Key Points
Question
Can high nodal burden (≥4 axillary metastases/≥N2) be predicted without completion axillary lymph node dissection (CALND) in patients with luminal ERBB2-negative tumors and, separately, in those with invasive lobular carcinoma, and 1 or 2 sentinel lymph node (SLN) macrometastases?
Findings
In this diagnostic/prognostic study including the CALND arm (n = 1010) of the randomized SENOMAC trial, 2 prediction models for the identification of patients with ≥N2 were developed, including number of SLN macrometastases, additional SLN micrometastases, SLN ratio, extracapsular extension, and tumor size (only luminal model).
Meaning
The prediction models may be used to identify patients at risk of high nodal burden eligible for intensified systemic treatment strategies without performing CALND.
This diagnostic/prognostic study evaluates prediction models for high nodal burden in luminal ERBB2-negative breast cancer.
Abstract
Importance
In patients with clinically node-negative (cN0) breast cancer and 1 or 2 sentinel lymph node (SLN) macrometastases, omitting completion axillary lymph node dissection (CALND) is standard. High nodal burden (≥4 axillary nodal metastases) is an indication for intensified treatment in luminal breast cancer; hence, abstaining from CALND may result in undertreatment.
Objective
To develop a prediction model for high nodal burden in luminal ERBB2-negative breast cancer (all histologic types and lobular breast cancer separately) without CALND.
Design, Setting, and Participants
The prospective Sentinel Node Biopsy in Breast Cancer: Omission of Axillary Clearance After Macrometastases (SENOMAC) trial randomized patients 1:1 to CALND or its omission from January 2015 to December 2021 among adult patients with cN0 T1-T3 breast cancer and 1 or 2 SLN macrometastases across 5 European countries. The cohort was randomly split into training (80%) and test (20%) sets, with equal proportions of high nodal burden. Prediction models were developed by multivariable logistic regression in the complete luminal ERBB2-negative cohort and a lobular breast cancer subgroup. Nomograms were constructed. The present diagnostic/prognostic study presents the results of a prespecified secondary analysis of the SENOMAC trial. Herein, only patients with luminal ERBB2-negative tumors assigned to CALND were selected. Data analysis for this article took place from June 2023 to April 2024.
Exposure
Predictors of high nodal burden.
Main Outcomes and Measures
High nodal burden was defined as ≥4 axillary nodal metastases. The luminal prediction model was evaluated regarding discrimination and calibration.
Results
Of 1010 patients (median [range] age, 61 [34-90] years; 1006 [99.6%] female and 4 [0.4%] male), 138 (13.7%) had a high nodal burden and 212 (21.0%) had lobular breast cancer. The model in the training set (n = 804) included number of SLN macrometastases, presence of SLN micrometastases, SLN ratio, presence of SLN extracapsular extension, and tumor size (not included in lobular subgroup). Upon validation in the test set (n = 201), the area under the receiver operating characteristic curve (AUC) was 0.74 (95% CI, 0.62-0.85) and the calibration was satisfactory. At a sensitivity threshold of ≥80%, all but 5 low-risk patients were correctly classified corresponding to a negative predictive value of 94%. The prediction model for the lobular subgroup reached an AUC of 0.74 (95% CI, 0.66-0.83).
Conclusions and Relevance
The predictive models and nomograms may facilitate systemic treatment decisions without exposing patients to the risk of arm morbidity due to CALND. External validation is needed.
Trial Registration
ClinicalTrials.gov Identifier: NCT02240472
Introduction
Sentinel lymph node (SLN) biopsy is the standard procedure for axillary staging in patients with clinically node-negative (cN0) breast cancer. While completion axillary lymph node dissection (CALND) was previously routine care in patients with positive SLNs, it is today safely omitted in patients with 1 or 2 SLN metastases.1,2,3 However, some systemic treatment recommendations in estrogen receptor (ER)–positive Erb-B2 receptor tyrosine kinase 2 (ERBB2)–negative breast cancer rely on the identification of high nodal burden (ie, ≥4 metastatic axillary lymph nodes [ALNs]), which can only be determined by CALND.4 The recent Abemaciclib Plus Endocrine Therapy for Hormone Receptor–Positive, HER2-Negative, Node-Positive, High-Risk Early Breast Cancer (MONARCHE) trial5,6 showed that patients with high-risk early ER-positive breast cancer, defined as 4 or more metastatic ALNs or 1 to 3 metastatic ALNs together with either tumor size of 5 cm or greater or histologic grade 3, had improved invasive disease-free survival after 2 years of adjuvant CDK 4/6 inhibitor treatment in addition to endocrine therapy compared to the control group. In the Microarray in Node-Negative and 1 to 3 Positive Lymph Node Disease May Avoid Chemotherapy (MINDACT) trial,7 axillary nodal burden was indicative of systemic treatment. In addition, screening for distant metastases and locoregional radiotherapy target extension are considered in breast cancer with high nodal burden.8 High nodal burden can thus alter adjuvant treatment decisions. Therefore, CALND omission may lead to undertreatment.
Negative outcomes of CALND, such as arm lymphedema (affecting up to 25% of patients), numbness, and restricted shoulder mobility, are well documented.3 Arm morbidity is considerably worse after CALND than after SLN biopsy only.9,10 Therefore, alternative strategies to correctly identify high nodal burden without exposing patients to the risk of long-term arm morbidity are warranted.
Known indicators of high nodal burden are larger tumor size and a higher number of suspicious nodes at axillary imaging.11 Risk factors for additional non-SLN metastases in patients with metastatic SLNs (ie, not necessarily a high nodal burden) also include a high ratio of metastatic to excised SLNs (SLN ratio), extracapsular extension, and lymphovascular invasion in the primary tumor.12
Invasive lobular carcinoma (ILC) is most often of luminal A subtype and accounts for 10% to 15% of all breast cancer cases.13,14,15,16 In comparison to invasive breast cancer of no special type (NST), ILC is associated with a higher nodal burden and a higher rate of non-SLN metastases.17,18,19 In ILC, axillary ultrasonography, fine-needle aspiration cytology, and core needle biopsy have a lower sensitivity for detection of nodal metastases than in NST.20,21,22 In addition, SLN biopsy has a higher false negative rate in ILC than in NST.23 Unfortunately, no published axillary de-escalation trials have presented subgroup analyses addressing ILC.1,2,3 Additionally, ILC is associated with late recurrences, which may not be fully captured by follow-up times ranging from 5.0 to 9.3 years.1,2,3
Prediction models aimed at detecting high nodal burden offer a tool to identify individuals who require intensified adjuvant treatments while avoiding CALND.24,25,26,27,28 However, existing models are based on retrospective data encompassing any histopathological and molecular subtypes. None of the models have focused on luminal breast cancer, where nodal burden influences adjuvant strategies and no distinction between NST and ILC has been made. The primary aim of the present study was to develop prediction models for high nodal burden in patients with 1 or 2 SLN macrometastases and luminal ERBB2-negative breast cancer and ILC, respectively, which could reduce the risk of undertreatment without exposing patients to CALND.
