Abstract
Purpose
To investigate subconjunctival administration of a single-stranded, adeno-associated virus, serotype 2, engineered to express stanniocalcin-1 with a FLAG tag (ssAAV2-STC-1-FLAG) as a novel sustained (IOP) lowering agent with a reduced ocular surface side effect profile.
Design
In vivo preclinical investigation in mice.
Subjects
C57BL/6J, DBA/2J, prostaglandin F (FP) receptor knockout mice.
Methods
Normotensive C57BL/6J mice were treated with a subconjunctival injection of ssAAV2-STC-1-FLAG (2 μL; 6 × 109 viral genomes [VGs]) in 1 eye and the same volume and concentration of ssAAV2-green fluorescent protein (GFP) or the same volume of phosphate-buffered saline in the fellow eye. Ocular hypertensive DBA/2J mice were subconjunctivally injected with 6 × 109 VGs of ssAAV2-STC-1-FLAG or ssAAV2-GFP. Steroid-mediated ocular hypertension was induced in C57BL/6J mice with weekly injections of dexamethasone into the conjunctival fornix, and mice were then injected subconjunctivally with 6 × 109 VGs of ssAAV2-STC-1-FLAG or ssAAV2-GFP. Prostaglandin F receptor knockout mice were injected subconjunctivally with 6 × 109 VGs of ssAAV2-STC-1-FLAG or phosphate-buffered saline. An identical vector was constructed without the FLAG tag (ssAAV2-STC-1) and evaluated in normotensive C57BL/6J mice. Intraocular pressure was assessed using the Tonolab tonometer for all experiments. Tumor necrosis factor alpha (TNFα), a marker of ocular surface inflammation, was compared between subconjunctivally delivered ssAAV2-STC-1-FLAG and other treatments including daily topical latanoprost.
Main Outcome Measures
Intraocular pressure assessment.
Results
Subconjunctival delivery of ssAAV2-STC-1-FLAG significantly reduced IOP for 10 weeks post injection in normotensive mice. Maximal IOP reduction was seen at week 3 postinjection (17.4%; 17.1 ± 0.8 vs. 14.1 ± 0.8 mmHg, P < 0.001). After the IOP-lowering effect had waned, a second injection restored the ocular hypotensive effect. Subconjunctivally delivered ssAAV2-STC-1-FLAG lowered IOP in DBA/2J mice (16.9%; 17.8 ± 2.0 vs. 14.8 ± 0.9 mmHg, P < 0.001) and steroid-mediated ocular hypertensive mice (20.0%; 19.0 ± 0.6 vs. 15.2 ± 0.7 mmHg, P < 0.001) over the experimental period. This construct also reduced IOP to a similar extent in wild-type (15.9%) and FP receptor knockout (15.7%) mice compared with the fellow eye. A related construct also lowered IOP without the FLAG tag in a similar manner. Reduction in conjunctival TNFα was seen when comparing subconjunctivally delivered ssAAV2-STC-1-FLAG to daily topical latanoprost.
Conclusions
Subconjunctival delivery of the STC-1 transgene with a vector system may represent a novel treatment strategy for sustained IOP reduction and improved ocular tolerability that also avoids the daily dosing requirements of currently available medications.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Glaucoma, Ocular hypertension, Stanniocalcin, Sustained intraocular pressure reduction
Glaucoma remains the leading cause of irreversible blindness worldwide.1 Though defined as an optic neuropathy, given the pressure sensitive nature of retinal ganglion cell loss, the only reliable treatment that reduces the onset or progression of glaucoma is intraocular pressure (IOP) reduction. Intraocular pressure reduction is achieved with medical therapy using topical eye drops or with procedural intervention. The therapeutic effects of medications are limited significantly because of poor patient compliance as less than half of patients use glaucoma eye drops as prescribed.2, 3, 4, 5, 6 Patients do not use the drops as recommended because of the burden of current dosing regimens coupled with side effects including ocular surface disease which occurs in over half of patients taking topical glaucoma medications.7,8
In order to provide patients with adequate levels of daily drug for IOP reduction, identification of agents or delivery systems that provide sustained release of medication are being investigated.9 We recently described a scaffold-free approach that resulted in sustained IOP reduction using stanniocalcin-1 (STC-1).10 Stanniocalcin-1 is a 50 kDa disulfide-linked dimer that functions in a hormonal fashion as a secreted protein.11 Stanniocalcin-1 is a stress-response protein that has low basal levels of expression but is upregulated by a variety of stresses including inflammation,12 oxidation,13 and hypoxia.14, 15, 16 Stanniocalcin-1 is neuroprotective in cerebral neurons16,17 and photoreceptors,18,19 likely by mechanisms of reducing inflammation20, 21, 22, 23 and oxidative stress.23, 24, 25, 26, 27, 28
Our laboratory identified STC-1 as a protein whose expression is induced by prostaglandin F2α (PGF2α) analogs, whose presence is required for the IOP-lowering properties of PGF2α analogs, and that is equivalent to PGF2α analogs for IOP reduction as a stand-alone drug. Furthermore, its mechanism of action is independent of the cellular receptor for PGF2α analogs, the prostaglandin F (FP) receptor.29,30 More recently, based upon its unique properties of being a secreted, naturally occurring protein, we demonstrated that sustained IOP reduction could be obtained for up to 6 months after a single injection into the anterior chamber of mice with a single-stranded, serotype 2 adeno-associated virus containing the transgene for STC-1 fused to a FLAG tag (ssAAV2-STC-1-FLAG).10 In order to determine whether a less invasive approach with periocular delivery could be developed, we sought to evaluate whether subconjunctival injection of ssAAV2-STC-1-FLAG would provide sustained IOP reduction in normotensive and ocular hypertensive mice.
Methods
Adeno-Associated Viral Vector Generation
Single-stranded-AAV2 was used to express ssAAV2-STC-1-FLAG as previously described.10 Vector constructs to express STC-1-FLAG using ssAAV2 were purchased from University of Florida and University of Iowa. An identical vector was generated for a control, which expressed green fluorescent protein (GFP) (ssAAV2-GFP). Additionally, to ensure the IOP lowering effects of the constructs were not due to the FLAG tag, we generated a similar construct using the same methodology but without the FLAG tag (ssAAV2-STC-1).
Measurement of IOP
Intraocular pressure was measured with a handheld rebound tonometer (Icare TonoLab; Colonial Medical Supply) as previously described.31,32 Briefly, conscious mice were restrained in a modified decapicone (Braintree Scientific, Inc) with the tonometer probe placed perpendicular to the corneal surface. For each recorded measurement, the probe contacts the cornea and rebounds 6 times and calculates IOP by using an algorithm based on probe incident velocity and deceleration. Baseline IOPs consisted of 2 to 4 days of daily measurements for all experiments. These values were averaged and reported as a single value. Subsequent experimental time points with IOP measured twice weekly were then averaged and presented as weekly values for summary graphs. All IOP measurements were obtained late-morning for consistency to minimize diurnal fluctuation. To ensure validity of data, a second laboratory member who was masked to experimental groups confirmed IOP reduction.
Subconjunctival Injection of AAV2 Vectors
All mouse studies received prior approval by the Mayo Clinic Institutional Animal Care, and Use Committee, followed the Association for Research in Vision and Ophthalmology guidelines, and adhered to the Declaration of Helsinki. Both male and female mice were used in our studies. All mice had full access to food and water ad libitum with 12-hour light and dark cycles. Light was provided from 7 am to 7 pm. After intraperitoneal anesthesia with a cocktail containing ketamine (80 mg/kg), xylazine (6 mg/kg), and acepromazine (1 mg/kg), mice were placed on the stage of a dissecting microscope. A 32-gauge needle (Hamilton Company) containing 2 μL of the AAV2 vector or phosphate-buffered saline (PBS) was inserted posterior to the limbus under the conjunctival tissue in the superotemporal quadrant. Once in the subconjunctival space, the injection volume was ejected to produce a subconjunctival bleb. The needle was slowly removed to minimize tissue damage and reflux. Following completion of experiment, mice were euthanized using carbon dioxide asphyxiation followed by cervical dislocation.
