Abstract
Cells of the monocyte lineage can be infected with human immunodeficiency virus type 1 (HIV-1) both during clinical infection and in vitro. The ability of HIV-1-based vectors to transduce human monocytes, monocyte-derived macrophages, and dendritic cells (DCs) was therefore examined, in order to develop an efficient protocol for antigen gene delivery to human antigen-presenting cells. Freshly isolated monocytes were refractory to HIV-1-based vector transduction but became transducible after in vitro differentiation to mature macrophages. This maturation-dependent transduction was independent of the HIV-1 accessory proteins Vif, Vpr, Vpu, and Nef in the packaging cells and of the central polypurine tract in the vector, and it was also observed with a vesicular stomatitis virus-pseudotyped HIV-1 provirus, defective only in envelope and Nef. The level and extent of reverse transcription of the HIV-1-based vector was similar after infection of immature monocytes and of mature macrophages. However, 2LTR vector circles could not be detected in monocytes, suggesting a block to vector nuclear entry in these cells. Transduction of freshly isolated monocytes exposed to HIV-1-based vector could be rescued by subsequent differentiation into DCs. This rescue was induced by fetal calf serum in the DC culture medium, which promoted vector nuclear entry.
Cells of the monocyte lineage are generally the first to be infected with human immunodeficiency virus (HIV-1) during viral transmission (12, 40, 50), and human monocyte-derived macrophages (MDMs) (16, 33, 34, 38) and peripheral blood-derived dendritic cells (DCs) (2, 4, 7, 42, 46) are susceptible to HIV-1 infection in vitro. The chemokine receptor CCR5 serves as a coreceptor for entry of macrophagetropic (M-tropic), or nonsyncytium-inducing, HIV-1 strains into CD4+ macrophages (43, 47). T-cell line-tropic (T-tropic), or syncytium-inducing, HIV-1 strains can also infect monocytes and macrophages, using CXCR4 as a coreceptor (45, 48).
This infection of cells of the monocyte lineage by HIV-1 suggests that HIV-1-based vectors may be particularly suitable for antigen gene delivery to antigen-presenting cells. Professional antigen-presenting cells, such as macrophages and DCs, scavenge antigens in tissue and upon inflammatory stimuli present peptides derived from these antigens to T cells (3). Patient DCs loaded in vitro with tumor antigens have shown great promise in the treatment of advanced-stage cancer (28, 29), and DCs expressing tumor antigen genes have been demonstrated to be superior to such antigen-loaded DCs in treatment of metastatic tumors in animals (21). Both DCs and macrophages can be differentiated in vitro from adherent peripheral blood monocytes, DCs by culture in the presence of fetal calf serum and the cytokines interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF) for 7 days (35), and macrophages by culture in medium containing human serum for at least 5 days (9). HIV-1-based vectors, pseudotyped with vesicular stomatitis virus G proteins (VSV-G), have previously been reported to transduce both monocyte-derived DCs (8, 18, 37) and MDMs (37, 51).
The requirements for HIV-1 infection in nondividing cells, such as those of the monocyte lineage, remain an area of controversy. Unlike in infections with simple retroviruses, after the completion of reverse transcription the preintegration complex containing the proviral DNA can traverse the nuclear membrane in the absence of mitosis. Various viral proteins have been implicated in this process. The accessory protein Vpr contains a nuclear localization sequence (19), as do integrase (13) and matrix (6). A subset of phosphorylated matrix proteins has also been described as having a role in nuclear entry (14, 15). More recently, a second primer binding site within the pol gene of the virus, the central polypurine tract (cPPT), has been proposed to act as a regulator of nuclear entry through the formation of triple-stranded DNA flap during reverse transcription (49). However, it is unclear to what extent each of these factors is responsible for nuclear entry in any particular cell type. Furthermore, in resting cells such as naive primary T cells, activation stimuli are required for the efficient completion of HIV-1 reverse transcription (25). This was thought to be due to the lack of deoxyribonucleotides (dNTPs) in resting cells, but an artificial increase in dNTP levels failed to rescue infection (24). However, CD28 costimulation and activation of the transcription factor NFAT have been shown to be required for HIV-1 infection of primary, resting T cells (20, 25, 44).
For clinical applications, in vitro manipulation of antigen-presenting cells should be kept to a minimum. We therefore used HIV-1-based vectors to transduce freshly isolated monocytes and then differentiated them into either macrophages or DCs. We found a postentry block to HIV vector transduction at the level of nuclear entry that was regulated by macrophage maturation or culture in fetal calf serum.
MATERIALS AND METHODS
Plasmids.
HIV-1-based plasmids were kindly provided by D. Trono (Geneva, Switzerland) and are described elsewhere (31, 51). The packaging plasmids pCMVΔR8.2 and pCMVΔR8.9 carry gag, pol, tat, and rev, and pCMVΔR8.2 also carries the accessory genes vif, vpr, vpu, and nef. The vector plasmid pHR′-CMV-eGFP contains a cytomegalovirus (CMV)-driven emerald green fluorescent protein (eGFP) expression cassette, and pMDG encodes VSV-G. Murine leukemia virus (MLV) vectors based on the pHIT system (pHIT60 carrying MLV gag-pol, pCNCG vector genome encoding a CMV-driven eGFP, and pHIT456 encoding the amphotropic MLV envelope) were kindly provided by Oxford Biomedica (Oxford, United Kingdom) (39). pHR′ plasmids containing Rous sarcoma virus (RSV), human elongation factor 1α (EF1α), or human β-actin promoters in place of CMV were constructed by Y. Ikeda (unpublished data), and the pHR′-cPPT-CMV-eGFP vector containing the cPPT was constructed as described previously (49). The envelope-defective HIV-1 clone NL4-3 harboring GFP in place of nef was obtained from P. Clapham (London, United Kingdom).
Virus production.
