Abstract
Earlier studies have shown that the d120 mutant of herpes simplex virus 1, which lacks both copies of the α4 gene, induces caspase-3-dependent apoptosis in HEp-2 cells. Apoptosis was also induced by the α4 rescuant but was blocked by the complementation of rescuant with a DNA fragment encoding the US3 protein kinase (R. Leopardi and B. Roizman, Proc. Natl. Acad. Sci. USA 93:9583–9587, 1996, and R. Leopardi, C. Van Sant, and B. Roizman, Proc. Natl. Acad. Sci. USA 94:7891–7896, 1997). To investigate its role in the apoptotic cascade, the US3 open reading frame was cloned into a baculovirus (Bac-US3) under the control of the human cytomegalovirus immediate-early promoter. We report the following. (i) Bac-US3 blocks processing of procaspase-3 to active caspase. Procaspase-3 levels remained unaltered if superinfected with Bac-US3 at 3 h after d120 mutant infection, but significant amounts of procaspase-3 remained in cells superinfected with Bac-Us3 at 9 h postinfection with d120 mutant. (ii) The US3 protein kinase blocks the proapoptotic cascade upstream of mitochondrial involvement inasmuch as Bac-US3 blocks release of cytochrome c in cells infected with the d120 mutant. (iii) Concurrent infection of HEp-2 cells with Bac-US3 and the d120 mutant did not alter the pattern of accumulation or processing of ICP0, -22, or -27, and therefore US3 does not appear to block apoptosis by targeting these proteins.
In earlier reports, this laboratory showed that the herpes simplex virus 1 (HSV-1) mutant d120, lacking both copies of the α4 gene, induced classical apoptosis in infected cells (13). In subsequent reports, it was shown that rescuants of the missing α4 genes continued to induce apoptosis but that DNA fragments sharing the US3 gene blocked apoptosis (14). Other laboratories have since confirmed that US3 is required to block apoptosis, but the site of action of the US3 protein kinase remains obscure (2, 12). In the studies reported here, we show that in HEp-2 cells US3 blocks a step upstream of mitochondrial activation. In these experiments, we cloned the US3 gene in a baculovirus vector under the control of the immediate-early promoter of human cytomegalovirus (HCMV). This vector, designated Bac-US3, expressed the protein kinase in infected cells. In HEp-2 cells infected with the d120 mutant, cytochrome c is released from mitochondria, procaspase-3 is activated by cleavage, and caspase-3 accumulates in infected cells (10). In cells doubly infected with d120 mutant and Bac-US3, cytochrome c was not released and the remainder of the apoptotic cascade was not activated.
Relevant to this report are the following. (i) The d120 mutant virus is highly cytotoxic to the cells it infects. The pattern of infection is highly reminiscent of classical apoptosis and includes condensation of chromatin, fragmentation of cellular DNA, and extensive vacuolization of the cytoplasm (13). The proapoptotic cascade induced by d120 virus is cell type dependent (10). In human SK-N-SH cells derived from a malignant glioma, the programmed cell death induced by the d120 mutant was caspase-3 independent in that caspase inhibitors were ineffective and caspase-3 activity was not detected (10, 11). The same virus induced caspase-3-dependent apoptosis in HEp-2 cells. Apoptosis was blocked in a HEp-2 cell line (Vax-3) transformed to constitutively overexpress Bcl-2 (9).
(ii) Cells infected with the d120 mutant express predominantly α gene products (7). Of these, ICP0 is of particular interest, since the cytopathic effects of a mutant lacking ICP0 in addition to other α proteins have been reported to be grossly reduced (17). ICP0 is overproduced in d120 mutant virus-infected cells (9). The protein accumulates initially in nuclei, but early in infection it is translocated into vesicle-like structures in association with proteasomes (15, 19). The distribution of ICP0 in Vax-3 cells remains unaltered but the amounts are actually increased most likely because the cytopathic effects of the d120 virus are grossly diminished in Vax-3 cells compared to the untransformed HEp-2 cells (9). The data support the conclusion that Bcl-2 blocks apoptosis induced by d120 mutants and reduces the cytopathic effects of the virus. The mechanisms by which d120 induces apoptosis and the role of US3 in blocking apoptosis induced by this virus remain unclear.
MATERIALS AND METHODS
Cells and viruses.
