Abstract
Objectives
This study was undertaken to identify potential predictors of atrial fibrillation after cardiac surgery (AFACS) through a modified Delphi process and expert consensus. These will supplement predictors identified through a systematic review and cohort study to inform the development of two AFACS prediction models as part of the PARADISE project (NCT05255224). Atrial fibrillation is a common complication after cardiac surgery. It is associated with worse postoperative outcomes. Reliable prediction of AFACS would enable risk stratification and targeted prevention. Systematic identification of candidate predictors is important to improve validity of AFACS prediction tools.
Design
This study is a Delphi consensus exercise.
Setting
This study was undertaken through remote participation.
Participants
The participants are an international multidisciplinary panel of experts selected through national research networks.
Interventions
This is a two-stage consensus exercise consisting of generating a long list of variables, followed by refinement by voting and retaining variables selected by at least 40% of panel members.
Results
The panel comprised 15 experts who participated in both stages, comprising cardiac intensive care physicians (n=3), cardiac anaesthetists (n=2), cardiac surgeons (n=1), cardiologists (n=4), cardiac pharmacists (n=1), critical care nurses (n=1), cardiac nurses (n=1) and patient representatives (n=2). Our Delphi process highlighted candidate AFACS predictors, including both patient factors and those related to the surgical intervention. We generated a final list of 72 candidate predictors. The final list comprised 3 demographic, 29 comorbidity, 4 vital sign, 13 intraoperative, 10 postoperative investigation and 13 postoperative intervention predictors.
Conclusions
A Delphi consensus exercise has the potential to highlight predictors beyond the scope of existing literature. This method proved effective in identifying a range of candidate AFACS predictors. Our findings will inform the development of future AFACS prediction tools as part of the larger PARADISE project.
Trial registration number
Keywords: cardiac surgery, risk factors, pacing & electrophysiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Engagement of an international, multidisciplinary expert panel ensured a range of perspectives on predictors of atrial fibrillation after cardiac surgery (AFACS), informing the larger PARADISE Project (NCT05255224).
Remote participation allowed for efficient engagement across different geographical regions.
The relatively small panel may not have captured the full diversity of expertise, opinions and experiences found in the broader community of professionals managing patients with AFACS.
Inclusion of patient and public representatives in the Delphi panel added valuable perspectives to the consensus process.
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia resulting in an irregular and often rapid heart rhythm. AF affects 30–40% of patients after cardiac surgery.1 AF after cardiac surgery (AFACS) is strongly associated with worse outcomes, including longer hospital and intensive care unit (ICU) stays and increased risks of stroke, persistent AF and mortality.2,4
Prophylactic treatments administered during the perioperative period have proven effective at lowering the incidence of AFACS.5 However, these treatments are not risk-free. Consequently, it is important to identify high-risk patients to target prophylaxis at those most likely to benefit. However, prediction of AFACS remains challenging. The pathophysiology of AFACS is multifactorial, involving comorbidity factors, surgical stress and the postoperative inflammatory response.6 Accurate AFACS prediction therefore requires consideration of a range of patient and intervention factors.
There are no reliable AFACS prediction models in widespread use despite multiple models being developed in recent years.7,10 The lack of effective tools for estimating AFACS risk has prevented the implementation of targeted prophylaxis protocols. Interventions to prevent AFACS may lead to improved outcomes.
The systematic identification of candidate AFACS predictors is important for the validity of any prediction model.11 While systematic reviews can identify candidate predictors from existing literature, a Delphi consensus exercise has the potential to highlight additional untested predictors based on mechanistic understanding or through clinical experience.
The Delphi method involves a structured communication technique to gather consensus among experts.12 This iterative process involves rounds of surveys where experts provide their independent opinions which are then collated by a facilitator and fed back to the panel.13 Delphi processes have previously been used to inform critical care practice, including management of COVID-1914 and identification of core outcome measures after respiratory failure.15
This study aimed to identify candidate AFACS predictors through expert consensus. These variables will supplement those identified through a systematic review of existing literature16 and a national cohort study.17 The final consolidated list of variables will inform two AFACS prediction models as part of the larger National Institute of Health Research-funded ‘Predicting AF after Cardiac Surgery–the PARADISE Score’ project (NCT05255224). The PARADISE project aims to develop two validated clinical prediction models to determine the risk of a patient developing AFACS: one in the preoperative assessment clinic or on admission for surgery (PARADISE-1) and another for the postoperative period (PARADISE-2).