Methods
Patient Characteristics
The Sentinel Node Biopsy in Breast Cancer: Omission of Axillary Clearance After Macrometastases (SENOMAC) trial is a randomized (1:1), prospective, international, noninferiority trial. The study protocol was approved by the ethics committee at Karolinska Institutet, Stockholm, Sweden, and the trial was conducted according to good clinical practice. Enrollment was from January 2015 to December 2021. Randomized assignment was to CALND or its omission. Adjuvant treatment was according to national guidelines. Informed written consent was obtained from all individual participants prior to study inclusion.
The SENOMAC trial design and end points have been previously described, and initial results have been presented.29,30 Adult patients diagnosed with primary invasive cN0 T1-T3 breast cancer and 1 or 2 SLN macrometastases were eligible. Additional SLN micrometastases were allowed. Preoperative axillary ultrasonography was mandatory. While patients having an SLN biopsy prior to preoperative systemic therapy were eligible, 55 such patients were excluded from the present analysis since their CALND results, obtained after systemic therapy, may not reflect the initial nodal status. Overall, 1010 patients with luminal ERBB2-negative breast cancer randomized to CALND constituted the present study cohort, 212 of whom (21.0%) had ILC (eFigure in Supplement 1). Data analysis for this article took place from June 2023 to April 2024. The Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline was followed.
Important Definitions
The TNM classification system was used. Thus, pathological nodal status (pN) was pN1 in case of 1 to 3 nodal metastases, pN2 for 4 to 9, and pN3 for 10 or more metastases.31 High nodal burden was defined as pN2 or pN3. Histopathological tumor type was categorized into 3 groups: NST, ILC, or other/mixed.32
Statistical Analysis
Sample size calculations were performed for the primary and secondary outcomes of the SENOMAC trial, but not for the present analysis.29 Patient and tumor demographic characteristics were summarized using standard descriptive statistical measures. Differences between the subgroups were evaluated using χ2 tests for categorical variables, linear trend tests for characteristics measured on an ordinal scale, and t tests for continuous variables.
Two prediction models for pN2-3 vs pN1 were developed: one for the entire luminal ERBB2-negative cohort and another for the ILC subgroup. For the development of the prediction model for the luminal ERBB2-negative cohort, patients were randomly split into a training set (80%) and a test set (20%) for validation. The split was stratified on outcome (≥pN2) to get the same prevalence of high nodal burden in both datasets. To minimize the risk of overfitting, the prediction model was developed in the training set and evaluated in the test set. The ILC subgroup was not split due to its smaller size.
Routinely accessible variables after SLN biopsy were selected based on published literature and considered as candidates for the prediction models (eAppendix 1 in Supplement 1). Univariable logistic regression analysis was used to estimate associations between candidate predictors and high nodal burden in the luminal ERBB2-negative cohort and the ILC subgroup, respectively.
The model development steps are described in detail in eAppendix 1 in Supplement 1. Briefly, backward logistic regression analysis was combined with bootstrap to select a subset of predictor variables for the final model. Since the fraction of missing data was low, models were developed using data from patients with complete data.
The diagnostic performance of the prediction model for the luminal ERBB2-negative cohort was evaluated in terms of discrimination (area under the receiver operating characteristic curve [AUC]) and calibration (Hosmer-Lemeshow plots, calibration slope, and intercept). The model for the luminal ERBB2-negative cohort was validated in the test set. Since no test set was set aside for the ILC subgroup, this model was not internally validated and should be regarded as exploratory. The equations for the final prediction models are presented in eAppendix 2 in Supplement 1. Nomograms were created. Sensitivity, specificity, positive predictive value, negative predictive value, true negative, true positive, false negative, and false positive rates for the models were calculated for clinically relevant predetermined cutoffs.
The prognostic value of the model for the luminal ERBB2-negative cohort was evaluated with recurrence-free survival (RFS) as the end point, using Kaplan-Meier estimates together with 1-df log-rank test for trend over the risk groups, and Cox regression. RFS was defined according to the Standardized Definitions for Efficacy End Points (STEEP) criteria.33 The proportional hazards assumption in the Cox models was tested using Schoenfeld residuals.
The evidence against each null hypothesis was quantified by a P value and the word significant used for 2-sided P < .05. The reported P values have not been adjusted for multiple comparisons. R version 4.2.2 (R Foundation) was used for all statistical analyses.
Results
Descriptive Results
High vs Low Nodal Burden
Of 1010 patients with luminal ERBB2-negative breast cancer assigned to CALND in the SENOMAC trial (luminal cohort; median [range] age, 61 [34-90] years; 1006 [99.6%] female and 4 [0.4%] male), 138 (13.7%) had a high nodal burden (Table). In 846 patients with 1 SLN macrometastasis, 83 (9.8%) had ≥pN2, while in 164 with 2 SLN macrometastases, 55 had ≥pN2 (33.5%) (P < .001). The median (range) tumor size was significantly smaller in patients with a low nodal burden (19.0 mm [1.1-136.0] vs 25.0 mm [4.0-155.0]; P < .001). Additional SLN micrometastases were less common in the low nodal burden group (84 of 872 [9.6%] vs 22 of 138 [15.9%]; P = .07), and SLN extracapsular extension significantly less prevalent (296 of 868 [34.1%] vs 66 of 137 [48.2%]; P = .002). No difference was seen between low vs high nodal burden for lymphovascular invasion in the primary tumor (242 of 870 [27.8%] vs 38 of 137 [27.7%]; P > .99).
