Abstract
Introduction
Preterm delivery (PTD) is the leading cause of death in children under 5 years of age. Cervical shortening detected by ultrasound can be used to predict PTD, but prediction is not perfect, and complementary diagnostic markers are needed. Recently, specific plasma microribonucleic acid (miRNAs) detected in early second trimester were shown to be associated with spontaneous PTD in high‐risk women with a singleton pregnancy. The aim of this study was to explore to what extent these miRNAs are associated with spontaneous PTD and cervical length in a general population.
Material and Methods
This study is a nested case–control study within the CERVIX study. The CERVIX study evaluated the ability of cervical length screening with transvaginal ultrasound to identify women at risk of PTD. In the present study, women who delivered spontaneously <34 weeks (n = 61) were compared with a control group of women who delivered at full term (39 + 0 to 40 + 6 gestational weeks, n = 205). Archived serum samples were analyzed with RT‐qPCR for miRNA expression levels of let‐7a‐5p, miR‐150‐5p, miR‐15b‐5p, miR‐185‐5p, miR‐191‐5p, miR‐19b‐3p, miR‐23a‐3p, miR‐374a‐5p, and miR‐93‐5p. The mean relative expression was compared between the groups. Sub‐analyses were performed for women delivering <32, <30, and <28 weeks vs the full‐term group.
Results
The analyzed miRNAs were not significantly differentially expressed in women delivering <34 weeks compared to those delivering at full term. MiR‐191‐5p and miR‐93‐5p were significantly overexpressed in women who delivered <32 weeks, and further increase in fold change was observed with decreasing gestational age at delivery. The level of miR‐15b‐5p was significantly higher in women delivering at <30 weeks compared to those delivering at full term.
Conclusions
Our study shows that overexpression of miR‐93‐5p, miR‐15b‐5p, and miR‐191‐5p in serum at early gestation is associated with spontaneous PTD in a general population. Further research is needed to evaluate the potential of these miRNAs as future biomarkers for spontaneous PTD, as well as their pathophysiological role in spontaneous PTD.
Keywords: cervical length measurement, gene expression profiles, maternal serum screening tests, MicroRNA, preterm birth
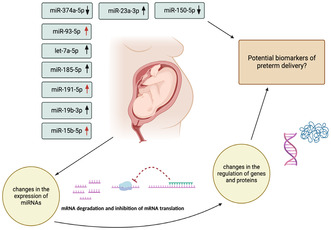
Microribonucleic acids (miRNAs) are small, single‐stranded, non‐protein‐coding RNAs with an important posttranscriptional gene regulation function. Because of their stable molecular structure, miRNAs have been suggested as potential biomarker candidates (e.g., cancer and inflammatory diseases). Nine specific miRNAs have previously been shown to be correlated with spontaneous preterm delivery in a high‐risk population of singleton pregnancies. In this study, these nine miRNAs were investigated in serum in a low‐risk population. An upright arrow indicates an upregulation of the miRNA in those delivering preterm in comparison with those delivering at term, while a downward‐facing arrow indicates a downregulation of the miRNA in those delivering preterm versus at term. A red arrow indicates a statistically significant up‐ or downregulation of the investigated miRNA in those delivering early preterm in our low‐risk population.
Abbreviations
- Cx1
cervical length measurement at 18–20 gestational weeks
- Cx2
cervical length measurement at 21–23 gestational weeks
- miRNA/miR
microribonucleic acid
- mRNA
messenger ribonucleic acid
- PTD
preterm delivery
Key message.
Preterm birth is the leading cause of death in children under 5 years of age. Improved diagnostic tools to identify women at increased risk of spontaneous preterm delivery are needed. Specific microRNAs show promise as possible biomarkers.
1. INTRODUCTION
Globally, 13.4 million babies were estimated to be born preterm (<37 + 0 weeks of gestation) in 2020 with an occurrence ranging from 5% to 6% in northern Europe to 16% in some Asian countries. 1 , 2 , 3 Preterm delivery (PTD) accounts for more than 1 million perinatal deaths annually and is the leading cause of death under 5 years of age. 4 , 5 , 6 Both the risk of death and long‐term impairment, including neurologic and developmental disabilities, are inversely related to gestational age at birth. 7 , 8
Spontaneous PTD is a complex syndrome with multiple etiologies, 9 and large parts of its pathophysiology are still insufficiently understood. 10 Cervical remodeling is essential in the parturition process and is associated with an inflammatory response including upregulation of prostaglandins, chemokines, cytokines, inflammatory cell infiltration, and an increased matrix metalloproteinase activity. 11 This process of cervical ripening can anticipate labor by several weeks 12 and can be observed as cervical shortening with transvaginal ultrasound. A short cervix (often defined as ≤25 mm), as measured by transvaginal ultrasound in the second trimester, is associated with increased risk of spontaneous PTD 13 , 14 , 15 , 16 , 17 , 18 in both low‐ and high‐risk singleton pregnancies. 19 Therefore, universal second‐trimester cervical length screening with transvaginal ultrasound has been proposed 20 and shown to be cost‐effective in several cost‐effectiveness analyses. 21 , 22 , 23 , 24 , 25 However, cervical length screening is expensive and requires ultrasound expertise. Moreover, the ability of cervical length to discriminate between women who deliver preterm vs at term is at best moderate. 17 , 25 It would be economically and practically advantageous if there was an easily accessible biomarker to predict spontaneous PTD that could be used as a complement to cervical length measurement.
Microribonucleic acids (miRNAs) are small, single‐stranded, non‐protein‐coding RNAs with an important posttranscriptional gene‐regulating function. Most miRNAs predominantly repress gene expression by inhibiting the translation of target messenger ribonucleic acids (mRNAs), and they are estimated to regulate up to 60% of the human genome expression. 26 , 27 The expression of miRNAs differs between tissue types and tends to show significant modification in response to pathological processes. Additionally, miRNAs are shed into the circulation where they remain stable and measurable. This highlights their potential as diagnostic and prognostic biomarkers in a variety of diseases. 28 , 29
Cook et al. recently reported an association between the expression of nine specific miRNAs in plasma (let‐7a‐5p, miR‐374a‐5p, miR‐15b‐5p, miR‐19b‐3p, miR‐23a‐3p, miR‐93‐5p, miR‐150‐5p, miR‐185‐5p, and miR‐191‐5p) and spontaneous PTD <34 + 0 gestational weeks (weeks) in a high‐risk population of singleton pregnancies. The samples were taken as early as 12 weeks of gestation, emphasizing their potential as early biomarkers of spontaneous PTD. 30 This could allow for earlier detection and intervention in those at risk of spontaneous PTD and potentially improve neonatal outcomes. With this as a background, the aim of this study is to evaluate the association between these nine miRNAs, cervical length, and spontaneous PTD in a general population with singleton pregnancies including those at low risk of PTD.
