Abstract
Background
Staphylococcus aureus bacteremia (SAB) is a high-risk condition associated with high morbidity and mortality. In the presence of cardiac implantable electronic devices (CIEDs), SAB may cause or clinically indicate device infection. We aimed to estimate the 10-year absolute risk of SAB in adult Danish first-time CIED carriers. Secondary aims included identification of risk factors associated with SAB.
Methods
A registry-based study utilizing Danish nationwide registers and including consecutive Danish patients undergoing first CIED implantation between 2000 and 2020 was conducted. The primary outcome was first-time SAB after CIED implantation.
Results
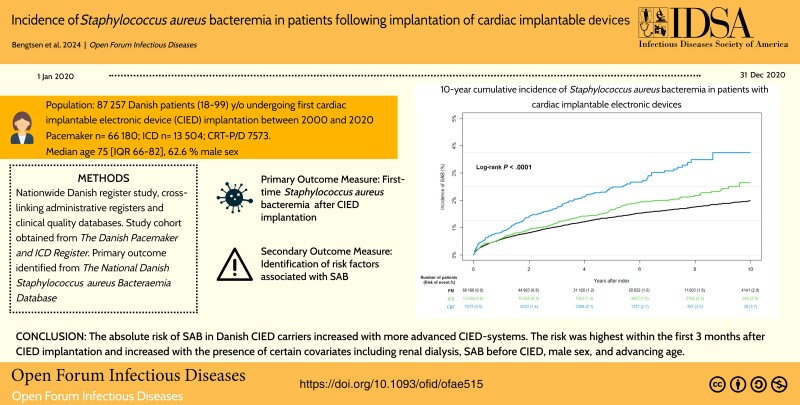
A total of 87 257 patients with first CIED implantation in the study period were identified (median age, 75 years; 62.6% were male; median follow-up, 3.8 years). Patients with pacemakers (PMs) were older and with more noncardiovascular comorbidities compared to patients with implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy devices with or without defibrillator capacity (CRTs). In total, 1366 patients (1.6%) developed SAB. The 10-year absolute risk (95% confidence interval) of SAB was 2.0% (1.9%–2.1%) for PM, 2.6% (2.2%–3.1%) for ICD, and 3.7% (3.0%–4.5%) for CRT. A multivariable Cox analysis identified hemodialysis (hazard ratio [HR], 8.51), SAB before CIED (HR, 2.76), liver disease (HR, 2.35), and carrying a CRT device (HR, 1.68) among the covariates associated with increased risk of SAB.
Conclusions
The absolute risk of SAB in Danish CIED carriers increased with more advanced CIED systems. The risk was highest within the first 3 months after CIED implantation and increased with the presence of certain covariates including renal dialysis, SAB before CIED, male sex, and advancing age.
Keywords: Staphylococcus aureus bacteremia, Cardiac resynchronization therapy, CIED, Implantable cardioverter defibrillator, Pacemaker
In a nationwide Danish cohort of first-time cardiac implantable electronic device recipients, the risk of Staphylococcus aureus bacteraemia increased with more advanced CIED-systems. The risk was highest within the first 3 months after device implantation.
Graphical Abstract
Graphical Abstract.
Device infection is the most feared infectious complication in patients with permanent cardiac implantable electronic devices (CIEDs), as it is associated with high morbidity and mortality and in general requires device extraction [1]. The most common pathogen encountered in CIED infections is Staphylococcus aureus (SA), accounting for approximately 30% of all infections [1–3]. In the case of Staphylococcus aureus bacteremia (SAB) in patients with CIEDs, studies have shown a risk of up to 50% for developing clinically verified device infection [4]. However, there is a paucity of data examining the risk of developing SAB in patients carrying a CIED.
During the past 20 years, the implantation rate of CIEDs has increased significantly [5], as has the burden of infections related to CIEDs [6, 7]. SA may cause biofilm formation on implanted foreign bodies and thus, bacteremia with SA may lead to device infection in CIED patients. Preventive measures to avoid infection post–CIED implantation include careful patient selection, adequate training of surgical staff, proper skin disinfection, prophylactic antibiotic, implementation of antibiotic envelopes, and post-procedure wound care [1, 8]. Previous studies within the field have focused on the prevalence of definite device infection in patients with CIEDs and SAB [9–11]. However, the occurrence of SAB in patients with CIEDs on a population-based level is not well described. Given the serious nature and potential clinical consequences of SAB in CIED patients, it is of utmost importance to quantify the incidence and risk of SAB in this population to help guide the risk stratification and treatment in real-world clinical practice.
Utilizing Danish nationwide registers, we aimed to estimate the risk of SAB in adult Danish patients following de novo CIED implantation through the years 2000–2020. Furthermore, we aimed to identify factors associated with SAB in patients with CIEDs.
METHODS
The present study was carried out as a nationwide register study, cross-linking administrative Danish nationwide registers and clinical quality databases. All data contained within the registers have been prospectively collected over time.
Data Sources
All Danish residents are equipped with a personal and unique social security number, which serves as personal identifier across official administrative systems and healthcare registers. Anonymized, this number makes it possible to cross-link the nationwide registers on an individual level without compromising patient anonymity and integrity. We used data obtained from 5 different registers:
The Danish Pacemaker and ICD [implantable cardioverter defibrillator] Register, which contains information on all Danish CIED procedures starting from 1982 [12].
The National Danish Staphylococcus aureus Bacteraemia Database hosted by Statens Serum Institut. Starting from 1957, it contains data on >90% of all microbiologically verified SABs in Denmark [13, 14].
The Danish National Patient Register contains data on all hospital admissions and contacts (inpatient and outpatient) classified according to the International Classification of Diseases [15]. The register obtained complete coverage in 1978.
The Danish Register of Medicinal Product Statistics holds information on all prescriptions dispersed from Danish pharmacies classified according to the Anatomical Therapeutic Chemical classification system [16].
The Danish Civil Registration System, which contains data on date of birth, date of death, vital status, sex, and migration [17].
