Abstract
Background
Neonatal encephalopathy (NE) is a multi-organ condition potentially leading to death or long-term neurodisability. Therapeutic hypothermia is the standard treatment for NE; however, long-term impairments remain common. Studies of new treatments for NE often measure and report different outcomes. Core outcome sets (COSs), a minimum set of outcomes to be measured and reported in all studies for a condition, address this problem. This paper aimed to identify outcomes reported (primary, secondary, adverse events and other reported outcomes) in (1) randomised trials and (2) systematic reviews of randomised trials of interventions for the treatment of NE in the process of developing a COS for interventions for the treatment of NE.
Methods
We completed a systematic search for outcomes used to evaluate treatments for NE using MEDLINE, Embase, Cochrane CENTRAL, the Cochrane Database of Systematic Reviews and the WHO International Clinical Trials Registry Platform. Two reviewers screened all included articles independently. Outcomes were extracted verbatim, similar outcomes were grouped and outcome domains were developed.
Results
386 outcomes were reported in 116 papers, from 85 studies. Outcomes were categorised into 18 domains. No outcome was reported by all studies, a single study reported 11 outcomes and it was not explicitly stated that outcomes had input from parents.
Discussion
Heterogeneity in reported outcomes means that synthesis of studies evaluating new treatments for NE remains difficult. A COS, that includes parental/family input, is needed to ensure consistency in measuring and reporting outcomes, and to enable comparison of randomised trials.
Keywords: Neonatology
WHAT IS ALREADY KNOWN ON THIS TOPIC
There are numerous randomised trials and systematic reviews of randomised trials for interventions for treating neonatal encephalopathy.
WHAT THIS STUDY ADDS
We have identified 66 unique outcomes from 85 studies. Previously, a systematic review identifying the outcomes reported in randomised trials and systematic reviews of randomised trials of interventions for treating neonatal encephalopathy did not exist.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Identifying the heterogeneity in outcomes reported will help inform the development of a core outcome set to help standardise the outcomes measured and reported in future studies.
Introduction
Neonatal encephalopathy (NE) is a neurological syndrome that can occur in late preterm and term infants.1 Consequences of NE may include the development of cerebral palsy, seizure disorders, cognitive impairments, developmental impairments, multi-organ dysfunction and death.2 3 The causes of NE can vary, including hypoxic-ischaemic injury (resulting in hypoxic-ischaemic encephalopathy), metabolic disorders, neonatal stroke, infection, maternal risk factors, placental complications and other genetic and epigenetic factors.4 It is estimated that the incidence of NE is between 3 and 8.5 per 1000 live births.5 6
A systematic review of 11 randomised controlled trials of therapeutic hypothermia in late preterm and term neonates with moderate-to-severe encephalopathy found that this therapy reduces the risk of death and neurodevelopmental disability by 25%.7 Therapeutic hypothermia is the standard treatment for moderate-to-severe NE in many countries in the developed world.8 9 However, evidence for the efficacy of therapeutic hypothermia is limited to a narrow therapeutic window of 6 hours from birth10 requiring an early diagnosis. Furthermore, long-term neurodevelopmental impairment still occurs in many survivors with moderate-to-severe encephalopathy treated with therapeutic hypothermia7 resulting in the development of novel neuroprotective treatment strategies. Recent clinical trials of other treatments include erythropoietin and darbepoetin,11,17 magnesium sulfate,18 2-iminobiotin19 and xenon gas.20
There are an increasing number of clinical trials of treatments for NE. Yet, a significant challenge arises in comparing the effectiveness of different interventions from different studies. Many studies measure and report different outcomes to determine the effectiveness of treatments. The lack of standardisation of outcomes measured means that researchers conducting evidence synthesis in reviews and meta-analyses cannot compare interventions and truly determine if a new intervention is better than the standard treatment or not.
To overcome this, the Core Outcome Measures in Effectiveness Trials (COMET) initiative recommends developing a standardised set of outcomes, known as a core outcome set (COS). A COS is an agreed, standardised set of outcomes that should be measured as a minimum in all studies for a health condition.21 This allows researchers to compare the outcomes reported in the results of trials. Although researchers may measure other outcomes relevant to their trial, the COS should always be measured and reported. Developing a COS involves presenting key stakeholders with the outcomes commonly measured, typically identified by a systematic review, and asking them to prioritise the most critical outcomes using consensus techniques.
The objective of this paper was to identify outcomes reported (primary, secondary, adverse events and other reported outcomes) in (1) randomised trials and (2) systematic reviews of randomised trials of interventions for the treatment of NE as the initial step in the development of a COS for interventions for the treatment of NE.
Methods
The study consisted of a systematic review of completed randomised trials and systematic reviews of randomised trials of interventions for the treatment of NE. The literature search was conducted in December 2019 and WHO registry trials were added in April 2020. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA)22 and COMET21 guidelines are used to report the conduct and findings of this review.
Protocol and registration
The protocol was registered with the PROSPERO database (CRD42020170265) and is contained within the protocol ‘COHESION: Core Outcomes in Neonatal Encephalopathy (Protocol)’.23
Eligibility criteria
The criteria for eligible studies were developed using the PICOS method (Population, Intervention, Comparator, Outcome and Study Design):
Population
Infants diagnosed and treated for NE, hypoxic-ischaemic or perinatal asphyxia/birth asphyxia encephalopathy; infants greater than or equal to 35 weeks’ gestation. Where mixed gestational age of at least 80% of infants must be greater than or equal to 35 weeks’ gestation.
Intervention
Any intervention for treating NE, hypoxic-ischaemic encephalopathy or perinatal/birth asphyxia.
Control/comparator
Comparing any intervention for the treatment of NE/hypoxic-ischaemic encephalopathy, or perinatal asphyxia with another treatment, placebo, standard care or nothing.
Outcome
All outcomes (related to the effect of the intervention) were included.
Study design
Randomised trials (last 20 years); systematic reviews of randomised trials (last 20 years).
Language
Only papers available in English were included.
Where there was insufficient information available in the reported study(ies) or review(s) to allow judgements as to whether it met the inclusion criteria, the study/review was excluded.
