Abstract
Studies utilizing various immunodeficient mouse models of rotavirus (RV) infection demonstrated significant roles of RV-specific secretory immunoglobulin A (IgA), CD4+ T cells, and CD8+ T cells in the clearance of RV and protection from secondary infection. Secretion of small but detectable amounts of IgA in RV-infected αβ T-cell receptor knockout mice (11) and distinctive anatomical localization and physiology of B1 cells suggested that B1 cells might be capable of producing RV-specific intestinal IgA in a T-cell-independent fashion and, therefore, be responsible for ablation of RV shedding. We investigated the role of B1 cells in the resolution of primary RV infection using a SCID mouse model. We found that the adoptive transfer of unseparated peritoneal exudate cells ablates RV shedding and leads to the production of high levels of RV-specific intestinal IgA. In contrast, purified B1 cells do not ablate RV shedding and do not induce a T-cell-independent or T-cell-dependent, RV-specific IgA response but do secrete large amounts of polyclonal (total) intestinal IgA. Cotransfer of mixtures of purified B1 cells and B1-cell-depleted peritoneal exudate cells differing in IgA allotypic markers also demonstrated that B2 cells (B1-cell-depleted peritoneal exudate cells) and not B1 cells produced RV-specific IgA. To our knowledge, this is the first observation that B1 cells are unable to cooperate with CD4+ T cells and produce virus-specific intestinal IgA antibody. We also observed that transferred CD4+ T cells alone are capable of resolving RV shedding, although no IgA is secreted. These data suggest that RV-specific IgA may not be obligatory for RV clearance but may protect from reinfection and that effector CD4+ T cells alone can mediate the resolution of primary RV infection. Reconstitution of RV-infected SCID mice with B1 cells results in the outgrowth of contaminating, donor CD4+ T cells that are unable to clear RV, possibly because their oligoclonal specificities may be ineffective against RV antigens.
Immunodeficient mice provide valuable models for persistent rotavirus (RV) shedding. Reconstitution of severe combined immunodeficient (SCID), recombination activating gene (RAG) 2 knockout, B-cell- or T cell-depleted animals with different subsets of immunocompetent cells allows one to examine the potential roles of these cells in the ablation of viral shedding as well as in the protection against reinfection. C.B-17 SCID and RAG 2 (C57BL/6 × 129) knockout mice become chronically infected with murine RV, suggesting that acquired immunity is required to clear the infection (12, 32). However, 40% of C57BL/6 SCID mice cleared primary RV infection, suggesting a role for the genetic background and innate mechanisms in the resolution of murine RV (11).
The importance of virus-specific intestinal immunoglobulin A (IgA), cytotoxic T lymphocytes (CTLs), or both in the resolution of the disease is supported by several findings. First, it has been observed that B-cell-deficient μMt knockout (genetically modified IgM transmembrane domain mutant) mice showed a significant delay before they cleared the infection (10, 26). Second, some secretory IgA antibodies against a RV protein (VP6) were capable of preventing primary infection and resolving chronic murine RV infection, as demonstrated by using “backpack tumor” transplantation of the IgA hybridomas (4). Third, RV-specific CTLs appeared at the intestinal mucosal surface of mice within the first week of the infection (31). Fourth, mice lacking CD8+ T cells (β2 microglobulin knockout or anti-CD8 antibody depleted) had a several-day delay in resolution of RV shedding (10, 12).
CD4+ T cells have been shown to be essential for complete clearance of RV infection. Thus, the depletion of CD8+ T cells from μMt −/− mice only slowed complete clearance of RV (26), whereas the depletion of CD4+ T cells from μMt −/− or immunocompetent mice led to chronic, high-level or low-level shedding of RV, respectively (25, 26).
Recently, Franco and Greenberg (11) have suggested the importance of T-cell-independent, RV-specific intestinal IgA in the clearance of primary infection. They have reported that although αβ T-cell receptor (TCR) knockout mice (devoid of αβ TCR+ T cells) cleared RV with a delay, they developed a low but detectable amount of RV-specific IgA that resolved the infection. A similarly reduced level of intestinal RV-specific IgA was observed in CD4+ T-cell-depleted immunocompetent mice, suggesting that T-cell-independent IgA is also present in normal mice.
B1 cells are potentially capable of producing IgA in a T-cell-independent fashion. The B1 cells differ from conventional B cells in many aspects (13, 15, 18). Mouse B1 cells are the major source of low-affinity “natural” IgM antibodies (18). Furthermore, in some experimental systems, about 40% of IgA-secreting cells in the gut can be derived from B1 cells that migrated from the peritoneal cavity into the lamina propria (3, 5, 20, 21, 22). T-cell dependence in vivo of B1 cells or their participation in germinal center reactions has not been demonstrated. Therefore, B1 cells might be capable of producing T-cell-independent, virus-specific IgA and of clearing primary RV infection.
We investigated the role of B1 cells in the clearance of primary RV infection using a SCID mouse model. We have found that the adoptive transfer of B1 cells purified by fluorescence-activated cell sorting (FACS) did not result in either production of RV-specific IgA or clearance of the infection. In contrast, reconstitution of SCID mice with unseparated peritoneal exudate cells resulted in both high levels of RV-specific intestinal IgA and ablation of RV shedding. Cotransfer of B1 cells and splenic CD4+ T cells did not result in the secretion of noticeable amounts of RV-specific IgA, although RV was cleared. Purified splenic CD4+ T cells alone were able to resolve the infection, although no RV-specific IgA was produced. Finally, cotransfer of mixtures of FACS-purified B1 cells and B1 cell-depleted peritoneal exudate cells differing in IgA allotypic markers demonstrated that B2 cells rather than B1 cells produced the RV-specific IgA. Therefore, B1 cells seem unable to produce either T-cell-dependent or T-cell-independent, RV-specific intestinal IgA. Taken together, these results suggest that RV-specific IgA may not be obligatory for clearance of primary RV infection and that effector CD4+ T cells alone can mediate the resolution of primary rotavirus infection.
MATERIALS AND METHODS
Mice and virus infection.
Recipient mice were conventionally reared C.B-17 (H-2d) SCID mice (Wistar Institute or Department of Biology, University of Pennsylvania, Philadelphia, Pa.). Eight- to twelve-week-old mice were inoculated orally with 100 μl of murine RV strain EDIM (9 × 103 50% shedding dose) per mouse by proximal esophageal intubation. EDIM was originally obtained from R. Ward (Children's Hospital Research Foundation, Cincinnati, Ohio) and passaged in suckling mice as described previously (7, 35). All donor mice were 8 to 12 weeks old, unprimed conventionally reared males with an H-2d background: BALB/c, C.B-17, or BALBc/dm2-H-2Ld−/− (Jackson Laboratories).