Animal Models
For studies in normotensive mice, C57BL/6J mice were injected at age 3 months with 6 × 109 viral genomes [VGs]) of ssAAV2-STC-1-FLAG in 1 eye and the same volume and titer of ssAAV2-GFP or the same volume of PBS in the fellow eye as a control. For ocular hypertensive studies, DBA/2J and steroid-induced ocular hypertensive mice were used.33 DBA2/J mice were injected at 5 months of age, prior to their reported increase in IOP, with 6 × 109 VGs of ssAAV2-STC-1-FLAG in 1 eye and the same volume and titer of ssAAV2-GFP in the fellow eye. For steroid-induced ocular hypertension, the increase in IOP was induced as previously described.33,34 Briefly, after anesthesia, a dexamethasone acetate suspension (200 μg in 20 μl) was injected weekly into the inferior conjunctival fornix of 1 eye in a slow-release formulation (sodium chloride [0.667 g/100 mL], edetate disodium United States Pharmacopeia dehydrate [0.05 g/100 mL], sodium bisulfate [0.1 g/100 mL], and creatinine [0/5 g/100 mL], pH 7).33,34 The fellow eye received a weekly injection with the slow-release formulation (vehicle) without the dexamethasone. Steroid-induced ocular hypertensive mice were injected with 6 × 109 viral VGs of ssAAV2-STC-1-FLAG or the same volume and titer of ssAAV2-GFP in a separate cohort after 6 weeks of weekly steroid injections since the ocular hypertension was induced in 1 eye. For studies in FP receptor knockout mice, mice developed in our laboratory as previously described were used.29 Prostaglandin F receptor knockout mice or wild-type littermate control mice were injected in a subconjunctival manner at age 3 months with 6 × 109 VGs of ssAAV2-STC-1-FLAG in 1 eye and the same volume of PBS in the fellow eye.
Assessment of Ocular Anatomy and Transgene Expression after ssAAV2-STC-1-FLAG Treatment
Normotensive C57BL/6J mice were treated with subconjunctival injections with ssAAV2-STC-1-FLAG (6 × 109 VGs) in 1 eye and same volume of PBS in the fellow eye. One week after injection, animals were euthanized, and eyes were enucleated and fixed in 10% formalin. Tissue was processed in paraffin, sectioned at 5 microns, and placed on Superfrost Plus slides. Sections were deparaffinized in xylene, and rehydrated in a series of descending alcohol concentrations followed by a rinse in PBS. Tissue sections were hematoxylin and eosin stained. Sections were dehydrated through a series of alcohol and xylene incubations, and cover slipped with a xylene based mounting medium (Toluene Solution, Permount, Thermo Fisher Scientific Inc). Images were captured using a Nikon Eclipse Ci microscope (Nikon).
For expression studies, conjunctiva was dissected using an Olympus SZX16 surgical microscope. Conjunctiva from 3 eyes of the same treatment group were pooled (e.g., = 4 represents 12 eyes total with 3 pooled eyes per sample). Total RNA was extracted using PicoPure RNA Isolation Kit (Thermo Fisher Scientific) and quantified using a DeNovix DS-11 spectrophotometer (DeNovix). cDNA was synthesized by reverse transcription using iScript cDNA Synthesis Kit (Bio-Rad). Real-time polymerase chain reaction was performed on a Roche Light Cycler 480 (Roche) using SYBR Green Universal Master Mix, and TaqMan Universal polymerase chain reaction Master Mix (Thermo Fisher Scientific). TaqMan Gene Expression Assay probes (Applied Biosystems) were used to measure gene expression (Mm03928990 g1 Rn18s and a custom-designed, STC-1-FLAG primer set [Forward: 5ʹ-cttcaacaggagacgcaccaatg-3ʹ; Reverse: 5ʹ-cttgtcatcgtcgtccttgtagtcg-3ʹ]). Delta cycle threshold was presented as the difference between 18s RNA and STC-1-FLAG expression.
Evaluation of Ocular Surface Inflammation Comparing Topical and Subconjunctival Therapies
Twenty-five C57BL/6J wild-type mice (3–4 months old) were randomized into 5 groups (n = 5 per group). Group 1 was treated with subconjunctival injections of ssAAV2-STC-1-FLAG (6 × 109 VGs). Group 2 was treated with subconjunctival injections of ssAAV2-GFP (6 × 109 VGs). Mice in the subconjunctival treatment groups were treated with a single injection 28 days prior to tissue collection. Fellow eyes served as untreated controls. The remaining groups were treated topically, once daily for 28 consecutive days, with a 5 μL eyedrop of latanoprost ophthalmic solution 0.005% (Group 3; Xalatan, Pfizer), recombinant human STC-1 with a FLAG tag (Group 4; recombinant human STC-1 [rhSTC-1]; 0.5 μg/μL; Biovender Research and Diagnostic Products),29,30 and latanoprost-free acid (Group 5; latanoprost-free acid [LFA]; 10−4 M; Cayman Chemical).29,30 Contralateral eyes received vehicle in LFA and rhSTC-1 groups. The contralateral eye was untreated in the latanoprost group.
After treatment, whole globes were removed, and conjunctiva was dissected. Protein was extracted using a mortar and pestle in cell lysis buffer (Triton X-100, 10% sodium dodecyl sulfate, 10× PBS, 1M Tris, pH 8.0) containing protease (Complete Mini, Roche) and phosphatase inhibitors (PhosSTOP, Roche). Samples were centrifuged at 13000 g for 10 minutes at 4°C, and supernatant was collected and quantified using a Bradford assay (Bio-Rad). Tumor necrosis factor alpha (TNFα) was quantified using an enzyme-linked immunosorbent assay kit (Invitrogen Life Technologies) according to manufacturer’s instructions.
Statistics
Student t test was used to compare treatment groups with controls for all experiments when a single comparison was made. For steroid-induced ocular hypertension experiments, separate cohorts of mice were used to compare treatment and control since ocular hypertension was induced in only 1 eye of the mouse. For the remainder of experiments, paired statistics were used because the IOP of the treated eye was compared with the fellow control eye. Analysis of variance was used for multigroup comparison with post hoc analysis. After multigroup comparison, pairwise comparison was performed to compare specific relevant groups. Values were expressed as mean ± standard deviation, and P values < 0.05 were considered significant.
Results
Sustained IOP Reduction with Subconjunctival ssAAV-2-STC-1-FLAG in Normotensive Mice
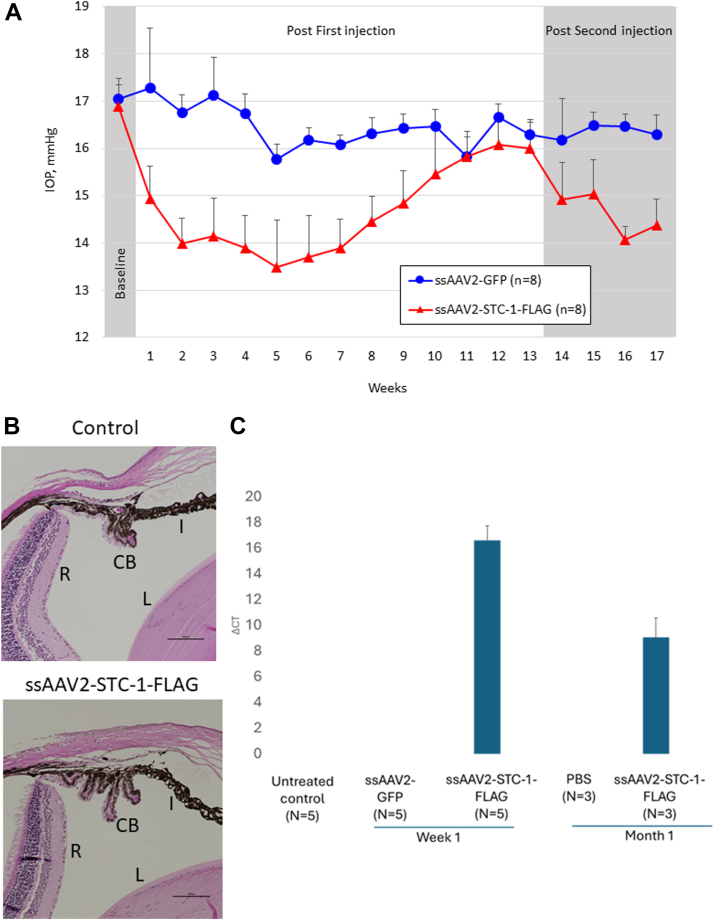
To determine whether subconjunctival administration of ssAAV2-STC-1-FLAG reduces IOP, C57BL/6J mice with similar baseline IOP between fellow eyes (0.9%, 17.0 ± 0.5 vs. 16.9 ± 0.5 mmHg, P = 0.4, n = 8) received a single subconjunctival injection of ssAAV2-STC-1-FLAG (2 μL; 6 × 109 VG) in 1 eye and the same volume and concentration of ssAAV2-GFP in the fellow eye. Averaged weekly IOP measurements revealed a significant reduction in IOP in the ssAAV2-STC-1-FLAG treatment group starting at week 1 (13.5%, 17.3 ± 1.3 vs. 14.9 ± 0.7 mmHg, P < 0.001, Fig 1A). Significant IOP reduction persisted through week 10 post injection (6.1%, 16.5 ± 0.4 vs. 15.5 ± 1.0 mmHg, P < 0.05). Maximal IOP lowering as assessed by difference between treatment groups was seen at week 3 (17.4%, 17.1 ± 0.8 vs. 14.1 ± 0.8 mmHg, P < 0.001). With no significant difference in IOP between fellow eyes at week 13 (1.8%, 16.3 ± 0.3 mmHg vs. 16.0 ± 0.5 mmHg, P = 0.3), animals were reinjected with the same treatments. Significant IOP reduction was restored in eyes that received ssAAV2-STC-1-FLAG treatment at week 14 (7.7%, 16.2 ± 0.9 vs. 14.9 ± 0.8 mmHg, P < 0.05, n = 6), 1 week after the second injection, and persistent IOP reduction was maintained through week 17 (11.7%, 16.3 ± 0.9 ± vs. 14.4 ± 0.8 mmHg, P < 0.001) when the experiment ended.
