., Hilbert J.L., Halama P., Siah A., Sahpaz S. Antifungal activity of hop extracts and compounds against the wheat pathogen Zymoseptoria tritici. Ind. Crops Prod. 2018;122:290–297. doi: 10.1016/j.indcrop.2018.05.061. [DOI] [Google Scholar]
- 35.Chiancone B., Guarrasi V., Leto L., Del Vecchio L., Galaverni M., Cirlini M. Vitro-derived hop (Humulus lupulus L.) leaves and roots as source of bioactive compounds: antioxidant activity and polyphenolic prole. 2022. [DOI]
- 36.Caffrey A.J., Lafontaine S., Dailey J., Varnum S., Lerno L.A., Zweigenbaum J., Heymann H., Ebeler S.E. Characterization of Humulus lupulus glycosides with porous graphitic carbon and sequential high performance liquid chromatography quadrupole time-of-flight mass spectrometry and high performance liquid chromatography fractionation. J. Chromatogr. A. 2022;1674 doi: 10.1016/j.chroma.2022.463130. [DOI] [PubMed] [Google Scholar]
- 37.Negri G., di Santi D., Tabach R. Bitter acids from hydroethanolic extracts of Humulus lupulus L., Cannabaceae, used as anxiolytic. Revista Brasileira de Farmacognosia. 2010;20:850–859. doi: 10.1590/S0102-695X2010005000051. [DOI] [Google Scholar]
- 38.Magalhães P.J., Vieira J.S., Gonçalves L.M., Pacheco J.G., Guido L.F., Barros A.A. Isolation of phenolic compounds from hop extracts using polyvinylpolypyrrolidone: characterization by high-performance liquid chromatography-diode array detection-electrospray tandem mass spectrometry. J. Chromatogr. A. 2010;1217:3258–3268. doi: 10.1016/j.chroma.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 39.Arruda T.R., Pinheiro P.F., Silva P.I., Bernardes P.C. A new perspective of a well-recognized raw material: phenolic content, antioxidant and antimicrobial activities and α- and β-acids profile of Brazilian hop (Humulus lupulus L.) extracts. LWT. 2021;141 doi: 10.1016/j.lwt.2021.110905. [DOI] [Google Scholar]
- 40.Choi J.Y., Desta K.T., Lee S.J., Kim Y.H., Shin S.C., Kim G.S., Lee S.J., Shim J.H., Hacımüftüoğlu A., Abd El-Aty A.M. LC-MS/MS profiling of polyphenol-enriched leaf, stem and root extracts of Korean Humulus japonicus Siebold & Zucc and determination of their antioxidant effects. Biomed. Chromatogr. 2018;32 doi: 10.1002/bmc.4171. [DOI] [PubMed] [Google Scholar]
- 41.Islam MdT., Streck L., Correia Jardim Paz M.F., de Castro e Sousa J.M., Oliveira Barros de Alencar M.V., Oliveira Ferreira da Mata A.M., Melo de Carvalho R., de Oliveira Santos J.V., da Silva-Junior A.A., Pinheiro Ferreira P.M., de Carvalho Melo-Cavalcante A.A. Preparation of phytol-loaded nanoemulsion and screening for antioxidant capacity. Int. Arch. Med. 2016 doi: 10.3823/1941. [DOI] [Google Scholar]
- 42.Pérez-González M.Z., Nieto-Trujillo A., Gutiérrez-Rebolledo G.A., García-Martínez I., Estrada-Zúñiga M.E., Bernabé-Antonio A., Jiménez-Arellanes M.A., Cruz-Sosa F. Lupeol acetate production and antioxidant activity of a cell suspension culture from Cnidoscolus chayamansa leaves. South Afr. J. Bot. 2019;125:30–38. doi: 10.1016/j.sajb.2019.06.030. [DOI] [Google Scholar]
- 43.Liu K., Zhang X., Xie L., Deng M., Chen H., Song J., Long J., Li X., Luo J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: molecular mechanisms and therapeutic efficacy. Pharmacol. Res. 2021;164 doi: 10.1016/j.phrs.2020.105373. [DOI] [PubMed] [Google Scholar]
- 44.Zhang P., Liu N., Xue M., Zhang M., Xiao Z., Xu C., Fan Y., Liu W., Qiu J., Zhang Q., Zhou Y. Anti-inflammatory and antioxidant properties of squalene in copper sulfate-induced inflammation in zebrafish (Danio rerio) Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24108518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keskin, Şirin Y., Çakir H.E., Keskin M. An investigation of Humulus lupulus L.: phenolic composition, antioxidant capacity and inhibition properties of clinically important enzymes. South Afr. J. Bot. 2019;120:170–174. doi: 10.1016/j.sajb.2018.04.017. [DOI] [Google Scholar]
- 46.Muzykiewicz A., Nowak A., Zielonka-Brzezicka J., Florkowska K., Duchnik W., Klimowicz A. Comparison of antioxidant activity of extracts of hop leaves harvested in different years. Herba Pol. 2019;65:1–9. doi: 10.2478/hepo-2019-0013. [DOI] [Google Scholar]
