Abstract
SNARE functions during membrane docking and fusion are regulated by Sec1/Munc18 (SM) chaperones and Rab/Ypt GTPase effectors. These functions for yeast vacuole fusion are combined in the six-subunit HOPS complex. HOPS facilitates Ypt7p nucleotide exchange, is a Ypt7p effector, and contains an SM protein. We have dissected the associations and requirements for HOPS, Ypt7p, and Sec17/18p during SNARE complex assembly. Vacuole SNARE complexes bind either Sec17p or the HOPS complex, but not both. Sec17p and its co-chaperone Sec18p disassemble SNARE complexes. Ypt7p regulates the reassembly of unpaired SNAREs with each other and with HOPS, forming HOPS·SNARE complexes prior to fusion. After HOPS·SNARE assembly, lipid rearrangements are still required for vacuole content mixing. Thus, Sec17p and HOPS have mutually exclusive interactions with vacuole SNAREs to mediate disruption of SNARE complexes or their assembly for docking and fusion. Sec17p may displace HOPS from SNAREs to permit subsequent rounds of fusion.
Keywords: fusion, HOPS, membrane, Rab, SNARE
Introduction
In eukaryotic cells, transport between membrane-enclosed compartments occurs by a conserved mechanism of vesicle budding, trafficking, and fusion. Vesicle docking and fusion requires SNAREs, a family of membrane proteins that assemble into characteristic bundles. SNARE interacting proteins of the Sec1/Munc18 (SM) family, small Rab/Ypt GTPases, and a diverse group of Rab-interacting proteins, termed effectors, are also essential. SNARE complexes in cis (in the same membrane) are disassembled by α-SNAP and NSF chaperones, permitting trans (between vesicles) interactions (Sollner et al, 1993; Mayer et al, 1996; Ungermann et al, 1998b). Rabs coordinate this assembly process through GTP-dependent associations with effectors prior to trans-SNARE complex formation, although how this occurs remains unclear. One postulate is that Rabs and their effector complexes catalyze the assembly of cognate SNARE complexes (Sogaard et al, 1994; Shorter et al, 2002).
Yeast vacuole SNAREs are associated with each other in cis-SNARE complexes and with the priming co-chaperone Sec17p (α-SNAP) (Ungermann et al, 1998a). Vacuoles have at least five abundant SNAREs: Vam3p, Vam7p, Vti1p, Nyv1p, and Ykt6p (Nichols et al, 1997; Ungermann et al, 1999). Most known SNARE complexes have a ‘3Q:1R' composition, where the Q or R residues at the center of the coiled-coil ‘SNARE' motif interact in four helix bundles (Fasshauer et al, 1998). Vam3p, Vti1p, and Vam7p are Q-SNAREs, and Nyv1p and Ykt6p are R-SNAREs. While most SNAREs are integral membrane proteins, Vam7p is water soluble. It is bound to membranes by its affinities for other SNAREs and for phosphatidylinositol 3-phosphate via its PX (Phox homology) domain (Cheever et al, 2001; Boeddinghaus et al, 2002). Vam7p may also interact with the Rab GTPase Ypt7p (Uetz et al, 2000; Ungermann et al, 2000).
The functions of Ypt7p and SNAREs are coupled by the HOPS (homotypic fusion and vacuole protein sorting)/Class C Vps complex (Price et al, 2000a; Sato et al, 2000; Seals et al, 2000). The HOPS complex contains six polypeptides, Vps11p, 16p, 18p, 33p, 39p, and 41p, identified genetically through screens for vps (Rothman and Stevens, 1986), pep (Jones, 1977), and vam (Wada et al, 1992) phenotypes of defective vacuole trafficking, function, and structure. Homologous genes in other organisms regulate pigment transport to lysosomes and storage granule compartments (Warner et al, 1998; Sevrioukov et al, 1999; Suzuki et al, 2003). Distinct phenotypes arise upon deletion of the genes encoding yeast HOPS subunits. Class B mutants (Vps39 and 41) have moderate vacuole fragmentation, while Class C (Vps11, 16, 18, and 33) mutants cause severe vacuole fragmentation. The complex of the four Class C Vps proteins associates with accessory factors such as either Vps39p/Vps41p or Vps8p to mediate distinct trafficking and fusion events (Sato et al, 2000; Seals et al, 2000; Richardson et al, 2004; Subramanian et al, 2004). HOPS can stimulate nucleotide exchange on Ypt7p and specifically associates with GTP-bound Ypt7p (Seals et al, 2000; Wurmser et al, 2000). Since one HOPS subunit, Vps33p, is an SM family member, Ypt7p interactions may regulate HOPS associations with vacuole SNAREs. The integration of these functions in a single protein complex makes HOPS a unique model to study the intersection between Rab and SNARE functions.
Yeast vacuole fusion, reconstituted in vitro, proceeds through experimentally defined subreactions. Sec18p (NSF) disassembles cis-SNARE complexes during priming, releasing Sec17p and the soluble SNARE Vam7p from vacuoles (Mayer et al, 1996; Boeddinghaus et al, 2002). Docking, the association of primed vacuoles prior to fusion, occurs in three stages: Ypt7p-dependent vacuole tethering, the assembly of fusion factors into a specific membrane microdomain termed the ‘vertex' ring, and SNARE pairing in trans (Mayer and Wickner, 1997; Ungermann et al, 1998b; Wang et al, 2002). SNARE complex formation leads to calcium release from lumenal stores, bilayer fusion, and aqueous compartment mixing (Peters and Mayer, 1998; Weber et al, 1998; Merz and Wickner, 2004b).
To explore the interactions between SNAREs and their chaperones, we have isolated vacuolar SNARE-associated complexes. Although we have previously shown that HOPS associates with Vam3p and Nyv1p (Price et al, 2000a), only a small fraction of HOPS or SNAREs associates in this manner. We now report that vacuole SNAREs are found in four distinct pools. The first is dissociated SNAREs. The remaining SNARE complexes are associated with Sec17p, HOPS, or neither. These SNARE complex associations have distinct functions during vacuole fusion. Sec17p-containing SNARE complexes are disassembled by Sec18p to establish a pool of unpaired SNAREs, which are required for subsequent interactions in trans. Ypt7p regulates the assembly of HOPS-containing SNARE complexes for vacuole docking prior to fusion. Sec17p can displace HOPS from SNARE complexes; this may allow disassembly by Sec18p into free SNAREs for subsequent rounds of docking and fusion. Thus, Sec17p and HOPS have distinct cycles of associations with SNAREs, controlling either SNARE complex disruption or SNARE complex assembly for vacuole docking and fusion.
Results
SNARE complexes from yeast vacuoles
We have characterized the spatial distribution of several green fluorescent protein (GFP)-tagged proteins during yeast vacuole docking (Wang et al, 2002, 2003b). These fusion proteins, expressed from their native promoters, permit normal vacuole structure in vivo and function normally for vacuole fusion in vitro (Wang et al, 2002, 2003b). We now use these GFP tags to study the physical associations of SNAREs. Vacuoles purified from strains expressing GFP-tagged proteins or from their untagged parental strains were solubilized with Triton X-100 and immunoprecipitated with antibodies to GFP. Immunoisolated complexes of three GFP-tagged SNAREs (Vam7p, Vti1p, and Vam3p) were subjected to SDS–PAGE and silver staining (Figure 1, top). A total of 11 bands were found specifically in immunoprecipitates from tagged Vam7p and Vam3p strains (10 for Vti1p). Other specifically associated polypeptides were breakdown products of tagged SNAREs.
Figure 1.
