Abstract
G protein-coupled receptors are regulated by ligand stimulation, endocytosis, degradation of recycling to the cell surface. Little information is available on the molecular mechanisms underlying G protein-coupled receptors recycling. We have investigated recycling of the G protein-coupled thyroid stimulating hormone receptor (TSHR) and found that it relies on hScrib, a membrane-associated PDZ protein. hScrib directly binds to TSHR, inhibits basal receptor endocytosis and promotes recycling, and thus TSHR signalling, at the cell membrane. We previously demonstrated that hScrib is associated with a βPIX–GIT1 complex comprised of a guanine nucleotide exchange factor and a GTPase-activating protein for ADP ribosylation factors that is involved in vesicle trafficking. We used dominant-negative constructs and small interfering RNA to show that TSHR recycling is regulated by the interaction between hScrib and βPIX, and by the activity of GIT1. In addition, ARF6, a major target for GIT1, is activated during TSH stimulation of HEK293 and FRTL-5 thyroid cells, and plays a key role in TSHR recycling. Thus, we have uncovered an hScrib–βPIX–GIT1–ARF6 pathway devoted to TSHR trafficking and function.
Keywords: ARF6, PDZ, recycling, Scrib, TSH receptor
Introduction
G protein-coupled receptors (GPCRs) play central roles in multiple physiological and pathological processes (Pierce et al, 2002). GPCRs are activated upon ligand binding, endocytosed, and may return to the cell surface via a recycling pathway that promotes receptor resensitisation. Alternatively, endocytosed GPCRs may be redirected to lysosomes where they are degraded. The latter process leads to long-term attenuation of receptor signalling, a process known as downregulation. Although the endocytosis of these receptors has been well studied, little information is available on the sorting pathways and, in particular, on the molecular mechanisms involved in GPCRs recycling to the plasma membrane.
The GPCR thyrotropin (TSH) receptor (TSHR) plays a key role in the physiological control of thyroid growth and function (Vassart and Dumont, 1992). This receptor belongs to a GPCRs subgroup that also includes the luteinising hormone (LH) and follicle stimulating hormone (FSH) receptors (Misrahi and Milgrom, 1997). Hormone binding leads to receptor activation and cAMP production via the adenylate cyclase pathway (Vassart and Dumont, 1992).
The localisation and density of TSHRs on thyroid membranes are critical for TSH responsiveness and thyroid function. Studies of TSHR trafficking in various thyroid and nonthyroid cells have shown that the receptor is rapidly internalised by clathrin-coated pits upon stimulation by TSH (Baratti-Elbaz et al, 1999; Singh et al, 2004). The human TSHR displays poor downregulation in contrast to the porcine LH receptor (pLHR). Indeed, most endocytosed TSHRs recycle back to the plasma membrane by an unknown mechanism, ready for a new round of activation by TSH.
The TSHR has a carboxy-terminal TVL (T for threonine, V for valine and L for leucine) motif, a sequence consistent with a canonical binding site for class I PDZ (PSD-95/Discs large/ZO1) domains. PDZ domain proteins are cytoplasmic scaffolding adaptors that play specialised roles in the transport and the localisation of receptors, and in the assembly of supramolecular signalling complexes (Nourry et al, 2003). Recent data have highlighted the important role played by the PDZ protein hScrib (named Scribble in Drosophila) in the development of vertebrates and invertebrates (Bilder and Perrimon, 2000; Murdoch et al, 2003). hScrib belongs to the LAP (LRR And PDZ) protein family, all members of which have 16 amino-terminal leucine-rich repeats (LRR) and none to four copies of carboxy-terminal PDZ domains. hScrib was originally identified as a partner for the oncogenic human papilloma virus-16 E6 protein (Nakagawa and Huibregtse, 2000). We have recently shown that hScrib is associated with the βPIX–GIT1 cytoplasmic complex and that this interaction plays an important role in regulated exocytosis in neuroendocrine cells (Audebert et al, 2004). βPIX (PAK-interacting exchange factor) is a guanine nucleotide exchange factor for RHO proteins, whereas GIT1 (G protein-coupled receptor kinase-interacting protein 1) has a GAP (GTPase-activating protein) domain specific for the ARF1 and ARF6 small GTPases. While hScrib is anchored at the basolateral membrane by its LRR domain (Legouis et al, 2003), the identity of the cell-surface receptors binding to hScrib remains unknown.
We provide here evidence on the direct interaction between TSHR and hScrib, and on the function of the hScrib–βPIX–GIT1–ARF6 pathway in the regulation of TSHR recycling and signalling.
Results
The PDZ motif of the TSHR is involved in receptor trafficking and interacts with hScrib
PDZ domain binding motifs are involved in the regulation of protein trafficking (Nourry et al, 2003). We therefore compared the intracellular trafficking of TSHR and TSHR-ΔTVL, a TSH receptor lacking the PDZ motif, in HEK293 cell lines stably expressing these receptors. Cell-surface expression of the TSHR was quantified in cells that had been incubated at 4°C with the biotinylated R5T-34 monoclonal antibody as described previously (Baratti-Elbaz et al, 1999; Quellari et al, 2003). Only ∼10% of the wild-type receptors were internalised in the absence of TSH (Figure 1) (Baratti-Elbaz et al, 1999). In the presence of TSH, ∼40% of the receptors were internalised after 40 min. Approximately 95% of the receptors were recycled back to the cell surface after 120 min (Figure 1) (Baratti-Elbaz et al, 1999). The kinetics of TSHR recycling is very similar to that reported for other GPCRs expressed in HEK293 cells (Innamorati et al, 2001). In contrast, the TSHR-ΔTVL mutant was constitutively internalised in the presence or absence of the hormone, and no TSHR-ΔTVL recycling was observed, even after 120 min at 37°C with TSH (Figure 1). TSHR-ΔTVL trafficking was similar to that observed for the wild-type TSHR in the presence of TSH and monensin, a recycling inhibitor, reinforcing the idea that the TVL motif is required for the proper recycling of the TSHR.
Figure 1.

The PDZ motif of the TSHR regulates receptor trafficking. Receptor expression at the cell surface in HEK293-TSHR (◊, ⧫, •) or HEK293-TSHR-ΔTVL (▪, ▴) cells. Cells were incubated with the biotinylated R5T-34 monoclonal antibody. Cell-surface receptor–antibody complexes were quantified as described previously (Baratti-Elbaz et al, 1999). TSH (10 IU/l) and monensin (40 μM) were also added to the incubation medium as indicated. Bars show the s.d. of duplicate experiments.
We next used two-hybrid assay in yeast to test the interaction between the TSHR intracellular domain (Figure 2A) and class I PDZ domain proteins, including hScrib (Figure 2B). We found that the full-length hScrib (hScrib) or the four PDZ domains of hScrib (hScrib-PDZ) interact with the whole TSHR intracellular domain (TSHR-IC1, residues 685–764) or its C-terminal part (TSHR-IC2, residues 747–764) (Figure 2C). No interaction was detected for hScrib lacking the PDZ domains (hScrib-ΔPDZ; Figure 2C). Neither hScrib nor hScrib-PDZ interacts with the TSHR lacking the TVL motif (TSHR-IC1-ΔTVL and TSHR-IC2-ΔTVL) (Figure 2C). No binding occurred between hScrib and the intracellular domains of the LH (LHR-IC) or FSH (FSHR-IC) receptors. Furthermore, we did not detect any interaction between the TSHR and the class I Erbin or PSD95 PDZ domains (Figure 2C). Finally, we demonstrated that the first and third PDZ domains are required for the interaction between hScrib and the TSHR (Figure 2D).
