Abstract
Major depressive disorder (MDD), affecting over 264 million individuals globally, is associated with immune system dysregulation and chronic neuroinflammation, potentially linked to neurodegenerative processes. This review examines blood-brain barrier (BBB) dysfunction in MDD, focusing on key regulators like matrix metalloproteinase 9 (MMP9), aquaporin-4 (AQP4), and ATP-binding cassette subfamily B member 1 (ABCB1). We explore potential mechanisms by which compromised BBB integrity in MDD may contribute to neuroinflammation and discuss the therapeutic potential of omega-3 polyunsaturated fatty acids (n-3 PUFAs). n-3 PUFAs have demonstrated anti-inflammatory and neuroprotective effects, and potential ability to modulate MMP9, AQP4, and ABCB1, thereby restoring BBB integrity in MDD. This review aims to elucidate these potential mechanisms and evaluate the evidence for n-3 PUFAs as a strategy to mitigate BBB dysfunction and neuroinflammation in MDD.
Keywords: Aquaporin-4, blood-brain barrier, inflammation, major depressive disorder, metalloproteinase-9, omega-3 polyunsaturated fatty acids
Introduction
Major depressive disorder (MDD) exerts a significant global burden, impacting individuals, communities, and healthcare infrastructures [1]. MDD stands as a primary cause of disability worldwide [2], carrying substantial economic and social repercussions. The World Health Organization estimates that over 264 million individuals across all age groups are affected by depression globally [3]. Depression is also important in neurodegenerative processes as it can both exacerbate the progression of diseases like Alzheimer’s Disease and Parkinson’s Disease and be an early indicator of these conditions [4,5]. Despite extensive research efforts spanning decades, the precise etiology of MDD remains elusive, necessitating continued investigation into its underlying mechanisms. Recent evidence suggests that dysregulation of the immune system, characterized by elevated levels of pro-inflammatory cytokines, plays a significant role in the pathogenesis of MDD [6]. This is because patients with MDD exhibit significantly higher levels of pro-inflammatory mediators such as interleukin-1 beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α) than healthy controls [7]. Moreover, treatment with pro-inflammatory cytokines or their derivatives resulted in the onset of depressive symptoms in individuals with no previous history of depression [8,9]. Pro-inflammatory mediators disrupt the integrity of the blood-brain barrier (BBB), thus initiating neuroinflammatory processes within the central nervous system (CNS) [10]. Impairment in BBB structure and functions is an emerging phenomenon in the pathology of depression and other CNS disorders [11].
The integrity of BBB can be impaired in pathological states through several mechanisms such as matrix metalloproteinase 9 (MMP9) dysregulation and impaired regulation of aquaporin 4 (AQP4), or ATP-binding cassette subfamily B member 1 (ABCB1) gene. MMP9 participates in diverse physiological processes, including tissue repair, angiogenesis, and modulation of immune responses [12]. Dysregulation of MMP9 occurs in disease states such as inflammation [12], where it compromises the structural integrity of BBB [13]. Mounting evidence associated elevated MMP9 levels with BBB disruption in diverse disease conditions such as acute ischemic stroke [14] and traumatic brain injury [15]. AQP4, a water channel protein in astrocytic end feet, facilitates water movement between blood vessels and the brain parenchyma, crucial for brain homeostasis and metabolic waste clearance through the glymphatic system [16]. The glymphatic system depends on AQP4-mediated water flux for cerebrospinal fluid movement and waste removal [17]. Similarly, ABCB1 gene, encoding P-glycoprotein (P-gp), functions as an efflux transporter at the BBB [18]. P-gp actively transports toxins and xenobiotics out of the brain, protecting neural tissues from harmful compounds [19]. Taken together, strict regulation of MMP9, AQP4, and ABCB1 is paramount to maintaining the optimal structural and functional integrity of the BBB.
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) have emerged as promising candidates for depression treatment and prevention with epidemiological and clinical studies reporting their beneficial effects [20-23] with a high degree of tolerability and safety [24,25]. Despite these promising findings, the precise mechanisms underlying the antidepressant effects of n-3 PUFAs remain incompletely understood. Available evidence shows that n-3 PUFAs exert antidepressant effects through the modulation of inflammatory pathways and antioxidant defense systems [26,27], neuroplasticity promotion [28], and the modulation hypothalamus-pituitary-adrenal (HPA) axis [29]. However, it is not known if n-3 PUFAs can exert their antidepressant effects by preserving the integrity of the BBB. Consequently, in this review, we aim to discuss the evidence of BBB dysfunction in MDD pathophysiology and the modulation of BBB integrity and functions as a potential therapeutic target for n-3 PUFAs in MDD.