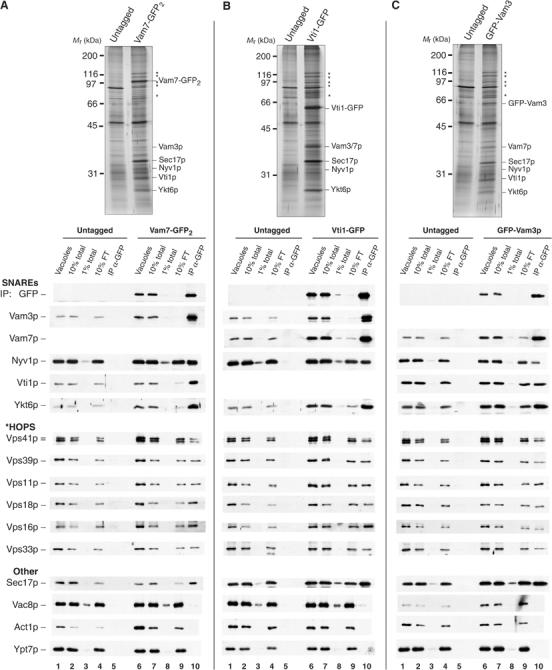
Isolation of SNARE complexes from yeast vacuoles. Vacuoles isolated from (A) untagged (DKY6281) and Vam7-GFP2, (B) untagged (BJ2168) and Vti1-GFP, and (C) untagged (BJ3505) and GFP-Vam3p-tagged yeast strains were solubilized and immunoprecipitated with antibodies to GFP (see Materials and methods). Immunoprecipitates were analyzed by SDS–PAGE, silver staining (top), and immunoblotting (bottom). Vacuoles were prepared by large-scale fermentation and batch purification (Ungermann et al, 1999; Seals et al, 2000). Vacuoles in PS buffer (120 μg) were reisolated by centrifugation (11 000 g, 20 min, 4°C). Pellets were suspended in solubilization buffer (450 μl; see Materials and methods). In all, 10% of the suspension (vacuoles, lanes 1 and 6) and high-speed supernatants (10% total, lanes 2 and 7; 1% total, lanes 3 and 8) were removed prior to immunoprecipitation (overnight, 4°C) with immobilized antibodies to GFP. The beads were collected, and 10% of the flow-through supernatant was removed (10% FT, lanes 4 and 9). Collected beads were resuspended five times with solubilization buffer and bound material was eluted by heating in sample buffer (IP, lanes 5 and 10). The samples were separated by SDS–PAGE and silver stained (top) or transferred to nitrocellulose and decorated with antibodies (bottom). Polypeptides identified by immunoblot are indicated; HOPS subunits are denoted by asterisks.
The identities of the SNARE complex proteins were established by immunoblot (Figure 1, bottom). Immunoprecipitates of Vam7-GFP2 and Vti1-GFP contained between 50 and 90% of the vacuole SNAREs Vam3p, Vti1p, Vam7p, and Ykt6p (Figure 1A and B, compare lanes 7 to 9 and 10). Over 90% of Nyv1p remained in the immunodepleted extract, consistent with its higher abundance relative to Vam3p (Wang et al, 2002; Huh et al, 2003). Qualitatively similar results were seen for immunoprecipitates of GFP-Vam3p, but a smaller proportion of other SNAREs were found in complex with Vam3p (Figure 1C). The efficiency of SNARE crossprecipitation showed considerable variation that depended upon the background strain used for the vacuole isolation and which SNARE was epitope tagged. However, in each case, almost all the Vam7p was found in complex with other SNAREs.
Vacuole SNARE proteins also interact with Sec17p and HOPS. About 10% of the vacuolar Sec17p was associated with each immunoprecipitated SNARE (Figure 1). The high-molecular-weight polypeptides associated with the SNAREs (Figure 1, top, asterisks) are the HOPS complex, as confirmed by immunoblotting (Figure 1A–C, bottom). Approximately 10% of each of the six HOPS subunits was specifically immunoprecipitated with the three GFP-tagged SNARE proteins (Figure 1A–C, compare lanes 7 and 10). Previous studies had suggested that HOPS interacts exclusively with unpaired Vam3p (Sato et al, 2000). However, immunoprecipitates of either Vam7p or Vti1p have as much HOPS as is recovered in association with Vam3p (Figure 1A–C, lane 10). Vac8p, an armadillo repeat-containing protein, can also associate with vacuole SNAREs (Veit et al, 2001), but only low levels (<1%) of Vac8p were recovered with GFP-tagged SNARE proteins under our isolation conditions. Actin and Ypt7p, although present in detergent extracts of vacuole membranes, were only recovered in immunoprecipitates at levels seen from control extracts (Figure 1, bottom, compare lanes 5 and 10). Other conditions of vacuole solubilization may reveal associations that are not preserved here, and further optimization may allow the resolution of substoichiometric components of vacuole SNARE complexes.
Sec17p and HOPS have distinct SNARE associations
Reciprocal immunoprecipitation showed that HOPS and Sec17p are not in the same SNARE complexes. Detergent extracts of vacuoles bearing no fusion protein tag, Vps33-GFP, or GFP-Vam3p were incubated with immobilized antibodies to either GFP or Sec17p (Figure 2A). The other subunits of the HOPS complex were specifically and efficiently co-immunoprecipitated with Vps33-GFP (Figure 2A, lane 2). Sec17p immunoprecipitates from the same vacuole detergent extract had a similar level of SNARE complex as found with GFP-Vam3p but had little detectable HOPS (Figure 2A, compare lane 3 with lanes 4–6). To confirm this, vacuoles bearing either no GFP fusion, Vps33-GFP, or GFP-Vps39p were solubilized in detergent and immunoprecipitated with antibodies to GFP (Figure 2B). Similar levels of other HOPS subunits were recovered with either Vps33-GFP or GFP-Vps39p, and both specifically associated with each of the vacuole SNAREs (Figure 2B, compare lane 7 to lanes 8 and 9). In contrast, we found little or no Sec17p or Ypt7p co-immunoprecipitating with HOPS (Figure 2B, lanes 8 and 9). Immunoprecipitation with antibodies to Sec17p or Vam3p yielded similar levels of SNARE complex (Figure 2B, lanes 10 and 11). Strikingly, Sec17p and its associated SNAREs bore no HOPS (lane 10), while antibody to Vam3p with the capacity to co-immunoprecipitate all SNARE complexes yielded both Sec17p and HOPS (lane 11).
Figure 2.
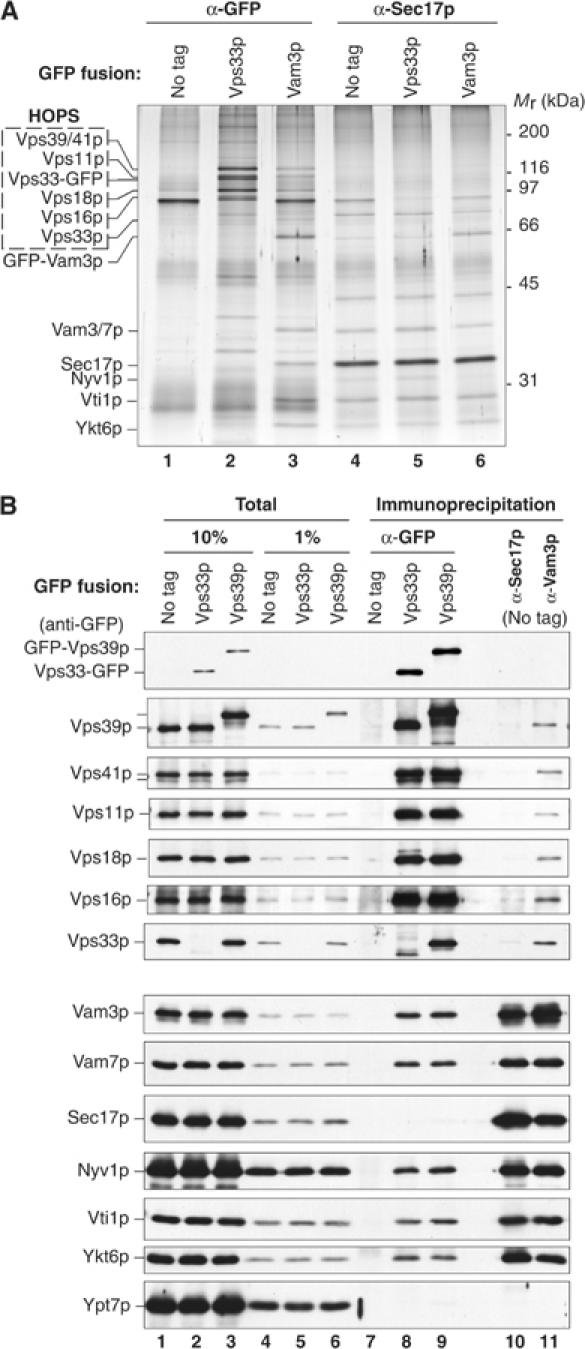
Complexes of HOPS and multiple vacuole SNAREs lack Sec17p. (A) Vacuoles isolated from untagged (BJ2168), Vps33-GFP-tagged, and GFP-Vam3p-tagged yeast strains (300 μg each) were sedimented (11 000 g, 20 min, 4°C) and suspended in 1 ml of solubilization buffer (see Materials and methods). A portion of each extract and an equivalent volume of the high-speed supernatant were removed, and the remaining extracts were divided in half for immunoprecipitation (1 h, 4°C) with affinity-purified antibodies to GFP (lanes 1–3) or Sec17p (lanes 4–6). The immunoprecipitated material was separated by SDS–PAGE and silver stained. (B) Vps33p and Vps39p are similarly associated with vacuole SNAREs but not Sec17p. Vacuoles (120 μg) bearing no GFP fusion protein (DKY6281), or Vps33-GFP or GFP-Vps39p were solubilized (see Materials and methods) and immunoprecipitated with affinity-purified antibodies to GFP (lanes 7–9), Sec17p (lane 10), or Vam3p (lane 11).
