Abstract
We previously demonstrated that the ability of foot-and-mouth disease virus (FMDV) to form plaques in cell culture is associated with the suppression of alpha/beta interferon (IFN-α/β). In the present study, we used Escherichia coli-expressed porcine and bovine IFN-α or -β individually to demonstrate that each was equally effective in inhibiting FMDV replication. The block in FMDV replication appeared to be at the level of protein translation, suggesting a role for double-stranded RNA-dependent protein kinase (PKR). In support of these findings, treatment of porcine and bovine cells with 2-aminopurine, an inhibitor of PKR, increased the yield of virus 8.8- and 11.2-fold, respectively, compared to that in untreated infected cells. In addition, results of FMDV infection in mouse embryonic fibroblast cells derived from gene knockout mice lacking the gene for RNase L−/− or PKR−/− or both indicated an important role for PKR in the inhibition of FMDV replication.
Alpha/beta interferon (IFN-α/β) is the first line of host cell defense against virus infection. Virus-infected cells are induced to express and secrete IFN-α/β, which primes neighboring cells to a virus-resistant state via a series of events leading to activation of IFN-α/β-stimulated genes (ISGs) (8, 26). ISGs that have been extensively characterized include the genes for double-stranded RNA-dependent protein kinase (PKR), 2′-5′A synthetase/RNase L, and Mx (26, 27). The products of these genes affect viruses at different stages of their replication cycle, and different viruses are susceptible to different ISG products (8, 26).
Foot-and-mouth disease virus (FMDV) is a positive-stranded RNA virus in the family Picornaviridae (22). Viral RNA is translated into a polyprotein which is co- and posttranslationally processed by virus-encoded proteinases into mature viral proteins (22, 25). L proteinase, the first viral protein to be translated, cleaves itself from the polyprotein at its carboxy terminus (24). L proteinase also cleaves host translation initiation factor eIF-4G resulting in the shutoff of host cap-dependent mRNA translation (7, 10, 13, 15). Consequently, FMDV RNA, which initiates translation in a cap-independent fashion via an internal ribosome entry site (IRES) and does not require intact eIF-4G, can freely use the host protein synthesis machinery for viral protein production (6, 10, 14, 20, 22).
In a recent study, it was proposed that L proteinase is an FMDV virulence factor since its cleavage of eIF-4G suppresses translation of IFN-α/β mRNA (6, 9). It was demonstrated that FMDV infection induces IFN-α/β mRNA synthesis but the L proteinase inhibits cap-dependent IFN-α/β mRNA translation. The suppression of IFN-α/β protein production allows wild-type (WT) FMDV (A12-IC) to rapidly grow and spread in host cells, whereas a mutant FMDV lacking L proteinase (A12-LLV2) grows poorly in cells capable of an IFN-α/β response. The inhibitory effect of IFN-α/β on FMDV is further supported in this report. A pig kidney cell line (IBRS2), possessing IFN-α/β genes which are not inducible by FMDV, became resistant to both A12-IC and A12-LLV2 viruses when treated with supernatant from secondary pig kidney (PK) cells containing IFN-α/β or with Escherichia coli-expressed porcine IFN-α or IFN-β. IFN-α/β treatment of IBRS2 cells resulted in a block in FMDV protein synthesis, suggesting PKR involvement. The PKR inhibitory effect was also demonstrated in secondary cells from target species (PK and embryonic bovine kidney [EBK] cells) by the use of the PKR inhibitor 2-aminopurine (2-AP). The cellular IFN-inducible antiviral mechanism involved in the inhibition of FMDV replication was further investigated using ISG knockout mouse embryonic fibroblast (EF) cells. The results indicate an important role for PKR in host cell responses to FMDV infection.
MATERIALS AND METHODS
Cells and viruses.
Baby hamster kidney (BHK-21) cells (clone 13) were used to propagate virus stocks and to measure virus titers in plaque assays. Secondary PK and EBK cells and IBRS2 cells were provided by the Animal Plant and Health Inspection Service, National Veterinary Service Laboratory, Ames, Iowa, and the Foreign Animal Disease Diagnostic Laboratory, Plum Island Animal Disease Center, Greenport, N.Y. (11). Mouse EF cells derived from RNase L−, PKR−, double-knockout, or wild-type C57BL/6J mice lacking Mx1 (RNase L−/−, PKR−/−, RNase L−/− PKR−/−, or WT) were provided by Robert H. Silverman (27). RNase L−/− PKR−/− EF cells were referred to as triple deficient (TD) in this report since they lacked all three major ISG components (27).