Study Population
We identified all Danish patients with a CIED-related procedure between 1 January 2000 and 31 December 2020. Procedures in which the patient were aged <18 or >99 years were excluded from the study cohort. Index was defined as date of first CIED procedure and follow-up continued until occurrence of the primary endpoint (first-time SAB), death, emigration, end-of-study (31 December 2020), or date of next CIED-related procedure, whichever came first. To ensure follow-up, we excluded foreign citizens, patients who emigrated prior to CIED implantation, patients with missing data, and patients with a solitary lead-related procedure as first database entry due to missing information on date and type of first CIED implant.
Baseline Characteristics
Comorbidities and operative procedures at index were defined according to International Classification of Diseases codes registered as primary or secondary diagnosis related to hospital contacts. Conditions were assessed as ever registered or in a timespan covering up to 5 years prior to index date, whichever was clinically relevant (Supplementary Table 1). For the diagnoses of diabetes and chronic obstructive pulmonary disease, the definition included redeemed prescriptions for disease-relevant drugs. We defined baseline pharmacotherapy as at least 1 filled prescription for a given remedy within 6 months prior to index date.
Outcome and Analyses
The primary outcome was defined as first episode of SAB after CIED implantation. Date of SAB was identified from the National Danish SAB Database. The primary analyses estimating the cumulative risk of SAB were carried out on all de novo CIED procedures and were stratified by CIED type and sex. Patients carrying cardiac resynchronization therapy (CRT) devices were combined in 1 group, irrespective of ICD capability due to the same number of leads. Up-/downgrade procedures were defined according to changes between device types—that is, upgrade procedures were defined as shifts from pacemaker (PM) to ICD/CRT or from ICD to CRT, while downgrade procedures were defined as shifts from CRT to ICD/PM or from ICD to PM. Secondary analyses estimating the cumulative incidence of SAB across all eligible procedures were stratified by procedure type (de novo implants, generator exchanges, or upgrades/downgrades) and cumulative number of CIED-related procedures (de novo implants, hardware replacements including up- and downgrades, revisions without hardware change, and solitary extractions). Patients with >1 procedure were allowed multiple entries in the secondary analyses. We defined index as the date of a given procedure. Follow-up for the specific procedure ended at time of first-time SAB, death, emigration, end of study (31 December 2020), or date of next procedure in the study period, whichever came first. A new entry was allowed at the date of next procedure. Only procedures including hardware changes were incorporated in the analysis of procedure type while all procedures were integrated when analyzing cumulative number of procedures.
Statistical Analyses
Baseline characteristics of the primary study population were stratified according to device type. Data are presented as crude numbers and percentages for categorical variables and median with interquartile range (IQR) for continuous variables. The primary outcome of first-time SAB after CIED implantation is reported as cumulative incidence estimated by Aalen-Johansen estimator accounting for the competing risk of death. All-cause mortality from date of de novo CIED implantation was estimated and reported as absolute risk. Incidence rates were calculated using days at risk for every patient. Factors associated with SAB in all de novo CIED implants were estimated through an adjusted multivariable Cox proportional hazard regression. We examined the covariates: sex, age, device type, implantation year, selected comorbidities (eg, diabetes, renal dialysis, SAB before CIED), and prior relevant surgical procedures (coronary artery bypass grafting, prosthetic heart valves, surgery (thoracic not including CIED procedures, abdominal, orthopedic, gynecologic, dermatological, or urologic) within 1 year prior to CIED implantation. The proportional hazard assumption was tested by scaled Schoenfeld residuals and by visual inspection of log(-log) curves and were found valid. Interaction between device type and sex and age, respectively, was assessed by likelihood ratio test. Data management and statistical analyses were carried out through the secure research facilities of Statistics Denmark using SAS statistical software version 9.4 (SAS Institute, Cary, North Carolina) and R Studio software (version 2022.7.0) [18].
Ethics
Register-based studies do not need ethical approval in Denmark. Data access was approved and granted by the data responsibility institute in the Capital Region of Denmark (approval number P-2019-191)
RESULTS
Study Population and Baseline Characteristics
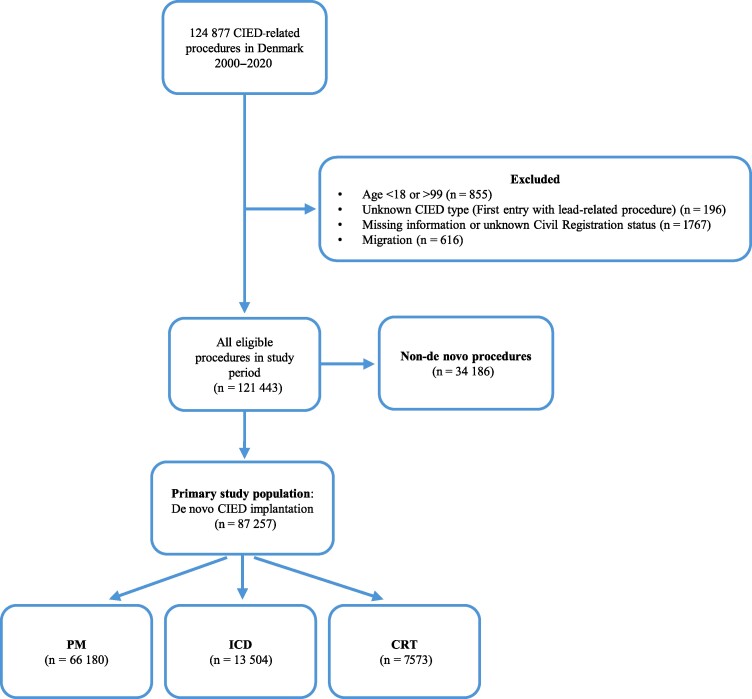
We identified 124 877 CIED-related procedures in Denmark between 2000 and 2020. Based on the exclusion criteria, 3434 procedures were excluded. Of the remaining procedures, 87 257 were de novo implantations, which formed our primary study population (Figure 1). Median device follow-up for de novo implants was 3.8 years (IQR, 1.5–6.8 years).