If HOPS and Sec17p had been in the same SNARE complex, it would have been detectable, based on the comparable efficiencies of Sec17p and Vam3p co-immunoprecipitation (Figure 2B, lanes 10 and 11). Approximately 20% of Vam3p co-immunoprecipitated with Sec17p (Figure 2B, lanes 1 and 10) and 10% of Sec17p co-immunoprecipitated with Vam3p (Figure 2B, lanes 1 and 11). If the complex of HOPS and Vam3p had also contained Sec17p, immunoprecipitation of HOPS should recover a similar proportion of Sec17p and Vam3p. We found that about 5% of the Vam3p was associated with Vps33-GFP/HOPS, but less than 1% of Sec17p was found associated with HOPS (Figure 2B, lanes 8 and 9). Thus, HOPS is associated with less Sec17p than would have been expected if the HOPS-associated Vam3p bore the same proportion of Sec17p seen for the total Vam3p population. This demonstrates that Vam3p has unique and separate associations with HOPS or Sec17p.
To test whether the complexes of other SNAREs with HOPS also lack Sec17p, we determined whether quantitative immunodepletion of Sec17p removed any of the SNAREs that are associated with the HOPS complex. Detergent extracts were prepared from vacuoles bearing Vps33-GFP or Vam7-GFP. One-half of each extract was immunoprecipitated with antibodies to GFP, and the other half was twice immunodepleted with antibodies to Sec17p and then immunoprecipitated with antibodies to GFP (Figure 3A). At each immunoprecipitation step, the antibody-bound (IP) and flow-through samples (FT) were examined for HOPS, SNARE, and Sec17p content. This experiment was designed to determine whether a thorough depletion of Sec17p-containing SNARE complexes would also diminish the HOPS-associated SNARE complexes. Immunodepletion with Sec17p antibodies removed approximately 90% of Sec17p and 70–80% of Vam7p, but only 10% of the other SNAREs and little, if any, of the HOPS from the detergent extracts (Figure 3B and C, compare lanes 1 and 5). The level of SNAREs recovered with Vps33-GFP was unaffected by Sec17p depletion (Figure 3B, compare lanes 7 and 10), and identical results were obtained with GFP-Vps39p vacuoles (data not shown). Of the 10% of Sec17p that remained in the Sec17p-depleted extract, little was associated with Vps33-GFP or Vam7-GFP (Figure 3B and C, compare lanes 5, 6, and 10). Although a high proportion of the vacuole-bound Vam7p associates with Sec17p, a substantial portion (≈90%) of the other vacuole SNAREs were not found in complex with Sec17p or Vam7p (Figure 3C, compare lanes 1, 6, and 10). Because it lacks a transmembrane anchor, Vam7p only stably associates with vacuoles via interactions with factors such as the SNAREs. More Vam3p, Vti1p, and Ykt6p remained associated with Vam7-GFP after Sec17p depletion than was associated with Vps33-GFP/HOPS (Figure 3B and C, compare lanes 2–10), showing that there is a pool of SNARE complexes that lack both Sec17p and HOPS. Four distinct vacuole SNARE associations are delineated: in this experiment, approximately 85% of SNAREs are unpaired, 10% are in Sec17p·SNARE complexes, 1% are in HOPS·SNARE complexes, and 4% are in SNARE complexes with neither Sec17p nor HOPS. The absolute percentage of SNAREs that are unpaired or associate with HOPS varies among yeast strains and vacuole preparations (compare Figures 1, 2, 3 and 4).
Figure 3.
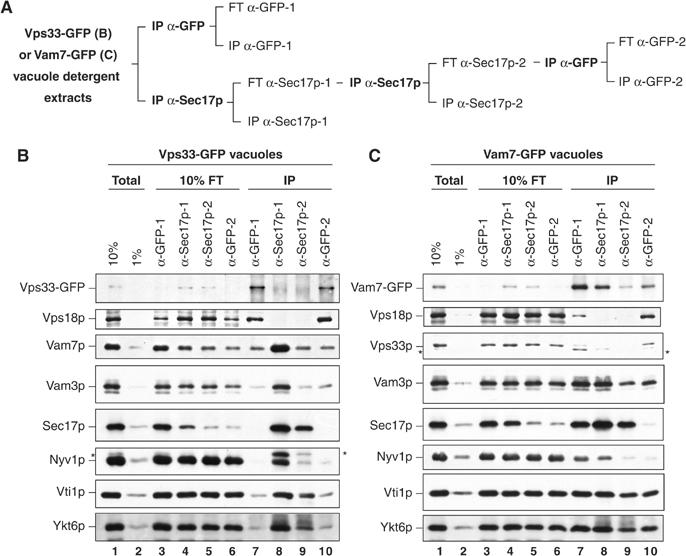
Immunodepletion of Sec17p does not affect the proportion of SNAREs found associated with HOPS. (A) The Sec17p immunodepletion strategy. Detergent extracts of vacuoles bearing either Vps33-GFP (B) or Vam7-GFP (C) were prepared (Total, lanes 1 and 2) and immunoprecipitated with antibodies to GFP (IP α-GFP-1, lane 7) or Sec17p (IP α-Sec17p-1, lane 8). The immunodepleted extracts from the first anti-GFP and anti-Sec17p immunoprecipitations were designated flow-through (FT α-GFP-1, lane 3; FT α-Sec17p-1, lane 4). The FT α-Sec17p-1 extract was immunodepleted again with fresh antibodies to Sec17p (IP α-Sec17p-2, lane 9). The twice depleted material was decanted, and an aliquot saved as FT α-Sec17p-2 (lane 5). The extract was then immunoprecipitated with antibodies to GFP (IP α-GFP-2, lane 10), and an aliquot of the material remaining after this immunoprecipitation was removed (FT α-GFP-2, lane 6). After immunoprecipitations, bound material was eluted by heating in reducing sample buffer for SDS–PAGE and immunoblotting. Asterisks indicate inefficiently stripped immunoreactive material leftover from previous antibody incubations.
Figure 4.