Figure 1.
Subconjunctival ssAAV2-STC-1-FLAG lowers IOP in a sustained fashion. A, C57BL/6J mice (n = 8) were injected subconjunctivally with ssAAV2-STC-1-FLAG (2μL; 6 × 109 VG) in 1 eye and ssAAV2-GFP (2μL; 6 × 109 VG) in the fellow eye. Significant, sustained IOP lowering was seen in the eye injected with ssAAV2-STC-1-FLAG. At the end of experimental week 13 when IOP reduction waned, all surviving mice (n = 6) received a second injection. Eyes injected with a second dose of ssAAV2-STC-1-FLAG showed a restored, significant, and sustained IOP reduction until experiment was ended. B, No difference in angle anatomy was seen between mice injected with ssAAV2-STC-1-FLAG and PBS-injected controls. Iris, ciliary body and peripheral retina, and lens. C, Eyes injected with ssAAV2-STC-1-FLAG showed transgene expression at week 1 and month 1 postinjection while no expression was seen in any control eyes whether untreated, PBS-treated, or ssAAV2-GFP-treated. CB = ciliary body; GFP = green fluorescent protein; I = iris; IOP = intraocular pressure; L = lens; PBS = phosphate-buffered saline; R = retina; ssAAV2-STC-1-FLAG = single-stranded, adeno-associated virus, serotype 2, engineered to express stanniocalcin-1 with a FLAG tag; VG = viral genome.
One week after injection with ssAAV2-STC-1-FLAG, histologic analysis showed normal angle anatomy of the iris, ciliary body and peripheral retina, and lens visible with no difference compared with a PBS-injected control (Fig 1B). Quantitative polymerase chain reaction of conjunctival tissue revealed an induction of STC-1-FLAG transgene in ssAAV2-STC-1-FLAG injected eyes at week 1 and month 1 postinjection (Fig 1C). Of note, no STC-1-FLAG expression was detected in ssAAV2-GFP-injected eyes, PBS-injected eyes, or untreated control eyes.
Sustained IOP Reduction with Subconjunctival ssAAV-2-STC-1-FLAG in DBA/2J Mice
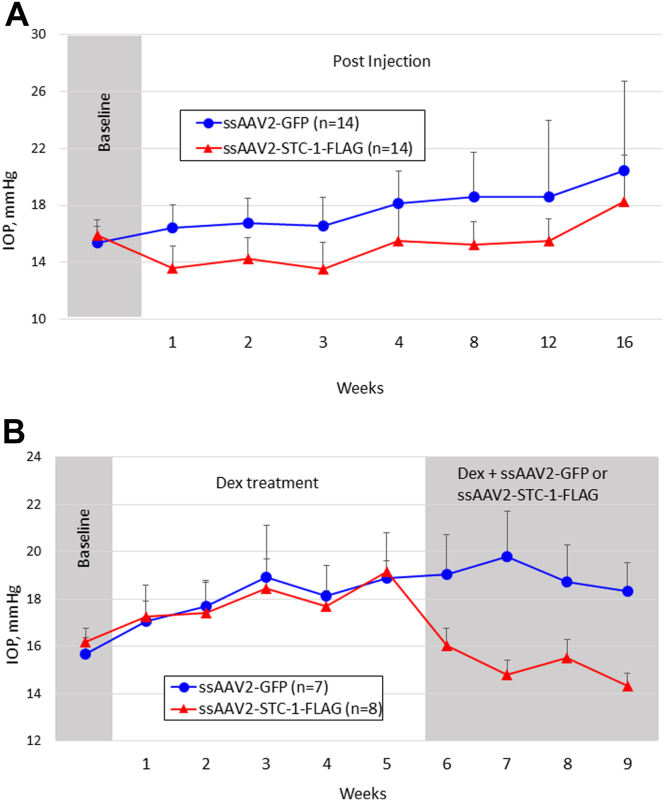
To determine whether subconjunctival administration of ssAAV2-STC-1-FLAG reduces IOP in a model of acquired pigment dispersion, 5-month old DBA/2J mice with similar baseline IOP between fellow eyes (3.2%, 15.4 ± 1.1 vs. 15.9 ± 1.1 mmHg, P = 0.2, n = 14, Fig 2A) received a single subconjunctival injection of ssAAV2-STC-1-FLAG (2 μL; 6 × 109 VG), prior to naturally occurring IOP rise, in 1 eye and the same volume and concentration of ssAAV2-GFP in the fellow eye. Significant IOP reduction was seen in the ssAAV2-STC-1-FLAG treatment group compared with the fellow eye by week 1 (19.1%, 16.6 ± 1.8 vs. 14.4 ± 1.5 mmHg, P < 0.001) and persisted through week 12 (12.9%, 18.6 ± 5.3 vs. 15.5 ± 1.5, P < 0.05). The average IOP reduction during this experimental period was 16.9% (17.8 ± 2.0 vs. 14.8 ± 0.9 mmHg, P < 0.001). In the ssAAV2-GFP-injected control eyes, the naturally occurring rise in IOP expected with the DBA/2J model occurred. Compared with baseline, a significant increase in IOP was first seen at week 3 postinjection (6 months of age; 18.8 ± 15.1%, P < 0.01) consistent with the iris atrophy and beginnings of IOP increase seen in this model35 and was seen through the end of the experiment at week 20 (77.0 ± 48%, P < 0.001). However, in the AAV2-STC-1-FLAG-injected eyes, the expected IOP increase was not observed. Instead, a significant decrease in IOP was seen through week 7 of the experiment compared with baseline (−5.5 ± 10.4%, P < 0.05). As the statistically significant IOP reduction compared with baseline was lost with the expected naturally occurring pigment dispersion and IOP rise, no significant change in IOP compared with baseline was seen through week 14 (11.4 ± 14.3%, P = 0.1) suggesting that ssAAV2-STC-1-FLAG delayed the onset pigment dispersion-induced elevated IOP 11 additional weeks compared with control. Of note, to date no increase in IOP with ssAAV2-GFP-injected eyes has been observed with any treatment of wild-type mice.
Figure 2.
Subconjunctival ssAAV2-STC-1-FLAG lowers IOP in pigment dispersion and steroid-induced ocular hypertension mice. A, DBJ/2J mice (n = 14) were injected subconjunctivally with ssAAV2-STC-1-FLAG (2μL; 6 × 109 VG) in 1 eye and ssAAV2-GFP (2μL; 6 × 109 VG) in the fellow eye. Significant, sustained IOP lowering was seen in the eye injected with ssAAV2-STC-1-FLAG through week 12. Of note, ssAAV2-STC-1 blunted the naturally occurring rise in IOP in the DBA/2J model. B, Steroid-induced ocular hypertension was induced in 1 eye of C57BL/6J mice (n = 15). Mice were injected subconjunctivally in the ocular hypertension eye with ssAAV2-STC-1-FLAG (2μL; 6 × 109 VG, n = 8) or ssAAV2-GFP (2μL; 6 × 109 VG, n = 7). Significant sustained IOP lowering was seen in the eye injected with ssAAV2-STC-1-FLAG. GFP = green fluorescent protein; IOP = intraocular pressure; ssAAV2-STC-1-FLAG = single-stranded, adeno-associated virus, serotype 2, engineered to express stanniocalcin-1 with a FLAG tag; VG = viral genome.
Sustained IOP Reduction with Subconjunctival ssAAV2-STC-1-FLAG in Steroid-Induced Hypertensive Mice
To determine whether subconjunctival administration of ssAAV2-STC-1-FLAG reduces IOP in a model of steroid-induced ocular hypertension, C57BL/6J mice with similar baseline IOP between fellow eyes (2.2%, 15.7 ± 0.6 vs. 16.0 ± 0.5 mmHg, P = 0.1, n = 15) received a weekly injection of dexamethasone acetate for 6 consecutive weeks to induce ocular hypertension. Since ocular hypertension was induced in only 1 eye of each mouse, mice were randomized to receive a single subconjunctival injection of ssAAV2-STC-1-FLAG (2 μL; 6 × 109 VG, n = 8) or the same volume and concentration of ssAAV2-GFP (n = 7) in the steroid-induced ocular hypertension eye. Significant IOP reduction was seen by week 1 postinjection in the ssAAV2-STC-1-FLAG group (15.9%, 19.1 ± 0.8 vs. 16.0 ± 0.8 mmHg, P < 0.001, Fig 2B) and persisted through the end of the experiment at 4 weeks posttreatment (21.7% 18.3 ± 1.2 vs. 14.3 ± 0.5 mmHg, P < 0.001). Maximal IOP reduction was seen at week 2 post treatment (25.2%, 19.8 ± 1.9 vs. 14.8 ± 0.7 mmHg, P < 0.001). Averaged weekly IOP measurements during the treatment period revealed a significant reduction in IOP of 20.0% (19.0 ± 0.6 vs. 15.2 ± 0.7 mmHg, P < 0.001).