Figure 1.
Flowchart of study population. The primary study population consisted of 87 257 patients with a de novo cardiac implantable electronic device. Secondary analyses were carried out on all eligible procedures. Abbreviations: CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; PM, pacemaker.
Patient characteristics at baseline varied between device types (Table 1). The majority of the primary study population consisted of patients with PMs (75.8%), who overall were older with more noncardiovascular comorbidities compared to patients with ICDs and CRT. Male sex was more frequently encountered among the patients with ICDs and CRTs. We observed less ischemic heart disease and associated interventional procedures among patients with PMs compared to those with ICDs and CRT. Previous SAB and hemodialysis were equally distributed across device types. Surgical procedures (thoracic not including CIED procedures, abdominal, orthopedic, gynecologic, dermatological, or urologic) within 1 year prior to CIED implantation was less frequently observed in patients with PMs compared to those with ICDs and CRTs. Patients in the ICD and CRT groups received more heart-specific medication, anticoagulants, and diuretics compared to PM patients at baseline.
Table 1.
Baseline Characteristics of the Primary Study Cohort Consisting of Patients With De Novo Cardiac Implantable Electronic Devices
| Characteristic | PM (n = 66 180) |
ICD (n = 13 504) |
CRT (n = 7573) |
Total (N = 87 257) |
|---|---|---|---|---|
| Age, y, median (IQR) | 77 (70–84) | 64 (55–71) | 69 (61–76) | 75 (66–82) |
| Male sex | 37 910 (57.3) | 10 997 (81.4) | 5697 (75.2) | 54 604 (62.6) |
| Previous SAB | 555 (0.8) | 154 (1.1) | 78 (1.0) | 787 (0.9) |
| Comorbidity | ||||
| AMI | 8489 (12.8) | 6295 (46.6) | 2224 (29.4) | 17 008 (19.5) |
| IHD | 23 242 (35.1) | 10 135 (75.1) | 4972 (65.7) | 38 349 (43.9) |
| Congestive heart failure | 12 321 (18.6) | 8453 (62.6) | 7038 (92.9) | 27 812 (31.9) |
| Aortic valve disease | 8302 (12.5) | 788 (5.8) | 972 (12.8) | 10 062 (11.5) |
| Mitral valve disease | 2892 (4.4) | 670 (5.0) | 658 (8.7) | 4220 (4.8) |
| Atrial fibrillation | 26 102 (39.4) | 3208 (23.8) | 2381 (31.4) | 31 691 (36.3) |
| Stroke | 9013 (13.6) | 1337 (9.9) | 827 (10.9) | 11 177 (12.8) |
| COPD | 10 113 (15.3) | 2036 (15.1) | 1661 (21.9) | 13 810 (15.8) |
| Diabetes | 10 096 (15.3) | 2348 (17.4) | 1697 (22.4) | 14 141 (16.2) |
| Cancer | 7532 (11.4) | 803 (5.9) | 632 (8.3) | 8967 (10.3) |
| Impaired renal function | 4220 (6.4) | 884 (6.5) | 701 (9.3) | 5805 (6.7) |
| End-stage renal disease | 591 (0.9) | 127 (0.9) | 71 (0.9) | 789 (0.9) |
| Dementia | 1691 (2.6) | 30 (0.2) | 23 (0.3) | 1744 (2.0) |
| Alcohol abuse with hospital contact | 1193 (1.8) | 312 (2.3) | 162 (2.1) | 1667 (1.9) |
| Liver disease | 667 (1.0) | 131 (1.0) | 101 (1.3) | 899 (1.0) |
| Procedures | ||||
| Hemodialysis within 6 mo | 490 (0.7) | 102 (0.8) | 55 (0.7) | 647 (0.7) |
| PCI | 6033 (9.1) | 5730 (42.4) | 1915 (25.3) | 13 678 (15.7) |
| CABG | 3846 (5.8) | 2860 (21.2) | 1147 (15.1) | 7853 (9.0) |
| Prosthetic heart valve | 3631 (5.5) | 423 (3.1) | 585 (7.7) | 4639 (5.3) |
| Surgery within 1 y | 15 460 (23.4) | 6441 (47.7) | 2324 (30.7) | 24 225 (27.8) |
| Pharmacotherapy | ||||
| Anticoagulant therapy | 16 564 (25.0) | 2844 (21.1) | 2364 (31.2) | 21 772 (25.0) |
| Antiplatelet therapy | 5009 (7.6) | 2739 (20.3) | 1070 (14.1) | 8818 (10.1) |
| RAAS inhibition | 29 373 (44.4) | 8431 (62.4) | 6417 (84.7) | 44 221 (50.7) |
| Thiazide diuretics | 11 654 (17.6) | 1159 (8.6) | 717 (9.5) | 13 530 (15.5) |
| β-blockers | 21 780 (32.9) | 7772 (57.6) | 5721 (75.5) | 35 273 (40.4) |
| Anti-arrhythmic medication | 2171 (3.3) | 617 (4.6) | 605 (8.0) | 3393 (3.9) |
| Lipid lowering | 23 217 (35.1) | 7574 (56.1) | 4470 (59.0) | 35 261 (40.4) |
| Loop diuretics | 17 969 (27.2) | 4520 (33.5) | 4824 (63.7) | 27 313 (31.3) |
| Systemic corticosteroids | 4167 (6.3) | 572 (4.2) | 537 (7.1) | 5276 (6.0) |
| Antipsychotics | 2043 (3.1) | 258 (1.9) | 154 (2.0) | 2455 (2.8) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; IHD, ischemic heart disease; IQR, interquartile range; PCI, percutaneous coronary intervention; PM, pacemaker; RAAS, renin-angiotensin-aldosterone system; SAB, Staphylococcus aureus bacteremia.