Detection of RV in feces.
To detect the presence of RV in SCID mice, fecal samples (three pellets from each mouse) were collected before and after transferring cells, suspended in 0.5 ml of Earl's balanced salt solution (BSS), and the quantities of RV antigen were measured by enzyme-linked immunosorbentassay (ELISA) as described previously (27). Aliquots of the blocking buffer added to wells instead of fecal homogenates were used as a negative control. Samples were considered positive if the optical density (OD) in the experimental well was both >0.1 OD units and twofold greater than the OD in the corresponding negative well.
Cell preparation and sorting for reconstitution of SCID mice.
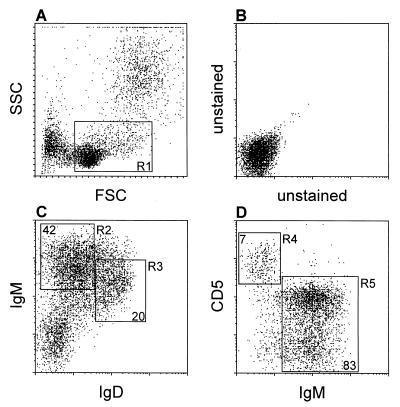
When chronic shedding of RV was established (3 to 4 weeks after RV inoculation), reconstitution of mice with immunocompetent cells was performed. Transferred cells included unseparated peritoneal exudate cells, FACS-purified B1 cells, FACS-purified CD4+ T cells, or B1 cells combined with CD4+ T cells. In reciprocal recombination experiments, B1 cells from BALB/c mice (Igha, the a allotype of immunoglobulin heavy chain) were mixed with non-B1 cells from C.B-17 mice (Ighb, the b allotype of immunoglobulin heavy chain) and vice versa and transferred. B1 and non-B1 cells were sorted from peritoneal lymphocytes, and CD4+ T cells were sorted from the spleen, respectively, by FACS (FACStar Plus; Becton Dickinson Immunocytometry Systems, Mountain View, Calif.). Sorted cells were 94 to 98% pure as confirmed by purity check. B1 cells were selected as IgMhi IgDlow cells and comprised approximately 42% of a peritoneal lymphocyte population, while T cells (IgM− CD5+) comprised 7% (Fig. 1). Non-B1 cells were all B1-cell-depleted peritoneal lymphocytes. Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-labeled monoclonal antibodies against mouse IgD and CD4 (clones 11-26c.2a and GK1.5, respectively) were purchased from PharMingen; PE-labeled polyclonal goat anti-mouse IgM antibody was from Southern Biotechnology Associates, Inc. (Birmingham, Ala.). Cells were suspended in 1 ml of RPMI 1640 medium containing 10% fetal bovine serum (FBS). RV-infected SCID mice were injected with cells intraperitoneally with either 3 × 106 peritoneal exudate cells, 5 × 105 FACS-purified B1 cells, 2 × 105 splenic CD4+ T cells, or a combination of 5 × 105 B1 and 2 × 105 splenic CD4+ T cells. For reciprocal recombination experiments (see Table 2), 4 × 105 B1 of one allotype were injected in combination with 2 × 106 non-B1 cells of the other allotype. Two to four months later, mice were sacrificed and examined for the expansion of B and T cells, as well as for the secretion of intestinal and serum immunoglobulin and specific antibodies.
FIG. 1.
FACS identification of B1, B2, and T cells among lymphocytes in the peritoneal exudate of BALB/c mice. Lymphocytes are defined as R1 according to their forward light scattering (FSC) and side light scattering (SSC) (A) and the lack of autofluorescence (B). B1 cells are defined as IgMhi IgDlow (R2), and B2 cells are defined as IgMintermed IgDhi (R3) (C). T cells are defined as CD5hi IgM− (R4), and all B cells (B1 and B2 cells) are defined as IgM+ cells (CD5intermed or CD5−; R5) (D). The percentages of B1, B2, and T cells are shown in corresponding regions.
TABLE 2.
Reciprocal recombination experimenta
| Cell type | Fragmentb | RVS IgAa (OD units) | RVS IgAb (OD units) | Total IgAa (ng/ml) | Total IgAb (ng/ml) |
|---|---|---|---|---|---|
| B1a/non-B1b | SI | 0 | 1.68 ± 0.54 | 160 ± 32 | 306 ± 93 |
| LI | 0 | 1.31 ± 0.60 | 212 ± 58 | 374 ± 136 | |
| B1b/non-B1a | SI | 2.07 ± 0.23 | 0 | 285 ± 70 | 122 ± 13 |
| LI | 2.05 ± 0.50 | 0 | 367 ± 114 | 201 ± 63 | |
| PECa | SI | 3.25 ± 0.60 | 0 | 204 ± 63 | 0 |
| LI | 3.22 ± 0.43 | 0 | 228 ± 62 | 0 | |
| PECb | SI | 0 | 1.82 ± 0.04 | 0 | 156 ± 1 |
| LI | 0 | 1.78 ± 0.28 | 0 | 165 ± 6 |
The production of RV-specific and total IgA in small and large intestinal lymphoid tissues of RV-infected SCID mice reconstituted with peritoneal B1 and non-B1 cells of a versus b allotypes is shown. SCID mice were inoculated with EDIM RV at 12 weeks of age and 3 weeks later were reconstituted with reciprocal combinations of peritoneal B1 and non-B1 cells of allotypes a versus b (indicated as roman superscripts). Unseparated peritoneal exudate cells (PEC) of each allotype served as positive controls. Cultures of small and large intestine fragments were performed 2 to 3 months later, and supernatants were tested by ELISA for the presence of RV-specific and total IgA of allotypes a and b. The data represent the means ± the SEM. The number of fragments per tissue per group of mice ranged from 12 to 24.
SI, small intestine; LI, large intestine.
Isolation of cells from recipient mice.