Table. Baseline Characteristics of Patients.
| Characteristic | No. (%) | P value | Trend test | ||
|---|---|---|---|---|---|
| Low nodal burden, pN1 (n = 872) | High nodal burden, ≥pN2 (n = 138) | Overall (N = 1010) | |||
| Sex | |||||
| Female | 868 (99.5) | 138 (100) | 1006 (99.6) | .95 | NA |
| Male | 4 (0.5) | 0 | 4 (0.4) | ||
| Age at randomization, y | |||||
| <40 | 19 (2.2) | 2 (1.4) | 21 (2.1) | .92 | >.99 |
| 40-49 | 137 (15.7) | 24 (17.4) | 161 (15.9) | ||
| 50-64 | 346 (39.7) | 55 (39.9) | 401 (39.7) | ||
| 65-74 | 262 (30.0) | 38 (27.5) | 300 (29.7) | ||
| ≥75 | 108 (12.4) | 19 (13.8) | 127 (12.6) | ||
| Mean (SD) | 61.19 (11.49) | 60.91 (11.43) | 61.15 (11.47) | .79 | NA |
| Median (range) | 61.00 (34.00-90.00) | 60.00 (34.00-88.00) | 61.00 (34.00-90.00) | NA | NA |
| T stage | |||||
| pT1 | 501 (57.5) | 56 (40.6) | 557 (55.1) | <.001 | <.001 |
| pT2 | 331 (38.0) | 61 (44.2) | 392 (38.8) | ||
| pT3 | 40 (4.6) | 21 (15.2) | 61 (6.0) | ||
| Tumor size, mm | |||||
| Mean (SD) | 22.78 (15.61) | 31.14 (21.67) | 23.92 (16.81) | <.001 | NA |
| Median (range) | 19.00 (1.10-136.00) | 25.00 (4.00-155.00) | 19.00 (1.10-155.00) | NA | NA |
| Tumor type | |||||
| NST | 673 (77.2) | 92 (66.7) | 765 (75.7) | .01 | NA |
| ILC | 170 (19.5) | 42 (30.4) | 212 (21.0) | ||
| Other/mixed | 29 (3.3) | 4 (2.9) | 33 (3.3) | ||
| Histological grade (NHG) | |||||
| 1 | 175 (20.2) | 31 (22.5) | 206 (20.5) | .78 | .48 |
| 2 | 559 (64.5) | 88 (63.8) | 647 (64.4) | ||
| 3 | 133 (15.3) | 19 (13.8) | 152 (15.1) | ||
| Missing | 5 (0.6) | 0 | 5 (0.5) | ||
| Lymphovascular invasion | |||||
| No | 628 (72.2) | 99 (72.3) | 727 (72.2) | >.99 | NA |
| Yes | 242 (27.8) | 38 (27.7) | 280 (27.8) | ||
| Missing | 2 (0.2) | 1 (0.7) | 3 (0.3) | ||
| Breast surgery performed | |||||
| Breast-conserving surgery | 592 (67.9) | 73 (52.9) | 665 (65.8) | <.001 | NA |
| Mastectomy | 280 (32.1) | 65 (47.1) | 345 (34.2) | ||
| Suspicious lymph nodes on ultrasonography | |||||
| No | 760 (87.2) | 113 (81.9) | 873 (86.4) | .12 | NA |
| Yes | 112 (12.8) | 25 (18.1) | 137 (13.6) | ||
| SLNs removed, No. | |||||
| 1-2 | 618 (70.9) | 102 (73.9) | 720 (71.3) | .57 | .35 |
| 3-4 | 221 (25.3) | 33 (23.9) | 254 (25.1) | ||
| >4 | 33 (3.8) | 3 (2.2) | 36 (3.6) | ||
| Mean (SD) | 2.10 (1.15) | 2.03 (1.03) | 2.09 (1.13) | .47 | NA |
| Median (range) | 2.00 (1.00-9.00) | 2.00 (1.00-6.00) | 2.00 (1.00-9.00) | NA | NA |
| SLN macrometastases, No. | |||||
| 1 | 763 (87.5) | 83 (60.1) | 846 (83.8) | <.001 | NA |
| 2 | 109 (12.5) | 55 (39.9) | 164 (16.2) | ||
| SLN micrometastases, No. | |||||
| 0 | 788 (90.4) | 116 (84.1) | 904 (89.5) | .07 | .02 |
| 1 | 79 (9.1) | 20 (14.5) | 99 (9.8) | ||
| 2 | 5 (0.6) | 2 (1.4) | 7 (0.7) | ||
| Total SLN metastases, No. | |||||
| 1 | 685 (78.6) | 67 (48.6) | 752 (74.5) | <.001 | <.001 |
| 2 | 176 (20.2) | 63 (45.7) | 239 (23.7) | ||
| 3 | 11 (1.3) | 8 (5.8) | 19 (1.9) | ||
| Mean (SD) | 1.23 (0.45) | 1.57 (0.60) | 1.27 (0.49) | <.001 | NA |
| Median (range) | 1.00 (1.00-3.00) | 2.00 (1.00-3.00) | 1.00 (1.00-3.00) | NA | NA |
| SLN ratio (SLN metastases/excised SLN) | |||||
| Mean (SD) | 0.70 (0.29) | 0.85 (0.23) | 0.72 (0.29) | <.001 | NA |
| Median (range) | 0.67 (0.11-1.00) | 1.00 (0.25-1.00) | 0.67 (0.11-1.00) | NA | NA |
| Extracapsular extension SLN | |||||
| No | 572 (65.9) | 71 (51.8) | 643 (64.0) | .002 | NA |
| Yes | 296 (34.1) | 66 (48.2) | 362 (36.0) | ||
| Missing | 4 (0.5) | 1 (0.7) | 5 (0.5) | ||
| Total lymph nodes removed, No. | |||||
| Mean (SD) | 15.09 (6.86) | 17.72 (8.00) | 15.45 (7.08) | <.001 | NA |
| Median (range) | 14.00 (1.00-51.00) | 17.00 (4.00-50.00) | 14.00 (1.00-51.00) | NA | NA |
| Metastases, No. | |||||
| Mean (SD) | 1.52 (0.67) | 7.54 (6.00) | 2.34 (3.09) | <.001 | NA |
| Median (range) | 1.00 (1.00-3.00) | 5.00 (4.00-42.00) | 1.00 (1.00-42.00) | NA | NA |
| Final nodal stage | |||||
| pN1 | 872 (100) | 0 | 872 (86.3) | <.001 | <.001 |
| pN2 | 0 | 108 (78.3) | 108 (10.7) | ||
| pN3 | 0 | 30 (21.7) | 30 (3.0) | ||
Abbreviations: ILC, invasive lobular carcinoma; NA, not applicable; NHG, Nottingham Histological Grade; NST, invasive breast cancer of no special type; SLN, sentinel lymph node.
Invasive Lobular Cancer
ILC constituted 21.0% of the entire luminal ERBB2-negative cohort. High nodal burden was more common in ILC than other histopathological tumor types (42 of 212 [19.8%] vs 96 of 798 [12.0%]; P = .005) (eTable in Supplement 1). The median (range) tumor size was significantly larger in patients with ILC (32.0 mm [6.0-155.0] vs 18.0 mm [1.1-97.0]; P < .001).