Figure 2.
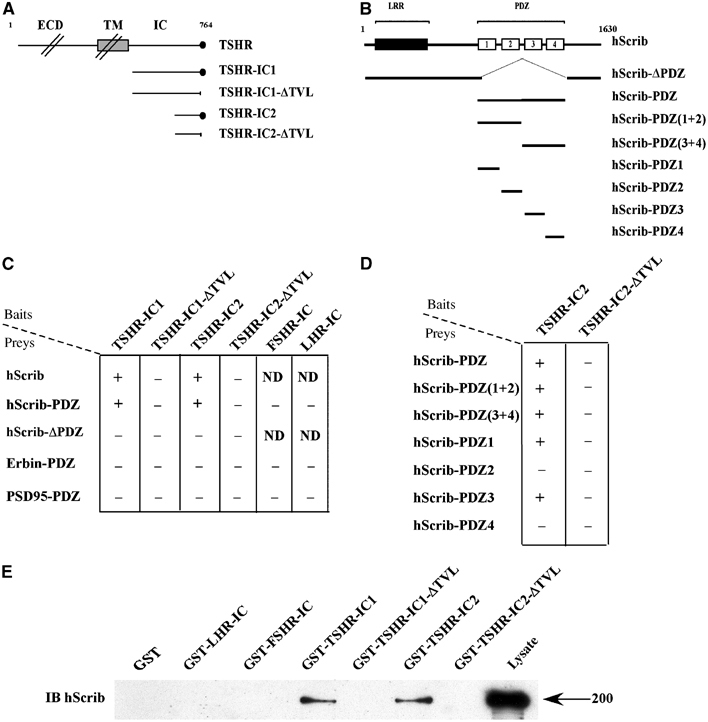
Direct interaction between the PDZ domain of hScrib and the C-terminus of the human TSHR. (A) Schematic representation of the human TSHR constructs used as baits in the yeast two- hybrid assay. ECD: extracellular domain; TM: transmembrane domain; IC: intracellular domain; •: PDZ motif of the TSHR. (B) Schematic representation of the hScrib constructs used as preys. LRR: leucine-rich repeats; PDZ: four PDZ domains (1–4) of hScrib. (C, D) PDZ-dependent interaction between hScrib and TSHR detected by yeast two-hybrid analysis. Interactions were revealed by a growth on −HIS plates and a positive β-galactosidase activity (+if positive, − if negative). ND: nondetermined. (E) Interaction between endogenous hScrib and the intracellular domain of the TSHR by GST pull-down assay. HEK293 cell extracts were incubated with the indicated GST fusion proteins. Bound proteins were revealed by immunoblotting (IB) with the anti-hScrib antibody. The position of the 200 kDa molecular weight marker is shown.
This interaction was confirmed using glutathione-S-transferase (GST) pull-down assays (Figure 2E). The assays were performed using HEK293 cell extracts containing endogenous hScrib (Figure 2E, lysate). We found that hScrib was retained by the GST-TSHR-IC1 and GST-TSHR-IC2 fusion proteins but not by the truncated proteins (GST-TSHR-IC1-ΔTVL and GST-TSHR-IC2-ΔTVL) (Figure 2E). No interaction was detected between hScrib and the control proteins (GST alone, GST-LHR-IC or GST-FSHR-IC), demonstrating that the interaction between the PDZ domains of hScrib and the C-terminus of the TSHR is specific.
Binding of hScrib to the TSHR is detected in HEK293 and FRTL-5 thyroid cells
Co-immunoprecipitation assays were performed using HEK293 cells transiently transfected with expression vectors encoding either TSHR or TSHR-ΔTVL. In Western blot, the T5U-317 antibody recognises two monomeric precursors (∼95 and ∼120 kDa) and an ∼60 kDa mature extracellular α subunit of the cleaved heterodimeric TSHR (Misrahi and Milgrom, 1997) (Figure 3A, IB TSHR). hScrib co-immunoprecipitates with the TSHR in the absence of TSH (Figure 3A, lane 2, IB hScrib). The amount of co-immunoprecipitated hScrib was ∼2.5±0.5-fold higher (mean±s.d., n=3) when cells were treated with TSH (Figure 3A, lane 3, IB hScrib, and Figure 3B). hScrib did not co-immunoprecipitate with TSHR-ΔTVL in the presence or absence of TSH (Figure 3A, lanes 5 and 6, IB hScrib) or with the FSHR (Figure 3A, lanes 8 and 9, IB hScrib). In Western blot, the FSHR-323 antibody recognises an ∼62 kDa precursor and the ∼80 kDa mature FSH receptor (Figure 3A, IB FSHR) (Nechamen and Dias, 2003). To demonstrate the existence of an endogenous complex containing TSHR and hScrib, we performed co-immunoprecipitations using protein extracts of the FRTL-5 rat thyroid cell line, an untransformed and TSH-dependent cell line that expresses several features of thyroid differentiation (Ambesi-Impiombato et al, 1980). The endogenous interaction between TSHR and hScrib was observed in FRTL-5 cells, confirming our results in transfected cells (Figure 3C, lane 2, IB hScrib). In contrast to HEK293 cells, we observed no significant increase of TSHR-hScrib co-immunoprecipitation in FRTL-5 cells upon addition of TSH (n=3) (Figure 3C, lane 3, IB hScrib), suggesting that this effect may be cell context-dependent.
Figure 3.

Binding of hScrib to the TSHR in HEK293 and FRTL-5 thyroid cells. (A) HEK293 cells were transiently transfected with expression vectors encoding TSHR, TSHR-ΔTVL or FSHR. Immunoprecipitations (IP) were performed using the anti-TSHR (T5U-51) and anti-FSHR (FSHR-323) monoclonal antibodies. Western blots (IB) were carried out using the following antibodies: polyclonal anti-hScrib (IB hScrib); monoclonal anti-TSHR, T5U-317 (IB TSHR); or monoclonal anti-FSHR, FSHR-323 (IB FSHR). Lanes 1, 4, 7: control lysates; lanes 2, 5, 8: cells incubated in the absence of hormone; lanes 3, 6, 9: cells stimulated by 10 IU/l of TSH or 3 IU/l of FSH. Black arrows indicate molecular weight markers (kDa). (B) The increased co-immunoprecipitation between TSHR and hScrib in cells stimulated by TSH was quantified (mean±s.d., n=3) using the Scion Image software. (C) FRTL-5 cells were lysed, and immunoprecipitation and Western blots were performed as described in (A). Lane 1: control lysate; lane 2: cells incubated in the absence of hormone; lane 3: cells incubated with 10 IU/l of TSH.
hScrib is targeted to the basolateral membrane of MDCK and thyroid cells and colocalises with the TSHR
The TSHR is present at the basolateral membrane of thyroid cells (Loosfelt et al, 1992) as well as of epithelial Madin-Darby canine kidney (MDCK) cells that ectopically express the receptor (Beau et al, 2004). hScrib is a membrane-associated protein also retained at the basolateral membrane of polarised epithelial cells (Dow et al, 2003). Accordingly, hScrib and the TSHR colocalised at the basolateral membrane domain of polarised MDCK cells (Figure 4A). Immunocytochemical and immunofluorescence confocal analysis also revealed that hScrib was restricted to the basolateral membrane of rat thyrocytes and colocalised with the TSHR (Figure 4B).