BBB Dysfunction in MDD
The BBB is a sophisticated structure primarily composed of endothelial cells that line the blood vessels within the brain. These endothelial cells are specialized and tightly interconnected by tight junction proteins (TJPs) including junctional adhesion molecules, claudins, and occludins, forming a physical barrier that regulates the passage of molecules between the bloodstream and brain tissue [30]. The BBB is further supported by astrocytes surrounding the blood vessels, providing structural reinforcement [31]. Together, these cells create a selective barrier that permits essential nutrients like glucose and amino acids to enter the brain while preventing the entry of harmful substances, toxins, and pathogens [32]. TJPs between endothelial cells restrict the free diffusion of molecules across the BBB, necessitating passage through specialized transport systems like carrier-mediated transporters and receptor-mediated endocytosis for specific molecules such as amino acids, ions, and glucose while excluding others [33]. BBB also plays a critical role in maintaining brain microenvironment homeostasis by limiting the passage of immune cells and inflammatory mediators into the brain [11]. Claudin-5 is the most expressed TJP within endothelial cells of the CNS, and preclinical studies have shown that depletion of Claudin-5 is adequate to enhance BBB permeability [34,35]. Overall, preserving BBB integrity is crucial for shielding the brain from harmful substances, facilitating the passage of essential nutrients, and maintaining proper CNS function. Conversely, BBB dysfunction can profoundly impact brain health and is implicated in various neurological and psychiatric disorders, including MDD [36].
Undoubtedly, AQP4 and ABCB1 are pivotal in upholding the integrity and function of the BBB, particularly concerning astrocytes and the glymphatic system [37,38]. AQP4, a water channel protein primarily found in astrocytic end feet, plays a crucial role in facilitating the efficient movement of water between blood vessels and the brain parenchyma [16,39,40]. This regulation is of paramount importance in maintaining brain homeostasis, especially in the clearance of metabolic waste through the glymphatic system [41]. The glymphatic system heavily relies on AQP4-mediated water flux to drive CSF movement and waste clearance [42]. On the other hand, ABCB1, which encodes P-gp, is a key efflux transporter at the BBB [43,44]. P-gp actively transports various substances, including toxins and xenobiotics, out of the brain, thereby safeguarding neural tissues from potentially harmful compounds [45]. In astrocytes, ABCB1 modulates the cellular environment and influences the distribution and clearance of endogenous and exogenous molecules [46]. The combined functions of AQP4 and ABCB1 are instrumental in the dynamic regulation of the BBB, where AQP4 supports fluid balance and waste removal, and ABCB1 ensures neuroprotection by preventing the accumulation of toxic aggregates. Their coordinated efforts are indispensable for maintaining neural health and optimizing the efficiency of the glymphatic system in preserving cerebral homeostasis.
Emerging evidence suggests that dysfunction of the BBB plays a role in the pathogenesis of MDD [36,47]. Notably, individuals with MDD demonstrate impaired endothelial cell function, characterized by a reduced relative uptake ratio of blood flow in the brachial artery following hyperemic challenge using dynamic nuclear imaging, indicative of compromised endothelial function [48]. Shang et al. found that MDD patients exhibited elevated BBB leakage, evidenced by higher mean volume transfer constant (Ktrans) values in the thalamus, caudate, and olfactory regions compared to healthy controls, while Ktrans values in the thalamus and hippocampus were positively correlated with depression severity [49]. Additionally, MDD patients exhibit elevated plasma levels of endothelial dysfunction markers, including soluble intercellular adhesion molecule (ICAM), soluble vascular cell adhesion molecule (VCAM), soluble E-selectin, and von Willebrand factor [50-52]. Consistent with this, serum samples from MDD patients show increased apoptotic potential on endothelial cells in vitro compared to non-depressed controls [53]. MDD is associated with reduced expression of TJPs in various brain regions, such as reduced Claudin-5 mRNA levels in the nucleus accumbens and hippocampus [54,55]. Furthermore, reduced Claudin-5 expression correlates with the age of onset and duration of depressive episodes in MDD patients [55]. Serum Claudin-5 levels are elevated in MDD patients, suggesting degradation of the protein [56,57]. Collectively, these findings underscore disruptions in BBB integrity in depression, although whether these disruptions precede or result from depressive states remains to be fully elucidated in humans. BBB dysfunction in MDD may result from several mechanisms such as dysregulations in MMP9, astrocytic and AQP4, and ABCB1.
MMP9 and BBB Dysregulation in MDD
Mounting evidence suggests the involvement of MMP9 in MDD pathophysiology. Indeed, elevated levels of MMP9 have been consistently observed in individuals with depression compared to control subjects across various studies. For instance, Domenici et al. (2010) reported higher MMP9 levels in patients with depression compared to controls [58], while Bobińska et al. reported elevated MMP9 mRNA transcripts in depressed individuals [59]. Similarly, Hamed et al. (2020) discovered higher MMP9 and reduced tissue inhibitor of metalloproteinase 1 (TIMP-1) levels in the blood of clinically diagnosed MDD patients [60]. Garvin et al. demonstrated a notable correlation between depressive symptoms and plasma MMP9 levels in a cohort of middle-aged individuals from Sweden [61]. Moreover, electroconvulsive therapy was found to reduce serum MMP9 levels in MDD patients who are responders but not in patients who relapsed [62]. Peripheral MMP9 levels appear to correlate with depression severity, as evidenced by studies indicating associations between MMP9 serum levels and depression severity [63]. Supporting these results, Bijata et al. observed increased MMP9 activity in the hippocampus of individuals with MDD who died by suicide in a post-mortem study [64].