Prediction Models
Luminal ERBB2-Negative Cohort
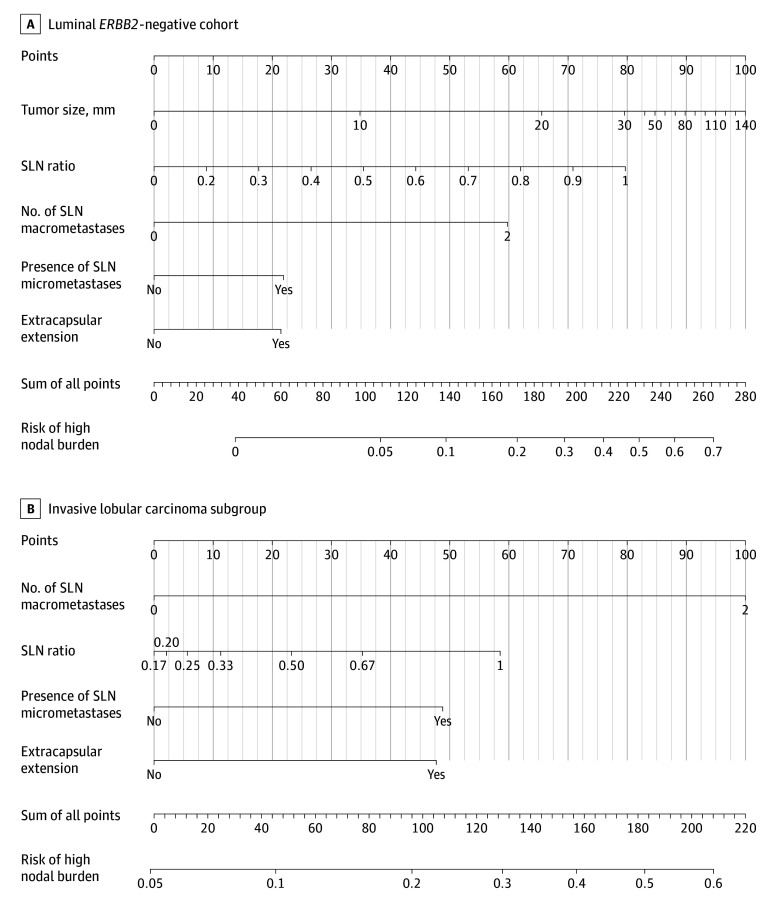
Two vs 1 SLN macrometastases, higher SLN ratio, additional SLN micrometastases, SLN extracapsular extension, and larger tumor size were independently associated with high nodal burden and selected for the multivariable prediction model (eAppendix 2 in Supplement 1), which reached an AUC of 0.77 (95% CI, 0.72-0.82) in the training set and 0.74 (95% CI, 0.62-0.85) in the test set (Figure 1A and B). The calibration plot showed that the mean predicted probabilities of high nodal burden did not deviate systematically from observed fractions in deciles based on the predicted probabilities of high nodal burden in the test set (minor deviations from the dashed identity line, which indicates perfect calibration). This was also supported by the summary measures calibration intercept 0.05 and calibration slope 0.83, which are close to the optimal values 0 and 1.00, respectively, for a perfectly calibrated model (Figure 1C). At a sensitivity threshold of 80% or greater, 111 of the 201 patients in the test set (55.2%) were identified as high risk (Figure 1B). While this proportion seems high compared to the observed prevalence for ≥pN2 of 13.4%, the negative predictive value was 94.4% (85/90), correctly classifying all but 5 patients as low risk at this threshold. The model is visualized in a nomogram (Figure 2A).
Figure 1. Discrimination and Calibration of the Prediction Models for High Nodal Burden.
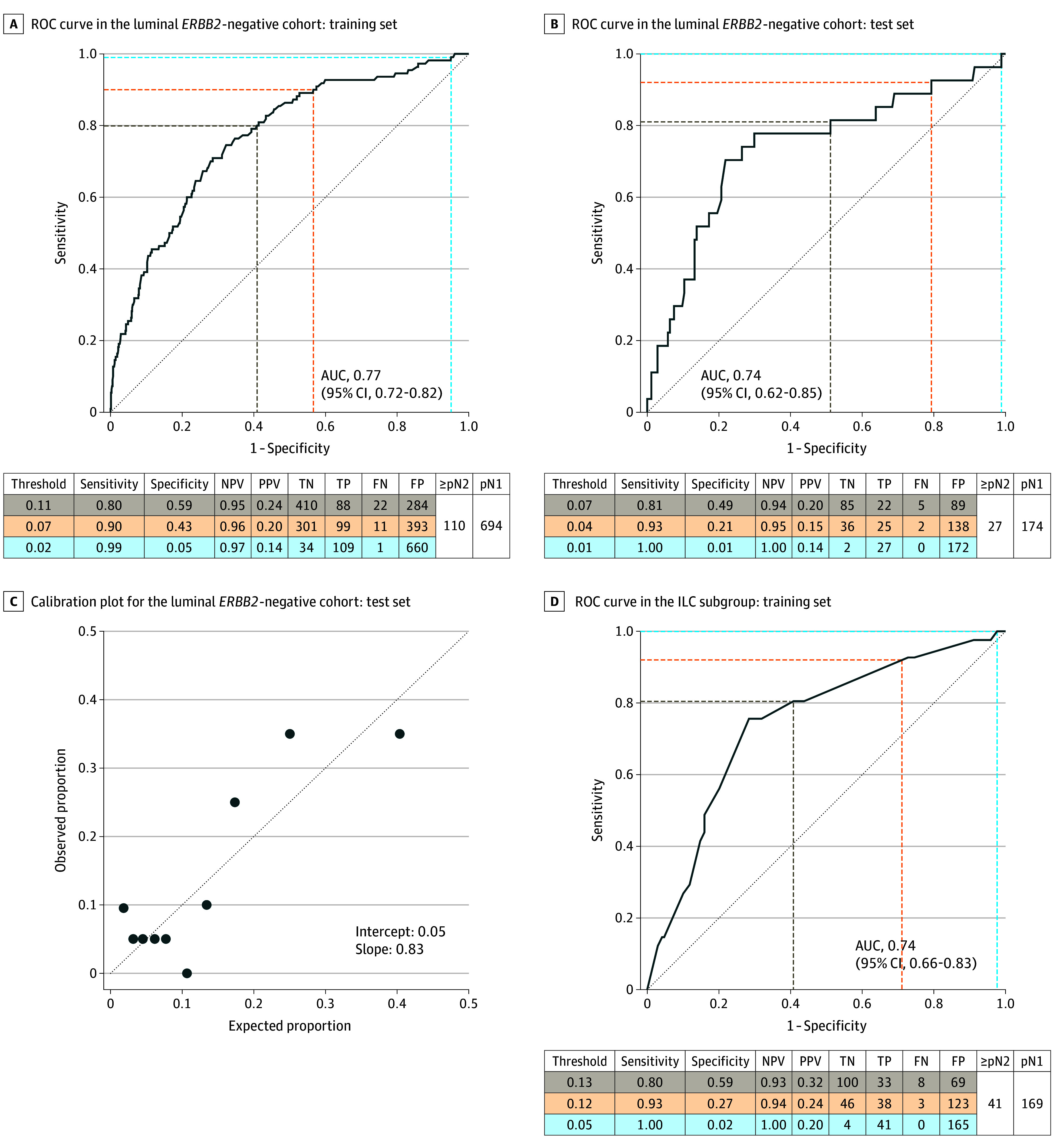
Threshold refers to the predicted probability of high nodal burden (≥pN2). In the calibration plot, the dashed oblique reference line indicates perfect calibration with an intercept of 0 and a slope of 1. The cluster of points in the lower left corner of the figure indicates that observed, as well as predicted, risk of high nodal burden is low for most patients in the cohort. In addition, the absence of a linear trend at this end of the risk spectrum suggests that the discrimination is poor for low-risk patients. However, the points in the upper right corner indicate that the model might be useful for identification of patients at high risk of high nodal burden, which was the aim of the present work. AUC indicates area under the ROC curve; FN, false negative; FP, false positive; N, nodal status; ILC, invasive lobular carcinoma; NPV, negative predicted value; PPV, positive predicted value; TN, true negative; TP, true positive; ROC, receiver operating characteristic curve.