HEp-2 cells were obtained from the American Type Culture Collection (Manassas, Va.). The cells were grown in Dulbecco's modified Eagle medium supplemented with 5% newborn calf serum. HSV-1(F) is the prototype HSV-1 strain used in the laboratory (8). The HSV-1(KOS) d120 mutant, a kind gift of N. DeLuca, lacks both copies of the α4 gene and was grown in a Vero-derived cell line (E5) expressing the α4 gene (7). The recombinant virus HSV-1(F) R7041 is described elsewhere (16). It lacks the PstI-BamHI fragment encoding amino acids 69 to 357 of the US3 gene (16).
Plasmids.
The entire US3 open reading frame (ORF) was amplified by PCR from pRB3446 and cloned into the BglII site of the baculovirus transfer vector MTS-1 (9). MTS-1 contains a CMV promoter inserted into the XhoI-EcoRI sites of pAcSG2 (Pharmingen, San Diego, Calif.).
Baculoviruses.
The construction of Bac-US5 and Bac-α22, recombinant baculoviruses expressing glycoprotein J and ICP22, respectively, was described elsewhere (16). Briefly, the US5 or ICP22 ORF was cloned into the MTS-1 transfer vector and cotransfected with baculogold DNA (Pharmingen) to create a recombinant baculovirus that expresses either US5 or ICP22 under the control of the constitutive CMV promoter. The US3-expressing baculovirus was created by cotransfecting the US3-MTS-1 transfer plasmid with baculogold DNA (Pharmingen) in accordance with the manufacturer's instructions. Efficient baculovirus gene expression in mammalian cells requires treatment with sodium butyrate, a histone deacetylase inhibitor (6). In all experiments in which HEp-2 cells were infected with baculoviruses, all infected or treated cultures were exposed to medium containing 10 mM sodium butyrate after 2 h of incubation at 37°C with approximately 15 PFU of the indicated recombinant baculoviruses per cell.
Immunoblot assays.
Cells were harvested by scraping cells into their medium, pelleted by low-speed centrifugation, rinsed twice with phosphate-buffered saline A (PBS A) (0.14 M NaCl, 3 mM KCl, 10 mM Na2HPO4, 1.5 mM KH2PO4), lysed in RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate in PBS A), and stored on wet ice for 10 min before centrifugation at 14,000 × g for 10 min. The protein concentration of the supernatant fluids was determined with the aid of the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) according to directions provided by the manufacturer. Protein samples (100 μg of protein per lane) were electrophoretically separated in a 12% denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, blocked, and reacted with a rabbit polyclonal antibody specific for either caspase-3 (1 μg/ml; Santa Cruz Biotechnologies, Santa Cruz, Calif.) or ICP22 (1:500) as previously described (1) or a monoclonal antibody to cytochrome c (1:500; Pharmingin), ICP0 (1:1,000; Goodwin Institute, Plantation, Fla.), or ICP27 (1:500; Goodwin Institute). Protein bands were visualized with either alkaline phosphatase or through enhanced chemiluminescent detection, according to the instructions of the manufacturer (Pierce, Rockford, Ill.).
Production of rabbit polyclonal antiserum against US3.
A SalI fragment of the US3 DNA sequence encoding amino acids 98 through 364 of US3 protein kinase was fused in frame to glutathione S-transferase (GST). The resulting fusion protein was expressed and purified from Escherichia coli BL21 as recommended by the manufacturer (Pharmacia). Two rabbits were inoculated at the Josman Laboratories (Napa, Calif.) with five subcutaneous injections of 1 mg of purified fusion protein at 14-day intervals. Sera were collected at 2-week intervals after the last injection. The serum was diluted 1:2,000 for all immunoblots.
Subcellular fractionation.
HEp-2 cells grown in 25-cm2-diameter flask cultures (4 × 106 cells) were either mock infected or infected with 10 PFU of HSV-1(F), R7041, or the d120 mutant virus per cell. Where indicated, the cells were infected with approximately 15 PFU of Bac-US3 or Bac-US5 recombinant baculovirus per cell. After 18 h of infection, the cells were collected and resuspended in 0.8 ml of ice-cold buffer A (20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 250 mM sucrose, and 0.1 mM phenylmethylsulfonyl fluoride). After 15 min on ice, the cells were homogenized in a Dounce homogenizer and then centrifuged for 10 min at 750 × g in order to remove unlysed cells and nuclei. The supernatant fluids were transferred to new tubes and centrifuged again at 10,000 × g for 20 min. The pellets were resuspended in buffer A and represent the mitochondrial fractions. The supernatants were then centrifuged for 30 min at 100,000 × g. The supernatant fluids represent the cytosolic fractions.