2. MATERIAL AND METHODS
2.1. Study design
The study is a nested case–control study within the CERVIX study, a prospective observational blinded multicenter study conducted at six university hospitals and one regional hospital in Sweden between 2014 and 2017. The aim of the CERVIX study was to investigate the ability of cervical length as measured with transvaginal ultrasound in the second trimester in an unselected population of women with a singleton pregnancy to correctly discriminate between those delivering preterm vs at full term (39 + 0 to 40 + 6 weeks). The study population consisted of 11 072 women with transvaginal cervical length measurement at 18 + 0 to 20 + 6 weeks (Cx1), 6288 women with cervical length measurements at 21 + 0 to 23 + 6 weeks (Cx2), and 6179 women with measurements taken at both Cx1 and Cx2, all with complete delivery data (https://doi.org/10.1186/ISRCTN18093885). The details of the CERVIX study have been described in a previous publication. 17 In short, asymptomatic women (i.e., without signs of preterm labor) with a singleton pregnancy were consecutively recruited to the CERVIX study at their routine second‐trimester fetal ultrasound examination, which included fetal biometry for estimation of gestational age and fetal anatomy scanning. Those ≥18 years old with a live singleton pregnancy between 18 + 0 and 20 + 6 weeks were invited to participate. Gestational age was estimated on the basis of ultrasound measurement of the fetal biparietal diameter, 31 , 32 or on the day of embryo transfer in case of in vitro fertilization according to Swedish guidelines (https://www.sfog.se/media/336451/fetometri.pdf). Women with fetal malformations detected at the ultrasound scan, ruptured membranes, symptoms or findings indicating ongoing miscarriage, current use of progesterone or cerclage in situ, and difficulties with understanding written or oral information about the study were not included in the study. Women with missing information about pregnancy outcomes were also excluded.
Transvaginal cervical length measurements were performed by specially trained midwife sonographers. They were certified to perform transvaginal cervical length measurements after standardized theoretical and practical training, and after having passed a theoretical and practical test. Cervical length was measured with the study participants in the lithotomy position with an empty urinary bladder. The measurement results were recorded in a web‐based electronic case record form (MedSciNet AB, Stockholm, Sweden, www.medscinet.se) together with standardized anamnestic information obtained from the women at enrollment. Details about training, certification, measurement procedure, and quality control of the cervical length measurements have been described in a previous publication. 33 Cervical length results presented in this study are the shortest of three measurements of endocervical length taken during at least 3 min.
In Sweden, most pregnant women undergo serological screening for human immunodeficiency virus (HIV), syphilis, and hepatitis C in early pregnancy (between 10 and 14 weeks of gestation) as recommended by the National Board of Health and Welfare. 34 Serological samples taken for screening for infectious diseases during pregnancy are routinely stored in a biobank for retrospective analysis if clinically indicated, and for research purposes when possible. 35 The Regional Ethical Committee in Gothenburg approved the use of the saved serological samples for those who participated in the CERVIX study at Sahlgrenska University Hospital in Gothenburg and Skåne University Hospital in Lund‐Malmö and who gave birth before 34 + 0 weeks, as well as for a control group of women who gave birth at full term (39 + 0 to 40 + 6 weeks).
2.2. Reference standard and group composition
The reference standard was gestational age at delivery based on fetal biometry (see above). Our primary outcome was spontaneous PTD <34 + 0 weeks (including late miscarriage occurring after inclusion in the study, i.e., spontaneous delivery at 18 + 0 to 21 + 6 weeks of a fetus showing no signs of life). Our secondary outcomes were spontaneous PTD <32 + 0 weeks, spontaneous PTD <30 + 0 weeks, and spontaneous PTD <28 + 0 weeks. A PTD was defined as spontaneous either after spontaneous onset of labor or after preterm prelabor rupture of membranes, the latter regardless of whether labor was induced or not. The diagnosis of spontaneous PTD in the index pregnancy was validated by scrutiny of medical records. For each case of spontaneous PTD or late miscarriage, three controls were selected. 36 The controls were chosen using simple random selection of women who had completed the study with spontaneous start of delivery at 39 + 0 to 40 + 6 gestational weeks, either after spontaneous onset of labor or after prelabor rupture of membranes. The interval of 39 + 0 to 40 + 6 weeks was chosen because delivery at early term, as well as at late term and postterm, is associated with a slightly higher risk of complications. 37 , 38 , 39 , 40 , 41
2.3. Clinical data collection
Information about maternal characteristics was retrieved from three Swedish national health registers hosted by the Swedish National Board of Health and Welfare (www.socialstyrelsen.se), as well as from the electronic case record form (standardized anamnestic information obtained from the women at enrollment). The procedure has been described in previous publications. 17 , 19 Information about pregnancy outcomes was obtained from the Swedish Pregnancy Register (www.graviditetsregistret.se), a Swedish national quality register. 42 If delivery data were missing in the Swedish Pregnancy Register, medical records were scrutinized. If no information was found in the records, the participants were contacted by mail or telephone.
2.4. Statistical analysis of clinical data
We present results as mean, standard deviation, and standard error of the mean, or as median, interquartile range, minimum, and maximum for continuous variables. For categorical variables, we present results as numbers and percentages. The Statistical software SAS System Version 9.4, SAS‐Institute, Cary, NC, USA, was used for analyses.
2.5. Serum sample collection
Blood samples were collected between 2014 and 2017. They were taken at the antenatal care clinic at gestational weeks 10 to 14 and were handled according to clinical routine for screening of HIV, syphilis, and hepatitis C as recommended by the Swedish National Board of Health and Welfare. 34 After collection, blood samples were stored in a refrigerator and centrifuged on the same day (800×g, 10 min, 4°C) to obtain serum. Thereafter, aliquots of serum were frozen and stored at −20°C in a biobank until analysis.
2.6. RNA isolation and real‐time quantitative polymerase chain reaction for miRNA expression and quantification
Samples were prepared for analysis in 2022. After thawing, the serum was centrifuged (12 000×g, 15 min at room temperature) to remove cell debris and precipitates. The risk of hemolysis in serum samples was assessed by measuring the absorbance of hemoglobin using spectrophotometry at 414 nm (Thermo Scientific™ NanoDrop™ 2000). 43 Cell‐free RNA from serum (100‐200 μL) was prepared with Quick‐cfRNA™ Serum & Plasma Kit (catalog No. R1059, Zymo Research, Irvine, USA). RNA was reversed transcribed to cDNA by using miRCURY LNA RT kit (catalog No. 339340, Qiagen, Hilden, Germany). Quality control spike‐ins were used to monitor RNA extraction (cel‐miR‐254‐3p; Integrated DNA Technologies BVBA, Leuven, Belgium) and cDNA synthesis (UniSp6; Qiagen, Hilden, Germany) efficacy. 30 The RT‐qPCR was performed using miRCURY LNA miRNA Custom PCR Panels (catalog No. 339330, Qiagen, Hilden, Germany), and miRCURY LNA SYBR Green PCR Kit and ROX dye as a reference (Catalogue No. 339347, Qiagen, Hilden, Germany) on QuantStudio 7Pro PCR machine (Applied Biosystems, Waltham, USA). Custom PCR panels consisted of primers for let‐7a‐5p, miR‐150‐5p, miR‐15b‐5p, miR‐185‐5p, miR‐191‐5p, miR‐19b‐3p, miR‐23a‐3p, miR‐374a‐5p, miR‐93‐5p, cel‐miR‐254‐3p, UniSp6, and UniSp3 (interplate control).