Methods
We undertook a Delphi consensus exercise to identify candidate AFACS predictors as part of the larger PARADISE project. We used the ACcurate COnsensus Reporting Document (ACCORD) guidance to report our Delphi process.18 We used an online survey tool (Survey Monkey) for data collection and analysis.
Panel selection
We identified potential expert participants through national research networks. We selected participants to reflect a multidisciplinary group of health professionals and patient representatives. Participants were invited via email and were not known to each other. We conducted this Delphi study entirely through electronic contact and data capture. We aimed for a panel size of 10–15 participants to achieve content validity.19 20
Stage 1
Panel members were asked to spontaneously list factors that affect AFACS risk in critically ill patients. We permitted potential protective factors as suggestions. We offered panel members six categories (demographics, comorbidities, vital signs, intraoperative variables, postoperative interventions and postoperative investigation results) to provide structure to responses.
Stage 2
The variables generated in stage 1 were re-presented to the panel. We asked each panel member to select variables that they felt affect AFACS risk. We refined the list by only retaining variables selected by at least 40% of panel members, which was consistent with previous work identifying candidate risk factors.21 The facilitating study team had no voting rights nor influence over panel responses. The Delphi exercise was facilitated by a member of the study team with domain knowledge and consensus exercise experience.
Patient and public involvement
Patient and public representatives were included in the Delphi panel.
Results
Participants