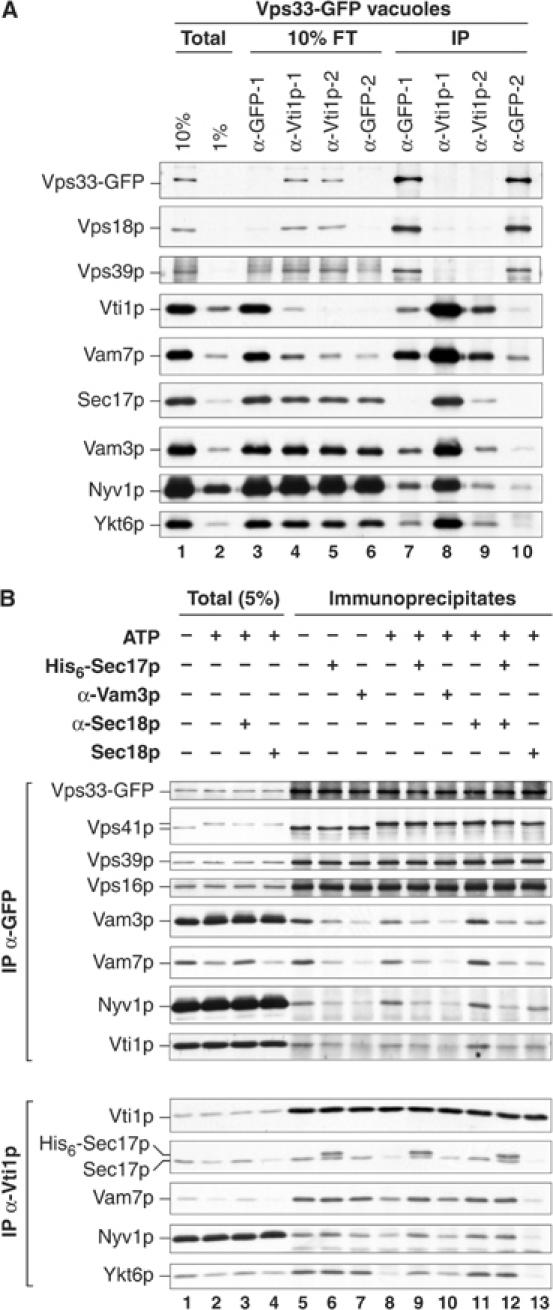
Sec17p can displace HOPS from interactions with SNARE complexes. (A) HOPS interacts with vacuole SNARE complexes. As outlined in Figure 3A, detergent extracts of vacuoles bearing Vps33-GFP (300 μg) were prepared and samples representing 10 and 1% of the extract (Total, lanes 1 and 2) were removed. The extracts were then divided. One-half was immunoprecipitated with antibodies to GFP (IP α-GFP-1, lane 7). The other extract was sequentially immunodepleted (1 h each) with immobilized antibodies to Vti1p (IP α-Vti1p-1, lane 8; IP α-Vti1p-2, lane 9) and then immunoprecipitated with antibodies to GFP (IP α-GFP-2, lane 10). After each step, samples were assayed for the extent of immunodepletion (see Figure 3A). After immunoprecipitation, bound material was eluted by boiling in sample buffer for SDS–PAGE and immunoblotting. (B) Sec17p displaces SNAREs from HOPS. Vacuoles (60 μg) bearing Vps33-GFP were incubated with buffer, anti-Sec18p Fab, anti-Vam3p Fab, or Sec18p (10 μg/ml) for 5 min on ice. Sec17p (24 μg/ml) was added where indicated and the reactions were further incubated for 5 min. Samples were normalized to fusion reaction salt conditions (all treatments) and an ATP regenerations system (see Materials and methods) was added where indicated. Reactions were incubated at 27°C (15 min), placed on ice (5 min), and vacuoles were reisolated (11 000 g, 10 min, 4°C). Pellets were extracted with solubilization buffer (400 μl), and insoluble material was removed (see Materials and methods). A sample of each solubilized extract was heated in sample buffer (Total, 5%). From one-half of the extract, Vps33-GFP was immunoprecipitated with antibodies to GFP and the other half was immunoprecipitated with antibodies to Vti1p (16 h, 4°C).
Sec17p can displace HOPS from SNARE complex interactions
While HOPS interacts with SNARE proteins, it was unclear whether this represents a direct affinity of HOPS for each of several unpaired vacuole SNAREs, for SNARE complexes lacking Sec17p, or for both. To assess this, we used an immunodepletion approach as outlined in Figure 3 to test whether removal of the SNARE Vti1p affected the associations of other SNAREs found associated with Vps33-GFP (Figure 4A). Depletion of greater than 90% of Vti1p caused the loss of Vam7p, Vam3p, Nyv1p, and Ykt6p associations with HOPS. Immunodepletion of Vti1p had only a modest effect on the overall levels of HOPS, Vam3p, Nyv1p, and Ykt6p in the detergent extract, in accord with the large portion of HOPS that is not SNARE associated and of SNAREs that are not in complex (Figures 2 and 3). Thus, most HOPS·SNARE complexes represent simultaneous interactions of HOPS with multiple vacuole SNAREs. However, it was unclear whether HOPS interacts with several unpaired SNAREs or only with intact SNARE complexes. The observed lack of Sec17p in HOPS·SNARE complexes (Figure 2) could result from either of these modes of interaction.
To clarify the nature of the HOPS·SNARE interaction, we tested the ability of different SNARE ligands to alter the association of each SNARE with HOPS. Incubation of vacuoles bearing Vps33-GFP with ATP did not affect the associations of HOPS subunits with each other or with the vacuole membrane (Figure 4B top, compare lanes 1–4 and 5 to 8). Similarly, ATP had little effect on the Vam3p, Vti1p, Vam7p, and Nyv1p recovered with HOPS (Figure 4B, compare lanes 5 and 8), as reported (Wang et al, 2003a). Immunoprecipitation of Vti1p revealed a loss of Sec17p and SNARE associations upon ATP treatment (Figure 4B, bottom, compare lanes 5 and 8). This disassembly was prevented by antibodies to Sec18p (lane 11). Given the exclusive interactions of SNARE complexes with HOPS or Sec17p, we determined whether added Sec17p affects the yield of HOPS·SNARE complexes. The addition of an elevated concentration of Sec17p (≈800 nM) inhibits vacuole fusion through recapture of primed SNAREs into cis-SNARE complexes (Wang et al, 2000). Added His6-Sec17p associated with vacuole SNARE complexes and decreased the levels of SNAREs recovered with HOPS (Figure 4B, compare lanes 5 and 6). The reduction of SNARE associations with HOPS occurred even when priming was blocked by antibodies to Sec18p or by omission of ATP (Figure 4B, compare lanes 5, 6, 8, 9, 11, and 12). Other factors may also be needed for Sec17p to convert HOPS·SNARE complexes to Sec17p·SNARE complexes. Nevertheless, these results provide further evidence that Sec17p and HOPS do not simultaneously interact with SNAREs.
Incubation with Fab fragments of antibody to Vam3p also caused a clear reduction in the amount of SNAREs bound to HOPS (Figure 4B, top, compare lanes 5–7 and 8–10). This antibody does not disrupt interactions between the SNAREs in pre-existing SNARE complexes (Figure 4B, bottom, compare lanes 5 and 7). If a substantial fraction of the HOPS·SNARE complexes had reflected associations with unpaired SNAREs, antibodies to individual SNAREs would not have perturbed the binding of other SNAREs with HOPS. While the binding of polyclonal Fab fragments to Vam3p may sterically impinge on other, closely associated proteins, any such inhibition would still require the other SNARE proteins to be present in a complex. This shared sensitivity of the associations of HOPS with multiple SNAREs to Vam3p antibody shows that HOPS has affinity for vacuole SNARE complexes rather than for separate, unpaired SNAREs. Although physiological levels of Sec17p are sufficient to displace SNAREs from HOPS in the absence of Sec18p function (Figure 4B, lane 12), added Sec18p may mobilize catalytic amounts of vacuolar Sec17p to mediate HOPS release from SNARE complexes (lane 13).