Preclinical models of depression reinforce the relationship between MMP9 and depression. The corticosterone mice model of depression showed heightened MMP9 activity and protein concentrations in both the hippocampus and cortex, which correlated positively with behavioral impairment [65]. Additionally, increased MMP9 was accompanied by a decreased expression of nectin-3, an important MMP9 substrate in these brain regions [65]. Similarly, heightened MMP9 activity was found in the hippocampal CA1 region of mice, suggesting its implication in chronic stress-induced social and cognitive alterations [66]. However, treatment with an MMP9 blocker has alleviated chronic stress-induced depression-like social and cognitive alterations [66]. In another study, chronic mild stress resulted in the elevation of MMP9 levels in the brain [67]. Cumulatively, these findings suggest a complex involvement of MMP9 in the pathophysiology of depression, highlighting its potential as a biomarker for depressive symptoms severity and treatment response. Thus, MMP9 may offer a novel therapeutic target for BBB-impairment-induced depression.
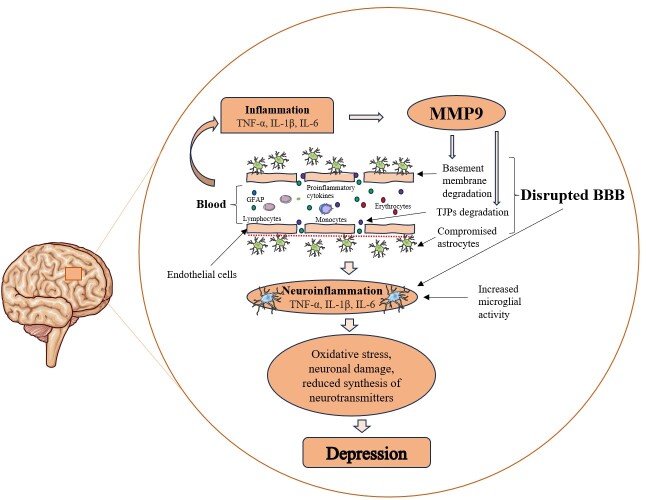
Indeed, the association between MMP9 and depression reported in several studies may be related to the negative impact of MMP9 on BBB integrity, however, evidence supporting this in the context of MDD is generally lacking. Specifically, MMP9 plays a crucial role in BBB disruption by degrading the TJPs and the basement membrane of the BBB [14]. This breakdown permits the passage of inflammatory cells and other harmful substances from the bloodstream into the brain, which triggers glial activation, initiating neuroinflammatory reactions [13]. Neuroinflammation has been linked to oxidative stress-induced damage to neurons [26]; decrease in the synthesis of neurotransmitters crucial for mood regulation such as dopamine and serotonin [68]; and BBB impairment [47], further exacerbating neuroinflammation, perpetuating a cycle of neuronal injury and immune activation. These processes collectively contribute to the development of depression (Figure 1).
Figure 1.
Role of MMP9 in Depression. During inflammation, pro-inflammatory cytokines such as NF-κβ, IL1β, and IL6 stimulate the synthesis of MMP9. MMP9 can impair the integrity of BBB via the degradation of basement membrane of the BBB and tight junction proteins, allowing the entry of inflammatory cytokines and other neurotoxic substances into the brain, which leads to neuroinflammation. Neuroinflammation leads to depression through neuronal damage induced by oxidative stress and reduced availability of neurotransmitters. BBB: Blood-brain barrier; GFAP, Glial fibrillary acidic protein; IL, Interleukin; MMP9, Matrix metalloproteinase 9; TJPs, Tight junction proteins; TNF-α, Tumor necrosis factor alpha.
Astrocytic and AQP4 Dysfunction in MDD
The intricate relationship between astrocytic dysfunction and depression has been increasingly elucidated through various studies, highlighting the pivotal roles of astrocytes and AQP4 in the pathophysiology of depressive disorders. The study by González-Arias et al. [69] reveals that astrocytes exhibit dysfunctional Ca2+ signaling in a chronic corticosterone mouse model of stress, characterized by altered dynamics and reduced serotonin-mediated responses, contributing to depressive-like behaviors. Similarly, Cobb et al. [70] found that the density of glial fibrillary acidic protein-(GFAP)-immunoreactive astrocytes is notably reduced in the hippocampus of individuals with MDD who were not undergoing antidepressant treatment. Gittins and Harrison [71] also noted a reduction in glial cell density, particularly astrocytes, in the anterior cingulate cortex (ACC) of subjects with mood disorders, suggesting a potential astrocytic pathology contributing to the altered functionality observed in depression. Inflammation plays a significant role in these findings, with Xie et al. [72] demonstrating that astrocyte-derived extracellular vesicles (ADEs) show increased inflammatory markers in MDD patients compared to healthy controls. This supports the inflammatory glial hypothesis of depression and highlights ADEs as valuable tools for assessing astrocyte activity in vivo.