Information sources and search strategy
Studies were identified by searching the following databases: Excerpta Medica database (Embase), MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR) and the WHO International Clinical Trials Registry Platform (ICTRP).
Databases were searched from December 2019 to April 2020 using a comprehensive search strategy modified for each database based on database-specific medical subject headings. The search strategies used are available in the online supplemental information.
Identification of studies
FQ and DD carried out independent screening of titles and abstracts for eligibility. Full-text papers were retrieved and reviewed to determine their eligibility for inclusion independently by DD and FQ with any disagreements discussed with a third reviewer, PH. We excluded protocols and ongoing trials from this review.
Data extraction
The data extracted from each study included study design, author details, year and journal of publication, the country in which the study was conducted, targeted condition, criteria for the diagnosis of NE, interventions under investigation and all outcomes as they are reported in the studies (including the time points). Extracting the country where the trial was conducted or the country where the lead author lived allowed us to determine any potential differences in outcomes between high and low- to middle-income countries (LMICs). FQ extracted the data while the second reviewer (EF) conducted a verification check of 50% of the outcomes extracted against the source paper. Any discrepancies found were verified by DD. Disagreement was resolved through discussion with a third reviewer (DD).
Data analysis and presentation
Data were tabulated so that each study and all outcomes measured in each study were displayed separately. Outcomes were grouped with similar outcomes under major domains (ie, neurological outcomes, cardiovascular outcomes, respiratory outcomes, gastrointestinal outcomes, motor development, other organ outcomes, cognitive development, development (special senses), development (speech and social), development (psychosocial), long-term disability, hospitalisation, adverse events, survival/living outcomes, healthcare utilisation, patient-reported outcomes, infection outcomes, parent-important outcomes), as guided by similar COS in this area.24 25 Outcomes and their domains identified from the systematic review were reviewed by the COHESION Steering Group, which includes public and patient involvement representatives. Time points of when outcomes were measured and ‘how’ the outcomes were measured were beyond the scope of this review and were not extracted.
Results
Study selection
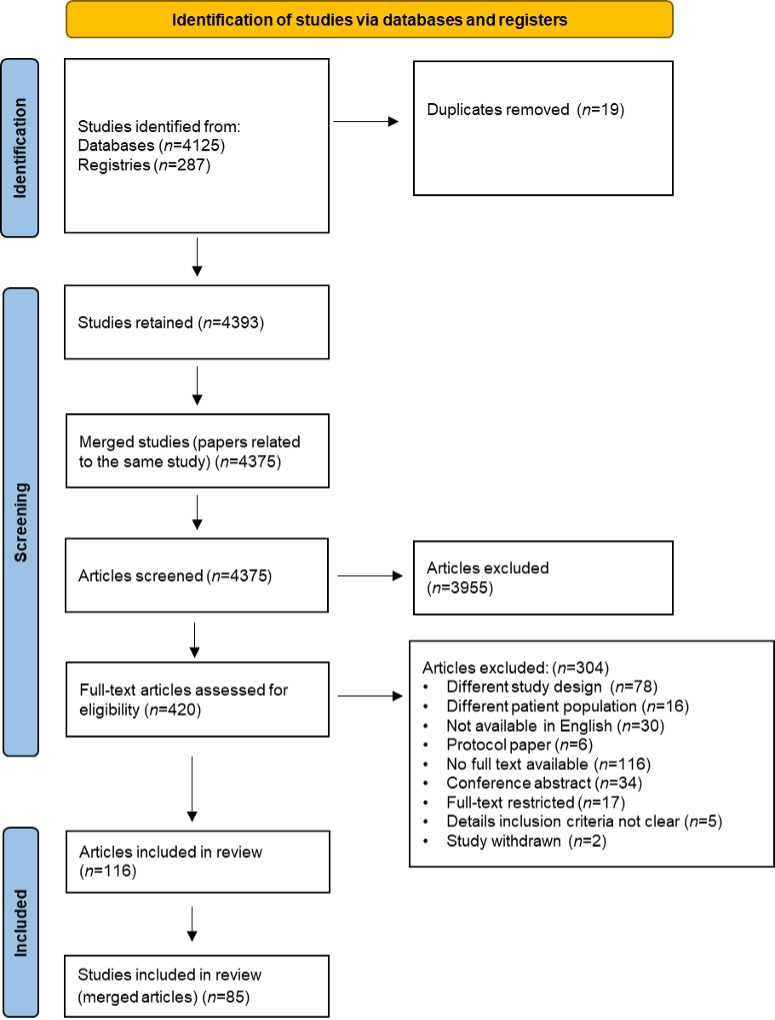
Overall, 4412 citations were imported for screening from MEDLINE, Embase, CENTRAL, CDSR and the WHO-ICTRP registry, 19 of which were removed as duplicates. These were initially grouped into 4375 studies. At title and abstract screening, 3955 titles were excluded as not meeting the PICOS inclusion criteria, and 420 articles were deemed eligible for full-text screening. A further 304 full-texts were excluded for various reasons including different study designs (n=78); different patient populations (n=16); not available in English (n=30); protocol paper (n=6); no free full text available or reply from the author in response to request for full text (n=116); conference abstract (n=34); full-text restricted (n=17); details inclusion criteria not clear (n=5); study withdrawn (n=2). There were 116 articles comprising 85 studies in the data extraction process as summarised in the COHESION PRISMA diagram (figure 1).
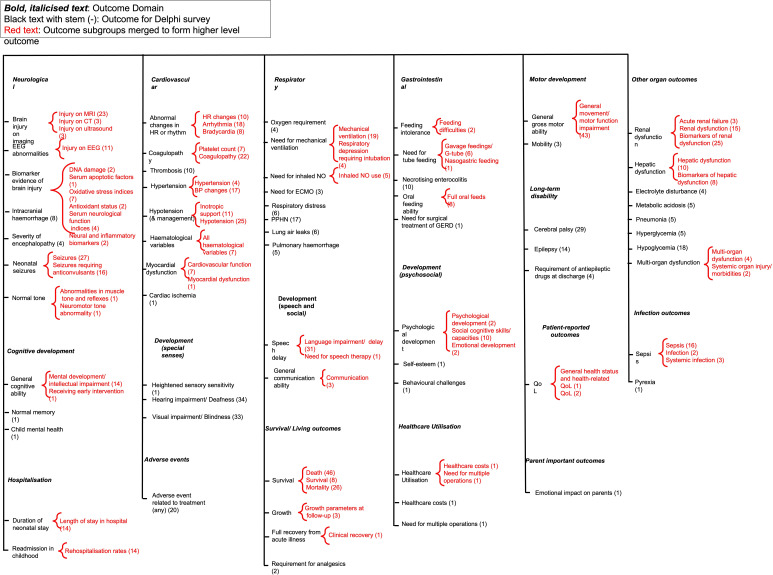
Figure 1. COHESION Preferred Reporting Items for Systematic reviews and Meta-Analyses flow diagram.
