Abstract
Purpose
To assess the feasibility of a second-generation (44-channel) suprachoroidal retinal prosthesis for provision of functional vision in recipients with end-stage retinitis pigmentosa (RP) over 2.7 years.
Design
Prospective, single-arm, unmasked interventional clinical trial.
Participants
Four participants, with advanced RP and bare-light perception vision.
Methods
The 44-channel suprachoroidal retinal prosthesis was implanted in the worse-seeing eye. Device stability, functionality, and adverse events were investigated at approximately 12-week intervals up to 140 weeks (2.7 years) postdevice activation.
Main Outcome Measures
Serious adverse event (SAE) reporting, visual response outcomes, functional vision outcomes, and quality-of-life outcomes.
Results
All 4 participants (aged 39–66 years, 3 males) were successfully implanted in 2018, and there were no device-related SAEs over the duration of the study. A mild postoperative subretinal hemorrhage was detected in 2 recipients, which cleared spontaneously within 2 weeks. OCT confirmed device stability and position under the macula. Improvements in localization abilities were demonstrated for all 4 participants in screen-based, tabletop, and orientation and mobility tasks. In addition, 3 of 4 participants recorded improvements in motion discrimination and 2 of 4 participants recorded substantial improvements in spatial discrimination and identification of tabletop objects. Participants reported their unsupervised use of the device included exploring new environments, detecting people, and safely navigating around obstacles. A positive effect of the implant on participants’ daily lives in their local environments was confirmed by an orientation and mobility assessor and participant self-report. Emotional well-being was not impacted by device implantation or usage.
Conclusions
The completed clinical study demonstrates that the suprachoroidal prosthesis raises no safety concerns and provides improvements in functional vision, activities of daily living, and observer-rated quality of life.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Suprachoroidal retinal prosthesis, Functional vision, Bionic eye, Retinitis pigmentosa, Activities of daily living
Visual prostheses can restore some visual function to those with profound vision loss due to retinal degenerative diseases such as retinitis pigmentosa (RP).1 A range of technologies exist but, with >500 recipients to date, retinal prostheses incorporating an implantable neurostimulator and an intraocular electrode array are the most common type.2 Of these, 3 devices have received regulatory approval: the Argus II epiretinal implant (Cortigent Inc, formerly Second Sight Medical Products),3,4 the Alpha IMS/AMS subretinal implant (Retina Implant AG),5, 6, 7 and the IRIS II epiretinal implant (Pixium Vision),2 although none of these are commercially available any longer. Several other devices are at the clinical trial stage, with the key differentiations being in the surgical placement of the electrode array: epiretinal, subretinal, suprachoroidal, or within visual cortex.2,8,9 In response to processed images from an external image sensor, retinal prostheses can stimulate residual bipolar and retinal ganglion cells to provide rudimentary vision to profoundly blind recipients and, with appropriate visual rehabilitation, aid object localization and navigation.2,8
We have previously reported successful results from a first-in-human clinical trial with a first-generation suprachoroidal retinal prosthesis (Clinicaltrials.gov NCT01603576; N = 3; 2012–2014)10 and interim results from a clinical trial with a second-generation suprachoroidal retinal prosthesis (Clinicaltrials.gov NCT03406416; N = 4; 2018–2020)11 in participants with end-stage RP. Findings demonstrated the surgical procedure was feasible and functionally useful with all participants showing improvement on functional vision tasks when compared with device off.10, 11, 12
In our interim report on the findings from the second-generation retinal prosthesis clinical trial,11 we reported the device stability, visual function, and functional vision outcomes in 4 participants with profound vision loss from end-stage RP for the first year of follow-up from device implantation (56 weeks). The aim of this report is to provide data on device stability, visual function, functional vision, and improvements in quality of life at the completion of the second-generation retinal prosthesis clinical trial, approximately 2.7 years after device implantation.
Methods
Participants and Eligibility Criteria
Participants with bare light perception vision due to RP, aged 39 to 66 years (mean 54 years), were enrolled (Clinicaltrials.gov NCT03406416) after providing written informed consent. The study was conducted according to the tenets of the Declaration of Helsinki and was approved by the Human Research Ethics committee from the Royal Victorian Eye and Ear Hospital (16/1266H).
Diagnosis was confirmed by the lead vitreoretinal surgeon (P. J. A.) after assessment of clinical history, a dilated fundus examination, and full-field electroretinography (Espion, Diagnosys LLC).13 Enrolled participants had a history of ≥10 years useful form vision, with further details of screening and baseline assessments having been previously reported.11 The full list of inclusion and exclusion criteria is shown in Supplementary Table S1 (available at https://aaojournal.org). All 4 participants received the retinal prosthesis monocularly and hence were unmasked. None of the participants dropped out of the study, although participant S4 did not complete all outcome measures at every time point because of personal events unrelated to the study, and there were coronavirus diease 2019 (COVID-19)-pandemic-related impacts on outcome measure data collection time points due to restrictions set by the human research ethics committee and hospital policy.
Bionic Vision Technologies Suprachoroidal Retinal Prosthesis
The intra-ocular component of the Bionic Vision Technologies suprachoroidal retinal prosthesis is a 19 × 8 mm silicone substrate containing 44 platinum disc electrodes (each 1 mm diameter) arranged in a staggered-grid as reported previously in the interim report.11 The 44 electrodes cover 10 × 7.5 mm of retina or approximately 38 × 28° of visual field.14 Two large return electrodes (2 mm diameter) are at the temporal edge of the substrate. A silicone cable containing 44 individually insulated helically coiled platinum wires bifurcates and runs to 2 hermetically sealed stimulation units, implanted under the postauricular scalp and wirelessly connected to externally-worn transmission coils. A CMOS video camera on custom-molded spectacles and a body-worn portable video processor together enable image filtering (Lanczos2 filter) to improve edge-detection and contrast.15 Electrodes were stimulated with charge-balanced, anodic-first, biphasic pulse waveforms with 500 μs phase width, 500 μs interphase gap, and 1700 μs shorting period. Video frames were acquired and processed at 120 frames-per-second. Stimulation per-electrode was limited to a maximum of 250 nanocoulombs per phase and 50 pulses per second on the basis of results from preclinical chronic stimulation studies.16 During mobility assessments, image intensity was always inverted such that the strongest stimulation corresponded to the darkest spots in the image. However, during home use, participants could select between noninverted and inverted modes.
Implantation Surgery
The 4 participants had the device implanted in the worse-seeing eye (3 right, 1 left) under general anesthesia by experienced vitreoretinal surgeons (P. J. A. and J. Y.) in collaboration with an otolaryngologist (R. B.) at the Royal Victorian Eye and Ear Hospital between February and August 2018. The surgical procedure followed the methods previously refined and described.10,11,17 The stimulator packages were placed on the squamous temporal bone. A lateral orbitotomy was created to provide a notch for the lead and grommet. A custom trocar was utilized to pass forward the electrode array. A temporal peritomy exposed the sclera and lateral rectus muscle before the electrode array was inserted into the dissected suprachoroidal pocket. The integrity of the device and overlying retina was confirmed intraoperatively with fundus examination and electrical impedance testing. The lead was secured in the orbitotomy using a silicone grommet.
Study Timeline and Clinical Outcomes
At 7 to 9 weeks postsurgery, all wounds were sufficiently healed, and devices were successfully switched on. From this point forward, all stated time-points are relative to device switch-on (week 1). Participants commenced device fitting at week 1 and training from weeks 2 to 16, with details described previously.11 Serious adverse events (SAEs) and adverse events were recorded for the duration of the study to monitor safety as per the study protocol (section 8) and in line with Good Clinical Practice standards, with the event description, time of onset postprocedure, severity, causality, and outcome collected and assessed by the principal investigator (P. J. A.). The primary efficacy outcome variable was visual perception threshold following stimulation of individual electrodes. Visual response thresholds were measured throughout the study using a 2-down 1-up modified staircase procedure.18 Lower threshold values indicate visual perception at lower levels of stimulation. Seven secondary efficacy outcome measure assessments of visual function and functional vision with Device On compared with Device Off (“test condition”) were performed at week 17 (after training was completed) and week 20, and thereafter every 12 weeks until study end point (defined as the last time point in the study where assessments could be achieved [week 110 for S1, S2, and S3, and week 140 for S4]). Assessments from week 68 to 140 were impacted by the COVID-19 pandemic and include subsets of data as feasible (Table 2). Between outcome measure assessments, participants were engaged by the research team via phone and email and were provided equipment servicing using “COVID-Safe” procedures. The outcome measures chosen complied with the Recommendations of the Task Force for the Harmonization of Outcomes and Vision Endpoints in Vision Restoration Trials.19
Table 2.
Study Visits Attended by Each Participant
| Participant ID | Study Outcome Measure Time-point (Weeks Postdevice Switch-On) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 17 | 20 | 32 | 44 | 56 | 68 | 80 | 92 | 110∗ | 140∗ | |
| S1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| S2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| S3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| S4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
Study end.
Screen-Based Assessments
The screen-based assessments have been previously described,11 and they were designed to assess localization and spatial discrimination (Fig 1). A scrambled condition with nonretinotopic mapping between points in the visual field and specific electrodes was included in the localization and motion discrimination tasks to assess interelectrode (retinotopic) discrimination.2,20,21 In the Scrambled condition, the presence of a stimulus is still noticeable, but its precise location and internal structure are disrupted. Participants were masked to the test condition; however, they all indicated they could tell when the Device Off condition was in use, whereas only 1 participant (S2) indicated any awareness of the otherwise undisclosed Scrambled condition. Viewing distance to the 40-inch touchscreen was measured prior to each task, and stimulus sizes were adjusted by the test program accordingly. For a typical viewing distance of arm’s length (approximately 43 cm), the 40-inch monitor spanned 95 × 63° of visual arc. Nonimplanted eyes were occluded and participants were free to move their head.