Sustained IOP Reduction with Subconjunctival ssAAV2-STC-1-FLAG in FP Receptor Knockout Mice
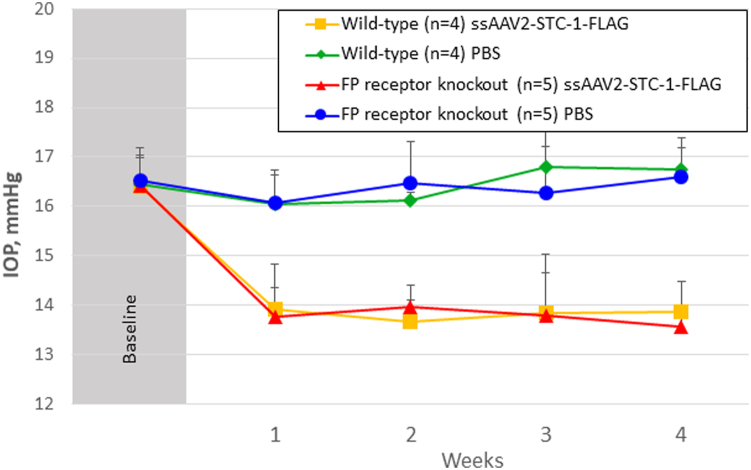
To determine whether subconjunctival administration of ssAAV2-STC1-FLAG reduced IOP in FP receptor knockout receptor mice, wild-type littermate control mice (n = 4) with no difference in IOP between fellow eyes at baseline (0.0%, 16.5 ± 0.5 vs. 16.4 ± 0.6 mmHg, P = 0.8, Fig 3) and FP receptor knockout mice (n = 5) with no difference in fellow eyes at baseline (0.0%, 16.5 ± 0.5 vs. 16.4 ± 0.7 mmHg, P = 0.6) were treated with a single subconjunctival injection of ssAAV2-STC-1-FLAG (2 μL; 6 × 109 VG) in 1 eye and the same volume of PBS in the fellow eye. Both FP receptor knockout (15.7%, 16.4 ± 0.7 vs. 13.8 ± 0.9 mmHg, P < 0.001) and wild-type (15.9%, 16.4 ± 0.7 vs. 13.8 ± 0.7 mmHg, P = 0.001) mice showed significant reduction in IOP compared with the fellow control eyes over the duration of the experiment. When comparing ssAAV2-STC-1-FLAG-treated littermate controls with ssAAV2-STC-1-FLAG-treated FP receptor knockout mice, no difference was seen over the duration of the experiment (0.0%, 13.8 ± 0.7 vs. 13.8 ± 0.9 mmHg, P = 0.9).
Figure 3.
Sustained IOP reduction with subconjunctival ssAAV2-STC-1-FLAG in FP receptor knockout mice. Wild-type littermate control mice (n = 4) and FP receptor knockout mice (n = 5) were injected subconjunctivally with ssAAV2-STC-1-FLAG (2μL; 6 × 109 VG) in 1 eye and the same volume of PBS in the fellow eye. Significant, sustained IOP lowering was seen in the eye injected with ssAAV2-STC-1-FLAG in both wild-type and FP receptor knockout mice starting in week 1 and persisting through week 4. Of note, there was no significant difference in IOP between treated eyes of wild-type and FP receptor knockout mice. FP = prostaglandin F; IOP = intraocular pressure; PBS = phosphate-buffered saline; ssAAV2-STC-1-FLAG = single-stranded, adeno-associated virus, serotype 2, engineered to express stanniocalcin-1 with a FLAG tag; VG = viral genome.
Assessment of IOP Reduction with ssAAV2-STC-1 with No FLAG Tag
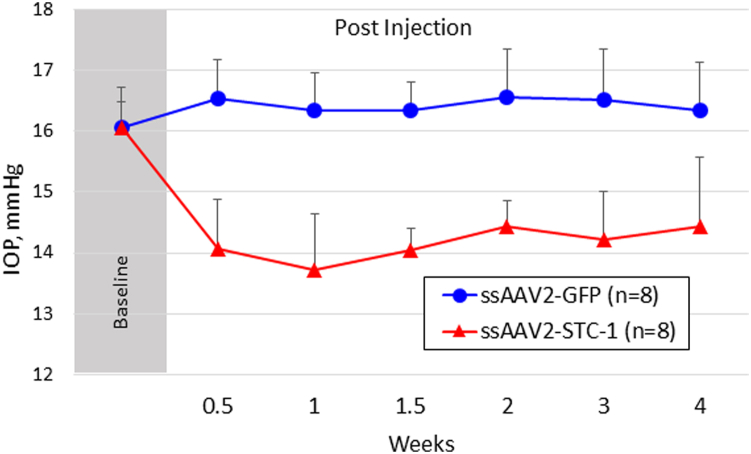
In order to determine whether the FLAG tag contributed to IOP reduction with ssAAV2-STC-1-FLAG, we generated an identical construct to deliver STC-1 without the FLAG tag, ssAAV2-STC-1. After baseline IOP measurements showed no significant difference between fellow eyes (0.0%, 16.1 ± 0.4 vs. 16.1 ± 0.7 mmHg, n = 8, P = 0.9, Fig 4), C57BL/6J mice received a single subconjunctival injection (2 μL; 6 × 109 VGs) of ssAAV2-STC-1 in 1 eye. The fellow eye of each mouse received a single subconjunctival injection with the same concentration and volume of ssAAV2-GFP. Mice that received a subconjunctival injection of ssAAV2-STC-1 showed a significant decrease in IOP compared with fellow control eyes starting at week 1 (15.0%, 16.5 ± 0.6 vs. 14.1 ± 0.8 mmHg, P < 0.001, Fig 4), similar to that seen with ssAAV-STC-1-FLAG in other experiments. Significant IOP reduction persisted until the end of the experiment at week 4 in eyes treated with ssAAV2-STC-1 (11.5%, 16.3 ± 0.7 vs. 14.4 ± 1.1 mmHg, P < 0.01).
Figure 4.
Subconjunctivally injected ssAAV2-STC-1 without the FLAG tag reduced IOP in a sustained fashion. After baseline IOP measurements, ssAAV2-STC-1 without a FLAG tag (2μL; 6 × 109 VG) was subconjunctivally injected in 1 eye and ssAAV2-GFP (2μL; 6 × 109 VG) was subconjunctivally injected into the fellow eye of 3-month-old C57BL/6J mice (n = 8). Significant sustained IOP lowering was seen in the eye injected with ssAAV2-STC-1 until the experiment was ended. GFP = green fluorescent protein; IOP = intraocular pressure; ssAAV2-STC-1 = single-stranded, adeno-associated virus, serotype 2, engineered to express stanniocalcin-1; VG = viral genome.
Comparison of ssAAV2-STC-1-FLAG with Topical Latanoprost for IOP Reduction and Ocular Surface Inflammation
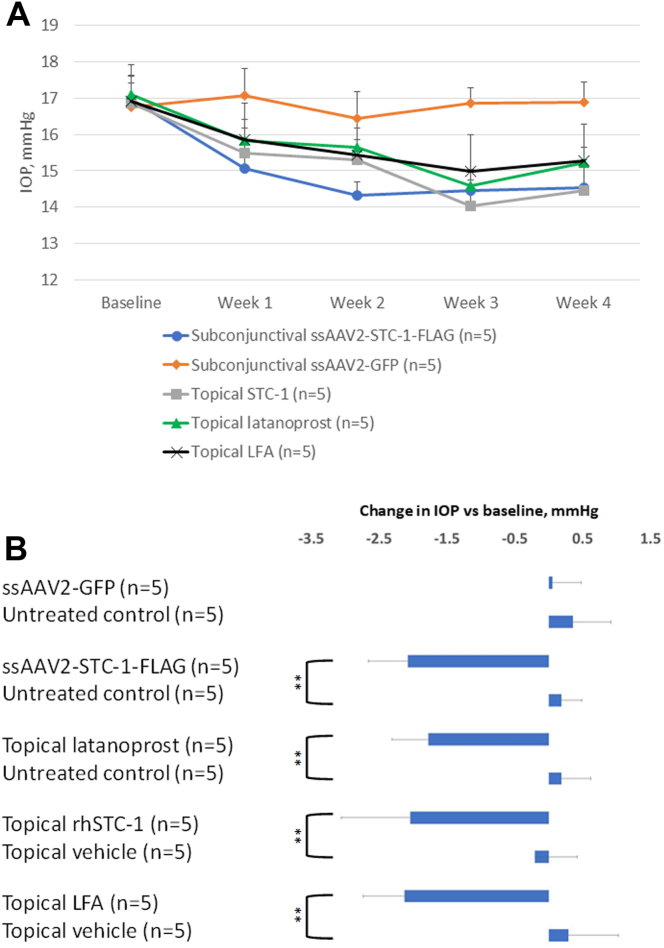
It has been reported that ocular surface inflammation is induced in the conjunctiva of C57BL/6J mice receiving daily topical latanoprost treatment for 4 weeks or 4 times daily topical latanoprost treatment for 1 week as assessed by increased levels of TNFα.36 Based on clinical dosing we selected once daily dosing for 1 month in order to determine if ssAAV-STC-1-FLAG induces ocular inflammation similar to what had been previously reported with topical latanoprost. Twenty-five 3-month-old C57BL/6J wild-type mice were randomized into 5 groups: subconjunctival injection of ssAAV2-STC-1-FLAG (6 × 109 VGs; n = 5), subconjunctival injection of ssAAV2-GFP (6 × 109 VG; n = 5), topical latanoprost 0.005% (n = 5), topical rhSTC-1 (2.5 μg; n = 5), or topical LFA (10-4 M; n = 5). For this experiment, animals that received topical treatment were treated with once daily drops, and animals that were treated with an injection received a single injection and tissues were collected 4 weeks later.