Human 293T cells were maintained in Dulbecco's modified Eagle's medium (Gibco, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS) and penicillin and streptomycin, and grown at 37°C in a humidified atmosphere with 10% CO2. Viruses were produced, essentially as described previously (31), by transient transfection of 293T cells with a weight ratio of 3:2:1 of vector to packaging to envelope plasmids using Lipofectamine (Gibco) per the manufacturer's instructions. Cells were then washed and grown for 48 h in serum-free OptiMEM (Gibco) at 37°C. Supernatants were harvested, passed through a 0.45-μm-pore-size filter, and concentrated by ultracentrifugation, 100,000 × g for 90 min (VSV-G pseudotypes) or low-speed centrifugation, 4,000 × g for 5 h (MLV amphotropic strain [MLV-A] pseudotypes). Viruses were aliquoted, their titers were determined on 293T cells, and the viruses were stored at −80°C prior to use.
Isolation of human MDMs.
Blood was drawn from consenting healthy donors into heparinized syringes, and peripheral blood mononuclear cells (PBMCs) were isolated on Ficoll gradients. Monocytes were allowed to adhere for 2 h on non-tissue culture-treated plates and washed extensively. Monocytes were then removed in EDTA, washed, and replated at the desired density on tissue culture plates and grown in RPMI medium plus 10% heat-inactivated human AB serum (Harlan, Loughborough, United Kingdom) and antibiotics. Cells isolated were >90% CD14 positive by FACScan.
Isolation of human monocyte-derived DCs.
Monocytes were grown in RPMI medium supplemented with 10% FCS, antibiotics, IL-4, and GM-CSF (1,000 U/ml) as described previously (35). Four days later, DC cultures were depleted of CD2-, CD3-, and CD19-expressing cells by negative immunomagnetic selection and replated at the desired density. After infection studies, DCs were identified by expression of CD1a and HLA-DR by FACScan.
Monocyte, MDM, and DC transductions.
On the desired day postisolation, monocytes, MDMs, or DCs were washed and exposed to virus or vector at the required multiplicity of infection (MOI), as standardized on 293T cells, for 12 h, and then the cells were washed thoroughly. We have observed that eGFP in viral supernatants can lead to pseudotransduction of phagocytic cells, with cells displaying punctate staining due to phagocytosed eGFP for up to 3 days (unpublished data). Therefore, cells were cultured for 7 days posttransduction, until cells displayed uniform cytoplasmic eGFP. Percent cell transduction was then determined by FACScan and analyzed by the Cellquest software. Transduced MDMs were stained with anti-human CD14-phycoerythrin (PE), and transduced DCs were stained with anti-human CD1a-PE (Harlan), HLA-DR-PE (Harlan), or CD83-PE (Becton Dickinson). Titers of NL4-3-pseudotyped viruses were determined in the same way; infection was detected by in situ p24 capsid expression using an anti-p24 monoclonal (EVA365/66; NIBSC, London, United Kingdom) and a secondary anti-mouse immunoglobulin β-galactosidase conjugate, obtained from Southern Technical Association (Birmingham, Ala.). Staining was developed using X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside).
PCR of reverse transcription intermediates.
A total of 2 × 105 cells were plated to detect reverse transcription intermediates, and 1 × 106 cells were plated for 2LTR circle detection. Viral stocks were treated with 20 U of DNase I/ml at 37°C for 1 h to remove contaminating plasmid DNA. Cells were then exposed to vector at an MOI of 10, 5, or 1 and harvested at 2-h intervals postinfection. Cells were washed and resuspended in 1× PCR buffer containing 5 mM MgCl2, 0.5% Tween 20, 0.5% NP-40, 0.1% gelatin, and 100 μg of proteinase K/ml, incubated at 56°C for at least 2 h, and then heated to 95°C for 10 min. Lysate (25 μl or a dilution) was used for PCRs and mixed 1:1 with 1× PCR buffer containing 1 mM concentrations of dNTPs, 1 μl of each primer (10 mM stock) and 1.5 U of Taq polymerase (Promega). Primers (Sigma, Poole, United Kingdom) were eGFP and β-actin, primers for LTR/gag, and 2LTR circles and have been described elsewhere (36). PCR conditions for eGFP and 2LTR were 94°C for 30 s, 62°C for 1 min, and 72°C for 45 s for 35 cycles; those for LTR/gag were 94°C for 30 s, 57°C for 1 min, and 72°C for 45 s for 35 cycles; and those for β-actin were 94°C for 30 s, 68°C for 1 min, and 72°C for 30 s for 35 cycles.
RESULTS
Maturation-dependent transduction of MDMs by HIV vectors.
Delivery of antigen genes to antigen-presenting cells may represent a powerful method of inducing potent, long-lasting immunity. Lentiviral vectors are particularly suitable as delivery vehicles because they stably transduce nondividing cells and do not express any viral proteins. We therefore tested the ability of HIV-based vectors to transduce human monocytes and MDMs. Adherent PBMCs were isolated from whole blood and allowed to differentiate in human serum. On various days postisolation, cells were exposed to HIV vectors, pseudotyped with either VSV-G or MLV-A envelopes, at an MOI of 10 as determined by titer on human 293T cells. Vectors were produced from packaging constructs either encoding all HIV-1 accessory genes (CMVΔR8.2) or containing deletions of vif, vpr, vpu, and nef (CMVΔR8.9). VSV-G-pseudotyped MLV, which does not infect nondividing cells, was used as a control.