Figure 2. Nomogram for Predicting the Probability of High Nodal Burden.
SLN ratio refers to (SLN micrometastases + SLN marcrometastases) / (all removed lymph nodes at SLN biopsy). SLN indicates sentinel lymph node.
Lobular Subgroup
In the ILC subgroup, 2 SLN macrometastases vs 1 had an odds ratio (OR) of 4.96 (95% CI, 2.17-11.47) in the final multivariable model for high vs low nodal burden (eAppendix 2 in Supplement 1). All independent factors identified in the luminal ERBB2-negative cohort, apart from tumor size, were associated with high nodal burden in ILC also and were included in the lobular prediction model with an AUC of 0.74 (95% CI, 0.66-0.83) in the development set (Figure 1D). The model is visualized in a nomogram (Figure 2B).
RFS
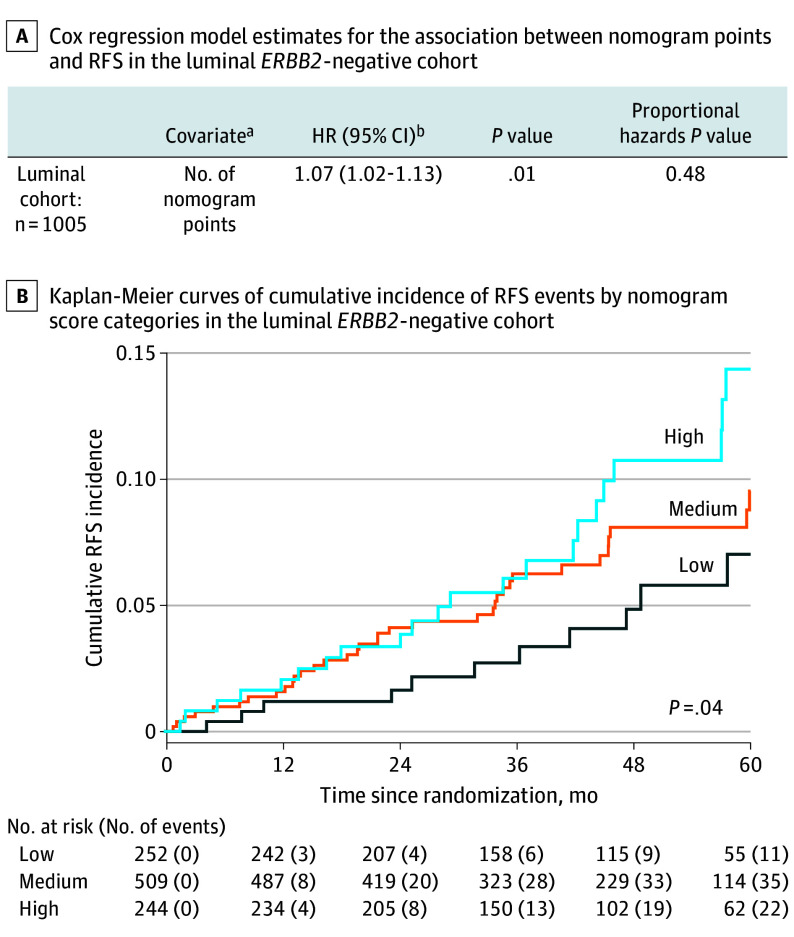
To investigate the prognostic potential of the prediction model, the total nomogram score was related to RFS in the luminal ERBB2-negative cohort. Cox regression analysis revealed that a 10-unit increase in the score corresponded to a 7% increase in the incidence of RFS events (hazard ratio [HR], 1.07; 95% CI, 1.02-1.13; P = .01) (Figure 3A). When categorizing the score into quartiles, the HR for the high-risk group (quartile 4) vs the low-risk group (quartile 1) was 2.11 (95% CI, 1.02-4.35; P = .04) and the HR for the medium-risk group (quartiles 2-3) vs quartile 1 was 1.59 (95% CI, 0.81-3.14; P = .18). The corresponding Kaplan-Meier estimates of cumulative incidence of RFS events are shown in Figure 3B.
Figure 3. Prognostic Value of the High Nodal Burden Prediction Model.
Patients were followed up from randomization to the date of first recurrence or death, defined according to the Standardized Definitions for Efficacy End Points (STEEP) criteria or, for event-free patients, to the date of last follow-up. Contralateral breast cancer was not considered a recurrence-free survival (RFS) event. Follow-up times for recurrence-free and alive participants were censored at the date of last visit. No follow-up times were censored for other reasons. The proportional hazards assumption was fulfilled for both Cox models (P = .48 in model with continuous score and P = .41 with score categories). Risk groups were created by dividing the total nomogram score into 3 groups: low (quartile 1), medium (quartiles 2-3), and high (quartile 4). The hazard ratio (HR) was calculated for a 10-unit nomogram score increase.
aDifferent spline functions were assessed using Akaike Information Criterion (AIC), but a linear model for total points gave the best fit to the data after adjustment for model complexity.
bInterpretation of this HR: for each 10-step increase of total nomogram points, the incidence of RFS events increases with 7%.