We approached 32 experts located in the UK, Germany, Belgium, Canada and North America. Of these, 15 (47%) agreed to participate in the Delphi process. The final panel included cardiac intensive care physicians (n=3), cardiac anaesthetists (n=2), cardiac surgeons (n=1), cardiologists (n=4), cardiac pharmacists (n=1), critical care nurses (n=1), cardiac nurses (n=1) and patient representatives (n=2).
Stage 1
Over the six domains of demographics, comorbidities, vital signs, intraoperative factors, postoperative interventions and postoperative investigation results, the panel generated 122 distinct candidate variables.
Stage 2
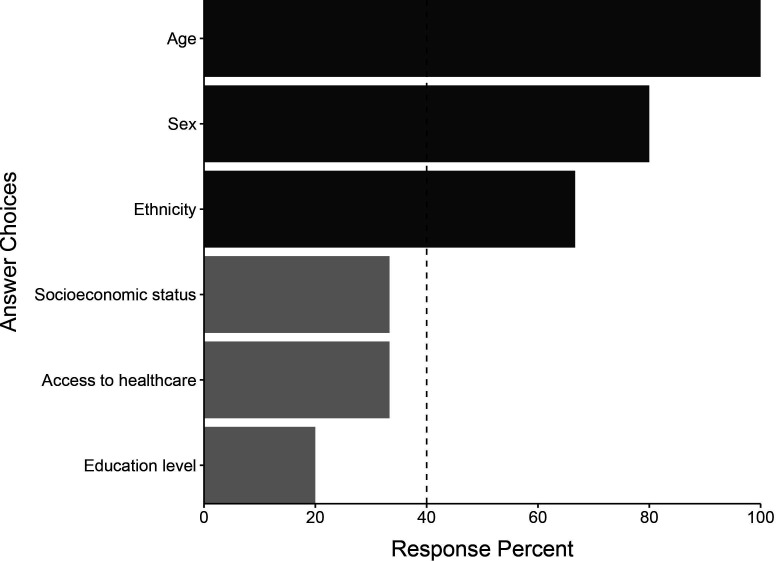
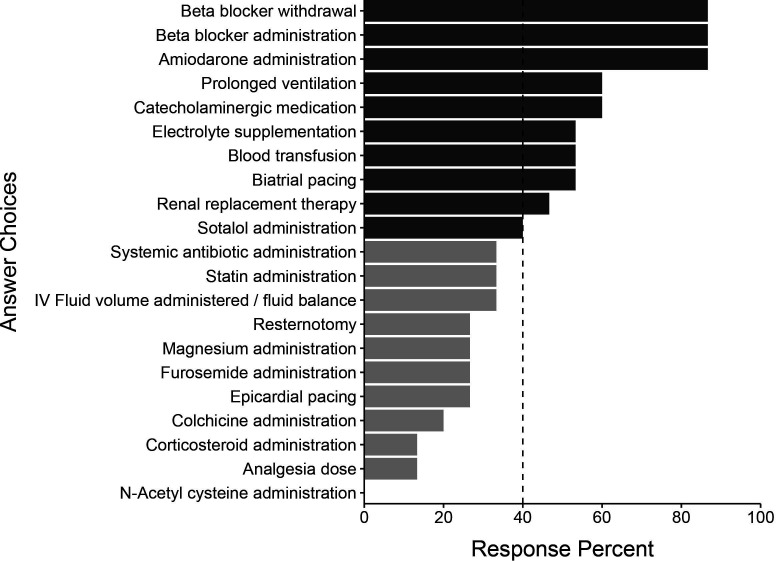
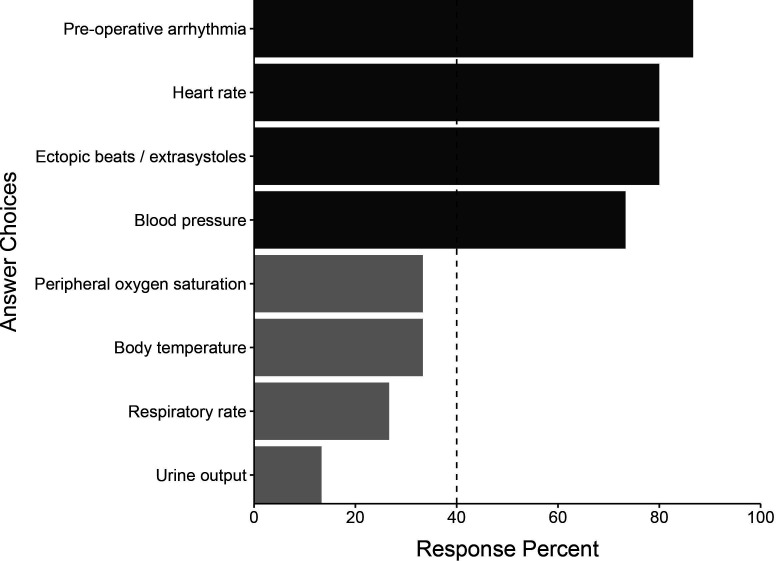
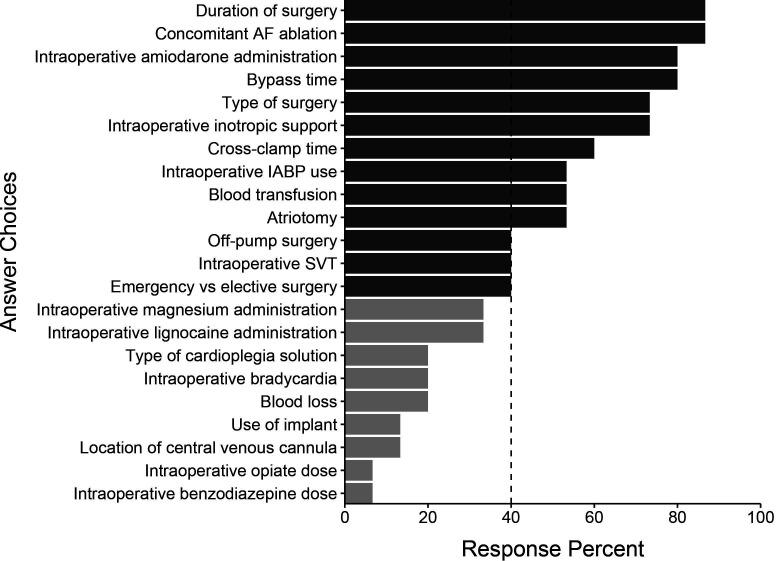
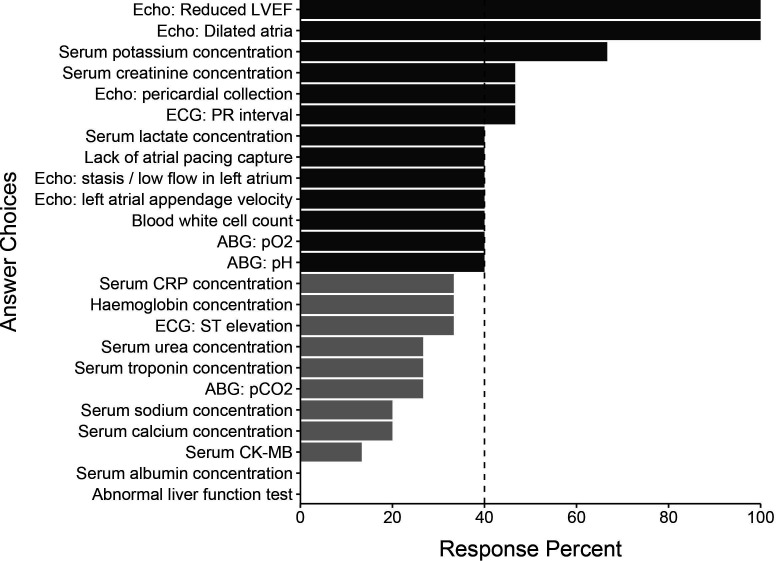
All 15 of the initial participants responded in round 2. The initial list of 122 variables was subject to a consensus vote where predictors were excluded if selected by at least 40% of panel members. We generated a final list of 72 candidate variables. This process resulted in 3 demographic variables, 29 comorbidity variables, 4 vital sign variables, 13 intraoperative variables, 10 postoperative investigation variables and 13 postoperative intervention variables. These variables and their associated response percentages are shown in figures15. Comorbidity variables are shown in online supplemental figure 1.
Figure 1. Demographic predictors identified by the Delphi panel in round 1. Light grey bars represent predictors not selected by 40% of the panel in round 2 and therefore not selected for the final list.
Figure 5. Postoperative intervention predictors by the Delphi panel in round 1. Light grey bars represent predictors not selected by 40% of the panel in round 2 and therefore not selected for the final list.
Figure 2. Vital sign predictors identified by the Delphi panel in round 1. Light grey bars represent predictors not selected by 40% of the panel in round 2 and therefore not selected for the final list.