Intraocular pressure was assessed in all groups over a 4-week experimental period (Fig 5A). When comparing the average IOP over this time, there was no significant difference in IOP with ssAAV2-GFP compared with the fellow control eye (1.9%, 16.9 ± 0.2 vs. 16.8 ± 0.5 mmHg, P = 0.6, n = 5, Fig 5B). All other treatment groups showed significant IOP reduction compared with the fellow eye: ssAAV2-STC-1-FLAG (16.2%, 17.1 ± 0.1 vs. 14.6 ± 0.3 mmHg, n = 5, P < 0.001), topical latanoprost (14.1%, 17.1 ± 0.3 vs. 15.3 ± 0.09, n = 5, P < 0.001), topical LFA (15.6%, 17.2 ± 0.5 vs. 14.8 ± 0.5 mmHg, n = 5, P < 0.001), and topical rhSTC-1 (14.7%, 17.0 ± 0.4 vs. 14.8 ± 0.8, n = 5, P < 0.001). There was no significant difference in IOP reduction when comparing the LFA, latanoprost, rhSTC-1, or ssAAV2-STC-1-FLAG treatment groups (P = 0.2).
Figure 5.
Subconjunctival ssAAV2-STC-1-FLAG lowers IOP in an equivalent manner to latanoprost. A, Graph showing IOPs of individual treatments over 4 weeks. B, Bar graph showing no significant difference in IOP between subconjunctival delivered ssAAV2-GFP (2μL; 6 × 109 VG) and fellow untreated control eye. Single-strand-AAV2-STC-1-FLAG (2μL; 6 × 109 VG), topical latanoprost (0.005%), topical LFA (10−4 M), and topical rhSTC-1 (5 μL; 0.5μg/μL) all showed a significant decrease in IOP compared with the fellow eye. No significant difference was seen between topical latanoprost, topical LFA, topical rhSTC-1, or ssAAV2-STC-1-FLAG. ∗∗P < 0.01. GFP = green fluorescent protein; IOP = intraocular pressure; LFA = latanoprost-free acid; rhSTC-1 = recombinant human stanniocalcin-1; ssAAV2-STC-1-FLAG = single-stranded, adeno-associated virus, serotype 2, engineered to express stanniocalcin-1 with a FLAG tag; STC-1 = stanniocalcin-1; VG = viral genome.
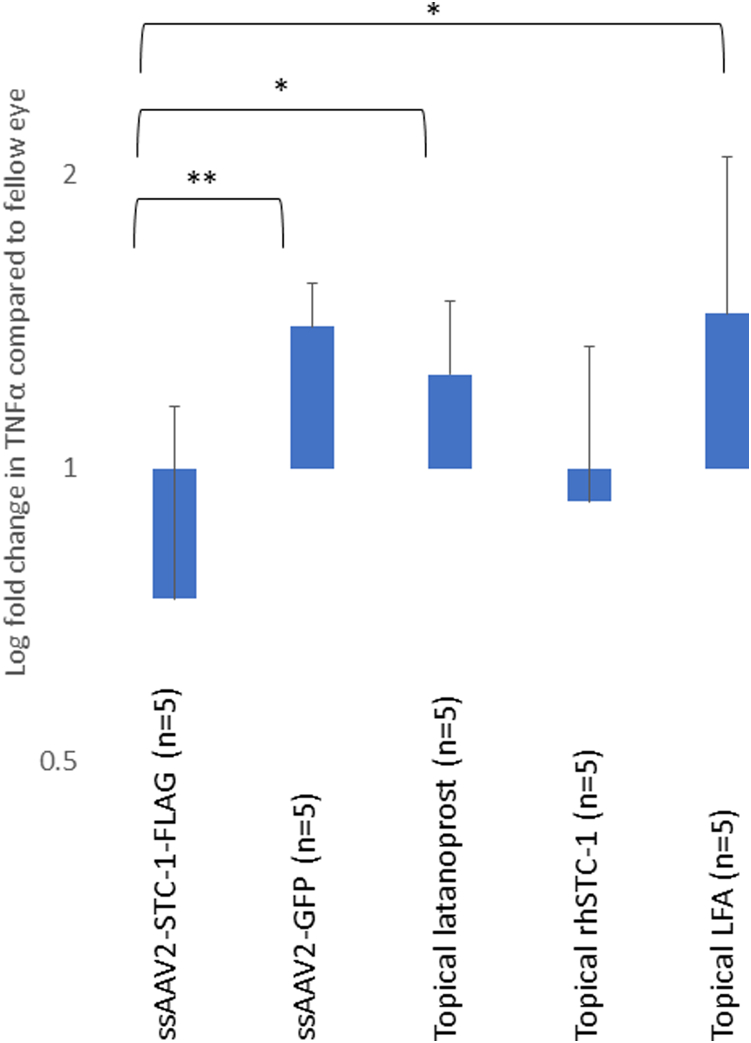
To assess ocular surface inflammation, we evaluated levels of TNFα in the conjunctiva. Single-strand-AAV2-GFP, topical latanoprost, and topical LFA all showed a trend of TNFα induction compared with the fellow control eye (Fig 6). In contrast, eyes treated topically with rhSTC-1 or with a subconjunctival injection of ssAAV2-STC-1-FLAG showed lower levels of TNFα compared with the fellow control eye. Specifically, there was a significant decrease in TNFα comparing ssAAV2-STC-1-FLAG treated eyes with ssAAV2-GFP (P = 0.01), latanoprost (P < 0.05), and LFA (P < 0.05) treated eyes.
Figure 6.
Subconjunctival delivered ssAAV2-STC-1-FLAG and topical STC-1 do not induce TNFα. Single-strand-AAV2-GFP, topical latanoprost, and topical LFA all showed a significantly increased concentration of TNFα at the protein level in conjunctiva compared with ssAAV2-STC-1-FLAG. ∗P < 0.05, ∗∗P < 0.01. GFP = green fluorescent protein; LFA = latanoprost-free acid; rhSTC-1 = recombinant human stanniocalcin-1; ssAAV2-STC-1-FLAG = single-stranded, adeno-associated virus, serotype 2, engineered to express stanniocalcin-1 with a FLAG tag; STC-1 = stanniocalcin-1; TNFα = tumor necrosis factor alpha.
Discussion
There is a need to enhance patient treatment compliance by developing novel IOP-lowering therapeutics that require less frequent administration and have minimal ocular side effect profiles. Our study demonstrates proof of concept that an IOP-lowering protein can be expressed following subconjunctival injection of a viral vector. Subconjunctival ssAAV2-STC-1-FLAG lowered IOP in normotensive mice, pigment dispersion mice, and steroid-induced ocular hypertension mice. Furthermore, IOP lowering was confirmed without the presence of the FLAG tag. Additionally, though IOP reduction was equivalent between topical latanoprost, topical LFA, and subconjunctival ssAAV2-STC-1-FLAG, expression of STC-1-FLAG via subconjunctival viral delivery showed reduced expression of conjunctival TNFα. Taken together, these data suggest a potentially viable, repeatable, minimally invasive, periocular delivery approach for STC-1, that can be used to achieve sustained IOP reduction while reducing ocular surface inflammation, a side effect of many current glaucoma medications.