Peritoneal exudate cells were prepared by rinsing a peritoneal cavity with 10 ml of cold Hank's BSS. Spleen and mesenteric lymph nodes were excised and teased to release single cells. Lamina propria cells from the small intestine were prepared as described elsewhere (34). Briefly, immediately after removal a small intestine was open longitudinally, cut into small pieces, and washed sequentially in phosphate-buffered saline (PBS), Ca2+-Mg2+-free PBS, and Ca2+-Mg2+-free PBS containing 1 mM EDTA and 1 mM dithiothreitol (DTT) until the medium was clear. This was followed by two 30-min (37°C) incubations at constant stirring in Ca2+-Mg2+-free PBS containing 1 mM EDTA and 1 mM DTT or containing 1 mM EDTA only, respectively, to remove epithelial and intraepithelial cells. A 45-min (37°C) incubation in RPMI 1640 containing 10% FBS and 0.5 mg of collagenase (from Clostridium histolyticum [Worthington Biochemicals]), 1.5 mg of dispase (Boehringer Mannheim), 0.15 mg of DNase (Sigma), and 0.5 mg of soybean trypsin inhibitor (Sigma) per ml resulted in a release of lamina propria lymphocytes. Cells were filtered through a cotton wool column, resuspended in RPMI 1640–10% FBS, and centrifuged over a Percoll gradient (40 to 70% Percoll in RPMI 1640; 650 × g, 20 min, 4°C). Lamina propria lymphocytes localized in the interface between the 40 and 70% Percoll layers.
FACS analysis.
Peritoneal exudate, mesenteric lymph node, spleen, and lamina propria cells were labeled for 30 min on ice with FITC- or PE-labeled monoclonal antibodies against mouse IgM (clone II/41); CD5 (clone 53-7.3); CD3e (clone 145-2C11); CD4 (clone GK1.5); CD8 (clone 53-6.7); TCR Vβ 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, and 17 (clones B20.6, KJ25, MR9-4, RR4-7, TR310, F23.1, MR10-2, B21.5, RR3-15, MR11-1, MR12-3, 14-2, and KJ23, respectively); H-2Ld (clone 28-14-8) (PharMingen); or polyclonal antibodies against IgA and the immunoglobulin kappa chain (Southern Biotechnology Associates, Inc.). After being stained, cells were washed in PBS and fixed in 1% paraformaldehyde solution. The fluorescence of 10,000 cells from every stained cell sample was measured by FACScan (Becton Dickinson) and analyzed using CellQuest Software.
Fragment cultures.
Fragment cultures of small and large intestines were prepared as previously described (24). Briefly, the intestines were cut longitudinally and washed sequentially in PBS, Ca2+-Mg2+-free PBS, Ca2+-Mg2+-free PBS–0.5 mM EDTA, and RPMI 1640–10% FBS. Square fragments (2 to 3 mm) were placed into wells of 24-well plates (Costar, Cambridge, Mass.) containing 1 ml of Kennett's HY medium (GIBCO) containing 10% FBS, 1% l-glutamine, 0.01% gentamicin, and 1% antibiotic solution (100 U of penicillin, 1% streptomycin, and 0.25 μg of amphotericin B [Fungizone] per ml) (GIBCO) and incubated for 7 days at 37°C in 95% O2 and 5% CO2. Typically, six replicate cultures were made from each intestinal segment from each mouse. Culture supernatants were stored at −20°C prior to the assay.
RIA.
Radioimmunoassay (RIA) was performed as previously described (17, 24). Goat anti-mouse (Fab′)2 (Jackson Immunoresearch Laboratories, Inc.) was a capture antibody for total immunoglobulin. Plates were coated with purified simian RV (strain SA11; originally obtained from N. Schmidt [Berkeley, Calif.]) at 0.2 μg per well for binding of RV-specific immunoglobulin. After the plates were blocked with PBS–1% bovine serum albumin (BSA), 20 μl of undiluted (for RV-specific immunoglobulin) or diluted (for total immunoglobulin) supernatants of fragment cultures or diluted sera were added. Detection antibodies were radiolabeled goat anti-mouse IgA, IgM, or IgG1, respectively (Southern Biotechnology Associates). Purified mouse IgA, IgM, or IgG1 served as standards. Linear portions of standard curves (usually from 0 to 20 ng per well) were used for the calculation of concentration of total IgA. Only curves with correlation coefficients of greater than 0.90 were used. Due to the lack of purified RV-specific IgA to use as a standard, RV-specific IgA is expressed in counts per minute (cpm).
Detection of allotype-specific IgA.
Supernatants from small and large intestinal fragment cultures of RV-infected SCID mice reconstituted with B1 and non-B1 cells in the reciprocal recombination experiments were examined for the presence of total and RV-specific IgA of allotypes a and b as previously described (8). Biotinylated monoclonal antibodies (clones HY-16 [from M. Pawlita, National Institutes of Health; see reference 22] and HISM2 [21]), were used for the detection of IgAa and IgAb, respectively.
ELISPOT assay.
Flat-bottomed 96-well plates (Nunc-Immuno plate; Maxisorp, Roskilde, Denmark) were coated overnight with rat anti-mouse immunoglobulin kappa light-chain antibody (clone R8-140 [PharMingen]) at 10 μg/ml in PBS, at 100 μl/well for detection of total immunoglobulin-secreting cells (IgSC) and with purified simian RV (strain SA11) at 0.2 μg/well at 100 μl/well for the detection of RV-specific antibody-secreting cells (ASC), respectively. Plates were washed with PBS and saturated with PBS–1% BSA (45 min, 37°C). Cells were suspended in cold RPMI 1640–1% BSA, a five-times serial dilution of cells starting at 106 cells per well was performed, and plated cells were incubated for 4 h at 37°C undisturbed. Cells were flicked off, and the plates were rinsed with deionized H2O–0.05% Tween 20 and washed with PBS–0.05% Tween 20. Alkaline phosphatase-labeled goat anti-mouse IgA antibody (Southern Biotechnology Associates, Inc.) was added at 1:500 in PBS–1% BSA–0.05% Tween 20 for 1 h. The substrate was 1 mg of 5-bromo-4-chloro-3-indolylphosphate (BCIP; Sigma) solution in the buffer containing 9.62 ml of 1 M 2-amino-2-methyl-1-propanol, 15 mg of MgCl2 · 6H2O, 0.01 ml of Triton X-450, and 0.01 g of sodium azide per liter (pH 10.25). The substrate was mixed with a 3% solution of low-melting-point agarose (Low E.E.O.; Sigma) in deionized H2O at the ratio 4:1. A 100-μl portion of the warm (42°C) mixture was added to develop the assay. After the agarose solidified, the plates were incubated for 1 h at 37°C. The spots were counted in all wells where their density did not exceed 100 per well, and the frequencies of IgSC and ASC per 106 cells were calculated. The mean IgSC or ASC levels for the tested populations of cells were determined as averages of the IgSC or ASC counts, respectively, determined at different concentrations of cells.