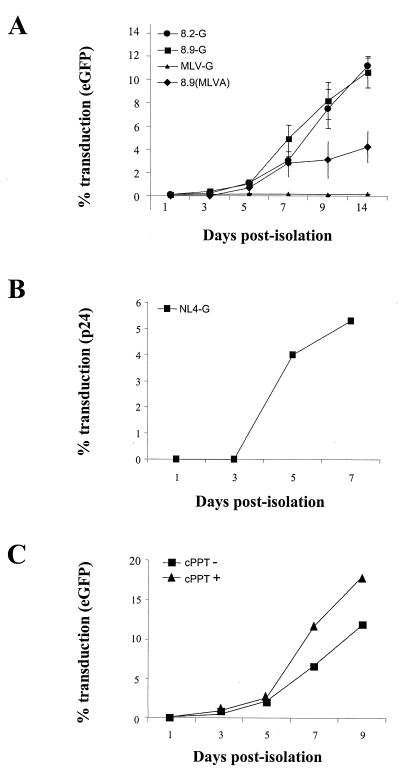
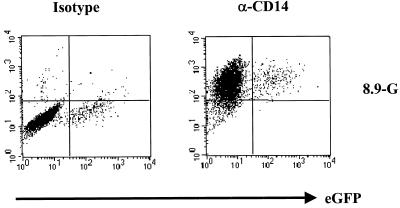
Freshly isolated monocytes were refractory to transduction by HIV vectors pseudotyped with either VSV-G or MLV-A envelopes. When these vectors were used at an MOI of 10, less than 0.5% of cells became transduced (Fig. 1A). Increasing the MOI above 50 led to cellular toxicity and did not result in increased transduction above 1% (not shown). However, after 5 days of culture in human serum the MDMs became progressively more susceptible to infection (Fig. 1A). The presence of the accessory proteins Vif, Vpr, Vpu, and Nef had no effect on this maturation-dependent transduction (Fig. 1A). A similar result was obtained with CMVΔR8.9 packaged vectors pseudotyped with the MLV-A envelope, which, unlike the pH-dependent VSV-G, fuses at the cell surface, indicating that this maturation dependence was not affected by route of entry. As expected, MLV vectors pseudotyped with VSV-G failed to transduce MDMs at any stage of differentiation. We confirmed that the cells which were transduced after exposure to vector at day 7 postisolation were macrophages by staining with an antibody to the lipopolysaccharide (LPS) binding protein receptor CD14. Figure 2 shows that over 90% of cells within the culture were CD14 positive and that the cells expressing eGFP were within the CD14-positive population.
FIG. 1.
Maturation dependence of HIV-1 infection in human MDMs. (A) HIV vector transduction. Adherent PBMCs were plated at 104 cells/well of a 96-well plate and infected with CMVΔR8.2(VSV-G) and CMVΔR8.9(VSV-G) packaged vectors (8.2-G and 8.9-G, respectively) or CMVΔR8.9(MLV-A) packaged vector [8.9(MLVA)] at an MOI of 10, as standardized on human 293T cells, on various days postisolation. Cells were cultured for 7 days posttransduction; percent cell transduction was then quantified by fluorescence-activated cell sorting. MLV vectors pseudotyped with VSV-G (MLV-G) were used as a control at the same MOI. Values are means ± standard errors of the means for three separate experiments. (B) HIV-1 infection. Titers of NL4-3 pseudotyped with VSV-G (NL4-G) were determined as for panel A, with the MOI standardized on 293T cells. Infection was scored by in situ p24 immunoassay. (C) Effect of cPPT on vector transduction (cPPT− or cPPT+). Titers of CMVΔR8.9(VSV-G) particles packaging pHR′-CMV-eGFP or HR′-cPPT-CMV-eGFP were determined as for panel A. The levels of p24 in both supernatants were equal (not shown), and infections were performed with an equivalent volume for which the cPPT− vector gave an MOI of 10 to standardize for virion input.
FIG. 2.
Identification of infected cells as macrophages Macrophages transduced with a CMVΔR8.9(VSV-G) packaged vector on day 7 postisolation were analyzed on day 14 for eGFP expression and stained with a PE-conjugated monoclonal antibody against the monocyte/macrophage marker CD14 or the equivalent PE-conjugated isotype control (y axis).
To demonstrate that there were no sequences within HIV-1 but absent from the HIV-based vectors which were required for monocyte transduction, we used the HIV-1 clone NL4-3, which is defective only in envelope and Nef, to produce virions pseudotyped with VSV-G. Infection was measured by in situ p24 expression. This virus also showed a similar dependence on monocyte differentiation for infection (Fig. 1B). A recently identified element within the HIV-1 pol gene, the cPPT, has been proposed to act as a second primer binding site during reverse transcription and lead to the formation of a DNA “flap,” which facilitates nuclear entry in nondividing cells (49). Inclusion of this sequence in vector genomes has allowed greater efficiency of transduction in a variety of nondividing primary cells, including mature macrophages (11). Vectors containing the cPPT were more efficient in transduction of mature macrophages; however, inclusion of the cPPT failed to allow transduction prior to day 5 of culture (Fig. 1C). This increase in transduction efficiency also explains that observed for the full-length NL4-3 pseudotype, which contains a cPPT, on day 5 of culture (Fig. 1B).
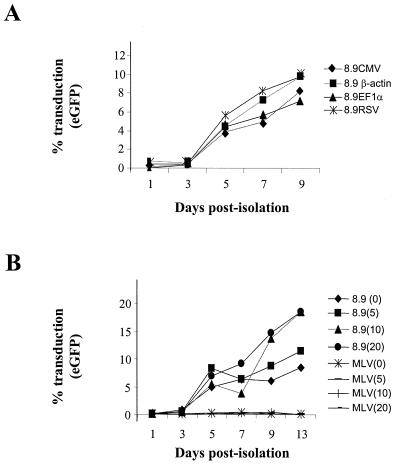
The marker gene for eGFP is driven from a minimal CMV promoter in the pHR′-CMV-eGFP vector. To assess whether the transgene promoter had any effect on the maturation-dependent transduction, VSV-G-pseudotyped vectors containing eGFP driven from the human β-actin or EF1α promoters or from the Rous sarcoma virus LTR (RSV) were generated. While the level of expression varied in permissive macrophages, none of these promoters allowed eGFP expression in monocytes (Fig. 3A). This suggested that lentiviral transduction of monocytes was blocked prior to proviral establishment and expression. It has previously been reported that pretreatment of vector stocks with dNTPs can increase transduction of some nondividing cells (30). To test whether this was also true for monocytes, vector stocks were treated with concentrations of dNTPs up to 20 mM for an hour prior to infection. Again, although the higher dNTP concentrations could increase titers on permissive macrophages, none had any effect on monocyte transduction (Fig. 3B). MLV(VSV-G) pseudotypes failed to transduce either monocytes or macrophages after dNTP treatment (Fig. 3B).
FIG. 3.
Maturation-dependent transduction is independent of transgene promoter and dNTP concentration. (A) MDMs were transduced as for Fig. 1A at an MOI of 10 with CMVΔR8.9(VSV-G) packaged vector with eGFP driven from either CMV, human β-actin, human EF1α, or RSV promoters. Cells were analyzed for eGFP expression 7 days posttransduction. (B) CMVΔR8.9(VSV-G) or MLV(VSV-G) vector stocks were treated for 1 h prior to infection with 0, 5, 10, or 20 μM concentrations of dNTPs as shown in parentheses and then used to infect MDMs at an MOI of 10 at various days postisolation. Cells were analyzed for eGFP expression 7 days posttransduction.