Figure 4.
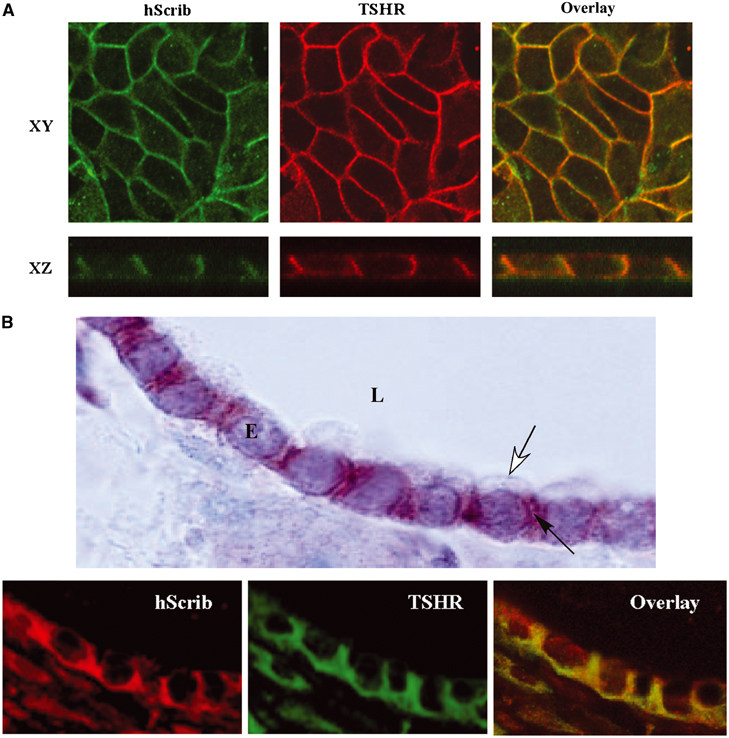
Localisation of hScrib in MDCK and thyroid cells. (A) Confocal microscopy analysis of the distribution of the ectopically expressed TSHR (red) and endogenous hScrib (green) in polarised MDCK cells. XY (upper panel, taken in the plane of the lateral membrane domain) and XZ (lower panel): sections of a selected area of labelled cells. (B) Immunocytochemistry and confocal analysis on thyroid glands. In the top panel is shown an immunocytochemical localisation of hScrib at the lateral membrane (black arrow) of the epithelial cells (E) and its exclusion of the apical membrane (white arrow). L: glandular lumen. Magnification: × 100. Lower panel: immunofluorescence and confocal microscopy analysis showing colocalisation between TSHR and hScrib in rat thyroid cells.
hScrib inhibits basal TSHR internalisation and promotes recycling of the receptor
The role of hScrib in TSHR trafficking was studied in HEK293-TSHR cells transfected with a dominant-negative form of hScrib (hScrib-PDZ) containing the four PDZ domains fused to the green fluorescent protein (GFP) (Audebert et al, 2004). As shown in Figure 5A, TSHR was constitutively internalised in these cells and no receptor recycling was observed (Figure 5A). Addition of TSH did not modify TSHR trafficking. In contrast, expression of hScrib-ΔPDZ, a mutant protein unable to interact with the TSHR, did not influence TSHR trafficking.
Figure 5.
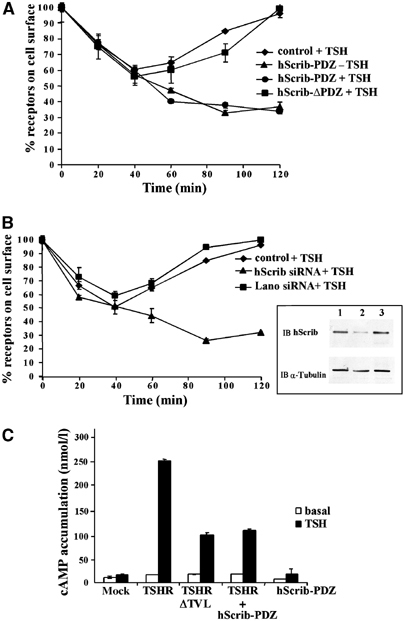
hScrib inhibits basal TSHR internalisation and promotes TSHR recycling and signalling at the plasma membrane. (A) TSHR trafficking was measured in HEK293-TSHR cells (control, ♦) expressing the GFP protein fused to the four PDZ domains of hScrib (hScrib-PDZ, •, ▴) or to an hScrib mutant lacking the PDZ domains (hScrib-ΔPDZ, ▪). (B) TSHR trafficking was measured in HEK293-TSHR cells (control, ♦) transfected with hScrib siRNA (▴) or Lano siRNA (▪). Inset: Western blot analysis using anti-hScrib antibody (IB hScrib) on protein extracts of mock transfection (lane 1), transfection with hScrib siRNA (lane 2) or transfection with Lano siRNA (lane 3; control). The blot was reprobed with an anti-α-tubulin antibody (IB α-tubulin) to evaluate sample loading. In (A) and (B), cell-surface receptor–antibody complexes were quantified as described in Figure 1. Bars show the s.d. of duplicate experiments. (C) The effect of TSHR trafficking on signal transduction. HEK293 cells were transiently transfected with the pSG5 vector (mock), pSG5-TSHR (TSHR), pSG5-TSHR-ΔTVL (TSHR-ΔTVL), GFP-hScrib-PDZ (hScrib-PDZ), or cotransfected with pSG5-TSHR and GFP-hScrib-PDZ (TSHR+hScrib-PDZ). cAMP concentrations were measured before (white bars) and after stimulation with 3 IU/l of TSH (black bars). The intracellular cAMP concentrations are expressed in nmol/l.
We next used a 21-bp small interfering RNA (siRNA) designed to decrease the expression of hScrib. HEK293-TSHR cells transfected with hScrib siRNA expressed low amounts of hScrib protein at 72 h post-transfection (Figure 5B). In the absence of TSH, the decrease of hScrib expression led to the constitutive internalisation of TSHR (data not shown). The recycling of TSHR was also suppressed upon addition of TSH. TSHR trafficking was not affected by the expression of the Lano siRNA control (Figure 5B).
These results show that in the absence of TSH, hScrib inhibits basal receptor internalisation and stabilises TSHR expression at the plasma membrane. After hormone stimulation, hScrib is required for redirecting the internalised TSHR into the recycling pathway.
hScrib controls TSHR signalling at the plasma membrane
Given the impact of hScrib on TSHR expression at the cell surface, we examined hormone-induced cAMP accumulation in cells when the interaction between hScrib and TSHR is prevented. cAMP concentrations were measured in HEK293 transfectants exposed to cycloheximide, an inhibitor of protein synthesis, and then stimulated with 3 IU/l of TSH for 20 min before being lysed. cAMP levels increased ∼15-fold in cells expressing TSHR after incubation with TSH (Figure 5C). In contrast, cells expressing TSHR-ΔTVL or coexpressing TSHR and GFP-hScrib-PDZ mutant showed an ∼60% decrease of hormone-induced cAMP accumulation when compared to cells expressing TSHR alone (Figure 5C). The decrease of cAMP concentration is probably an effect of the downregulation of cell-surface TSHR in these cells (Figures 1 and 5A). These results show that the interaction between hScrib and TSHR is important to promote receptor signal transduction at the plasma membrane.