AQP4 emerges as a crucial player in the regulation of neurogenesis and depressive behaviors. Kong et al. [73] showed that AQP4 knockout mice exhibited disrupted fluoxetine-induced enhancement of hippocampal neurogenesis and behavioral improvements, underscoring the essential role of AQP4 in mediating antidepressant effects. Liu et al. [74] found that chronic social defeat stress significantly elevated hippocampal AQP4 levels, and its knockdown alleviated depression and enhanced the expression of N-methyl D-aspartate (NMDA) receptor subtype 2B and postsynaptic density protein 95. Additionally, the study by Westermair et al. [75] identified a genetic variant associated with the AQP4 locus that increases the risk of lifetime depression, further implicating AQP4 in the etiology of depression. Moreover, the role of AQP4 in mitigating corticosterone-induced depression by maintaining astrocyte function and hippocampal neurogenesis is highlighted in studies like Kong et al. [76], which showed that AQP4 knockout mice displayed more severe depressive-like behaviors and reduced astrocyte density. Xia et al. [77] demonstrated that chronic stress impairs the glymphatic pathway and decreases AQP4 expression, leading to reduced Aβ clearance and increased accumulation, suggesting a mechanism linking depression to Alzheimer’s Disease. Finally, the exploration of AQP4 autoantibodies in treatment-resistant depression, as presented by Iorio [78], underscores the potential autoimmune component in certain forms of depression, highlighting the need for considering autoimmune factors in treatment-resistant cases. Despite some studies, such as Gur et al. [79], not finding significant associations between AQP4-IgG autoantibodies and major depressive episodes, the overall evidence points to the significant role of AQP4 in mood disorders through various mechanisms. These studies collectively emphasize the critical involvement of astrocytic dysfunction and AQP4 in the development and progression of depression, pointing to their potential as therapeutic targets. Understanding the intricate interplay between astrocytic dysfunction, inflammation, and AQP4 can pave the way for novel treatments for depression and its comorbid conditions.
ABCB1 and Treatment Resistance in MDD
The ABCB1 gene, encoding P-gp, significantly affects the concentration of antidepressants in the brain by modulating the BBB. Variations in this gene can lead to differences in P-gp expression, which in turn influences individual responses to antidepressant treatments [80]. Several studies highlight that specific polymorphism in the ABCB1 gene, such as rs2235040, rs9282564, and G2677T, are associated with higher remission rates and faster time to remission in patients treated with P-gp substrate antidepressants. These findings suggest that genotyping for these single nucleotide polymorphisms (SNPs) could potentially guide personalized treatment plans, although more research is needed to confirm their clinical utility [81-83]. Some ABCB1 variants, particularly the G2677T allele, are associated with an increased risk of suicide attempts, indicating that genetic screening could also help in identifying patients at higher risk for adverse psychiatric outcomes [84]. Studies in different populations, such as the Chinese Han population, reveal that certain ABCB1 polymorphisms (eg, rs6946119, rs28401781, rs4148739, and rs3747802) do not significantly predispose individuals to MDD but may still influence treatment responses, underscoring the importance of considering ethnic and genetic backgrounds in research and treatment [85,86].
Variants like C3435T and rs1045642 have been linked to differences in remission times and required dosages for achieving remission. Patients with specific genotypes (eg, TT at C3435T and rs1045642) tend to have faster remission times and require lower doses of certain antidepressants, highlighting the potential for genotype-guided dosing strategies [87,88]. Certain ABCB1 gene SNPs (eg, 1236 T, 3435 T, 2677 T/A) correlate with higher initial severity of depressive symptoms and decreased efficacy of antidepressants, indicating these genetic markers could be used to predict and monitor treatment response and adjust strategies accordingly [89]. Research indicates that while ABCB1 variants may affect antidepressant efficacy, they do not appear to influence cortisol regulation in patients undergoing treatment for MDD, suggesting that their role is more directly related to drug transport and response rather than broader endocrine effects [81]. Some studies found no significant association between certain ABCB1 polymorphisms and the predisposition to MDD, suggesting that these genetic variations may primarily influence treatment outcomes rather than the likelihood of developing depression [85]. Overall, these studies underscore the importance of ABCB1 genetic variations in influencing antidepressant efficacy, treatment response, and potential adverse outcomes. They suggest that genotyping for ABCB1 variants could enhance personalized treatment strategies for depression, although further research is needed to solidify these findings and implement them in clinical practice.