Figure 3. Intraoperative predictors identified by the Delphi panel in round 1. Light grey bars represent predictors not selected by 40% of the panel in round 2 and therefore not selected for the final list. AF, atrial fibrillation; IABP, intra-aortic balloon pump; SVT, supraventricular tachycardia.
Figure 4. Postoperative investigation predictors identified by the Delphi panel in round 1. Light grey bars represent predictors not selected by 40% of the panel in round 2 and therefore not selected for the final list. ABG, arterial blood gas; CK-MB, creatine kinase-myocardial band; CRP, C-reactive protein; Echo, echocardiogram; LVEF, left ventricular ejection fraction; pCO2, partial pressure of carbon dioxide; pH, potential of hydrogen; pO2, partial pressure of oxygen.
These variables were then combined with those identified in a parallel cohort study and systematic review16 17 to generate a final list of candidate variables to inform the PARADISE project.
The most consistently identified demographic risk factor was patient age. Multiple comorbidities were identified. Those specific to AF risk such as prior AF and left atrial size were ranked highest. Other pre-existing conditions with demonstrable associations, including heart failure and chronic obstructive pulmonary disease, were also identified.
Many intraoperative predictors were highlighted, including cross-clamp time, the presence of intraoperative inotropic support, type of surgery, bypass time and intraoperative amiodarone administration. Echocardiographic parameters, including reduced left ventricular ejection fraction (LVEF) and left atrial dilation, ranked highest in the postoperative investigation domain. Laboratory findings felt to be important candidates included serum potassium, creatinine and lactate concentrations. White cell count was also felt to be an important predictor. Heart rate and blood pressure were highlighted as vital sign variables, along with any identified arrhythmia or ectopic beats.