HIV-based vectors undergo reverse transcription but not nuclear entry in monocytes.
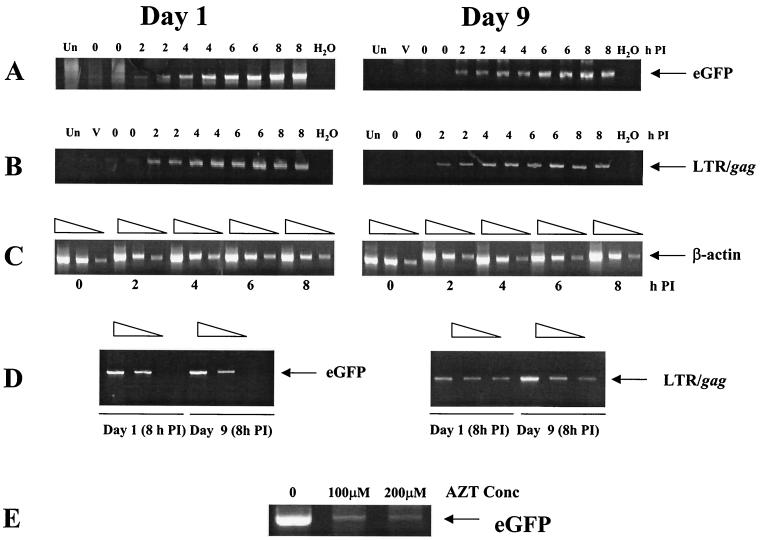
Since the VSV-G receptor is present on most human cell types, we reasoned that virion entry was unlikely to be the defect in vector transduction of monocytes. We therefore analyzed whether reverse transcription of vector genomes could occur in monocytes. To do this, 2 × 105 day 1 monocytes or day 9 mature macrophages were infected with CMVΔR8.9 packaged vector pseudotyped with VSV-G at an MOI of 10. At 2-h intervals after exposure to vector, cells were harvested and lysed, and PCR was performed for reverse transcription intermediates. PCR products of intermediate reverse transcripts containing eGFP sequences increased similarly over time in both monocytes and macrophages, indicating that reverse transcription initiation was not impaired in monocytes (Fig. 4A). Products resulting from reverse transcripts generated after the second-strand transfer (LTR/gag) also increased over time in both cell types (Fig. 4B). Human β-actin primers were used to demonstrate that similar amounts of cellular DNA was present in all samples (Fig. 4C). Serial dilution of the sample from the 8-h time point showed similar levels of eGFP and LTR/gag products in both immature and mature cells (Fig. 4D). PCR of an amount of viral supernatant equivalent to that used to infect the cells was negative (Fig. 4A and B). Furthermore, addition of the reverse transcriptase inhibitor zidovudine greatly inhibited generation of eGFP reverse transcripts (Fig. 4E). These data demonstrate that monocytes can support efficient reverse transcription and that the block to transduction in these cells must occur at a later stage in the viral life cycle.
FIG. 4.
Analysis of reverse transcription in monocytes (day 1) or mature macrophages (day 9). Cells were harvested at 2-h intervals after exposure to CMVΔR8.9(VSV-G) packaged vector at an MOI of 10. PCR was then performed for intermediate reverse transcripts containing eGFP sequences (A) and second-strand-transfer products containing LTR/gag sequences (B), and serial twofold dilutions were used for PCR of cellular β-actin for a loading control (C). PCR was also performed on supernatants containing an equivalent amount of virus to check for DNA contamination in the viral preparations (lanes V). Serial twofold dilutions of samples from the the 8-h time points of days 1 and 9 were used for PCR to estimate relative amounts of eGFP and LTR/gag transcripts (D). The reverse transcriptase inhibitor zidovudine (AZT) was added to day 1 cells exposed to CMVΔR8.9(VSV-G) packaged vector at an MOI of 10. After 14 h, PCR was performed on cell lysates to detect eGFP transcripts (E). Un, uninfected cells; PI, postinfection.
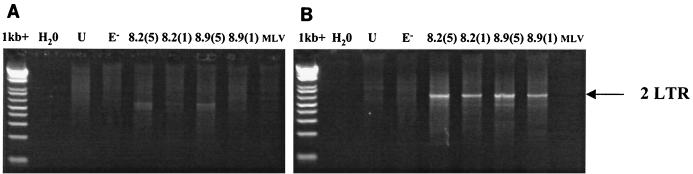
After completion of reverse transcription, HIV preintegration complexes traverse the cell and enter the nucleus. Ligases within the nucleus can circularize proviral DNA, before integration into the host chromosome can occur. Although these circles are believed to be nonfunctional, they can serve as measure of viral nuclear entry (41). To assess whether vector DNA enters the nucleus in monocytes, 106 day 1 monocytes or day 9 macrophages were infected with CMVΔR8.2 or CMVΔR8.9 packaged vector pseudotyped with VSV-G at an MOI of 5 or 1. At 48 h after exposure to vector, cells were lysed and PCR was performed to detect 2LTR circles. While circles were detected in mature macrophages, neither vector produced circles after infection of monocytes (Fig. 5). A smaller PCR product of the incorrect size can be seen at a low level in both cell types (Fig. 5). These data suggest that the block to transduction in monocytes is at a level between the completion of reverse transcription and nuclear entry. This block is not relieved by accessory viral gene products but appears to be regulated during monocyte-to-macrophage differentiation.
FIG. 5.
Analysis of nuclear 2LTR circles in infected monocytes (day 1 [A]) and mature macrophages (day 9 [B]). Cells were exposed to CMVΔR8.2(VSV-G) or CMVΔR8.9(VSV-G) packaged vectors overnight at MOIs of 5 and 1 (8.2 and 8.9, respectively). MLV(VSV-G), unenveloped CMVΔR8.2 (E−) vectors, and uninfected cell lysates (U) were used as controls. Cells were then washed, cultured for 48 h, and lysed. PCR with primers specific for 2LTR circles (36) was performed on an equivalent of 1.5 × 105 cells. The 2LTR product of 680 bp is indicated.