n-3 PUFAs in MDD
n-3 PUFAs are increasingly recognized to confer health benefits in several diseases, including neuropsychiatric disorders [90-92]. Indeed, mounting evidence suggests that n-3 PUFAs deficiency resulting from inadequate dietary intake, disease, or both, is a clinical feature of depression [93] and several interventional studies report that supplementation with n-3 PUFAs demonstrated benefits in improving depression severity [94-96]. Specifically, n-3 PUFAs demonstrated a significant prophylactic effect on bipolar depression [20] and interferon-(IFN)-alpha-induced depression [22]. Moreover, n-3 PUFAs have been documented to exhibit higher tolerability and safety as evidenced by few adverse effects associated with their therapy compared to conventional antidepressant therapies [24,97]. Several mechanisms have been postulated to be responsible for the observed antidepressant efficacy of n-3 PUFAs and their ability to modulate inflammatory pathways and antioxidant defense systems as important mechanisms [26,27]. Indeed, n-3 PUFAs reduce interferon-(IFN)-γ- induced expressions of TNF-α, IL-6, nitric oxide synthase (NOS), and cyclo-oxygenase-2 (COX-2), while also promoting the upregulation of heme oxygenase-1 (HO-1) in BV-2 microglia [98]. It was further found that treatment of astrocytes from mice with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) showed a dose-dependent decrease in reactive oxygen species (ROS) generation and significantly enhanced reduced glutathione (GSH) and glutathione peroxidase 4 (GPX4) levels [99]. These effects were mediated via the modulation of nuclear factor erythroid 2-related factor 2 (nrf2) [99], the master regulator of antioxidant enzymes.
Emerging evidence suggests that n-3 PUFAs antidepressant efficacy may be mediated by their metabolites, specialized pro-resolvin mediators (SPMs). Recently, Borsini et al. showed that metabolites from EPA and DHA produced by the lipoxygenase (LOX) and cytochrome (CYP) P450 enzymes; 4-hydroxydocosahexaenoic acid, 5-hydroxyeicosapentaenoic acid, 18-hydroxyeicosapentaenoic acid (18-HEPE), 17(18) epoxyeicosatetraenoic acid, 19(20)-epoxydocosapentaenoic acid, and 20-hydroxydocosahexaenoic acid showed a significant increase in MDD patients following EPA and DHA supplementation [100]. Moreover, the levels of these metabolites were negatively correlated with depression severity [100]. Similarly, Yang et al. found a significant increase in plasma eicosapentaenoylethanolamide which correlated positively with clinical remission following supplementation with EPA in patients with MDD [101]. In addition, EPA resulted in a dose- and time-dependent increase in plasma EPA and 18-HEPE in patients with MDD and chronic inflammation [102]. Further corroborating these findings, higher clinical response to EPA was noted in MDD patients who demonstrated a greater ability to activate the synthesis of 18-HEPE [103]. n-3 PUFAs may also exert antidepressant effects via the modulation of the HPA axis [29], prevention of neurodegeneration [104], and promotion of neuronal plasticity [28]. With the increasing evidence that BBB disruption is found in MDD [36,49], it is currently unclear whether n-3 PUFAs can exert their antidepressant efficacy by maintaining the integrity of BBB via the modulation of key players in BBB disruption, such as MMP9.
n-3 PUFAs, BBB, and Antidepressant Action
Effect of n-3 PUFAs on MMP9
There is currently no direct evidence of the modulatory effects of n-3 PUFAs on MMP9 in the context of depression. However, several lines of evidence demonstrate that n-3 PUFAs can modulate MMP9 in both preclinical and clinical models of several diseases.
The effects of n-3 PUFAs on MMP9 have been widely studied in the context of cancer as this enzyme is crucial for tumor angiogenesis and metastasis [105]. In the castrate-resistant chronic cancer progression model, Liang et al. found that treatment with n-3 PUFAs demonstrated a significant reduction in MMP9 expression in tumor infiltrating M2-like macrophages [106]. Similarly, Yin et al. demonstrated a dose-dependent downregulation of MMP9 in non-small cell lung cancer cells by DHA [107]. Furthermore, suppression of cancer-associated fibroblasts MMP9 activity was reported following treatment with n-3 PUFAs [108]. In addition, it was reported that DHA inhibits the metastasis of breast cancer cell by targeting metalloproteinases, particularly, MMP9 [109]. Furthermore, DHA prevented cell invasion following exposure to 12-O-tetradecanoylphorbol-13-acetate by inhibiting MMP9 expression in MCF-7 breast cancer cell lines [110]. In a separate study, colon cancer cells were exposed to a cytokine-enriched medium, resulting in the upregulation of MMP9-dependent neurogenic locus notch homolog protein 1 (NOTCH1) signaling [111]. However, treatment with EPA attenuated the observed effects of inflammatory stimulus on NOTCH1 signaling via the reduction of MMP9 activity [111]. Other studies similarly reported reduced MMP9 activity or reduction in protein levels in other tumor types following treatment with n-3 PUFAs [112,113]. Collectively, these findings indicate that n-3 PUFAs might exert anti-tumor effects by modulating MMP9 within the tumor microenvironment. Moreover, these results underscore the potential of n-3 PUFAs to target other pathological conditions linked to MMP9 dysregulation.