HOPS·SNARE complexes assemble during vacuole docking prior to membrane fusion
We next sought to differentiate the functional roles of the HOPS·SNARE and Sec17p·SNARE complexes, both kinetically and through reactions synchronized by reversible arrest. Vacuole fusion is sensitive to certain inhibitors when added from the beginning of the reaction, and reactions acquire resistance to these inhibitors when their targets have fulfilled their functions. The curves of resistance indicate the stage of fusion after which that factor is no longer required. Vacuole fusion achieves resistance to antibodies against Vps33p with similar kinetics as to antibodies against Vam3p but after Sec17/18p functions have been fulfilled (Figure 5A). This suggests that Vps33p functions at a similar stage of vacuole fusion as Vam3p, consistent with data indicating that two other HOPS components, Vps39p and Vps41p, also function during docking (Price et al, 2000b).
Figure 5.

HOPS·SNARE complexes assemble during docking and prior to fusion. (A) Vps33p functions during docking. Standard vacuole fusion reactions were begun and, at indicated times, aliquots were dispensed to tubes on ice or to tubes with buffer or antibodies to Sec18p, Vps33p, or Vam3p. The reactions were further incubated at 27°C and assayed for alkaline phosphatase activity after 90 min. (B) Testing Ypt7p and HOPS requirements for fusion and SNARE complex assembly in the absence of Sec17/18p function. Fusion reactions (180 μl) contained anti-Sec17p antibodies and ATP. After 15 min, inhibitors (Gdi1p/Gyp1-46p, affinity-purified antibodies to Vps33p, Fab fragments of antibody to Vam3p, or MED) were added and incubated (5 min) before GST-Vam7p (190 ng) was added where indicated. Reactions were continued for a total of 70 min and then placed on ice. An aliquot (30 μl) was removed to assay matured alkaline phosphatase activity (C), and the associations of the added GST-Vam7p were assessed by precipitation from vacuole detergent extracts using immobilized glutathione (D). Vacuoles were sedimented from the remainder of the reactions (11 000 g, 10 min, 4°C) and the pellets were extracted on ice with solubilization buffer with 1 mM dithiothreitol (DTT) (200 μl; see Materials and methods). Detergent-insoluble material was removed by ultracentrifugation, and GST-Vam7p was retrieved with glutathione Sepharose (20 μl beads, 1 h, 4°C). Unbound material was removed as described for immunoprecipitations (see Materials and methods), and bound material was eluted by heating in reducing sample buffer.
Since Sec17p is not found in HOPS·SNARE complexes, these complexes may form after Sec18p drives Sec17p release and SNARE complex disassembly. Vacuole fusion normally requires priming by Sec17/18p to release Vam7p from cis-SNARE complexes for subsequent trans-SNARE interactions (Mayer et al, 1996; Boeddinghaus et al, 2002; Thorngren et al, 2004). Added recombinant Vam7p combines with unpaired SNAREs, bypassing the SNARE complex disassembly requirement for in vitro vacuole fusion (Thorngren et al, 2004). This provides an opportunity to examine the role of HOPS during SNARE complex assembly and vacuole fusion in the absence of Sec17/18p-mediated disassembly. Fusion reactions can be driven by added Vam7p in the continued presence of anti-Sec17p, which traps any free Sec17p and prevents SNARE complex disassembly (Boeddinghaus et al, 2002). We used these conditions and a GST-Vam7p fusion protein to study the associations of SNARE proteins from the unpaired state prior to vacuole fusion (Thorngren et al, 2004). Fusion reactions were performed in the presence of ATP and antibodies to Sec17p. Added GST-Vam7p bypassed the anti-Sec17p block and allowed fusion (Figure 5B and C). The added GST-Vam7p formed a complex with Vam3p, Vti1p, Nyv1p, and HOPS, but not with untagged Vam7p (Figure 5D, lane 8). SNARE complex assembly and fusion was blocked by extraction of Ypt7p or by inhibitory antibodies to Vps33p or Vam3p when added prior to GST-Vam7p (Figure 5C and D, lanes 9–11). However, addition of MARCKS effector domain, a ligand of phosphoinositides and acidic phospholipids that localizes to vertex docking junctions (Fratti et al, 2004), allowed formation of this complex (Figure 5D, lane 12) even though fusion was still prevented (Figure 5C). Approximately 10% of Vam3p, and a lower percentage of Nyv1p, Vti1p, and HOPS, interacted with the added GST-Vam7p. Collectively, these data show that Ypt7p and its effector HOPS function during SNARE complex formation, that this does not require Sec17p, and that the HOPS complex remains associated with SNAREs.
Inhibition of fusion by the MED lipid ligand suggested that this inhibitor prevented vacuole fusion at a late stage that might be separable from reaction resistance to Vam3p antibodies. Fusion reactions blocked with Sec17p antibodies are sensitive to Vam3p antibodies or to either of two phosphoinositide ligands, MED and the Epsin N-terminal homology domain (ENTH), when added immediately prior to Vam7p addition (Figure 6A, left arrowhead) even though these reactions were incubated for the full 70 min after Vam7p addition. If the inhibitors were added after Vam7p addition, fusion reactions rapidly acquire resistance to Vam3p antibody while the lipid ligands maintained greater inhibition of vacuole fusion (Figure 6A). Vacuole morphometry provided an independent measure of the inhibition of fusion by MED and ENTH. The extent of vacuole fusion can be examined by fluorescent microscopy, since fusion results in a quantifiable change in vacuole surface area (Merz and Wickner, 2004a, 2004b). Fusion reactions were initiated in the presence of Sec17p antibodies as above. After 20 min, Vam7p was added to bypass the block. While not all vacuoles fuse under these conditions, Vam7p addition caused a clear shift in the vacuole size distribution (no Vam7p, Figure 6B; with Vam7p, Figure 6C). MED or ENTH, if added immediately after Vam7p, inhibits the production of large vacuoles (Figure 6D and E). Thus, by two independent assays, MED and ENTH inhibit vacuole fusion, suggesting that phosphatidylinositide phosphates such as PI(4,5)P2 and/or lipid rearrangements are required for vacuole fusion even after Ypt7p- and HOPS-mediated SNARE complex assembly.
Figure 6.

Resolution of SNARE complex assembly and vacuole fusion. (A) Separation of SNARE and lipid ligand inhibitor sensitivity during fusion reactions driven by added Vam7p. Fusion reactions contained antibodies to Sec17p and ATP. Vam7p (6.3 μg/ml) was added after 20 min. Reactions received inhibitors (anti-Vam3p IgG, MED, ENTH, or placed on ice) at indicated times (19.5, 20.25, 20.75, 22, 25, 35, or 90 min), and alkaline phosphatase activity was measured after 90 min. PMSF (7 mM final concentration) was added to reactions placed on ice to terminate further proteolytic maturation of Pho8p. (B–E) Lipid ligands prevent vacuole fusion. As in (A), fusion reactions were begun at 27°C in the presence of anti-Sec17p antibody (B). The block was bypassed with Vam7p (C) after 20 min, and after 15 s, MED (D) or ENTH (E) was added. After 90 min, fusion was assayed by alkaline phosphatase activity (ALP) or fluorescence microscopy. Reactions (30 μl) were mixed with low-melt agarose (50 μl) containing the lipophilic dye MDY-64 and 10 μl of the suspension was mounted on a glass slide. Fluorescent micrographs were taken from random fields, and the surface areas of individual vacuoles were determined (see Materials and methods). A representative image from one field is shown (inset; scale bar is 5 μm).
Discussion
Previous work has described associations of Rabs, their effector complexes, and SM proteins with SNARE proteins, but the order and functional significance of these interactions during each stage of fusion has been unclear. We propose a working model that frames our experimental observations of distinct vacuole SNARE complexes and integrates the physiological cycles of SNARE complex disassembly by Sec17/18p and assembly by HOPS (Figure 7). Sec17p is released early in the vacuole fusion reaction during Sec18p-mediated SNARE complex disassembly (Figure 7, stage 1; Mayer et al, 1996). Sec17p release is required for cis-SNARE complex disassembly and to provide sufficient unpaired SNAREs for fusion (Wang et al, 2000), but released Sec17p is not required in vitro for downstream events prior to fusion (Mayer et al, 1996). HOPS interacts with Ypt7p and unpaired SNAREs, perhaps sequentially, to mediate SNARE complex assembly (Figure 7, stage 2; Price et al, 2000a; Sato et al, 2000). Ypt7p functions at stage 2 may entail tethering, vertex ring enrichment of proteins and lipids, and SNARE complex assembly. After SNARE complex assembly, HOPS can be released (stage 3). It is unclear whether HOPS·SNARE complexes or SNARE complexes without HOPS promote late stages of fusion, but lumenal compartment mixing can still be blocked by MED even after complex assembly (Figure 5D). Sec17p binds to the SNARE complex (stage 4) to allow Sec18p-mediated disassembly. Stages of this working model may represent conserved features of an association pathway of Rabs and their effectors with SNAREs and their chaperones.