RESULTS
Peritoneal exudate cells, B1 cells plus splenic CD4+ T cells, or splenic CD4+ T cells alone but not B1 cells alone clear RV infection.
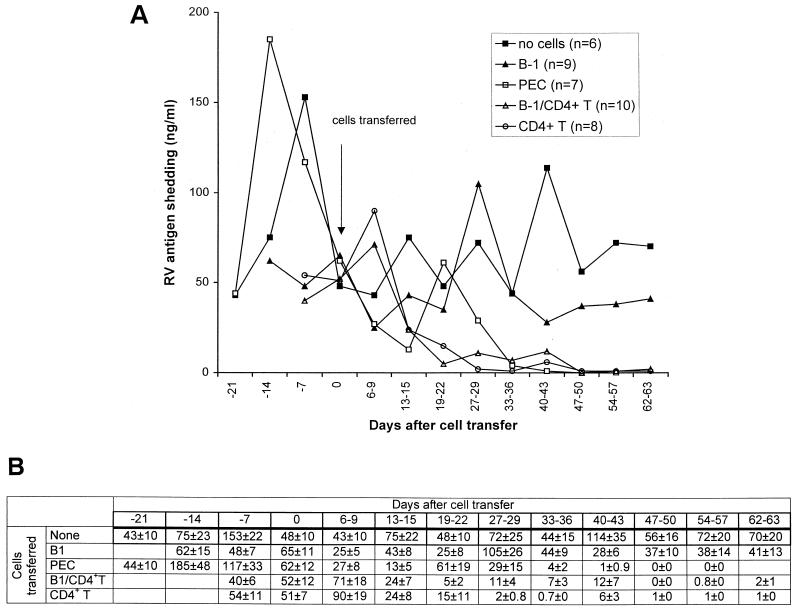
Conventionally reared SCID mice that chronically shed RV were reconstituted with either unseparated peritoneal exudate cells (3 × 106) or FACS-purified B1 cells (5 × 105), splenic CD4+ T cells (2 × 105), or a combination of B1 cells (5 × 105) and splenic CD4+ T cells (2 × 105) from BALB/c mice. Following cell transfer, fecal samples were collected weekly over a 3-month interval, and RV shedding was monitored by ELISA. The transfer of peritoneal exudate cells, B1 cells plus CD4+ T cells, or CD4+ T cells alone ablated RV shedding (Fig. 2). In contrast, mice that received either purified B1 cells or no cells continued shedding RV. In the groups of mice that cleared RV, clearance occurred between days 19 and 50 after reconstitution with cells (Fig. 2). The fact that some mice stopped shedding RV but then showed a low-level, short-term shedding before RV was completely eliminated suggests that these mice became reinfected with RV from their neighbors in the cage but then rapidly cleared the virus.
FIG. 2.
Fecal RV antigen shedding curves of conventionally reared SCID mice, as determined by ELISA (A) and the means ± the standard error of the means at every time point of shedding curves (B). Mice were inoculated with EDIM strain of murine RV at 8 weeks of age and received unseparated peritoneal exudate cells, B1 cells, B1 plus CD4+ T cells, CD4+ T cells, or no cells 3 to 4 weeks later.
Transfer of B1 cells results in secretion of total but not RV-specific intestinal IgA.
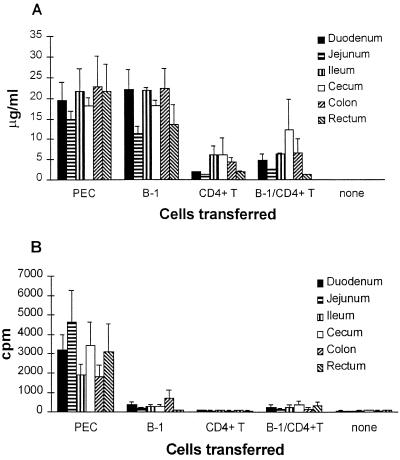
Analysis of intestinal fragment cultures demonstrated that recipients of either peritoneal exudate cells or B1 cells developed high levels of total IgA in all major portions of the small and large intestines (Fig. 3A). In contrast, considerable levels of RV-specific IgA developed only in small and large intestines of mice reconstituted with peritoneal exudate cells (Fig. 3B). This development of RV-specific antibodies correlated with the clearance of RV infection and suggested a T-cell-dependent mechanism of clearance. However, the cotransfer of B1 cells and splenic CD4+ T cells did not induce production of RV-specific IgA (Fig. 3B). This finding is consistent with the results of the ELISPOT analysis. High frequencies of ASC were observed only in the lamina propria of mice reconstituted with peritoneal exudate cells (Table 1). Only in peritoneal exudate cell-reconstituted mice, did ASC comprise a noticeable proportion of the IgSC (6%). Nevertheless, some ASC were found in mice injected with B1 cells plus CD4+ T cells, but the frequencies were very low. In all three groups of mice, lamina propria and mesenteric lymph node cells contained considerable numbers of IgSC, whereas very few IgSC were detected in the spleen (Table 1). The frequencies of IgSC in the lamina propria of recipients of B1 cells or B1 plus CD4+ T cells were lower than in the recipients of peritoneal exudate cells (Table 1), although the amounts of secreted total IgA were similar (Fig. 3A). Furthermore, the sera of these mice did not contain detectable levels of either RV-specific or total IgA (Fig. 4). This suggests a mucosal association of IgA production during RV infection.
FIG. 3.
Production of IgA by major segments of small and large intestinal lymphoid tissues. RV-infected SCID mice were reconstituted with unseparated peritoneal exudate cells, B1 cells, B1 cells plus CD4+ T cells, CD4+ T cells from BALB/c mice, or no cells. Cultures of small and large intestinal fragments were performed 2 to 3 months after reconstitution. Supernatants were tested by RIA for the presence of total IgA (A) and RV-specific IgA (B). Data represent the mean ± the standard error of the mean (SEM) in micrograms/milliliter for the total IgA and in cpm for the RV-specific IgA. The numbers of fragments per tissue per group of mice that received a certain type of cells ranged from 8 to 36.
TABLE 1.