Characteristics of studies
This review included 62 randomised trials, 10 systematic reviews, 6 systematic reviews and meta-analysis, 6 meta-analysis and 1 review of systematic reviews. In the reviews and/or meta-analyses, 11 of the first authors were from LMICs and 12 were from high-income countries. Treatments from the 62 randomised trials included therapeutic hypothermia alone, adjuvants to therapeutic hypothermia (melatonin, xenon, darbepoetin, allopurinol, magnesium sulfate, erythropoietin and topiramate), alternative treatments to therapeutic hypothermia (melatonin, ascorbic acid and ibuprofen, phenobarbital, allopurinol, magnesium sulfate, magnesium sulfate and melatonin, erythropoietin, topiramate, monosialoganglioside vs citicoline, pyritinol) or alternative therapy such as fluid restriction.
In total, 386 outcomes were reported across 116 papers.826,128 Once duplicates were removed, 66 outcomes deemed similar were categorised under 18 domains as illustrated in figure 2. The number of domains and the number of outcomes identified in this review reflect the heterogeneity of outcomes reported in trials for the treatment of NE. Some outcomes were more commonly reported in studies. For example, brain injury on imaging was reported in 29 studies, survival was reported in 80 studies and general gross motor ability was reported in 43 studies. In contrast some outcomes, for example, the need for surgical treatment of gastro-oesophageal reflux disease and healthcare costs were reported in single studies.
Figure 2. Outcomes extracted from the 85 studies, outcomes merged and outcome domains identified and developed. BP, blood pressure; COS, core outcome set; GERD, gastro-oesophageal reflux disease; HR, heart rate; QoL, quality of life; NO - nitric oxide; ECMO, extracorporeal membrane oxygenation; PPHN, Persistent pulmonary hypertension of the newborn.
Although time points were not extracted, it was noted that for some outcomes, for example, injury indicated on MRI, multiple different time points were reported for measuring the outcome, leading to further heterogeneity in outcome reporting. The terminology used to describe outcomes also varied as death, mortality and survival were used to describe the same outcome across many studies.
‘Adverse events’ were reported in 20 studies. Some adverse events were reported as outcomes in some studies, for example, renal dysfunction. Among the 85 studies included in this review, only one meta-analysis49 reported outcomes of interest to patients and clinicians, described as cerebral palsy or survival with normal neurological function. However, the authors did not state how they determined these outcomes were important to patients and clinicians.
Discussion
This systematic review, identifying the outcomes measured and reported in treating NE/perinatal asphyxia/hypoxic-ischaemic encephalopathy across 85 studies, illustrates the differences in outcomes reported among studies. No outcome was measured in all studies, and 11 outcomes were only measured in a single study. This level of heterogeneity in the outcomes measured and reported in studies contributes to large amounts of research waste. It also hinders the ability to synthesise trial results to compare and contrast interventions.
Systematic reviews are used in COS development to identify the outcomes considered important to clinicians and researchers.21 However, the benefits of including the perspectives of patients and/or parents and caregivers of patients in this process are also recognised to ensure the inclusion of outcomes they consider important for their health and the health of their children.129 We found that only one meta-analysis included data on outcomes of interest to both patients and clinicians. Parents/caregivers of patients have different priorities on outcomes they consider to be important130 and it is therefore essential that patients’/parents’ views should be included in the selection of outcomes for randomised trials. In our qualitative interviews,130 25 parents identified 21 outcomes as important that were not measured and reported in the randomised trials included in this systematic review. For this qualitative study, saturation was defined as when no new outcomes were identified as the interviews and analysis progressed.
These findings highlight the need to develop a COS to be used in all studies evaluating interventions for the treatment of NE. A COS will ensure consistency in reporting the results of trials for the treatment of NE. Developing a COS ensures that measured outcomes are relevant to healthcare providers, researchers, patients and the parents of infants diagnosed with and treated for NE.
While we acknowledge that the search strategy for our systematic review was conducted in 2019, we suggest that the extent of heterogeneity in outcomes reported is unlikely to have changed substantially in more recent trials (Wu et al, 2022; Salamah et al 2023).17 131 First, the absence of a COS for NE means that researchers need more guidance on what outcomes to select and report, making it unlikely that the heterogeneity has decreased spontaneously over a short period. Second, the issue of outcome heterogeneity is deeply rooted in the diverse clinical presentations of the condition and the varied methodologies employed across studies. These factors are unlikely to have changed significantly in a short time frame. Third, our review serves as a baseline assessment that underscores the pressing need for developing a COS for interventions for treating NE. This need would remain pertinent even if some heterogeneity reduction has occurred in recent years. Therefore, while the age of the search may be considered a limitation, the fundamental issue of outcome heterogeneity we have identified will likely remain a persistent challenge in the field until a COS is developed and implemented.
Conclusion
This review highlights the heterogeneity in outcome reporting across studies looking at interventions for treating NE. The manner in which outcomes are described also varied across studies, highlighting the need for consistency. This heterogeneity warrants the development of a COS for interventions for treating NE. This review demonstrates the benefits of a COS in studies evaluating treatments for NE, as this will support the reduction of research waste and allow improved synthesis of trial results.
supplementary material
Footnotes
Funding: This research was funded by the Health Research Board, Neonatal Encephalopathy PhD Training Network (HRB-NEPTuNE), grant number CDA-FA-2018-008.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Fiona Quirke, Email: 22306056@studentmail.ul.ie.