A TSHR lacking the PDZ motif is targeted to late endosomal and lysosomal compartments
The impairment of TSHR recycling by disruption of the interaction with hScrib suggests that the internalised receptor is sequestered in intracellular compartments or redirected to the lysosomes. We compared the intracellular routes taken by the wild-type and mutated TSHR by immunofluorescence and confocal analysis. Receptor subcellular localisation was followed in HEK293 cells expressing TSHR or TSHR-ΔTVL using the following intracellular compartment markers: early endosomal antigen 1 (EEA-1) for the early endosomes, GTPase Rab11 for the perinuclear recycling compartment and lysosome-associated membrane protein 2 (LAMP2) for the late endosome and lysosome. TSHR and TSHR-ΔTVL were initially present at the plasma membrane (data not shown), and were internalised and transported to EEA1-positive vesicles after 30 min of incubation at 37°C with TSH (Figure 6A and B). After 60 min with TSH, the TSHR was detected in the perinuclear recycling compartment where it partially colocalised with Rab11. In contrast, the TSHR-ΔTVL did not colocalise with Rab11 and accumulated extensively in the LAMP2-positive vesicles. Colocalisation with LAMP2 was minimal in the case of the TSHR. Cell-surface biotinylation experiments were carried out and the proteolysis rate of TSHR and TSHR-ΔTVL was quantified. As shown in Figure 7A, the ∼60 kDa α subunit of the mature heterodimeric receptor was heavily biotinylated. Overexposure of the autoradiograms also allows the detection of the mature monomeric precursor (not shown). The TSHR-ΔTVL receptor was significantly proteolysed after incubation of 4 h at 37°C, whereas the expression of the wild-type receptor remained stable during the same period of time (Figure 7B). These results are consistent with the hypothesis that the C-terminal PDZ binding motif of the TSHR plays a role in the sorting of internalised receptors to the recycling compartment. Receptors lacking association with hScrib are misrouted to the late endosomal and the lysosomal pathway where they are degraded.
Figure 6.

The TSHR lacking the PDZ target motif is targeted to the late endosomal and lysosomal compartment. Intracellular trafficking of TSHR (A) and TSHR-ΔTVL (B) receptors in HEK293 cells was monitored by confocal microscopy. The receptors were detected using the R5T-34 monoclonal antibody. Labelled cells were incubated at 37°C for 30 min and stained with anti-EEA1 antibody, or for 60 min and stained with anti-Rab11 and anti-LAMP2 antibodies.
Figure 7.

The TSHR-ΔTVL receptor is degraded more rapidly than the wild-type TSHR. (A) Cell-surface biotinylation of TSHR and TSHR-ΔTVL was performed in stably transfected HEK293 cells. After incubation at 37°C for the indicated times, biotinylated receptors were retained on streptavidin-agarose and revealed with the T5U-317 monoclonal antibody. PD: pull-down; IB: immunoblot. A representative immunoblot is shown from three independent experiments. The size of the molecular weight markers is indicated on the right in kDa. (B) Biotinylated TSHR was quantified using the Scion Image software. Values are expressed as the percentage of the initial value (mean±s.d., n=3 for TSHR, n=4 for TSHR-ΔTVL).
TSHR recycling requires a functional hScrib–βPIX–GIT-1 protein complex
We have previously described an hScrib–βPIX–GIT1 complex in mammalian cells (Audebert et al, 2004). βPIX is an exchange factor for Cdc42 and Rac small GTPases, whereas GIT1 contains an active ARF (ADP ribosylation factor) GAP domain (Premont et al, 2004). βPIX and GIT1 are strongly associated through a direct protein–protein interaction and act as both enzymes and scaffold proteins (Premont et al, 2004). We hypothesised that βPIX and GIT1 are involved in TSHR recycling. Overexpression of Flag-βPIX did not affect TSHR trafficking (Figure 8A). Expression of a mutant unable to interact with hScrib (Flag-βPIX-ΔTNL) (Audebert et al, 2004) did not increase the basal levels of receptor internalisation (Figure 8A), arguing against a role of βPIX in receptor retention at the plasma membrane. In contrast, TSHR recycling was impaired in cells treated with TSH. The exchange activity of βPIX for Rac or Cdc42 was not required for TSHR recycling since overexpression of the βPIX L238R-L239S mutant that lacks enzymatic activity (Lee et al, 2001) did not affect hormone-activated TSHR recycling (Supplementary Figure S1). We confirmed the role of βPIX using βPIX siRNA, which almost completely suppressed βPIX expression in HEK293-TSHR cells (Figure 8B, inset) and abolished receptor recycling (Figure 8B). Our data demonstrate that βPIX plays a role in TSHR recycling and suggest that its interaction with hScrib, but not its enzymatic activity, is required for TSHR recycling.
Figure 8.

The hScrib–βPIX–GIT1 complex is necessary for TSHR recycling. In (A–C), cell-surface receptor–antibody complexes were quantified as described in Figure 1. Bars show the s.d. of duplicate experiments. (A) HEK293-TSHR cells (control, ◊) were transfected with an expression vector encoding βPIX (•) or a βPIX-ΔTNL mutant (○). TSH (10 IU/l) was added to the medium for the control (⧫) or the cells expressing βPIX-ΔTNL (□). (B) HEK293-TSHR cells (control, ⧫) were transfected with βPIX siRNA (▪) or Lano siRNA (▴). Inset: Western blot analysis using the anti-βPIX antibody (IB βPIX) on cellular extracts of mock transfection (lane 1), transfection with βPIX siRNA (lane 2) or transfection with Lano siRNA (lane 3; control). The blot was reprobed with an anti-α-tubulin antibody (IB α-tubulin) to evaluate sample loading. (C) HEK293-TSHR cells (control, ⧫) were transfected with expression vectors encoding GIT1 (•), ARNO (▪) or mutant proteins (GIT1 R39A, ○; ARNO E156K, ▴). TSH (10 IU/l) was added to the medium.
We then examined the role of GIT1 in receptor trafficking. GIT1 has both ARF6 and ARF1-GAP activity. ARF6 and ARF1 are small GTPases involved in vesicle trafficking whose activity is positively regulated by GEF proteins including ARNO (ARF nucleotide binding site opener) (Chavrier and Goud, 1999). Overexpression of GIT1 promoting ARF-GDP loading suppressed postendocytotic recycling of the TSHR (Figure 8C). The ARF-GAP activity of GIT1 is necessary for this inhibition because overexpression of GIT1 R39A, a mutant devoid of GAP activity (N Vitale and RT Premont, unpublished data), failed to do so (Figure 8C).
Overexpression of ARNO did not modify receptor trafficking, whereas overexpression of ARNO E156K, a catalytically inactive mutant (Frank et al, 1998), totally suppressed receptor recycling (Figure 8C). These results suggest that ARNO promotes TSHR recycling and that the guanine nucleotide exchange activity of ARNO is necessary for this process. Taken together, our data suggest that the recycling of TSHR not only requires hScrib, but also the proteins that modulate the activity of the ARF small GTPases, such as GAP (GIT1) and GEF (ARNO) proteins.
TSHR recycling requires the activation of ARF6
GIT1 and ARNO regulate the activity of ARF6 and ARF1. To determine which of these small G proteins is implicated in TSHR recycling, we overexpressed GDP-bound forms of ARF6, ARF6 T27N (D'Souza-Schorey et al, 1995), and of ARF1, ARF1 T31N (Peters et al, 1995), in HEK293-TSHR cells. Overexpression of ARF6 T27N, but not of wild-type ARF6 or ARF1 T31N, strongly inhibited receptor recycling (Figure 9A). Interestingly, activated ARF1 is mainly found on intracellular membranes and in the Golgi apparatus, whereas activated ARF6 is mainly localised at the plasma membrane (Chavrier and Goud, 1999). These results suggest that activation of ARF6 regulates the recycling of the TSHR after hormone-induced internalisation.