FMDV A12-IC was derived from the full-length serotype A12 infectious clone, pRMC35 (21), and A12-LLV2 was derived from the infectious clone lacking the Lb coding region, pRM-LLV2 (19). Heparan sulfate-binding FMDV chimeras containing a serotype O1 capsid in an A12 background with or without L proteinase (vCRM48-KGE or vLLCRM48-KGE, respectively), provided by Peter W. Mason, were used in mouse cell experiments (1, 17, 23). In all assays, unless indicated, the multiplicity of infection (MOI) used was based on titration in BHK-21 cells.
IFN-α/β PCR and RT-PCR.
PCR and reverse transcription-PCR (RT-PCR) for IFN-α/β were performed using a previously described RT-PCR protocol and porcine-specific primers (6). The same primers were also used in PCR with DNA extracted from uninfected PK or IBRS2 cells to demonstrate the presence of the IFN-α/β genes. Sets of primers designed from the 5′ ends, directly downstream of the signal sequences, and the 3′ ends of porcine and bovine IFN-α or IFN-β sequence were used in PCR to amplify full-length IFN-α or IFN-β genes to produce constructs for E. coli expression.
E. coli-expressed porcine and bovine IFN.
mRNA was harvested from PK or EBK cells infected with A12-LLV2 at 6 h postinfection (hpi) and amplified by RT-PCR using IFN-α- or -β-specific primer sets for porcine or bovine species and Pfu DNA polymerase (Stratagene, La Jolla, Calif.) for 20 to 25 cycles. PCR products were cloned into the plasmid pCR-BluntII-TOPO (Invitrogen, Carlsbad, Calif.) and subcloned into pET-15b (Novagen, Madison, Wis.) downstream of a T7 promoter to produce pET-porcine-IFN-α or -β and pET-bovine-IFN-α or -β. These IFN-containing plasmids were sequenced to confirm specificity and used to transform BL21(DE3)-competent bacteria for high-level T7 promoter-driven expression. The expression of IFN was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) and confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using reticulocyte lysate-expressed IFNs obtained from an in vitro transcription and translation system (TNT kit; Promega, Madison, Wis.) as markers. Kinetics of IPTG induction for each protein were analyzed and the time of maximal yield was used in later experiments. These E. coli-expressed IFNs were acid-treated to pH 2.0, neutralized, and tested for biological activity in a standard IFN assay as described below. As a control, pET-15b containing the FMDV 3C proteinase coding region was expressed in E. coli, treated as above, and tested in a standard IFN assay.
IFN assay.
Cells were treated with supernatant containing IFN or E. coli-expressed IFN for 16 to 24 h and then infected with A12-IC or A12-LLV2 viruses. Cells were overlaid with gum tragacanth at 1 hpi and stained for plaques at 24 to 36 hpi (6).
Northern blot hybridization.
IBRS2 cells were treated with PK supernatant containing IFN-α/β for 16 h and infected at an MOI of 10 with A12-IC virus. RNA was harvested at 1, 2, and 3 hpi using an RNeasy Mini Kit (Qiagen, Valencia, Calif.) and run on a 1% formaldehyde agarose gel. RNA was transferred to a nylon membrane, fixed with UV light, and hybridized to a peroxidase-labeled full-length FMDV probe from the plasmid pRMC35 (21), and the signal was detected using a chemiluminescent technique (4).
Radioimmunoprecipitation assay.
IBRS2 cells were treated with PK supernatant containing IFN-α/β for 16 h and infected at an MOI of 10 with A12-IC virus. At 0.5, 1, 1.5, 2, 2.5, and 3 hpi, the supernatant was replaced with media without methionine for 0.5 h and then cells were radiolabeled with [35S]methionine for 0.5 h. Cells were harvested, and the viral proteins or viral 3D and its precursor proteins were detected in cell lysates by radioimmunoprecipitation using a convalescent-phase serum or polyclonal antibody against 3D, respectively, as described previously (5).
Inhibition of PKR activity by 2-AP.