Figure 1.
Screen-based tasks. (A) Square Localization task. (B) Grating Acuity task. The participant must describe the orientation of the lines as being either horizontal or vertical. (C) Tabletop Search task. (D) Photos of the objects used for Tabletop Search (from left): placemat, plate, bowl, cup, can, and fork.
For the square localization task, performance (“pointing error”) was quantified for each trial as the distance (vector magnitude) between the center of a 10° wide square and the point touched, without consideration of the direction of the error. Twenty-four trials per device condition (Device On, Device Off, and Scrambled) were administered in 8 trial blocks using a balanced randomized design. If a trial exceeded 30 seconds, a repetitive alarm compelled a prompt response.
For the motion discrimination task, the participant used a keypad to indicate direction-of-movement of a single 5° wide bar moving perpendicularly across the screen in 1 of 4 cardinal directions. Twenty-four trials per device condition were administered in 8 trial blocks as above. This task was repeated at up to 3 speeds; 7, 15, and 30°/sec, with participants progressing to the next speed if their score significantly exceeded chance (25%) and they consented to continue. All trials were completed within 30 seconds.
Spatial discrimination was assessed using the Basic Grating Acuity test program (Fig 1B).22 The participant was required to identify the line orientation of evenly spaced horizontal or vertical black stripes (2 alternative forced choice, 24 trials per condition), firstly at 0.033 cycles-per-degree (cpd) and then repeated at a higher spatial frequency (0.1 cpd), if the participant exceeded the passing criterion (75% accuracy), or at a lower spatial frequency (0.01 cpd) if they did not. If a trial exceeded 30 seconds, it was scored as “incorrect” by the test program.
Modified Door Task
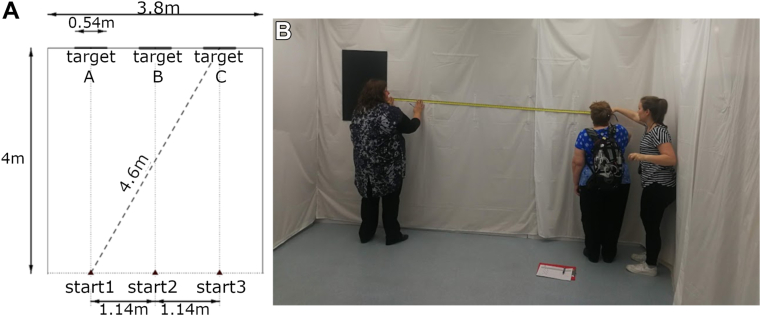
This orientation and mobility task11 assessed whether participants could detect, walk toward, and touch a black, high-contrast target (54 × 70 cm) on the far wall of a white-walled room measuring 3.8 × 4.8 meters (Fig 2B). The required traversal distance between start position (1, 2, 3) and target location (A, B, C) varied between 4 and 4.6 m (Fig 2A). The participant announced “stop” when they thought they were within arms-reach of the target prior to actually reaching for the target. Participants were scored across 10 randomized trials per condition for the time to complete each trial and the distance from finger-tip to nearest edge of the target. If the participant successfully touched the target, the outcome was ascribed a distance of zero.
Figure 2.
Modified Door task. (A) Configuration of the task with all measurements in cm. A black high-contrast target, representing a darkened window or doorway, was randomly positioned at location A, B, or C. The target measured 54 × 70 cm (W × H) and the top-edge was approximately 2 meters above the floor. Participants started each trial at a random selection of starting points 1, 2, or 3. (B) Measuring fingertip distance to the target in the task.
Tabletop Search
The 6 common household objects (plate, bowl, placemat, cup, can, or fork) were utilized in a search task as shown in Figure 1C, D. One object was randomly selected and placed within a 3 × 3 tabletop grid measuring 109 × 81 cm total (each grid cell measuring 36 × 27 cm, 9 grid cells in total). This task has been previously described and validated to stratify low-vision participants according to visual acuity and remaining visual field.11,23 Participants were scored across 20 randomized trials per condition on 4 outcomes; accuracy of verbalized object location, object identification, successful touch of the object, and distance from fingertip to nearest edge of the object. If the participant successfully touched the object, the outcome was ascribed a distance of zero.
Obstacle Avoidance
Participants were assessed in this orientation and mobility task on their ability to detect and avoid obstacles at 2.55 m intervals while walking down a 20-meter corridor (1.75 m wide). There was 1 obstacle at each interval, randomly placed at left, center, or right, with obstacle type randomly selected from 6 possible types, as described previously.11 Participants were scored across 10 randomized trials per condition on object detection (verbal indication), speed (as percentage of predetermined preferred walking speed), frequency of obstacle contact, and percentage of trials with zero collisions or contact.
Functional Low-Vision Observer-Rated Assessment
The Functional Low-Vision Observer-Rated Assessment (FLORA, Second Sight Medical Products)24 was used to compare nonlaboratory Device On versus Device Off performance in functional vision, activities of daily living, and orientation and mobility. These evaluations and associated questionnaires were administered by an orientation and mobility specialist at the participant’s local environments, and were restricted to tasks identified as being relevant to each participant’s self-reported goals and activities.12 Each completed task was graded as being easy to impossible and graphed as the mean participant score across 4 categories: orientation, mobility, activities of daily living, and interacting with others. Furthermore, the specific contribution of vision (as opposed to other senses) to completing the task was assessed for these same categories.
Quality-of-Life Assessments
The impact of vision impairment–very low vision (IVI-VLV)25 questionnaire was used to assess vision-related quality of life. There are 2 subscales: activities of daily living, mobility, and safety (16 items) and emotional well-being (EWB; 12 items). The Patient Health Questionnaire-926 was additionally used as an independent assessment of EWB.
Device Stability
Color retinal fundus photography (TRC-50EX, Topcon Medical Systems, Clarus 500, Carl Zeiss Meditec AG) and OCT (Spectralis® OCT, Heidelberg Engineering GmbH) were conducted throughout the study to monitor device stability. Any relative changes to position of the array or electrode-to-retina distance (middle of electrode to inner boundary of the retinal pigment epithelium) observed in OCT B-scans were quantified using previously described methods that have been found to be reliable.27
Statistical Analysis
For the localization and motion discrimination tasks, efficacy with Device On was compared with Device Off and with the Scrambled condition. Device conditions were compared at the participant level using the nonparametric Friedman test,28 which allows for differences across multiple test dates. Conover nonparametric equivalent of Fisher least significance difference method was used for posthoc comparisons.29 When an outcome measure was missed (because of participant absence or fatigue), or block sizes were unequal because of procedural error, within-participant comparisons were performed with Wittkowski variant of the Friedman test (which is tolerant of incomplete blocking).28
Performance in the spatial discrimination task was considered above chance if accuracy was >75% (ie, P < 0.05 for a binomial distribution of 24 trials). Family-wise errors within-participant for each task were controlled using Holm’s sequential correction.30 As functional vision tasks in this longitudinal study were repeated at multiple time-points, a leave-one-out Kruskal–Wallis analysis was used to assess whether a participant’s score at their last study visit differed from the distribution of scores from their earlier visits at each of the functional assessments. Statistical analyses were performed in R, version 3.6.3 (R Foundation for Statistical Computing).
Results
All 4 enrolled participants (Table 3) were successfully unilaterally implanted with the suprachoroidal implant. The surgical procedures were uncomplicated, taking between 204 and 260 minutes. Postsurgical impedance testing showed that all electrodes were functional in all participants. At the end of the study, 97% of all electrodes were functional, equating to a loss of 1 electrode for participant S1, 3 electrodes for S2, 0 electrodes for S3, and 1 electrode for S4. A subset of remaining electrodes exhibited short, infrequent periods of high-impedance, suggestive of stretching in the affected leadwire.31
Table 3.
Baseline Participant Characteristics
| Feature | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| Gender | Male | Male | Female | Male |
| Age at implant (years) | 47 | 63 | 66 | 39 |
| Eye condition | Retinitis pigmentosa (rod cone dystrophy) | Retinitis pigmentosa (rod cone dystrophy) | Retinitis pigmentosa (rod cone dystrophy) | Retinitis pigmentosa (cone rod dystrophy, diagnosis as infant) |
| Observed nystagmus | Mild | Intermittent | None | Mild |
| Visual acuity | Light perception both eyes | Light perception both eyes | Light perception both eyes | Light perception both eyes |
| Electroretinogram stimulus light threshold (candela secs/m2) | 0.1 | 0.1 | 0.001 | 0.001 |
| Age when legally blind (years) | 20 | 34 | 41 | 13 |
| Approximate years of useful form vision | 34 | 43 | 56 | 19 |
| Primary mobility aid | Cane | Cane | Guide Dog | Cane |
| Implanted eye | Left | Right | Right | Right |
Clinical Outcomes
The primary study outcome objective of safety was met, with no device-related SAEs over the duration of this trial. Adverse events related to surgery were all expected events: swollen eyelid, pain around the stimulator region, conjunctival injection, minor inflammation of the anterior chamber, ocular pressure sensation, and tenderness of operated eye and lateral canthus. As anticipated from our first-in-human trial,10 2 recipients had a small trace of subretinal hemorrhage that presented as mild obscuration of the electrodes on color fundus photograph and cleared spontaneously within 2 weeks.11 A full discussion of adverse events will follow in a subsequent publication.