Multiple postoperative interventions were identified. Beta blocker administration and beta blocker withdrawal were most consistently identified, along with amiodarone administration. Prolonged ventilation and the use of catecholaminergic medication were also highlighted.
Discussion
In this consensus exercise, we aimed to identify important variables affecting AFACS risk. Highlighted variables may be used in the development of predictive models to inform future randomised trials or as covariates in prognostic studies. They will inform the development of two AFACS prediction models in the PARADISE project.
Remote participation promoted international involvement. Our approach allowed experts from different settings to contribute effectively, ensuring a comprehensive array of perspectives and maximising the advantages of the group consensus model. The expert panel identified several factors across a range of categories.
Age was the most frequently identified demographic AFACS predictor. Increasing age is associated with molecular changes in atrial tissue that drive electrical and structural changes, leading to AF initiation. These changes affect normal conduction pathways, promoting non-uniform conduction, electrical dissociation and re-entry circuits, leading to arrhythmia.22 23 Increasing age has been consistently identified in other studies of AFACS predictors.9 10 24
Multiple comorbidities and preadmission measurements were highlighted as candidate AFACS predictors. These predominantly reflected cardiovascular, pulmonary and metabolic diseases. Left atrial size was the most consistently identified candidate predictor. Atrial dilatation is a strong predictor of AF in the community.25 Many comorbidities lead to elevated atrial pressure and subsequent atrial stretch. Atrial stretch alters ion channel function and causes a decrease in atrial effective refractory period and an increase in AF inducibility.26 27 Increasing left atrial diameter is associated with AF risk in the general ICU and after cardiac surgery.28 Obesity was highlighted as an important risk factor. This is consistent with existing literature suggesting that each 1 kg/m2 increase corresponds to a 1% increase in the risk of AFACS.29 This association may represent secondary changes in left atrial volume, autonomic tone and neurohormonal activation associated with obesity.
The type of surgery was identified as an important predictor. This finding is consistent with previous studies demonstrating that patients undergoing coronary artery bypass graft surgery face a 15% to 40% risk of developing AF. This risk increases up to around 50% after isolated valve surgery and up to 60% in combined procedures.1 30
Furthermore, the complexity and duration of the cardiac surgery itself also play a significant role in the development of AF. Surgical trauma induces a systemic inflammatory response proportional to the degree of surgical stress, which is influenced by the duration of the surgery.31 32 Cardiopulmonary bypass duration was highlighted, which is consistent with existing literature. Bypass itself may increase AFACS risk sevenfold versus off-pump procedures.33 Potential mechanisms include the duration of myocardial ischaemia, atrial cannulation, increased inflammatory response and the sequelae of cardioplegia.34
Echocardiographic and laboratory measurements featured highly in the postoperative investigations domain. Consistent with preoperative findings, the presence of postoperative atrial dilatation was felt to be an important predictor of AFACS. Postoperative-reduced LVEF was also identified. Heart failure shares many common comorbidities and pathophysiological pathways with AF. As such, they are likely to coexist. Beyond common precursors, systolic heart failure itself may increase the risk of AF through many pathways, including increased atrial stretch, neurohormonal alterations and cellular remodeling.35
Of the identified laboratory measurements, serum potassium had the highest level of agreement. Hypokalaemia reduces the outward repolarising current and increases intracellular calcium, thereby increasing the risk of afterdepolarisations.36 Marginally lower serum potassium concentrations are associated with a higher risk of developing AF in patients in the community.37 Although no causal association has been demonstrated, high-normal serum potassium concentrations are often targeted after cardiac surgery with the intention of preventing AFACS.38 Routine potassium supplementation is not risk-free, and optimal target potassium levels are yet to be determined. Ongoing trials should inform this area in the future.39
Postoperative factors included medications such as beta blockers (administration and withdrawal), amiodarone and catecholaminergic drugs. Perioperative beta blocker therapy has been consistently shown to reduce the incidence of AFACS.5 Its use as AFACS prophylaxis is recommended in national guidelines.40,42 However, these guidelines acknowledge the biases of existing literature and the limited evidence of improvement in patient-centred outcomes. As such, they assert that perioperative prophylaxis must be weighed against potential side effects and administered on an individualised basis. Continuation of beta blockers appears important in those patients routinely taking them preoperatively—beta blocker withdrawal was associated with a doubling of the odds of AFACS in a 2-centre study of 743 patients normally taking beta blockers undergoing cardiac surgery.43 Its continuation in these patients on the first postoperative day is accordingly recommended in national guidelines.38
Prolonged ventilation was identified as a candidate AFACS predictor. Ventilation lasting over 24 hours postoperatively has been found to be strongly associated with AFACS.8 However, its inclusion in any predictive tool postpones the tool’s utility until 24 hours postoperatively. Indeed, as much of AFACS occurs early in the postoperative period,9 when developing any AFACS prediction model, the benefit of including any postoperative variables must be weighed against the necessary delay in prediction while these variables are being measured.