PK or EBK cells were mock treated or treated with 2-AP at a concentration of 10 or 3 mM, respectively, for 4 h prior to infection (12). PK or EBK cells were infected with A12-IC or A12-LLV2 at an MOI of 0.05 (based on PK or EBK titer) for 1 h and treated with 150 mM NaCl and 20 mM 2-(N-morpholino)ethanesulfonic acid (MES), pH 6.0, to inactivate free virus and then were incubated with minimum essential medium in the absence or presence of 2-AP for 24 h. Supernatant samples were collected at 1 and 24 hpi for titration on BHK-21 cells.
Virus growth in cells from ISG knockout mice.
RNase L−/−, PKR−/−, TD, or WT EF cells were infected with heparan sulfate binding vCRM48-KGE and vLLCRM48-KGE viruses at an MOI of 1 (equivalent to an MOI of 0.1 in EF cells as measured by an infective-center assay [6]). At 1 hpi, cells were treated with MES. Viruses were harvested at 1 and 24 hpi and titrated on BHK-21 cells, and the increase of titer over 24 h was determined as virus growth for each cell type. Two independent experiments were performed and the ratio of the growths of vLLCRM48-KGE and vCRM48-KGE in each cell type was reported as a percentage.
RESULTS
Expression of porcine and bovine IFN-α and -β.
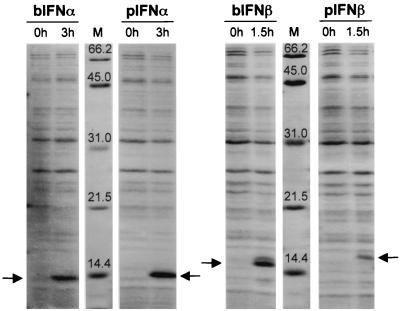
It had previously been shown that supernatant containing porcine or bovine IFN-α/β inhibited FMDV replication (6). To more directly study the effects of IFN-α and -β on FMDV replication, we amplified and cloned these IFN genes from PK and EBK cells using primers with consensus sequences specific to IFN-α and -β for each species. A clone of each IFN was sequenced and expressed in E. coli using the pET system (Fig. 1). At maximal induction, bovine IFN-α and -β and porcine IFN-α were expressed at similar levels, while porcine IFN-β was expressed at lower levels (Fig. 1). When treated at pH 2.0, serially diluted, and tested for biological activity, porcine and bovine IFN-α or -β produced similar inhibitory effects on FMDV replication in cells from homologous species including a pig kidney cell line (IBRS2) and EBK cells (Table 1). However, the control E. coli-expressed FMDV protein 3C had no inhibitory effect on virus replication (data not shown). Expressed porcine and bovine IFN-α or -β also had similar antiviral activities against vesicular stomatitis virus, encephalomyocarditis virus (EMCV), and classical swine fever virus (data not shown). In addition, we found that, unlike bovine IFN-β, porcine and bovine IFN-α and porcine IFN-β could inhibit FMDV replication in cells from the other species (Table 1).
FIG. 1.
Expression of bovine and porcine IFN-α or -β in E. coli. Lysates of BL21(DE3) cells containing bovine and porcine pET-IFN-α or -β (bIFN-α or -β and pIFN-α or -β), respectively, were collected at 0, 1.5, or 3 h after IPTG induction, run on an SDS-PAGE (15% acrylamide) and stained with Coomassie blue. Arrows indicate induced IFN proteins. Lanes M, protein molecular mass markers in kilodaltons.
TABLE 1.
Inhibitory effects of E. coli-expressed IFN on FMDV replication
| Cytokine | Highest dilution that reduced FMDV plaque no. by 50% in:
|
|
|---|---|---|
| EBK cells | IBRS2 cells | |
| Bovine IFN-α | 1:8,100 | 1:3,200 |
| Bovine IFN-β | >1:8,100 | –a |
| Porcine IFN-α | >1:8,100 | >1:8,100 |
| Porcine IFN-β | 1:1,600 | 1:4,050 |
–, less than 1:100.
Complementation of IBRS2 cells with IFN-α/β.