Figure 7.
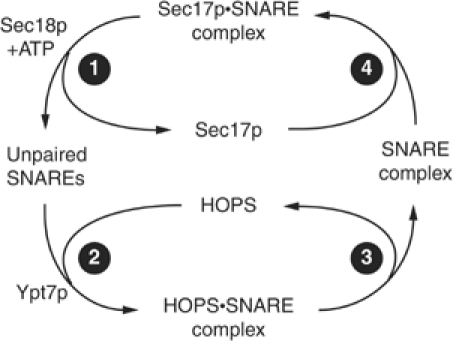
Working model of the HOPS and Sec17p cycles.
This cycle of Sec17p, HOPS, and SNAREs reflects altered protein associations, but each stage may also include regulated steps of protein recruitment to the vacuole membrane and release. Sec17p and Vam7p are released from vacuoles upon SNARE complex disassembly, but Vam7p reassociates with vacuole membranes through binding to phosphatidylinositide 3-phosphate via its PX domain (Cheever et al, 2001; Boeddinghaus et al, 2002) and possibly Ypt7p (Ungermann et al, 2000). Specific inhibitors of membrane fusion can disturb these steady-state associations of HOPS and Vam7p with membranes. Dilution of vacuole fusion reactions in the presence of ATP causes a portion of the vacuole-bound HOPS to be released (Price et al, 2000a). Premature activation of Ypt7p·GTP hydrolysis by Gyp7p inhibits vacuole fusion and causes HOPS release (Eitzen et al, 2000). Gdi1p extraction of Ypt7p affects the vacuole associations of both HOPS and Vam7p, but these effects are temporally distinct from both Ypt7p extraction and Sec17/18p-mediated SNARE complex disassembly (Price et al, 2000a; Ungermann et al, 2000; Boeddinghaus et al, 2002). GTP-bound Ypt7p may be required for the vacuole localization and function of HOPS. A Q68L mutant of Ypt7p deficient in GTP hydrolysis (Vollmer et al, 1999; Eitzen et al, 2000) has wild-type vacuole morphology and fusion (Vollmer et al, 1999; Eitzen et al, 2000), and SNARE associations with HOPS are unaffected (data not shown). Other Rabs regulate the associations of their tethering factors with membranes. Gdi1p extraction of Ypt1p (yeast Rab1) releases Uso1p (yeast p115), a tethering factor that functions in transport to the Golgi complex, and prevents p115 recruitment to ER-derived vesicles (Cao et al, 1998; Allan et al, 2000). Thus, interference with normal Rab function disrupts a conserved pathway of recruitment and membrane localization of tethering factors. However, because we detected that only a small percentage of HOPS is in association with Ypt7p or SNAREs (Figure 2) at any time, HOPS must maintain its vacuole localization via another, undefined receptor.
Despite sequence conservation, SM proteins use different mechanisms to interact with their cognate syntaxins (Gallwitz and Jahn, 2003). The presynaptic SM protein Munc18 associates with a ‘closed' conformation of Syntaxin1A that prevents Syntaxin1A from interacting with other SNAREs or α-SNAP (Pevsner et al, 1994; Hayashi et al, 1995; Yang et al, 2000). However, other SM proteins associate with SNARE complexes. Yeast Sec1p interacts with the fully assembled exocytic SNARE complex (Carr et al, 1999; Scott et al, 2004). Other yeast syntaxins, such as Tlg2p, Sed5p, and Ufe1p, contain an N-terminal peptide motif that may allow recruitment of their SM proteins immediately after SNARE complex disassembly (Yamaguchi et al, 2002). Interestingly, such binding can occur even when the syntaxin protein is found in SNARE complexes with α-SNAP (Gavin et al, 2002; Peng and Gallwitz, 2002). However, other SNAREs, including Vam3p and Sso1/2p, do not contain a similar peptide sequence that is sufficient to confer binding to their SM proteins (Dulubova et al, 2001; Yamaguchi et al, 2002). The SNARE motif of syntaxin mediates its binding to α-SNAP (Hanson et al, 1995; Hayashi et al, 1995; McMahon and Sudhof, 1995). Unlike Syntaxin1A, Vam3p does not autonomously fold into a closed conformation (Dulubova et al, 2001). Mutational analysis indicates that the N-terminal region of Vam3p is surprisingly dispensable for Vam3p function in vivo and in vitro (Laage and Ungermann, 2001; Wang et al, 2001b). Experiments employing immobilized domains of Vam3p indicated that its SNARE motif was sufficient to retrieve Vps33p from yeast extracts (Dulubova et al, 2001), although it is unclear which HOPS subunit mediates its interaction with SNAREs (Sato et al, 2000). Based on the expected affinities of both HOPS and Sec17p for the SNARE domain of Vam3p, and because they are in distinct SNARE complexes (Figure 2), we suggest a mode of interaction where Sec17p and HOPS binding to the SNARE motif of Vam3p in SNARE complexes is mutually exclusive. Post-translational modifications such as reversible phosphorylation may also contribute to a conserved mechanism of SM protein function that is not fully replicated in experiments using recombinant proteins (Bryant and James, 2003).
Our observations are relevant to SNARE and tethering factor interactions in other transport steps as well. Recent work on the mode of association of non-SM protein tethering factors with SNAREs has revealed that a coiled-coil motif may stabilize such interactions. Shorter et al (2002) tested the functional importance of this motif in p115, and found that it can interact with mammalian Sly1 protein and with Syntaxin 5 but not α-SNAP. This SNARE-interacting motif is required for Golgi biogenesis in vivo and Golgi reassembly in vitro (Shorter et al, 2002; Puthenveedu and Linstedt, 2004). This sequence mediated a specific interaction with several unpaired SNAREs and was required for p115 to catalyze their assembly (Shorter et al, 2002). While p115 remained associated with SNAREs after their assembly, its continued association was not required to maintain the stability of the SNARE complex. Because we see that HOPS·SNARE associations are a minority of SNARE associations formed during vacuole fusion, continued association of HOPS with SNAREs may not be required after SNARE assembly (Figure 7, stage 3). HOPS binding or its release may instead signal events downstream of SNARE complex formation. Collectively, our findings support a model where tethering factors and α-SNAP act as mutually exclusive chaperones to facilitate the stepwise assembly and function of distinct SNARE complexes.
Materials and methods
Antibodies
His6-GFP was expressed from XL1-Blue Escherichia coli cells containing pJK83, purified by Ni-NTA agarose (Seedorf et al, 1999), and injected into rabbits for antisera production. For affinity purification of IgG, his6-GFP (10 mg) was coupled to 1 ml of Affigel-10 resin (Bio-Rad) according to the manufacturer's instructions. Antibodies to Vam3p, Vam7p, Vti1p, Nyv1p, Ykt6p, and Sec17p were described (Haas and Wickner, 1996; Nichols et al, 1997; Ungermann et al, 1999; Merz and Wickner, 2004b). Secondary detection was with HRP-conjugated anti-mouse or anti-rabbit antibodies. Some immunoblots were decorated with biotinylated, affinity-purified antibodies. Labeling of these antibodies with NHS-LC-Biotin (Pierce) was performed according to the manufacturer's instructions, and NeutrAvidin-HRP (Pierce) was used at 10–40 ng/ml. Antibodies to HOPS subunits have been described (Price et al, 2000b; Seals et al, 2000).