The Delphi method leverages the collective expertise and insights of a panel of experts. This can highlight novel or less-documented AFACS risk factors not evident in existing literature. It also allows for the integration of clinical experience and subjective expert opinions, which is especially valuable in areas with limited research. Panellist anonymity was maintained with consensus determined by a priori criteria. However, the findings should be interpreted in the context of certain limitations. The Delphi method relies heavily on subjective evaluations, requiring a degree of trust in the anonymous expert panel essential. This method lacks standardised guidelines for consensus or panel selection. In our study, panellists were purposively chosen to ensure diverse and relevant experience in AFACS; however, no quantitative analysis of expertise was undertaken. No sensitivity analyses were performed, and our response rate to initial response was modest, although all respondents from round 1 continued to round 2. Though international, the panel was not global, potentially biasing and limiting the generalisability of our results.
Conclusion
This international consensus exercise facilitated the generation of candidate predictors to inform the development of AFACS prediction tools as part of the larger PARADISE project. The use of a consensus exercise allowed for the incorporation of diverse expert opinions, leading to a comprehensive list of candidate predictors encompassing patient and intervention factors with a strong evidence base or biological plausibility.
supplementary material
Acknowledgements
The authors wish to express a huge gratitude to the anonymous experts who gave up their precious time to take part in this study. JB was supported by an NIHR Doctoral Research Fellowship (NIHR300224) and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). OCR was supported by the NIHR Oxford BRC. PJW was supported by NIHR Oxford BRC. DAC was supported by the Pandemic Sciences Institute at the University of Oxford, the NIHR Oxford BRC, an NIHR Research Professorship, a Royal Academy of Engineering Research Chair and the InnoHK Hong Kong Centre for Cerebro-cardiovascular Engineering (COCHE).
Footnotes
Funding: This study is funded by the NIHR National Institute of Health Research, Health Technology Assessment Programme Health Technology Assessment Project (NIHR131227) and sponsored by the University of Oxford. This study is also funded by the National Institute of Health (R01HL149998).
Prepub: Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-086589).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants was approved by the PARADISE study and the NHS Health Research Authority (HRA) (IRAS 296508) with ethical approval by the HRA Research Ethics Committee Cambridge East (ref: 21/EE/0166). Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Jonathan Bedford, Email: Jonathan.Bedford@ndcn.ox.ac.uk.
Kara G Fields, Email: kgfields@bwh.harvard.edu.
Gary Stephen Collins, Email: gary.collins@csm.ox.ac.uk.
Gregory Y H Lip, Email: gregory.lip@liverpool.ac.uk.
David A Clifton, Email: david.clifton@eng.ox.ac.uk.
Benjamin O’Brien, Email: ben.obrien@dhzc-charite.de.
Jochen D Muehlschlegel, Email: dmuehlsch@jhu.edu.
Peter J Watkinson, Email: peter.watkinson@ndcn.ox.ac.uk.
Oliver C Redfern, Email: oliver.redfern@ndcn.ox.ac.uk.