In cell cultures, which produce and respond to IFN-α/β, including secondary PK, EBK, and lamb kidney cells, A12-LLV2 infection results in slower virus growth and yields titers lower than those of A12-IC, and the virus is unable to form plaques (6). Here, we demonstrate that A12-LLV2 can spread to form plaques in the pig kidney cell line IBRS2 (Fig. 2A). To identify factors which contribute to the difference in A12-LLV2 resistance between PK and IBRS2 cells, RNA was harvested from A12-IC, A12-LLV2, or mock-infected cells at 6 hpi, treated with DNase I to eliminate DNA contamination, and used in IFN-α/β RT-PCRs (Fig. 2B). DNA was also extracted from uninfected PK and IBRS2 cells and was used to confirm the presence of intact IFN-α/β genes. We found that both PK and IBRS2 cells had intact IFN-α/β genes. However, upon FMDV infection, IFN-β was induced only in PK cells (Fig. 2B). Thus, the lack of IFN mRNA expression in IBRS2 cells correlated with the ability of A12-LLV2 to form plaques in this cell line (Fig. 2A). Resistance to A12-LLV2 could be introduced into IBRS2 cells by complementing cells with A12-LLV2-infected PK supernatant containing IFN-α/β or E. coli-expressed porcine IFN-α or -β (Fig. 3), suggesting that IBRS2 cells had a defect in IFN-α/β induction.
FIG. 2.
(A) Plaque formation ability of A12-LLV2 on PK or IBRS2 cells. Cells were infected with approximately 100 PFU of A12-LLV2, overlaid, and stained at 36 h postinoculation. (B) Induction of IFN-α or IFN-β mRNA in PK or IBRS2 cells. Cells were infected with A12-IC, A12-LLV2, or mock infected for 6 h and used in RT-PCR as described in Materials and Methods. Aliquots from RT reactions were used in three separate PCR assays with IFN-α, IFN-β, and β-actin primers. DNA from uninfected PK or IBRS2 cells was used in PCR assays as target controls. IFN-α, IFN-β, and β-actin RT-PCR products are 379, 452, and 890 bp, respectively. Lanes MW, 1-kb-ladder DNA molecular weight markers.
FIG. 3.
Plaque assay on IBRS2 cells. Cells were treated for 16 h with supernatant from mock-infected PK (A) or A12-LLV2-infected PK containing IFN-α/β (B) or with E. coli-expressed porcine IFN-α (C) or IFN-β (D) and then infected with approximately 100 PFU of A12-LLV2. Cells were overlaid and stained at 36 h postinoculation.
Effects of IFN-α/β on FMDV RNA and protein syntheses in IBRS2 cells.
To examine the step(s) in FMDV replication affected by IFN-α/β, IBRS2 cells were treated overnight with IFN-α/β and infected with A12-IC. The syntheses of FMDV RNA and protein were analyzed by Northern blot hybridization and immunoprecipitation, respectively. In untreated IBRS2 cells, newly synthesized A12-IC full-length RNA was initially detected between 1 and 2 hpi (Fig. 4). Treatment of IBRS2 cells with PK supernatant containing IFN-α/β prior to A12-IC infection delayed viral RNA synthesis to between 2 and 3 hpi, although there was still a significant amount of viral RNA produced by 3 hpi. In IFN-α/β-pretreated cells, production of viral protein 3D and its precursors was both delayed from 1 or 1.5 hpi to 2 or 2.5 hpi and significantly inhibited compared to that of mock-treated cells (Fig. 4B). Similar results were obtained using a convalescent-phase serum that detected an array of FMDV structural and nonstructural proteins (data not shown). Thus, in IFN-α/β-pretreated cells, the increased amount of A12-IC RNA observed between 2 to 3 hpi was not paralleled by an increased amount of viral protein production even up to 4 hpi. In contrast, in the absence of IFN-α/β treatment, the increase in viral protein production did parallel the increased amount of viral RNA.
FIG. 4.
Synthesis of FMDV full-length RNA and protein. IBRS2 cells were treated for 16 h with supernatant from A12-LLV2-infected PK cells containing IFN-α/β (+) or mock-infected PK cells (−) and infected at an MOI of 10 with A12-IC virus. (A) RNA was harvested at 1, 2, and 3 hpi and run on a 1% formaldehyde agarose gel. RNA was transferred to a nylon membrane and hybridized to a peroxidase-labeled full-length FMDV probe, and the signal was detected using a chemiluminescent technique. The arrow indicates full-length FMDV RNA. (B) [35S]methionine-labeled proteins were harvested at 1.5, 2, 2.5, 3, 3.5, and 4 h postinoculation, immunoprecipitated using a polyclonal antibody against FMDV nonstructural protein 3D, and analyzed on an SDS-PAGE (15% acrylamide). Arrows indicate 3D and precursors. Lane c, A12-IC-infected IBRS2 lysate immunoprecipitated with 3D antiserum.
