Abstract
Immunity to human immunodeficiency virus virion-like structures or a polyprotein has been examined after DNA immunization with Rev-independent expression vectors. A Gag-Pol fusion protein stimulated cytotoxic T lymphocyte and antibody responses to Gag and Pol, while a Gag-Pol pseudoparticle did not elicit substantial Pol responses. This fusion protein may be useful for AIDS vaccines.
The development of a cytotoxic T-lymphocyte (CTL) response to viruses is often crucial to the outcome of infections. Lysis of infected cells prior to the production of progeny virions may limit virus burst size (27), and human immunodeficiency virus (HIV)-specific CD8+ CTLs have been shown to be important in viral clearance and in the control of initial HIV type 1 (HIV-1) spread (1, 11). CTL responses specific to HIV also contribute to reduction in viral load during acute and asymptomatic infection (10, 14) and may be involved in protection against the establishment of persistent HIV infections (19, 20), thus representing a desirable response in an HIV-1 vaccine. Early studies of DNA vaccination against HIV in mice required the inclusion of Rev in their expression vectors (13, 16, 25), but modification of INS has been shown to facilitate Rev-independent expression of HIV-1 Gag (18, 29), allowing detectable humoral and CTL responses against this protein (18). These modified HIV-1 Gag genes produced virus-like particles of the expected density and morphology and induced an immune response to HIV-1 Gag after DNA immunization in mice (29). We prepared synthetic HIV-1 clade B Gag and Pol expression vectors that are based on human (h) codon usage. These vectors encode hGag-Pol and its derivatives, hGag, hPol, and an hGag-Pol fusion protein. The synthetic Gag-Pol genes show little nucleotide homology to those of HIV-1, but the sequences of the associated proteins are the same. Here, the immunogenicities of these different forms of Gag in plasmid expression vectors were compared.
Expression of synthetic HIV-1 clade B Gag and Pol genes.
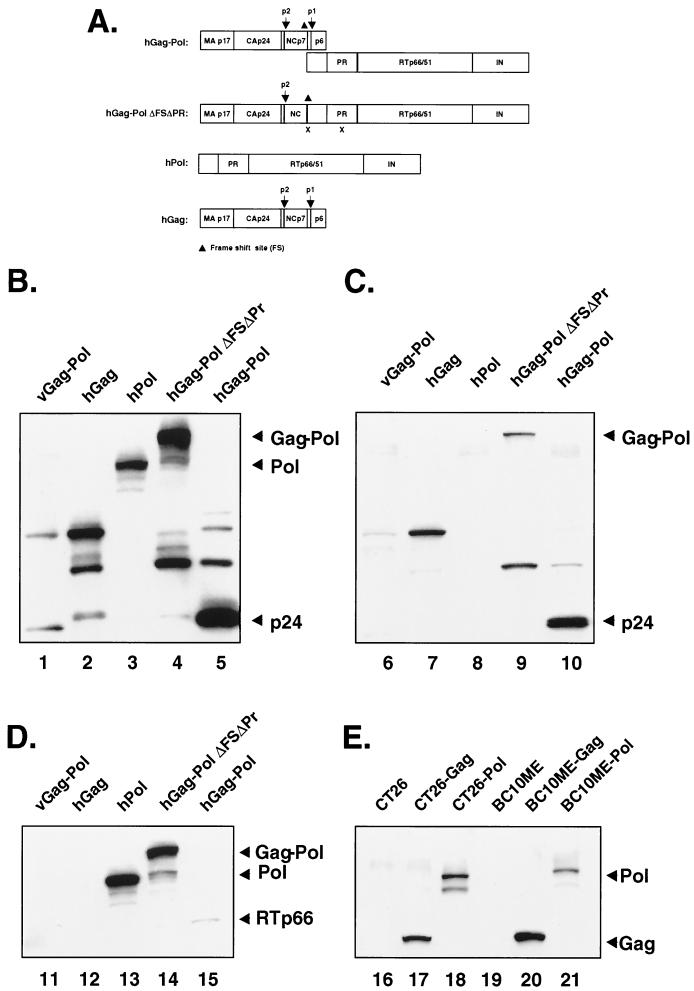