Figure 9.

The TSHR recycling pathway requires the activation of ARF6. (A) HEK293-TSHR cells (control, ⧫) were transfected with expression vectors encoding ARF6 (▴), a dominant-negative form of ARF6 (ARF6 T27N, ▪) or a dominant-negative form of ARF1 (ARF1 T31N, •). Quantification of cell-surface receptor–antibody complexes was determined in the presence of TSH, as described in Figure 1. Bars show the s.d. of duplicate experiments. (B) Activation of ARF6 is TSH-dependent and requires the PDZ motif of the TSHR. The amount of activated GTP-bound ARF6 was assessed at the indicated time points using a GST-MT2 pull-down assay on extracts of HEK293-TSHR (left panel, upper blot) and HEK293-TSHR-ΔTVL (left panel, lower blot) cells stimulated by TSH. Bound proteins were detected by immunoblotting with anti-ARF6 antibodies and quantification was carried out by densitometric analysis (right panel). Activation is expressed in arbitrary units for cells expressing TSHR (black bars) and TSHR-ΔTVL (white bars) and is plotted against the time of TSH exposure (min). The results shown are the means±s.d. (TSHR: n=4; TSHR-ΔTVL: n=2). (C) The amount of activated GTP-bound ARF6 (upper panel) was assessed as in (B) at 120 min in the absence (−) or in the presence (+) of TSH in HEK293-TSHR cells transfected with no siRNA (control), or with hScrib or Lano siRNA. Densitometric analysis (lower panel) was carried out as in (B). Black bars: +TSH; white bars: −TSH. The results shown are the means±s.d. (n=2).
To determine whether ARF6 is activated upon receptor stimulation by TSH, the amount of GTP-bound ARF6 produced in the HEK293-TSHR cells was quantified using a GST pull-down assay. A GST-MT2 fusion protein previously shown to bind GTP-bound ARF6 was used in this assay (Schweitzer and D'Souza-Schorey, 2002). The level of GTP-bound ARF6 increased markedly after stimulation by TSH, the maximal response occurring after 120 min of stimulation (Figure 9B). Densitometric analysis of Western blots revealed an ∼15-fold increase of the levels of activated ARF6 after 120 min of TSH treatment (Figure 9B). In contrast, no increase of GTP-bound ARF6 was detected in HEK293-TSHR-ΔTVL cells after TSH treatment, indicating that the interaction between hScrib and TSHR is critical for TSH-dependent ARF6 activation (Figure 9B). Furthermore, decreased amounts of GTP-bound ARF6 were recovered in HEK293-TSHR cells after transfection of hScrib siRNA and treatment with TSH when compared to controls (Figure 9C). The TSH-dependent activation of ARF6 was also confirmed in FRTL-5 thyroid cells. A 6.8±0.6-fold increase of the amount of GTP-bound ARF6 was observed after 120 min of TSH treatment (n=2) (Figure 10A). Transfection of hScrib siRNA yielded an 86±6% decrease of the expression of hScrib (n=2) (Figure 10A, inset) and a 71.3±12.4% reduction of the amount of ARF6-GTP (n=2) (Figure 10A). ARF6 activation is thus promoted by TSH, and hScrib appears to be a key element in this process (Figure 10B).
Figure 10.
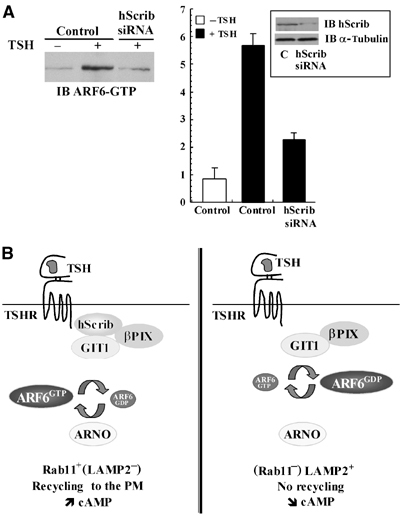
hScrib regulates ARF6 activity in FRTL-5 thyroid cells. (A) FRTL-5 cells were transfected with a vector encoding hScrib siRNA or a control vector. The amount of activated GTP-bound ARF6 (left panel) was assessed as in Figure 9 at 120 min in the absence (−TSH) or in the presence (+TSH, 120 min) of TSH. Densitometric analysis (right panel) of GTP-bound ARF6 was carried out as in Figure 9. The results shown are the means±s.d. (n=2). The inset shows Western blots performed with extracts from control (C) or hScrib siRNA-transfected FRTL-5 cells probed with anti-hScrib or anti-α-tubulin antibodies. (B) Model of the molecular machinery implicated in TSHR recycling and signalling. Upon TSH stimulation and binding to hScrib, the TSHR is targeted to the Rab11+ (LAMP2−) recycling compartment. TSH also promotes GTP loading of ARF6 and cAMP production (left panel). In the absence of binding to hScrib, TSHR-ΔTVL is directed to a LAMP2+ (Rab11−) late endosomal and lysosomal compartment and is downregulated. PM: plasma membrane.
Discussion
hScrib is expressed at the basolateral membrane of thyroid follicular cells, a site where the TSHR is also located (Loosfelt et al, 1992). Deletion of the C-terminal PDZ binding site of the TSHR does not alter the polarised targeting of the TSHR (Beau et al, 2004). We demonstrate that this region instead regulates TSHR internalisation and recycling to the cell surface, and that hScrib plays a key role in the intracellular trafficking of this GPCR. hScrib inhibits basal TSHR internalisation in the absence of TSH and promotes recycling of the internalised receptor upon hormone stimulation. Because the number of receptors recycled back to plasma membrane is a key element for TSH responsiveness and TSHR signalling, disruption of TSHR recycling by the expression of hScrib dominant-negative constructs impinges on the accumulation of cAMP (Figure 10B).
Previous works have already shown that PDZ proteins play a role in GPCR endocytosis. Indeed, PSD-95 inhibits β1-adrenergic receptor (Xiang and Kobilka, 2003), N-methyl-D-aspartate receptor (NMDA) (Roche et al, 2001) and 5HT2A receptor internalisation (Xia et al, 2003). The mechanism underlying the inhibition of basal TSHR endocytosis by hScrib is not clearly defined. One possibility is that hScrib mediates the assembly of a protein complex at the plasma membrane that prevents internalisation of the TSHR, possibly by masking an endocytotic signal. Exposure of TSHR to TSH promotes internalisation; a conformational change in receptor structure or post-translational modifications may unlock this effect.