PKR effect in IFN-α/β-competent PK and EBK cells.
The results from the IBRS2 cell experiment suggested that IFN-α/β pretreatment blocked translation of FMDV RNA, implicating a possible role for PKR. However, priming cells overnight with IFN-α/β prior to infection with FMDV may not accurately reflect the situation of a viral infection in which cells are infected before IFN-α/β induction. Since A12-LLV2 infection of PK and EBK cells results in expression and secretion of IFN-α/β, an experiment was performed without IFN pretreatment. IFN-α/β-competent PK and EBK cells were infected with A12-LLV2 at an MOI of 0.05 (based on the PK or EBK titer), to allow IFN-α/β produced in initially infected cells to induce an antiviral state in neighboring uninfected cells, and the impact of PKR on FMDV replication was determined using the PKR inhibitor 2-AP. Various concentrations (30, 10, and 3 mM) of 2-AP were tested on PK and EBK cells prior to the experiments to determine the amount that was not toxic to each cell type. PK and EBK cells were infected in the presence or absence of 2-AP. The titers at 24 hpi showed that 2-AP treatment at 10 and 3 mM concentrations increased the yield of A12-LLV2 in PK and EBK cells 8.8- and 11.2-fold, respectively, compared to the yield for untreated infected cells (from 4 × 102 to 3.5 × 103 PFU/ml in PK cells and from 2.5 × 102 to 2.8 × 103 PFU/ml in EBK cells). In contrast, 2-AP treatment increased the yield of A12-IC in PK cells only 2.3-fold (from 1.9 × 105 to 4.4 × 105 PFU/ml) and did not enhance replication in EBK cells (from 1.6 × 105 to 2.5 × 104 PFU/ml).
FMDV resistance is PKR and RNase L dependent.
To further characterize the IFN-induced antiviral defense that is responsible for the inhibition of FMDV replication, EF cells derived from ISG knockout mice were infected with WT FMDV or FMDV lacking the gene for L proteinase (“L-deleted FMDV”). Since FMDV A12 (A12-IC and A12-LLV2), which attaches to cells via the integrin αvβ3 (2, 17), could not productively infect mouse cells, we used another pair of genetically similar FMD viruses which attach to cells by binding to heparan sulfate and could productively infect EF cells. The yield of WT FMDV in these cells was approximately 104 to 105 PFU/106 cells at 24 hpi. The heparan sulfate-binding WT FMDV (vCRM48-KGE) and its L proteinase-deleted derivative (vLLCRM48-KGE) are chimeric viruses containing the capsid coding region from serotype O1 Campos in A12-IC and A12-LLV2 genetic backgrounds, respectively (1, 17, 23). The EF cells were infected with vCRM48-KGE or vLLCRM48-KGE virus at a low MOI (see Materials and Methods), and the yield of each virus was determined at 24 hpi. Three types of EF cells derived from ISG knockout mice, all of which are lacking Mx1, were used, including PKR−/−, RNase L−/−, and TD (PKR−/− RNase L−/−) cells (27). As expected, vLLCRM48-KGE virus yielded a titer which was only 3% of the vCRM48-KGE virus titer at 24 hpi in WT EF cells that contained both PKR and RNase L (Fig. 5). vLLCRM48-KGE virus yielded titers which were 76 or 19% of vCRM48-KGE virus titers in cells which were either PKR or RNase L deficient, respectively (Fig. 5). However, in TD cells, both viruses grew to similar titers (the vLLCRM48-KGE titer was 91% of the vCRM48-KGE titer) (Fig. 5). These results demonstrated that the presence of PKR significantly reduced the yield of vLLCRM48-KGE virus, while RNase L had a lesser effect.
FIG. 5.
Growth of FMDV on EF cells. WT, RNase L−/−, PKR−/− or TD EF cells were infected with vCRM48-KGE or vLLCRM48-KGE virus at a low MOI. At 1 hpi, cells were treated with MES. Viruses were harvested at 1 and 24 hpi, titrated on BHK-21 cells, and the increase of titer over 24 h was determined as virus growth. The ratio of the growths of vLLCRM48-KGE and vCRM48-KGE in each cell type was reported as a percentage.