Subconjunctival drug delivery has several inherent advantages over intraocular approaches. The most common clinically approved intraocular injection is intravitreal administration of anti-VEGF agents. Intravitreal injections are generally well-tolerated, and many patients receive numerous injections over a lifetime. However, potential complications of intravitreal injections do occur. These include vitreous hemorrhage, increased IOP, uveitis, traumatic cataract, and endophthalmitis.37 Overall, endophthalmitis is rare,38 and though the sequelae of endophthalmitis is broad, severe cases may lead to loss of the eye itself.39 A less invasive periocular approach would reduce the risk of adverse events seen with intraocular injections. Subconjunctival administration has the advantage of local delivery of medication without needle penetration into the intraocular space and is widely accepted and utilized in conjunction with other procedures such as anterior segment surgery. From the patient’s perspective, use of a subconjunctival delivery approach would provide a less invasive, lower risk procedure that could be delivered efficiently and safely at the slit lamp with minimal discomfort. Though subconjunctival delivery of transgenes by viral vectors has advantages over existing therapeutics and delivery methods,40 their testing in animal models has been limited to models of acute corneal injury41,42 and as adjuncts in glaucoma surgery.43 Our study is unique in that we target IOP reduction as a stand-alone therapeutic.
PGF2α topical therapeutics such as latanoprost are the first line pharmacologic therapy for IOP reduction to treat glaucoma or ocular hypertension in many practices. However, up to 20% of patients are either minimally responsive or unresponsive to PGF2α analogs, and side effects such as orbital fat atrophy, conjunctival hyperemia, ocular surface irritation, pigmentation of the iris and periocular skin, and hypertrichosis may be seen.44 Treatment with PGF2α analogs has induced expression of markers of ocular surface inflammation in patients45 and animal models.36 This is believed to be due to the binding to the FP receptor, whose activation initiates a number of cellular pathways including those that are proinflammatory.46, 47, 48 Inhibition of PGF2α by pharmacologic blockade of the FP receptor with competitive antagonist AL-8810 has been shown to reduce the inflammatory response49 and be therapeutic in animal models of stroke,50 traumatic brain injury,51 and multiple sclerosis.52 These results imply that proinflammatory side effects of PGF2α are a result of FP receptor activation. In the current study, we found that subconjunctivally administered ssAAV2-STC-1-FLAG is able to lower IOP in FP receptor knockout mice, unlike latanoprost, and similar to what we found with topical administration of rhSTC-129 and intracameral ssAAV2-STC-1-FLAG.10 We also found elevated levels of TNFα consistent with an induced inflammatory response after latanoprost and LFA treatment. In contrast, expression of STC-1-FLAG or topically delivered rhSTC-1 did not increase TNFα levels. While STC-1 is a downstream effector molecule of latanoprost signaling, it is a standalone IOP ocular hypotensive agent that does not utilize the FP receptor.29 We hypothesize that the reason for induction of conjunctival TNFα in LFA and latanoprost treated eyes is a result of FP receptor activation.
Therapeutics that offer sustained IOP lowering over weeks to months have potential advantages over conventional dosing regimens with topical medications. Studies estimate that less than half of patients use glaucoma eye drops as prescribed.2, 3, 4, 5, 6 An injectable medication, especially one that sustains IOP reduction over extended periods, has the potential to eliminate these barriers resulting in better adherence, compliance, and outcomes. Additionally, because of the pharmacokinetics and dosing regimens of current glaucoma medications, fluctuation in IOP is common and believed to be a key contributor to glaucoma.53,54 Therefore, sustained expression of an IOP-lowering protein, even for a finite period of time, has the potential to reduce rates of progression of glaucoma by providing a constant dose of medication that minimizes IOP fluctuation.
Intraocular pressure reduction was observed in normotensive mice as well as 2 mouse models that result in ocular hypertension, each with different mechanisms of outflow obstruction. Initial testing of novel compounds for IOP reduction are often performed in normotensive mice due to their ease of use and consistent IOP measurements before, during, and after treatment.55 Furthermore, results in normotensive mice have important implications for the significant number of patients with “normal” or “low” tension glaucoma.56 The steroid-induced ocular hypertension model is a relatively acute model of trabecular meshwork dysfunction resulting from the overexpression of extracellular matrix proteins leading to an increase in outflow resistance and IOP elevation.34 The DBA/2J mouse is a more chronic, inherited model of ocular hypertension and glaucomatous optic neuropathy57 resulting from pigment dispersion.35 Mice typically develop elevated IOP by 9 months of age secondary to multiple mechanisms including trabecular meshwork dysfunction, presence of posterior synechiae, and a late acquired secondary angle closure.35,58,59 It should be noted that in our study, DBA/2J mice were injected prior to the rise in IOP at 5 months of age. Therefore, DBA/2J mice were normotensive at the time of treatment and IOP reduction compared with the fellow eye which was maintained as ocular hypertension developed. Importantly, ssAAV2-STC-1-FLAG delayed the onset of ocular hypertension in the treated eye. Reduction of IOP following subconjunctival administration of ssAAV2-STC-1-FLAG in multiple animal models characterized by both normal and abnormal trabecular meshwork is consistent with our prior data that showed a decrease in IOP after once daily ophthalmic delivery of rhSTC-1.29,30,33
Several different hypotheses were evaluated in this study and therefore, maximum sustainability of IOP reduction after subconjunctival delivery was not investigated in every model system. For example, AAV2-STC-1 (without the FLAG tag) experiments were designed to test the hypothesis that the vector construct without the FLAG tag maintained IOP reduction seen with ssAAV2-STC-1-FLAG. Studies in FP receptor knockout mice were designed to test the hypothesis that this pathway is not needed for IOP reduction with ssAAV2-STC-1-FLAG. Additionally, evaluation of the effect of ssAAV2-STC-1-FLAG on IOP in steroid-induced ocular hypertension model was limited to 1 month as induced ocular hypertension can wane over time, as indicated in prior studies.34 In the present study, significant IOP lowering was observed for 10 weeks in normotensive mice with subconjunctival ssAAV2-STC-1-FLAG. It should be noted that intracameral administration of ssAAV2-STC-1-FLAG in a prior study10 showed sustainability of IOP reduction for up to 6 months. Because of the distinct locations that the virus is delivered, it is conceivable that different proportions of labile and stable cell types will be transduced. If 1 delivery technique results in transduction of more stable cells, it is feasible to hypothesize that this method will lead to longer expression of the STC-1 transgene and subsequent longer duration of reduction of IOP. Additional studies are required to evaluate the effectiveness of subconjunctival injection in comparison to intracameral injections.
One potential caveat with the use of AAVs in intraocular drug delivery is the potential for inciting a viral vector mediated inflammatory response. Much of the existing understanding and concerns around inflammatory responses come from studies using intravitreal and subretinal approaches.60 Though data are limited for both intracameral and subconjunctival delivery of AAV, to date, we have no evidence of intraocular or periocular inflammation as assessed by clinical examination or histopathology with intracameral or subconjunctival delivery of ssAAV2-STC-1-FLAG. In the current study, subconjunctival ssAAV2-GFP induced TNFα to a similar degree as topical latanoprost and LFA. However, subconjunctival delivery of ssAAV2-STC-1-FLAG and topical STC-1 both showed a reduction of TNFα in conjunctiva compared with the fellow eye. We hypothesize that the similar induction of TNFα seen in ssAAV2-GFP and latanoprost occurs by different mechanisms. We suspect that ssAAV2-GFP induces a small inflammatory response related to the viral vector while latanoprost and LFA induce TNFα secondary to FP receptor activation. Stanniocalcin-1 is an anti-inflammatory protein and reduces inflammation by multiple mechanisms including inhibition of macrophage chemotaxis, modulation of transendothelial migration of leukocytes, and reduction of T-cell infiltration.20, 21, 22, 23 We hypothesize that the anti-inflammatory functions of STC-1 blunt the induction of TNFα seen with subconjunctival administration of ssAAV2-GFP, topical latanoprost, and topical LFA. Therefore, expression of STC-1 that lowers IOP without inducing ocular surface inflammation has potential to treat patients with limited therapeutic options due to ocular surface side effects seen with existing medications.
Stanniocalcin-1 is a downstream effector molecule within the latanoprost pathway but is a standalone ocular hypotensive agent.29,30,33 Therefore, one may expect that latanoprost and STC-1 have a similar mechanism of action. We previously demonstrated that in mice STC-1 lowers IOP by increasing trabecular outflow when delivered as a recombinant protein topically (rhSTC-1) or when delivered intracamerally with ssAAV2.10 Likewise, latanoprost lowers IOP in mice by increasing trabecular outflow.10,61 This is in contrast to humans, where multiple mechanisms of action have been described for latanoprost. While the predominant effect is likely uveoscleral,62 ≥1 study found increases in both uveoscleral and trabecular outflow,63 and use of intraocular prostaglandin implants suggest a lowering of episcleral venous pressure.64 Future studies of subconjunctivally-delivered ssAAV2-STC-1-FLAG should include determination of mechanism of action of IOP reduction, ideally in a larger animal model, since species differences in contribution of outflow pathways exist.65
One potential limitation of our study is that we used the contralateral eye as a control, either injecting AAV2-GFP or the same volume of PBS. While we have not detected any measurable change in IOP or any measurable FLAG expression in control eyes compared with baseline in wild-type mice, we cannot exclude the possibility that virus enters the systemic circulation and could reach the fellow eye in small amounts. This may have an impact on the overall effect of the IOP reduction. Our results are consistent with other reports that AAV-GFP serves as a vector control that does not affect IOP.66,67
Another limitation is the selection of our ocular hypertensive models. While we tried to select mouse models that closely represent human disease (i.e., steroid response and pigment dispersion), we are aware that these models have limitations. For example, the steroid-induced model is relatively acute model (i.e., the ocular hypertensive effects are not long-lasting) while the DBA/2J mouse model has variability due to multiple mechanisms resulting in elevated IOP.35,58,59 While there is no specific glaucoma model in mice, to fully appreciate the maximal IOP lowering capacity of ssAAV2-STC-1-FLAG, additional testing is warranted in alternative models such as the myocilin model68 or use of microbeads to cause IOP elevation.69
In summary, subconjunctival administration of ssAAV2-STC-1-FLAG lowers IOP in a sustained manner. Additional preclinical studies are needed to assess the safety and efficacy of this therapeutic approach in appropriate large animal model systems to determine whether this treatment strategy may benefit the 80 million people worldwide afflicted by glaucoma.70
Manuscript no. XOPS-D-24-00071.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The authors have made the following disclosures:
D.K.: Financial support – Mayo Clinic.