While the mechanisms involved in GPCR endocytosis have been extensively studied, little is known about those governing their routing between recycling endosomes and late endosomes or lysosomes (von Zastrow, 2003). The mechanism underlying the sorting of internalised β2-adrenergic receptors between these compartments has been partially uncovered and is mediated by the NHERF/EBP50 PDZ protein (Cao et al, 1999). NSF (N-ethylmaleimide-sensitive factor), a non-PDZ domain protein, was initially considered an important determinant of β2-adrenergic receptor recycling (Cong et al, 2001), but a recent work attributes to NHERF/EBP50 the major role in this process (Gage et al, 2004). NHERF/EBP50 also binds to the C-terminus of the human κ opioid receptor and enhances its recycling rate (Li et al, 2002). In the case of the human LHR, the recycling is regulated by the binding of the GIPC (GAIP-interacting protein C-terminus) PDZ protein to the cytoplasmic tail of the receptor (Hirakawa et al, 2003). Our results, combined with those of recent studies (Gage et al, 2004), indicate that PDZ proteins are part of a general mechanism implicated in the recycling of endocytosed GPCRs. PDZ domain proteins most likely do not act alone in this process and it is important to gain more insights into the molecular networks associated to these proteins.
In this study, we have demonstrated that βPIX and GIT1, two proteins associated with hScrib (Audebert et al, 2004), are required for TSHR recycling. Expression of βPIX and interaction between hScrib and βPIX are required for TSHR recycling. hScrib probably scaffolds TSHR and βPIX when TSHR binds to PDZ1 and PDZ3 domains (Figure 2D) and βPIX binds to all four PDZ domains (Audebert et al, 2004) of hScrib. Overexpression of GIT1 did not modify TSH-mediated receptor endocytosis but led to an inhibition of receptor recycling. This result contrasts with previous studies, indicating that GIT1 is involved in the endocytosis of several GPCRs, including the β2-adrenergic receptor (Claing et al, 2000).
GAP and GEF proteins regulate the function of small GTPases that are required for multiple biological processes, including protein trafficking and signal transduction. Increased amounts of GTP-bound ARF6 probably promoted by ARNO were present in cell extracts following TSH stimulation. We showed that TSHR/hScrib interaction was required for ARF6 activation in epithelial HEK293 and thyroid FRTL-5 cells.
ARFs are ubiquitously expressed small GTP binding proteins. They are essential components of the machinery that regulates membrane trafficking along the endocytic and biosynthetic pathways (Chavrier and Goud, 1999). ARF6 plays a role in cell-surface receptor trafficking. It is involved in β2-adrenergic receptor endocytosis (Claing et al, 2001) and LH/CG receptor desensitisation (Mukherjee et al, 2000). ARF6 also regulates constitutive recycling of the transferrin receptor in CHO cells (D'Souza-Schorey et al, 1995). In addition, a novel plasma membrane–endosomal recycling pathway regulated by ARF6 distinct from transferrin-positive endosomes has been identified (Radhakrishna and Donaldson, 1997). Stimulation-dependent recycling of integrins is regulated by both ARF6 and Rab11 (Powelka et al, 2004). ARF6 is necessary to promote the endocytic process of a variety of GPCRs internalised via clathrin-coated and non-clathrin-coated vesicle pathways and the caveolae pathway (Houndolo et al, 2004). TSHR, which internalises through clathrin-coated vesicles (Baratti-Elbaz et al, 1999), is an exception since the expression of ARF6 T27N blocks TSHR recycling but not endocytosis. Similarly, the internalisation of the vasoactive intestinal peptide receptor is not modified by ARF T27N or overexpressed GIT1 (Claing et al, 2000; Houndolo et al, 2004).
In this study, we have identified a novel pathway devoted to TSHR trafficking and signalling that involves hScrib, βPIX, GIT1 and ARF6. Future directions will aim to unravel other components of this signalling pathway and to determine its role in the recycling and signalling of other GPCRs.
Materials and methods
Cells, antibodies and reagents
HEK293 and FRTL-5 cells were grown and transfected as described (Ambesi-Impiombato et al, 1980; Atger et al, 1999). The efficiency of cell transfection was ⩾50% in all experiments. HEK293 cells stably expressing the wild-type human TSHR (HEK293-TSHR) or the ΔTVL receptor (HEK293-TSHR-ΔTVL) were established as described (Atger et al, 1999). For siRNA transfection, HEK293-TSHR cells were transfected using oligofectamine reagent (Invitrogen Co., Cergy-Pontoise, France).
The monoclonal antibodies directed against the TSHR (R5T-34, T5U-317, T5U-51) and FSHR (FSHR-323) have been described previously (Loosfelt et al, 1992; Vannier et al, 1996; de Bernard et al, 1999). Anti-hScrib (C20 and K21), anti-EEA1, anti-Rab11, anti-LAMP2 and anti-α-tubulin polyclonal antibodies are from Santa-Cruz (Tebu-Bio, Le Perray en Yvelines, France). Anti-βPIX and anti-GIT1 polyclonal antibodies were obtained from BD Biosciences (Le Pont de Claix, France). Anti-ARF6 polyclonal antibody is a gift from J Donaldson (Laboratory of Cell Biology, NHLBI, NIH, USA).
Bovine TSH, monensin, cycloheximide and the anti-mouse Cy3-conjugated secondary antibody are from Sigma (St Quentin Fallavier, France). 125I-labelled streptavidin was obtained from Amersham Pharmacia Biosciences (Orsay, France). Recombinant human FSH (hFSH) was obtained from Serono (Boulogne-Billancourt, France). Anti-rabbit Alexa488-conjugated and anti-goat Alexa647-conjugated secondary antibodies were purchased from Molecular Probes (Netherlands).
Plasmids and siRNA
The pSG5-hTSHR-ΔTVL eukaryotic expression vector was constructed using pSG5-hTSHR as the template (Atger et al, 1999) by site-directed mutagenesis (QuickChange Mutagenesis kit, Stratagene).
For the two-hybrid screen in yeast, baits and preys were constructed using pBTM116 and pACT2 vectors, respectively. The hScrib, Erbin and PSD95 prey constructs have been described previously (Saito et al, 2001; Audebert et al, 2004). The following vectors were constructed: pBTM-hTSHR-IC1 (residues 685–764), pBTM-hTSHR-IC1-ΔTVL (residues 685–761), pBTM-hTSHR-IC2 (residues 747–764), pBTM-hTSHR-IC2-ΔTVL (residues 747–761), pBTM-pLHR-IC (residues 628–696) and pBTM-hFSHR (residues 633–695). The pDEST15 (Invitrogen) vector was used to produce the corresponding GST fusion proteins. hScrib, βPIX, GIT1 and ARNO constructs have already been described (Premont et al, 1998; Caumont et al, 2000; Audebert et al, 2004).
The following siRNAs were purchased from Qiagen (Courtaboeuf, France): AACGATCTGGAAGTGCTGCCA for human hScrib siRNA, TGACCTGGAAGTGCTGCCT for rat hScrib siRNA in pSuper vector (Oligoengine) and AAACGATTCCGGATGGCATTG for Lano siRNA. The human β-PIX siRNA has been described previously (Park et al, 2004).
Yeast two-hybrid system
The yeast strain L40 was cotransformed with the bait and prey plasmids using a polyethylene glycol–lithium acetate protocol and colonies were grown on selection plates lacking leucine and tryptophan to select for colonies containing both plasmids. After 3 days, colonies were streaked on plates lacking leucine, tryptophan and histidine. Filter-based β-galactosidase assays were performed according to the MATCHMAKER two-hybrid kit (Clontech) (Borg et al, 2000).