The effects of n-3 PUFAs on MMP9 were similarly reported in other conditions, including the periodontal diseases as this enzyme centrally participates in the destruction of organic matrix of the dentin [114]. Specifically, a significant reduction in periodontal tissue MMP9 expression was found in the murine periodontitis model following the treatment of with n-3 PUFAs [115]. Similarly, resolvin (Rv) E1 and D1, metabolites derived from EPA and DHA significantly dampened the expression of MMP9 in immortalized mouse cementoblasts [116]. It was further shown in both in vitro and ex vivo teeth studies that n-3 PUFAs inhibit the proteolytic activity of MMP9 [117]. On the other hand, Liuzzi et al. treated cultured rat microglial cells with different doses of n-3 PUFAs followed by lipopolysaccharide stimulation (LPS) [118], and a dose-dependent inhibition in LPS-induced synthesis of MMP9 was associated with n-3 PUFAs [118]. In addition, Chitranjali et al. treated LPS-stimulated peripheral blood mononuclear cells (PBMCs) with n-3 PUFAs concentrate obtained from Dunaliella salina (a marine microalgae), and revealed that n-3 PUFAs blocked the expression of MMP9 in the PBMCs [119]. Similarly, aspirin-triggered RvD1 suppresses MMP9 activity and reduces inflammation and oxidative stress in mice exposed to ultraviolet radiation [120]. It was further shown that n-3 PUFAs decrease MMP9 expression in the mice model of Duchenne muscular dystrophy [121]. Corroborating these findings, studies further showed that treatment with n-3 PUFAs reduces the aortas expression of MMP9 in the murine models of the abdominal aortic aneurysm model [122-125]. Overall, n-3 PUFAs have shown promising effects on inhibiting the expression and activity of MMP9, indicating their therapeutic effects in pathological conditions characterized by MMP9 dysregulation, such as MDD (Table 1).
Table 1. Effects of n-3 PUFAs on MMP9: Evidence from Pre-clinical Studies.
| S/N | Ref | Model | Main findings |
| 1 | [106] | Castrate-resistant chronic cancer progression model | n-3 PUFAs decreased the expression of MMP9 mRNA |
| 2 | [107] | In vitro non-small cell lung cancer | DHA reduces the levels of metastasis-associated proteins including MMP9 in a dose-dependent manner |
| 3 | [108] | In vitro / in vivo | n-3 PUFAs suppressed MMP9 activity in cancer-associated fibroblasts |
| 4 | [115] | Murine periodontitis model | n-3 PUFAs decreased the tissue expression of MMP9 |
| 5 | [116] | In vitro | Resolvin E1 and resolvin D1 significantly reduced the expression of MMP9 |
| 6 | [117] | In vitro | n-3 PUFAs inhibited the proteolytic activity of MMP9 |
| 7 | [118] | In vitro | n-3 PUFAs dose-dependently decreased MMP9 protein levels secreted from LPS-activated microglial cells |
| 8 | [119] | In vitro | n-3 PUFAs concentrate downregulated LPS-induced expression of MMP9 by peripheral blood mononuclear cells |
| 9 | [120] | In vivo | Aspirin-triggered resolvin D1 suppressed the activity of MMP9 |
| 10 | [121] | Duchenne muscular dystrophy model | n-3 PUFAs reduced MMP9 gene expression and improved myoblast engraftment, satellite cell activation, and muscle regeneration |
| 11 | [124] | Abdominal aortic aneurysm model | MMP9 levels in the aortas were reduced following EPA treatment |
| 12 | [125] | Abdominal aortic aneurysm model | EPA and DHA significantly decreased the expression of MMP9 in the aortas |
EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid; LPS, Lipopolysaccharide; MMP9, Matrix metalloproteinase 9; n-3 PUFAs, omega-3 polyunsaturated fatty acids; PBMCs, Peripheral blood mononuclear cells.
The effect of n-3 PUFAs on MMP9 was similarly studied in clinical studies (Table 2), corroborating findings from preclinical studies. Shinto et al. demonstrated in an open-label study that relapsing-remitting multiple sclerosis (RRMS) patients supplemented with n-3 PUFAs (9.6g/day) for three months exhibited significantly lower MMP9 secretion from the immune cells [126]. In the study, more than a 50% decrease in MMP9 secretion from the PBMCs was found after three months compared with the baseline [126]. In another study, PBMCs from healthy controls were treated with EPA and DHA and were stimulated with concanavalin A [127]. The study found a significant reduction in MMP9 protein levels and MMP9 activity in the PBMCs associated with both EPA and DHA [127]. Frew et al. further demonstrated a reduction in MMP9 activity in amnion exposed to EPA and DHA compared with control [128]. Epitropoulus et al. demonstrated in a randomized controlled trial involving patients with dry eye disease that supplementation with n-3 PUFAs, compared with placebo is associated with a significant decrease in MMP9 positivity (67.9% vs. 35.0%) [129].