Transduction of monocyte-derived DCs is not maturation dependent.
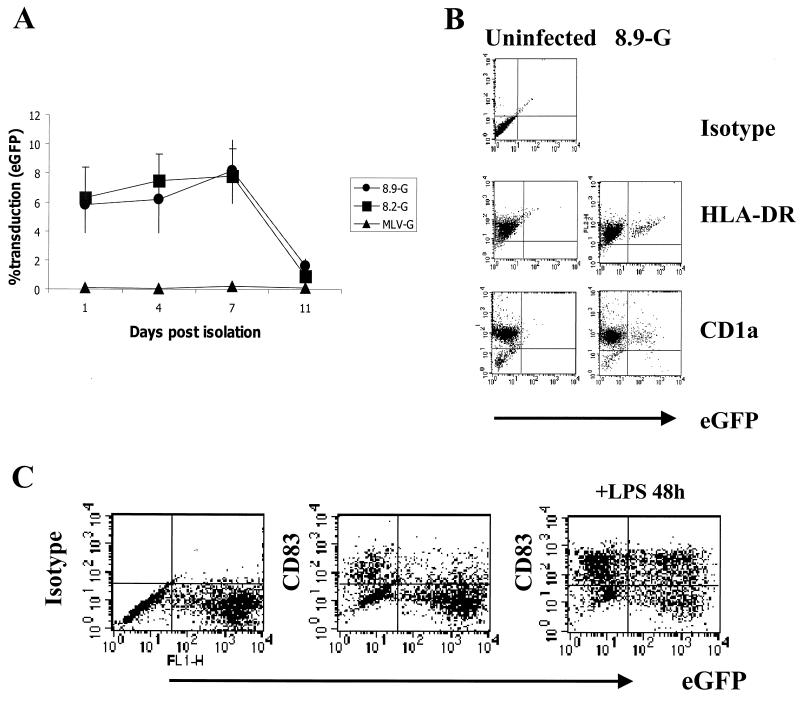
Recent reports show that human DCs can be transduced by lentiviral vectors (8, 18). Since DCs are differentiated from adherent monocytes in the presence of IL-4 and GM-CSF, we tested whether DC cultures exhibited maturation dependence similar to that observed for macrophages. Adherent monocytes were isolated as before, plated in RPMI medium plus 10% FCS and the DC differentiation-promoting cytokines IL-4 and GM-CSF, and transduced on day 1, 4, 7, or 11 postisolation as monocytes with both HIV-based vectors and MLV vectors pseudotyped with VSV-G. After washing, the cells were cultured for 7 days prior to analysis. Figure 6A shows that, unlike MDMs, monocyte-derived DC cultures were equally susceptible to transduction by HIV vectors on days 1, 4, and 7. The transduced cells were identified as DCs by the expression of the Langerhans cell marker CD1a and HLA-DR (Fig. 6B). Most transduced cells were CD83lo, indicating that they were immature DCs; they could be activated to CD83hi mature DCs by LPS stimulation (Fig. 6C). DCs cultured for 11 days showed a marked decrease in transduction efficiency (Fig. 6A), a result consistent with reported DC transduction with HIV vectors after prolonged culture (37). As shown earlier for macrophage transduction, DC transduction efficiency was independent of accessory gene function (Fig. 6A), and as expected, MLV-based vectors failed to transduce DCs at all time points.
FIG. 6.
Transduction of monocyte-derived DCs by HIV-based vectors. (A) Adherent monocytes were differentiated to DCs in RPMI medium containing 10% FCS, IL-4, and GM-CSF. Cells were exposed to CMVΔR8.2(VSV-G), CMVΔR8.9(VSV-G), or MLV(VSV-G) packaged vectors at an MOI of 10 for 12 h on various days after isolation as monocytes. Cells were the washed and cultured for 7 days and analyzed for eGFP expression by FACScan analysis. Results are means ± standard errors from three separate experiments. (B) Transduced cells were identified as DCs by expression of eGFP and staining with the Langerhans cell marker CD1a and HLA-DR (y axis). (C) Day 1 monocytes transduced with CMVΔR8.9(VSV-G) packaged HR′cPPTCMVeGFP vectors at at MOI of 10 were differentiated to DCs, cultured for a further 48 h with and without 50 ng of LPS/ml, and stained for surface expression of the DC activation marker CD83 (y axis). Cell populations shown were previously gated on HLA-DR+ CD1a+ DCs.
Monocyte transduction can be rescued by subsequent differentiation to DCs.