Incidence of SAB and All-Cause Mortality
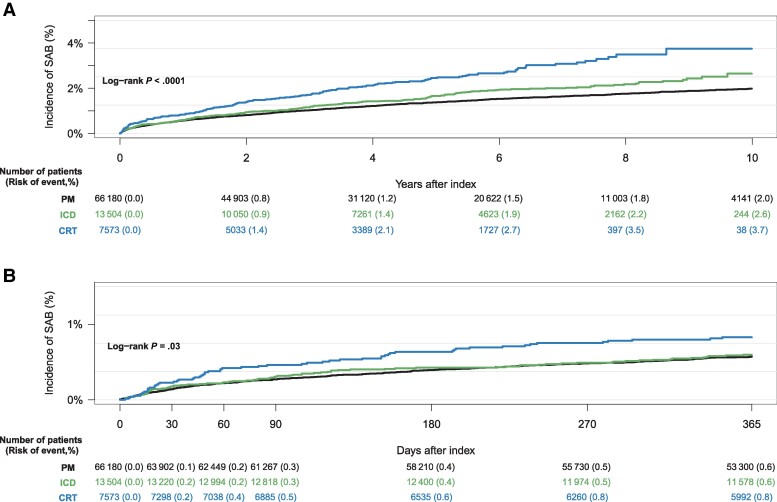
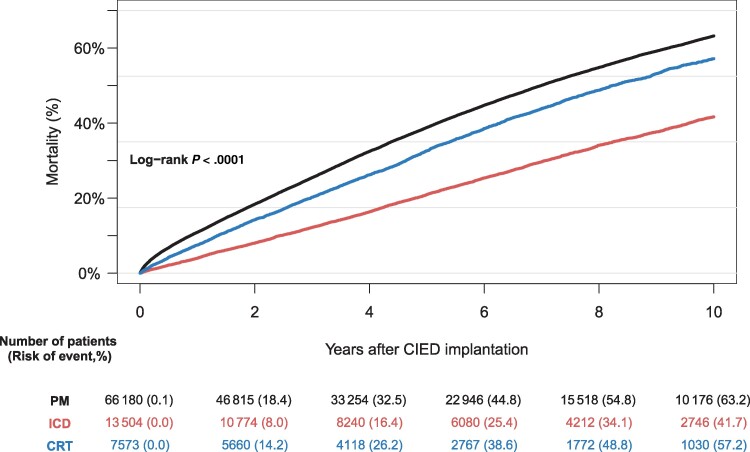
We recorded a total of 1366 SAB cases (PM, n = 974; ICD, n = 222; CRT, n = 170) following de novo CIED implantation across the study period covering 21 calendar years and 376 144 person-years. Median time to SAB was 1.8 years (IQR, 0.5–4.2 years). The 10-year cumulative incidence of SAB was 2.0% (95% confidence interval [CI], 1.9%–2.1%) for PM, 2.6% (95% CI, 2.2%–3.1%) for ICDs, and 3.7% (95% CI, 3.0%–4.5%) for CRTs (Figure 2A). The greatest increase in the rate of SAB was observed within the first 3 months after implantation and was greater for patients who had received a CRT device compared to patients with a PM or ICD (Figure 2B, Table 2). The 10-year all-cause mortality from date of de novo CIED implantation was highest among patients with a PM (63.2%) and lowest among patients with an ICD (41.7%) (Figure 3).
Figure 2.
Ten-year (A) and 360-day (B) cumulative incidence of Staphylococcus aureus bacteremia after de novo cardiac implantable electronic device implantation accounting for the competing risk of death, both stratified by device type. Abbreviations: CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; PM, pacemaker; SAB, Staphylococcus aureus bacteremia.
Table 2.
Incidence Rates of Staphylococcus aureus Bacteremia After De Novo Cardiac Implantable Electronic Device Implantation According to Device Type, 2000–2020
| Months From CIED Implantation | Total No. of SAB Cases | PM (IR/100 000 PY) |
ICD (IR/100 000 PY) |
CRT (IR/100 000 PY) |
Total (IR/100 000 PY) |
|---|---|---|---|---|---|
| 0–1 | 131 | 1707 (1390–2097) | 2097 (1394–3156) | 2784 (1731–4478) | 1862 (1569–2209) |
| 1–2 | 74 | 1021 (780–1337) | 650 (310–1363) | 2378 (1408–4015) | 1080 (860–1356) |
| 2–3 | 47 | 629 (447–884) | 1004 (556–1814) | 508 (164–1576) | 678 (509–902) |
| 3–6 | 106 | 526 (422–656) | 473 (285–784) | 711 (404–1253) | 534 (441–646) |
| 6–9 | 71 | 388 (298–506) | 264 (132–527) | 503 (251–1005) | 378 (300–477) |
| 9–12 | 70 | 383 (292–503) | 443 (257–763) | 328 (137–788) | 388 (307–491) |
| 12–24 | 215 | 285 (242–337) | 369 (271–504) | 635 (456–884) | 329 (287–376) |
| 24–36 | 155 | 279 (2323–336) | 235 (155–357) | 388 (245–616) | 281 (240–329) |
Abbreviations: CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; IR, incidence rate; PM, pacemaker; PY, person-years; SAB, Staphylococcus aureus bacteremia.
Figure 3.
Ten-year all-cause mortality from date of cardiac implantable electronic device implantation, stratified by device type. Abbreviations: CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; PM, pacemaker.