2.7. RT‐qPCR data analysis
The LinRegPCR program v2017.1 44 was used to determine quantification cycle (Cq) values and amplification efficiencies for all miRNA targets. Preprocessing of Cq data consisted of normalization to the interplate control (UniSp3), RNA extraction control (cel‐miR‐254‐3p), and the reverse transcription control (UniSp6). To evaluate the miRNA expression, Cq values of target miRNAs were used to obtain relative expression to endogenous controls (2−ΔCq).
The two endogenous miRNA controls were identified from the nCounter assay data by Cook et al. 30 using NormFinder 45 which incorporates the inter‐ and intragroup variances and calculates the stability value per miRNA target as a measure of expression stability. It also identifies the best combination of miRNA targets to provide the highest stability.
2.8. Statistical analysis of serum miRNA expression
Values obtained from the PTD cases and controls were subsequently used for univariate analyses with a non‐parametric Mann–Whitney U‐test (two tailed) to compare the mean relative expressions between the groups. A p‐value of <0.05 was considered statistically significant.
To test whether hemolysis influenced the results of the miRNA expression analyses, we performed interaction analyses using linear regression. The miRNAs were used as the dependent variable one at a time, and spontaneous PTD/full‐term delivery, hemolysis, and an interaction term between spontaneous PTD/full‐term delivery and hemolysis were used as independent variables.
The correlation between the normalized miRNA expression values and cervical length was calculated using Spearman's rank correlation. 46 Correlation was tested both for cervical length as a continuous variable and for specific cut‐offs from ≤25 mm and ≤30 mm. The correlation between the normalized miRNA expression values and gestational age was also tested using Spearman's rank correlation. 46 A p‐value of <0.05 was considered statistically significant.
All statistical analyses were performed using SAS 9.4 (SAS‐Institute, Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics and clinical outcomes
A flowchart showing the study design and study population is presented in Figure 1. We included 61 women with spontaneous PTD (<34 + 0 weeks) and 205 women with spontaneous full‐term delivery (39 + 0 to 40 + 6 weeks). Maternal characteristics are shown in Table 1. About 90% of the women were of white ethnicity and the majority had at least 12 years of education. The proportion of women with an educational level <12 years, low socioeconomic status, and a previous spontaneous PTD were higher in the preterm group compared to the term group. None of the included women had undergone cervical conization. Characteristics regarding pregnancy, delivery, and neonatal outcomes are described in Table 2. The mean gestational age at delivery was 31 + 3 weeks in the preterm group and 40 + 0 weeks in the term group. No woman in either group had a redeemed prescription of progesterone or had a cerclage inserted after inclusion in the study. Maternal characteristics and pregnancy outcomes for women who gave birth spontaneously at <32 + 0 weeks (n = 27), <30 + 0 weeks (n = 17), and < 28 + 0 weeks (n = 8) are also shown in Tables 1 and 2.
FIGURE 1.
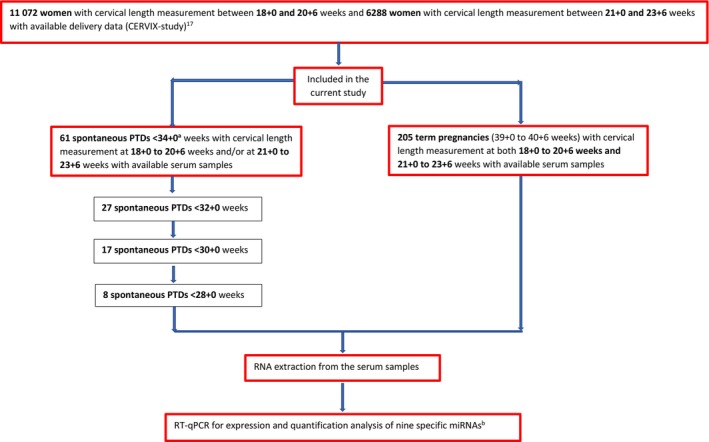
Flowchart showing the study design and group composition. PTD, preterm delivery; RT‐qPCR, quantitative reverse‐transcription polymerase chain reaction; miRNA, microRNA. aIncluding one spontaneous late miscarriage <22 + 0 weeks occurring after inclusion in the study. bExamined miRNAs: let‐7a‐5p, miR‐374a‐5p, miR‐15b‐5p, miR‐19b‐3p, miR‐23a‐3p, miR‐93‐5p, miR‐150‐5p, miR‐185‐5p, and miR‐191‐5p. 30
TABLE 1.
Baseline maternal characteristics.