DISCUSSION
FMDV L proteinase shuts off host cap-dependent mRNA translation, thus allowing the virus to use the host cell protein synthesis machinery with little competition (6, 7, 9, 10, 13, 15, 20, 22). In a previous report, it was demonstrated that by utilizing this strategy, FMDV suppresses IFN-α/β protein production, resulting in rapid virus growth and spread (6). In contrast, the infectivity of an L-deleted virus, A12-LLV2, which does not have the ability to shut off IFN-α/β protein production, was profoundly restricted in IFN-α/β-competent cells such as PK and EBK cells (6).
In this study, we demonstrate that FMDV growth can be artificially suppressed in a cell line, IBRS2, that cannot be induced to express IFN-α/β mRNA by complementation with IFN-α/β proteins. We also demonstrate that IFN-α of porcine or bovine origin and porcine IFN-β could exert an inhibitory effect on FMDV in cells from homologous and heterologous species, whereas bovine IFN-β exerted its effect only on bovine cells.
IFN-α/β presumably inhibits FMDV by binding to the IFN-α/β receptor and initiating a series of events leading to the activation of ISGs within these cells. The three most extensively studied ISG components include PKR, 2′-5′A synthetase/RNase L, and Mx. EMCV, another picornavirus, has been shown to be susceptible to the 2′-5′A synthetase RNase L pathway (26), although a role for PKR in the inhibition of EMCV replication was also demonstrated by constitutive expression of human PKR in mouse cells or by experiments in EF cells derived from ISG knockout mice (16, 27). Analysis of viral protein and RNA syntheses in infected IBRS2 cells showed that IFN-α/β treatment significantly inhibited viral protein synthesis even in the presence of newly synthesized viral RNA, suggesting the involvement of PKR in translation shutoff that blocked virus replication. A slower rate of viral RNA synthesis in IFN-α/β-treated cells could be due either to reduced levels of the viral RNA polymerase (3D) (demonstrated in Fig. 4B) because of translation inhibition (possibly by PKR) or to RNA degradation by the 2′-5′A synthetase RNase L pathway. However, we have not been able to detect FMDV RNA degradation by either Northern blot hybridization or sucrose gradient analysis (data not shown).
Utilizing our WT and L-deleted mutant pair system, we were able to demonstrate the significance of these ISGs in FMDV suppression in secondary PK and EBK cells and EF cells without the need to preexpose cells to IFN-α/β, thereby avoiding induction of ISGs prior to virus infection. We utilized a PKR inhibitor, 2-AP, which has been shown to inhibit activation of PKR (12). 2-AP treatment increased the yield of L-deleted FMDV 8.8- and 11.2-fold in PK and EBK cells, respectively. A similar result was also reported by 2-AP treatment of HeLa cells infected with a poliovirus 2Apro mutant that, similar to L-deleted FMDV, does not cleave eIF-4G and thus does not shut off host protein synthesis (18). As expected, 2-AP treatment had little or no effect on WT FMDV infection. The significance of PKR and/or 2′-5′A synthetase RNase L in the inhibition of FMDV replication was further investigated in EF cells derived from ISG knockout mice. In this experiment, approximately 10% of cells were initially infected and the infection continued for 24 h. We demonstrated that IFN-α/β mRNA expression was inducible in EF cells by WT and L-deleted FMDV infections (data not shown) and we expected to observe the impact of ISGs in L-deleted FMDV infection because of the translation of IFN-α/β mRNA. The yield of L-deleted FMDV was compared to WT FMDV in a percentage format to allow a relative quantitation of the involvement of each ISG product in the inhibition of virus replication. In WT EF cells, L-deleted viruses grew 33-fold more poorly than the L-expressing viruses, but in EF cells derived from PKR gene knockout mice, the L-deleted viruses grew almost as well as L-expressing viruses. Thus, PKR exerts a significant effect on FMDV suppression. Similarly, the role of RNase L was examined using this virus pair in EF cells derived from RNase L gene knockout mice, but the impact of RNase L may not be accurately assessed in this study because the 2′-5′A synthetase RNase L system is relatively weak in these cells (27). Nevertheless, our results indicated that PKR and 2′-5′A synthetase RNase L were mainly responsible for the inhibition of L-deleted FMDV replication. However, the effect of the Mx pathway on FMDV infection could not be determined with this system.