Funded by NIH K08 (G.W.R.), Mayo Foundation for Medical Education and Research (G.W.R.), and National Eye Institute grant EY031758 (G.W.R.).
HUMAN SUBJECTS: No human subjects were included in this study.
ANIMAL SUBJECTS: Animal subjects were used in this study. All mouse studies received prior approval by the Mayo Clinic (Rochester, MN) Institutional Animal Care, and Use Committee, followed the Association for Research in Vision and Ophthalmology guidelines, and adhered to the Declaration of Helsinki.
Author Contributions:
Conception and design: Roddy
Data collection: Roddy, Kohli, Niknam, Omer, Chowdhury, Anderson, Pacheco Marrero, Rinkoski
Analysis and interpretation: Roddy, Fautsch
Obtained funding: N/A
Overall responsibility: Roddy
References
- 1.Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajurkar K., Dubey S., Gupta P.P., et al. Compliance to topical anti-glaucoma medications among patients at a tertiary hospital in North India. J Curr Ophthalmol. 2018;30:125–129. doi: 10.1016/j.joco.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman-Casey P.A., Robin A.L., Blachley T., et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122:1308–1316. doi: 10.1016/j.ophtha.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordstrom B.L., Friedman D.S., Mozaffari E., et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 5.Hwang D.K., Liu C.J., Pu C.Y., et al. Persistence of topical glaucoma medication: a nationwide population-based cohort study in Taiwan. JAMA Ophthalmol. 2014;132:1446–1452. doi: 10.1001/jamaophthalmol.2014.3333. [DOI] [PubMed] [Google Scholar]
- 6.Feehan M., Munger M.A., Cooper D.K., et al. Adherence to glaucoma medications over 12 Months in two US community pharmacy chains. J Clin Med. 2016;5 doi: 10.3390/jcm5090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung E.W., Medeiros F.A., Weinreb R.N. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 8.Fechtner R.D., Godfrey D.G., Budenz D., et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618–621. doi: 10.1097/ICO.0b013e3181c325b2. [DOI] [PubMed] [Google Scholar]
- 9.Kompella U.B., Hartman R.R., Patil M.A. Extraocular, periocular, and intraocular routes for sustained drug delivery for glaucoma. Prog Retin Eye Res. 2020;82 doi: 10.1016/j.preteyeres.2020.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roddy G.W., Roy Chowdhury U., Anderson K.J., et al. Transgene expression of Stanniocalcin-1 provides sustained intraocular pressure reduction by increasing outflow facility. PLoS One. 2022;17 doi: 10.1371/journal.pone.0269261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner G.F., Dimattia G.E. The stanniocalcin family of proteins. J Exp Zool A Comp Exp Biol. 2006;305:769–780. doi: 10.1002/jez.a.313. [DOI] [PubMed] [Google Scholar]
- 12.Tang S.E., Wu C.P., Wu S.Y., et al. Stanniocalcin-1 ameliorates lipopolysaccharide-induced pulmonary oxidative stress, inflammation, and apoptosis in mice. Free Radic Biol Med. 2014;71:321–331. doi: 10.1016/j.freeradbiomed.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen A., Chang A.C., Reddel R.R. Stanniocalcin-1 acts in a negative feedback loop in the prosurvival ERK1/2 signaling pathway during oxidative stress. Oncogene. 2009;28:1982–1992. doi: 10.1038/onc.2009.65. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y., Zemans R., Correll K., et al. Stanniocalcin-1 is induced by hypoxia inducible factor in rat alveolar epithelial cells. Biochem Biophys Res Commun. 2014;452:1091–1097. doi: 10.1016/j.bbrc.2014.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi X., Wang J., Qin Y. Recombinant adeno-associated virus-delivered hypoxia-inducible stanniocalcin-1 expression effectively inhibits hypoxia-induced cell apoptosis in cardiomyocytes. J Cardiovasc Pharmacol. 2014;64:522–529. doi: 10.1097/FJC.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 16.Durukan T.A., Westberg J.A., Serlachius M., et al. Stanniocalcin 1 is important for poststroke functionality, but dispensable for ischemic tolerance. Neuroscience. 2013;229:49–54. doi: 10.1016/j.neuroscience.2012.10.062. [DOI] [PubMed] [Google Scholar]
- 17.Zhang K., Lindsberg P.J., Tatlisumak T., et al. Stanniocalcin: a molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci USA. 2000;97:3637–3642. doi: 10.1073/pnas.070045897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roddy G.W., Rosa R.H., Jr., Oh J.Y., et al. Stanniocalcin-1 rescued photoreceptor degeneration in two rat models of inherited retinal degeneration. Mol Ther. 2012;20:788–797. doi: 10.1038/mt.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roddy G.W., Yasumura D., Matthes M.T., et al. Long-term photoreceptor rescue in two rodent models of retinitis pigmentosa by adeno-associated virus delivery of Stanniocalcin-1. Exp Eye Res. 2017;165:175–181. doi: 10.1016/j.exer.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty A., Brooks H., Zhang P., et al. Stanniocalcin-1 regulates endothelial gene expression and modulates transendothelial migration of leukocytes. Am J Physiol Renal Physiol. 2007;292:F895–F904. doi: 10.1152/ajprenal.00219.2006. [DOI] [PubMed] [Google Scholar]
- 21.Huang L., Garcia G., Lou Y., et al. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2009;174:1368–1378. doi: 10.2353/ajpath.2009.080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanellis J., Bick R., Garcia G., et al. Stanniocalcin-1, an inhibitor of macrophage chemotaxis and chemokinesis. Am J Physiol Ren Physiol. 2004;286:F356–F362. doi: 10.1152/ajprenal.00138.2003. [DOI] [PubMed] [Google Scholar]
- 23.Sheikh-Hamad D. Mammalian stanniocalcin-1 activates mitochondrial antioxidant pathways: new paradigms for regulation of macrophages and endothelium. Am J Physiol Ren Physiol. 2010;298:F248–F254. doi: 10.1152/ajprenal.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L., Belousova T., Pan J.S., et al. AKI after conditional and kidney-specific knockdown of Stanniocalcin-1. J Am Soc Nephrol. 2014;25:2303–2315. doi: 10.1681/ASN.2013070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Huang L., Abdelrahim M., et al. Stanniocalcin-1 suppresses superoxide generation in macrophages through induction of mitochondrial UCP2. J Leukoc Biol. 2009;86:981–988. doi: 10.1189/jlb.0708454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyamandi V.Z., Johnsen V.L., Hughey C.C., et al. Enhanced stem cell engraftment and modulation of hepatic reactive oxygen species production in diet-induced obesity. Obesity. 2014;22:721–729. doi: 10.1002/oby.20580. [DOI] [PubMed] [Google Scholar]
- 27.Ohkouchi S., Block G.J., Katsha A.M., et al. Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol Ther. 2012;20:417–423. doi: 10.1038/mt.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalvin L.A., Hartnett M.E., Bretz C.A., et al. Stanniocalcin-1 is a modifier of oxygen-induced retinopathy severity. Curr Eye Res. 2019;45:46–51. doi: 10.1080/02713683.2019.1645184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roddy G.W., Rinkoski T.A., Monson K.J., et al. Stanniocalcin-1 (STC-1), a downstream effector molecule in latanoprost signaling, acts independent of the FP receptor for intraocular pressure reduction. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roddy G.W., Viker K.B., Winkler N.S., et al. Stanniocalcin-1 is an ocular hypotensive agent and a downstream effector molecule that is necessary for the intraocular pressure-lowering effects of latanoprost. Invest Ophthalmol Vis Sci. 2017;58:2715–2724. doi: 10.1167/iovs.16-21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy Chowdhury U., Bahler C.K., Holman B.H., et al. Ocular hypotensive effects of the ATP-sensitive potassium channel opener cromakalim in human and murine experimental model systems. PLoS One. 2015;10 doi: 10.1371/journal.pone.0141783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy Chowdhury U., Rinkoski T.A., Bahler C.K., et al. Effect of cromakalim prodrug 1 (CKLP1) on aqueous humor dynamics and feasibility of combination therapy with existing ocular hypotensive agents. Invest Ophthalmol Vis Sci. 2017;58:5731–5742. doi: 10.1167/iovs.17-22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roddy G.W., Chowdhury U.R., Monson K.J., Fautsch M.P. Stanniocalcin-1 reduced intraocular pressure in two models of ocular hypertension. Curr Eye Res. 2021;46:1525–1530. doi: 10.1080/02713683.2021.1899246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel G.C., Phan T.N., Maddineni P., et al. Dexamethasone-induced ocular hypertension in mice: effects of myocilin and route of administration. Am J Pathol. 2017;187:713–723. doi: 10.1016/j.ajpath.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.John S.W., Smith R.S., Savinova O.V., et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 36.Yang Y., Huang C., Lin X., et al. 0.005% preservative-free latanoprost induces dry eye-like ocular surface damage via promotion of inflammation in mice. Invest Ophthalmol Vis Sci. 2018;59:3375–3384. doi: 10.1167/iovs.18-24013. [DOI] [PubMed] [Google Scholar]