| Control (39 + 0 to 40 + 6 weeks) | sPTD <34 + 0 weeks | sPTD <32 + 0 weeks | sPTD <30 + 0 weeks | sPTD <28 + 0 weeks | |
|---|---|---|---|---|---|
| Variable | n = 205 | n = 61 | n = 27 | n = 17 | n = 8 |
| Maternal age at delivery (years) | 31.7 (4.3) | 31.4 (4.7) | 30.6 (5.1) | 31.7 (4.3) | 27.8 (4.6) |
| Ethnicity | |||||
| White | 190 (92.7%) | 55 (90.2%) | 23 (85.2%) | 14 (82.4%) | 6 (75.0%) |
| Black | 1 (0.5%) | 2 (3.3%) | 2 (7.7%) | 2 (11.8%) | 2 (25.0%) |
| Mixed White Black | 3 (1.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Middle East | 4 (1.9%) | 2 (3.3%) | 1 (3.8%) | 1 (5.9%) | 0 (0.0%) |
| India | 2 (1.0%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Southeast Asian | 1 (0.5%) | 1 (1.7%) | 1 (3.8%) | 0 (0.0%) | 0 (0.0%) |
| Other | 4 (1.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Maternal country of birth | |||||
| Sweden | 160 (86.5%) | 43 (91.4%) | 15 (71.4%) | 9 (64.3%) | 3 (50.0%) |
| Other European | 17 (9.1%) | 2 (4.3%) | 4 (19.0%) | 3 (21.4%) | 1 (16.7%) |
| Outside Europe | 8 (4.3%) | 2 (4.3%) | 2 (9.5%) | 2 (14.3%) | 2 (33.3%) |
| Highest level of education | |||||
| 9 years | 3 (1.7%) | 5 (10.0%) | 3 (14.3%) | 2 (14.3%) | 2 (33.3%) |
| 12 years | 38 (21.5%) | 12 (24.0%) | 6 (28.6%) | 4 (28.6%) | 2 (33.3%) |
| >12 years | 134 (76.6%) | 33 (66.0%) | 12 (57.1%) | 8 (57.1%) | 2 (33.3%) |
| Main occupation | |||||
| Employed | 154 (82.8%) | 42 (80.8%) | 16 (76.2%) | 9 (64.3%) | 2 (33.3%) |
| Student | 13 (6.9%) | 5 (9.6%) | 5 (23.8%) | 5 (35.7%) | 4 (66.7%) |
| Maternity leave | 7 (3.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Unemployed | 9 (4.8%) | 1 (1.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Sick leave | 2 (1.1%) | 3 (5.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Other | 1 (0.5%) | 1 (1.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Low socioeconomic status a | 12 (5.8%) | 7 (11.4%) | 3 (11.1%) | 2 (18.2%) | 2 (25.0%) |
| Height at first antenatal visit (cm) | 167.4 (5.9) n = 173 | 164.2 (6.2) n = 51 | 165.0 (5.1) n = 22 | 165.6 (5.1) n = 14 | 165.8 (2.2) n = 6 |
| Variable | n = 205 | n = 61 | n = 27 | n = 17 | n = 8 |
| Weight at first antenatal visit (kg) | 66.6 (10.8) n = 167 | 65.5 (11.3) n = 51 | 65.5 (11.3) n = 22 | 69.4 (11.1) n = 14 | 70.7 (11.5) n = 6 |
| BMI at first antenatal visit | 23.8 (3.9) n = 166 | 24.2 (4.1) n = 50 | 24.0 (3.9) n = 22 | 25.3 (4.1) n = 14 | 25.7 (4.4) n = 6 |
| Smoking or using snuff at first antenatal visit | 4 (1.9%) | 3 (5.3%) | 2 (8.0%) | 1 (6.3%) | 0 (0.0%) |
| Alcohol screening performance (AUDIT) b | |||||
| ≥6 points (risk behavior) | 8 (5.6%) | 3 (6.1%) | 1 (5.0%) | 1 (7.1%) | 0 (0.0%) |
| IVF in current pregnancy | 6 (3.0%) | 5 (8.6%) | 1 (4.0%) | 0 (0.0%) | 0 (0.0%) |
| Chronic hypertension at first antenatal visit | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Diabetes type 1 or 2 at first antenatal visit | 0 (0.0%) | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Renal disease at first antenatal visit | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Conization prior to inclusion date | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Parity | |||||
| Nullipara | 111 (56.9%) | 34 (55.7%) | 16 (59.3%) | 11 (40.7%) | 6 (75%) |
| Previous stillbirths ≥1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Previous late miscarriage | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Previous spontaneous PTD <37 weeks ≥1 | 3 (1.5%) | 6 (9.8%) | 1 (3.7%) | 0 (0.0%) | 0 (0.0%) |
Note: For categorical variables, n (%) is presented, and for continuous variables. mean (SD) is presented. Adapted from: Göransson et al. 47
Abbreviations: AUDIT, alcohol use disorder test; BMI, body mass index (kg/m2); IVF, in vitro fertilization; PTD, preterm delivery; sPTD, spontaneous preterm delivery; sPTD, spontaneous preterm delivery.
Low socioeconomic status defined as education ≤9 years, unemployment, and/or sick leave.
Alcohol screening by AUDIT (Alcohol Use Disorder Test) tool according to antenatal care routines. 47
TABLE 2.
Pregnancy, delivery, and neonatal outcome.
| Control (39 + 0 to 40 + 6 weeks) | sPTD <34 + 0 weeks | sPTD <32 + 0 weeks | sPTD <30 + 0 weeks | sPTD <28 + 0 weeks | |
|---|---|---|---|---|---|
| Variable | n = 205 | n = 61 | n = 27 | n = 17 | n = 8 |
| Redeemed prescription of vaginal progesterone after inclusion in the study | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Cerclage after inclusion | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Preeclampsia or gestational hypertension at delivery | 5 (2.4%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Chronic hypertension at delivery | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Diabetes type 1 or 2 or gestational diabetes at delivery | 0 (0.0%) | 2 (3.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Mode of delivery | |||||
| Cesarean delivery | 13 (6.3%) | 16 (26.2%) | 9 (33.3%) | 5 (29.4%) | 2 (25.0%) |
| Gestational age at delivery (days) | 280.6 (3.6) n = 205 | 219.6 (18.1) n = 61 | 203.5 (16.6) n = 27 | 191.5 (18.8) n = 17 | 175.6 (16.0) n = 8 |
| Birthweight (g) | 3538 (395) n = 205 | 1793 (506) n = 52 | 1426 (434) n = 24 | 1188 (325) n = 14 | 888 (188) n = 6 |
| Small for gestational age a | 2 (1.0%) | 3 (5.0%) | 1 (3.8%) | 0 (0.0%) | 0 (0.0%) |
| Apgar score at 5 min <7 | 2 (1.0%) | 42 (77.8%) | 14 (60.9%) | 7 (41.2%) | 4 (50%) |
| Perinatal mortality b | 0 (0.0%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Stillbirth after 22 + 0 weeks | 0 (0.0%) | 2 (3.3%) | 1 (3.8%) | 0 (0.0%) | 0 (0.0%) |
Note: For categorical variables, n (%) is presented, and for continuous variables, mean (SD) is presented. Adapted from: Marsál et al. 48
Abbreviation: sPTD, spontaneous preterm delivery.
Defined as ≥2 SDs below the Swedish gestational age‐ and sex‐specific growth standard. 48
Includes stillbirth after 22 + 0 weeks and death within 7 days after birth.
Cervical length at 18 + 0 to 20 + 6 weeks (Cx1) and 21 + 0 to 23 + 6 weeks (Cx2) are shown in Tables S1 and S2. At Cx1, the cervix was shorter in the preterm groups (mean 32.7 mm at <34 weeks, mean 32.1 mm at <32 weeks, and mean 32.8 mm at <30 weeks) than in the term group (mean 35.4 mm) and was shortest in those who gave birth at <28 + 0 weeks (mean 30.4 mm). The corresponding values for measurements taken at Cx2 were 30.1 mm (<34 weeks), 27.5 mm (<32 weeks), and 23.8 mm (<30 weeks) vs 35.8 mm (term). When considering the whole preterm group (<34 weeks, n = 61), the prevalence of short cervical length (defined as ≤25 mm) was 13.3% (8/61) at Cx1 and 22.2% (6/27) at Cx2 as compared to 2.0% (4/205) at Cx1 and 2.9% (6/205) at Cx2 in the term group.