Device Stability
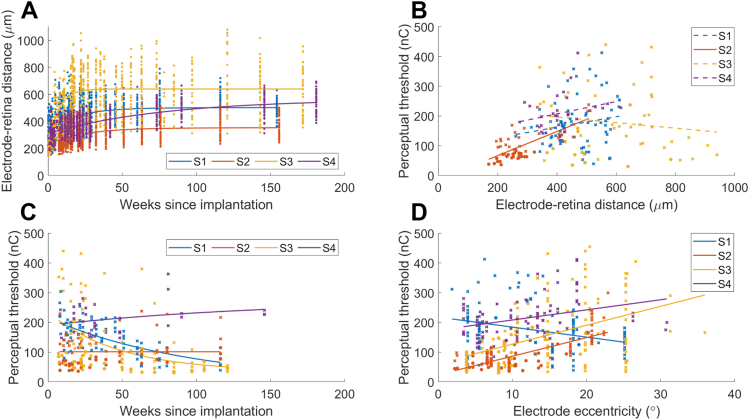
The electrode array was implanted under the macular region in all participants (Fig 3 and 4). There was no folding of or damage to the retinal layers. Longitudinal electrode-to-retina distance measures (from OCT B-scan analyses) are presented in Figure 5A. Data from all electrodes are included, but not all electrodes could be visualized at every clinical visit. The data are well described by an asymptotic exponential fit31 and demonstrate that electrode-to-retina distances increased immediately after surgery but then stabilized for 3 of 4 participants (S1 at 502 ± 21 μm by 51 weeks postoperative examination, S2 at 353 ± 23 μm by 63 weeks postoperative examination, and S3 at 639 ± 53 μm by 17 weeks postoperative examination). For the remaining participant (S4), the rate of increase was calculated from the derivative of the model at weekly intervals31 and had slowed to <0.33 μm/week by the end of the study (mean distance 566 ± 34 μm).
Figure 3.
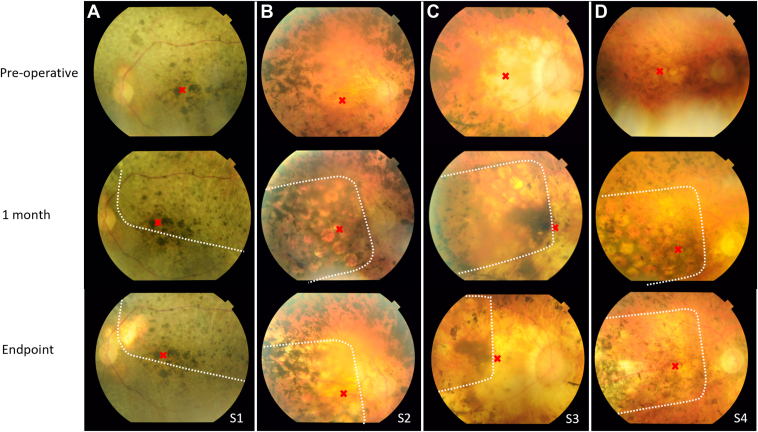
Color fundus photos preoperatively, at 1-month postsurgery and at study end point. (A) Participant S1. (B) Participant S2. (C) Participant S3. (D) Participant S4. Red cross indicates fovea. White dots indicate edge of silicone substrate. The electrodes can be seen in some participants better than others because of differences in pigmentation.
Figure 4.
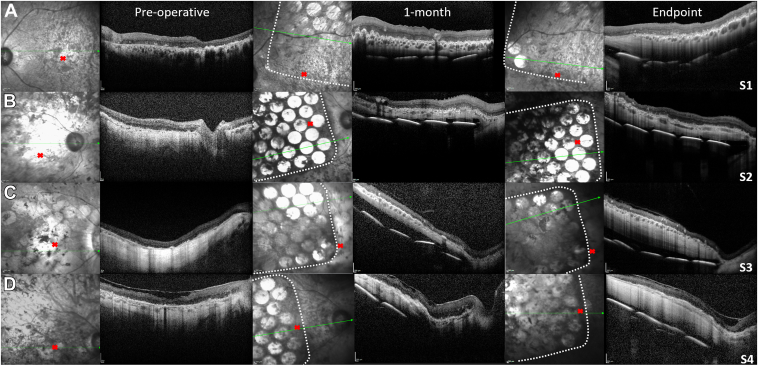
Near infrared (NIR) and OCT B-scan retinal imaging preoperatively, 1-month postsurgery and at study end point. (A) Participant S1. (B) Participant S2. (C) Participant S3. (D) Participant S4. White dots indicate edge of silicone substrate. B-scans through electrodes (visible as bright circles) are captured at the array tip. The electrodes can be seen in some participants better than others because of differences in pigmentation. Green arrows on NIR images indicate the matching OCT scan position. The position of the implant tip relative to the optic nerve head and fovea (red cross) in each participant is evident. S1 received a left eye implant and S2-S4 received a right eye implant. Scale bars = 200 μm.
Figure 5.
Electrode to retina distance and perceptual thresholds. (A) Longitudinal electrode-retina distance measures for all 44 stimulating electrodes for participants S1-S4. An asymptotic exponential model was fitted to the data (solid lines), suggesting an initial period of increasing distances which then settled to a stable value that was maintained for the rest of the study. (B) Perceptual thresholds and linear fit against electrode-retina distance. For shorted/synchronous pairs, the average distance for the 2 electrodes is shown. Regression models fitted to the data are represented by solid lines when the gradient was different from zero (P < 0.05) and dashed lines otherwise. (C) Perceptual thresholds for a subset of 5 electrodes per participant and exponential fit over time. (D) Perceptual thresholds and linear fit against electrode eccentricity from the fovea. For shorted/synchronous pairs, the eccentricity was calculated from the centroid of the 2 electrodes.
Analyses of visualized position of the leading edge of the implant versus the optic nerve head suggested some minimal rotation (15° for S1 at study end compared to baseline, range 0.4° to 3.7° for all other participants) and translation temporally (range 139 to 320 μm), but generally the implant remained stable throughout the trial after an initial period of settling. These small shifts did not necessitate any change to camera image sampling locations.
Device Thresholds
Average phosphene thresholds in the first 4 weeks post switch-on were 182 ± 96 nC (mean ± standard deviation, range 38 to 454 nC). Participants described phosphenes near the fovea as having defined shapes (e.g., described by S3 as “a crescent moon shape”), whereas phosphenes at the periphery were described as having less definition (e.g., “like the sunrise peeping over the top of a hill”). Electrodes at the periphery were used in a paired configuration to maintain safe charge limits, defined from our preclinical studies as 250 nC for a single electrode and 500 nC for a pair.16 As detailed previously,11 the phosphene yield obtained within safe limits was 27 of 44 electrodes (61.4%) for S1; 32 (72.7%) for S2; 24 (54.5%) for S3; and 25 (56.8%) for S4, comprising predominantly foveal electrodes with sparser density at the periphery. Short-term or long-term fading was not reported, although faint spontaneous visual activity was noticed following days with several hours of device usage.
Figure 5B–D details a subset of 5 phosphenes per participant that were selected for longitudinal monitoring, chosen to include a selection of foveal, para-foveal, and peripheral phosphenes. A correlation between perceptual thresholds and electrode-retina distances was observed only for S2 (0.41 nC/μm, P < 0.001, Fig 5B). Perceptual thresholds remained measurable for all of the tracked electrodes, and were sufficient to allow ≥2 dB of dynamic range within the predefined charge limits. Ordinary least-squares linear regression models (Fig 5C, detailed previously31) show that thresholds decreased over time for S1 (−1.35 nC/week, P < 0.001) and for S3 (−1.40 nC/week, P < 0.001), suggesting familiarization with the phosphenes over time or an improved ability to discriminate phosphenes from spontaneous retinal activity. Thresholds increased slightly over time for S2 (0.43 nC/week, P = 0.021), and there was no substantial change in thresholds over time for S4 (P = 0.13). Thresholds increased with eccentricity from the fovea in S2, S3, and S4, but decreased with eccentricity in S1 (Fig 5D). This result was not unexpected as, in contrast to the other 3 participants, electrode-to-retina distances for participant S1 were greatest at the fovea.
Screen-Based Assessments
Square-Localization Task
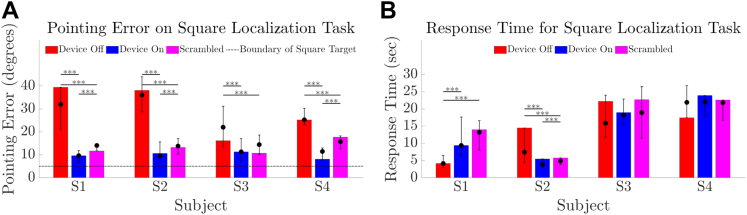
Touch accuracy in this localization task was better with Device On than Device Off for all participants (all P < 0.001, Fig 6A). Median pointing error from target center ranged from 9.5° to 11.4° (Device On) versus 22.0° to 36.0° (Device Off) versus 13.8° to 15.7° (Scrambled). Pointing error in the Scrambled condition was worse than Device On for participants S1, S2, and S4 (P < 0.001) but better than Device Off for all participants (all P < 0.001). There was no difference between the Device On and Scrambled conditions for participant S3 (P = 0.18). Response times were shortest for participants S1 and S2 (Fig 6B).
Figure 6.