Yeast strains
Saccharomyces cerevisiae strains containing gene fusions to GFP were described previously (Wang et al, 2002, 2003b). To minimize degradation of Vam7-GFP2, a Vam7-GFP strain was created in the protease-deficient BJ2168 background (MATa leu2 trp1 ura3-52 prb1-1122 pep4-3 prc1-407 gal2). A single EGFP and an adjacent TRP1 marker were placed at the 3′ end of the VAM7 gene by homologous recombination on the chromosome as described (Longtine et al, 1998; Wang et al, 2002). BJ3505 (MATα pep4∷HIS3 prb1-Δ1.6R his3-Δ200 lys2-801 trp1-Δ101 (gal3) ura3-52 gal2 can1) and DKY6281 (MATα pho8∷TRP1 leu2-3 leu2-112 ura3-52 his3-200 trp1-901 lys2-801 suc2-9) yeast strains were used to assay vacuole fusion.
Vacuole fusion and reagents
Yeast vacuoles were prepared by flotation through discontinuous Ficoll gradients (Haas, 1995). Fusion reactions were in PS buffer (20 mM PIPES/KOH, pH 6.8, 200 mM sorbitol) containing 125 mM KCl, 5 mM MgCl2, 10 μM coenzyme A, 38.6 μg/ml Pbi2p (IB2), and 3 μg each of vacuoles prepared from BJ3505 and DKY6281 yeast. Fusion reactions were performed at 27°C, and Pho8p activity was assayed (Haas, 1995). Alkaline phosphatase activity of 1 U yields 1 μmol of p-nitrophenol per minute per milligram of BJ3505 vacuole protein. An ATP regeneration system (1 mM ATP, 1 mM MgCl2, 0.5 mg/ml creatine kinase, 3 mM creatine phosphate) was added where indicated. Inhibitors of vacuole fusion were used at the following concentrations: his6-Sec17p (24 μg/ml), anti-Sec17p IgG (225 μg/ml), anti-Vam3p IgG (43 μg/ml), Gdi1p (130 μg/ml) and Gyp1-46p (260 μg/ml), affinity-purified anti-Sec18p (16.7 μg/ml), affinity-purified anti-Vps33p (23 μg/ml), or affinity-purified anti-Vam3p (12 μg/ml). Fab fragments to Vam3p were prepared by digestion of IgG by immobilized papain (Pierce) according to the manufacturer's instructions and used at 33 μg/ml. MARCKS effector domain (Wang et al, 2001a) was synthesized by the WM Keck Facility at Yale University and used at 10 μM. GST-ENTH (Hyman et al, 2000) was expressed in E. coli, cleaved with thrombin, and ENTH was used at 570 μg/ml to inhibit fusion.
Recombinant Vam7p was isolated as described (Merz and Wickner, 2004b) and used in fusion reactions at 6.3 μg/ml. Alternatively, GST-Vam7p was eluted from the resin with Buffer A (50 mM Tris–HCl, pH 8.0, 10% glycerol, 10 mM reduced glutathione, 1 mM DTT) and immediately applied to a Mono-Q HR 10/10 column (Amersham Biosciences) at 4°C in Buffer B (20 mM Tris–HCl, pH 8.0, 10% glycerol, 1 mM DTT). The column was developed with a 200 ml linear gradient from 50 to 250 mM KCl in Buffer B. Fractions were analyzed by SDS–PAGE, and the pooled peak was concentrated by Centriprep-30 (Millipore). Protein was dialyzed into PS buffer containing 125 mM KCl and 5 mM MgCl2, frozen in aliquots with liquid nitrogen, and used in fusion reactions at 1 μg/ml.
Immunoprecipitations
Sedimented vacuoles were overlaid with ice-cold solubilization buffer (20 mM HEPES/KOH, pH 7.4, 100 mM NaCl, 2 mM EDTA, 0.5% Triton X-100 (Anatrace), 20% glycerol, 1 × protease inhibitor cocktail (0.46 μg/ml leupeptin, 3.5 μg/ml pepstatin, 2.4 μg/ml pefabloc-SC, 1 mM PMSF)), resuspended on ice, and diluted to 0.15–0.3 mg vacuole protein/ml. After 20 min, detergent-insoluble material was removed (TLA 100 or TLA 120.2, 52 000 r.p.m., 11 min, 4°C). Clarified detergent extracts were incubated on a nutator for 1–16 h with affinity-purified antibodies covalently coupled to protein-A–Sepharose (100 μg antibody/ml packed bed of CL-4B beads; Amersham Biosciences) prepared as described (Harlowe and Lane, 1999). Beads (20 μl per immunoprecipitation) were collected by brief centrifugation (3000 g, 2 min, 4°C) and suspended 4–5 times with cold solubilization buffer (0.8–1 ml each). Bound proteins were eluted in SDS sample buffer (94°C, 5 min) for SDS–PAGE analysis (Ausubel et al, 1994). For silver staining and some immunoblots, nonreducing conditions were used for elution from immunoprecipitates to prevent coelution of coupled antibodies. Samples were reduced with β-mercaptoethanol prior to separation by SDS–PAGE.
Microscopy
Micrographs of stained vacuoles were acquired and analyzed (Merz and Wickner, 2004a, 2004b) using an Olympus BX51 upright microscope using a 100 W mercury arc lamp, Plan Apochromat objective (× 60, 1.4 NA) and a Sensicam QE CCD camera (Cooke) controlled by IP lab (Scanalytics) running under Mac OS X. Vacuole morphometry was performed using ImageJ 1.32 (http://rsb.info.nih.gov/ij) and analyzed with JMP 5.0.1a (SAS Institute).
Acknowledgments
We thank Alexey Merz, Christopher Stroupe, and Li Wang for important preliminary work, Pamela Silver for anti-GFP antibodies and antisera for initial experiments, and Charles Barlowe and Harry Higgs for comments. This work was supported by a grant from the National Institute of General Medical Sciences (NIGMS). KMC received predoctoral support from T32GM08704 (NIGMS). RAF was supported by a postdoctoral fellowship from the Helen Hay Whitney Foundation.