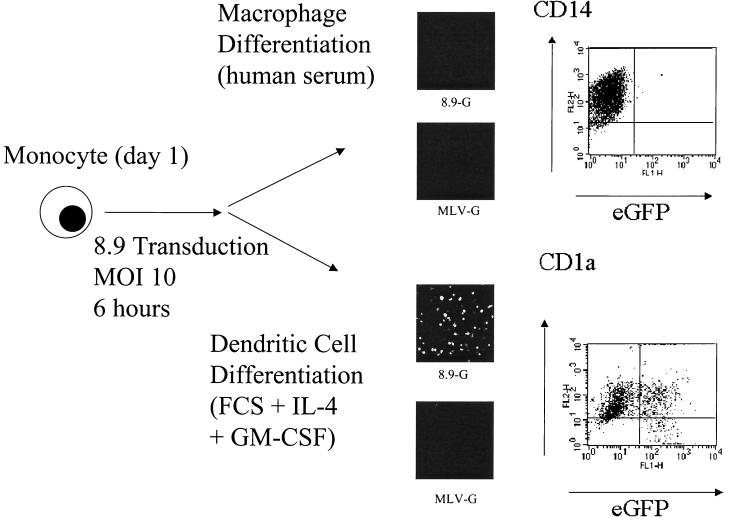
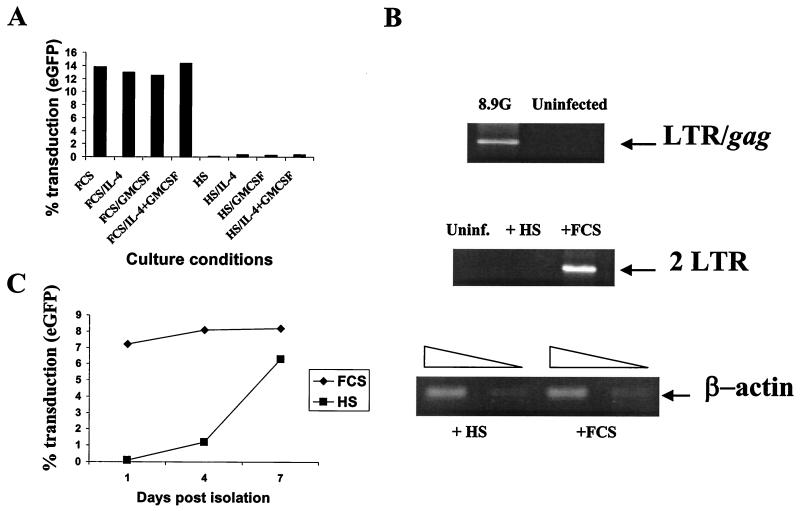
To assess whether DC differentiation could rescue vector transduction in monocytes postentry, adherent day 1 monocytes were exposed to CMVΔR8.9 pseudotyped VSV-G vectors at an MOI of 10 for 6 h in RPMI medium plus human serum. The cells were then washed thoroughly, replated, and differentiated to macrophages or DCs (Fig. 7). As expected, macrophage cultures did not express eGFP; however, the same monocytes differentiated to DCs became eGFP positive, demonstrating that vector rescue in these cells was at a postentry level. To assess the relative contribution of the factors required for rescue, day 1 monocytes transduced as described above for 6 h were replated in RPMI medium supplemented with FCS or human serum and with IL-4, GM-CSF, or both cytokines. As shown in Fig. 8A, all monocytes cultured in medium containing FCS became eGFP positive, whereas cells cultured in human serum showed little eGFP expression. Neither cytokine had a significant effect on vector rescue in monocytes cultured in human serum.
FIG. 7.
Monocyte differentiation to DCs rescues transduction. Adherent monocytes (106; day 1) were infected with CMVΔR8.9(VSV-G) or MLV(VSV-G) packaged vectors (8.9 and MLV, respectively) at an MOI of 10. At 6 h posttransduction, cells were washed and either plated in macrophage medium (RPMI medium plus 10% human serum) or DC differentiation medium (10% FCS plus IL-4 and GM-CSF). eGFP expression was assessed 7 days later by confocal microscopy of infected cells. Images were acquired on a Bio-Rad MRC 1064 confocal microscope at a magnification of ×200. eGFP expression in CMVΔR8.9-transduced cells was analyzed by fluorescence-activated cell sorting, and cells were phenotype stained for CD14 (macrophages) or CD1a (DCs).
FIG. 8.
FCS rescues transduction of monocytes by HIV-based vectors. (A) Monocytes exposed to CMVΔR8.9(VSV-G) packaged vectors at an MOI of 10 for 6 h were subsequently cultured in RPMI medium containing either FCS or human serum with IL-4, GM-CSF, or both cytokines. eGFP expression was analyzed 7 days later by fluorescence-activated cell sorting. (B) Effect of FCS on accumulation of 2LTR circles in monocytes. Day 1 monocytes were exposed to CMVΔR8.9(VSV-G) (8.9-G) packaged vectors at an MOI of 10 for 6 h in RPMI medium plus human serum. PCR was performed on a sample of these cells to detect second-strand-transfer reverse transcription products (LTR/gag). The monocytes were washed and replated in medium containing either human serum (HS) or FCS and cultured for a further 24 h. The cells were lysed, and PCR was performed to detect 2LTR circles and β-actin as a loading control. (C) DC culture in human serum renders these cells maturation dependent. DCs were cultured in either human serum or FCS and exposed to CMVΔR8.9(VSV-G) packaged vectors at an MOI of 10 on various days postisolation as monocytes, washed, and cultured for a further 7 days. eGFP expression was analyzed by fluorescence-activated cell sorting.
The rescue of the postentry block in monocytes by FCS treatment led us to study whether this had a positive effect on the accumulation of nuclear 2LTR circles in these cells. Day 1 monocytes were exposed to CMVΔR8.9 pseudotyped VSV-G vectors at an MOI of 10 for 6 h. At this time, second-strand-transfer PCR products could be detected in these cells (Fig. 8B). The cells were then replated in the presence of human serum or FCS for a further 24 h, whereupon the cells were lysed and PCR was performed to detect 2LTR circles. Figure 8B shows that FCS treatment was sufficient to rescue 2LTR circles in monocytes. DNA loading was assessed by actin PCR. Rescue by FCS treatment could not be achieved 48 h after exposure to vector, suggesting that functional preincubation complexes are degraded after this time (data not shown).
For clinical applications, DC culture in autologous human serum rather than FCS will be desirable. It must therefore be noted that DCs cultured in human serum plus IL-4 and GM-CSF showed the same maturation-dependent transduction as macrophages (Fig. 8C). The DCs which were transduced were phenotypically identical to those in the FCS–IL-4–GM-CSF cultures (data not shown).
DISCUSSION
In this study we found a block to HIV-1-based vector infection of monocytes at the level of nuclear entry that was not present after monocytes had matured into macrophages following 5 days of culture. Studies of HIV-1 infection have also reported a block in monocyte infection which could be relieved by macrophage differentiation (34). In the case of M-tropic strains of HIV-1, this has been explained by the up-regulation of the coreceptor CCR5 during macrophage differentiation, which relieves a block to viral entry (43). However, a postentry block to T-tropic HIV-1 infection of monocytes has also been described (36). Our work with VSV-G and MLV-A pseudotyped HIV-1-based vectors demonstrates that this block occurs irrespective of the viral route of entry and identifies nuclear translocation as the stage at which infection is inhibited. The extent to which our data can be extrapolated to the behavior of wild-type HIV-1 needs to be determined.
