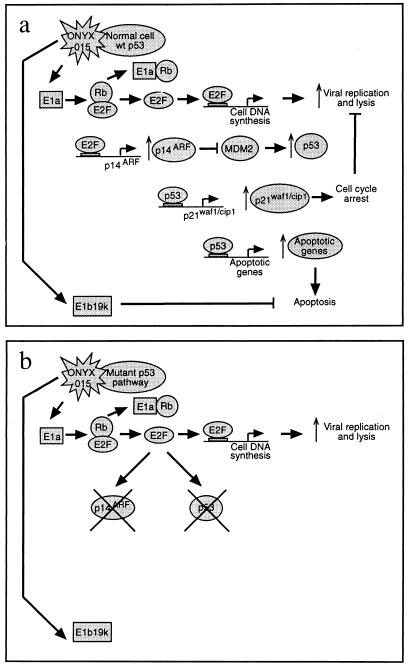
Increased knowledge of how normal cell growth is altered during tumorigenesis has led to the development of novel approaches to killing cancer cells. However, the application of novel tumor therapies, like conventional therapies, still depends on there being an effective means of selectively targeting tumors. One new approach uses an attenuated adenovirus (Ad) that has been designated ONYX-015 (4). Originally called dl1520 (2), ONYX-015 is attenuated by deletion of a large part of the coding sequence for the E1b55k gene product which, in association with another viral protein, E4ORF6, binds the tumor suppressor protein p53 (48, 49). This binding to p53 inhibits p53's transcriptional activity and causes its degradation (8). ONYX-015 was reported to selectively replicate in and kill cells with mutations in the p53 gene (4). Since approximately half of all human tumors contain defects in the p53 gene, ONYX-015 has the potential to be selective for many human malignancies. It is proposed that the selectivity of ONYX-015 for tumor cells is due to the loss of the E1b55k-E4ORF6-p53 interaction. The basis for this hypothesis is outlined below and in Fig. 1.
FIG. 1.
Proposed mechanism of action of ONYX-015. In normal cells (a), infection with ONYX-015 leads to displacement of E2F from pRb by E1a and a subsequent induction of p53. Overexpression of p53 will lead to cell cycle arrest by activation of p21waf1/cip1, while p53-mediated apoptosis is blocked by the Ad E1b19k protein. This is proposed to attenuate viral replication and prevent cell lysis. Conversely, in cells expressing mutant or no p53 or which are defective in the p53 pathway (b), viral replication and lysis should occur.
It has been known for many years that Ads alter cell cycle controls (6, 7, 50, 63), presumably to maximize virus replication, and that this is dependent on viral E1a gene products (6, 51). Following infection, products of the E1a gene bind to the cellular retinoblastoma protein (pRb) (60), which leads to displacement of the E2F transcription factor (Fig. 1) (40). The now active E2F then transactivates genes responsible for entry into S phase (40). E2F also transactivates the p14ARF gene (3), whose protein product binds to cellular MDM2 (42, 54), sequestering it to the nucleolus (59). During normal cell cycling, MDM2 regulates p53 levels through a negative feedback loop (37). As p53 levels rise above a threshold, p53 binds to the MDM2 promoter, stimulating transcription. MDM2 then interacts directly with p53 to cause its degradation and consequent down-regulation of MDM2. It is proposed that the result of MDM2 neutralization by p14ARF is that p53 levels will rise and may cause cell cycle arrest or apoptosis prior to completion of viral replication, thus generating an abortive virus infection. It is thought that, in order to counter this, Ads express three other proteins: E1b55k and E4ORF6, which together bind p53 facilitating its degradation (14), and E1b19k, a member of the Bcl-2 family of antiapoptotic proteins (9, 43). In this way, the virus ensures that a productive infection occurs.
If cells expressing wild-type (wt) p53 are infected with ONYX-015, p53 levels will rise as there is no E1b55k-E4ORF6 complex to stimulate degradation (Fig. 1a) (14). Increased p53 should lead to cell cycle arrest by activation of the p53-responsive gene p21waf1/cip1 (11, 20), but p53-induced apoptosis should be blocked, since ONYX-015 still expresses E1b19k (9, 43). It is proposed that the resultant cell cycle arrest will prevent (or significantly reduce) virus replication (4). In tumor cells defective in p53, cell cycle arrest should not occur and efficient viral replication and cell lysis should ensue (Fig. 1b). Thus, ONYX-015 should replicate and lyse p53-deficient tumor cells with an efficiency comparable to that of wt Ad while being strongly attenuated in cells expressing wt p53.