Incidence of SAB According to Subgroup
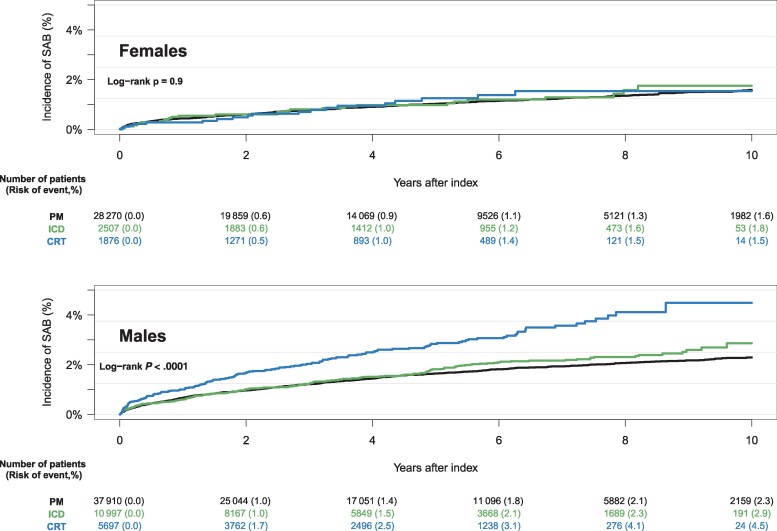
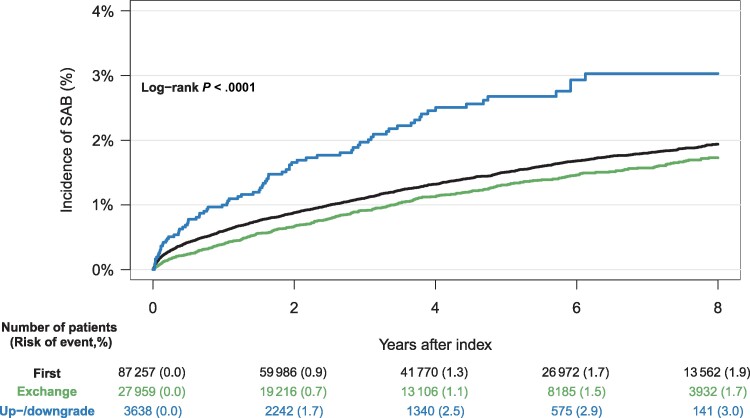
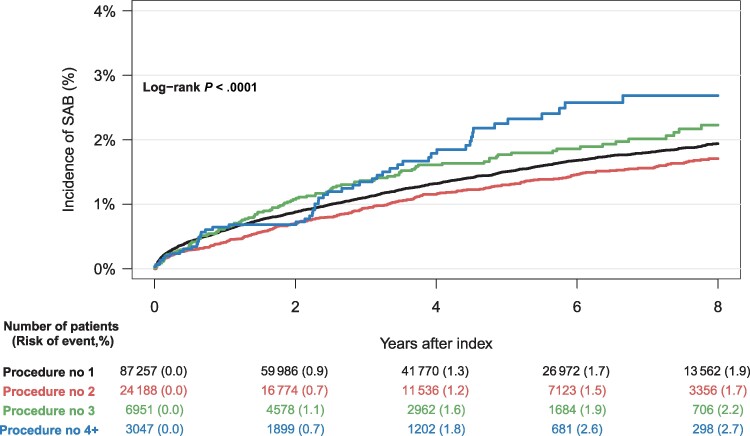
The cumulative incidence of SAB was higher for males compared to females, across all device types, with male CRT patients having the highest risk (4.4%) (Figure 4). Patients who underwent upgrade and downgrade procedures had a higher cumulative incidence of SAB compared to de novo implant procedures and generator exchanges (Figure 5). The risk of SAB increased with the cumulative number of CIED-related procedures (Figure 6).
Figure 4.
Ten-year cumulative incidence of Staphylococcus aureus bacteremia accounting for the competing risk of death, stratified by device type and sex. Abbreviations: CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; PM, pacemaker; SAB, Staphylococcus aureus bacteremia.
Figure 5.
Eight-year cumulative incidence of Staphylococcus aureus bacteremia (SAB) accounting for the competing risk of death, stratified by type of procedure. First = de novo implantation, Exchange = generator exchange, Up-/downgrade = cardiac implantable electronic device upgrade or downgrade.
Figure 6.
Eight-year cumulative incidence of Staphylococcus aureus bacteremia (SAB) accounting for the competing risk of death, stratified by cumulative number of cardiac implantable electronic device–related procedures.
Factors Associated With SAB
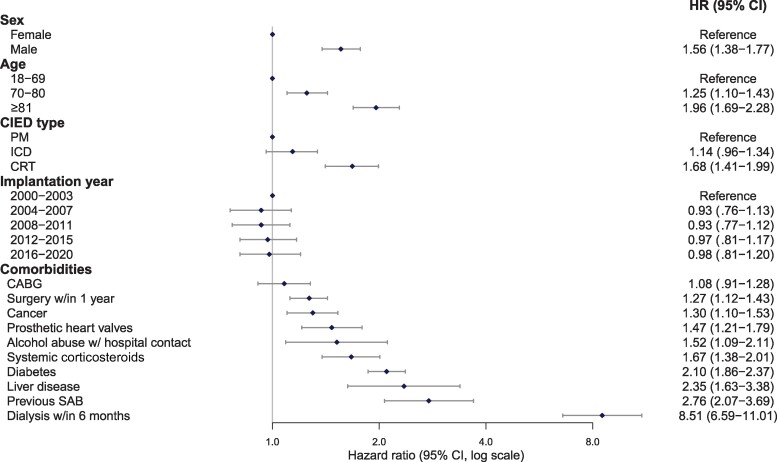
In adjusted analysis of all de novo CIED implantation procedures, a total of 12 factors were significantly associated with an increased hazard of SAB (Figure 7). Hemodialysis within 6 months of CIED implantation showed the highest hazard. Likewise, we observed that older age and male sex were associated with an increased hazard of SAB. No interaction between device type and age (P = .08) or device type and sex (P = .12) was demonstrated. Compared to PM, having a CRT implanted was associated with increased hazard of SAB, whereas ICDs showed no significantly increased hazard. We observed no difference in the hazard of SAB across the study years. Chronic medical conditions with a potential to compromise the immune system (cancerous disease, alcohol abuse, systemic corticosteroids, hemodialysis, liver disease, and diabetes) showed an increased hazard of SAB, as did the presence of prosthetic heart valves. Univariate analyses of the factors showed similar results (Supplementary Table 2). When evaluating covariates associated with SAB early after CIED implantation (within 0–90 days), we found that diabetes, surgery within 1 year of CIED implantation, SAB prior to CIED, renal dialysis within 6 months of CIED implantation, and the implantation of a CRT device compared to PM were associated with increased hazard of SAB (Supplementary Figure 1). In a landmark analysis, we evaluated covariates associated with late SAB (>90 days from CIED implantation) and found similar results as the main analysis (including overall SAB, irrespective of time from CIED implantation) (Supplementary Figure 2).