Differentiation of monocytes into DCs could rescue vectors that had already entered the cells. Surprisingly, the relief of this restriction was traced to the culture of these cells in FCS rather than cytokine treatment. Cytokine treatment is sufficient to rescue HIV vector transduction in quiescent T cells (44); however, these cells are blocked at the level of reverse transcription and T-cell activation causes a G0-to-G1b transition, a prerequisite for HIV-1 reverse transcription (24, 25). In monocytes which were not restricted at the level of reverse transcription, FCS treatment or macrophage differentiation had a positive effect on the accumulation of nuclear viral DNA. It will be of interest to determine which intracellular signaling pathway, either triggered or inhibited by FCS, relieves the block to monocyte infection. HIV-1 infection of primary T cells has been shown to be dependent on NFAT activation (20), whereas LPS stimulation of p38 has been shown to block HIV-1 infection of mature macrophages (52). The mechanism by which nuclear entry is regulated also remains unclear, although the HIV-1 accessory protein Vpr and the cPPT within the vector, which have been implicated in nuclear entry (19, 49), were not required for infection of macrophages or DCs. The HIV-1 envelope protein gp120 can activate intracellular signaling pathways via CD4 (5, 32) or chemokine receptors (1, 10, 27). Therefore, exposure of monocytes, MDMs, or DCs to some variants of gp120 may also affect their permissivity to HIV-1.
Kootstra and Schuitemaker (22) and Schuitemaker et al. (38) have proposed that HIV-1 infection of macrophages is restricted to proliferating cells. In their studies, block of the cell cycle in early G1 prevented reverse transcription due to an insufficient dNTP pool and G2 progression was associated with the detection of nuclear DNA (23). They described a block to HIV-1 infection in monocytes at the level of reverse transcription, suggesting an early G1 block (38). In contrast, we find that freshly isolated monocytes are competent for reverse transcription and that treatment of the virus with dNTPs fails to rescue transduction. Furthermore, infection can be rescued by DC differentiation, which does not involve proliferation as measured by Ki-67 staining or propidium iodide labeling of DNA content (17).
In order to transduce antigen-presenting cells with HIV-1-based vectors, the most rapid protocol will be to expose freshly isolated monocytes to the vector and then induce DC differentiation. Previous studies have induced DC differentiation for 3 to 8 days before HIV-1-based vector infection (8, 37). Transduction of freshly isolated cells will minimize the time of cell culture and allow DCs at various stages of differentiation to be compared for their ability to home to lymph nodes and persistently present antigen. Differentiation of DCs from lentivirus-transduced stem cells has been reported (26). Culture in FCS will, however, be undesirable. It will therefore be necessary to identify the signal which allows vector transduction and to stimulate this by another mechanism. Transduction of peripheral blood DCs will be a less invasive and probably cheaper clinical source of DCs for modification; however, the efficacy of immune response induction with DCs from these sources requires comparison.
This work was supported by the Medical Research Council, United Kingdom, and the Cancer Research Campaign, United Kingdom.
We are grateful to D. Trono and R. Zufferey for the supply of vector plasmids and technical advice. We thank Y. Takeuchi, A. McKnight, and P. Clapham for helpful comments and critical reading of the manuscript.
ACKNOWLEDGMENTS
S.N. and F.M. contributed equally to this study.
REFERENCES
- 1.Arthos J, Rubbert A, Rabin R L, Cicala C, Machado E, Wildt K, Hanbach M, Steenbeke T D, Swofford R, Farber J M, Fauci A S. CCR5 signal transduction in macrophages by human immunodeficiency virus and simian immunodeficiency virus envelopes. J Virol. 2000;74:6418–6424. doi: 10.1128/jvi.74.14.6418-6424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayehunie S, Garcia-Zepeda E, Hoxie J, Horuk R, Kupper T, Luster A, Ruprecht R. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood. 1997;90:1379–1386. [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Blauvert A, Asada H, Saville H W, Klaus-Kovtun V, Altman D, Yarchoan R, Katz S. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J Clin Investig. 1997;100:2043–2053. doi: 10.1172/JCI119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briant L, Robert-Hebmann V, Acquaviva C, Pelchen-Matthews A, Marsh M, Devaux C. The protein tyrosine kinase p56lck is required for triggering NF-kappaB activation upon interaction of human immunodeficiency virus type 1 envelope glycoprotein gp120 with cell surface CD4. J Virol. 1998;72:6207–6214. doi: 10.1128/jvi.72.7.6207-6214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinsky I M, Haggerty S, Dempsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of nondividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canque B, Rosenzwejg M, Camus S, Yagello M, Bonet M-L, Guigon M, Gluckman J. The effect of in vitro human immunodeficiency virus infection on dendritic cell differentiation and function. Blood. 1996;88:4215–4228. [PubMed] [Google Scholar]
- 8.Chinnasamy N, Chinnasamy D, Toso J F, Lapointe R, Candotti F, Morgan R A, Hwu P. Efficient gene transfer to human peripheral blood monocyte-derived dendritic cells using human immunodeficiency virus type 1-based lentiviral vectors. Hum Gene Ther. 2000;11:1901–1909. doi: 10.1089/10430340050129512. [DOI] [PubMed] [Google Scholar]
- 9.Collman R, Hassan N F, Walker R, Godfrey B, Cutilli J, Hastings J C, Friedman H, Douglas S D, Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989;170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis C, Dikic I, Unumatz D, Hill C, Arthos J, Siani M, Thompson D, Schlessinger J, Littman D. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 and CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Follenzi A, Ailles L, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 12.Frankel S, Wenig B, Burke M, Mannan P, Thompson L D, Abbondanzo S, Nelson A, Pope M, Steinman R. Replication of HIV-1 dendritic cell derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 13.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 15.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 16.Gendelman H, Orenstein J, Martin M, Ferrua C, Mitra R, Phipps T, Wahl L, Lane H, Fauci A, Burke D, Skillman D, Meltzer M. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman R M. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72:2733–2737. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber A, Kan-Mitchell J, Kuhen K L, Mukai T, Wong-Staal F. Dendritic cells transduced by multiply deleted HIV-1 vectors exhibit normal phenotypes and functions and elicit an HIV-specific cytotoxic T-lymphocyte response in vitro. Blood. 2000;96:1327–1333. [PubMed] [Google Scholar]