Table 2. Effects of n-3 PUFAs on MMP9: Evidence from Clinical Studies.
| S/N | Ref | Patients | Main finding |
| 1 | [126] | Relapsing-remitting multiple sclerosis patients | EPA and DHA resulted in a significant decrease in MMP9 protein and activity in the PBMCs |
| 2 | [127] | Healthy subjects | n-3 PUFAs significantly decreased MMP9 secretion from the PBMCs |
| 3 | [128] | Clinical | The activity of MMP9 in the amnion was significantly reduced by treatment with EPA and DHA compared to control |
| 4 | [129] | Dry eye disease patients | n-3 PUFAs decrease MMP9 positivity |
EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid; MMP9, Matrix metalloproteinase 9; n-3 PUFAs, omega-3 polyunsaturated fatty acids; PBMCs, Peripheral blood mononuclear cells.
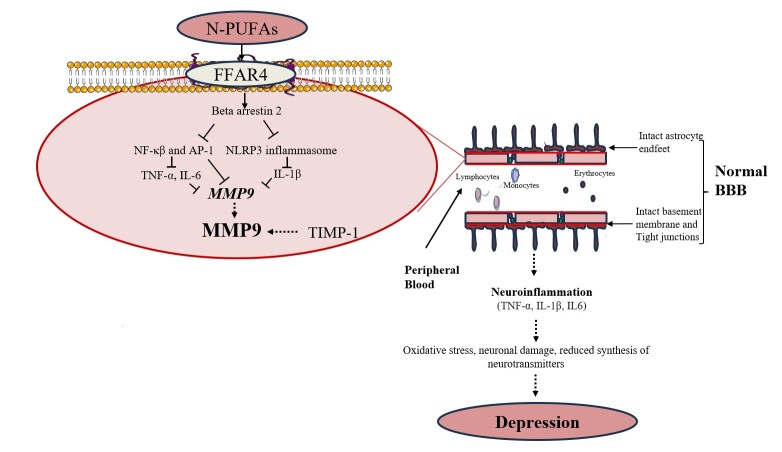
The reported inhibitory effects of n-3 PUFAs on MMP9 production in multiple studies may be likely linked to their ability to regulate inflammatory processes (Figure 2). Notably, n-3 PUFAs can inhibit the synthesis of MMP9 through several mechanisms. Specifically, n-3 PUFAs can inhibit the DNA binding activity of transcription factors such as activator protein (AP-1) and nuclear factor kappa-beta (NF-κβ). These transcription factors have binding sites on the MMP9 gene promoter [130,131], hence, their inhibition by n-3 PUFAs decreases the transcription of the MMP9 gene. Additionally, n-3 PUFAs may lower MMP9 levels by reducing the expression of proinflammatory cytokines such as IL-1β and IL-6. The ability of n-3 PUFAs to dampen the production of these cytokines has been well-established [132].
Figure 2.
Potential Mechanisms Associated with n-3 PUFAs Antidepressant Effects via the Improvement of BBB Integrity. n-3 PUFAs can decrease the expression of MMP9 via mechanisms including direct inhibition of NF-κβ and AP-1, resulting in reduced transcription of MMP9 gene. Further, n-3 PUFAs indirectly decrease MMP9 transcription by reducing the levels of NF-κβ, IL-1β, and IL-6, which stimulate MMP9 synthesis. Collectively, this reduces the extracellular levels of MMP9 thus limiting its proteolytic effects on the BBB and ultimately depression. AP-1, Activator protein 1; BBB, Blood-brain barrier; FFAR4, Free fatty acid receptor 4; IL, Interleukin; MMP9, Matrix metalloproteinase 9; n-3 PUFAs, Omega-3 polyunsaturated fatty acids; NF-κβ, Nuclear factor kappa beta; NLRP3, Nucleotide-binding oligomerization domain-like receptor protein 3; TIMP-1, Tissue inhibitors of metalloproteinase-1; TNF, Tumor necrosis factor.
Effects of n-3 PUFAs on AQP4 and ABCB1
n-3 PUFAs have a profound impact on AQP4 and the glymphatic system—crucial for brain health. Several studies underscore the pivotal role of n-3 PUFAs in enhancing the glymphatic clearance of neurotoxic substances, particularly Aβ, through mechanisms heavily reliant on AQP4 [133]. n-3 PUFAs significantly improved glymphatic function, reduced Aβ accumulation, and restored AQP4 expression and polarity, which are crucial for maintaining neurological health and function, especially during post-traumatic brain injury [134]. These fatty acids exhibit neuroprotective and anti-inflammatory effects, supporting cognitive function and potentially delaying or preventing neurodegenerative disorders such as AD [104]. Enriched n-3 PUFAs have been shown to enhance neuroprotection against microinfarctions by improving AQP4-mediated glymphatic clearance of interstitial solutes [135]. Additionally, it has been documented that n-3 PUFAs could protect against neuroinflammation and support cognitive function by promoting AQP4 polarization and enhancing glymphatic system efficiency [104]. The beneficial impact of n-3 PUFAs on the brain’s clearance system highlights their therapeutic potential in managing and preventing various neurological conditions by sustaining microvascular integrity and promoting efficient waste removal through the glymphatic pathway.