Discussion
In this diagnostic/prognostic study, the predictive models and corresponding nomograms for high nodal burden (≥pN2) in patients with 1 or 2 SLN macrometastases and luminal ERBB2-negative breast cancer and ILC, respectively, may help select patients for intensified adjuvant therapies while avoiding CALND. At a threshold corresponding to a sensitivity of at least 80%, all but 5 low-risk patients were correctly classified, corresponding to a negative predictive value of 94%. In the luminal ERBB2-negative cohort, the predicted probability of high nodal burden (≥pN2) was also prognostic of RFS, underscoring the clinical relevance of the prediction model as an alternative to CALND.
A minority of patients with 1 or 2 macrometastases on SLN biopsy have a high nodal burden: in the American College of Surgeons Oncology Group Z0011 (ACOSOG Z0011) trial,1 47 of 343 (13.7%) had 4 or more metastatic ALNs; in the European Organisation for Research and Treatment of Cancer 10981-22023 (AMAROS) trial,3 52 of 672 (8%) had 4 or more positive additional nodes (besides SLN metastasis); and in the Optimal Treatment of the Axilla—Surgery or Radiotherapy After Positive Sentinel Lymph Node Biopsy in Early-Stage Breast Cancer (OTOASOR) trial,34 54 of 244 (22%) had ≥pN2a. Despite the presence of ≥pN2 disease in these trials, the omission of CALND did not increase long-term locoregional recurrence rates. However, there are currently no data from randomized clinical trials comparing CALND vs its omission in a population of patients with verified ≥pN2 disease. The proportion of ≥pN2 status ranges from 3.5% to 16% in cN0 breast cancer.35,36 Thus, the therapeutic effect of CALND is debatable. Here we present a tool for procuring similar diagnostic information considering multiple risk factors of ≥pN2.
Prediction Models—Comparison to Previous Studies
In the multicenter tool for risk prediction of 4 or more ALN metastases in SLN-positive breast cancer developed by Meretoja et al,28 based on retrospective data from 675 patients, the AUC reached 0.77 (95% CI, 0.74-0.81) in the external validation using a retrospective cohort (n = 760). The included variables were site prevalence of ≥pN2, number of metastatic and benign SLNs, tumor size, and extracapsular extension. Murata et al25 presented a model for prediction of high nodal burden based on 804 patients who were cN0 SLN positive. The AUC was 0.89 (95% CI, 0.84-0.93) in the test set, with good discrimination; however, as this was a retrospective single-institution study, external validation is needed. In another prediction model by Kim et al,26 lymphovascular invasion, extranodal extension, T stage, and a high SLN ratio were independently associated with high nodal burden in a retrospective analysis of 1437 patients. The AUC was 0.82 in the test set (no CI provided; no external validation). In a nomogram by Yang et al24 incorporating the number of SLN metastases, extracapsular extension, pT stage, and tumor location in the breast in 1480 patients, the AUC was 0.80 (95% CI, 0.76-0.85) in the external validation set. In a small study by Corsi et al27 (n = 271), SLN ratio, extranodal extension, and multifocal disease were predictors of more than 2 metastatic ALNs, with an AUC of 0.74 (no CI provided; no external validation).
The prevalence of high nodal burden in the above-mentioned studies ranged from 5.7% to 20.7% (compared to 13.7% in this study). While the models based on retrospective cohorts by Meretoja et al and Yang et al were externally validated, none have been evaluated in terms of recurrence or survival analyses.
ILC
ILC is a distinct biological entity which warrants specific focus. The presented prediction model for ILC is, to our knowledge, the first model for high nodal burden in ILC and 1 or 2 SLN macrometastases. Studies are rarely analyzed by histopathological tumor type,37 and treatment recommendations usually address NST and ILC as 1 entity.4 Only in recent years, ILC-specific clinical trials have been initiated.16
Tumor size was selected in the luminal ERBB2-negative cohort but not in the ILC subgroup. Lee et al36 and Rivers et al38 found tumors greater than 20 mm to be associated with high nodal burden. However, in our study larger tumor size was independently associated with high nodal burden in the luminal ERBB2-negative but not the ILC subgroup. Considering the suggested threshold at greater than 20 mm, the significantly larger tumor size in ILC compared to other histopathological tumor types (median, 32 mm vs 18 mm) might explain why tumor size was not a strong independent predictive factor in the ILC subgroup.
Imaging is highly indicative of high nodal burden and can rule out stage ≥pN2 breast cancer with a negative predictive value of 96%.11,39 The sensitivity of axillary ultrasonography for predicting ≥3 metastatic ALN is, however, lower in ILC than in NST.20 Despite ILC being associated with more ALN metastases,40,41 nodal burden is more often underestimated preoperatively.42 Other imaging modalities, such as magnetic resonance imaging or 18F-fluorodeoxyglucose positron emission tomography/computed tomography, are of limited clinical value.42,43,44 These limitations underline the need for prediction models developed exclusively in ILC.
Altogether, ILC poses diagnostic staging challenges, and the risk of occult ALN involvement remains challenging. The use of a decision support tool might thus be more important in ILC than in NST.
Strengths and Limitations
A major strength of this study is that it is based on meticulously curated prospectively collected data from a large randomized clinical trial. Moreover, we address both global performance measures of discrimination and calibration. In addition, applied sensitivity cutoffs are derived from clinical reasoning rather than being data driven. We exclusively incorporated routinely accessible variables, available at the intended time point for using the nomogram. To further enable clinical use, a nomogram is presented for each target population. While prediction models were not developed to predict RFS, we show clinical validity by differentiation between prognostic strata based on nomogram scores. Longer follow-up will be necessary to monitor separation of RFS curves given the potential of luminal breast cancer to recur late.