3.2. Expression analysis of the nine miRNAs and spontaneous PTD
There was no statistically significant difference in the mean relative expression level of any of the nine analyzed miRNAs between the preterm group (spontaneous PTD <34 + 0 weeks) and the control group. miR‐191‐5p was significantly overexpressed in women who delivered <32 + 0 weeks with an increasing difference in fold change if the delivery occurred at <30 + 0 weeks or at <28 + 0 weeks (Table 3; Figure 2). Both miR‐93‐5p and miR‐15b‐5p were statistically significantly overexpressed in the group who delivered <30 + 0 weeks compared to women who delivered at full term, and for miR‐93‐5p, statistically significant difference was also seen for delivery <32 weeks (Table 3; Figure 2).
TABLE 3.
Mean relative expression of nine miRNAs in women who gave birth spontaneously <34 weeks, <32 weeks, <30 weeks, or <28 weeks, presented as fold change compared to a control group of women who gave birth at full term.
| miRNA | Control 39 + 0 to 40 + 6 weeks (n = 205) | sPTD <34 + 0 weeks (n = 61) | sPTD <32 + 0 weeks (n = 27) | sPTD <30 + 0 weeks (n = 17) | sPTD <28 + 0 weeks (n = 8) |
|---|---|---|---|---|---|
| Let‐7a‐5p | 1.00 (0.05) | 0.96 (0.06) | 1.10 (0.09) | 1.12 (0.10) | 1.09 (0.18) |
| miR‐150‐5p | 1.00 (0.06) | 0.92 (0.06) | 0.96 (0.09) | 0.91 (0.08) | 0.95 (0.14) |
| miR‐15b‐5p | 1.00 (0.05) | 1.10 (0.10) | 1.16 (0.12) | 1.28 (0.18)* | 1.35 (0.29) |
| miR‐185‐5p | 1.00 (0.06) | 1.14 (0.11) | 1.29 (0.18) | 1.24 (0.24) | 1.38 (0.44) |
| miR‐191‐5p | 1.00 (0.05) | 1.09 (0.09) | 1.34 (0.19)* | 1.53 (0.27)** | 1.85 (0.52)* |
| miR‐19b‐3p | 1.00 (0.04) | 1.09 (0.08) | 1.24 (0.14) | 1.27 (0.20) | 1.32 (0.36) |
| miR‐23a‐3p | 1.00 (0.05) | 0.98 (0.09) | 1.05 (0.12) | 1.03 (0.17) | 1.20 (0.34) |
| miR‐374a‐5p | 1.00 (0.46) | 0.65 (0.12) | 0.61 (0.05) | 0.59 (0.06) | 0.52 (0.10) |
| miR‐93‐5p | 1.00 (0.04) | 1.11 (0.07) | 1.27 (0.11)** | 1.26 (0.14)* | 1.13 (0.19) |
Note: Results are presented as mean relative expression (fold change) and SEM (standard error of the mean). A two‐tailed p‐value of <0.05 is considered statistically significant (non‐parametric Mann–Whitney U‐test).
Abbreviation: sPTD, spontaneous preterm delivery.
Signifies a p‐value of <0.05.
Signifies a p‐value of <0.01.
FIGURE 2.
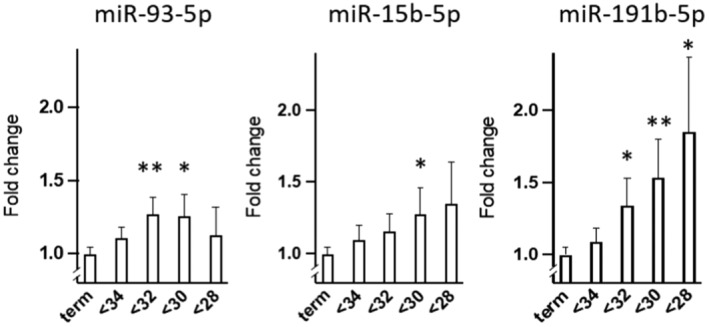
Expression of miR‐93‐5p, miR‐15b‐5p, and miR‐191b‐5p (fold change ± SEM) in women delivering preterm at <34, <32, <30, and <28 weeks of gestation compared to in women delivering at term (39 + 0 to 40 + 6 weeks). *p < 0.05, **p < 0.01 (Mann–Whitney U‐test). miR, microribonucleic acid; SEM, standard error of the mean.
Hemolysis in plasma and serum samples has been known to affect miRNA expression profiles. 49 , 50 , 51 To deal with this matter, interaction analyses were carried out to test whether the degree of hemolysis in the samples affected our results. No interaction effect was found (Table S3).
No statistically significant correlation was found between the expression level of the investigated miRNAs and cervical length (data not shown). Correlation was tested both for cervical length as a continuous variable and for two predefined cervical length cut‐offs (per mm from ≥25 to ≤30 mm). The relative expression level of miR‐191‐5p was negatively correlated with gestational age at delivery (p < 0.05).
4. DISCUSSION
In our cohort of mainly low‐risk women (mainly Caucasian women with >12 years of education, and of whom few had a previous spontaneous PTD and none had a history of cervical conization), none of the nine analyzed miRNAs 30 were significantly differentially expressed in women who gave birth <34 + 0 weeks compared to those who gave birth at full term (39 + 0 to 40 + 6 weeks). MiR‐191‐5p was significantly overexpressed in the group who delivered <32 + 0 weeks, with an increasing difference in fold change if the delivery occurred at <30 + 0 weeks or at <28 + 0 weeks. The relative expression level of miR‐191‐5p was also negatively correlated with gestational age at delivery. The relative expression level of miR‐15b‐5p and miR‐93‐5p were significantly higher in women delivering at <30 + 0 weeks compared to those delivering at full term, and for miR‐93‐5p, this association was also seen for spontaneous PTD <32 + 0 weeks. We found no correlation between the mean relative expression of any of the investigated miRNAs and cervical length measured either at 18–20 (Cx1) or at 21–23 (Cx2) gestational weeks.
The major strength of our study is that it is performed on a comparatively large patient cohort. Other strengths are the blinded prospective two‐center design of the study, 17 the meticulous confirmation of maternal characteristics and delivery outcomes using national health and quality registers, 17 , 19 the homogeneity regarding ethnicity and socioeconomic status of the study participants, and that no participant received preventive treatment for spontaneous PTD (progesterone or cerclage) between inclusion and outcome. The homogeneity of the population could also be seen as a limitation since the results may not be transferable to a different setting.