The proposed model for ONYX-015's tumor selectivity is reliant on there being a strict link between virus replication and cell cycle progression. However, it has been known for some years that Ad DNA synthesis occurs independently of cell cycle stage (12, 26, 27), and more recently, this has been shown for E1b55k-defective viruses (12). Despite the lack of restriction on viral DNA synthesis shown in this recent study, attenuation of virus production was evident in cells not actively synthesizing DNA (12), consistent with the proposed model and with known functions of E1b55k (1, 41). However, this attenuation was found to be independent of p53 (12).
The model also predicts that infection of wt p53-expressing cells with ONYX-015 virus should lead to cell cycle arrest, via up-regulation of p53 (Fig. 1a). While there is clear evidence that p53 is induced following infection with viruses defective in E1b55k (14) or in the E1b55k-E4ORF6-p53 complex (44, 53), there is no evidence that cell cycle arrest occurs. Indeed, the opposite has been shown (10, 56, 58). Disruption of pRb function, through binding of proteins such as Ad E1a, simian virus 40 large T antigen, and human papillomavirus E7, has been shown directly to overcome a p53-dependent cell cycle arrest (18, 35, 58). Such experimental findings are consistent with the fact that p21waf1/cip1, the major cell cycle target gene of p53, inhibits the phosphorylation activities of several cyclins, all of which function upstream of pRb (36), but they are inconsistent with the proposed mechanism of ONYX-015 attenuation in wt p53-expressing cells (Fig. 1a).
In addition, there is now substantial evidence demonstrating that infection with ONYX-015 results in neither selective replication nor selective death in cells with defective p53 genes. The original studies showed that ONYX-015 replicated less efficiently than wt Ad in cell lines expressing wt p53 (4). In cells that were p53 defective, ONYX-015 replicated with an efficiency comparable to that of wt Ad (4, 23). Similar results have also been seen with YKL-1, another Ad with an E1b55k deletion (33). However, these findings have not been supported by other studies, in which no correlation was found between p53 status and virus replication efficiency in a large panel of tumor-derived cell lines (13, 19, 47, 52, 56). In general, these reports suggest that differences in ONYX-015 replication efficiency in different cells are due to infectivity or permissiveness for replication, rather than the p53 status of the cell (52). Consistent with these data, cytopathic effect (CPE) analysis in a number of tumor cell lines showed that ONYX-015 does not cause any more CPE in p53-deficient cells than in cells expressing wt p53 (17, 23, 47). This indicates that ONYX-015 does not cause selective death of p53-deficient cells, which contrasts with early reports showing that cell death following ONYX-015 infection was less efficient in wt p53 cells than in mutant p53 cells (4, 23). It appears that differences in cell killing by ONYX-015 again may be due to infectivity or permissiveness (52), rather than p53 status.
Results from our laboratory have led to a different conclusion again, viz., that Ads actually require wt p53 for efficient cell death to occur (10, 17). Initial experiments showed that only tumor cells expressing wt p53 died after wt Ad infection (17), although viability was measured only out to 4 to 5 days postinfection. In a subsequent study, experiments were carried out to 10 days after infection with wt Ad and ONYX-015, comparing the rates of death of four tumor lines expressing wt p53 and four cell lines deficient in p53 (10). These data indicated that cells with wt p53 died faster than those that were p53 defective, with little death occurring before 4 to 5 days postinfection in the p53-defective cells, consistent with the earlier report. In all cases, irrespective of p53 status, ONYX-015 was less efficient at inducing cell death, a result also obtained by others (12, 15, 56). It was also shown, using a panel of Ad E1b55k mutants (30), that those able to bind p53 killed cells more efficiently than those that could not (10). We have therefore proposed that p53 (in complex) may actually promote Ad-induced cell death. Although ours is the only group to have proposed this, where there is an overlap of cell lines, the death kinetics observed are essentially identical to those in another study in which cells with wt p53 were shown to die faster than p53-defective cells (12) at similar doses of virus. These data are also supported by other work showing that p53-null cells were not killed at all by wt Ad (34) and that E1b55k-expressing Ads induced more CPE than those without E1b55k (61). Such data suggest that the E1b55k-E4ORF6-p53 complex influences the rate of cell death after Ad infection but may not be absolutely required.