Synthetic HIV-1 Gag and/or Pol expression vectors for hGag-Pol, hGag-PolΔFsΔPr, hPol, and hGag were prepared (Fig. 1A). Gag (amino acids 1 to 432) from HXB2 (GenBank accession no. K03455) and Pol (amino acids 3 to 1003) from NL4-3 (GenBank accession no. M19921) were reverse translated (Genetics Computer Group, Inc., Madison, Wis.) using codons expected for human cells. Eighty-six oligonucleotides of 75 bp with 25 nucleotides of overlap covering 4,325 DNA bp with 5′ SalI and 3′ EcoRI sites were synthesized. hGag-Pol was assembled by PCR with Pwo (Boehringer Mannheim) and Turbo Pfu (Stratagene) high-fidelity DNA polymerase, cloned into SalI-blunted BglII-digested pNGVL-3 (28), and confirmed by DNA sequencing. A 226-bp fragment spanning the frameshift site and the overlapping region of the two reading frames from NL4-3 was retained to allow expression of Gag and Gag-Pol precursor polyproteins. Three additional constructs were derived from the hGag-Pol gene. Five thymidines (Ts) in the frameshift site of the hGag-Pol gene were deleted (ΔFS), and the protease was inactivated by replacing AGG in the protease coding sequence with GGC (R42G) to create hGag-PolΔFSΔPr (8, 12). Codons for 432 amino acids of the NH2 terminal of hGag-Pol were deleted and an ATG start codon was added to create the hPol gene. Codons for 925 amino acids of the COOH terminal of hGag-Pol were deleted to create the hGag gene. pCMVΔ8.2, a kind gift from Inder Verma, expressed viral Gag-Pol (15). To confirm expression, the synthetic or viral Gag-Pol genes were transiently transfected into 293T cells, a human kidney-derived cell line. The expression of Gag precursor proteins from codon-altered vectors was 10- to 100-fold higher than that of viral Gag-Pol (Fig. 1B and C), as determined by quantitative phosphorimaging. When cell lysates were analyzed by immunoblotting with human anti-HIV-1 immunoglobulin G (IgG) (Fig. 1B), monoclonal anti-p24 (Fig. 1C), and rabbit anti-reverse transcriptase (RT) (Fig. 1D), Gag p55, Pol p110, and Gag-Pol p160 precursor proteins were detected in hGag, hPol, and hGag-Pol fusion plasmid-transfected 293T cells, as was expected. Mature virion proteins p24 and RT p66 were detected in the hGag-Pol gene-transfected cells (Fig. 1B to D). This processing might result from activation of intracellular protease(s) by high-level expression of Gag and Gag-Pol (9). Virus-like particles were detected by transmission electron microscopy from the hGag and hGag-Pol gene-transfected cells but not hGag-PolΔFsΔPr or hPol gene-transfected cells (data not shown). Stable expression of HIV-1 Gag and Pol proteins from codon-optimized genes in mouse CT26 and BH10ME cells was also observed (Fig. 1E), and no major differences in antibody reactivity compared to human cells were observed.
FIG. 1.