- 47.Baba S.A., Malik S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015;9:449–454. doi: 10.1016/j.jtusci.2014.11.001. [DOI] [Google Scholar]
- 48.Di Lodovico S., Menghini L., Ferrante C., Recchia E., Castro-Amorim J., Gameiro P., Cellini L., Bessa L.J. Hop extract: an efficacious antimicrobial and anti-biofilm agent against multidrug-resistant staphylococci strains and cutibacterium acnes. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolenc Z., Langerholc T., Hostnik G., Ocvirk M., Štumpf S., Pintarič M., Košir I.J., Čerenak A., Garmut A., Bren U. Antimicrobial properties of different hop (humulus lupulus) genotypes. Plants. 2023;12 doi: 10.3390/plants12010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anwar S., Almatroudi A., Allemailem K.S., Joseph R.J., Khan A.A., Rahmani A.H. Protective effects of ginger extract against glycation and oxidative stress-induced health complications: an in vitro study. Processes. 2020;8 doi: 10.3390/PR8040468. [DOI] [Google Scholar]
- 51.Feng G., Han K., Yang Q., Feng W., Guo J., Wang J., Yang X. Interaction of pyrogallol-containing polyphenols with mucin reinforces intestinal mucus barrier properties. J. Agric. Food Chem. 2022;70:9536–9546. doi: 10.1021/acs.jafc.2c03564. [DOI] [PubMed] [Google Scholar]
- 52.Lyu Y.L., Zhou H.F., Yang J., Wang F.X., Sun F., Li J.Y. Biological activities underlying the therapeutic effect of quercetin on inflammatory bowel disease. Mediat. Inflamm. 2022;2022 doi: 10.1155/2022/5665778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amevor F.K., Cui Z., Du X., Ning Z., Deng X., Xu D., Shu G., Wu Y., Cao X., Shuo W., Tian Y., Li D., Wang Y., Zhang Y., Du X., Zhu Q., Han X., Zhao X. Supplementation of dietary quercetin and vitamin E promotes the intestinal structure and immune barrier integrity in aged breeder hens. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.860889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu K.Y., Nakatsu C.H., Jones-Hall Y., Kozik A., Jiang Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic. Biol. Med. 2021;163:180–189. doi: 10.1016/j.freeradbiomed.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 55.Sasaoka N., Sakamoto M., Kanemori S., Kan M., Tsukano C., Takemoto Y., Kakizuka A. Long-term oral administration of hop flower extracts mitigates alzheimer phenotypes in mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ricci C., Maccarini M., Falus P., Librizzi F., Mangione M.R., Moran O., Ortore M.G., Schweins R., Vilasi S., Carrotta R. Amyloid β-peptide interaction with membranes: can chaperones change the fate? J. Phys. Chem. B. 2019;123:631–638. doi: 10.1021/acs.jpcb.8b11719. [DOI] [PubMed] [Google Scholar]
- 57.Vilasi S., Carrotta R., Ricci C., Rappa G.C., Librizzi F., Martorana V., Ortore M.G., Mangione M.R. Inhibition of aβ1-42 fibrillation by chaperonins: human Hsp60 is a stronger inhibitor than its bacterial homologue GroEL. ACS Chem. Neurosci. 2019;10:3565–3574. doi: 10.1021/acschemneuro.9b00183. [DOI] [PubMed] [Google Scholar]
- 58.Meisl G., Yang X., Hellstrand E., Frohm B., Kirkegaard J.B., Cohen S.I.A., Dobson C.M., Linse S., Knowles T.P.J. Differences in nucleation behavior underlie the contrasting aggregation kinetics of the Aβ40 and Aβ42 peptides. Proc Natl Acad Sci U S A. 2014;111:9384–9389. doi: 10.1073/pnas.1401564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pizzo F., Mangione M.R., Librizzi F., Manno M., Vilasi S., Martorana V., Noto R. The possible role of the type I chaperonins in human insulin self-association. Life. 2022;12 doi: 10.3390/life12030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levine H. Thioflavine T interaction with synthetic Alzheimer's disease β‐amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guarrasi V., Rappa G.C., Costa M.A., Librizzi F., Raimondo M., Di Stefano V., Germanà M.A., Vilasi S. Valorization of apple peels through the study of the effects on the amyloid aggregation process of κ-casein. Molecules. 2021;26 doi: 10.3390/molecules26082371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.