Although not all of the details are clear, most cell culture experiments suggest that wt Ad is more efficient at killing cells than ONYX-015, irrespective of p53 status, and moreover, wt p53 does not appear to attenuate ONYX-015 replication. Furthermore, another virus with an E1b55k-deletion, Ad338, has been shown to replicate poorly in tumor cell lines with mutant p53, but it replicates efficiently in a tumor cell line with wt p53 and in a normal lung fibroblast line (21). Such data are generally inconsistent with the proposed model for tumor selectivity by ONYX-015.
In vivo experiments in which human cancer cells were grown in immune-deficient mice (xenografts) have provided some support for the selective destruction of p53-deficient tumor cells by ONYX-015 (4, 24, 46, 57). However, where the comparison has been made, wt Ad (4, 57) or viruses with E1b55k (61) were more effective than ONYX-015. Such data are reminiscent of the studies in tissue culture and again indicate that the differences in tumor growth reduction may be simply a consequence of virus infectivity or permissiveness. Although in the above-mentioned studies (4, 24, 46, 57) little effect on the surrounding normal tissues was evident, this is most likely due to the fact that human cells are several orders of magnitude more susceptible to infection by human Ads than are mouse cells surrounding the xenograft (63).
A recent report (45) attempts to explain some of the experimental inconsistencies of the model. These new data show that loss of the p53 regulatory protein, p14ARF, facilitates ONYX-015 replication but has no effect on wt Ad and that loss of p14ARF expression is a common feature of tumor cells (45, 54). The implication of this observation is that in all (or most) tumors or tumor-derived cell lines, there is a defect either in p53 itself or in the p53 pathway. If true, this would seem to account for the ability of ONYX-015 to replicate in tumor cells with wt p53 (12, 47, 56), as all tumor cells must be effectively defective in the p53 response to Ad infection. If the new model is correct, then lack of cell cycle arrest after infection of wt p53-expressing cells by ONYX-015 (10, 56) is simply explained by the p53 pathway being defective. Thus, there would be no attenuation of virus replication in such cells. By contrast, in normal cells, such as primary cells in culture and normal tissue surrounding a tumor, ONYX-015 would be attenuated as outlined above and in Fig. 1a.
Despite this new information and modification of the model, not all of the deficiencies of the original model are explained. The fact that p21waf1/cip1 functions upstream of pRb to induce cell cycle arrest is not addressed and remains a fundamental problem, as the proposed mechanism of attenuation of ONYX-015 in normal cells depends on this occurring. Thus, if ONYX-015 infects a normal cell, E1a would bind pRb, thereby bypassing the p21waf1/cip1 checkpoint, and no cell cycle arrest would occur. Hence, even in normal cells, it is not clear how the attenuation of ONYX-015 would occur. Moreover, when several primary human cells were examined, it was found that there was no greater attenuation of ONYX-015 in the primary cells than in a panel of tumor-derived cell lines (47). The lack of a clear relationship between ONYX-015 replication and cell cycle progression (26, 56) also remains a problem for the proposed model.
The argument suggests that in cells which have defective p14ARF following infection, disruption of pRb by viral proteins such as E1a will have no downstream consequences because the pathway is nonfunctional (45). Hence, cell cycle arrest or apoptosis will not occur. However, there is evidence that E1a can activate p53 independently of the pRb pathway (5, 16, 45).
Additionally, if p14ARF is deleted, then MDM2 should be constitutively active, leading to degradation of p53. Thus, p14ARF-defective cells should not express p53. However, this is clearly not the case (45). In order to avoid this, such cells must also have a defect in MDM2, which has yet to be demonstrated. Even if there is a defect in MDM2 function, then far from being inactive, p53 should be permanently up-regulated and may therefore be activated. Significantly, transactivation-competent p53 proteins have been demonstrated in several wt p53-expressing tumor-derived and transformed cell lines (29, 47).