Measurement of DEVDase activity.
Cellular extracts were assayed for caspase-3 activity with a tetrapeptide conjugated to phenylnitraniline (DEVD-pNA). HEp-2 cells grown in 25-cm2-diameter flask cultures were either mock infected or infected with 10 PFU of HSV-1(F) or the d120 mutant and, where indicated, infected with Bac-US3 or Bac-US5 recombinant baculovirus as described above. At 18 h after infection, the cells were scraped in their medium, rinsed twice with PBS A, resuspended in 150 μl of lysis buffer {50 mM HEPES [pH 7.4], 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], 1 mM dithiothreitol, 0.1 mM EDTA}, and left on ice for 5 min. The lysates were then centrifuged at 10,000 × g for 10 min at 4°C, and the supernatant fluids were collected and tested for DEVDase activity as recommended by the manufacturer (BIOMOL). The released chromophore was measured in a spectrophotometer for absorbance at 405 nm after 2 h.
RESULTS
Characterization of the rabbit polyclonal antibody against US3 protein kinase and of the baculovirus expressing the US3 protein kinase.
To facilitate the study of the US3 protein kinase, an antibody was raised against the protein product of the US3 gene as described in Materials and Methods. As shown in Fig. 1, the antibody reacts with doublet bands with an apparent Mr of 68,000 and 69,000 in cells infected with the wild-type parent virus HSV-1(F) but not with mock-infected cells or with a mutant, R7041, from which the US3 gene had been deleted (Fig. 1).
FIG. 1.

Photograph of electrophoretically separated cell lysates reacted with polyclonal rabbit antibody to a GST-US3 fusion protein. Replicate 25-cm2 flask cultures of HEp-2 cells were mock infected or infected for 24 h at 37°C with 10 PFU of HSV-1(F) or R7041 per cell, harvested, solubilized, electrophoretically separated on denaturing gels, electrically transferred to a nitrocellulose sheet, and reacted with rabbit antibody to a GST-US3 fusion protein.
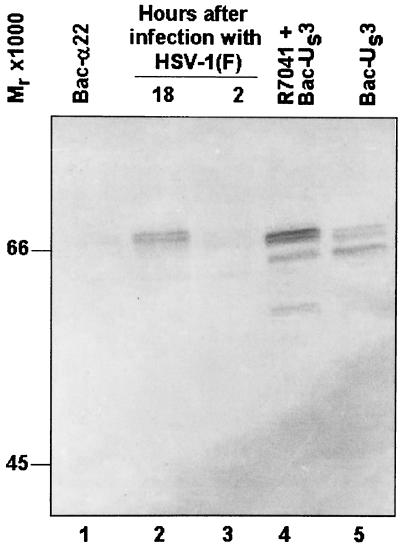
In the experiments described below it was critical to deliver to all HSV-infected cells, in a dose-dependent manner, a gene expressing the US3 protein kinase. We have chosen to deliver the gene by exposing cells to a recombinant baculovirus designated Bac-US3, which expresses the US3 ORF driven by the HCMV immediate-early promoter, as described in Materials and Methods. The results of an immunoblot assay for the expression of US3 protein is shown in Fig. 2. The results show that US3 protein expressed in cells infected with Bac-US3 alone or in cells doubly infected with R7041 and Bac-US3 could not be differentiated with respect to electrophoretic mobility from wild-type US3 protein expressed in cells infected with HSV-1(F). As expected, the US3 protein was not detected in mock-infected cells.
FIG. 2.
Photograph of electrophoretically separated cell lysates reacted with polyclonal rabbit antiserum to a GST-US3 fusion protein. Replicate 25-cm2 flask cultures of HEp-2 cells were mock infected or infected with 100 PFU of HSV-1(F) per cell for 2 h or 10 PFU of HSV-1(F) or R7041 per cell and, where indicated, coincubated with Bac-α22 or Bac-US3 for 18 h. Cultures were harvested, solubilized, electrophoretically separated on denaturing gels, transferred to a nitrocellulose sheet, and reacted with rabbit antiserum to a GST-US3 fusion protein.
US3 protein kinase blocks the processing of procaspase-3 induced by d120 mutant virus in HEp-2 cells.