Schematic representation of HIV-1 Gag-Pol expression constructs and expression in transfected 293T cells and stably transfected CT26 and BC10ME cells. (A) The protein sequences of Gag (amino acids 1 to 432) from HXB2 (GenBank accession no. K03455) and Pol (amino acids 3 to 1003) from NL4-3 (GenBank accession no. M19921) were used to create a synthetic version of hGag-Pol using codons found in human cells. Cell lysates from 293T cells transfected with pCMVΔR8.2 containing the coding sequences for viral Gag-Pol (vGag-Pol) (15), pNGVL-hGag, hPol, hGag-PolΔFSΔPr, and hGag-Pol were separated by sodium dodecyl sulfate–4 to 15% gradient polyacrylamide gel electrophoresis (SDS–4 to 15% PAGE), transferred to nitrocellulose filters, and analyzed by immunoblotting with human anti HIV-1-IgG (B), monoclonal anti-p24 (C), and rabbit anti-RT (D). (E) Cell lysates from CT26 and BC10ME cells stably transduced with either hGag or hPol were analyzed with human anti-HIV-1 IgG. 293T cells were transfected with 10 μg of pCMVdR8.2 plasmid (containing the viral Gag-Pol gene) or 5 μg of pVR1012s (containing the codon-altered genes) in a 10-cm-diameter dish using calcium phosphate (2). Three days after transfection, cell lysates were prepared with radioimmunoprecipitation assay buffer (Boehringer Mannheim), separated by SDS–4 to 15% gradient PAGE, and then transferred onto an Immobilon P membrane (Millipore). Membranes were then incubated with anti-HIV-1 IgG (AIDS Research and Reference Reagent Program, Rockville, Md.), monoclonal anti-p24 (ICN), or rabbit anti-RT (Intracel, Rockville, Md.). Bands were visualized using the ECL Western blotting detection reagent (Amersham Pharmacia Biotech, Piscataway, N.J.), as described by the manufacturer. Expression levels were determined using a phosphorimager. hGag-PolΔFSΔPr was made by modification of the frameshift site and inactivation of protease (see the text). For hPol, 432 amino acids were deleted from the NH2-terminal region of hGag-Pol and an ATG codon was added to the associated coding sequence. hGag was made by deletion of 925 amino acids from the COOH-terminal region of hGag-Pol. hGag-Pol, hGag-PolΔFSΔPr, hPol, and hGag are expressed from the pNGVL-3 vector backbone.
Induction of HIV-1 Gag and Pol CTL responses in mice by DNA vaccination.
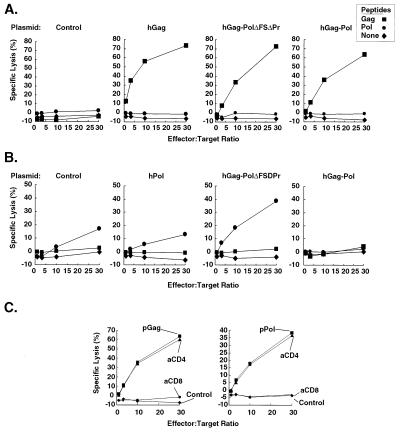
To evaluate the cellular immune response to HIV-1 Gag and Pol proteins, 6- to 8-week-old BALB/c female mice were injected intramuscularly four times at 2-week intervals (200 μl of 0.5-mg/ml DNA in saline). Two weeks after the final vaccination, CTL responses specific to HIV-1 Gag and/or Pol were analyzed using Gag or Pol peptide-pulsed BC10ME cells or mouse fibrosarcoma cell lines derived from B/C-N cells (3) after in vitro sensitization for 1 week. Immunization with hGag, hGag-PolΔFSΔPr, or hGag-Pol genes induced comparable responses specific to Gag (Fig. 2A); however, after immunization with hPol, hGag-PolΔFSΔPr, or hGag-Pol genes, only fusion protein hGag-PolΔFSΔPr, and hPol to a lesser extent, elicited a marked CTL response to Pol (Fig. 2B). To confirm that the specific killing in the CTL assays was induced by CD8+ CTLs, CD4+ or CD8+ cells were depleted from sensitized splenocytes by Dynal beads (Dynal, Inc., Lake Success, N.Y.). Depletion of CD8+ cells abolished the specific lysis in the hGag-PolΔFSΔPr gene-immunized mice, while depletion of CD4 had little effect on lysis (Fig. 2C).
FIG. 2.
Gag- or Pol-specific CTL response mediated by CD8-positive cells in immunized mice. Two weeks after mice were immunized with a control vector, hGag, hPol, hGag-PolΔFSΔPr, and hGag-Pol, splenic cells were harvested and sensitized with naive mouse splenic cells pulsed with Gag or Pol peptides. One week later, effector cells were tested for cytolytic activity in a 5-h 51Cr release assay using 51Cr-labeled BC10ME target cells that were pulsed for 2 h with either HIV-1 Gag peptides (A) or HIV-1 Pol peptides (B). (C) CD4+ or CD8+ lymphocytes were depleted from splenic cells of immunized mice with anti-mouse CD4+ or CD8+ Dynal beads according to the manufacturer's instructions. The peptides used for sensitizing cells are as follows: two from the Gag protein, P17(88–115) (VHQRIEIKDTKEALDKIEEEQNKSKKKA) and p24(62–76) (HQAAMQMLKETINEE), and seven peptides from Pol, P66(175–189) (NPDIVIYQYMDDLYV), P66(179–193) (VIYQYMDDLYVGSDL), P66(183–197) (YMDDLYVGSDLEIGQ), P66(187–201) (LYVGSDLEIGQHRTK), P66(223–237) (KEPPFLWMGYELHPD), P66(227–241) (FLWMGYELHPDKWTV), and P66(367–381) (QLTEAVQKIATESIV). The standard errors were ≤5% in these CTL assays and highly statistically significant.