Linda Biesty, Email: linda.biesty@universityofgalway.ie.
Malcolm Battin, Email: malcolmb@adhb.govt.nz.
Frank Harry Bloomfield, Email: f.bloomfield@auckland.ac.nz.
Mandy Daly, Email: mandy.daly@yahoo.co.uk.
Elaine Finucane, Email: elaine1finucane@gmail.com.
Patricia Healy, Email: patricia.healy@nuigalway.ie.
Tim Hurley, Email: tim.hurley@ucdconnect.ie.
Jamie J Kirkham, Email: jamie.kirkham@manchester.ac.uk.
Eleanor Molloy, Email: eleanor.molloy@tcd.ie.
David M Haas, Email: dahaas@iu.edu.
Shireen Meher, Email: smeher@liverpool.ac.uk.
Elaine Ní Bhraonáin, Email: elainenib@gmail.com.
Karen Walker, Email: karen.walker@health.nsw.gov.au.
James Webbe, Email: j.webbe@imperial.ac.uk.
Declan Devane, Email: declan.devane@universityofgalway.ie.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ task force on neonatal encephalopathy. Obstet Gynecol. 2014;123:896–901. doi: 10.1097/01.AOG.0000445580.65983.d2. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol. 2012;72:156–66. doi: 10.1002/ana.23647. [DOI] [PubMed] [Google Scholar]
- 3.Shah P, Riphagen S, Beyene J, et al. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89:F152–5. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslam S, Strickland T, Molloy EJ. Neonatal Encephalopathy: Need for Recognition of Multiple Etiologies for Optimal Management. Front Pediatr. 2019;7:142. doi: 10.3389/fped.2019.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–38. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Lee ACC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74 Suppl 1:50–72. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;2013:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankaran S, Laptook AR, Pappas A, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312:2629–39. doi: 10.1001/jama.2014.16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yıldız EP, Ekici B, Tatlı B. Neonatal hypoxic ischemic encephalopathy: an update on disease pathogenesis and treatment. Expert Rev Neurother. 2017;17:449–59. doi: 10.1080/14737175.2017.1259567. [DOI] [PubMed] [Google Scholar]
- 10.Williams CE, Gunn A, Gluckman PD. Time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke. 1991;22:516–21. doi: 10.1161/01.str.22.4.516. [DOI] [PubMed] [Google Scholar]
- 11.Razak A, Hussain A. Erythropoietin in perinatal hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. J Perinat Med. 2019;47:478–89. doi: 10.1515/jpm-2018-0360. [DOI] [PubMed] [Google Scholar]
- 12.Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–26. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- 13.Baserga MC, Beachy JC, Roberts JK, et al. Darbepoetin administration to neonates undergoing cooling for encephalopathy: a safety and pharmacokinetic trial. Pediatr Res. 2015;78:315–22. doi: 10.1038/pr.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Shimi MS, Awad HA, Hassanein SMA, et al. Single dose recombinant erythropoietin versus moderate hypothermia for neonatal hypoxic ischemic encephalopathy in low resource settings. J Matern Fetal Neonatal Med. 2014;27:1295–300. doi: 10.3109/14767058.2013.855894. [DOI] [PubMed] [Google Scholar]
- 15.Malla RR, Asimi R, Teli MA, et al. Erythropoietin monotherapy in perinatal asphyxia with moderate to severe encephalopathy: a randomized placebo-controlled trial. J Perinatol. 2017;37:596–601. doi: 10.1038/jp.2017.17. [DOI] [PubMed] [Google Scholar]
- 16.Avasiloaiei A, Dimitriu C, Moscalu M, et al. High-dose phenobarbital or erythropoietin for the treatment of perinatal asphyxia in term newborns. Pediatr Int. 2013;55:589–93. doi: 10.1111/ped.12121. [DOI] [PubMed] [Google Scholar]
- 17.Wu YW, Comstock BA, Gonzalez FF, et al. Trial of Erythropoietin for Hypoxic-Ischemic Encephalopathy in Newborns. N Engl J Med. 2022;387:148–59. doi: 10.1056/NEJMoa2119660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Farargy MS, Soliman NA. A randomized controlled trial on the use of magnesium sulfate and melatonin in neonatal hypoxic ischemic encephalopathy. NPM . 2019;12:379–84. doi: 10.3233/NPM-181830. [DOI] [PubMed] [Google Scholar]
- 19.Biselele T, Bambi J, Betukumesu DM, et al. A Phase IIa Clinical Trial of 2-Iminobiotin for the Treatment of Birth Asphyxia in DR Congo, a Low-Income Country. Paediatr Drugs. 2020;22:95–104. doi: 10.1007/s40272-019-00373-3. [DOI] [PubMed] [Google Scholar]
- 20.Azzopardi D, Robertson NJ, Bainbridge A, et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 2016;15:145–53. doi: 10.1016/S1474-4422(15)00347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson PR, Altman DG, Bagley H, et al. The COMET Handbook: version 1.0. Trials. 2017;18:280. doi: 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quirke FA, Healy P, Bhraonáin EN, et al. COHESION: core outcomes in neonatal encephalopathy (protocol) Trials. 2021;22:125. doi: 10.1186/s13063-021-05030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Healy P, Gordijn SJ, Ganzevoort W, et al. A Core Outcome Set for the prevention and treatment of fetal GROwth restriction: deVeloping Endpoints: the COSGROVE study. Am J Obstet Gynecol. 2019;221:339. doi: 10.1016/j.ajog.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 25.Webbe JWH, Duffy JMN, Afonso E, et al. Core outcomes in neonatology: development of a core outcome set for neonatal research. Arch Dis Child Fetal Neonatal Ed. 2020;105:425–31. doi: 10.1136/archdischild-2019-317501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad QM, Chishti AL, Waseem N. Role of melatonin in management of hypoxic ischaemic encephalopathy in newborns: A randomized control trial. J Pak Med Assoc. 2018;68:1233–7. [PubMed] [Google Scholar]