An important limitation of our study is that we used archived serum samples with limited control of how each sample was handled from sampling to storage. The handling of the samples may have affected the quality of the miRNAs. Furthermore, some samples had a degree of hemolysis which potentially could have affected the levels of the analyzed miRNAs. 49 , 50 , 52 We did, however, analyze the degree of hemolysis in each sample. Exclusion of samples with a high degree of hemolysis did not have a major impact on the results, and the interaction analyses did not reveal any association between hemolysis and the levels of the miRNAs analyzed 49 (Table S3). Another limitation is the low number of extreme spontaneous PTD (<28 weeks). The gapped design of the study (comparing spontaneous PTD with full‐term delivery) could also be seen as a limitation. However, we find this acceptable considering the exploratory nature of the study. 53
In our study, three of nine investigated miRNAs (miR‐93‐5p, miR‐15b‐5p, and miR‐191‐5p) were significantly overexpressed in women with very early spontaneous PTD (<30 weeks) as compared to in control women delivering at full term. These results are consistent with the results by Cook et al., even though their study demonstrated a larger difference in relative expression of miR‐93‐5p, miR‐15b‐5p, and miR‐191‐5p between the preterm and term group. 30 The results are also in agreement with a study by Bhati et al. 54 who found higher serum levels of miR‐191‐5p in cases born <37 weeks vs at term. In contrast to our findings, but similar to those of Cook et al. they also found elevations in miR‐150‐5p and miR‐185‐5p in PTD cases. However, Bhati et al. do not specify at what gestational age serum was sampled, which makes a direct comparison with our results difficult. Expression of miRNA in peripheral white blood cell fraction sampled in early pregnancy has also been shown to be associated with subsequent PTD. 55 However, the differences in miRNAs in white blood cells between preterm and term cases do not seem to match the changes in serum/plasma reported by Cook et al. 30 even though a direct comparison has not been made.
There are several reasons why our results are not fully consistent with those of Cook et al. Firstly, our study is performed in a mainly low‐risk Caucasian population while the study by Cook et al. is based on a high‐risk ethnically mixed population. In the study by Cook et al., women were recruited when attending a preterm prevention clinic, that is, they were already considered to be at increased risk of PTD based on previous PTD, previous mid‐trimester loss, or previous excisional treatment of cervical intraepithelial neoplasia. 30 In our study, none of the included women had experienced previous cervical conization or a previous mid‐trimester loss, and only 9/266 (3.4%) of the included women had a previous spontaneous PTD <37 + 0 weeks. Also, all women who delivered <34 + 0 weeks in the study by Cook et al. presented with cervical shortening (<25 mm), while only 8/61 (13%, Cx1) and 6/27 (22%, Cx2) of the women who delivered <34 + 0 weeks in our study were found to have cervical shortening (≤25 mm). Secondly, the samples used in this study were collected earlier in pregnancy (10–14 weeks of gestation), whereas Cook et al. used plasma samples taken at three timepoints (12–14, 15–18, and 18–21 weeks). 30 There is a possibility that some of the samples in our study were taken too early for it to be possible to distinguish spontaneous PTD cases from term controls. Thirdly, our study used archived serum samples which were kept at 4°C to coagulate, centrifuged on the same day, and stored at –20°C. The plasma samples used by Cook et al. were prospectively collected in EDTA tubes on ice and centrifuged within 30 min of blood collection and stored at –80°C. 30 Generally, miRNAs are stable in circulation and resistant to degradation by RNA activity thanks to binding to proteins and encapsulation within exosomes. 56 However, prolonged holding time of whole blood prior to plasma separation has been shown to affect miRNA expression profiles. 49 Prolonged and variable holding time of whole blood prior to serum separation may have a more pronounced effect on levels of miRNA as the blood is allowed to coagulate which could release miRNA from platelets and cellular constituents. 57 , 58 There is also some evidence that long‐term storage of plasma and serum may affect the miRNA yield. 59
As described, miRNAs are involved in posttranscriptional regulation through transcriptional blockade, or by mRNA degradation, leading to a decrease in the target protein. MiRNAs are involved in multiple biological processes including cell proliferation, differentiation, apoptosis, migration, and cellular invasion. 26 , 27 , 60 , 61 , 62 MiR‐191‐5p, miR‐93‐5p, and miR‐15‐5p have all been associated with various types of diseases, such as different types of cancers, type 2 diabetes, inflammatory diseases, myocardial diseases, as well as neurodegenerative diseases. 60 , 61 , 62 Still, there is very limited knowledge about the above‐described miRNAs and their potential pathophysiological role in spontaneous PTD. In a recently published systematic review by Subramanian et al., the authors concluded that there was an association between miR‐191‐5p and PTD, intrauterine growth restriction, and preeclampsia, although the mechanisms behind these findings are still unknown. 63 Interestingly, Celik et al. have proposed matrix metalloproteinase 3 (MMP3), a proteolytic enzyme with the capacity to break down collagen fibers also found in the fetal membranes, as a potential miR‐93‐5p target. 64 , 65 This could imply that an imbalance in the expression of MMP3, due to alteration of miR‐93‐5p, could lead to preterm premature rupture of membranes, although further investigation is needed. Furthermore, Zhang et al found miR‐15‐5p to be significantly elevated in follicular fluid in women undergoing in vitro fertilization with a poor ovarian response. 66 They suggest miR‐15‐5p to be involved in the pathogenesis of poor ovarian response by regulating oocytes, granulosa cell proliferation, and apoptosis. There is, however, no information on the pathophysiological role of miR‐15‐5p in preterm birth.
5. CONCLUSION
Others have found overexpression of miR‐93‐5p, miR‐15b‐5p, and miR‐191‐5p in plasma at early gestation to be associated with sPTD in women at high risk of sPTD. 30 We found overexpression of miR‐93‐5p, miR‐15b‐5p, and miR‐191‐5p in serum at early gestation to be associated with spontaneous PTD also in a mainly low‐risk population. However, the ability of let‐7a‐5p, miR‐374a‐5p, miR‐15b‐5p, miR‐19b‐3p, miR‐23a‐3p, miR‐93‐5p, miR‐150‐5p, miR‐185‐5p, and miR‐191‐5p in plasma to identify women at risk for spontaneous PTD could not be replicated using serum samples in our mainly low‐risk population. Still, it would be of great interest to perform a prospective study, based on plasma instead of serum, to further investigate the potential of using the described nine miRNAs as potential biomarkers for spontaneous PTD in a mainly low‐risk population. There is also a need to better understand the pathophysiological role of the investigated miRNAs. This requires further research.
AUTHOR CONTRIBUTIONS
Tove Wikström, Sung Hye Kim, Ulla‐Britt Wennerholm, Bo Jacobsson, Lil Valentin, Phillip R. Bennett, Vasso Terzidou, and Henrik Hagberg all conceived and designed the study. Tove Wikström collected all the samples, Anna‐Lena Leverin and Sung Hye Kim analyzed the miRNA and Sung Hye Kim performed the bioinformatic evaluation. Tove Wikström, Henrik Hagberg, and the statisticians Mattias Molin and Jacob Bramstang performed the statistical analyses. All authors contributed to the preparation of the manuscript and provided intellectual input.
FUNDING INFORMATION
The Swedish research council, grant/award numbers: Dnr 2014–06998, 2019–01320, and 2023–02035, the Swedish state under the agreement between the Swedish Government and the country councils, the ALF agreement, grant/award numbers: ALFGBG‐136431, ALFGBG‐426411, and ALFGBG‐718591. This work was supported by the March of Dimes and The National Institute of Health Research (NIHR) Imperial Biomedical Research Center (BRC).