Data availability statement
Data are available upon reasonable request.
References
- 1.Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061–73. doi: 10.7326/0003-4819-135-12-200112180-00010. [DOI] [PubMed] [Google Scholar]
- 2.Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–8. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Park-Hansen J, Greve AM, Clausen J, et al. New-onset of postoperative atrial fibrillation is likely to recur in the absence of other triggers. Ther Clin Risk Manag. 2018;14:1641–7. doi: 10.2147/TCRM.S165155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gialdini G, Nearing K, Bhave PD, et al. Perioperative Atrial Fibrillation and the Long-term Risk of Ischemic Stroke. JAMA . 2014;312:616. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arsenault KA, Yusuf AM, Crystal E, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;2013:CD003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrev D, Aguilar M, Heijman J, et al. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417–36. doi: 10.1038/s41569-019-0166-5. [DOI] [PubMed] [Google Scholar]
- 7.Amar D, Shi W, Hogue CW, Jr, et al. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J Am Coll Cardiol. 2004;44:1248–53. doi: 10.1016/j.jacc.2004.05.078. [DOI] [PubMed] [Google Scholar]
- 8.Magee MJ, Herbert MA, Dewey TM, et al. Atrial fibrillation after coronary artery bypass grafting surgery: development of a predictive risk algorithm. Ann Thorac Surg. 2007;83:1707–12. doi: 10.1016/j.athoracsur.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Mariscalco G, Biancari F, Zanobini M, et al. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF score. J Am Heart Assoc. 2014;3:e000752. doi: 10.1161/JAHA.113.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–9. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz JM, George M, Rossetti SC, et al. Factors Influencing Clinician Trust in Predictive Clinical Decision Support Systems for In-Hospital Deterioration: Qualitative Descriptive Study. JMIR Hum Factors. 2022;9:e33960. doi: 10.2196/33960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalkey N, Helmer O. An Experimental Application of the DELPHI Method to the Use of Experts. Manage Sci. 1963;9:458–67. doi: 10.1287/mnsc.9.3.458. [DOI] [Google Scholar]
- 13.Mahajan V, Linstone HA, Turoff M. The Delphi Method: Techniques and Applications. J Market Res. 1976;13:317. doi: 10.2307/3150755. [DOI] [Google Scholar]
- 14.Nasa P, Azoulay E, Khanna AK, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care. 2021;25:106. doi: 10.1186/s13054-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Needham DM, Sepulveda KA, Dinglas VD, et al. Core Outcome Measures for Clinical Research in Acute Respiratory Failure Survivors. An International Modified Delphi Consensus Study. Am J Respir Crit Care Med. 2017;196:1122–30. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields KG, Ma J, Petrinic T, et al. Multivariable prediction models for atrial fibrillation after cardiac surgery: a systematic review protocol. BMJ Open. 2023;13:e067260. doi: 10.1136/bmjopen-2022-067260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung S-C, O’Brien B, Lip GYH, et al. Prognostic model for atrial fibrillation after cardiac surgery: a UK cohort study. Clin Res Cardiol. 2023;112:227–35. doi: 10.1007/s00392-022-02068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gattrell WT, Logullo P, van Zuuren EJ, et al. ACCORD (ACcurate COnsensus Reporting Document): A reporting guideline for consensus methods in biomedicine developed via a modified Delphi. PLoS Med. 2024;21:e1004326. doi: 10.1371/journal.pmed.1004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35:382–5. [PubMed] [Google Scholar]
- 20.Linstone H, Turoff M. The delphi method. Techniques and applications. Addison-Wesley; 1975. [Google Scholar]