- 27.Aker K, Støen R, Eikenes L, et al. Therapeutic hypothermia for neonatal hypoxic-ischaemic encephalopathy in India (THIN study): a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2020;105:405–11. doi: 10.1136/archdischild-2019-317311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akisu M, Huseyinov A, Yalaz M, et al. Selective head cooling with hypothermia suppresses the generation of platelet-activating factor in cerebrospinal fluid of newborn infants with perinatal asphyxia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:45–50. doi: 10.1016/s0952-3278(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 29.Akula VP, Joe P, Thusu K, et al. A randomized clinical trial of therapeutic hypothermia mode during transport for neonatal encephalopathy. J Pediatr. 2015;166:856–61. doi: 10.1016/j.jpeds.2014.12.061. [DOI] [PubMed] [Google Scholar]
- 30.Aly H, Abd-Rabboh L, El-Dib M, et al. Ascorbic acid combined with ibuprofen in hypoxic ischemic encephalopathy: a randomized controlled trial. J Perinatol. 2009;29:438–43. doi: 10.1038/jp.2009.1. [DOI] [PubMed] [Google Scholar]
- 31.Aly H, Elmahdy H, El-Dib M, et al. Melatonin use for neuroprotection in perinatal asphyxia: a randomized controlled pilot study. J Perinatol. 2015;35:186–91. doi: 10.1038/jp.2014.186. [DOI] [PubMed] [Google Scholar]
- 32.Atıcı A, Çelik Y, Gülaşı S, et al. Comparison of selective head cooling therapy and whole body cooling therapy in newborns with hypoxic ischemic encephalopathy: short term results. Turk Pediatri Ars. 2015;50:27–36. doi: 10.5152/tpa.2015.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azzopardi D, Robertson NJ, Kapetanakis A, et al. Anticonvulsant effect of xenon on neonatal asphyxial seizures. Arch Dis Child Fetal Neonatal Ed. 2013;98:F437–9. doi: 10.1136/archdischild-2013-303786. [DOI] [PubMed] [Google Scholar]
- 34.Azzopardi D, Chew AT, Deierl A, et al. Prospective qualification of early cerebral biomarkers in a randomised trial of treatment with xenon combined with moderate hypothermia after birth asphyxia. EBioMedicine. 2019;47:484–91. doi: 10.1016/j.ebiom.2019.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azzopardi D, Strohm B, Marlow N, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371:140–9. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 37.Rivero-Arias O, Eddama O, Azzopardi D, et al. Hypothermia for perinatal asphyxia: trial-based resource use and costs at 6-7 years. Arch Dis Child Fetal Neonatal Ed. 2019;104:F285–92. doi: 10.1136/archdischild-2017-314685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell H, Eddama O, Azzopardi D, et al. Hypothermia for perinatal asphyxia: trial-based quality of life at 6–7 years. Arch Dis Child. 2018;103:654–9. doi: 10.1136/archdischild-2017-313733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 40.Battin MR, Dezoete JA, Gunn TR, et al. Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics. 2001;107:480–4. doi: 10.1542/peds.107.3.480. [DOI] [PubMed] [Google Scholar]
- 41.Battin MR, Penrice J, Gunn TR, et al. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics. 2003;111:244–51. doi: 10.1542/peds.111.2.244. [DOI] [PubMed] [Google Scholar]
- 42.Benders MJNL, Bos AF, Rademaker CMA, et al. Early postnatal allopurinol does not improve short term outcome after severe birth asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91:F163–5. doi: 10.1136/adc.2005.086652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaandorp JJ, van Bel F, Veen S, et al. Long-term neuroprotective effects of allopurinol after moderate perinatal asphyxia: follow-up of two randomised controlled trials. Arch Dis Child Fetal Neonatal Ed. 2012;97:F162–6. doi: 10.1136/archdischild-2011-300356. [DOI] [PubMed] [Google Scholar]
- 44.Bharadwaj SK, Bhat BV. Therapeutic hypothermia using gel packs for term neonates with hypoxic ischaemic encephalopathy in resource-limited settings: a randomized controlled trial. J Trop Pediatr. 2012;58:382–8. doi: 10.1093/tropej/fms005. [DOI] [PubMed] [Google Scholar]
- 45.Bhat MA, Charoo BA, Bhat JI, et al. Magnesium sulfate in severe perinatal asphyxia: a randomized, placebo-controlled trial. Pediatrics. 2009;123:e764–9. doi: 10.1542/peds.2007-3642. [DOI] [PubMed] [Google Scholar]
- 46.Çelik Y, Atıcı A, Gülaşı S, et al. The effects of selective head cooling versus whole-body cooling on some neural and inflammatory biomarkers: a randomized controlled pilot study. Ital J Pediatr. 2015;41:79. doi: 10.1186/s13052-015-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conway JM, Walsh BH, Boylan GB, et al. Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome - A systematic review. Early Hum Dev. 2018;120:80–7. doi: 10.1016/j.earlhumdev.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Das S, Sarkar N, Bhattacharya M, et al. Neurological Outcome at 30 Months of Age after Mild Hypothermia via Selective Head Cooling in Term Neonates with Perinatal Asphyxia Using Low-Cost CoolCap: A Single-Center Randomized Control Pilot Trial in India. J Pediatr Neurol. 2017;15:157–65. doi: 10.1055/s-0037-1603681. [DOI] [Google Scholar]
- 49.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–7. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: Safety outcomes. Pediatr Neurol. 2005;32:18–24. doi: 10.1016/j.pediatrneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Lowe DW, Hollis BW, Wagner CL, et al. Vitamin D insufficiency in neonatal hypoxic–ischemic encephalopathy. Pediatr Res. 2017;82:55–62. doi: 10.1038/pr.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans DJ, Levene MI. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia. Cochrane Database Syst Rev. 2000:CD001240. doi: 10.1002/14651858.CD001240. [DOI] [PubMed] [Google Scholar]
- 54.Evans DJ, Levene MI, Tsakmakis M. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia. Cochrane Database Syst Rev. 2007:CD001240. doi: 10.1002/14651858.CD001240.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Filippi L, Fiorini P, Catarzi S, et al. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI): a feasibility study. J Matern Fetal Neonatal Med. 2018;31:973–80. doi: 10.1080/14767058.2017.1304536. [DOI] [PubMed] [Google Scholar]