Results for square localization task comparing device on (blue) versus device off (red) versus a scrambled condition (magenta). (A) Pointing error (in degrees) from touch location to target center for 24 trials of each condition. The boundary of the 10 degree wide square target is indicated by a dotted horizontal line. (B) Response time (s) for 24 trials of each condition. The height of the bar is each participant’s result at the final time-point. Error bars denote the range of average scores across all time-points. Solid circles denote the median of average scores across all time-points. ∗∗∗P value for within-participant comparisons of < 0.001.
Motion Discrimination
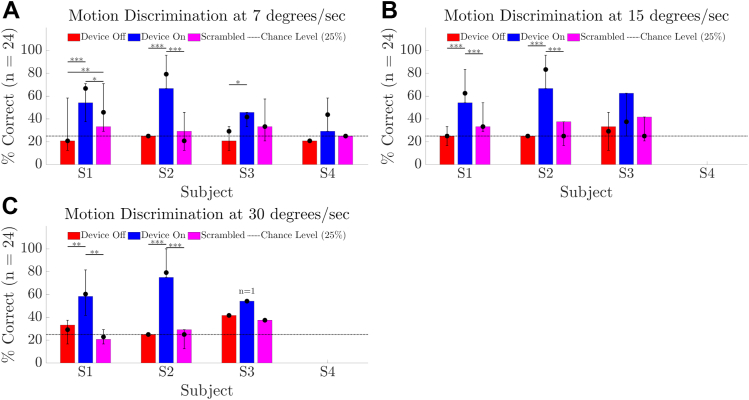
All participants completed the motion discrimination task at 7°/sec (Fig 7A) with participants S1, S2, and S3 medians for success rate being better with Device On (66.7%, 79.2%, and 41.7%) versus Device Off (20.8, 25, 29.2%; P < 0.001 for S1 and S2, P = 0.002 for S3). A subset completed the task at 15 and 30°/sec (Fig 7B-C): participants S1 and S2 continued to demonstrate a benefit of Device On (both P < 0.001 at 15°/sec, P = 0.001 at 30°/sec for S1, P < 0.001 at 30°/sec for S2) whereas the median score for participant S3 was near chance at 15°/sec, although this improved to 54.2% at week 90 and 62.5% at study end. S3 had a single attempt at 30°/sec at week 90, scoring 54.2%. Success rate with the positive-control (Scrambled) condition was poorer than with Device On for participants S1 and S2 (all P < 0.001, except P = 0.004 for S1 at 7°/sec and P < 0.001 for S1 at 30°/sec), with the greatest impact on performance observed for participant S2 at 30°/sec (25% median score with Scrambled versus 79.2% median score with Device On). Data for S4 are limited to weeks 20 and 44. S3 attempted 30°/sec at week 90 only. Unexpectedly, participant S1 scored 58.3% at 7°/sec with Device Off on 1 occasion (week 17); however, Device Off performance was at chance levels for all subsequent measurements.
Figure 7.
Results for motion discrimination task comparing device on (blue) versus device off (red) versus a scrambled condition (magenta). (A) Success rate at 7°/sec. (B) Success rate at 15°/sec. (C) Success rate at 30°/sec. Chance level (25%) is shown as a horizontal line. The height of the bar is each participant’s result at the final time-point. Error bars denote the range of average scores across all time-points. Solid circles denote the median of average scores across all time-points. P values for within-participant comparisons are indicated by ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05. Participant S3 had a single attempt at 30°/sec at week 90, denoted by the superscript “n = 1” in panel C.
Spatial Discrimination
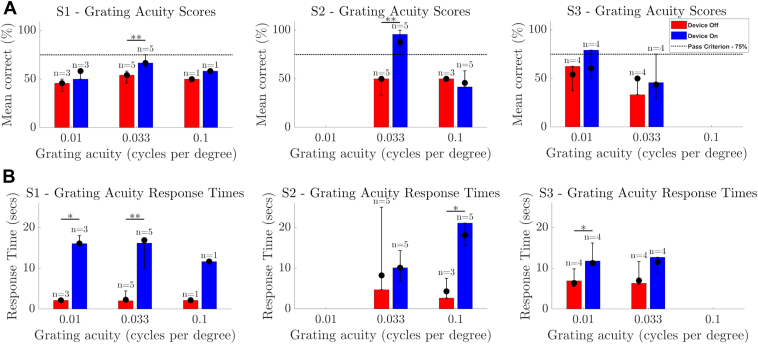
Participant S4 did not attempt the spatial discrimination task, participant S3 attempted at weeks 44 onward, and participants S1 and S2 attempted at weeks 32 onward. Incomplete data are due to the high levels of fatigue generated by this task, which limited its usefulness. Figure 8A demonstrates that the passing criterion of 75% on 24 trials of the two-alternative forced-choice Basic Grating Acuity spatial discrimination task with a grating spacing of 0.033 cpd was met or exceeded on 1 occasion for participant S1 (range 62.5%–75%), on 5 occasions for participant S2 (range 75%–100%), and on 1 occasion for participant S3 (range 29.2%–75%). S3 met the criterion for 0.01 cpd at study end (range 50%–75%). In general, the response times with Device Off (Fig 8B) are shorter than with Device On, as there was reduced task engagement when participants realized the camera was off. However, S2 gave a resolute effort with Device Off on 1 occasion, demonstrated by a 1-time increase in average response time to 25 seconds. The percentage of trials scored as “incorrect” because they exceeded 30 seconds was <3% for each participant (range 0.5%–2.9%). There was no evidence of a difference in screen-based task performance at study-end versus the intraparticipant distribution of scores from earlier visits (P > 0.05).
Figure 8.
Results for the spatial discrimination task comparing device on (blue) versus device off (red) for participants S1 to S3. (A) Percentage correct of 24 trials. (B) Mean response time (s). The height of the bar is each participant’s result at the final time-point. Error bars denote the range of average scores across all time-points. Solid circles denote the median of average scores across all time-points. P value for within-participant comparisons is indicated by ∗∗P < 0.01; ∗P < 0.05. Text above the error bars indicates n, the number of times each grating acuity spacing was attempted throughout the study. A horizontal dotted line indicates the passing criterion of 75%. S2 consistently achieved a passing criterion at 0.033 cpd and therefore was not administered the lower 0.01 cpd test configuration. Participant S4 did not attempt this task. cpd = cycles-per-degree.
Functional Assessments
There was no evidence of within-participant changes in performance over the study for the functional assessments (P > 0.05).
Modified Door Task
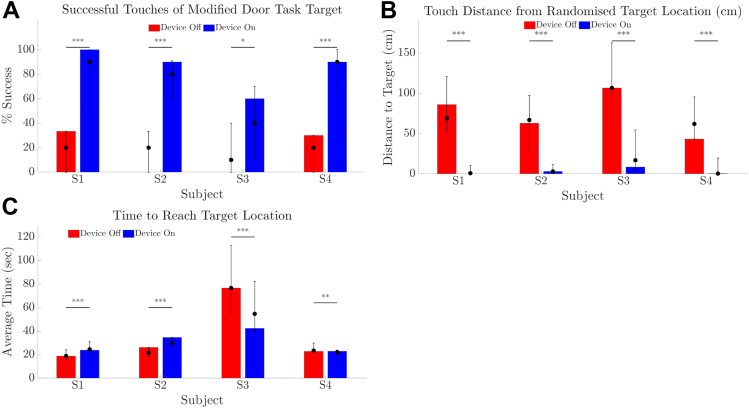
All participants could complete this orientation and mobility task and were more successful with Device On at reaching and touching the target (Fig 9A, P < 0.001 for participants S1, S2, S4, P = 0.02 for participant S3). Participants used the perceived width of the target to determine proximity, describing that phosphene activity was visible for a greater visual arc during head-scanning as they approached the target. Median distance between fingertip and target ranged from 62.0 to 106.6 cm with Device Off and from 0.3 to 16.9 cm with Device On (Fig 9B) and was better than Device Off in all cases (P < 0.001). Time to complete the task was longest for participant S3 (Fig 9C), who noted difficulty with distance estimation during the task.
Figure 9.
Results for modified door task comparing device on (blue) versus device off (red). (A) Rate of successful touches of target (%). (B) Touch distance from fingertip to target including successful touches. (C) Time taken to reach target. The height of the bar is each participant’s result at the final time-point, and indicates zero successful touches with Device Off at the final time-point for participants S2 and S3 (as per A). Error bars denote the range of average scores across all time-points. Solid circles denote the median of average scores across all time-points. P values of within-participant comparisons are shown; ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05.
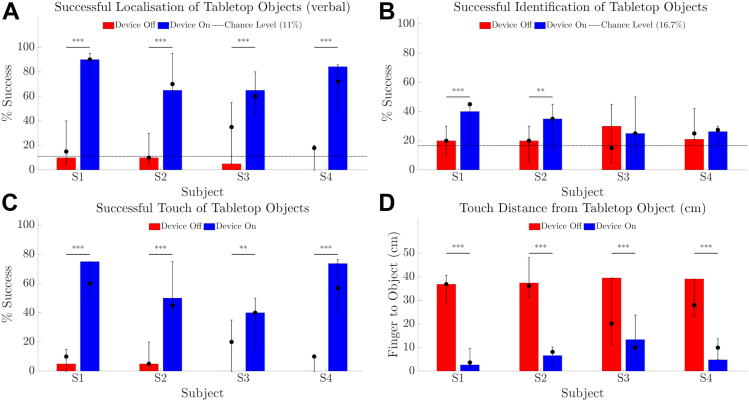
Tabletop Search
Verbal localization of objects was better with Device On than with Device Off for all participants (Fig 10A, participants medians = 60%–90%, all P < 0.001). Average object identification scores were also higher with Device On than with Device Off for participants S1 (P < 0.001) and S2 (P = 0.003), but did not exceed 45% for those participants (Fig 10B). There was higher success for all participants at touching the object with Device On than Off (P < 0.001 for S1, S2, and S4, P = 0.003 for S3, Fig 10C), indicating good camera to hand coordination. Median distance between fingertip and object was shorter with Device On (all P < 0.001, 3.7–10.0 cm with Device On vs. 20.2–36.9 cm with Device Off, Fig 10D).