Protein procedures
In the following methods, all experiments were reproduced at least three times in duplicate unless indicated. Western blot and GST pull-down assays were performed as described previously (Audebert et al, 2004). GTP-bound ARF6 was detected using a GST-MT2 pull-down assay (Schweitzer and D'Souza-Schorey, 2002). Equal amounts of cell extracts were incubated for 1 h at 4°C with GST-MT2 fusion protein conjugated to glutathione beads. Bound proteins were subjected to SDS–PAGE on a 12% gel. Western blots were performed using anti-ARF6 antibodies (Vitale et al, 2002). The density of the immunoreactive ARF6-GTP band was quantified using a Fuji phosphorimager and values are expressed in arbitrary units.
Transiently transfected HEK293 cells were stimulated by incubation for 30 min in DMEM containing 20 mM Hepes, 0.1% BSA and either 10 IU/l of TSH or 3 IU/l of recombinant hFSH. FRTL-5 cells grown in the absence of TSH for an overnight period were unstimulated or stimulated by 10 UI/l of TSH in Coon's medium. Cell extracts were prepared as previously described (Atger et al, 1999). TSHR was immunoprecipitated by overnight incubation at 4°C with 20 μg of a monoclonal antibody (T5U-51) recognising the extracellular domain of the receptor. Complexes were then harvested by incubation with protein G (Amersham Pharmacia, Orsay, France). Bound proteins were analysed by Western blotting using anti-hScrib (C20) antibody or monoclonal anti-TSHR (T5U-317) or anti-FSHR (FSHR-323) antibodies.
cAMP assay
Transiently transfected HEK293 cells were incubated with 3 IU/l of TSH and 70 μM cycloheximide. Total cAMP concentration was measured as described previously (Quellari et al, 2003).
Internalisation and recycling of receptor–antibody complexes
To measure the internalisation of the receptors (Baratti-Elbaz et al, 1999; Quellari et al, 2003), HEK293 cell lines stably expressing the wild-type and the mutant TSHR were incubated at 4°C, in the presence or absence of TSH, with the biotinylated R5T-34 monoclonal antibody recognising an extracellular epitope of the receptor (Baratti-Elbaz et al, 1999). After washing to remove unbound antibodies, the cells were incubated for different periods of time at 37°C to allow receptor–antibody complexes internalisation. The amount of biotinylated antibodies remaining at the cell surface was then quantified by measuring the binding of 125I-labelled streptavidin. Experiments were performed in duplicate and at least three times.
Immunocytochemistry and immunofluorescence analysis
Formol-fixed and paraffin-embedded frozen rat thyroid glands were sectioned (thickness: 5 μm) and immunolabelled as described (Loosfelt et al, 1992) with a goat anti-hScrib antibody (K21). Detection was by using the peroxidase technique.
For immunofluorescence analysis, HEK293-TSHR or HEK293-TSHR-ΔTVL cells (105 per well) were incubated for 30 min at 4°C with a monoclonal anti-TSHR antibody (R5T-34) in the presence of 3 IU/l of TSH. Cells were then incubated at 37°C for the indicated time periods. Cells were then fixed, permeabilised and incubated for 2 h with antibodies directed against EEA1, Rab11 and LAMP2. Fluorescent secondary antibodies were applied sequentially (Baratti-Elbaz et al, 1999). More than 60% of the cells display the colocalisation of TSHR with EEA1, Rab11 and LAMP2. Indirect immunofluorescence was performed on polarised MDCK cells stably expressing the TSHR (Beau et al, 2004) or on rat thyroid cells. The cells were observed with a Zeiss LSM-510 confocal scanning laser microscope equipped with a 25 mW argon laser, using a Plan Apochromat 40 objective.
Cell-surface biotinylation
Cell-surface biotinylation was carried out as described in transfected HEK-293 cells (Ren et al, 2003) using sulpho-NHS-SS-biotin and streptavidin-agarose from Pierce, except that biotinylated proteins were eluted from the beads using 20 mM Tris (pH 7.5), 50 mM NaCl and 50 mM dithiothreitol for 1 h at 37°C.
Supplementary Material
Supplementary Figure S1
Acknowledgments
We thank Philippe Chavrier (Institut Curie, Paris) for helpful discussions and for providing the ARF expression vectors. We thank Daniel Birnbaum and André LeBivic for reading the manuscript. We also thank Dr JG Donaldson (Laboratory of Cell Biology, NHLBI, NIH) for kindly providing the anti-ARF6 antibody. We are grateful to Philippe Leclerc and Olivier Trassard, Service de Microscopie Confocale IFR93, for technical assistance. This work was supported by INSERM (Institut National de la Santé et de la Recherche Médicale) and by University Paris XI (M.M). J-PB is funded by INSERM, Institut Paoli-Calmettes, La Ligue Nationale Contre Le Cancer (Label Ligue) and ACI ‘Jeune Chercheur'. MQ and CA are recipients of a grant from the Ministère de l'Education Nationale, de la Recherche et de la Technologie. CN and SN are recipients of fellowships from Conseil Général 13 and La Ligue Nationale Contre Le Cancer, respectively.
References
- Ambesi-Impiombato FS, Parks LA, Coon HG (1980) Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci USA 77: 3455–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atger M, Misrahi M, Young J, Jolivet A, Orgiazzi J, Schaison G, Milgrom E (1999) Autoantibodies interacting with purified native thyrotropin receptor. Eur J Biochem 265: 1022–1031 [DOI] [PubMed] [Google Scholar]
- Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, Dupont JL, Premont RT, Sempere C, Strub JM, Van Dorsselaer A, Vitale N, Borg JP (2004) Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol 14: 987–995 [DOI] [PubMed] [Google Scholar]
- Baratti-Elbaz C, Ghinea N, Lahuna O, Loosfelt H, Pichon C, Milgrom E (1999) Internalization and recycling pathways of the thyrotropin receptor. Mol Endocrinol 13: 1751–1765 [DOI] [PubMed] [Google Scholar]
- Beau I, Groyer-Picard MT, Desroches A, Condamine E, Leprince J, Tome JP, Dessen P, Vaudry H, Misrahi M (2004) The basolateral sorting signals of the thyrotropin and luteinizing hormone receptors: an unusual family of signals sharing an unusual distal intracellular localization, but unrelated in their structures. Mol Endocrinol 18: 733–746 [DOI] [PubMed] [Google Scholar]
- Bilder D, Perrimon N (2000) Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403: 676–680 [DOI] [PubMed] [Google Scholar]
- Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, Fournier E, Adelaide J, Margolis B, Birnbaum D (2000) ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol 2: 407–414 [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M (1999) A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401: 286–290 [DOI] [PubMed] [Google Scholar]
- Caumont AS, Vitale N, Gensse M, Galas MC, Casanova JE, Bader MF (2000) Identification of a plasma membrane-associated guanine nucleotide exchange factor for ARF6 in chromaffin cells. Possible role in the regulated exocytotic pathway. J Biol Chem 275: 15637–15644 [DOI] [PubMed] [Google Scholar]
- Chavrier P, Goud B (1999) The role of ARF and Rab GTPases in membrane transport. Curr Opin Cell Biol 11: 466–475 [DOI] [PubMed] [Google Scholar]
- Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, Lefkowitz RJ (2001) beta-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis. J Biol Chem 276: 42509–42513 [DOI] [PubMed] [Google Scholar]
- Claing A, Perry SJ, Achiriloaie M, Walker JK, Albanesi JP, Lefkowitz RJ, Premont RT (2000) Multiple endocytic pathways of G protein-coupled receptors delineated by GIT1 sensitivity. Proc Natl Acad Sci USA 97: 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong M, Perry SJ, Hu LA, Hanson PI, Claing A, Lefkowitz RJ (2001) Binding of the beta2 adrenergic receptor to N-ethylmaleimide-sensitive factor regulates receptor recycling. J Biol Chem 276: 45145–45152 [DOI] [PubMed] [Google Scholar]