Earlier studies from this laboratory have shown that d120 mutant-induced apoptosis in HEp-2 cells is caspase-3 dependent (10). Caspase-3, a downstream effector caspase, is translated as an inactive zymogen, procaspase-3, that has an apparent Mr of 32,000 (18). Upon upstream caspase activation, procaspase-3 is activated by proteolytic cleavage. Activation of caspase-3 correlates with the disappearance of the Mr 32,000 form (18).
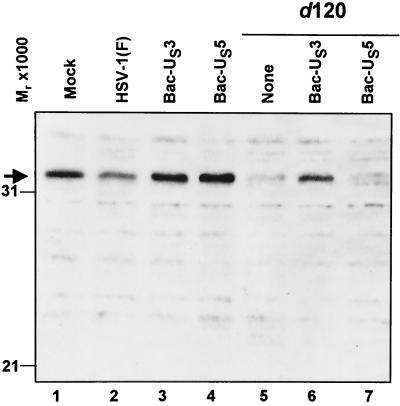
To address the question of whether US3 expression is sufficient to prevent d120 mutant-induced caspase-3 processing, HEp-2 cells were either mock infected or infected with HSV-1(F) or d120, with simultaneous infection with Bac-US3 or Bac-US5. The cells were harvested 18 h after infection, solubilized, electrophoretically separated in denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with antibody to caspase-3. In this instance, Bac-US5 served a dual function. This recombinant baculovirus blocks the induction of apoptosis by gD-minus mutants (20), but in preliminary experiments it had no effect on apoptosis induced by the d120 mutant virus. In these experiments, Bac-US5 was used as a negative control. The antibody reacts with procaspase-3 only. The results follow (Fig. 3). (i) Procaspase-3 levels in cells singly infected with Bac-US3 or Bac-US5 could not be differentiated from those in mock-infected cells (lanes 1, 3, and 4). (ii) Procaspase-3 levels in HSV-1(F)-infected cells were slightly reduced relative to those of mock-infected cells (lane 2), whereas the levels of procaspase-3 in cells infected with the d120 mutant were barely detectable (lane 5). (iii) The levels of procaspase-3 in cells doubly infected with d120 mutant and Bac-US3 were similar to those detected in HSV-1(F)-infected cells (lane 6), whereas procaspase-3 could not be detected in cells doubly infected with d120 mutant and Bac-US5 (lane 7). We conclude that the US3 protein kinase delivered in trans blocked the cleavage of procaspase-3.
FIG. 3.
Photograph of electrophoretically separated cell lysates reacted with polyclonal rabbit antibody to caspase-3. Replicate 25-cm2 flask cultures of HEp-2 cells were mock infected or infected with HSV-1(F) or d120 mutant virus and, where indicated, coinfected with Bac-US3 or Bac-US5 recombinant baculovirus. The cell cultures were harvested 18 h after infection, solubilized, electrophoretically separated on denaturing gels, electrically transferred to a nitrocellulose sheet, and reacted with rabbit antiserum to caspase-3.
US3 protein kinase blocks DEVDase activity of induced by d120 mutant virus infection in HEp-2 cells.
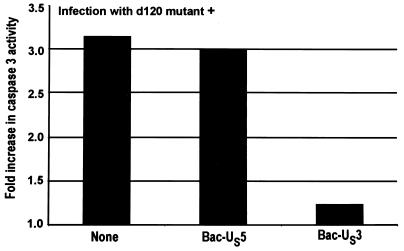
The results of the experiment described above indicated that the d120 mutant activates the processing of procaspase-3 and that this process is blocked by the US3 protein kinase. To verify that in cells infected with the d120 mutant caspase-3 is activated and that US3 blocks this activation, extracts from HEp-2 cells harvested 18 h after infection with the d120 mutant only or double infection with the d120 mutant and either Bac-US3 or Bac-US5 were tested for DEVDase activity. The results, normalized with respect to the level of caspase activity in mock-infected cells, are shown in Fig. 4 and were as follows. Consistent with the results described above, cells infected with d120 mutant alone or doubly infected with d120 mutant and Bac-US5 exhibited a greater than threefold increase in caspase-3 activity relative to mock-infected cells. In cells doubly infected with d120 mutant and Bac-US3, the DEVDase activity was only slightly (1.25-fold) higher than that of mock-infected cells. These results indicate that the US3, but not the US5, protein blocked the activation of caspase-3 in HEp-2 cells infected with the d120 mutant virus.