Figure 10.
Results for tabletop search task comparing device on (blue) versus device off (red). (A) Success rate (%) for verbally indicating object location on a 3 × 3 grid. (B) Success rate (%) for identifying object type (1 of 6). (C) Success rate (%) for contacting the object. (D) Distance from fingertip to object including successful touches. Chance level is indicated as a horizontal line in panels A and B. The height of the bar is each participant’s average of 20 trials at the final time-point. Error bars denote the range of average scores across all time-points. Solid circles denote the median of average scores across all time-points. P values of within-participant comparisons are shown; ∗∗∗P < 0.001; ∗∗P < 0.01.
Localization and touch results for participant S3 with Device Off ranged higher than for other participants but were still poorer than with Device On. Although participant S3 has no measurable islands of vision for either eye on Goldmann perimetry, they reported ability to detect tabletop objects by noticing changes in contrast (“shadows”) during head-scanning.
Comparisons between Reported Object and Actual Object (Supplementary Figure S11, available at https://aaojournal.org) show that responses cluster (dark band of cells from lower left to upper right of the matrix) in relation to the size of the actual object, and this was most pronounced for participants S1, S2, and S4. Confusion with Device On appeared more likely to be between objects of similar size (e.g., can vs. cup = 44.8% for S1) than dissimilar size (e.g., cup vs. plate = 4.2% for S1). With Device Off, participants tended to respond “cup” or “bowl” when uncertain, regardless of actual object size (Supplementary Figure S11).
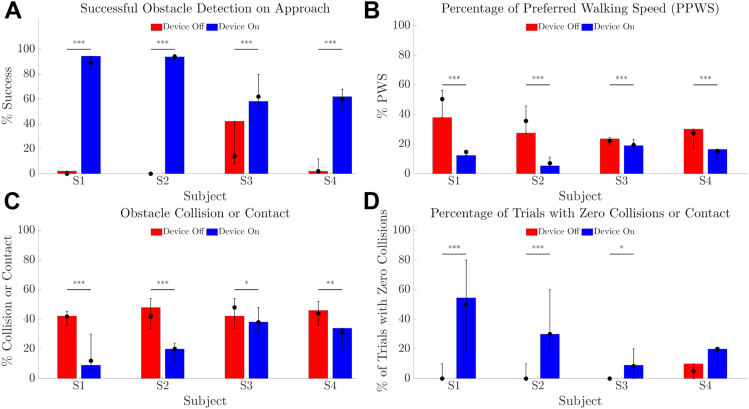
Obstacle Avoidance
Detection of obstacles was better with Device On (Fig 12A, all P < 0.001) and fewer contacts or collisions with obstacles were observed (Fig 12C, P < 0.001 for S1 and S2, P = 0.008 for S3, P = 0.001 for S4). The percentage of trials with zero collisions or contact were greater with Device On for S1 and S2 (both P < 0.001) and S3 (P = 0.01), and was <10% with Device Off for all participants (Fig 12D). However, the integrated use of head-scanning and spatial assessment reduced walking speed so that participants were slower to finish the course with Device On (Fig 12B, all P < 0.001).
Figure 12.
Results for obstacle avoidance task comparing device on (blue) versus device off (red). (A) Success rate (%) for verbally indicating object location on approach. (B) Walking speed for each trial, expressed as percentage of preferred walking speed (PPWS). (C) Collision rate (%) for each trial. (D) Percent of trials with zero collisions or contact. The height of the bar is each participant’s average of 10 trials at the final time-point. Error bars denote the range of average scores across all time-points. Solid circles denote the median of average scores across all time-points. P values of within-participant comparisons are shown; ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05.
Quality-of-Life Assessments
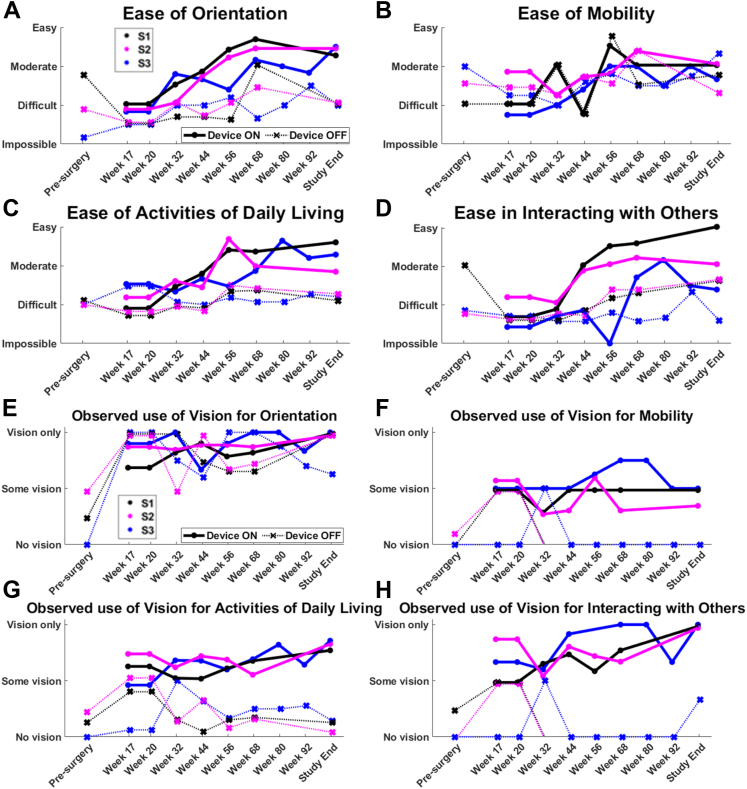
Functional Low-Vision Observer-Rated assessments to study-end have been previously reported in detail.12 In summary, FLORA assessments showed that orientation tasks (e.g., finding doorways) became easier with Device On over time, trending above “moderate” by study end, whereas they remained “difficult” with Device Off (Fig 13A). Ease of completing activities of daily living (e.g., locating items in a familiar environment and negotiating stairways independently) also trended to “moderate” with Device On, remaining “difficult” with Device Off (Fig 13C). Mobility tasks were rated as mostly “moderate” or “difficult,” with no difference between Device On and Off (Fig 13B). Interactions with others (e.g., visually locating or tracking a person) continuously improved over the study with Device On for S1 and S2, whereas participant S3 continued to find these tasks mostly “difficult” (Fig 13D). Participant S4 declined to complete FLORA assessments for reasons unrelated to the study.
Figure 13.
Participant means for Functional Low-Vision Observer-Rated Assessments in 4 task categories: (A) Orientation, (B) Mobility, (C) Activities of daily living, and (D) Interacting with others. (E–H) The relative contribution of vision (with respect to other senses) was determined for these same tasks. One participant is excluded from this analysis (see text). Participant averages are shown as circles with solid lines (Device On) and crosses with dotted lines (Device Off). The color key for individual participant data is described in the legend of panels A and E.
The degree to which the participant used their “vision” (i.e., the combination of residual vision and prosthetic vision) during the FLORA is shown in Figure 13E–H. Completing the task with “no vision” implies that other senses were used for that task (e.g., auditory and proprioception). “Vision” contributed to task performance more with Device On than with Device Off for mobility (week 32 onward), activities of daily living (week 17 onward), and social interactions (week 32 onward, Fig 13F–H). Visual orientation (Fig 13E) was the only task category in which participants appeared equally likely to use prosthetic vision (Device On) and residual vision (Device Off). Regular attempts and training on these tasks throughout the study, both with and without an orientation and mobility specialist, is likely to have contributed to Device On and Device Off improvements.
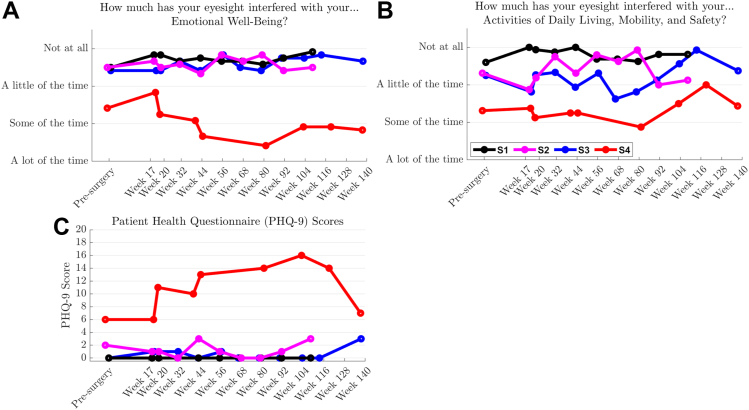
Scores for the EWB component of the IVI-VLV were stable for 3 of the 4 participants (Fig 14A), with a decrease for participant S4 being closely coupled with an increase in their Patient Health Questionnaire-9 score (Fig 14C). An independent psychologist worked with this participant to address psychosocial stressors that were not study related. Participants S1 to S3 described the impact of their vision loss on activities of daily living, mobility, and safety ranging from “a little of the time” to “not at all” (Fig 14B). Device logs indicated that participants S1 to S3 used their devices every 2 or 3 days initially, which decreased to around once per fortnight in later periods of the study, unfortunately coinciding with the COVID-19 pandemic and restrictions on movement outside of local environs and on social interactions. Participant S4 used their device rarely, and for <30 minutes on each occasion, which is linked to the previously mentioned psychosocial stressors and limited exploratory opportunities available. All participants described an exploratory approach in the initial period—trying the device in a variety of situations and environments. Following this exploration, these participants determined a set of activities for which they found the device provided most benefit, e.g., visiting a friend’s house or attending a woodworking class. On these occasions, participants used the device for around 2 to 3 hours at a time.