Picornaviruses “take over” cellular macromolecular synthesis by inactivating host cap-dependent protein synthesis and utilizing viral IRES-dependent translation. Therefore, for infected TD cells, in which all of the three major ISG components are absent, we hypothesized that WT virus infection would result in higher virus yields than L-deleted virus infection because of translation competition between cellular mRNAs and viral RNAs in L-deleted virus infected cells. Surprisingly, the growth of the L-deleted virus in TD cells approached that of WT virus (Fig. 5). This suggests that translation competition has only a small inhibitory effect on the growth of the L-deleted virus in TD cells and/or that other ISGs also have only a minor role in the suppression of FMDV replication.
We have demonstrated that, in FMDV infection of cells in culture, the main effect of host protein synthesis shutoff by the FMDV L proteinase is the suppression of the primary antiviral response. It is apparent that if host cells are allowed to express and secrete IFN-α/β, as observed with L-deleted FMDV infection, then PKR and, to some degree, 2′-5′A synthetase RNase L pathways will have an inhibitory effect on FMDV. PKR exerts its effect by phosphorylating eIF2α, thereby inhibiting protein translation of not only host-capped mRNA but also viral IRES-regulated mRNA.
As we have previously shown, L-deleted FMDVs are attenuated in their natural hosts, including swine and bovines (1, 3, 5, 14). Their attenuation appears related to the inability to block synthesis of cytokines such as IFN-α/β due to the absence of L proteinase activity. Thus, the identification of inhibitors of L proteinase or of ISG products, such as PKR, may be useful in developing antiviral strategies to block the spread and shedding of FMDV in infected animals.
ACKNOWLEDGMENTS
We thank Carole Harbison and Tracy DeMeola for assistance in construction of IFN-α/β plasmids. We acknowledge the Animal Plant and Health Inspection Service, National Veterinary Service Laboratory, Ames, Iowa, and the Foreign Animal Disease Diagnostic Laboratory, Plum Island Animal Disease Center, Greenport, N.Y., for EBK, PK, and IBRS2 cells; Robert H. Silverman, Aimin Zhou, and Bryan R.G. Williams, Cleveland Clinic Foundation, for EF cells derived from knockout mice; and Peter W. Mason, Plum Island Animal Disease Center, for heparan sulfate-binding vCRM48-KGE and vLLCRM48-KGE viruses.
REFERENCES
- 1.Almeida M R, Rieder E, Chinsangaram J, Ward G, Beard C, Grubman M J, Mason P W. Construction and evaluation of an attenuated vaccine for foot-and-mouth disease: difficulty adapting the leader proteinase-deleted strategy to the serotype O1 virus. Virus Res. 1998;55:49–60. doi: 10.1016/s0168-1702(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 2.Berinstein A, Roivainen M, Hovi T, Mason P W, Baxt B. Antibodies to the vitronectin receptor (integrin alpha V beta 3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J Virol. 1995;69:2664–2666. doi: 10.1128/jvi.69.4.2664-2666.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown C C, Piccone M E, Mason P W, McKenna T S, Grubman M J. Pathogenesis of wild-type and leaderless foot-and-mouth disease virus in cattle. J Virol. 1996;70:5638–5641. doi: 10.1128/jvi.70.8.5638-5641.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinsangaram J, Akita G Y, Osburn B I. Detection of bovine group B rotaviruses in feces by polymerase chain reaction. J Vet Diagn Investig. 1994;6:302–307. doi: 10.1177/104063879400600304. [DOI] [PubMed] [Google Scholar]
- 5.Chinsangaram J, Mason P W, Grubman M J. Protection of swine by live and inactivated vaccines prepared from a leader proteinase-deficient serotype A12 foot-and-mouth disease virus. Vaccine. 1998;16:1516–1522. doi: 10.1016/S0264-410X(98)00029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinsangaram J, Piccone M E, Grubman M J. Ability of foot-and-mouth disease virus to form plaques in cell culture is associated with suppression of alpha/beta interferon. J Virol. 1999;73:9891–9898. doi: 10.1128/jvi.73.12.9891-9898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devaney M A, Vakharia V N, Lloyd R E, Ehrenfeld E, Grubman M J. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J Virol. 1988;62:4407–4409. doi: 10.1128/jvi.62.11.4407-4409.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodbourn S, Didcock L, Randall R E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 9.Grubman M J, Chinsangaram J. Foot-and-mouth disease virus: the role of the leader proteinase in viral pathogenesis. Recent Res Dev Virol. 2000;2:123–134. [Google Scholar]