Frequencies of IgA-secreting cells in intestinal and nonintestinal lymphoid tissues of RV-infected SCID mice following reconstitution with peritoneal exudate cells, B1, or B1 plus CD4+ T cellsa
| Transferred cells | Analyzed cells | Mean no. ± SEM
|
% ASC/IgSC | |
|---|---|---|---|---|
| ASC count | IgSC count | |||
| PEC | LP | 1.2 × 104 ± 3 × 103 | 2 × 105 ± 6 × 103 | 6 |
| MLN | 200 ± 53 | 6 × 104 ± 2 × 104 | 0.4 | |
| Spleen | 25 ± 5 | 1.8 × 103 ± 940 | 1.4 | |
| B1 | LP | 0 | 1.5 × 104 ± 402 | 0 |
| MLN | 0 | 5.4 × 104 ± 8 × 103 | 0 | |
| Spleen | 0 | 342 ± 166 | 0 | |
| B1/CD4+ T | LP | 180 ± 53 | 3.4 × 104 ± 5.5 × 103 | 0.5 |
| MLN | 125 ± 4 | 3.6 × 104 ± 3.3 × 103 | 0.3 | |
| Spleen | 6 ± 1 | 195 ± 4 | 3 | |
PEC, peritoneal exudate cells; LP, lamina propria cells; MLN, mesenteric lymph node cells. RV-infected SCID mice were reconstituted with unseparated peritoneal exudate cells, sorted B1 cells, or B1 cells plus CD4+ T cells from BALB/c mice. At 2 to 3 months after reconstitution, the recipient's lamina propria, mesenteric lymph node, and spleen cells were isolated, ELISPOT analysis of total and RV-specific IgA production was performed, and the frequencies of IgA ASC and IgSC per 106 cells were determined. The data represent the means ± the SEM for every tested population of cells from each group of mice (three to five mice/group). ASC/IgSC ratios are also shown.
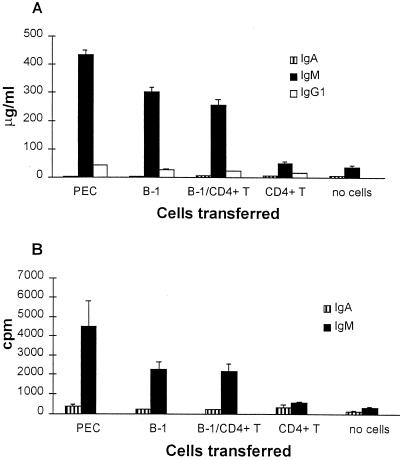
FIG. 4.
IgA, IgM, and IgG1 in the sera of RV-infected SCID mice. Mice were reconstituted with unseparated peritoneal exudate cells (n = 7), B1 cells (n = 9), B1 cells plus CD4+ T cells (n = 10), CD4+ T cells (n = 8) from BALB/c mice, or no cells (n = 6). At 2 to 3 months after reconstitution, the mice were bled and the sera were tested by RIA for the presence of total IgA, IgM, and IgG1 and for RV-specific IgA and IgM antibodies. The data represent the means ± the SEM in micrograms/milliliter for the total immunoglobulin (A) and in cpm for the RV-specific antibodies (B).
Very small amounts of IgM and IgG1 were found in intestinal fragment cultures in all groups of mice (data not shown). However, the sera of the mice that received peritoneal exudate cells, B1 cells, or B1 cells plus CD4+ T cells all contained considerable levels of both total and RV-specific IgM, compared to mice that received CD4+ T cells only or no cells (Fig. 4). The amounts of total serum IgG1 were very low in all groups of mice (Fig. 4A).
Taken together, these observations demonstrate that unseparated peritoneal exudate cells but not purified B1 cells produced RV-specific IgA, suggesting that B2 cells may be a source of RV-specific IgA.
Peritoneal non-B1 rather than B1 cells contribute RV-specific intestinal IgA.
In order to find out whether B1 or B2 cells were a source of RV-specific intestinal IgA in peritoneal exudate cell-reconstituted SCID mice, we performed reciprocal recombination and transfer experiments. Peritoneal lymphocytes of allotypes a (BALB/c) and b (C.B-17) were fractionated into B1-cell and non-B1-cell populations by FACS. Combinations of B1 cells (4 × 105) and non-B1 cells (2 × 106) of different allotypes were transferred into RV-infected recipient SCID mice, yielding a complete mixture of peritoneal lymphocytes. In control groups, 2 × 106 unseparated peritoneal exudate cells of allotypes a or b were transferred. In all four groups, the clearance of RV was observed, whereas unreconstituted mice continued shedding RV antigen (data not shown). Successful reconstitution of peritoneal cavity, lamina propria, mesenteric lymph nodes, and spleen was confirmed by FACS analysis (data not shown). Consistent with our previous data, total IgA had allotypes of both B1 and non-B1 cells (Table 2), suggesting that both B1 and B2 cells produced total IgA. In contrast, RV-specific IgA always had the allotype provided by non-B1 cells, suggesting that it was provided by B2 cells.
Expansion of B cells and T cells in RV-infected, cell-reconstituted SCID mice.
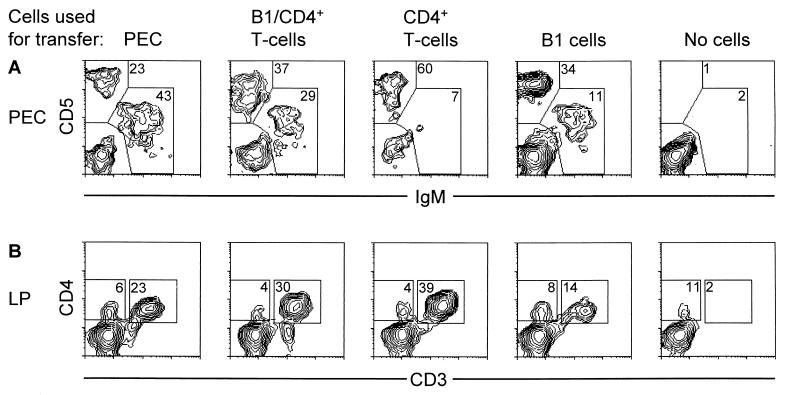
Following transfer of either peritoneal exudate cells or B1 cells, high proportions of B1 cells (IgMhi CD5+ and IgMhi CD5−) were found in the peritoneal cavity of reconstituted mice (Fig. 5A). Very few IgM+ cells were seen in the spleen and mesenteric lymph nodes (data not shown). The transfer of splenic CD4+ T cells resulted in effective repopulation of the peritoneal cavity (Fig. 5A), lamina propria (Fig. 5B), spleen, and mesenteric lymph nodes (not shown) with CD4+ T cells.