Results from clinical trials show some considerable potential in the use of ONYX-015 as a cancer therapy (4, 22, 23, 25, 31, 32, 39) and phase III trials are imminent. However, the results are probably not as good as anticipated. As a single agent, ONYX-015 treatment of head and neck cancers results in some degree of tumor regression in most cases, but the effect appears to be refractive once treatment is ceased (39). In contrast, combination therapy using both ONYX-015 and cisplatin plus 5-fluorouracil shows much more promising results than either agent alone in the treatment of head and neck cancers (31, 32) and ovarian carcinomas (22). This is supported by a report showing that ONYX-015 works synergistically with chemotherapeutic agents in lung cancer cells (62). In the clinical setting, the order in which the virus and chemotherapeutic agents are delivered is important: ONYX-015 treatment prior to or at the same time as chemotherapy is the most successful. It could be that the virus in some way renders tumor cells more susceptible to the chemotherapeutic agents. Nonetheless, results from single-agent trials show that there is little or no virus replication in surrounding normal tissue (39), implying that ONYX-015 is attenuated in normal cells.
But, how does ONYX-015 attenuation occur? If the evidence discussed above is correct, then attenuation of ONYX-015 in normal cells cannot be dependent on a functional p53 pathway as proposed. However, it is possible that the model is correct but that the contradictory evidence derives from experiments using artificial systems which are far from relevant to the clinical setting. For example, primary cell cultures, the closest approximation there is to normal cells, might also contain some of the same defects that are present in tumor-derived or transformed cell lines, such as a defect in the p53 pathway. Such cells have been selected for survival and growth in tissue culture, albeit short-term, which is a very different environment from that which exists in vivo. If so, then new experimental systems may need to be established to be able to test the model properly.
If, on the other hand, one accepts that the weight of available evidence suggests that ONYX-015 is not attenuated by p53, then a new model needs to be developed. Most of the data are consistent with the interpretation that both virus growth and cell death are not strictly dependent on p53 status but vary according to how permissive or infectible the different cell types are for Ads. The attenuation therefore would be due to the functional defect in the virus due to the absence of E1b55k (1, 41). This explanation, however, makes no fundamental distinction between normal and tumor cells, implying that the efficacy of ONYX-015 as an antitumor virus would vary dramatically according to the tumor type and the nature of the surrounding tissues. The evidence that ONYX-015 may have some applicability as a selective antitumor virus suggests, however, that there must be another level of attenuation in normal cells. One likely explanation for this comes from a recent study which has shown that the human coxsackievirus and Ad receptor (hCAR) (55) is important in efficient Ad infection (38). hCAR has been shown to be expressed at a high level in tumor cells whereas the only normal cells it is expressed in are basal epithelial cells (28); thus, the majority of normal cells are unable to be infected.
If ONYX-015 is to fulfill its current promise as a tumor therapy, then it is important to understand how it kills cells and how it is (if it is) selective for tumor cells. Only in this way can one be sure of its ability to discriminate one cell type from another. Clearly, there is much more work to be done.
ACKNOWLEDGMENTS
We thank P. Jackson (University of New South Wales) and M. Hibma (University of Otago) for comments on the manuscript.
This work was supported by grants from the Health Research Council and the Cancer Society of New Zealand.