CONFLICT OF INTEREST STATEMENT
V Terzidou and P Bennett are named inventors on a patent WO2016142688A1 (method for predicting cervical shortening and preterm birth) which covers some of the miRNA markers used in the study.
ETHICS STATEMENT
Ethical approval was given by the Regional Ethical Committee in Gothenburg: 825‐13 (November 11, 2013), T053‐14 (January 21, 2014), T691‐14 (September 19, 2014), T972‐15 (December 7, 2015), T122‐16 (February 25, 2016), T896‐17 (October 16, 2017), T‐645‐18 (July 9, 2018), T878‐18 (October 11, 2018), and T970‐18 (November 1, 2018).
Supporting information
Table S1.
Table S2.
Table S3.
ACKNOWLEDGMENTS
Mattias Molin and Jacob Bramstang, the Statistical Consulting Group, Gothenburg, performed the statistical analyses.
Wikström T, Kim SH, Leverin A‐L, et al. Association between miRNAs in serum at 10–14 gestational weeks and spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2024;103:2013‐2023. doi: 10.1111/aogs.14926
Vasso Terzidou and Henrik Hagberg contributed equally to this work.
REFERENCES
- 1. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162‐2172. [DOI] [PubMed] [Google Scholar]
- 2. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37‐e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Newborn and child health, United Nations Children's fund (UNICEF) & United Nations Population Fund. Born Too Soon: Decade of Action on Preterm Birth. World Health Organization. 2023. https://apps.who.int/iris/handle/10665/367620
- 4. Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katz J, Lee AC, Kozuki N, et al. Mortality risk in preterm and small‐for‐gestational‐age infants in low‐income and middle‐income countries: a pooled country analysis. Lancet. 2013;382(9890):417‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serenius F, Ewald U, Farooqi A, et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal Care in Sweden. JAMA Pediatr. 2016;170(10):954‐963. [DOI] [PubMed] [Google Scholar]
- 7. Ward RM, Beachy JC. Neonatal complications following preterm birth. BJOG. 2003;110(Suppl 20):8‐16. [DOI] [PubMed] [Google Scholar]
- 8. Johnson S, Marlow N. Early and long‐term outcome of infants born extremely preterm. Arch Dis Child. 2017;102(1):97‐102. [DOI] [PubMed] [Google Scholar]
- 9. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bezold KY, Karjalainen MK, Hallman M, Teramo K, Muglia LJ. The genomics of preterm birth: from animal models to human studies. Genome Med. 2013;5(4):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terzidou V. Preterm labour. Biochemical and endocrinological preparation for parturition. Best Pract Res Clin Obstet Gynaecol. 2007;21(5):729‐756. [DOI] [PubMed] [Google Scholar]
- 12. Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab. 2010;21(6):353‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18‐22 weeks' gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92(6):902‐907. [DOI] [PubMed] [Google Scholar]
- 14. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development maternal fetal medicine unit network. N Engl J Med. 1996;334(9):567‐572. [DOI] [PubMed] [Google Scholar]
- 15. Leung TN, Pang MW, Leung TY, Poon CF, Wong SM, Lau TK. Cervical length at 18‐22 weeks of gestation for prediction of spontaneous preterm delivery in Hong Kong Chinese women. Ultrasound Obstet Gynecol. 2005;26(7):713‐717. [DOI] [PubMed] [Google Scholar]
- 16. Grimes‐Dennis J, Berghella V. Cervical length and prediction of preterm delivery. Curr Opin Obstet Gynecol. 2007;19(2):191‐195. [DOI] [PubMed] [Google Scholar]
- 17. Kuusela P, Jacobsson B, Hagberg H, et al. Second trimester transvaginal ultrasound measurement of cervical length for prediction of preterm birth: a blinded prospective multicentre diagnostic accuracy study. BJOG. 2021;128(2):195‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Os MA, Kleinrouweler CE, Schuit E, et al. Influence of cut‐off value on prevalence of short cervical length. Ultrasound Obstet Gynecol. 2017;49(3):330‐336. [DOI] [PubMed] [Google Scholar]
- 19. Wikström T, Hagberg H, Jacobsson B, et al. Effect of second‐trimester sonographic cervical length on the risk of spontaneous preterm delivery in different risk groups: a prospective observational multicenter study. Acta Obstet Gynecol Scand. 2021;100(9):1644‐1655. [DOI] [PubMed] [Google Scholar]
- 20. Campbell S. Prevention of spontaneous preterm birth: universal cervical length assessment and vaginal progesterone in women with a short cervix: time for action! Am J Obstet Gynecol. 2018;218(2):151‐158. [DOI] [PubMed] [Google Scholar]
- 21. Werner EF, Han CS, Pettker CM, et al. Universal cervical‐length screening to prevent preterm birth: a cost‐effectiveness analysis. Ultrasound Obstet Gynecol. 2011;38(1):32‐37. [DOI] [PubMed] [Google Scholar]
- 22. Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost‐effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554‐556. [DOI] [PubMed] [Google Scholar]
- 23. Einerson BD, Grobman WA, Miller ES. Cost‐effectiveness of risk‐based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100‐107. [DOI] [PubMed] [Google Scholar]
- 24. Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol. 2010;202(6):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wikström T, Kuusela P, Jacobsson B, et al. Cost‐effectiveness of cervical length screening and progesterone treatment to prevent spontaneous preterm delivery in Sweden. Ultrasound Obstet Gynecol. 2022;59(6):778‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281‐297. [DOI] [PubMed] [Google Scholar]
- 27. Esteller M. Non‐coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861‐874. [DOI] [PubMed] [Google Scholar]
- 28. Basak I, Patil KS, Alves G, Larsen JP, Moller SG. microRNAs as neuroregulators, biomarkers and therapeutic agents in neurodegenerative diseases. Cell Mol Life Sci. 2016;73(4):811‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neudecker V, Brodsky KS, Kreth S, Ginde AA, Eltzschig HK. Emerging roles for MicroRNAs in perioperative medicine. Anesthesiology. 2016;124(2):489‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cook J, Bennett PR, Kim SH, et al. First trimester circulating MicroRNA biomarkers predictive of subsequent preterm delivery and cervical shortening. Sci Rep. 2019;9(1):5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Selbing A, Kjessler B. Conceptual dating by ultrasonic measurement of the fetal biparietal diameter in early pregnancy. Acta Obstet Gynecol Scand. 1985;64(7):593‐597. [DOI] [PubMed] [Google Scholar]
- 32. Saltvedt S, Almstrom H, Kublickas M, Reilly M, Valentin L, Grunewald C. Ultrasound dating at 12‐14 or 15‐20 weeks of gestation? A prospective cross‐validation of established dating formulae in a population of in‐vitro fertilized pregnancies randomized to early or late dating scan. Ultrasound Obstet Gynecol. 2004;24(1):42‐50. [DOI] [PubMed] [Google Scholar]
- 33. Kuusela P, Wennerholm UB, Fadl H, et al. Second trimester cervical length measurements with transvaginal ultrasound: a prospective observational agreement and reliability study. Acta Obstet Gynecol Scand. 2020;99(11):1476‐1485. [DOI] [PubMed] [Google Scholar]
- 34. Socialstyrelsen . Accessed Feb 13, 2024. https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelkatalog/foreskrifter‐och‐allmanna‐rad/2004‐10‐13_2004_13.pdf
- 35. Biobanksverige . Accessed Feb 13, 2024. https://biobanksverigese/wp‐content/uploads/G2c‐Till‐dig‐som‐lämnar‐mödravårdsprov_utskriftwebpdf.