FIG. 5.
Two-color FACS analysis of peritoneal exudate (PEC) and lamina propria (LP) cells from RV-infected SCID mice reconstituted with unseparated peritoneal exudate cells, B1 cells, B1 cells plus CD4+ T cells, CD4+ T cells from BALB/c mice, or no cells. (A) Peritoneal exudate cells were isolated from the recipient mice 2 to 3 months after their reconstitution. The percentages of IgM+ B cells and CD5+ T cells among gated lymphocytes are shown. (B) Lamina propria cells were isolated from the recipient mice 2 to 3 months after their reconstitution. The percentages of CD4+/CD3+ T cells among gated lymphocytes are shown. The data shown are typical for each group of mice (not less than three mice per group were examined) and were reproduced two to three times.
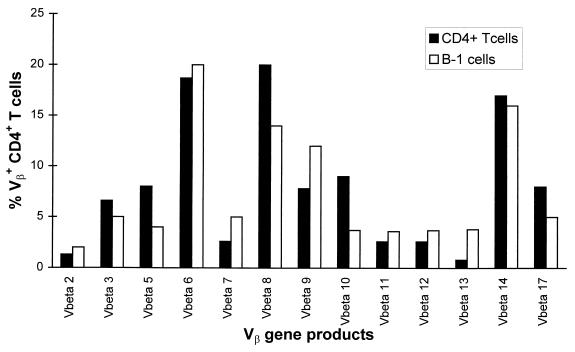
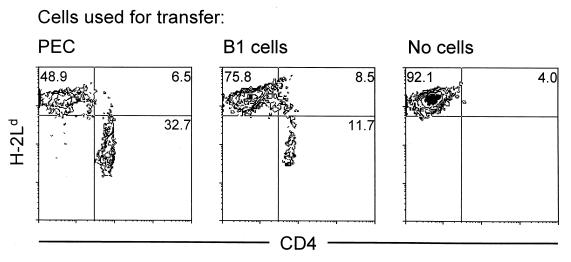
Strikingly, not only mice that were given unseparated peritoneal exudate cells or splenic. CD4+ T cells but also mice that received B1 cells alone had considerable proportions of CD5hi T cells in the peritoneal cavity (Fig. 5A). Most of these cells in the lamina propria were CD4+ and CD3+ (Fig. 5B). CD4+ T cells were also found in the spleen and mesenteric lymph nodes (data not shown). Despite the fact that CD4+ T cells arose from minor contaminants in FACS-purified B1 cells, they appeared to be polyclonal, as judged by the expression of different TCR Vβ gene products (Fig. 6). However, they could not clear RV infection. In order to find out if these CD4+ T cells had donor origin or derived from “leaky” SCID recipients, we transferred 2.5 × 105 FACS-purified B1 cells or 106 unseparated peritoneal exudate cells from H-2Ld−/− donor mice. The lack of H-2Ld in these mice served as a genetic marker that allowed discrimination between donor and recipient cells following cell transfer. As shown in Fig. 7, the majority of CD4+ T cells that repopulated the mesenteric lymph nodes of both recipients of B1 cells and peritoneal exudate cells were H-2Ld−, whereas the proportions of H-2Ld+ CD4+ T cells in these mice were similar to those in unreconstituted SCID mice. Therefore, most CD4+ T cells that expanded in recipients of B1 cells had donor origin.
FIG. 6.
Two-color FACS analysis of mesenteric lymph node cells from RV-infected SCID mice reconstituted with FACS-purified B1 cells or CD4+ T cells from BALB/c mice. Mesenteric lymph node cells were isolated from the recipients 2 to 3 months after their reconstitution. Each bar represents the percentage of CD4+ T cells that expresses a particular TCR Vβ gene product among total CD4+ T cells.
FIG. 7.
Two-color FACS analysis of mesenteric lymph node cells from RV-infected SCID mice. Mice received B1 cells or unseparated peritoneal exudate cells from H-2Ld −/− mice or no cells. Mesenteric lymph node cells were isolated from the recipients at 26 days after reconstitution. The percentages of H-2Ld− and H-2Ld+ subpopulations of CD4+ T cells are shown.
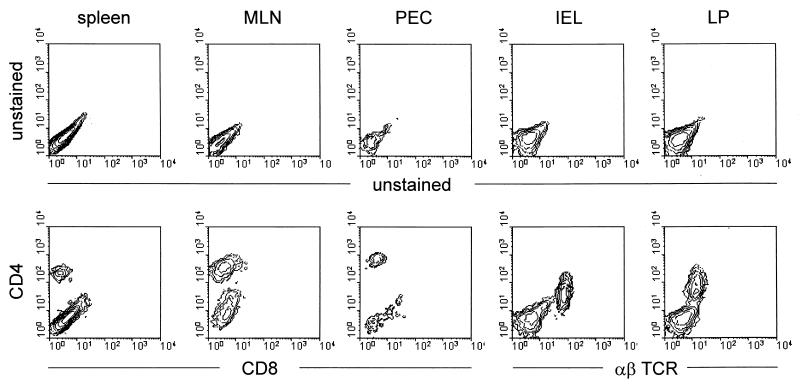
Our findings that appreciable numbers of CD4+ T cells grew out of the FACS-purified B1 cell inocula in vivo raise the reasonable concern that CD8+ T-cell inocula account for the cessation of virus shedding in the infected recipients. Therefore, we have included other relevant FACS analyses from various tissues taken from the recipients of FACS-purified CD4+ T cells used in the experiments recorded in Fig. 2, 3, 4, and 5 (Fig. 8). These analyses show that 2 to 3 months after the transfer of FACS-purified CD4+ T cells, the recipients showed no convincing presence of CD8+ T cells (or CD4−, α/β TCR+ T cells) in the spleen, mesenteric lymph nodes, peritoneal cavity exudate, intra-epithelial leukocytes, or gut lamina propria cells (see also Fig. 5B). The absence of any appreciable CD8+ T-cell outgrowth and the prompt cessation of viral shedding (in about 3 weeks) support a role for CD4+ T cells in viral clearance from the gut.
FIG. 8.