REFERENCES
- 1.Babiss L E, Ginsberg H S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984;50:202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker D D, Berk A J. Adenovirus proteins from both E1b reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 3.Bates S, Phillips A C, Clark P A, Stott F, Peters G, Ludwig R L, Vousden K H. p14ARF links the tumour suppressors Rb and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff J R, Kirn D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J A, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumour cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite A, Nelson C, Skulimowski A, McGovern J, Pigott D, Jenkins J. Transactivation of the p53 oncogene by E1a gene products. Virology. 1990;177:595–605. doi: 10.1016/0042-6822(90)90525-v. [DOI] [PubMed] [Google Scholar]
- 6.Braithwaite A W, Cheetham B F, Li P, Parish C R, Waldron-Stevens L K, Bellett A J D. Adenovirus-induced alterations of the cell growth cycle: a requirement for expression of E1A but not of E1B. J Virol. 1983;45:192–199. doi: 10.1128/jvi.45.1.192-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braithwaite A W, Murray J D, Bellett A J D. Alterations to controls of cellular DNA synthesis by adenovirus infection. J Virol. 1981;39:331–340. doi: 10.1128/jvi.39.2.331-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cathomen T, Weitzman M D. A functional complex of adenovirus proteins E1B–55kDa and E4orf6 is necessary to modulate the expression level of p53 but not its transcriptional activity. J Virol. 2000;74:11407–11412. doi: 10.1128/jvi.74.23.11407-11412.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiou S-K, Tseng C-C, Rao L, White E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J Virol. 1994;68:6553–6566. doi: 10.1128/jvi.68.10.6553-6566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dix B R, O'Carroll S J, Myers C J, Edwards S J, Braithwaite A W. Efficient induction of cell death by adenoviruses requires binding of E1B55k and p53. Cancer Res. 2000;60:2666–2672. [PubMed] [Google Scholar]
- 11.El-Deiry W, Tokino T, Velculescu V, Levy D, Parsons R, Trent J, Lin D, Mercer E, Kinzler K, Vogelstein B. WAF1, a potential mediator of p53 tumour suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 12.Goodrum F D, Ornelles D A. The early region 1B 55-kilodalton oncoprotein of adenovirus relieves growth restrictions imposed on viral replication by the cell cycle. J Virol. 1997;71:548–561. doi: 10.1128/jvi.71.1.548-561.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodrum F D, Ornelles D A. p53 status does not determine outcome of E1b 55-kilodalton mutant adenovirus lytic infection. J Virol. 1998;72:9479–9490. doi: 10.1128/jvi.72.12.9479-9490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grand R, Grant M, Gallimore P. Enhanced expression of p53 in human cells infected with mutant adenoviruses. Virology. 1994;203:229–240. doi: 10.1006/viro.1994.1480. [DOI] [PubMed] [Google Scholar]
- 15.Grand R J, Owen D, Rookes S M, Gallimore P H. Control of p53 expression by adenovirus 12 early region 1A and early region 1B 54K proteins. Virology. 1996;218:23–34. doi: 10.1006/viro.1996.0162. [DOI] [PubMed] [Google Scholar]
- 16.Hale T K, Braithwaite A W. The adenovirus oncoprotein E1a stimulates binding of transcription factor ETF to transcriptionally activate the p53 gene. J Biol Chem. 1999;274:23777–23786. doi: 10.1074/jbc.274.34.23777. [DOI] [PubMed] [Google Scholar]
- 17.Hall A R, Dix B R, O'Carroll S J, Braithwaite A W. p53-dependent cell death/apoptosis is required for a productive adenovirus infection. Nat Med. 1998;4:1068–1072. doi: 10.1038/2057. [DOI] [PubMed] [Google Scholar]
- 18.Hansen R, Reddel R, Braithwaite A. The transforming oncoproteins determine the mechanism by which p53 suppresses cell transformation: pRb-mediated growth arrest or apoptosis. Oncogene. 1995;11:2535–2545. [PubMed] [Google Scholar]
- 19.Harada J N, Berk A J. p53-independent and -dependent requirements for E1B–55K in adenovirus type 5 replication. J Virol. 1999;73:5333–5344. doi: 10.1128/jvi.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper W, Adami G, Wei N, Keyomarsi K, Elledge S. The p21 CDK-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 21.Hay J G, Shapiro N, Sauthoff H, Heitner S, Phupakdi W, Rom W N. Targeting the replication of adenoviral gene therapy vectors to lung cancer cells: the importance of the adenoviral E1b–55kD gene. Hum Gene Ther. 1999;10:579–590. doi: 10.1089/10430349950018652. [DOI] [PubMed] [Google Scholar]