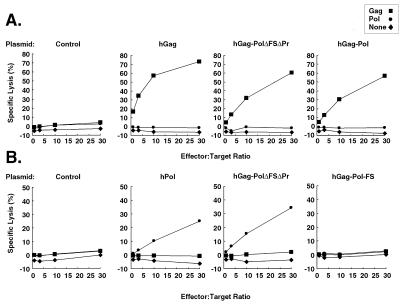
The responses were further analyzed and confirmed with CT26 and BC10ME cell lines stably expressing hGag or hPol. Responses to Gag in the mice immunized with hGag, hGag-PolΔFSΔPr, or hGag-Pol genes were similar to responses to peptide-pulsed targets (Fig. 3A). Mice immunized with the fusion protein hGag-PolΔRTΔPr gene generated the highest specific response to HIV-1 Pol on BC10ME cell lines stably expressing Pol as target cells (Fig. 3B), comparable to the response to hPol alone. These stably transfected cell lines were therefore more sensitive as target cells than peptide-pulsed cells in the Pol CTL assays.
FIG. 3.
Gag- or Pol-specific CTL response mediated by CD8-positive cells in immunized mice using stable expressing cell lines as target cells. Two weeks after immunization in mice, splenic cells were harvested and sensitized with naive mouse splenic cells pulsed with Gag or Pol peptides. One week later, effector cells were tested for cytolytic activity in a 5-h 51Cr release assay using 51Cr-labeled BC10ME target cells expressing either HIV-1 Gag (A) or Pol protein (B). To prepare target cell lines, hGag and hPol genes were individually subcloned into the XhoI and EcoRI sites of retroviral vector pPGS-CITE-Neo. Three plasmids were used to produce recombinant retroviruses containing the hGag or hPol genes (28). The supernatants were collected 48 h after transfection to transduce CT26 and BC10ME (3), which are syngeneic to BALB/c mice, and selected in 0.8 mg of G418/ml 2 days after infection. The positive clones were screened and confirmed by Western blotting and maintained in 10% fetal calf serum-supplemented RPMI (GIBCO-BRL) with 0.5 mg of G418/ml.
Antibody response in the immunized mice.
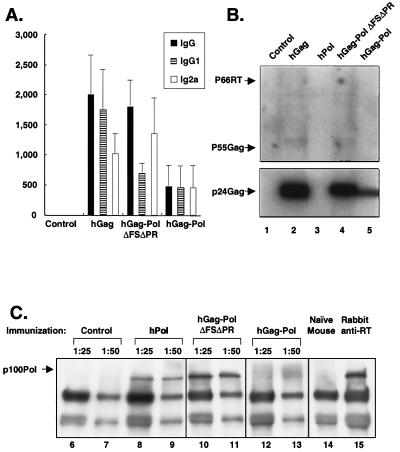
Sera from mice immunized with different plasmids were analyzed with a p24 enzyme-linked immunosorbent assay (ELISA). hGag-immunized mice demonstrated the highest p24 antibody titers (Fig. 4A). Unexpectedly, hGag-Pol virus-like particles elicited the lowest levels of p24 antibody. Subtype analysis of anti-p24 antibodies revealed that IgG2a was the predominant isotype in mice immunized with hGag-PolΔRTΔPr, indicating a possible Th1 response (Fig. 4A). Similar results were observed by Western blotting to p24 Gag with pooled sera, but Pol antibodies were not detected with a commercial Western blotting kit (Fig. 4B). In contrast, antibodies to Pol were detected in mice immunized with hPol and hGag-PolΔFSΔPr by immunoprecipitation and Western blotting (Fig. 4C). Presumably, this assay is more sensitive and better able to detect native conformational epitopes. Though both the hGag-Pol and hGag-Pol fusion proteins elicited similar Gag responses, the hGag-Pol fusion protein was more effective in the stimulation of CTL and antibody responses to Pol.