- 19.Heinzinger N K, Bukinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita S, Chen B, Kaneshima H, Nolan G. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- 21.Klein C, Bueler H, Mulligan R C. Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J Exp Med. 2000;191:1699–1708. doi: 10.1084/jem.191.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kootstra N A, Schuitemaker H. Proliferation-dependent replication in primary macrophages of macrophage-tropic HIV type 1 variants. AIDS Res Hum Retrovir. 1998;14:339–345. doi: 10.1089/aid.1998.14.339. [DOI] [PubMed] [Google Scholar]
- 23.Kootstra N A, Zwart B M, Schuitemaker H. Diminished human immunodeficiency virus type 1 reverse transcription and nuclear transport in primary macrophages arrested in early G1 phase of the cell cycle. J Virol. 2000;74:1712–1717. doi: 10.1128/jvi.74.4.1712-1717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korin Y, Zack J. Progression to G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korin Y, Zack J. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J Virol. 1999;73:6526–6532. doi: 10.1128/jvi.73.8.6526-6532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Mukai T, Young D, Frankel S, Law P, Wong-Staal F. Transduction of CD34+ cells by a vesicular stomach virus protein G (VSV-G) pseudotyped HIV-1 vector. Stable gene expression in progeny cells, including dendritic cells. J Hum Virol. 1998;1:346–352. [PubMed] [Google Scholar]
- 27.Liu Q H, Williams D A, McManus C, Baribaud F, Doms R W, Schols D, De Clercq E, Kotlikoff M I, Collman R G, Freedman B D. HIV-1 gp120 and chemokines activate ion channels in primary macrophages through CCR5 and CXCR4 stimulation. Proc Natl Acad Sci USA. 2000;97:4832–4837. doi: 10.1073/pnas.090521697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackensen A, Herbst B, Chen J L, Kohler G, Noppen C, Herr W, Spagnoli G C, Cerundolo V, Lindemann A. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34(+) hematopoietic progenitor cells. Int J Cancer. 2000;86:385–392. doi: 10.1002/(sici)1097-0215(20000501)86:3<385::aid-ijc13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Nair S K, Hull S, Coleman D, Gilboa E, Lyerly H K, Morse M A. Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T-lymphocyte responses in vitro using autologous dendritic cells loaded with CEA peptide or CEA RNA in patients with metastatic malignancies expressing CEA. Int J Cancer. 1999;82:121–124. doi: 10.1002/(sici)1097-0215(19990702)82:1<121::aid-ijc20>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Naldini L, Blomer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 32.Popik W, Hesselgesser J, Pitha P. Binding to human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signalling pathway. J Virol. 1998;72:6406–6413. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potts B, Maury W, Martin M. Replication of HIV-1 in primary monocyte cultures. Virology. 1990;175:465–476. doi: 10.1016/0042-6822(90)90431-p. [DOI] [PubMed] [Google Scholar]
- 34.Rich E, Chen I, Zack J, Leonard M, O'Brien W. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Investig. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroers R, Sinha I, Segall H, Schmidt-Wolf I G, Rooney C M, Brenner M K, Sutton R E, Chen S Y. Transduction of human PBMC-derived dendritic cells and macrophages by an HIV-1-based lentiviral vector system. Mol Ther. 2000;1:171–179. doi: 10.1006/mthe.2000.0027. [DOI] [PubMed] [Google Scholar]
- 38.Schuitemaker H, Kootstra N A, Koppelman M H, Bruisten S M, Huisman H G, Tersmette M, Miedema F. Proliferation-dependent HIV-1 infection of monocytes occurs during differentiation into macrophages. J Clin Investig. 1992;89:1154–1160. doi: 10.1172/JCI115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soto-Ramirez L E, Renjifo B, McLane M F, Marlink R, O'Hara C, Sutthent R, Wasi C, Vithayasai P, Vithayasai V, Apichartpiyakul C, Auewarakul P, Pena Cruz V, Chui D S, Osathanondh R, Mayer K, Lee T H, Essex M. HIV-1 Langerhans' cell tropism associated with heterosexual transmission of HIV. Science. 1996;271:1291–1293. doi: 10.1126/science.271.5253.1291. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Pinchuk L M, Agy M B, Clark E A. Nuclear import of HIV-1 DNA in resting CD4+ T cells requires a cyclosporin A-sensitive pathway. J Immunol. 1997;158:512–517. [PubMed] [Google Scholar]
- 42.Tsunestugu-Yokata Y, Akagawa K, Kimoto K, Suzuki K, Iwasaki M, Yasuda S, Hausser G, Hultgren C, Meyerhans A, Takemori T. Monocyte-derived cultured dendritic cells are susceptible to human immunodeficiency virus infection and transmit virus to resting T cells in the process of normal antigen presentation. J Virol. 1995;69:4544–4547. doi: 10.1128/jvi.69.7.4544-4547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuttle D L, Harrison J K, Anders C, Sleasman J W, Goodenow M M. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol. 1998;72:4962–4969. doi: 10.1128/jvi.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unumatz D, KewalRamani V, Marmon S, Littman D. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valentin A, Trivedi H, Lu W, Kostrikis L G, Pavlakis G N. CXCR4 mediates entry and productive infection of syncytia-inducing (X4) HIV-1 strains in primary macrophages. Virology. 2000;269:294–304. doi: 10.1006/viro.1999.0136. [DOI] [PubMed] [Google Scholar]
- 46.Weissman D, Li Y, Ananworanich J, Zhou L-J, Adelsberger J, Tedder T, Baseler M, Fauci A. Three populations of cells with dendritic cell morphology exist in the peripheral blood, only one of which is infectable [sic] with human immunodeficiency type 1. Proc Natl Acad Sci USA. 1995;92:826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu L, Gerard N, Wyatt R, Choe C, Parolin C, Ruffing N, Borsetti A, Cardoso A, Desjardin E, Newman W, Gerard C, Sodrowski J. CD4 induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 48.Yi Y, Isaacs S N, Williams D A, Frank I, Schols D, De Clercq E, Kolson D L, Collman R G. Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism. J Virol. 1999;73:7117–7125. doi: 10.1128/jvi.73.9.7117-7125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 50.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 51.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 52.Zybarth G, Reiling N, Schmidtmayerova H, Sherry B, Bukrinsky M. Activation-induced resistance of human macrophages to HIV-1 infection in vitro. J Immunol. 1999;162:400–406. [PubMed] [Google Scholar]