- de Bernard S, Misrahi M, Huet JC, Beau I, Desroches A, Loosfelt H, Pichon C, Pernollet JC, Milgrom E (1999) Sequential cleavage and excision of a segment of the thyrotropin receptor ectodomain. J Biol Chem 274: 101–107 [DOI] [PubMed] [Google Scholar]
- Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, Trapani JA, Russell SM, Richardson HE, Humbert PO (2003) hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene 22: 9225–9230 [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Li G, Colombo MI, Stahl PD (1995) A regulatory role for ARF6 in receptor-mediated endocytosis. Science 267: 1175–1178 [DOI] [PubMed] [Google Scholar]
- Frank S, Upender S, Hansen SH, Casanova JE (1998) ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6. J Biol Chem 273: 23–27 [DOI] [PubMed] [Google Scholar]
- Gage RM, Matveeva EA, Whiteheart SW, von Zastrow M (2004) Type I PDZ ligands are sufficient to promote rapid recycling of G protein-coupled receptors independent of binding to NSF. J Biol Chem 280: 3305–3313 [DOI] [PubMed] [Google Scholar]
- Hirakawa T, Galet C, Kishi M, Ascoli M (2003) GIPC binds to the human lutropin receptor (hLHR) through an unusual PDZ domain binding motif, and it regulates the sorting of the internalized human choriogonadotropin and the density of cell surface hLHR. J Biol Chem 278: 49348–49357 [DOI] [PubMed] [Google Scholar]
- Houndolo T, Boulay PL, Claing A (2004) G protein-coupled receptor endocytosis in ARF6-depleted cells. J Biol Chem 280: 5598–5604 [DOI] [PubMed] [Google Scholar]
- Innamorati G, Le Gouill C, Balamotis M, Birnbaumer M (2001) The long and the short cycle. Alternative intracellular routes for trafficking of G-protein-coupled receptors. J Biol Chem 276: 13096–13103 [DOI] [PubMed] [Google Scholar]
- Lee SH, Eom M, Lee SJ, Kim S, Park HJ, Park D (2001) BetaPix-enhanced p38 activation by Cdc42/Rac/PAK/MKK3/6-mediated pathway. Implication in the regulation of membrane ruffling. J Biol Chem 276: 25066–25072 [DOI] [PubMed] [Google Scholar]
- Legouis R, Jaulin-Bastard F, Schott S, Navarro C, Borg JP, Labouesse M (2003) Basolateral targeting by leucine-rich repeat domains in epithelial cells. EMBO Rep 4: 1096–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JG, Chen C, Liu-Chen LY (2002) Ezrin–radixin–moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J Biol Chem 277: 27545–27552 [DOI] [PubMed] [Google Scholar]
- Loosfelt H, Pichon C, Jolivet A, Misrahi M, Caillou B, Jamous M, Vannier B, Milgrom E (1992) Two-subunit structure of the human thyrotropin receptor. Proc Natl Acad Sci USA 89: 3765–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misrahi M, Milgrom E (1997) The TSH receptor. In Handbook of Experimental Pharmacology, Weetman AP, Grossman A (eds) pp 33–73. Heidelberg: Springer-Verlag Berlin [Google Scholar]
- Mukherjee S, Gurevich VV, Jones JC, Casanova JE, Frank SR, Maizels ET, Bader MF, Kahn RA, Palczewski K, Aktories K, Hunzicker-Dunn M (2000) The ADP ribosylation factor nucleotide exchange factor ARNO promotes beta-arrestin release necessary for luteinizing hormone/choriogonadotropin receptor desensitization. Proc Natl Acad Sci USA 97: 5901–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, Arkell R, Stanier P, Copp AJ (2003) Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet 12: 87–98 [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM (2000) Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol 20: 8244–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechamen CA, Dias JA (2003) Point mutations in follitropin receptor result in ER retention. Mol Cell Endocrinol 201: 123–131 [DOI] [PubMed] [Google Scholar]
- Nourry C, Grant SG, Borg JP (2003) PDZ domain proteins: plug and play!. Sci STKE 2003: E7. [DOI] [PubMed] [Google Scholar]
- Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, Rhee SG, Bae YS (2004) Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol 24: 4384–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Hsu VW, Ooi CE, Finazzi D, Teal SB, Oorschot V, Donaldson JG, Klausner RD (1995) Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J Cell Biol 128: 1003–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650 [DOI] [PubMed] [Google Scholar]
- Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW (2004) Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 5: 20–36 [DOI] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Freeman JL, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ (1998) beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc Natl Acad Sci USA 95: 14082–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Perry SJ, Schmalzigaug R, Roseman JT, Xing Y, Claing A (2004) The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal 16: 1001–1011 [DOI] [PubMed] [Google Scholar]
- Quellari M, Desroches A, Beau I, Beaudeux E, Misrahi M (2003) Role of cleavage and shedding in human thyrotropin receptor function and trafficking. Eur J Biochem 270: 3486–3497 [DOI] [PubMed] [Google Scholar]
- Radhakrishna H, Donaldson JG (1997) ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol 139: 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Riley NJ, Needleman LA, Sanders JM, Swanson GT, Marshall J (2003) Cell surface expression of GluR5 kainate receptors is regulated by an endoplasmic reticulum retention signal. J Biol Chem 278: 52700–52709 [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ (2001) Molecular determinants of NMDA receptor internalization. Nat Neurosci 4: 794–802 [DOI] [PubMed] [Google Scholar]
- Saito H, Santoni MJ, Arsanto JP, Jaulin-Bastard F, Le Bivic A, Marchetto S, Audebert S, Isnardon D, Adelaide J, Birnbaum D, Borg JP (2001) Lano, a novel LAP protein directly connected to MAGUK proteins in epithelial cells. J Biol Chem 276: 32051–32055 [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, D'Souza-Schorey C (2002) Localization and activation of the ARF6 GTPase during cleavage furrow ingression and cytokinesis. J Biol Chem 277: 27210–27216 [DOI] [PubMed] [Google Scholar]
- Singh SP, McDonald D, Hope TJ, Prabhakar BS (2004) Upon thyrotropin binding the thyrotropin receptor is internalized and localized to endosome. Endocrinology 145: 1003–1010 [DOI] [PubMed] [Google Scholar]
- Vannier B, Loosfelt H, Meduri G, Pichon C, Milgrom E (1996) Anti-human FSH receptor monoclonal antibodies: immunochemical and immunocytochemical characterization of the receptor. Biochemistry 35: 1358–1366 [DOI] [PubMed] [Google Scholar]
- Vassart G, Dumont JE (1992) The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13: 596–611 [DOI] [PubMed] [Google Scholar]
- Vitale N, Chasserot-Golaz S, Bailly Y, Morinaga N, Frohman MA, Bader MF (2002) Calcium-regulated exocytosis of dense-core vesicles requires the activation of ADP-ribosylation factor (ARF)6 by ARF nucleotide binding site opener at the plasma membrane. J Cell Biol 159: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M (2003) Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci 74: 217–224 [DOI] [PubMed] [Google Scholar]
- Xia Z, Gray JA, Compton-Toth BA, Roth BL (2003) A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem 278: 21901–21908 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Kobilka B (2003) The PDZ-binding motif of the beta2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc Natl Acad Sci USA 100: 10776–10781 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1