Figure 7.
Adjusted multivariable Cox regression model showing risk factors associated with Staphylococcus aureus bacteremia after de novo cardiac implantable electronic device implantation. Abbreviations: CABG, coronary artery bypass grafting; CI, confidence interval; CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; HR, hazard ratio; ICD, implantable cardioverter defibrillator; PM, pacemaker; SAB, Staphylococcus aureus bacteremia.
DISCUSSION
Incidence and Risk of SAB
In this Danish nationwide cohort study, we investigated the risk of SAB after CIED implantation in adult Danish patients between 2000 and 2020. The main finding of our study was that the risk of SAB in CIED patients varied with device type. The crude risk of SAB in patients with a CRT was significantly increased compared to other device types, and almost twice as high compared to patients with a PM. The adjusted analysis showed a significant association between occurrence of SAB and device complexity, with increased hazard of SAB in patients with CRTs compared with PMs. These observations are in line with previous findings regarding infective complications following CIED implantation. A previous Danish study evaluated CIED infections of all microbiological origins in 97 750 patients and found a hazard ratio of 2.22 and 1.68 for CRT-Defibrillator (CRT-D) and CRT-Pacemaker (CRT-P), respectively, compared to PMs [19]. Likewise, an analysis of a subpopulation from the "World-wide Randomized Antibiotic Envelope Infection Prevention" Trial (WRAP-IT) showed increased risk of microbiological unspecified CIED infection with CRT-P and CRT-D compared to PMs and ICDs [20]. SA has been observed as the main pathogen in up to 30% of CIED-related infections, and thus contributes substantially to the infective burden related to CIED implantations [1, 3]. Although we observed a trend (hazard ratio [HR], 1.14 [95% CI, 0.96–1.34]) toward increased risk of SAB in patients implanted with an ICD compared to patients implanted with a PM, we were unable to show a statistically significant association. This latter finding contrasts with the observations of Olsen and colleagues who evaluated confirmed CIED infections of all microbiological origins [19]. Even though SAB in CIED patients may not per se equal device infection, the risk is reported as high as 50% [4, 9]. Thus, bloodstream infections with SA should be prevented whenever possible. Our data confirm the trend toward an increased risk of SAB with more implanted hardware; thus, the more advanced the device, the higher the risk of infection. It is well established that implantation of foreign bodies increases the risk of systemic infections [21]. Our findings support this fact with respect to CIEDs. However, one must bear in mind the observational nature of the present study and thus only an association can be demonstrated.
The 10-year all-cause mortality was observed to be significantly higher among patients with a PM (63.2%) compared to patients carrying a CRT device (57.2%) or an ICD (41.7%). This undoubtedly reflects the higher median age at the time of CIED implantation among the patients with PMs compared to patients with ICDs and CRTs. Even with a high 10-year all-cause mortality among the patients with PM, the risk of SAB remained high compared to a nonselected patient population [22], in which the 3-year incidence rate of SAB in patients >75 years old was observed to range between 107.7 and 126.7 per 100 000 person-years. The high mortality and relatively higher risk of SAB among patients with a CRT compared to patients with a PM draws attention to the difference in age at the time of CIED implantation and comorbidity burden of the groups in-between. Diabetes and recent surgery are reported as risk factors for SAB in non-CIED patients [23], and the higher prevalence of these 2 factors observed in the patients with CRTs could contribute to the higher risk of SAB compared to patients with PMs. When interpreting the results of the present study, one must bear in mind the ever-present challenge of competing risk of death in observational studies. Given a relatively higher age at baseline compared to patients with CRTs and ICDs, the patients implanted with a PM might simply die before they contract SA and develop SAB.
Timing of Infection
It is suggested that infection in close timely relation to CIED procedures might be associated to procedural factors, while infection separated in time from the operational procedure could be the result of hematogenous infection or spreading from peripheral primary infection [24]. In previous studies, the cut-off between early and late infection has been set at different time points from last CIED procedure, varying from 30 days to 1 year [24–26]. We observed the highest incidence rate of SAB to occur within the first 3 months from CIED implantation, and more profound for CRTs compared to ICDs and PMs (Table 2). Our findings suggest that the previous definition of surgical site infection, defined as infections occurring up to 1 year after surgery in patients receiving implants [27], might not be applicable in the case of SAB in CIED patients. Rather, the cut-off between early and late SAB following CIED implantations could appropriately be set at 3 months, corresponding to the updated definitions and recommendations of the Centers for Disease Control and Prevention’s National Healthcare Safety Network, which recommends a 90-day surveillance period for surgical site infection following CIED procedures [28].
In our study, we observed an increased risk of SAB with male sex compared to female sex, regardless of device type. The higher risk of SAB among males compared to females is well-known and previously described [23, 29, 30]. Additionally, we observed a tendency toward a higher risk of SAB with more advanced devices among males. Interestingly, we did not see the same tendency among the female patients, and the hierarchy within the complexity of the CIEDs and associated risk of SAB seemed to equalize.