References
- Allan BB, Moyer BD, Balch WE (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289: 444–448 [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current Protocols in Molecular Biology. New York: John Wiley & Sons Inc. [Google Scholar]
- Boeddinghaus C, Merz AJ, Laage R, Ungermann C (2002) A cycle of Vam7p release from and PtdIns 3-P-dependent rebinding to the yeast vacuole is required for homotypic vacuole fusion. J Cell Biol 157: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, James DE (2003) The Sec1p/Munc18 (SM) protein, Vps45p, cycles on and off membranes during vesicle transport. J Cell Biol 161: 691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J 17: 2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Grote E, Munson M, Hughson FM, Novick PJ (1999) Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol 146: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever ML, Sato TK, de Beer T, Kutateladze TG, Emr SD, Overduin M (2001) Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat Cell Biol 3: 613–618 [DOI] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Wang Y, Sudhof TC, Rizo J (2001) Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol 8: 258–264 [DOI] [PubMed] [Google Scholar]
- Eitzen G, Will E, Gallwitz D, Haas A, Wickner W (2000) Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J 19: 6713–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT, Jahn R (1998) Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA 95: 15781–15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W (2004) Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol 167: 1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D, Jahn R (2003) The riddle of the Sec1/Munc-18 proteins—new twists added to their interactions with SNAREs. Trends Biochem Sci 28: 113–116 [DOI] [PubMed] [Google Scholar]
- Gavin AC, Bosche M, Krause R, Grandi P, Marzioch M, Bauer A, Schultz J, Rick JM, Michon AM, Cruciat CM, Remor M, Hofert C, Schelder M, Brajenovic M, Ruffner H, Merino A, Klein K, Hudak M, Dickson D, Rudi T, Gnau V, Bauch A, Bastuck S, Huhse B, Leutwein C, Heurtier MA, Copley RR, Edelmann A, Querfurth E, Rybin V, Drewes G, Raida M, Bouwmeester T, Bork P, Seraphin B, Kuster B, Neubauer G, Superti-Furga G (2002) Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415: 141–147 [DOI] [PubMed] [Google Scholar]
- Haas A (1995) A quantitative assay to measure homotypic vacuole fusion. Methods Cell Sci 17: 283–294 [Google Scholar]
- Haas A, Wickner W (1996) Homotypic vacuole fusion requires Sec17p (yeast alpha-SNAP) and Sec18p (yeast NSF). EMBO J 15: 3296–3305 [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Otto H, Barton N, Jahn R (1995) The N-ethylmaleimide-sensitive fusion protein and alpha-SNAP induce a conformational change in syntaxin. J Biol Chem 270: 16955–16961 [DOI] [PubMed] [Google Scholar]
- Harlowe E, Lane D (1999) Using Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H (1995) Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J 14: 2317–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK (2003) Global analysis of protein localization in budding yeast. Nature 425: 686–691 [DOI] [PubMed] [Google Scholar]
- Hyman J, Chen H, Di Fiore PP, De Camilli P, Brunger AT (2000) Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH(2)-terminal homology (ENTH) domain, structurally similar to Armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn(2)+ finger protein (PLZF). J Cell Biol 149: 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EW (1977) Proteinase mutants of Saccharomyces cerevisiae. Genetics 85: 23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laage R, Ungermann C (2001) The N-terminal domain of the t-SNARE Vam3p coordinates priming and docking in yeast vacuole fusion. Mol Biol Cell 12: 3375–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961 [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W (1997) Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF). J Cell Biol 136: 307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A (1996) Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell 85: 83–94 [DOI] [PubMed] [Google Scholar]
- McMahon HT, Sudhof TC (1995) Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. J Biol Chem 270: 2213–2217 [DOI] [PubMed] [Google Scholar]
- Merz AJ, Wickner WT (2004a) Resolution of organelle docking and fusion kinetics in a cell-free assay. Proc Natl Acad Sci USA 101: 11548–11553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz AJ, Wickner WT (2004b) Trans-SNARE interactions elicit Ca2+ efflux from the yeast vacuole lumen. J Cell Biol 164: 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HR, Wickner WT, Haas A (1997) Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature 387: 199–202 [DOI] [PubMed] [Google Scholar]
- Peng R, Gallwitz D (2002) Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J Cell Biol 157: 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Mayer A (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396: 575–580 [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu SC, Scheller RH (1994) n-Sec1: a neural-specific syntaxin-binding protein. Proc Natl Acad Sci USA 91: 1445–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A, Seals D, Wickner W, Ungermann C (2000a) The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J Cell Biol 148: 1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A, Wickner W, Ungermann C (2000b) Proteins needed for vesicle budding from the Golgi complex are also required for the docking step of homotypic vacuole fusion. J Cell Biol 148: 1223–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, Linstedt AD (2004) Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proc Natl Acad Sci USA 101: 1253–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SC, Winistorfer SC, Poupon V, Luzio JP, Piper RC (2004) Mammalian late vacuole protein sorting orthologues participate in early endosomal fusion and interact with the cytoskeleton. Mol Biol Cell 15: 1197–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JH, Stevens TH (1986) Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell 47: 1041–1051 [DOI] [PubMed] [Google Scholar]
- Sato TK, Rehling P, Peterson MR, Emr SD (2000) Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell 6: 661–671 [DOI] [PubMed] [Google Scholar]
- Scott BL, Van Komen JS, Irshad H, Liu S, Wilson KA, McNew JA (2004) Sec1p directly stimulates SNARE-mediated membrane fusion in vitro. J Cell Biol 167: 75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price A (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA 97: 9402–9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedorf M, Damelin M, Kahana J, Taura T, Silver PA (1999) Interactions between a nuclear transporter and a subset of nuclear pore complex proteins depend on Ran GTPase. Mol Cell Biol 19: 1547–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukov EA, He JP, Moghrabi N, Sunio A, Kramer H (1999) A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol Cell 4: 479–486 [DOI] [PubMed] [Google Scholar]
- Shorter J, Beard MB, Seemann J, Dirac-Svejstrup AB, Warren G (2002) Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol 157: 45–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Sollner T (1994) A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell 78: 937–948 [DOI] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE (1993) A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75: 409–418 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Woolford CA, Jones EW (2004) The Sec1/Munc18 protein, Vps33p, functions at the endosome and the vacuole of Saccharomyces cerevisiae. Mol Biol Cell 15: 2593–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Oiso N, Gautam R, Novak EK, Panthier JJ, Suprabha PG, Vida T, Swank RT, Spritz RA (2003) The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc Natl Acad Sci USA 100: 1146–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorngren N, Collins KM, Fratti RA, Wickner W, Merz AJ (2004) A soluble SNARE drives rapid docking, bypassing ATP and Sec17/18p for vacuole fusion. EMBO J 23: 2765–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, Qureshi-Emili A, Li Y, Godwin B, Conover D, Kalbfleisch T, Vijayadamodar G, Yang M, Johnston M, Fields S, Rothberg JM (2000) A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae. Nature 403: 623–627 [DOI] [PubMed] [Google Scholar]
- Ungermann C, Nichols BJ, Pelham HR, Wickner W (1998a) A vacuolar v–t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol 140: 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Price A, Wickner W (2000) A new role for a SNARE protein as a regulator of the Ypt7/Rab-dependent stage of docking. Proc Natl Acad Sci USA 97: 8889–8891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W (1998b) Defining the functions of trans-SNARE pairs. Nature 396: 543–548 [DOI] [PubMed] [Google Scholar]
- Ungermann C, von Mollard GF, Jensen ON, Margolis N, Stevens TH, Wickner W (1999) Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J Cell Biol 145: 1435–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Laage R, Dietrich L, Wang L, Ungermann C (2001) Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion. EMBO J 20: 3145–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer P, Will E, Scheglmann D, Strom M, Gallwitz D (1999) Primary structure and biochemical characterization of yeast GTPase-activating proteins with substrate preference for the transport GTPase Ypt7p. Eur J Biochem 260: 284–290 [DOI] [PubMed] [Google Scholar]
- Wada Y, Ohsumi Y, Anraku Y (1992) Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae I. Isolation and characterization of two classes of vam mutants. J Biol Chem 267: 18665–18670 [PubMed] [Google Scholar]
- Wang CW, Stromhaug PE, Kauffman EJ, Weisman LS, Klionsky DJ (2003a) Yeast homotypic vacuole fusion requires the Ccz1–Mon1 complex during the tethering/docking stage. J Cell Biol 163: 973–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Arbuzova A, Hangyas-Mihalyne G, McLaughlin S (2001a) The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J Biol Chem 276: 5012–5019 [DOI] [PubMed] [Google Scholar]
- Wang L, Merz AJ, Collins KM, Wickner W (2003b) Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J Cell Biol 160: 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Seeley ES, Wickner W, Merz AJ (2002) Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell 108: 357–369 [DOI] [PubMed] [Google Scholar]
- Wang L, Ungermann C, Wickner W (2000) The docking of primed vacuoles can be reversibly arrested by excess Sec17p (alpha-SNAP). J Biol Chem 275: 22862–22867 [DOI] [PubMed] [Google Scholar]
- Wang Y, Dulubova I, Rizo J, Sudhof TC (2001b) Functional analysis of conserved structural elements in yeast syntaxin Vam3p. J Biol Chem 276: 28598–28605 [DOI] [PubMed] [Google Scholar]
- Warner TS, Sinclair DA, Fitzpatrick KA, Singh M, Devlin RH, Honda BM (1998) The light gene of Drosophila melanogaster encodes a homologue of VPS41, a yeast gene involved in cellular-protein trafficking. Genome 41: 236–243 [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE (1998) SNAREpins: minimal machinery for membrane fusion. Cell 92: 759–772 [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Sato TK, Emr SD (2000) New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol 151: 551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Sudhof TC (2002) Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell 2: 295–305 [DOI] [PubMed] [Google Scholar]
- Yang B, Steegmaier M, Gonzalez LC Jr, Scheller RH (2000) nSec1 binds a closed conformation of syntaxin1A. J Cell Biol 148: 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]