- 56.Galvao TF, Silva MT, Marques MC, et al. Hypothermia for perinatal brain hypoxia-ischemia in different resource settings: a systematic review. J Trop Pediatr. 2013;59:453–9. doi: 10.1093/tropej/fmt047. [DOI] [PubMed] [Google Scholar]
- 57.Gane BD, Bhat V, Rao R, et al. Effect of therapeutic hypothermia on DNA damage and neurodevelopmental outcome among term neonates with perinatal asphyxia: a randomized controlled trial. J Trop Pediatr. 2014;60:134–40. doi: 10.1093/tropej/fmt098. [DOI] [PubMed] [Google Scholar]
- 58.Garg B, Sharma D, Bansal A. Systematic review seeking erythropoietin role for neuroprotection in neonates with hypoxic ischemic encephalopathy: presently where do we stand. J Matern Fetal Neonatal Med. 2018;31:3214–24. doi: 10.1080/14767058.2017.1366982. [DOI] [PubMed] [Google Scholar]
- 59.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 60.Gunn AJ, Wyatt JS, Whitelaw A, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55–8. doi: 10.1016/j.jpeds.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–21. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 62.Lingappan K, Kaiser JR, Srinivasan C, et al. Relationship between PCO2 and unfavorable outcome in infants with moderate-to-severe hypoxic ischemic encephalopathy. Pediatr Res. 2016;80:204–8. doi: 10.1038/pr.2016.62. [DOI] [PubMed] [Google Scholar]
- 63.Guillet R, Edwards AD, Thoresen M, et al. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr Res. 2012;71:205–9. doi: 10.1038/pr.2011.30. [DOI] [PubMed] [Google Scholar]
- 64.Groenendaal F, Rademaker CMA, Toet MC, et al. Effects of magnesium sulphate on amplitude-integrated continuous EEG in asphyxiated term neonates. Acta Paediatr. 2002;91:1073–7. doi: 10.1080/080352502760311575. [DOI] [PubMed] [Google Scholar]
- 65.Gunes T, Ozturk MA, Koklu E, et al. Effect of allopurinol supplementation on nitric oxide levels in asphyxiated newborns. Pediatr Neurol. 2007;36:17–24. doi: 10.1016/j.pediatrneurol.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Horn AR, Woods DL, Thompson C, et al. Selective cerebral hypothermia for post-hypoxic neuroprotection in neonates using a solid ice cap. S Afr Med J. 2006;96:976–81. [PubMed] [Google Scholar]
- 67.Ichiba H, Tamai H, Negishi H, et al. Randomized controlled trial of magnesium sulfate infusion for severe birth asphyxia. Pediatr Int. 2002;44:505–9. doi: 10.1046/j.1442-200x.2002.01610.x. [DOI] [PubMed] [Google Scholar]
- 68.Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 69.Jacobs SE, Hunt R, Tarnow-Mordi WO, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- 70.Jacobs S, Hunt R, Tarnow-Mordi W, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2003;4:CD003311. doi: 10.1002/14651858.CD003311. [DOI] [PubMed] [Google Scholar]
- 71.Joy R, Pournami F, Bethou A, et al. Effect of Therapeutic Hypothermia on Oxidative Stress and Outcome in Term Neonates with Perinatal Asphyxia: A Randomized Controlled Trial. J Trop Pediatr (Lond) 2013;59:17–22. doi: 10.1093/tropej/fms036. [DOI] [PubMed] [Google Scholar]
- 72.Kariholu U, Montaldo P, Markati T, et al. Therapeutic hypothermia for mild neonatal encephalopathy: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2020;105:225–8. doi: 10.1136/archdischild-2018-315711. [DOI] [PubMed] [Google Scholar]
- 73.Laptook AR, Shankaran S, Tyson JE, et al. Effect of Therapeutic Hypothermia Initiated After 6 Hours of Age on Death or Disability Among Newborns With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA. 2017;318:1550–60. doi: 10.1001/jama.2017.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li T, Xu F, Cheng X, et al. Systemic Hypothermia Induced within 10 Hours After Birth Improved Neurological Outcome in Newborns with Hypoxic-Ischemic Encephalopathy. Hosp Pract (1995) 2009;37:147–52. doi: 10.3810/hp.2009.12.269. [DOI] [PubMed] [Google Scholar]
- 75.Liang SP, Chen Q, Cheng YB, et al. Comparative Effects of Monosialoganglioside versus Citicoline on Apoptotic Factor, Neurological Function and Oxidative Stress in Newborns with Hypoxic-Ischemic Encephalopathy. J Coll Physicians Surg Pak. 2019;29:324–7. doi: 10.29271/jcpsp.2019.04.324. [DOI] [PubMed] [Google Scholar]
- 76.Lin Z-L, Yu H-M, Lin J, et al. Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia: an experience from a single neonatal intensive care unit. J Perinatol. 2006;26:180–4. doi: 10.1038/sj.jp.7211412. [DOI] [PubMed] [Google Scholar]
- 77.Liu Z, Xiong T, Meads C. Clinical effectiveness of treatment with hyperbaric oxygen for neonatal hypoxic-ischaemic encephalopathy: systematic review of Chinese literature. BMJ. 2006;333:374. doi: 10.1136/bmj.38776.731655.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv H-Y, Wu S-J, Wang Q-L, et al. Effect of erythropoietin combined with hypothermia on serum tau protein levels and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Neural Regen Res. 2017;12:1655–63. doi: 10.4103/1673-5374.217338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maiwald CA, Annink KV, Rüdiger M, et al. Effect of allopurinol in addition to hypothermia treatment in neonates for hypoxic-ischemic brain injury on neurocognitive outcome (ALBINO): study protocol of a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III) BMC Pediatr. 2019;19:210. doi: 10.1186/s12887-019-1566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nair MKC, George B, Jeyaseelan L. Pyritinol for post asphyxial encephalopathy in term babies-- a randomized double-blind controlled trial. Indian Pediatr. 2009;46 Suppl:s37–42. [PubMed] [Google Scholar]
- 81.Nuñez-Ramiro A, Benavente-Fernández I, Valverde E, et al. Topiramate plus Cooling for Hypoxic-Ischemic Encephalopathy: A Randomized, Controlled, Multicenter, Double-Blinded Trial. Neonatology. 2019;116:76–84. doi: 10.1159/000499084. [DOI] [PubMed] [Google Scholar]