When exploring factors associated with SAB after de novo CIED implantation, we found that hemodialysis within 6 months of CIED implantation and prior SAB were associated with a large increase in the HR of SAB. Additionally, implantation of prosthetic heart valves and chronic comorbidities with the potential to weaken the immune system were associated with a statistically significantly increased hazard of SAB, as were operative procedures within 1 year prior to CIED implantation. Most of the identified factors were nonmodifiable and comparable to known risk factors for SAB in non-CIED patients [23, 31]. When analyzing factors associated with SAB dependent on time from CIED implantation, we found differences in associated factors for early SAB (within 90 days of CIED implantation) and late SAB (>90 days after CIED implantation). Early infection was associated with prior SAB and covariates related to breach of the skin barrier (dialysis and surgery within 1 year of CIED), whereas late infection showed the same associations as the time-independent analysis. It is plausible that early infection primarily reflects contamination in relation to the device procedure or other recent interventions with penetration of the skin barrier, that is, direct contamination. On the other hand, late infections might reflect other entrance points of infection and a chronic susceptibility to infections highlighted by the association to covariates previously shown to increase the risk of systemic infections (eg, male sex, high age, diabetes) [23, 31].
Our observation that the risk of SAB increases with advanced age does not correlate with previous results on definite CIED infections across all microbiological origins [32] where increasing age was observed to lower the infection risk. This discrepancy might indicate that bloodstream infections with SA differentiate from the pool of infections analyzed by Olsen and colleagues [32], and that chronic comorbidities and frailty of the elderly have more impact than the cumulated time with an implanted CIED. Additionally, the difference probably reflects the outcomes of the studies: SAB versus definite CIED infections, defined as device removal due to infection. The decision about device removal is again impacted by age and frailty so that younger patients with less comorbidity are more prone to get extracted.
Our secondary analyses estimated a greater risk of SAB with increasing number of CIED-related procedures. This observation supports the previous findings of Olsen et al and Tarakji et al, underscoring the need for minimizing the number of invasive procedures whenever possible [19, 20], as well as securing correct treatment indication and appropriate choice of device. Additionally, we observed that up- and downgrade procedures were associated with increased risk of SAB compared to de novo implants. We were not able to show a statistically significant difference between de novo implants and generator exchanges. These results are in opposition to the findings of Olsen and colleagues [19] who, through a multivariable regression analysis, found increased infection risk with both upgrade/downgrade procedures and replacements compared to de novo implantations (HR, 4.39 and 4.93 for up-/downgrade and replacements, respectively). A likely explanation for this inconsistency is the afore-mentioned difference in endpoints: SAB versus definite device infection by all microorganisms assessed by device explant. Furthermore, it is reasonable to expect that generator exchange increases the risk of localized pocket infection, which leads to device extraction but does not increase the risk of SAB. Our results suggest that procedures involving leads and intravascular material including abandoned hardware carry a greater risk of SAB than procedures involving only the generator.
Strengths and Limitations
Data on CIED procedures were derived from the Danish Pacemaker and ICD Register, which is a clinical quality database, organized under the Danish Clinical Registries (RKKP). The register is updated by the treating physician at every procedure undertaken. All 14 implantation centers in Denmark report to the register and the coverage is considered complete regarding procedures and the data of high quality. The National Danish SAB Database contains >90% of all microbiologically verified SABs in Denmark. However, the reporting is voluntary, and we cannot exclude the possibility that some cases might be missing. Overall, we consider the study's selection bias minimal. We consider the size and nationwide coverage of data a great strength. From the definitions and type of data included in the study, we cannot conclude if definite device endocarditis was present in the SAB patients. This question is central in the discussion of infective complications after CIED implantations, but would require clinical, imaging, and/or autopsy data. Observational studies have inherent limitations including confounding by indication and the lack of possibilities for establishing a causal relationship. The present study should be considered descriptive of the population in question. Further studies within the subject are encouraged, preferably in the form of randomized trials, to uncover potential causality.
CONCLUSIONS
In this nationwide cohort study of Danish patients with cardiac implatable electronic devices from 2000 to 2020, the 10-year cumulative incidence of Staphylococcus aureus bacteremia was 2.0% for patients with a PM, 2.7% for patients with an ICD, and 3.7% for patients carrying a CRT. The risk was highest within the first 3 months after implantation and increased with more advanced devices, male sex, advancing age, and cumulative number of procedures.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Kasper Høtoft Bengtsen, Department of Cardiology, Zealand University Hospital, Roskilde, Denmark.
Alexander Christian Falkentoft, Department of Cardiology, Zealand University Hospital, Roskilde, Denmark.
Melanie Vuong Le, Department of Cardiology, Zealand University Hospital, Roskilde, Denmark.
Ketil Haugan, Department of Cardiology, Zealand University Hospital, Roskilde, Denmark.
Berit Thornvig Philbert, Department of Cardiology, Rigshospitalet, Copenhagen, Denmark.
Jens Brock Johansen, Department of Cardiology, Odense University Hospital, Odense, Denmark.
Christian Torp-Pedersen, Department of Cardiology, Nordsjællands Hospital, Hillerød, Denmark.
Sam Riahi, Department of Cardiology, Aalborg University Hospital, Aalborg, Denmark.
Jens Cosedis Nielsen, Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Charlotte Larroudé, Department of Cardiology, Herlev and Gentofte University Hospital, Copenhagen, Denmark.
Andreas Petersen, Department of Bacteria, Fungi and Parasites, National Reference Laboratory for Antimicrobial Resistance, Statens Serum Institut, Copenhagen, Denmark.
Anders Rhod Larsen, Department of Bacteria, Fungi and Parasites, National Reference Laboratory for Antimicrobial Resistance, Statens Serum Institut, Copenhagen, Denmark.
Lauge Østergaard, Department of Cardiology, Rigshospitalet, Copenhagen, Denmark.
Emil Fosbøl, Department of Cardiology, Rigshospitalet, Copenhagen, Denmark.
Niels Eske Bruun, Department of Cardiology, Zealand University Hospital, Roskilde, Denmark.
Anne-Christine Ruwald, Department of Cardiology, Zealand University Hospital, Roskilde, Denmark; Department of Cardiology, Rigshospitalet, Copenhagen, Denmark.
Notes
Financial support. This work was supported by the Arvid Nilsson Foundation (salary-covering grant to K. H. B.).