FIG. 4.
Effect of HSV-1 infection on DEVDase activity of infected cells. Replicate 25-cm2 flask cultures of HEp-2 cells were either mock infected or infected with d120 and, where indicated, coinfected with Bac-US3 or Bac-US5 recombinant baculovirus. Cultures were harvested at 18 h after infection and assayed for DEVDase activity colorimetrically at 405 nm as described in Materials and Methods. The results are expressed as fold increase in activity over that of mock-infected cells.
US3 blocks activation of procaspase-3 at least in part as late as 9 h after infection with d120 mutant.
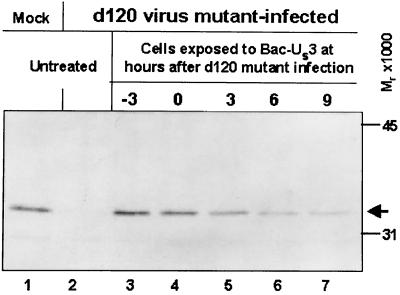
The question raised in these studies is whether US3 must be administered at the time of or prior to d120 mutant infection or whether the US3 protein can block activation of procaspase-3 when expressed several hours after d120 infection. To answer this question, HEp-2 cells were mock infected or infected with the d120 mutant. At various times after d120 mutant infection, the HEp-2 cells were exposed to Bac-US3. The cells were harvested at 18 h after infection with the d120 mutant virus, solubilized, electrophoretically separated in denaturing polyacrylamide gels, transferred to a nitrocellulose sheet, and reacted with the anti-procaspase-3 antibody. The results (Fig. 5) follow. Procaspase-3 was not detected in HEp-2 cells infected with d120 mutant virus only (lane 2). The levels of procaspase-3 in cells infected with d120 and exposed to Bac-US3 3 h before infection or at the time of infection could not be differentiated from those of mock-infected cells. Exposure of HEp-2 cells to Bac-US3 at 3, 6, or 9 h after infection resulted in gradual diminution of procaspase levels. However, procaspase-3 was readily detected in cells exposed to Bac-US3 as late as 9 h after d120 infection.
FIG. 5.
Photograph of electrophoretically separated cell lysates reacted with polyclonal antibody to caspase-3. Replicate 25-cm2 flask cultures of HEp-2 cells were either mock infected or infected with d120 and, where indicated, coinfected with Bac-US3 at the indicated times relative to d120 infection. Cultures were harvested 18 h after infection, solubilized, electrophoretically separated on denaturing gels, electrically transferred to a nitrocellulose sheet, and reacted with rabbit antiserum to caspase-3.
Us3 prevents d120-induced apoptosis upstream of the mitochondrial activation.
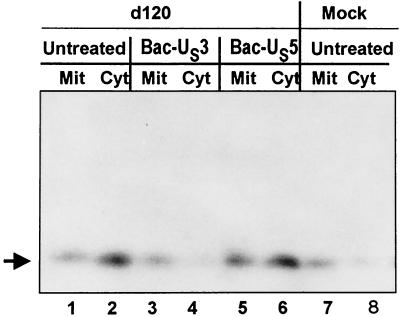
Earlier studies from this laboratory have shown that the d120 mutant induced the release of cytochrome c from mitochondria in all of the cell lines tested (13). Extensive studies have shown that the release of cytochrome c precedes the activation of the cascade that leads to the activation of caspase-9 and, subsequently, activation of caspase-3 (16). Since US3 blocked d120 mutant virus-induced activation of caspase-3, it was of interest to determine if the US3 protein kinase acts upstream or downstream of cytochrome c release from mitochondria. To resolve this question, HEp-2 cells were either mock or d120 infected in the presence or absence of Bac-US3 or Bac-US5, harvested at 18 h after infection, fractionated, and analyzed to determine the subcellular localization of cytochrome c as described in Materials and Methods. As shown in Fig. 6, cytochrome c from mock-infected cells cofractionated primarily with the mitochondrial fraction, whereas cytochrome c from the d120 mutant infection cofractionated primarily with the cytosolic fraction (lanes 1, 2, 7, and 8). Bac-US5 had no effect on the displacement of cytochrome c from mitochondria to cytosol (lanes 5 and 6), whereas Bac-US3 blocked the release of cytochrome c from mitochondria into the cytosolic fraction (lanes 3 and 4). These results indicate that the US3 protein kinase acts upstream of the cytochrome c release and of the activation of the proapoptotic cascade that follows its release.