The foremost limitation of this study is the lack of validation in an external cohort and the fact that no internal validation was conducted for the ILC subgroup due to limited sample size. Although the inclusion criteria in SENOMAC are considered broad, generalization beyond this population should be used with caution. Another limitation is the absence of some variables of putative relevance for modeling, such as mode of detection (eg, screening or clinically detected), race, ethnicity, and body mass index. Progesterone receptor status and proliferation rate (Ki67) were not considered because of poor standardization and varying availability across centers. The 21-gene Oncotype DX Breast Recurrence Score may inform decisions on adjuvant chemotherapy in postmenopausal luminal ERBB2-negative breast cancer with 1 to 3 metastatic ALNs without CALND. However, the clinical value of Oncotype DX for guidance of adjuvant abemaciclib is not yet determined, and a nomogram can be applied irrespective of the availability of genomic tests.45 In the SENOMAC trial, multigene signatures were not registered. While acknowledging this as a limitation, we emphasize that the presented models are clinically feasible also in economically diverse conditions and not dependent on the availability of tissue for analysis. No imaging-derived variables were considered for the models, enabling application in routine clinical settings where axillary imaging is not part of diagnostic workup for patients with cN0 T1-T2 breast cancer eligible for upfront surgery.11,46
Conclusions
The presented model predicts high nodal burden in patients with luminal ERBB2-negative T1-T3 breast cancer and 1 or 2 SLN macrometastases with good performance measures. Use of the nomogram may facilitate systemic treatment decisions without exposing patients to CALND, thus minimizing the risk of arm morbidity. External validation is necessary.
eFigure. Patient Flow Chart
eAppendix 1. Prediction Model – Variable Selection
eAppendix 2. Multivariable Logistic Regression Model
eTable. Baseline Characteristics of the Included Patients Stratified on Invasive Lobular Carcinoma (ILC) vs Other Histopathological Tumor Types
Data sharing statement
References
- 1.Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918-926. doi: 10.1001/jama.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galimberti V, Cole BF, Zurrida S, et al. ; International Breast Cancer Study Group Trial 23-01 investigators . Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297-305. doi: 10.1016/S1470-2045(13)70035-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303-1310. doi: 10.1016/S1470-2045(14)70460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein HJ, Curigliano G, Loibl S, et al. ; Members of the St. Gallen International Consensus Panel on the Primary Therapy of Early Breast Cancer 2019 . Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541-1557. doi: 10.1093/annonc/mdz235 [DOI] [PubMed] [Google Scholar]
- 5.Johnston SRD, Harbeck N, Hegg R, et al. ; MONARCHE Committee Members and Investigators . Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (MONARCHE). J Clin Oncol. 2020;38(34):3987-3998. doi: 10.1200/JCO.20.02514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rastogi P, O’Shaughnessy J, Martin M, et al. Adjuvant abemaciclib plus endocrine therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative, high-risk early breast cancer: results from a preplanned MONARCHE overall survival interim analysis, including 5-year efficacy outcomes. J Clin Oncol. 2024;42(9):987-993. doi: 10.1200/JCO.23.01994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso F, van’t Veer LJ, Bogaerts J, et al. ; MINDACT Investigators . 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717-729. doi: 10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- 8.Cardoso F, Kyriakides S, Ohno S, et al. ; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org . Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194-1220. doi: 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 9.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95(3):279-293. doi: 10.1007/s10549-005-9025-7 [DOI] [PubMed] [Google Scholar]
- 10.Appelgren M, Sackey H, Wengström Y, et al. ; SENOMAC Trialists’ Group . Patient-reported outcomes one year after positive sentinel lymph node biopsy with or without axillary lymph node dissection in the randomized SENOMAC trial. Breast. 2022;63:16-23. doi: 10.1016/j.breast.2022.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang JM, Leung JWT, Moy L, Ha SM, Moon WK. Axillary nodal evaluation in breast cancer: state of the art. Radiology. 2020;295(3):500-515. doi: 10.1148/radiol.2020192534 [DOI] [PubMed] [Google Scholar]
- 12.van la Parra RF, Peer PG, Ernst MF, Bosscha K. Meta-analysis of predictive factors for non-sentinel lymph node metastases in breast cancer patients with a positive SLN. Eur J Surg Oncol. 2011;37(4):290-299. doi: 10.1016/j.ejso.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 13.Pramod N, Nigam A, Basree M, et al. Comprehensive review of molecular mechanisms and clinical features of invasive lobular cancer. Oncologist. 2021;26(6):e943-e953. doi: 10.1002/onco.13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Baelen K, Geukens T, Maetens M, et al. Current and future diagnostic and treatment strategies for patients with invasive lobular breast cancer. Ann Oncol. 2022;33(8):769-785. doi: 10.1016/j.annonc.2022.05.006 [DOI] [PubMed] [Google Scholar]
- 15.McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and 'omics. Breast Cancer Res. 2015;17(1):12. doi: 10.1186/s13058-015-0519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCart Reed AE, Kalinowski L, Simpson PT, Lakhani SR. Invasive lobular carcinoma of the breast: the increasing importance of this special subtype. Breast Cancer Res. 2021;23(1):6. doi: 10.1186/s13058-020-01384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narbe U, Bendahl PO, Fernö M, Ingvar C, Dihge L, Rydén L. St Gallen 2019 guidelines understage the axilla in lobular breast cancer: a population-based study. Br J Surg. 2021;108(12):1465-1473. doi: 10.1093/bjs/znab327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adachi Y, Sawaki M, Hattori M, et al. Comparison of sentinel lymph node biopsy between invasive lobular carcinoma and invasive ductal carcinoma. Breast Cancer. 2018;25(5):560-565. doi: 10.1007/s12282-018-0852-x [DOI] [PubMed] [Google Scholar]
- 19.Caudle AS, Kuerer HM, Le-Petross HT, et al. Predicting the extent of nodal disease in early-stage breast cancer. Ann Surg Oncol. 2014;21(11):3440-3447. doi: 10.1245/s10434-014-3813-4 [DOI] [PubMed] [Google Scholar]
- 20.Morrow E, Lannigan A, Doughty J, et al. Population-based study of the sensitivity of axillary ultrasound imaging in the preoperative staging of node-positive invasive lobular carcinoma of the breast. Br J Surg. 2018;105(8):987-995. doi: 10.1002/bjs.10791 [DOI] [PubMed] [Google Scholar]
- 21.Topps A, Clay V, Absar M, et al. The sensitivity of pre-operative axillary staging in breast cancer: comparison of invasive lobular and ductal carcinoma. Eur J Surg Oncol. 2014;40(7):813-817. doi: 10.1016/j.ejso.2014.03.026 [DOI] [PubMed] [Google Scholar]