FIG. 4.
HIV-1 p24 antibody ELISAs, HIV-1 immunoblotting, and immunoprecipitation (IP) Western blotting. (A) An HIV-1 p24 antibody ELISA was performed by coating 96-well plates with 50 μl of purified recombinant HIV-1IIIB p24 antigen at a concentration of 2 μg/ml in phosphate-buffered saline (PBS), pH 7.4; controls were less than 1:100. The anti-p24 ELISA was performed in Immulon 96-well plates (Dynex Technologies, Inc., Chantilly, Va.). The plates were coated with 50 μl of purified recombinant HIV-1IIIB p24 antigen (Intracel) at 2 μg/ml in PBS buffer, pH 7.4 (GIBCO-BRL), with 0.05% sodium azide and washed twice with PBS, and then 200 μl of blocking buffer (containing 3% bovine serum albumin and 0.05% Tween 20) was added to each well and incubated for 2 h. Mouse sera were serially diluted from 1:100 to 1:12,800 in blocking buffer, added to the p24-coated plates, and incubated overnight at 4°C. Plates were then washed four times with PBS (0.05% Tween 20) and incubated with goat anti-mouse IgG (1:2,000 dilution; Roche) or IgG1 (1:4,000) or IgG2a (1:4,000) for 2 h at room temperature. Plates were washed four times, and then ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] peroxidase substrate (100 μl; KPL, Gaithersburg, Md.) was added to each well. The reaction was stopped after 30 min by addition of 1% SDS (100 μl). The plates were read on an ELISA reader at 405 nm, and titers were calculated at a cutoff optical density of 0.4. (B) Strips produced by HIV-1 immunoblotting containing HIV-1 proteins were incubated with pooled mouse sera at a dilution of 1:25. Bands were visualized using the ECL Western blotting detection reagent. Strips containing HIV-1 proteins (Immunetics, Inc., Cambridge, Mass.) were incubated with pooled mouse sera at a dilution of 1:25. Purified human anti-HIV IgG (AIDS Research and Reference Reagent Program) was used as a positive control. Bands were visualized using the ECL Western blotting detection reagent (Amersham Pharmacia Biotech). (C) IP and Western blotting of hPol gene-transfected 293T cell lysates 3 days after transfection with radioimmunoprecipitation assay buffer. The pooled mouse serum was diluted with IP buffer. After 10 μg of the cell lysate containing HIV-1 Pol protein was added, the reaction mixtures were incubated overnight on a rotator at 4°C. The next day, 250 μl of protein G- and A-Sepharose beads (10% [vol/vol] in IP buffer) was added, and the reaction mixtures were incubated on a rotator for 2 h at 4°C. The reaction mixtures were washed four times with IP buffer, resuspended with 30 μl of 1× sample buffer, and then loaded onto an SDS-polyacrylamide gel. The reaction mixtures were transferred to an Immobilon P membrane and then incubated with anti-HIV-1 IgG. Bands were visualized using the ECL Western blotting detection reagent.
In this study, the immunogenicities of different Gag and Pol expression vectors were compared. Particularly, we sought to compare the immune responses to Gag alone, a Gag-Pol fusion protein, and a naturally frameshifted Gag-Pol expression vector in which pseudoparticles were generated. A significant Pol response was elicited only in mice immunized with hGag-PolΔFSΔPr or Pol alone. Because immunization with the hGag-Pol gene failed to induce detectable cellular or humoral responses to the HIV-1 Pol protein, these findings suggest that the Gag-Pol fusion protein induces a range of responses and allows delivery of an immunogen with a larger number of epitopes than the native protein, encoded by a single continuous open reading frame. During viral replication, viral gag-pol produces the Gag precursor protein and the Gag-Pol fusion protein by frameshifting in a 20:1 ratio (26). The deletion of a frameshift site in hGag-PolΔFSΔPr results in production of only the Gag-Pol fusion protein. Expression of Gag-Pol proteins alone in human cells is not adequate to form releasable viral particles because HIV-1 viral assembly requires Gag precursor proteins (17, 23). The ability of hGag-PolΔFsΔPr to elicit strong Gag- and Pol-specific CTL responses in mice may be explained by high-level expression of the Gag-Pol fusion protein and its retention within cells, not normally seen during normal viral replication, which could provide more protein for antigen presentation. Moreover, mutation of viral protease prevents the viral protein from causing intracellular damage and increasing cellular toxicity. Overexpression of this polyprotein is also likely to affect its intracellular localization and transport and may improve antigen presentation.