- 22.Heise C, Lemmon M, Kirn D. Efficacy with a replication-selective adenovirus plus cisplatin-based chemotherapy: dependence on sequencing but not p53 functional status or route of administration. Clin Cancer Res. 2000;6:4908–4914. [PubMed] [Google Scholar]
- 23.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff D D, Kirn D H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 24.Heise C C, Williams A, Olesch J, Kirn D H. Efficacy of a replication-competent adenovirus (ONYX-015) following intratumoral injection: intratumoral spread and distribution effects. Cancer Gene Ther. 1999;6:499–504. doi: 10.1038/sj.cgt.7700071. [DOI] [PubMed] [Google Scholar]
- 25.Heise C C, Williams A M, Xue S, Propst M, Kirn D H. Intravenous administration of ONYX-015, a selectively replicating adenovirus, induces antitumoral efficacy. Cancer Res. 1999;59:2623–2628. [PubMed] [Google Scholar]
- 26.Hodge L D, Scharff M D. Effect of adenovirus on host cell DNA synthesis in synchronized cells. Virology. 1969;37:554–564. doi: 10.1016/0042-6822(69)90273-6. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz M S. Intermediates in the synthesis of type 2 adenovirus deoxyribonucleic acid. J Virol. 1971;8:675–683. doi: 10.1128/jvi.8.5.675-683.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchin M E, Pickles R J, Yarbrough W G. Efficiency of adenovirus-mediated gene transfer to oropharyngeal epithelial cells correlates with cellular differentiation and human coxsackie and adenovirus receptor expression. Hum Gene Ther. 2000;11:2365–2375. doi: 10.1089/104303400750038471. [DOI] [PubMed] [Google Scholar]
- 29.Hutton F G, Turnell A S, Gallimore P H, Grand R J. Consequences of disruption of the interaction between p53 and the larger adenovirus early region 1B protein in adenovirus E1 transformed human cells. Oncogene. 2000;19:452–462. doi: 10.1038/sj.onc.1203316. [DOI] [PubMed] [Google Scholar]
- 30.Kao C C, Yew P, Berk A J. Domains required for in vitro association between the cellular p53 and the adenovirus 2 E1b 55kDa protein. Virology. 1990;179:806–814. doi: 10.1016/0042-6822(90)90148-k. [DOI] [PubMed] [Google Scholar]
- 31.Khuri F R, Nemunaitis J, Ganly I, Arseneau J, Tannock I F, Romel L, Gore M, Ironside J, MacDougall R H, Heise C, Randlev B, Gillenwater A M, Bruso P, Kaye S B, Hong W K, Kirn D H. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 32.Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging. Nat Med. 1998;4:1341–1342. doi: 10.1038/3902. [DOI] [PubMed] [Google Scholar]
- 33.Lee H, Kim J, Lee B, Chang J W, Ahn J, Park J O, Choi J, Yun C O, Kim B S, Kim J H. Oncolytic virus potential of E1b 55kDa-deleted YKL-1 recombinant adenovirus: correlation with p53 functional status. Int J Cancer. 2000;88:454–463. doi: 10.1002/1097-0215(20001101)88:3<454::aid-ijc19>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 34.Marcellus R C, Lavoie J N, Boivin D, Shore G C, Ketner G, Branton P E. The early region 4 orf4 protein of human adenovirus type 5 induces p53-independent cell death by apoptosis. J Virol. 1998;72:7144–7153. doi: 10.1128/jvi.72.9.7144-7153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy S A, Symonds H S, Van Dyke T. Regulation of apoptosis in transgenic mice by simian virus 40 T antigen-mediated inactivation of p53. Proc Natl Acad Sci USA. 1994;91:3979–3983. doi: 10.1073/pnas.91.9.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittnacht S. Control of pRB phosphorylation. Curr Opin Genet Dev. 1998;8:21–27. doi: 10.1016/s0959-437x(98)80057-9. [DOI] [PubMed] [Google Scholar]
- 37.Momand J, Zambetti G, Olson D, George D, Levine A. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 38.Mori T, Arakawa H, Tokino T, Mineura K, Nakamura Y. Significant increase of adenovirus infectivity in glioma cell lines by extracellular domain of hCAR. Oncol Res. 1999;11:513–521. [PubMed] [Google Scholar]
- 39.Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, Landers S, Maples P, Romel L, Randlev B, Reid T, Kaye S, Kirn D. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B–55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- 40.Nevins J R. Cell cycle targets of the DNA tumor viruses. Curr Opin Genet Dev. 1994;4:130–134. doi: 10.1016/0959-437x(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 41.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B–58K transforming polypeptide modulates transport or cytoplasmic stabilization of viral host cell mRNAs. Mol Cell Biochem. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pomerantz J, Schreiber-Agus N, Liegeois N J, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee H W, Cordon-Cardo C, DePinho R A. The INK4a tumor suppressor gene product, p19ARF, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 43.Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridgway P J, Hall A R, Myers C J, Braithwaite A W. p53/E1b58kDa complex regulates adenovirus replication. Virology. 1997;237:404–413. doi: 10.1006/viro.1997.8782. [DOI] [PubMed] [Google Scholar]