Two-color FACS analysis of spleen, mesenteric lymph node, peritoneal exudate cells, intraepithelial lymphocytes, and lamina propria lymphocytes of SCID mice reconstituted with CD4+ T cells from BALB/c mice. Cells were isolated from recipients 2 to 3 months after their reconstitution.
DISCUSSION
These results further define which components of the adaptive immune system are important in the clearance of RV infection. Earlier studies have shown that CD8+ T cells and RV-specific intestinal IgA can clear the virus (10, 12, 26). RV-specific secretory IgA derived from a transplanted “backpack” IgA hybridoma against VP6 protein successfully protects from primary infection and resolves chronic murine RV shedding (3). Furthermore, it was shown that even in the absence of T cells some RV-specific intestinal IgA could be produced (11), suggesting that B1 cells could be responsible. We now show that B1 cells are not capable of mounting a T-cell-independent, RV-specific IgA response but are capable of generating large amounts of polyclonal (total) intestinal IgA. In the presence of purified CD4+ T cells or non-B1-cell components of peritoneal exudate (containing CD4+ T cells), B1 cells still did not produce RV-specific IgA. Moreover, when B1 and non-B1 cells (B2 cell enriched) with different IgA allotypes were transplanted into RV-infected SCID mice, RV-specific IgA had a non-B1, and therefore, B2-cell origin. To our knowledge, this is the first observation that B1 cells are unable to cooperate with CD4+ T cells and produce virus-specific intestinal IgA antibody.
We observed a surprising phenomenon: the expansion of CD4+ T cells in recipients of FACS-purified B1 cells. Using B1 cells from mice with a genetic marker (H-2Ld−/−), most of these CD4+ T cells have been shown to have donor origin (Fig. 7). These cells seem to derive from a few peritoneal CD4+ T cells—perhaps, fewer than one to three thousand—that contaminate sorted B1 cells. Furthermore, these cells appear to be polyclonal, similar to their counterparts in mice reconstituted with splenic CD4+ T cells (Fig. 6) and, therefore, their reactivity against RV should be the same. However, these CD4+ T cells never cleared the RV infection. Perhaps serologic assessment is simply not sufficiently sensitive to detect oligoclonality that may occur within individual Vβ subsets. Matsuda et al. (29) have used nucleotide sequence analysis to demonstrate selection for particular CDRs (complementarity-determining regions of TCR) sequences within individual Vβ subsets. Thus, the expanded CD4+ T cells contaminating the FACS-purified B1-cell inocula may possibly lack RV-specific cells but still contain some specificities reactive with antigens present in the normal microbiota. Such interactions of these residual CD4+ T cells may account for the expression of “natural” IgA in the guts of conventionally reared recipients of B1 cells.
Moreover, we also show that CD4+ T cells themselves are capable of resolving RV shedding. This suggests that RV-specific IgA may not be obligatory for RV clearance but may protect from reinfection. This is in agreement with the recent finding that C57BL/6 IgA knockout mice cleared primary RV infection as effectively as IgA normal mice (30). The levels of CD4+ T cells and CD8+ T cells in these mice were equivalent to IgA normal mice (14), suggesting that they are fully functional to resolve RV infection. These T cells, however, did not mediate the protection of these mice from secondary RV infection, whereas IgG antibodies appeared to play a significant role. There are several possible mechanisms by which CD4+ T cells might clear RV infection. Cytokines released by RV-activated CD4+ T cells, such as interferons (IFNs) might activate intraepithelial natural killer cells which, in turn, would kill RV-infected epithelial cells. Thus, IFN was shown to activate cytotoxicity of human natural killer cells against RV-infected target cells (19). On the other hand, IFN-γ released from activated natural killer cells (especially in the SCID microenvironment) or CD4+ T cells themselves might kill infected epithelial cells directly. Moreover, IFN-γ and interleukin-1, but not IFN-α, were shown to inhibit RV entry into human intestinal epithelial cell lines (1). There is a growing evidence for CD4+ T-cell-mediated cytotoxicity during viral infections. Thus, influenza (9), vaccinia (33), Sendai (16), lymphocytic choriomeningitis (23, 28) virus infections were successfully cleared by cytotoxic CD4+ T cells in the absence of CD8+ T cells. CD4+ T-cell-mediated cytotoxicity may be both major histocompatibility complex class II restricted (28) and unrestricted (6). In addition, CD4+ T cells were shown to acquire a cytotoxic reactivity in the large intestinal lamina propria of CD4+ T-cell-reconstituted SCID mice with inflammatory bowel disease. The cytotoxicity was Fas-FasL dependent (2). To find out if this mechanism operates in RV clearance, we are planning to infect primary cultures of mouse intestinal epithelial cells with RV to determine whether RV upregulates the expression of Fas on epithelial cells. If this occurs, these cells will be used as targets for FasL+, potentially cytotoxic, intestinal CD4+ T cells.
ACKNOWLEDGMENTS
This work was supported by grants AI-23970 and AI-37108 to J.J.C. from the National Institute of Allergy and Infectious Diseases. A.W.Z. was supported by a Stipendium für angehende Forschende from the Swiss National Science Foundation.
We thank Hank Pletcher for assistance with the FACStar Plus sorter, the Lucille P. Markey Trust for funding of the Flow Cytometry Facility of the Cancer Center at the University of Pennsylvania, and Alec McKay for assistance with the FACScan analyzer. We thank Ann Snyder and Don Baumann for technical assistance and Ethel Cebra for secretarial assistance. We thank the Wistar Institute for allowing us to use their animal facility.