References
- 1. Blomström-Lundqvist C, Traykov V, Erba PA, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections. Europace 2020; 22:515–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miyagi Y, Sakamoto S, Kawase Y, et al. Temporal and microbiological analysis of cardiac implantable electrical device infections—a retrospective study. Circ Rep 2021; 3:488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Urien JM, Camus C, Leclercq C, et al. The emergence of Staphylococcus aureus as the primary cause of cardiac device–related infective endocarditis. Infection 2021; 49:999–1006. [DOI] [PubMed] [Google Scholar]
- 4. Nakajima I, Narui R, Tokutake K, et al. Staphylococcus bacteremia without evidence of cardiac implantable electronic device infection. Heart Rhythm 2021; 18:752–9. [DOI] [PubMed] [Google Scholar]
- 5. Raatikainen MJP, Arnar DO, Merkely B, et al. A decade of information on the use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology countries: 2017 report from the European Heart Rhythm Association. Europace 2017; 19:II1–90. [DOI] [PubMed] [Google Scholar]
- 6. Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J Am Coll Cardiol 2011; 58:1001–6. [DOI] [PubMed] [Google Scholar]
- 7. Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010; 33:414–9. [DOI] [PubMed] [Google Scholar]
- 8. Frausing MHJP, Kronborg MB, Johansen JB, Nielsen JC. Avoiding implant complications in cardiac implantable electronic devices: what works? Europace 2021; 23:163–73. [DOI] [PubMed] [Google Scholar]
- 9. Chamis AL, Peterson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 2001; 104:1029–33. [DOI] [PubMed] [Google Scholar]
- 10. Snygg-Martin U, Ruus C, Skovbjerg S, Studahl M, Andersson LM. Does extraction of cardiac implantable electronic devices improve outcome in patients with Staphylococcus aureus bacteraemia? Infect Dis 2020; 52:877–82. [DOI] [PubMed] [Google Scholar]
- 11. Berge A, Carlsén C, Petropoulos A, Gadler F, Rasmussen M. Staphylococcus aureus bacteraemia, cardiac implantable electronic device, and the risk of endocarditis: a retrospective population-based cohort study. Eur J Clin Microbiol Infect Dis 2023; 42:583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johansen J. Danish pacemaker and ICD register-annual report 2022. 2022. Available at: https://www.sundhed.dk/content/cms/21/109821_dpir_annual_report_2022_07082023_final_offentlig-version.pdf. Accessed 12 March 2024.
- 13. Jessen O, Rosendal K, Bülow P, Faber V, Eriksen KR. Changing staphylococci and staphylococcal infections. N Engl J Med 1969; 281:627–35. [DOI] [PubMed] [Google Scholar]
- 14. Petersen A, Skov R, Larsen AR, Benfield T, Schønheyder HC. Staphylococcus aureus bacteraemia cases in Denmark 2015. 2016. Available at: https://antibiotika.ssi.dk/-/media/arkiv/indhold/dk-dansk/smitteberedskab/referencelaboratorier/stafylokoklaboratoriet/sab-2015-final.pdf. Accessed 6 August 2024.
- 15. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol 2017; 46:798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pedersen CB. The Danish civil registration system. Scand J Public Health 2011; 39:22–5. [DOI] [PubMed] [Google Scholar]
- 18. R Core Team . R Foundation for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2024. [Google Scholar]
- 19. Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB. Incidence of device-related infection in 97 750 patients: clinical data from the complete Danish device-cohort (1982–2018). Eur Heart J 2019; 40:1862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarakji KG, Krahn AD, Poole JE, et al. Risk factors for CIED infection after secondary procedures: insights from the WRAP-IT trial. JACC Clin Electrophysiol 2022; 8:101–11. [DOI] [PubMed] [Google Scholar]
- 21. von Eiff C, Jansen B, Kohnen W, Becker K. Infections associated with medical devices. Drugs 2005; 65:179–214. [DOI] [PubMed] [Google Scholar]
- 22. Mejer N, Westh H, Schønheyder HC, et al. Stable incidence and continued improvement in short term mortality of Staphylococcus aureus bacteraemia between 1995 and 2008. BMC Infect Dis 2012; 12:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daneman N, Homenauth E, Saskin R, Ng R, Ha A, Wijeysundera HC. The predictors and economic burden of early-, mid- and late-onset cardiac implantable electronic device infections: a retrospective cohort study in Ontario, Canada. Clin Microbiol Infect 2020; 26:255.e1–6. [DOI] [PubMed] [Google Scholar]
- 25. Sohail MR, Hussain S, Le KY, et al. Risk factors associated with early-versus late-onset implantable cardioverter-defibrillator infections. J Interv Card Electrophysiol 2011; 31:171–83. [DOI] [PubMed] [Google Scholar]
- 26. Welch M, Uslan DZ, Greenspon AJ, et al. Variability in clinical features of early versus late cardiovascular implantable electronic device pocket infections. Pacing Clin Electrophysiol 2014; 37:955–62. [DOI] [PubMed] [Google Scholar]
- 27. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 1999; 20:247–80. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention National Healthcare Safety Network . Surgical site infection event (SSI). 2024. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual. Accessed 6 May 2024.
- 29. Humphreys H, Fitzpatick F, Harvey BJ. Gender differences in rates of carriage and bloodstream infection caused by methicillin-resistant Staphylococcus aureus: are they real, do they matter and why? Clin Infect Dis 2015; 61:1708–14. [DOI] [PubMed] [Google Scholar]
- 30. Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med 2007; 167:834–9. [DOI] [PubMed] [Google Scholar]
- 31. Chaudry MS, Gislason GH, Kamper A, et al. Increased risk of Staphylococcus aureus bacteremia in hemodialysis—a nationwide study. Hemodial Int 2019; 23:230–8. [DOI] [PubMed] [Google Scholar]
- 32. Olsen T, Jørgensen OD, Nielsen JC, et al. Risk factors for cardiac implantable electronic device infections: a nationwide Danish study. Eur Heart J 2022; 43:4946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.