As early as 1988, CTLs specific for HIV-1 RT were found in blood samples from HIV-1-infected individuals (7, 24). Relatively strong Gag-specific CTL responses have been shown in numerous nonhuman primate and human studies using DNA vaccines or a live recombinant vector containing viral Gag-Pol constructs (4–6, 21, 22), but fewer Pol-specific CTL responses have been reported. The detection of significant CTL responses specific to Pol in our study may be attributed in part to establishment of stable Pol-expressing cell lines, in which codon alteration and inactivation of FS and PR in the Pol gene allow high-level expression of the Pol protein without cellular toxicity. Though it remains possible that hGag-Pol or a combination of hGag and hPol may exert similar effects with appropriate adjuvants or with different prime-boost regimens, the Rev-independent Gag-Pol fusion protein stimulates HIV-1 Gag- and Pol-specific CTL responses as a DNA vaccine in mice. Because it allows more epitopes encoded by one open reading frame to be presented, the Gag-Pol fusion protein may prove useful in the development of AIDS vaccines.
Acknowledgments
We thank Nancy Barrett for preparation of the figures, Bimal Chakrabarti and Judith Stein for advice, and Cherilyn Davis and Ati Tislerics for manuscript preparation.
Yue Huang was partially supported by a postdoctoral fellowship from the Medical Research Council, Canada.
REFERENCES
- 1.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins J L, Patek P Q, Cohn M. Tumorigenicity and lysis by natural killers. J Exp Med. 1981;153:89–106. doi: 10.1084/jem.153.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans T G, Keefer M C, Weinhold K J, Wolff M, Montefiori D, Gorse G J, Graham B S, McElrath M J, Clements-Mann M L, Mulligan M J, Fast P, Walker M C, Excler J L, Duliege A M, Tartaglia J. A canarypox vaccine expressing multiple human immunodeficiency virus type 1 genes given alone or with rgp120 elicits broad and durable CD8+ cytotoxic T lymphocyte responses in seronegative volunteers. J Infect Dis. 1999;180:290–298. doi: 10.1086/314895. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari G, Berend C, Ottinger J, Dodge R, Bartlett J, Toso J, Moody D, Tartaglia J, Cox W I, Paoletti E, Weinhold K J. Replication-defective canarypox (ALVAC) vectors effectively activate anti-human immunodeficiency virus-1 cytotoxic T lymphocytes present in infected patients: implications for antigen-specific immunotherapy. Blood. 1997;90:2406–2416. [PubMed] [Google Scholar]
- 6.Gorse G J, Patel G B, Mandava M D, Belshe R B. Vaccine-induced cytotoxic T lymphocytes against human immunodeficiency virus type 1 using two complementary in vitro stimulation strategies. Vaccine. 1999;18:835–849. doi: 10.1016/s0264-410x(99)00323-0. [DOI] [PubMed] [Google Scholar]
- 7.Hosmalin A, Clerici M, Houghten R, Pendleton C D, Flexner C, Lucey D R, Moss B, Germain R N, Shearer G M, Berzofsky J A. An epitope in human immunodeficiency virus 1 reverse transcriptase recognized by both mouse and human cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1990;87:2344–2348. doi: 10.1073/pnas.87.6.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung M, Patel P, Davis S, Green S R. Importance of ribosomal frameshifting for human immunodeficiency virus type 1 particle assembly and replication. J Virol. 1998;72:4819–4824. doi: 10.1128/jvi.72.6.4819-4824.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karacostas V, Wolffe E J, Nagashima K, Gonda M A, Moss B. Overexpression of the HIV-1 gag-pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology. 1993;193:661–671. doi: 10.1006/viro.1993.1174. [DOI] [PubMed] [Google Scholar]