Figure 14.
Participants reported on the impact of vision impairment–very low vision on their (A) emotional well-being and (B) activities of daily living, mobility, and safety in the month prior to each assessment. Participant averages are shown as solid lines. (C) Totals for the Patient Health Questionnaire-9, an independent assessment of emotional well-being. Assessments for participant S4 were markedly influenced by external stressors that were unrelated to the device or the study.
Anecdotal Experiences
All participants demonstrated improvements in localization which were foundational to their successful use of the device in daily life. Participants reported greater confidence in navigation, such as increased tendency to explore new environments and reduced need for assistance when traveling to local shops.12 Additional feedback was that the device supplemented cane-use—enabling safe navigation around people and obstacles, and enabling identification of waypoints (e.g., trees or lamp posts) along a known route.12 Combining participant self-reports with the FLORA quality-of-life assessments, the orientation and mobility specialist confirmed a positive impact of the retinal prosthesis for all participants at all time-points, except for 1 neutral experience recorded for participant S3 at week 20.
Discussion
The primary safety objective was successfully achieved in this study, with no SAEs occurring throughout the trial. In all 4 participants, the device was implanted with no intraoperative complications and minimal (3%) loss of electrode functionality during the study. A small trace of subretinal hemorrhage in postoperative imaging was present as expected10 in 2 of the 4 participants and resolved without intervention. The electrode array remains in position under the macula for all 4 recipients (2.7+ years postimplantation), with minimal lateral movement at study-end (maximum 320 μm, which is 32% of 1 electrode diameter), some rotation (maximum 15°), and no signs of extrusion or retinal trauma. These results affirm the feasibility and stability of the suprachoroidal surgical approach for retinal prostheses.
The suprachoroidal approach is attractive because of reduced surgical complexity and therefore reduced risk of intra- and postoperative complications. In contrast, the surgical procedures for alternative surgical approaches are more complex and several surgery-related SAEs have been reported. Device insertion of an epiretinal device, such as the Argus II, requires a pars plana vitrectomy and sclerotomy.32 Further steps are required for subretinal devices such as the Alpha AMS and PRIMA (Pixium Vision), including the creation of a subretinal bleb and retinotomy to introduce the device.5,33 Reported SAEs for epiretinal and subretinal devices include retinal detachment,5,33,34 conjunctival and scleral erosions,5,32,34 hypotony,32 and endophthalmitis.32 In further support of the reduced risk of SAEs in the suprachoroidal approach, other human studies using suprachoroidal10 and intrascleral35 electrodes report an absence of intraocular SAEs, aside from iridocyclitis in the intrascleral participants that resolved after changing the stimulation parameters, although the total number of people implanted with these devices is comparatively low, so further multicenter studies will be needed to fully assess device safety. Studies involving suprachoroidal or intrascleral devices have implanted 12 participants to-date, versus >350 with epiretinal devices and >100 with subretinal devices.2
Device stability in the present trial was quantified with longitudinal assessments of electrode-to-retina distances27 and perceptual thresholds. Electrode-to-retina distances increased in the months immediately after implantation before plateauing for 3 participants and slowing to a small rate of increase (<0.33 microns/week, equivalent to 17 microns/year) for the remaining participant (S4).31 Although we were able to generate visual percepts in all 4 participants within a safe charge limit, participants S2 and S4 exhibited a slight increase in perceptual thresholds, which may relate to the longer time-course of increasing electrode-to-retina distance observed in these 2 participants.31 Perceptual thresholds decreased over time in participants S1 and S3, suggesting familiarization with the phosphenes or masking of spontaneous retinal activity. All participants continue to readily discern phosphenes and to use the system to perform functional vision tasks.
We observed that electrodes up to 36 degrees from the fovea could safely produce a phosphene in this cohort, consistent with a theoretical visual field of 38 × 28 degrees14 subtended by the active electrodes (versus approximately 11 × 19 degrees for the epiretinal Argus II36 and approximately 15 degrees on the diagonal for the subretinal Alpha AMS5). Thresholds were higher at more eccentric (peripheral) electrodes for 3 participants (S2–S4)—which is expected because of lower ganglion cell density and greater retinal degeneration and remodeling.37 The opposite relationship observed for thresholds versus eccentricity for participant S1 may be attributed to their comparatively greater electrode-to-retina distances at the fovea.31
The secondary efficacy outcomes were also well met, demonstrated by improvements in functional vision, orientation and mobility, and activities of daily living in controlled environments as well as in local environments. All participants were more accurate in the square localization task with Device On, with participant averages for error ranging from 9.5° to 11.4°. These results are similar to reported accuracy of approximately 8° on average for 27 Argus II recipients38 and reported best accuracy of approximately 8° for 1 of 3 suprachoroidal-transretinal device (Nidek Co) recipients.35 Our results for motion discrimination are also comparable: participants S1 and S2 exceeded the 62.5% pass criterion with Device On at speeds up to 30 degrees/sec and participant S3 demonstrated a benefit of Device On at 7 degrees/sec (although not exceeding 62.5%). A similar task in Argus II recipients, quantized to 15 degree arcs rather than the 4 cardinal directions in the present study, demonstrated benefit of Device On in 54% of 28 participants (at speeds up to 31.6 degrees/sec), comparable to our findings.39
We have previously published the relationship between directional confusion in the motion discrimination task and the surgical position of the electrode array in the mediolateral and inferior-superior dimensions.40 Comparing diminished performance with the Scrambled (nonretinotopic) condition in motion discrimination for participants S1 and S2, versus little impact of the same condition for participant S3, led us to conclude that S3 had less retinotopic discrimination and depended on head scanning cues to determine the direction of motion. Additionally, as was noted from comparatively higher scores in the Device Off condition for the Tabletop search task, participant S3 may have some immeasurable residual contrast vision that can contribute to these tasks. Our recent report of phosphene positions derived from eye-saccades further confirmed interparticipant differences in retinotopic discrimination.41 Although motion discrimination can be achieved with head-directed gaze at lower speeds, S1 and S2 were able to use retinotopic cues at speeds up to 30 degrees/sec in the normal Device On condition and could perform the task without head scanning.40 These same 2 participants were more successful at spatial discrimination (0.033 cpd on the Basic Grating Acuity assessment versus one-time success at 0.01 cpd for S3), indicating a probable association between successful motion discrimination and spatial discrimination. Motion discrimination is also possible with the Alpha IMS and AMS subretinal devices—with 1 exceptional recipient able to discriminate motion up to 35 degrees/sec (and grating acuity of 3.3 cpd), but otherwise only 18% to 21% of recipients passing the motion task at any speed.6,7 Despite the limited spatial acuity of relatively large suprachoroidal electrodes, it is unsurprising that increasing the area of retina covered provides a spatiotemporal benefit and allows recipients to observe the progression of moving stimuli across a larger field-of-view for a longer time period. Importantly, spatial acuity is not a prerequisite for the improvements in localization demonstrated by all participants in this study.
The tabletop task in the present study combined visual search and reaching accuracy with object identification. Localization and touch accuracy were better with Device On. Object identification scores in the tabletop task were modest but better with Device On for participants S1 and S2, and some clustering according to object size suggests an ability for the device to provide size discrimination. We have previously provided some interpretation of the user-experience in this task, relating to physical difficulty when reaching for the back row and mild confusion on the horizontal plane because of the side-mounted camera position on the glasses frame.11 Behavioral confounds in reaching tasks, including compromised performance for eccentric targets, have been reported previously,42 and it is expected that a stereo-camera version of the suprachoroidal system will address depth and azimuth confounds. Object identification is reported to be a challenging task in all retinal prosthesis studies.6,7,35,43
Many visual prosthesis recipients identify improvements in social interactions, activities of daily living, and orientation and mobility as important quality-of-life benefits. Success in these outcomes was demonstrated in controlled functional vision tasks (modified door task, obstacle avoidance) as well as in local environments (FLORA). All participants were more successful with Device On at touching the target in the modified door task, with averages for 3 participants (S1, S2, and S4) exceeding 80% and touch distances for these 3 participants <3 cm from the target on average. This compares favorably to mean task success of 50% in Argus II recipients (N = 28) at 12 months postimplantation.44 Obstacle detection was better with Device On, although participants contacted ≥1 obstacle on average per traversal (but less than with Device Off). Estimating angles and distances to visual targets has been described as “effortful and subject to inaccuracies” in Argus II participants.45
Outside the lab environment, the effect of the implant on participants’ daily lives was assessed using the FLORA instrument, developed to evaluate the Argus II in everyday environments.24 A study of 26 Argus II recipients at approximately 3 years postimplant demonstrated the strongest device benefit in tasks related to visual orientation.46 Here, we also observed that rated performance in visual orientation tasks with Device On improved from “difficult” on average to “moderate” by study-end, compared with “difficult” with Device Off.12 Mobility tasks were also observed to be “difficult” on average at the beginning of the study and were rated as “moderate” on average by study-end, but these ratings applied equally to both Device On and Device Off. Regular attempts and training on these tasks throughout the study, both with and without an orientation and mobility specialist, is likely to have contributed equally to Device On and Device Off improvements. Importantly, the suprachoroidal device does not obstruct incidental light from reaching the retina, so it was anticipated that both Device On and Device Off performance could include contributions from residual light perception. Also, the increased scene complexity in outdoor scenarios can make mobility with Device On substantially more challenging than for high-contrast indoor tasks.47 We are currently addressing these challenges with stereo-camera image processing, to preferentially highlight proximal objects and filter the remaining scene complexity.