FIG. 6.
Immunoblot showing cytochrome c distribution in HEp-2 cells. HEp-2 cells were either mock infected or infected with the viruses, as indicated, and harvested 18 h after infection. The fractionation procedure was as described in Materials and Methods. Mit, mitochondrial fraction; Cyt, cytosolic fraction.
Expression of US3 protein kinase does not affect the accumulation of ICP0, ICP22, or ICP27 in d120 mutant virus-infected cells.
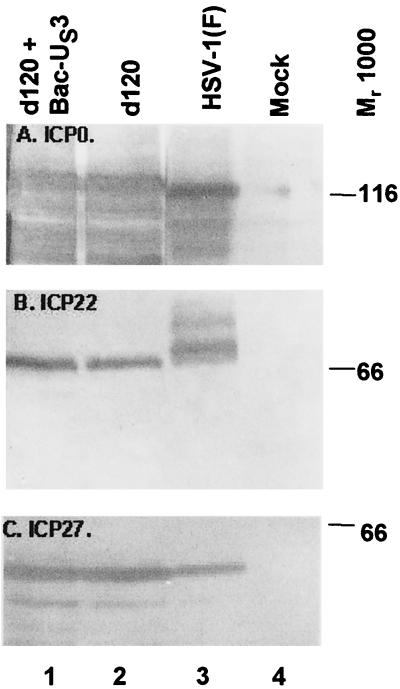
Cells infected with the d120 mutant express primarily α proteins, and at least in the cell lines tested, the level of accumulation of these proteins is higher than that observed in cells productively infected with wild-type virus (9). Among the various mechanisms by which d120 mutant could induce apoptosis is the overexpression of α proteins; conversely, the US3 protein kinase could block apoptosis by precluding excessive accumulation of these proteins. To determine the effect of US3 protein kinase on the accumulation of α proteins, HEp-2 cells were either mock infected or infected with HSV-1(F) or with d120 mutant virus alone or in the presence of Bac-US3. The cells were harvested at 18 h after infection and processed as described in Materials and Methods. The electrophoretically separated denatured lysates of the infected cells were reacted with monoclonal antibody to ICP0 (Fig. 7A), ICP27 (panel C), or ICP22 (panel B). The results follow. HEp-2 cells infected with the d120 mutant accumulated only slightly more ICP0 than wild-type virus-infected cells. At this time after infection, however, a large fraction of ICP0 was degraded and formed numerous faster migrating forms. The key observation is that there was no significant difference in the accumulation of ICP0 in cells infected with ICP0 alone or in doubly infected cells (Fig. 7A, compare lanes 1 and 2). ICP22 is processed primarily by the UL13 protein kinase (14). In d120-infected cells, ICP22 formed a single band that migrated faster than the fastest migrating band of ICP22 in lysates of wild-type virus-infected cells (Fig. 7B, compare lanes 2 and 3). Again, the US3 protein kinase had no effect on the accumulation or processing of ICP22. Finally, although ICP27 accumulated in larger amounts in d120 mutant virus-infected cells, the presence of US3 protein kinase had no effect on the accumulation of ICP27 (Fig. 7C, compare lanes 1 and 2).
FIG. 7.
Photograph of electrophoretically separated cell lysates reacted with serum to HSV-1(F) α proteins. Replicate 25-cm2 flask cultures of HEp-2 cells were mock infected or infected with 10 PFU of HSV-1(F) or the d120 mutant per cell and, where indicated, coinfected with Bac-US3. The cells were harvested at 18 h after infection, solubilized, electrophoretically separated on denaturing gels, transferred to a nitrocellulose sheet, and reacted with antibodies specific for ICP0 (A), ICP22 (B), or ICP27 (C).
We conclude that US3 protein kinase had no effect on accumulation of the α proteins tested in this study.
Characterization of Us3 protein kinase expression kinetics.