- 10.Klein R M, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeb D D, Swanstrom R, Everitt L, Manchester M, Stamper S E, Hutchison C A., III Complete mutagenesis of the HIV-1 protease. Nature. 1989;340:397–400. doi: 10.1038/340397a0. [DOI] [PubMed] [Google Scholar]
- 13.Lu S, Santoro J C, Fuller D H, Haynes J R, Robinson H L. Use of DNAs expressing HIV-1 Env and noninfectious HIV-1 particles to raise antibody responses in mice. Virology. 1995;209:147–154. doi: 10.1006/viro.1995.1238. [DOI] [PubMed] [Google Scholar]
- 14.Moss P A H, Rowland-Jones S L, Frodsham P M, McAdam S, Giangrande P, McMichael A J, Bell J I. Persistent high frequency of human immunodeficiency virus-specific cytotoxic T cells in peripheral blood of infected donors. Proc Natl Acad Sci USA. 1995;92:5773–5777. doi: 10.1073/pnas.92.13.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 16.Okuda K, Bukawa H, Hamajima K, Kawamoto S, Sekigawa K, Yamada Y, Tanaka S, Ishi N, Aoki I, Nakamura M. Induction of potent humoral and cell-mediated immune responses following direct injection of DNA encoding the HIV type 1 env and rev gene products. AIDS Res Hum Retrovir. 1995;11:933–943. doi: 10.1089/aid.1995.11.933. [DOI] [PubMed] [Google Scholar]
- 17.Park J, Morrow C D. The nonmyristylated Pr160gag-pol polyprotein of human immunodeficiency virus type 1 interacts with Pr55gag and is incorporated into viruslike particles. J Virol. 1992;66:6304–6313. doi: 10.1128/jvi.66.11.6304-6313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu J-T, Song R, Dettenhofer M, Tian C, August T, Felber B, Pavlakis G, Yu X-F. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J Virol. 1999;73:9145–9152. doi: 10.1128/jvi.73.11.9145-9152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowland-Jones S L, Nixon D F, Aldhous M C, Gotch F, Ariyoshi K, Hallam N, Kroll J S, Froebel K, McMichael A. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 20.Rowland-Jones S L, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 21.Seth A, Ourmanov I, Kuroda M J, Schmitz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seth A, Ourmanov I, Schmitz J E, Kuroda M J, Lifton M A, Nickerson C E, Wyatt L, Carroll M, Moss B, Venzon D, Letvin N L, Hirsch V M. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J Virol. 2000;74:2502–2509. doi: 10.1128/jvi.74.6.2502-2509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith A J, Srinivasakumar N, Hammarskjold M L, Rekosh D. Requirements for incorporation of Pr160gag-pol from human immunodeficiency virus type 1 into virus-like particles. J Virol. 1993;67:2266–2275. doi: 10.1128/jvi.67.4.2266-2275.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker B D, Flexner C, Paradis T J, Fuller T C, Hirsch M S, Schooley R T, Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988;240:64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Ugen K E, Srikantan V, Agadjanyan M G, Dang K, Refaeli Y, Sato A I, Boyer J, Williams W V, Weiner D B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson W, Braddock M, Adams S E, Rathjen P D, Kingsman S M, Kingsman A J. HIV expression strategies: ribosomal frameshifting is directed by a short sequence in both mammalian and yeast systems. Cell. 1988;55:1159–1169. doi: 10.1016/0092-8674(88)90260-7. [DOI] [PubMed] [Google Scholar]
- 27.Yang O, Kalams S, Rosenzweig M, Trocha A, Jones N, Koziel M, Walker B, Johnson R P. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Delgado R, Xu L, Todd R F, Nabel E G, Sanchez A, Nabel G J. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- 29.zur Megede J, Chen M-C, Doe B, Schaefer M, Greer C E, Selby M, Otten G R, Barnett S W. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J Virol. 2000;74:2628–2635. doi: 10.1128/jvi.74.6.2628-2635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]