Activities of daily living tasks were improved with the device, such as navigating the home, locating objects on tables, and sorting light from dark laundry.12 These benefits extended to social interactions (“interacting with others”), such as locating ones spouse in a café, making eye contact with students in a classroom, and detecting people moving at a train station—confirming feasibility of the device in local environments.12 Activities of daily living tasks were facilitated by the contrast information provided by the device, although the location and angle of light sources proved important. The benefit of conducting laundry sorting tasks against a black-colored cloth to increase contrast has been previously reported with the Argus II.48 Laundry sorting with the Alpha IMS/AMS has not been reported directly, but may also be possible since they report discrimination of up to 6 levels of grayscale.6,7
Self-reported vision-related quality of life was monitored using the validated IVI-VLV instrument.25 This differs from other instruments that are aimed at populations with mild to moderate vision loss, as patients eligible for retinal prostheses typically do not perform many of the activities queried by such instruments.46 Similar to reports in Argus II recipients assessed with the Vision and Quality of Life Index,49,50 the IVI-VLV data in the present study showed EWB was not impacted by device implantation or usage.
A limitation of the study is that only 4 patients were enrolled. It is customary to have limited enrollment for a novel surgical approach and this has been the case for prototype studies of epiretinal4 and subretinal5 devices also. These groups implanted more participants in subsequent trials.3 The strengths of the study include the intensive period of fitting and rehabilitation provided, a multidisciplinary team including assessments by an orientation and mobility specialist, regular follow up of outcome measures for >2 years, and that all participants remained involved in the study. Although device usage was impacted by COVID-19 restrictions on social interactions and travel outside local communities in Melbourne, Australia, participants reported that the device effectiveness did not decline over time (and this is corroborated by the sustained performance in lab-based tasks) but that some local environments were visually complex and difficult to interpret. Indeed, with feedback arising from the present study, all 4 participants are now actively evaluating further device improvements that will aid usage in their local environments (NCT05158049). In conclusion, this report has shown the 44-channel retinal prosthesis can be implanted in the suprachoroidal space without harm, with no SAEs recorded and 97% of electrodes still functional at study-end. The completed clinical study demonstrates that the prosthesis provides substantial improvements in functional vision, activities of daily living, and observer-rated quality of life, with similar functional outcomes to retinal prostheses that have reached the market. The excellent feasibility profile, device longevity outcomes, and functionality results make the suprachoroidal approach a feasible option to improve functional vision in end-stage RP.
Manuscript no. XOPS-D-23-00296.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors made the following disclosures:
C. J. A.: Support - National Health and Medical Research Council (NHMRC) of Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
P. J. A.: Support - NHMRC of Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Grants - Victorian Government, Australia (Operational Infrastructure Support funding); Patents - Centre for Eye Research Australia; Advisory Board - Bionic Vision Technologies Pty Ltd (Australia) (unpaid).
L. N. A.: Support - NHMRC of Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
E. K. B.: Support - NHMRC of Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
N. B.: Support - NHMRC of Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Patents - Australian National University (Australia).
L. K.: Support - NHMRC of Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
W. G. K.: Support - NHMRC, Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia).
M. K.: Support - NHMRC, Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
J. K.: Support - NHMRC, Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia), Estate of the late Dr Brian Entwisle; Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
C. D. L.: Support - NHMRC, Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
M. B. M.: Support - NHMRC, Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
D. A. X. N.: Support - NHMRC, Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia), Estate of the late Dr Brian Entwisle; Grants - Victorian Government, Australia (Operational Infrastructure Support funding); Patents - Bionics Institute.
M. A. P.: Support - NHMRC, Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia), Estate of the late Dr Brian Entwisle; Grants - Victorian Government, Australia (Operational Infrastructure Support funding); Patents - Bionics Institute.
S. A. T.: Support - NHMRC, Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia), Estate of the late Dr Brian Entwisle; Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
N. B.: Support - NHMRC, Australia (Grant 1082358), Bionic Vision Technologies Pty Ltd (Australia); Grants - Victorian Government, Australia (Operational Infrastructure Support funding).
The Bionics Institute and the Centre for Eye Research Australia wish to acknowledge the support of the Victorian Government through its Operational Infrastructure Support Program (VIC, Australia), and for generous support from the estate of the late Dr Brian Entwisle. The sponsor and/or funding organizations had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. Participants with bare light perception vision due to retinitis pigmentosa, aged 39 to 66 years (mean 54 years), were enrolled (Clinicaltrials.gov NCT03406416) after providing written informed consent. The study was conducted according to the tenets of the Declaration of Helsinki and was approved by the Human Research Ethics committee from the Royal Victorian Eye and Ear Hospital (16/1266H).
No animal subjects were included in this study.
Author Contributions:
Conception and design: Petoe, Abbott, Titchener, Kentler, Nayagam, Barnes, Walker, Ayton, Luu, Allen
Analysis and interpretation: Petoe, Abbott, Titchener, Kolic, Nayagam, Baglin, Barnes, Walker, Karapanos, McGuinness, Ayton, Luu, Allen
Data collection: Petoe, Titchener, Kolic, Kentler, Nayagam, Baglin, Kvansakul, Allen
Obtained funding: Petoe, Ayton, Luu, Allen
Overall responsibility: Petoe, Abbott, Titchener, Kolic, Kentler, Nayagam, Baglin, Kvansakul, Barnes, Walker, Karapanos, McGuinness, Ayton, Luu, Allen
Presented at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, May 1-7, 2021, San Francisco, USA.
See appendix A for the members of the Bionics Institute and Centre for Eye Research Australia Retinal Prosthesis Consortium.
Writing Group Members:
Matthew A. Petoe; Carla J. Abbott; Samuel A. Titchener; Maria Kolic; William G. Kentler; David A.X. Nayagam; Elizabeth K. Baglin; Jessica Kvansakul; Nick Barnes; Janine G. Walker; Lewis Karapanos; Myra B. McGuinness; Lauren N. Ayton; Chi D. Luu; Penelope J. Allen.
Study Group Members:
Peter J. Blamey; Robert J. Briggs; Owen Burns; Stephanie B. Epp; Dean Johnson; Hugh J. McDermott; Ceara McGowan; Rodney E. Millard; Peter M. Seligman; Robert K. Shepherd; Mohit N. Shivdasani; Nicholas C. Sinclair; Patrick C. Thien; Joel Villalobos; Chris E. Williams; Jonathan Yeoh; Kiera A. Young.
Contributor Information
Penelope J. Allen, Email: pjallen@melbourneretina.com.au.