- 45.Ries S J, Brandts C H, Chung A S, Biederer C H, Hann B C, Lipner E M, McCormick F, Korn W M. Loss of p14ARF in tumor cells facilitates replication of the adenovirus mutant dl 1520 (ONYX-015) Nat Med. 2000;6:1128–1133. doi: 10.1038/80466. [DOI] [PubMed] [Google Scholar]
- 46.Rogulski K R, Freytag S O, Zhang K, Gilbert J D, Paielli D L, Kim J H, Heise C C, Kirn D H. In vivo antitumor activity of ONYX-015 is influenced by p53 status and is augmented by radiotherapy. Cancer Res. 2000;60:1193–1196. [PubMed] [Google Scholar]
- 47.Rothmann T, Hengstermann A, Whitaker N J, Scheffner M, zur Hausen H. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarnow P, Hearing P, Anderson C W, Halbert D N, Shenk T, Levine A J. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J Virol. 1984;49:692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarnow P, Ho Y S, Williams J, Levine A J. Adenovirus E1b–58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kD cellular protein in transformed cells. Cell. 1982;28:387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- 50.Shimojo H, Yamashita T. Induction of DNA synthesis by adenoviruses in contact-inhibited hamster cells. Virology. 1968;36:422–433. doi: 10.1016/0042-6822(68)90167-0. [DOI] [PubMed] [Google Scholar]
- 51.Stabel S, Argos P, Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985;4:2329–2336. doi: 10.1002/j.1460-2075.1985.tb03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steegenga W T, Riteco N, Bos J L. Infectivity and expression of the early adenovirus proteins are important regulators of wild-type and DeltaE1B adenovirus replication in human cells. Oncogene. 1999;18:5032–5043. doi: 10.1038/sj.onc.1202886. [DOI] [PubMed] [Google Scholar]
- 53.Steegenga W T, Riteco N, Jochemsen A G, Fallaux F J, Bos J L. The large E1B protein together with the E4ORF6 protein target p53 for active degradation in adenovirus infected cells. Oncogene. 1998;16:349–357. doi: 10.1038/sj.onc.1201540. [DOI] [PubMed] [Google Scholar]
- 54.Stott F J, Bates S, James M C, McConnell B B, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden K H, Peters G. The alternative product from the human CDKN2A locus, p14ARF, participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turnell A S, Grand R J A, Gallimore P H. The replicative capacities of large E1B-null group A and group C adenoviruses are independent of host cell p53 status. J Virol. 1999;73:2074–2083. doi: 10.1128/jvi.73.3.2074-2083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vollmer C M, Ribas A, Butterfield L H, Dissette V B, Andrews K J, Eilber F C, Montejo L D, Chen A Y, Hu B, Glaspy J A, McBride W H, Economou J S. p53 selective and nonselective replication of an E1B-deleted adenovirus in hepatocellular carcinoma. Cancer Res. 1999;59:4369–4374. [PubMed] [Google Scholar]
- 58.Vousden K H, Vojtesek B, Fisher C, Lane D. HPV-16 E7 or adenovirus E1A can overcome the growth arrest of cells immortalized with a temperature-sensitive p53. Oncogene. 1993;8:1697–1702. [PubMed] [Google Scholar]
- 59.Weber J D, Taylor L J, Roussel M F, Sherr C J, Bar-Sagi D. Nucleolar ARF sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 60.Whyte P, Buchkovich K J, Horowitz J M, Friend S H, Raybuck M, Weinberg R A, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- 61.Wildner O, Morris J C. The role of the E1B 55 kDa gene product in oncolytic adenoviral vectors expressing herpes simplex virus-tk: assessment of antitumor efficacy and toxicity. Cancer Res. 2000;60:4167–4174. [PubMed] [Google Scholar]
- 62.You L, Yang C T, Jablons D M. ONYX-015 works synergistically with chemotherapy in lung cancer cell lines and primary cultures freshly made from lung cancer patients. Cancer Res. 2000;60:1009–1013. [PubMed] [Google Scholar]
- 63.Younghusband H B, Tyndall C, Bellett A J. Replication and interaction of virus DNA and cellular DNA in mouse cells infected by a human adenovirus. J Gen Virol. 1979;45:455–467. doi: 10.1099/0022-1317-45-2-455. [DOI] [PubMed] [Google Scholar]