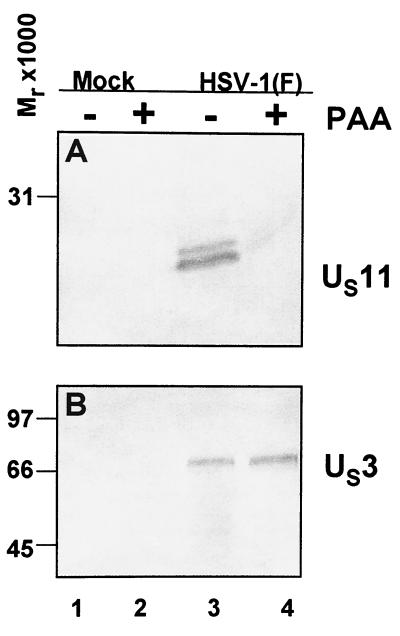
Since Bac-Us3 administered as late as 9 h to d120-infected cultures still showed a reduction of caspase-3 processing, it became of interest to determine the kinetics of Bac-Us3 expression during infection with HSV-1(F). To address this issue, HEp2 cells were mock infected or HSV-1(F) infected in the presence or absence of phosphonoacetic acid (PAA), which prevents expression of γ2 genes. As shown in Fig. 8, the γ2 protein US11 was expressed in untreated HSV-1(F)-infected cells but not in cells exposed to 300 μg of PAA per ml of medium during and after infection. In contrast, the US3 protein kinase was efficiently and equally expressed in untreated as well as PAA-treated cells (Fig. 8, lanes 3 and 4). These results indicate that the US3 protein kinase meets the criteria for inclusion in the β group of HSV-1 proteins.
FIG. 8.
Photograph of electrophoretically separated cell lysates reacted with serum to US11 or GST-US3. Replicate 25-cm2 flask cultures of HEp-2 cells were mock infected or infected with 10 PFU of HSV-1(F) per cell in the presence or absence of 300 μg of PAA per ml of medium. The cells were harvested 18 h after infection, solubilized, electrophoretically separated on a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with antibodies specific for US11 (A) or US3 (B).
DISCUSSION
In the studies presented here we examined the role of the HSV-1 US3 protein kinase in blocking apoptosis induced by the d120 mutant. We have selected for these studies HEp-2 cells in which the proapoptotic cascade leading to morphologic changes of classical apoptosis and the fragmentation of viral DNA was clearly delineated. The key features of the data follow.
(i) As reported earlier (11) for infected HEp-2 cells, d120 mutant virus activated the proapoptotic cascade initiated by the release of cytochrome c. This led to the cleavage activation of procaspase-3 and to accumulation of active caspase-3 in the infected cells. US3 but not US5, delivered in trans, blocked the release of cytochrome c, cleavage activation of procaspase-3, and accumulation of active caspase. The results indicate that in HEp-2 cells, US3 blocks a step leading to the activation of proapoptotic changes in mitochondria. The target on which it acts is unknown. The same evidence argues that the d120 mutant activates a mitochondrial stress response that leads to programmed cell death. The observation that Bcl-2 blocks apoptosis (9) is consistent with this conclusion.
(ii) The results presented in this report indicate that US3 ORF either blocks a mitochondrial stress response that leads to apoptosis or acts on an as-yet-unidentified viral gene product capable of triggering a mitochondrial stress response. The HSV-1 recombinant virus from which the US3 ORF had been deleted does not, however, induce apoptosis (12; J. Munger and V. Roizman, unpublished studies). The implications of this observation are that the US3 protein kinase acts to prevent apoptosis and that its absence does not per se induce programmed cell death. To induce programmed cell death there must occur a specific stimulus induced by an HSV gene product expressed in d120 mutant virus. Alternatively, HSV-1 encodes both an inducer and another blocker, and it is the latter that is damaged and not expressed in either d120 mutant or in the virus in which the α4 gene had been rescued.
(iii) To date, a relatively large number of mutants have been shown to induce apoptosis. These include mutants lacking the genes encoding gD and gJ (12), mutants lacking α4 or α27 genes, and a mutant carrying a temperature-sensitive lesion in the UL36 gene (2–4, 9–11, 14, 16). In cells infected with the latter mutant, apoptosis is induced only at the nonpermissive temperature (11). With the exception of virus stocks of a gD− mutant grown in cells expressing the gD gene (gD−/+ virus stocks), apoptosis induced by these mutants can be specifically related to the failure of the mutant viruses to express β or γ genes normally expressed later in infection and which would be expected to block apoptosis induced by the initial interaction of the virus with the infected cell. There is no specificity to either the absence of ICP4 or ICP27, since the Δα4 mutant expresses ICP27 and the Δα27 mutant expresses ICP4. The notable exception is gD−/+ virus stock. In cells infected with this stock, all genes except the missing gD or gJ are expressed and yet the cells undergo apoptosis, suggesting that US3 protein kinase cannot alone protect the infected cell from undergoing programmed cell death. Indeed, either gJ or gD delivered in trans by baculovirus vectors blocked the apoptosis induced by gD−/+ stocks (12). The conclusion of these studies is that US3 protein kinase may be necessary but is not sufficient to block apoptosis induced by all injuries to the infected cells caused by viral mutants.