- 10.Guarne A, Tormo J, Kirchweger R, Pfistermueller D, Fita I, Skern T. Structure of the foot-and-mouth disease virus leader protease: a papain-like fold adapted for self-processing and eIF4G recognition. EMBO J. 1998;17:7469–7479. doi: 10.1093/emboj/17.24.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.House C, House J A. Evaluation of techniques to demonstrate foot-and-mouth disease virus in bovine tongue epithelium: comparison of the sensitivity of cattle, mice, primary cell cultures, cryopreserved cell cultures and established cell lines. Vet Microbiol. 1989;20:99–109. doi: 10.1016/0378-1135(89)90033-3. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Conway T W. 2-Aminopurine inhibits the double-stranded RNA-dependent protein kinase both in vitro and in vivo. J Interferon Res. 1993;13:323–328. doi: 10.1089/jir.1993.13.323. [DOI] [PubMed] [Google Scholar]
- 13.Kirchweger R, Ziegler E, Lamphear B J, Waters D, Liebig H D, Sommergruber W, Sobrino F, Hohenadl C, Blaas D, Rhoads R E, Skern T. Foot-and-mouth disease virus leader proteinase: purification of the Lb form and determination of its cleavage site on eIF-4 gamma. J Virol. 1994;68:5677–5684. doi: 10.1128/jvi.68.9.5677-5684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason P W, Piccone M E, McKenna T S, Chinsangaram J, Grubman M J. Evaluation of a live-attenuated foot-and-mouth disease virus as a vaccine candidate. Virology. 1997;227:96–102. doi: 10.1006/viro.1996.8309. [DOI] [PubMed] [Google Scholar]
- 15.Medina M, Domingo E, Brangwyn J K, Belsham G J. The two species of the foot-and-mouth disease virus leader protein, expressed individually, exhibit the same activities. Virology. 1993;194:355–359. doi: 10.1006/viro.1993.1267. [DOI] [PubMed] [Google Scholar]
- 16.Meurs E F, Watanabe Y, Kadereit S, Barber G N, Katze M G, Chong K, Williams B R, Hovanessian A G. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J Virol. 1992;66:5804–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neff S, Sa-Carvalho D, Rieder E, Mason P W, Blystone S D, Brown E J, Baxt B. Foot-and-mouth disease virus virulent for cattle utilizes the integrin alpha(v)beta3 as its receptor. J Virol. 1998;72:3587–3594. doi: 10.1128/jvi.72.5.3587-3594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Neill R E, Racaniello V R. Inhibition of translation in cells infected with a poliovirus 2Apro mutant correlates with phosphorylation of the alpha subunit of eucaryotic initiation factor 2. J Virol. 1989;63:5069–5075. doi: 10.1128/jvi.63.12.5069-5075.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccone M E, Rieder E, Mason P W, Grubman M J. The foot-and-mouth disease virus leader proteinase gene is not required for viral replication. J Virol. 1995;69:5376–5382. doi: 10.1128/jvi.69.9.5376-5382.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccone M E, Zellner M, Kumosinski T F, Mason P W, Grubman M J. Identification of the active-site residues of the L proteinase of foot-and-mouth disease virus. J Virol. 1995;69:4950–4956. doi: 10.1128/jvi.69.8.4950-4956.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieder E, Bunch T, Brown F, Mason P W. Genetically engineered foot-and-mouth disease viruses with poly(C) tracts of two nucleotides are virulent in mice. J Virol. 1993;67:5139–5145. doi: 10.1128/jvi.67.9.5139-5145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rueckert R R. Picornaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P H, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 609–654. [Google Scholar]
- 23.Sa-Carvalho D, Rieder E, Baxt B, Rodarte R, Tanuri A, Mason P W. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J Virol. 1997;71:5115–5123. doi: 10.1128/jvi.71.7.5115-5123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strebel K, Beck E. A second protease of foot-and-mouth disease virus. J Virol. 1986;58:893–899. doi: 10.1128/jvi.58.3.893-899.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vakharia V N, Devaney M A, Moore D M, Dunn J J, Grubman M J. Proteolytic processing of foot-and-mouth disease virus polyproteins expressed in a cell-free system from clone-derived transcripts. J Virol. 1987;61:3199–3207. doi: 10.1128/jvi.61.10.3199-3207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilcek J, Sen G C. Interferons and other cytokines. In: Fields B N, Knipe D M, Howley P H, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 375–399. [Google Scholar]
- 27.Zhou A, Paranjape J M, Der S D, Williams B R, Silverman R H. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]