- 36. Kang M‐S, Choi S‐H, Koh I‐S. The effect of increasing control‐to‐case ratio on statistical power in a simulated case‐control SNP association study. Genom Informat. 2009;7(3):148‐151. [Google Scholar]
- 37. Wennerholm UB, Saltvedt S, Wessberg A, et al. Induction of labour at 41 weeks versus expectant management and induction of labour at 42 weeks (SWEdish Post‐term induction study, SWEPIS): multicentre, open label, randomised, superiority trial. BMJ. 2019;367:l6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alkmark M, Keulen JKJ, Kortekaas JC, et al. Induction of labour at 41 weeks or expectant management until 42 weeks: a systematic review and an individual participant data meta‐analysis of randomised trials. PLoS Med. 2020;17(12):e1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ACOG practice bulletin no . 127: management of preterm labor. Obstet Gynecol. 2012;119(6):1308‐1317. [DOI] [PubMed] [Google Scholar]
- 40. Murray SR, Shenkin SD, McIntosh K, et al. Long term cognitive outcomes of early term (37‐38 weeks) and late preterm (34‐36 weeks) births: a systematic review. Wellcome Open Res. 2017;2:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muglu J, Rather H, Arroyo‐Manzano D, et al. Risks of stillbirth and neonatal death with advancing gestation at term: a systematic review and meta‐analysis of cohort studies of 15 million pregnancies. PLoS Med. 2019;16(7):e1002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stephansson O, Petersson K, Bjork C, Conner P, Wikstrom AK. The Swedish pregnancy register ‐ for quality of care improvement and research. Acta Obstet Gynecol Scand. 2018;97(4):466‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah JS, Soon PS, Marsh DJ. Comparison of methodologies to detect low levels of hemolysis in serum for accurate assessment of serum microRNAs. PLoS One. 2016;11(4):e0153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruijter JM, Ramakers C, Hoogaars WM, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37(6):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Andersen CL, Jensen JL, Ørntoft TF. Normalization of real‐time quantitative reverse transcription‐PCR data: a model‐based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64(15):5245‐5250. [DOI] [PubMed] [Google Scholar]
- 46. Spearman C. The proof and measurement of association between two things. By C. Spearman, 1904. Am J Psychol. 1987;100(3–4):441‐471. [PubMed] [Google Scholar]
- 47. Göransson M, Magnusson A, Heilig M. Identifying hazardous alcohol consumption during pregnancy: implementing a research‐based model in real life. Acta Obstet Gynecol Scand. 2006;85:657‐662. [DOI] [PubMed] [Google Scholar]
- 48. Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843‐848. [DOI] [PubMed] [Google Scholar]
- 49. Kim SH, MacIntyre DA, Sykes L, Arianoglou M, Bennett PR, Terzidou V. Whole blood holding time prior to plasma processing alters microRNA expression profile. Front Genet. 2021;12:818334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng HH, Yi HS, Kim Y, et al. Plasma processing conditions substantially influence circulating microRNA biomarker levels. PLoS One. 2013;8(6):e64795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. MacLellan SA, MacAulay C, Lam S, Garnis C. Pre‐profiling factors influencing serum microRNA levels. BMC Clin Pathol. 2014;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras‐Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem. 2011;57(6):833‐840. [DOI] [PubMed] [Google Scholar]
- 53. Boniface JJ, Burchard J, Saade GR. Effects of selective exclusion of patients on preterm birth test performance. Obstet Gynecol. 2019;134(6):1333‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bhati T, Ray A, Arora R, Siraj F, Parvez S, Rastogi S. Immunomodulation of cytokine signalling at feto‐maternal interface by microRNA‐223 and− 150–5p in infection‐associated spontaneous preterm birth. Mol Immunol. 2023;160:1‐11. [DOI] [PubMed] [Google Scholar]
- 55. Winger EE, Reed JL, Ji X. Early first trimester peripheral blood cell microRNA predicts risk of preterm delivery in pregnant women: proof of concept. PLoS One. 2017;12(7):e0180124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Glinge C, Clauss S, Boddum K, et al. Stability of circulating blood‐based MicroRNAs—pre‐analytic methodological considerations. PLoS One. 2017;12(2):e0167969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang K, Yuan Y, Cho JH, McClarty S, Baxter D, Galas DJ. Comparing the MicroRNA spectrum between serum and plasma. PLoS One. 2012;7(7):e41561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Max KEA, Bertram K, Akat KM, et al. Human plasma and serum extracellular small RNA reference profiles and their clinical utility. Proc Natl Acad Sci USA. 2018;115(23):E5334‐e5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Andreu Z, Rivas E, Sanguino‐Pascual A, et al. Comparative analysis of EV isolation procedures for miRNAs detection in serum samples. J Extracell Vesicles. 2016;5:31655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nagpal N, Kulshreshtha R. miR‐191: an emerging player in disease biology. Front Genet. 2014;5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hussen BM, Abdullah SR, Rasul MF, et al. MiRNA‐93: a novel signature in human disorders and drug resistance. Cell Commun Signal. 2023;21(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghafouri‐Fard S, Khoshbakht T, Hussen BM, Jamal HH, Taheri M, Hajiesmaeili M. A comprehensive review on function of miR‐15b‐5p in malignant and non‐malignant disorders. Front Oncol. 2022;12:870996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Subramanian A, Weiss D, Nyhan K, Dewan A, Jukic AMZ. Circulating miRNAs in the first trimester and pregnancy complications: a systematic review. Epigenetics. 2023;18(1):2152615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Celik ZB, Tural S, Cengiz AK, Kara N, Alayli G. Upregulation of microRNA‐93‐5p/microRNA‐4668‐5p, and promoter methylation of matrix metalloproteinase‐3 and interleukin‐16 genes in Turkish patients with rheumatoid arthritis. Egypt Rheumatol. 2021;43(1):35‐39. [Google Scholar]
- 65. Demir‐Weusten AY, Seval Y, Kaufmann P, Demir R, Yucel G, Huppertz B. Matrix metalloproteinases‐2, −3 and −9 in human term placenta. Acta Histochem. 2007;109(5):403‐412. [DOI] [PubMed] [Google Scholar]
- 66. Zhang K, Zhong W, Li WP, Chen ZJ, Zhang C. miR‐15a‐5p levels correlate with poor ovarian response in human follicular fluid. Reproduction. 2017;154(4):483‐496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.