- 22.Hackney L, Williams S, Bajwa S, Morley-Davies AJ, Kirby RM, Britton I. Influence of tumor histology on preoperative staging accuracy of breast metastases to the axilla. Breast J. 2013;19(1):49-55. doi: 10.1111/tbj.12042 [DOI] [PubMed] [Google Scholar]
- 23.Guo R, Brabham CE, Fahrner-Scott K, et al. Accuracy of sentinel lymph node biopsy in invasive lobular carcinoma of the breast. J Clin Oncol. 2020;38(15)(suppl):e12604-e12604. doi: 10.1200/JCO.2020.38.15_suppl.e12604 [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Lan X, Huang Z, et al. Development and external validation of a nomogram to predict four or more positive nodes in breast cancer patients with one to three positive sentinel lymph nodes. Breast. 2020;53:143-151. doi: 10.1016/j.breast.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murata T, Watase C, Shiino S, et al. Development and validation of a pre- and intra-operative scoring system that distinguishes between non-advanced and advanced axillary lymph node metastasis in breast cancer with positive sentinel lymph nodes: a retrospective study. World J Surg Oncol. 2022;20(1):314. doi: 10.1186/s12957-022-02779-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim I, Ryu JM, Kim JM, et al. Development of a nomogram to predict N2 or N3 stage in T1-2 invasive breast cancer patients with no palpable lymphadenopathy. J Breast Cancer. 2017;20(3):270-278. doi: 10.4048/jbc.2017.20.3.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corsi F, Sorrentino L, Albasini S, et al. Prediction of nodal staging in breast cancer patients with 1-2 sentinel nodes in the Z0011 era. Medicine (Baltimore). 2020;99(35):e21721. doi: 10.1097/MD.0000000000021721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meretoja TJ, Audisio RA, Heikkilä PS, et al. International multicenter tool to predict the risk of four or more tumor-positive axillary lymph nodes in breast cancer patients with sentinel node macrometastases. Breast Cancer Res Treat. 2013;138(3):817-827. doi: 10.1007/s10549-013-2468-3 [DOI] [PubMed] [Google Scholar]
- 29.de Boniface J, Frisell J, Andersson Y, et al. ; SENOMAC Trialists’ Group . Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer. 2017;17(1):379. doi: 10.1186/s12885-017-3361-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Boniface J, Filtenborg Tvedskov T, Rydén L, et al. ; SENOMAC Trialists’ Group; SENOMAC Trialists’ Group . Omitting axillary dissection in breast cancer with sentinel-node metastases. N Engl J Med. 2024;390(13):1163-1175. doi: 10.1056/NEJMoa2313487 [DOI] [PubMed] [Google Scholar]
- 31.Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. Wiley-Blackwell; 2009. [Google Scholar]
- 32.Canas-Marques R, Schnitt SJ. E-cadherin immunohistochemistry in breast pathology: uses and pitfalls. Histopathology. 2016;68(1):57-69. doi: 10.1111/his.12869 [DOI] [PubMed] [Google Scholar]
- 33.Tolaney SM, Garrett-Mayer E, White J, et al. Updated Standardized Definitions for Efficacy End Points (STEEP) in adjuvant breast cancer clinical trials: STEEP version 2.0. J Clin Oncol. 2021;39(24):2720-2731. doi: 10.1200/JCO.20.03613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sávolt Á, Péley G, Polgár C, et al. Eight-year follow up result of the OTOASOR trial: the Optimal Treatment of the Axilla—Surgery or Radiotherapy After Positive Sentinel Lymph Node Biopsy in Early-Stage Breast Cancer: a randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol. 2017;43(4):672-679. doi: 10.1016/j.ejso.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 35.Rocco N, Ghilli M, Curcio A, et al. Is routine axillary lymph node dissection needed to tailor systemic treatments for breast cancer patients in the era of molecular oncology? a position paper of the Italian National Association of Breast Surgeons (ANISC). Eur J Surg Oncol. 2024;50(2):107954. doi: 10.1016/j.ejso.2024.107954 [DOI] [PubMed] [Google Scholar]
- 36.Lee MK, Montagna G, Pilewskie ML, Sevilimedu V, Morrow M. Axillary staging is not justified in postmenopausal clinically node-negative women based on nodal disease burden. Ann Surg Oncol. 2023;30(1):92-97. doi: 10.1245/s10434-022-12203-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mouabbi JA, Hassan A, Lim B, Hortobagyi GN, Tripathy D, Layman RM. Invasive lobular carcinoma: an understudied emergent subtype of breast cancer. Breast Cancer Res Treat. 2022;193(2):253-264. doi: 10.1007/s10549-022-06572-w [DOI] [PubMed] [Google Scholar]
- 38.Rivers AK, Griffith KA, Hunt KK, et al. Clinicopathologic features associated with having four or more metastatic axillary nodes in breast cancer patients with a positive sentinel lymph node. Ann Surg Oncol. 2006;13(1):36-44. doi: 10.1245/ASO.2006.03.080 [DOI] [PubMed] [Google Scholar]
- 39.Neal CH, Daly CP, Nees AV, Helvie MA. Can preoperative axillary US help exclude N2 and N3 metastatic breast cancer? Radiology. 2010;257(2):335-341. doi: 10.1148/radiol.10100296 [DOI] [PubMed] [Google Scholar]
- 40.Chen BF, Tsai YF, Lien PJ, et al. Clinical characteristics and treatment outcomes of invasive ductal and lobular carcinoma: analyses of 54,832 Taiwan cancer registry index cases. Breast Cancer Res Treat. 2023;201(3):547-560. doi: 10.1007/s10549-023-07044-5 [DOI] [PubMed] [Google Scholar]
- 41.Oesterreich S, Nasrazadani A, Zou J, et al. Clinicopathological features and outcomes comparing patients with invasive ductal and lobular breast cancer. J Natl Cancer Inst. 2022;114(11):1511-1522. doi: 10.1093/jnci/djac157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawaguchi S, Kinowaki K, Tamura N, et al. High-accuracy prediction of axillary lymph node metastasis in invasive lobular carcinoma using focal cortical thickening on magnetic resonance imaging. Breast Cancer. 2023;30(4):637-646. doi: 10.1007/s12282-023-01457-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilardi L, Airò Farulla LS, Curigliano G, Corso G, Leonardi MC, Ceci F. FDG and Non-FDG radiopharmaceuticals for PET imaging in invasive lobular breast carcinoma. Biomedicines. 2023;11(5):1350. doi: 10.3390/biomedicines11051350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schumacher K, Inciardi M, O’Neil M, et al. Is axillary imaging for invasive lobular carcinoma accurate in determining clinical node staging? Breast Cancer Res Treat. 2021;185(3):567-572. doi: 10.1007/s10549-020-06047-w [DOI] [PubMed] [Google Scholar]
- 45.Kalinsky K, Barlow WE, Gralow JR, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385(25):2336-2347. doi: 10.1056/NEJMoa2108873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saksena M, Jimenez R, Coopey S, Harris K. Axillary ultrasound evaluation in breast cancer patients: a multidisciplinary viewpoint and middle ground. J Breast Imaging. 2021;3(6):672-675. doi: 10.1093/jbi/wbab070 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Patient Flow Chart
eAppendix 1. Prediction Model – Variable Selection
eAppendix 2. Multivariable Logistic Regression Model
eTable. Baseline Characteristics of the Included Patients Stratified on Invasive Lobular Carcinoma (ILC) vs Other Histopathological Tumor Types
Data sharing statement