REFERENCES
- 1.Bass D M. Interferon gamma and interleukin 1, but not interferon alpha, inhibit rotavirus entry into human intestinal cell lines. Gastroenterology. 1997;113:81–89. doi: 10.1016/S0016-5085(97)70083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonhagen K, Thoma S, Bland P, Bregenholt S, Rudolphi A, Claesson M H, Reimann J. Cytotoxic reactivity of gut lamina propria CD4+ alpha beta T cells in SCID mice with colitis. Eur J Immunol. 1996;26:3074–3083. doi: 10.1002/eji.1830261238. [DOI] [PubMed] [Google Scholar]
- 3.Bos N A, Bun J C, Bijma H, Cebra E R, Cebra J J, Deenen G J, van der Cammen M J, Kroese F G. Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect Immun. 1996;64:616–623. doi: 10.1128/iai.64.2.616-623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns J W, Siadat-Pajouh M, Krishnaney A A, Greenberg H B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 5.Cebra J J, Bos N A, Cebra E R, Cuff C F, Deenen G J, Kroese F G, Shroff K E. Development of components of the mucosal immune system in SCID recipient mice. Adv Exp Med Biol. 1994;355:255–259. doi: 10.1007/978-1-4615-2492-2_43. [DOI] [PubMed] [Google Scholar]
- 6.Choi S H, Splitter G A. Induction of MHC-unrestricted cytolytic CD4+ T cells against virally infected target cells by cross-linking CD4 molecules. J Immunol. 1994;153:3874–3881. [PubMed] [Google Scholar]
- 7.Coffin S E, Moser C A, Cohen S, Clark H F, Offit P A. Immunologic correlates of protection against rotavirus challenge after intramuscular immunization of mice. J Virol. 1997;71:7851–7856. doi: 10.1128/jvi.71.10.7851-7856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffin S E, Clark S L, Bos N A, Brubaker J O, Offit P A. Migration of antigen-presenting B cells from peripheral to mucosal lymphoid tissues may induce intestinal antigen-specific IgA following parenteral immunization. J Immunol. 1999;163:3064–3070. [PubMed] [Google Scholar]
- 9.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty P C. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991;174:875–880. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco M A, Greenberg H B. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800–7806. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco M A, Greenberg H B. Immunity to rotavirus in T cell deficient mice. Virology. 1997;238:169–179. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- 12.Franco M A, Tin C, Rott L S, VanCott J L, McGhee J R, Greenberg H B. Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, fas, and gamma interferon. J Virol. 1997;71:479–486. doi: 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy R R, Carmack C E, Li Y S, Hayakawa K. Distinctive developmental origins and specificities of murine CD5+ B cells. Immunol Rev. 1994;137:91–118. doi: 10.1111/j.1600-065x.1994.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 14.Harriman G R, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, Mbawuike I N. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol. 1999;162:2521–2529. [PubMed] [Google Scholar]
- 15.Herzenberg L A, Stall A M, Lalor P A, Sidman C, Moore W A, Parks D R, Herzenberg L A. The Ly-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 16.Hou S, Doherty P C, Zijlstra M, Jaenisch R, Katz J M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 17.Hurwitz J L, Tagart V B, Schweitzer P A, Cebra J J. Patterns of isotype expression by B cell clones responding to thymus-dependent and thymus-independent antigens in vitro. Eur J Immunol. 1982;12:342–348. doi: 10.1002/eji.1830120416. [DOI] [PubMed] [Google Scholar]
- 18.Kantor A B, Herzenberg L A. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 19.Kohl S, Harmon M W, Tang J P. Cytokine-stimulated human natural killer cytotoxicity: response to rotavirus-infected cells. Pediatr Res. 1983;17:868–872. doi: 10.1203/00006450-198311000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kroese F G, Ammerlaan W A, Kantor A B. Evidence that intestinal IgA plasma cells in mu, kappa transgenic mice are derived from B-1 (Ly-1 B) cells. Int Immunol. 1993;5:1317–1327. doi: 10.1093/intimm/5.10.1317. [DOI] [PubMed] [Google Scholar]
- 21.Kroese F G, Ammerlaan W A, Deenen G J, Adams S, Herzenberg L A, Kantor A B. A dual origin for IgA plasma cells in the murine small intestine. Adv Exp Med Biol. 1995;371A:435–440. doi: 10.1007/978-1-4615-1941-6_91. [DOI] [PubMed] [Google Scholar]
- 22.Kroese F G, Butcher E C, Stall A M, Lalor P A, Adams S, Herzenberg L A. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1:75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann-Grube F, Lohler J, Utermohlen O, Gegin C. Antiviral immune responses of lymphocytic choriomeningitis virus-infected mice lacking CD8+ T lymphocytes because of disruption of the beta 2-microglobulin gene. J Virol. 1993;67:332–339. doi: 10.1128/jvi.67.1.332-339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logan A C, Chow K P, George A, Weinstein P D, Cebra J J. Use of Peyer's patch and lymph node fragment cultures to compare local immune responses to Morganella morganii. Infect Immun. 1991;59:1024–1031. doi: 10.1128/iai.59.3.1024-1031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeal M M, Rae M N, Ward R L. Evidence that resolution of rotavirus infection in mice is due to both CD4 and CD8 cell-dependent activities. J Virol. 1997;71:8735–8742. doi: 10.1128/jvi.71.11.8735-8742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNeal M M, Barone K S, Rae M N, Ward R L. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virol. 1995;214:387–397. doi: 10.1006/viro.1995.0048. [DOI] [PubMed] [Google Scholar]
- 27.Moser C A, Cookinham S, Coffin S E, Clark H F, Offit P A. Relative importance of rotavirus-specific effector and memory B cells in protection against challenge. J Virol. 1998;72:1108–1114. doi: 10.1128/jvi.72.2.1108-1114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller D, Koller B H, Whitton J L, LaPan K E, Brigman K K, Frelinger J A. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science. 1992;255:1576–1578. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda J L, Gapin L, Sydora B C, Bryne F, Binder S, Kronenberg M, Aranda R. Systemic activation and antigen-driven oligoclonal expansion of T cells in a mouse model of colitis. J Immunol. 2000;164:2797–2806. doi: 10.4049/jimmunol.164.5.2797. [DOI] [PubMed] [Google Scholar]
- 30.O'Neal C M, Harriman G R, Conner M E. Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A. J Virol. 2000;74:4102–4109. doi: 10.1128/jvi.74.9.4102-4109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Offit P A, Dudzik K I. Rotavirus-specific cytotoxic T lymphocytes cross-react with target cells infected with different rotavirus serotypes. J Virol. 1988;62:127–131. doi: 10.1128/jvi.62.1.127-131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riepenhoff-Talty M, Dharakul T, Kowalski E, Michalak S, Ogra P L. Persistent rotavirus infection in mice with severe combined immunodeficiency. J Virol. 1987;61:3345–3348. doi: 10.1128/jvi.61.10.3345-3348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spriggs M K, Koller B H, Sato T, Morrissey P J, Fanslow W C, Smithies O, Voice R F, Widmer M B, Maliszewski C R. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Heijden P J, Stok W. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J Immunol Methods. 1987;103:161–167. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- 35.Ward R L, McNeal M M, Sheridan J F. Development of an adult mouse model for studies on protection against rotavirus. J Virol. 1990;64:5070–5075. doi: 10.1128/jvi.64.10.5070-5075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]