- 82.Pauliah SS, Shankaran S, Wade A, et al. Therapeutic hypothermia for neonatal encephalopathy in low- and middle-income countries: a systematic review and meta-analysis. PLoS One. 2013;8:e58834. doi: 10.1371/journal.pone.0058834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perrone S, Szabó M, Bellieni CV, et al. Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury. Pediatr Neurol. 2010;43:236–40. doi: 10.1016/j.pediatrneurol.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Prakash R, Savitha MR, Krishnamurthy B. Neurodevelopmental Outcome at 12 Months of Postnatal Magnesium Sulphate Therapy for Perinatal Asphyxia. J Nepal Paedtr Soc. 2017;36:256–62. doi: 10.3126/jnps.v36i3.15565. [DOI] [Google Scholar]
- 85.Rahman S, Canpolat F, Oncel M, et al. Multicenter randomized controlled trial of therapeutic hypothermia plus magnesium sulfate versus therapeutic hypothermia plus placebo in the management of term and near-term infants with hypoxic ischemic encephalopathy (The Mag Cool study): A pilot study. J Clin Neonatol. 2015;4:158. doi: 10.4103/2249-4847.159863. [DOI] [Google Scholar]
- 86.Rakesh K, Vishnu Bhat B, Adhisivam B, et al. Effect of therapeutic hypothermia on myocardial dysfunction in term neonates with perinatal asphyxia - a randomized controlled trial. J Matern Fetal Neonatal Med. 2018;31:2418–23. doi: 10.1080/14767058.2017.1344633. [DOI] [PubMed] [Google Scholar]
- 87.Robertson NJ, Nakakeeto M, Hagmann C, et al. Therapeutic hypothermia for birth asphyxia in low-resource settings: a pilot randomised controlled trial. Lancet. 2008;372:801–3. doi: 10.1016/S0140-6736(08)61329-X. [DOI] [PubMed] [Google Scholar]
- 88.Rossouw G, Irlam J, Horn AR. Therapeutic hypothermia for hypoxic ischaemic encephalopathy using low-technology methods: a systematic review and meta-analysis. Acta Paediatr. 2015;104:1217–28. doi: 10.1111/apa.12830. [DOI] [PubMed] [Google Scholar]
- 89.Schulzke SM, Rao S, Patole SK. A systematic review of cooling for neuroprotection in neonates with hypoxic ischemic encephalopathy - are we there yet? BMC Pediatr. 2007;7:30. doi: 10.1186/1471-2431-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med. 2010;15:238–46. doi: 10.1016/j.siny.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 92.Shankaran S, Pappas A, Laptook AR, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122:e791–8. doi: 10.1542/peds.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mietzsch U, Parikh NA, Williams AL, et al. Effects of hypoxic-ischemic encephalopathy and whole-body hypothermia on neonatal auditory function: a pilot study. Am J Perinatol. 2008;25:435–41. doi: 10.1055/s-0028-1083842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laptook AR, Shankaran S, Ambalavanan N, et al. Outcome of term infants using apgar scores at 10 minutes following hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:1619–26. doi: 10.1542/peds.2009-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shankaran S, Laptook AR, McDonald SA, et al. Temperature profile and outcomes of neonates undergoing whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatr Crit Care Med. 2012;13:53–9. doi: 10.1097/PCC.0b013e31821926bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. Erratum in: N Engl J Med. 367(11):1073 (2012) N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shankaran S, Barnes PD, Hintz SR, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Erratum in: Arch Dis Child Fetal Neonatal Ed. 99(3):301 (2014) Arch Dis Child Fetal Neonatal Ed. 2012;97:F398–404. doi: 10.1136/archdischild-2011-301524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Natarajan G, Shankaran S, Laptook AR, et al. Apgar scores at 10 min and outcomes at 6-7 years following hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2013;98:F473–9. doi: 10.1136/archdischild-2013-303692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pappas A, Shankaran S, McDonald SA, et al. Cognitive outcomes after neonatal encephalopathy. Pediatrics. 2015;135:e624–34. doi: 10.1542/peds.2014-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shankaran S, Laptook AR, McDonald SA, et al. Acute Perinatal Sentinel Events, Neonatal Brain Injury Pattern, and Outcome of Infants Undergoing a Trial of Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy. J Pediatr. 2017;180:275–8. doi: 10.1016/j.jpeds.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Natarajan G, Shankaran S, Laptook AR, et al. Association between sedation-analgesia and neurodevelopment outcomes in neonatal hypoxic-ischemic encephalopathy. J Perinatol. 2018;38:1060–7. doi: 10.1038/s41372-018-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shankaran S, Laptook AR, Pappas A, et al. Effect of Depth and Duration of Cooling on Death or Disability at Age 18 Months Among Neonates With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA. 2017;318:57–67. doi: 10.1001/jama.2017.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sheng L, Li Z. Adjuvant treatment with monosialoganglioside may improve neurological outcomes in neonatal hypoxic-ischemic encephalopathy: A meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0183490. doi: 10.1371/journal.pone.0183490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shepherd E, Salam RA, Middleton P, et al. Neonatal interventions for preventing cerebral palsy: an overview of Cochrane Systematic Reviews. Cochrane Database Syst Rev. 2018;6:CD012409. doi: 10.1002/14651858.CD012409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh D, Kumar P, Majumdar S, et al. Effect of phenobarbital on free radicals in neonates with hypoxic ischemic encephalopathy--a randomized controlled trial. J Perinat Med. 2004;32:278–81. doi: 10.1515/JPM.2004.052. [DOI] [PubMed] [Google Scholar]