Bionics Institute and Centre for Eye Research Australia Retinal Prosthesis Consortium:
Peter J. Blamey, Robert J. Briggs, Owen Burns, Stephanie B. Epp, Dean Johnson, Hugh J. McDermott, Ceara McGowan, Rodney E. Millard, Peter M. Seligman, Robert K. Shepherd, Mohit N. Shivdasani, Nicholas C. Sinclair, Patrick C. Thien, Joel Villalobos, Chris E. Williams, Jonathan Yeoh, and Kiera A. Young
Supplementary Data
References
- 1.Dias M.F., Joo K., Kemp J.A., et al. Molecular genetics and emerging therapies for retinitis pigmentosa: basic research and clinical perspectives. Prog Retin Eye Res. 2018;63:107–131. doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Ayton L.N., Barnes N., Dagnelie G., et al. An update on retinal prostheses. Clin Neurophysiol. 2020;131:1383–1398. doi: 10.1016/j.clinph.2019.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostad-Ahmadi Z., Daemi A., Modabberi M.R., et al. Safety, effectiveness, and cost-effectiveness of Argus II in patients with retinitis pigmentosa: a systematic review. Int J Ophthalmol-Chi. 2021;14:310–316. doi: 10.18240/ijo.2021.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanai D., Weiland J.D., Mahadevappa M., et al. Visual performance using a retinal prosthesis in three subjects with retinitis pigmentosa. Am J Ophthalmol. 2007;143:820–827. e822. doi: 10.1016/j.ajo.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Edwards T.L., Cottriall C.L., Xue K., et al. Assessment of the electronic retinal implant Alpha AMS in restoring vision to blind patients with end-stage retinitis pigmentosa. Ophthalmology. 2018;125:432–443. doi: 10.1016/j.ophtha.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stingl K., Bartz-Schmidt K.U., Besch D., et al. Subretinal visual implant Alpha IMS – clinical trial interim report. Vis Res. 2015;111:149–160. doi: 10.1016/j.visres.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Stingl K., Schippert R., Bartz-Schmidt K.U., et al. Interim results of a multicenter trial with the new electronic subretinal implant Alpha AMS in 15 patients blind from inherited retinal degenerations. Front Neurosci. 2017;11:445. doi: 10.3389/fnins.2017.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen P.J. Retinal prostheses: where to from here? Clin Exp Ophthalmol. 2021;49:418–429. doi: 10.1111/ceo.13950. [DOI] [PubMed] [Google Scholar]
- 9.Yanovitch L., Raz-Prag D., Hanein Y. A new high-resolution three-dimensional retinal implant: system design and preliminary human results. bioRxiv. 2022 doi: 10.1101/2022.09.14.507901. [DOI] [Google Scholar]
- 10.Ayton L.N., Blamey P.J., Guymer R.H., et al. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petoe M.A., Titchener S.A., Kolic M., et al. A second-generation (44-channel) suprachoroidal retinal prosthesis: interim clinical trial results. Transl Vis Sci Technol. 2021;10:12. doi: 10.1167/tvst.10.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karapanos L., Abbott C.J., Ayton L.N., et al. Functional vision in the real-world environment with a second-generation (44-channel) suprachoroidal retinal prosthesis. Transl Vis Sci Technol. 2021;10:7. doi: 10.1167/tvst.10.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayton L.N., Apollo N.V., Varsamidis M., et al. Assessing residual visual function in severe vision loss. Invest Ophthalmol Vis Sci. 2014;55:1332–1338. doi: 10.1167/iovs.13-12657. [DOI] [PubMed] [Google Scholar]
- 14.Dacey D.M., Petersen M.R. Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proc Natl Acad Sci USA. 1992;89:9666–9670. doi: 10.1073/pnas.89.20.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes N., Scott A.F., Lieby P., et al. Vision function testing for a suprachoroidal retinal prosthesis: effects of image filtering. J Neural Eng. 2016;13:15. doi: 10.1088/1741-2560/13/3/036013. [DOI] [PubMed] [Google Scholar]
- 16.Nayagam D.A., Thien P.C., Abbott C.J., et al. A pre-clinical model for safe retinal stimulation. Invest Ophthalmol Vis Sci. 2017;58:4204. [Google Scholar]
- 17.Saunders A.L., Williams C.E., Heriot W., et al. Development of a surgical procedure for implantation of a prototype suprachoroidal retinal prosthesis. Clin Exp Ophthalmol. 2014;42:665–674. doi: 10.1111/ceo.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivdasani M.N., Sinclair N.C., Dimitrov P.N., et al. Factors affecting perceptual thresholds in a suprachoroidal retinal prosthesis. Invest Ophthalmol Vis Sci. 2014;55:6467–6481. doi: 10.1167/iovs.14-14396. [DOI] [PubMed] [Google Scholar]
- 19.Ayton L.N., Rizzo J.F., III, Bailey I.L., et al. Harmonization of outcomes and vision endpoints in vision restoration trials: recommendations from the international HOVER taskforce. Transl Vis Sci Technol. 2020;9:25. doi: 10.1167/tvst.9.8.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahuja A.K., Behrend M.R. The Argus™ II retinal prosthesis: factors affecting patient selection for implantation. Prog Retin Eye Res. 2013;36:1–23. doi: 10.1016/j.preteyeres.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Petoe M.A., McCarthy C.D., Shivdasani M.N., et al. Determining the contribution of retinotopic discrimination to localization performance with a suprachoroidal retinal prosthesis. Invest Ophthalmol Vis Sci. 2017;58:3231–3239. doi: 10.1167/iovs.16-21041. [DOI] [PubMed] [Google Scholar]
- 22.Bach M., Wilke M., Wilhelm B., et al. Basic quantitative assessment of visual performance in patients with Very low vision. Invest Ophthalmol Vis Sci. 2010;51:1255–1260. doi: 10.1167/iovs.09-3512. [DOI] [PubMed] [Google Scholar]
- 23.Finger R.P., McSweeney S.C., Deverell L., et al. Developing an instrumental activities of daily living tool as part of the low vision assessment of daily activities protocol. Invest Ophthalmol Vis Sci. 2014;55:8458–8466. doi: 10.1167/iovs.14-14732. [DOI] [PubMed] [Google Scholar]
- 24.Geruschat D.R., Flax M., Tanna N., et al. FLORA™: phase I development of a functional vision assessment for prosthetic vision users. Clin Exp Optom. 2015;98:342–347. doi: 10.1111/cxo.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finger R.P., Tellis B., Crewe J., et al. Developing the impact of vision impairment-very low vision (IVI-VLV) questionnaire as part of the LoVADA protocol. Invest Ophthalmol Vis Sci. 2014;55:6150–6158. doi: 10.1167/iovs.14-14731. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbott C.J., Baglin E.K., Kolic M., et al. Interobserver agreement of electrode to retina distance measurements in a second-generation (44-channel) suprachoroidal retinal prosthesis. Transl Vis Sci Technol. 2022;11:4. doi: 10.1167/tvst.11.9.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wittkowski K.M. Friedman-type statistics and consistent multiple comparisons for unbalanced designs with missing data. J Am Stat Assoc. 1988;83:1163–1170. [Google Scholar]
- 29.Conover W.J. John Wiley & Sons; New York, NY: 1998. Practical nonparametric statistics. [Google Scholar]
- 30.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 31.Titchener S.A., Nayagam D.A.X., Kvansakul J., et al. A second-generation (44-channel) suprachoroidal retinal prosthesis: long-term observation of the electrode–tissue interface. Transl Vis Sci Technol. 2022;11:12. doi: 10.1167/tvst.11.6.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghodasra D.H., Chen A., Arevalo J.F., et al. Worldwide Argus II implantation: recommendations to optimize patient outcomes. BMC Ophthalmol. 2016;16:52. doi: 10.1186/s12886-016-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muqit M.M.K., Hubschman J.P., Picaud S., et al. PRIMA subretinal wireless photovoltaic microchip implantation in non-human primate and feline models. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaffrath K., Schellhase H., Walter P., et al. One-year safety and performance assessment of the Argus II retinal prosthesis: a postapproval study. JAMA Ophthalmol. 2019;137:896–902. doi: 10.1001/jamaophthalmol.2019.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujikado T., Kamei M., Sakaguchi H., et al. One-year outcome of 49-channel suprachoroidal–transretinal stimulation prosthesis in patients with advanced retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2016;57:6147–6157. doi: 10.1167/iovs.16-20367. [DOI] [PubMed] [Google Scholar]
- 36.Yue L., Weiland J.D., Roska B., et al. Retinal stimulation strategies to restore vision: fundamentals and systems. Prog Retin Eye Res. 2016;53:21–47. doi: 10.1016/j.preteyeres.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Jones B.W., Pfeiffer R.L., Ferrell W.D., et al. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016;150:149–165. doi: 10.1016/j.exer.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahuja A.K., Dorn J.D., Caspi A., et al. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br J Ophthalmol. 2011;95:539–543. doi: 10.1136/bjo.2010.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorn J.D., Ahuja A.K., Caspi A., et al. The detection of motion by blind subjects with the epiretinal 60-electrode (Argus II) retinal prosthesis. JAMA Ophthalmol. 2013;131:183–189. doi: 10.1001/2013.jamaophthalmol.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Titchener S.A., Kvansakul J., Shivdasani M.N., et al. Oculomotor responses to dynamic stimuli in a 44-channel suprachoroidal retinal prosthesis. Transl Vis Sci Technol. 2020;9:31. doi: 10.1167/tvst.9.13.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Titchener S.A., Goossens J., Kvansakul J., et al. Estimating phosphene locations using eye movements of suprachoroidal retinal prosthesis users. Transl Vis Sci Technol. 2023;12:20. doi: 10.1167/tvst.12.3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endo T., Kanda H., Hirota M., et al. False reaching movements in localization test and effect of auditory feedback in simulated ultra-low vision subjects and patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2016;254:947–956. doi: 10.1007/s00417-015-3253-2. [DOI] [PubMed] [Google Scholar]
- 43.Luo Y.H.-L., Zhong J., Merlini F., et al. The use of Argus® II retinal prosthesis to identify common objects in blind subjects with outer retinal dystrophies. Invest Ophthalmol Vis Sci. 2014;55:1834. [Google Scholar]
- 44.da Cruz L., Dorn J.D., Humayun M.S., et al. Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology. 2016;123:2248–2254. doi: 10.1016/j.ophtha.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia S., Petrini K., Rubin G.S., et al. Visual and non-visual navigation in blind patients with a retinal prosthesis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geruschat D.R., Richards T.P., Arditi A., et al. An analysis of observer-rated functional vision in patients implanted with the Argus II Retinal Prosthesis System at three years. Clin Exp Optom. 2016;99:227–232. doi: 10.1111/cxo.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kartha A., Sadeghi R., Barry M.P., et al. Prosthetic visual performance using a disparity-based distance-filtering system. Transl Vis Sci Technol. 2020;9:27. doi: 10.1167/tvst.9.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dagnelie G., Christopher P., Arditi A., et al. Performance of real-world functional vision tasks by blind subjects improves after implantation with the Argus® II retinal prosthesis system. Clin Exp Ophthalmol. 2017;45:152–159. doi: 10.1111/ceo.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misajon R., Hawthorne G., Richardson J., et al. Vision and quality of life: the development of a utility measure. Invest Ophthalmol Vis Sci. 2005;46:4007–4015. doi: 10.1167/iovs.04-1389. [DOI] [PubMed] [Google Scholar]
- 50.Duncan J.L., Richards T.P., Arditi A., et al. Improvements in vision-related quality of life in blind patients implanted with the Argus II Epiretinal Prosthesis. Clin Exp Optom. 2017;100:144–150. doi: 10.1111/cxo.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.