- 37.Zehden J.A., Mortensen X.M., Reddy A., Zhang A.Y. Systemic and ocular adverse events with intravitreal anti-VEGF therapy used in the treatment of diabetic retinopathy: a review. Curr Diab Rep. 2022;22:525–536. doi: 10.1007/s11892-022-01491-y. [DOI] [PubMed] [Google Scholar]
- 38.Sigford D.K., Reddy S., Mollineaux C., Schaal S. Global reported endophthalmitis risk following intravitreal injections of anti-VEGF: a literature review and analysis. Clin Ophthalmol. 2015;9:773–781. doi: 10.2147/OPTH.S77067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J.J., Jo Y.J., Lee J.S. Clinical characteristics and risk factors for visual prognosis according to the types of infectious endophthalmitis. PLoS One. 2022;17 doi: 10.1371/journal.pone.0278625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bower J.J., Song Z., Song L. Subconjunctival administration of adeno-associated virus vectors in small animal models. J Vis Exp. 2022 doi: 10.3791/63532. [DOI] [PubMed] [Google Scholar]
- 41.Cheng H.C., Yeh S.I., Tsao Y.P., Kuo P.C. Subconjunctival injection of recombinant AAV-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol Vis. 2007;13:2344–2352. [PubMed] [Google Scholar]
- 42.Lai L.J., Xiao X., Wu J.H. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J Biomed Sci. 2007;14:313–322. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- 43.Lee E.J., Han J.C., Park D.Y., et al. Effect of connective tissue growth factor gene editing using adeno-associated virus-mediated CRISPR-Cas9 on rabbit glaucoma filtering surgery outcomes. Gene Ther. 2021;28:277–286. doi: 10.1038/s41434-020-0166-4. [DOI] [PubMed] [Google Scholar]
- 44.Toris C.B., Gabelt B.T., Kaufman P.L. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008;53(Suppl1):S107–S120. doi: 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigues Mde L., Felipe Crosta D.P., Soares C.P., et al. Immunohistochemical expression of HLA-DR in the conjunctiva of patients under topical prostaglandin analogs treatment. J Glaucoma. 2009;18:197–200. doi: 10.1097/IJG.0b013e31818153f4. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Yan S., Xiao B., et al. Prostaglandin F2alpha facilitates hepatic glucose production through CaMKIIgamma/p38/FoxO1-signaling pathway in fasting and obesity. Diabetes. 2018;67:1748–1760. doi: 10.2337/db17-1521. [DOI] [PubMed] [Google Scholar]
- 47.Yamagishi-Kimura R., Honjo M., Aihara M. Contribution of prostanoid FP receptor and prostaglandins in transient inflammatory ocular hypertension. Sci Rep. 2018;8 doi: 10.1038/s41598-018-29273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maehara T., Higashitarumi F., Kondo R., Fujimori K. Prostaglandin F(2alpha) receptor antagonist attenuates LPS-induced systemic inflammatory response in mice. FASEB J. 2020;34:15197–15207. doi: 10.1096/fj.202001481R. [DOI] [PubMed] [Google Scholar]
- 49.Sharif N.A., Klimko P.G. Prostaglandin FP receptor antagonists: discovery, pharmacological characterization and therapeutic utility. Br J Pharmacol. 2018;176:1059–1078. doi: 10.1111/bph.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Y.T., Moon S.K., Maruyama T., et al. Prostaglandin FP receptor inhibitor reduces ischemic brain damage and neurotoxicity. Neurobiol Dis. 2012;48:58–65. doi: 10.1016/j.nbd.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glushakov A.V., Robbins S.W., Bracy C.L., et al. Prostaglandin F2alpha FP receptor antagonist improves outcomes after experimental traumatic brain injury. J Neuroinflammation. 2013;10:132. doi: 10.1186/1742-2094-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwasa K., Yamamoto S., Takahashi M., et al. Prostaglandin F2alpha FP receptor inhibitor reduces demyelination and motor dysfunction in a cuprizone-induced multiple sclerosis mouse model. Prostaglandins Leukot Essent Fatty Acids. 2014;91:175–182. doi: 10.1016/j.plefa.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Kim J.H., Caprioli J. Intraocular pressure fluctuation: is it important? J Ophthalmic Vis Res. 2018;13:170–174. doi: 10.4103/jovr.jovr_35_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musch D.C., Gillespie B.W., Lichter P.R., et al. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–207. doi: 10.1016/j.ophtha.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowdhury U.R., Holman B.H., Fautsch M.P. ATP-sensitive potassium (K(ATP)) channel openers diazoxide and nicorandil lower intraocular pressure in vivo. Invest Ophthalmol Vis Sci. 2013;54:4892–4899. doi: 10.1167/iovs.13-11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao J., Solano M.M., Oldenburg C.E., et al. Prevalence of normal-tension glaucoma in the Chinese population: a systematic review and meta-analysis. Am J Ophthalmol. 2019;199:101–110. doi: 10.1016/j.ajo.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 57.Sheldon W.G., Warbritton A.R., Bucci T.J., Turturro A. Glaucoma in food-restricted and ad libitum-fed DBA/2NNia mice. Lab Anim Sci. 1995;45:508–518. [PubMed] [Google Scholar]
- 58.Chang B., Smith R.S., Hawes N.L., et al. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21:405–409. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- 59.Turner A.J., Vander Wall R., Gupta V., et al. DBA/2J mouse model for experimental glaucoma: pitfalls and problems. Clin Exp Ophthalmol. 2017;45:911–922. doi: 10.1111/ceo.12992. [DOI] [PubMed] [Google Scholar]
- 60.Ail D., Ren D., Brazhnikova E., et al. Systemic and local immune responses to intraocular AAV vector administration in non-human primates. Mol Ther Methods Clin Dev. 2022;24:306–316. doi: 10.1016/j.omtm.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Millar J.C., Clark A.F., Pang I.H. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011;52:685–694. doi: 10.1167/iovs.10-6069. [DOI] [PubMed] [Google Scholar]
- 62.Weinreb R.N., Toris C.B., Gabelt B.T., et al. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47(Suppl 1):S53–S64. doi: 10.1016/s0039-6257(02)00306-5. [DOI] [PubMed] [Google Scholar]
- 63.Lim K.S., Nau C.B., O'Byrne M.M., et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008;115:790–795.e4. doi: 10.1016/j.ophtha.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S.S., Robinson M.R., Weinreb R.N. Episcleral venous pressure and the ocular hypotensive effects of topical and intracameral prostaglandin analogs. J Glaucoma. 2019;28:846–857. doi: 10.1097/IJG.0000000000001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson M., McLaren J.W., Overby D.R. Unconventional aqueous humor outflow: a review. Exp Eye Res. 2017;158:94–111. doi: 10.1016/j.exer.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buie L.K., Rasmussen C.A., Porterfield E.C., et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 2010;51:236–248. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee S.H., Sim K.S., Kim C.Y., Park T.K. Transduction pattern of AAVs in the trabecular meshwork and anterior-segment structures in a rat model of ocular hypertension. Mol Ther Methods Clin Dev. 2019;14:197–205. doi: 10.1016/j.omtm.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patil S.V., Kaipa B.R., Ranshing S., et al. Lentiviral mediated delivery of CRISPR/Cas9 reduces intraocular pressure in a mouse model of myocilin glaucoma. Sci Rep. 2024;14:6958. doi: 10.1038/s41598-024-57286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morgan J.E., Tribble J.R. Microbead models in glaucoma. Exp Eye Res. 2015;141:914. doi: 10.1016/j.exer.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 70.Tham Y.C., Li X., Wong T.Y., et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]