- 21.Bedford JP, Garside T, Darbyshire JL, et al. Risk factors for new-onset atrial fibrillation during critical illness: A Delphi study. J Intensive Care Soc . 2022;23:414–24. doi: 10.1177/17511437211022132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anyukhovsky EP, Sosunov EA, Plotnikov A, et al. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovasc Res. 2002;54:462–9. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 23.Anyukhovsky EP, Sosunov EA, Chandra P, et al. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res. 2005;66:353–63. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 24.El-Chami MF, Kilgo PD, Elfstrom KM, et al. Prediction of new onset atrial fibrillation after cardiac revascularization surgery. Am J Cardiol. 2012;110:649–54. doi: 10.1016/j.amjcard.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 25.Vaziri SM, Larson MG, Benjamin EJ, et al. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–30. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 26.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96:1686–95. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 27.Bode F, Katchman A, Woosley RL, et al. Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation. 2000;101:2200–5. doi: 10.1161/01.cir.101.18.2200. [DOI] [PubMed] [Google Scholar]
- 28.Karimi A, Goodarzynejad H, Mortazavi SH, et al. Left Atrial Size; a Missing Component in Scoring Systems for Predicting Atrial Fibrillation Following Coronary Artery Bypass Surgery. Acta Cardiol Sin. 2020;36:456–63. doi: 10.6515/ACS.202009_36(5).20181023A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zacharias A, Schwann TA, Riordan CJ, et al. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation. 2005;112:3247–55. doi: 10.1161/CIRCULATIONAHA.105.553743. [DOI] [PubMed] [Google Scholar]
- 30.Creswell LL, Schuessler RB, Rosenbloom M, et al. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 31.Takenaka K, Ogawa E, Wada H, et al. Systemic inflammatory response syndrome and surgical stress in thoracic surgery. J Crit Care. 2006;21:48–53. doi: 10.1016/j.jcrc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Baigrie RJ, Lamont PM, Kwiatkowski D, et al. Systemic cytokine response after major surgery. Br J Surg. 1992;79:757–60. doi: 10.1002/bjs.1800790813. [DOI] [PubMed] [Google Scholar]
- 33.Ascione R, Caputo M, Calori G, et al. Predictors of atrial fibrillation after conventional and beating heart coronary surgery: A prospective, randomized study. Circulation. 2000;102:1530–5. doi: 10.1161/01.cir.102.13.1530. [DOI] [PubMed] [Google Scholar]
- 34.Ferro CRC, Oliveira DC de, Nunes FP, et al. Postoperative atrial fibrillation after cardiac surgery. Arq Bras Cardiol. 2009;93:59–63. doi: 10.1590/s0066-782x2009000700011. [DOI] [PubMed] [Google Scholar]
- 35.Koniari I, Artopoulou E, Velissaris D, et al. Atrial fibrillation in patients with systolic heart failure: pathophysiology mechanisms and management. J Geriatr Cardiol. 2021;18:376–97. doi: 10.11909/j.issn.1671-5411.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss JN, Qu Z, Shivkumar K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ Arrhythm Electrophysiol. 2017;10:e004667. doi: 10.1161/CIRCEP.116.004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krijthe BP, Heeringa J, Kors JA, et al. Serum potassium levels and the risk of atrial fibrillation: the Rotterdam Study. Int J Cardiol. 2013;168:5411–5. doi: 10.1016/j.ijcard.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien B, Burrage PS, Ngai JY, et al. Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthetists Practice Advisory for the Management of Perioperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery. J Cardiothorac Vasc Anesth. 2019;33:12–26. doi: 10.1053/j.jvca.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 39.Campbell NG, Allen E, Sanders J, et al. The impact of maintaining serum potassium ≥3.6 mEq/L vs ≥4.5 mEq/L on the incidence of new-onset atrial fibrillation in the first 120 hours after isolated elective coronary artery bypass grafting - study protocol for a randomised feasibility trial for the proposed Tight K randomized non-inferiority trial. Trials. 2017;18:618. doi: 10.1186/s13063-017-2349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neumann F-J, Sousa-Uva M. Ten commandments’ for the 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur Heart J. 2019;40:79–80. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 41.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 42.Lawton JS, Tamis-Holland JE, Writing Committee Members 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:197–215. doi: 10.1016/j.jacc.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Younis A, Orvin K, Nof E, et al. The effect of periprocedural beta blocker withdrawal on arrhythmic risk following transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2019;93:1361–6. doi: 10.1002/ccd.28017. [DOI] [PubMed] [Google Scholar]