- 106.Singh D, Kumar P, Narang A. A randomized controlled trial of phenobarbital in neonates with hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2005;18:391–5. doi: 10.1080/13895260500327979. [DOI] [PubMed] [Google Scholar]
- 107.Simbruner G, Mittal RA, Rohlmann F, et al. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–8. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 108.Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG Seizures in Hypoxic Ischemic Encephalopathy: A Randomized Controlled Trial. Pediatrics. 2015;136:e1302–9. doi: 10.1542/peds.2014-3777. [DOI] [PubMed] [Google Scholar]
- 109.Sun J, Li J, Cheng G, et al. Effects of hypothermia on NSE and S-100 protein levels in CSF in neonates following hypoxic/ischaemic brain damage. Acta Paediatr. 2012;101:e316–20. doi: 10.1111/j.1651-2227.2012.02679.x. [DOI] [PubMed] [Google Scholar]
- 110.Tagin MA, Woolcott CG, Vincer MJ, et al. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–66. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 111.Tagin M, Shah PS, Lee KS. Magnesium for newborns with hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. J Perinatol. 2013;33:663–9. doi: 10.1038/jp.2013.65. [DOI] [PubMed] [Google Scholar]
- 112.Tanigasalam V, Bhat V, Adhisivam B, et al. Does therapeutic hypothermia reduce acute kidney injury among term neonates with perinatal asphyxia?--a randomized controlled trial. J Matern Fetal Neonatal Med. 2016;29:2545–8. doi: 10.3109/14767058.2015.1094785. [DOI] [PubMed] [Google Scholar]
- 113.Tanigasalam V, Plakkal N, Vishnu Bhat B, et al. Does fluid restriction improve outcomes in infants with hypoxic ischemic encephalopathy? A pilot randomized controlled trial. J Perinatol. 2018;38:1512–7. doi: 10.1038/s41372-018-0223-7. [DOI] [PubMed] [Google Scholar]
- 114.Thayyil S, Shankaran S, Wade A, et al. Whole-body cooling in neonatal encephalopathy using phase changing material. Arch Dis Child Fetal Neonatal Ed. 2013;98:F280–1. doi: 10.1136/archdischild-2013-303840. [DOI] [PubMed] [Google Scholar]
- 115.Thoresen M, Whitelaw A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics. 2000;106:92–9. doi: 10.1542/peds.106.1.92. [DOI] [PubMed] [Google Scholar]
- 116.van Rooij LGM, Toet MC, van Huffelen AC, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125:e358–66. doi: 10.1542/peds.2009-0136. [DOI] [PubMed] [Google Scholar]
- 117.Velaphi S, Mokhachane M, Mphahlele R, et al. Effect of prophylactic phenobarbital on seizures, encephalopathy and mortality in neonates with perinatal asphyxia. S Afr J CH . 2013;7:17–21. doi: 10.7196/sajch.494. [DOI] [Google Scholar]
- 118.Whitelaw A. Systematic review of therapy after hypoxic-ischaemic brain injury in the perinatal period. Semin Neonatol. 2000;5:33–40. doi: 10.1053/siny.1999.0113. [DOI] [PubMed] [Google Scholar]
- 119.Wong V, Cheuk DKL, Chu V. Acupuncture for hypoxic ischemic encephalopathy in neonates. Cochrane Database Syst Rev. 2013;2013:CD007968. doi: 10.1002/14651858.CD007968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu L, Yi B, Hu Y, et al. The efficacy of hypothermia in hypoxic-ischemic encephalopathy at 18 mo or more. Indian J Pediatr. 2012;79:1342–6. doi: 10.1007/s12098-011-0673-9. [DOI] [PubMed] [Google Scholar]
- 121.Wu YW, Mathur AM, Chang T, et al. High-Dose Erythropoietin and Hypothermia for Hypoxic-Ischemic Encephalopathy: A Phase II Trial. Pediatrics. 2016;137:e20160191. doi: 10.1542/peds.2016-0191. [DOI] [PubMed] [Google Scholar]
- 122.Yang CS, Lin YZ, Guo Q, et al. Chinese Herbal Medicine Xingnaojing Injection for Hypoxic Ischemic Encephalopathy in Newborns: A Systematic Review and Meta-Analysis. Chin J Integr Med. 2018;24:147–55. doi: 10.1007/s11655-015-1974-z. [DOI] [PubMed] [Google Scholar]
- 123.Young L, Berg M, Soll R. Prophylactic barbiturate use for the prevention of morbidity and mortality following perinatal asphyxia. Cochrane Database Syst Rev. 2016;2016:CD001240. doi: 10.1002/14651858.CD001240.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee CYZ, Chakranon P, Lee SWH. Comparative Efficacy and Safety of Neuroprotective Therapies for Neonates With Hypoxic Ischemic Encephalopathy: A Network Meta-Analysis. Front Pharmacol. 2019;10:1221. doi: 10.3389/fphar.2019.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang W, Lu M, Zhang C, et al. Therapeutic hypothermia increases the risk of cardiac arrhythmia for perinatal hypoxic ischaemic encephalopathy: A meta-analysis. PLoS ONE. 2017;12:e0173006. doi: 10.1371/journal.pone.0173006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang W, Ma J, Danzeng Q, et al. Safety of Moderate Hypothermia for Perinatal Hypoxic-Ischemic Encephalopathy: A Meta-analysis. Pediatr Neurol. 2017;74:51–61. doi: 10.1016/j.pediatrneurol.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 127.Zhou WH, Shao XM, Cao y., et al. Safety study of hypothermia for treatment of hypoxic-ischemic brain damage in term neonates. Acta Pharmacol Sin. 2002;23:64–8. [Google Scholar]
- 128.Zhou W, Cheng G, Shao X, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–72. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 129.Kirkham JJ, Boers M, Tugwell P, et al. Outcome measures in rheumatoid arthritis randomised trials over the last 50 years. Trials. 2013;14:324. doi: 10.1186/1745-6215-14-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quirke F, Ariff S, Battin M, et al. Core outcomes in neonatal encephalopathy: a qualitative study with parents. BMJ Paediatr Open . 2022;6:e001550. doi: 10.1136/bmjpo-2022-001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salamah A, El Amrousy D, Elsheikh M, et al. Citicoline in hypoxic ischemic encephalopathy in neonates: a randomized controlled trial. Ital J Pediatr. 2023;49:55. doi: 10.1186/s13052-023-01452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]