(iv) The US3 protein kinase is packaged in the virion (J. Munger, M. T. Sciotrino, and B. Roizman, unpublished data). In the experiments described in this report, US3 protein synthesis was not blocked by PAA and therefore meets the definition of a β protein. Consistent with this kinetic class, US3 ORF would be expected to to be expressed between 4 and 6 h after infection. The data presented in this report indicate that the levels of procaspase-3 were undiminished relative to those of wild-type virus controls in cells in which Bac-US3 infection was delayed by 3 h. More provocative is the evidence that significant levels of procaspase-3 were detected in cells infected with Bac-US3 9 h after infection with the d120 mutant. Since the expression of genes carried by baculovirus vectors takes several hours, the results suggest that the evolution of d120-induced apoptosis is a relatively slow event and that US3 protein kinase expressed late in infection can still block apoptosis even at the mitochondrial level.
HSV has evolved a large number of genes that block host responses to infection. Included in this list are a number of viral functions designed to block the numerous pathways that lead to programmed cell death—a key host response to an invading microorganism. The data presented in this report strengthen the evidence that the US3 protein kinase has a specific role in thwarting host responses to infection by blocking apoptosis at a premitochondrial level. Studies now in progress are designed to identify the target of the US3 protein kinase.
ACKNOWLEDGMENTS
These studies were aided by Public Health Service grants from the National Cancer Institute (CA87761, CA83939, CA71933, and CA78766).
REFERENCES
- 1.Ackermann M, Sarmiento M, Roizman B. Application of antibody to synthetic peptides for the characterization of the intact and truncated α22 protein specified by herpes simplex virus 1 and the R325 α22− deletion mutant. J Virol. 1985;56:207–215. doi: 10.1128/jvi.56.1.207-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano S, Honda T, Goshima F, Watanabe D, Miyake Y, Sugiura Y, Nishiyama Y. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J Gen Virol. 1999;80:51–56. doi: 10.1099/0022-1317-80-1-51. [DOI] [PubMed] [Google Scholar]
- 3.Auber M, Blaho J A. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J Virol. 1999;73:2803–2813. doi: 10.1128/jvi.73.4.2803-2813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubert M, O'Toole J, Blaho J A. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J Virol. 1999;73:10359–10370. doi: 10.1128/jvi.73.12.10359-10370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batterson W, Furlong D, Roizman B. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983;45:397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Condreay J P, Witherspoon S M, Clay W C, Kost T A. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLuca N A, McCarth A, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 9.Galvan V, Brandimarti R, Munger J, Roizman B. Bcl-2 blocks a caspase-dependent pathway of apoptosis activated by herpes simplex virus 1 infection in HEp-2 cells. J Virol. 2000;74:1931–1938. doi: 10.1128/jvi.74.4.1931-1938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galvan V, Brandimarti R, Roizman B. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J Virol. 1999;73:3219–3226. doi: 10.1128/jvi.73.4.3219-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerome K R, Fox R, Chen Z, Sears A E, Lee H-Y, Corey L. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J Virol. 1999;73:8950–8957. doi: 10.1128/jvi.73.11.8950-8957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leopardi R, Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez P, Van Sant C, Roizman B. The requirements for the nuclear-cytoplasmic translocation of infected-cell protein 0 of herpes simplex virus 1. J Virol. 2001;75:3832–3840. doi: 10.1128/JVI.75.8.3832-3840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purves F C, Longnecker R M, Leader D P, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samaniego L A, Wu N, DeLuca N A. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 19.Van Sant C, Lopez P, Advani S J, Roizman B. Role of cyclin D3 in the biology of herpes simplex virus 1 ICP0. J Virol. 2001;75:1888–1898. doi: 10.1128/JVI.75.4.1888-1898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou G, Galvan V, Campadelli-Fiume G, Roizman B. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J Virol. 2000;74:11782–11791. doi: 10.1128/jvi.74.24.11782-11791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]