There are few studies that specifically document the effect of n-3 PUFAs on ABCB1. n-3 PUFAs can suppress the gene expression and pump activity of ABCB1 thus improving the efficacy of cancer chemotherapy [136,137]. We hypothesize that these fatty acids may downregulate ABCB1 expression through various signaling pathways, including those involving peroxisome proliferator-activated receptors and nuclear factor kappa-light-chain-enhancer of activated B cells. This downregulation could reduce the efflux activity of P-gp, potentially increasing the intracellular concentration of various drugs, particularly antidepressant medications. Further, n-3 PUFAs are integrated into cellular membranes, changing their fluidity and structure [138,139]. This alteration can potentially impact the function of membrane-bound proteins, including P-gp. Thus, changes in membrane fluidity can modulate the activity of ABCB1, potentially enhancing or inhibiting its drug transport capabilities.
Conclusion
BBB dysfunction is a pathological phenomenon in MDD. Dysfunctional BBB allows immune cell infiltration into the CNS and ultimately neuroinflammation. Interestingly, n-3 PUFAs can potentially enhance the integrity of BBB through mechanisms such as diminished expression and activity of MMP9, enhanced AQP4-dependent glymphatic clearance of neurotoxic substances, particularly Aβ, and downregulation of the ABCB1 gene. Given the consistent evidence of antidepressant effects associated with n-3 PUFAs, it is conceivable that their antidepressant efficacy may be partly attributable to their ability to reduce MMP9 levels or inhibit its activity, and improve the glymphatic clearance of waste, thus enhancing BBB integrity and function. However, empirical support for this hypothesis is currently lacking, underscoring the need for studies in this area. Future studies should focus on clarifying the connection between MMP9, AQP4, and ABCB1 with BBB dysfunction in MDD and assess whether n-3 PUFAs can exert their antidepressant efficacy by modulating these markers. Additionally, research should explore the specific relationships between n-3 PUFAs, MMP9, AQP4, ABCB1 expression, and depressive symptoms. These insights would furnish an additional mechanistic understanding of the antidepressant effects attributed to n-3 PUFAs, potentially facilitating the development of more effective antidepressant therapies.
Acknowledgments
The authors of this work were supported by the following grants: NSTC 113-2314-B-039-046 and 113-2923-B-039-001-MY3 from the National Science and Technology Council (NSTC), Taiwan; and ANHRF 110-13, 111-52, 112-24, 112-47, 113-24, 113-38, 113-40 from An-Nan Hospital, China Medical University, Tainan, Taiwan
Glossary
- 18-HEPE
18-hydroxyeicosapentaenoic acid
- ACC
anterior cingulate cortex
- AP-1
Activator protein 1
- BBB
Blood-brain barrier
- CNS
Central nervous system
- COX-2
cyclo-oxygenase-2
- CYP
Cytochrome
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- GFAP
glial fibrillary acidic protein
- GPX4
Glutathione peroxidase 4
- GSH
Reduced glutathione
- HPA
Hypothalamus-Pituitary-Adrenal
- ICAM
Intercellular adhesion molecule
- IL-1β
Interleukin 1β
- IL-6
Interleukin 6
- IFN
Interferon
- Ktrans
volume transfer constant
- LOX
Lipoxygenase
- LPS
Lipopolysaccharide
- MDD
Major depressive disorder
- MMP9
Metalloproteinase 9
- n-3 PUFAs
Omega-3 polyunsaturated fatty acids
- NMDA
N-methyl D-aspartate
- NOS
nitric oxide synthase
- NOTCH1
Neurogenic locus notch homolog protein 1
- Nrf-2
Nuclear factor erythroid 2-related factor 2
- NF-κβ
Nuclear factor kappa-beta
- PBMCs
Peripheral blood mononuclear cells
- ROS
Reactive oxygen species
- RvD
Resolvin D
- RvE
Resolvin E
- SNPs
Single nucleotide polymorphisms
- SPM
Specialized pro-resolving mediator
- TIMP
Tissue inhibitors of metalloproteinase
- TJPs
Tight junction proteins
- TNF-α
Tumor necrosis factor-alpha
- VCAM
Vascular cell adhesion molecule
- ZO-1
Zonula occludens 1
Author Contributions
HZ: Conceptualization, investigation, methodology, writing–original draft, writing–review and editing. WLW: Writing–review and editing, SKS: Writing–original draft, writing–review and editing. WCC: Writing–review and editing. WCL: Writing–review and editing. YSS: Writing–review and editing. JPC: Writing–review and editing. KPS: Conceptualization, supervision, writing–review and editing.
References
- Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700-12. Epub 20211008. https://doi.org/ 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MJ. Depression Is the Leading Cause of Disability Around the World. JAMA. 2017. Apr;317(15):1517. 10.1001/jama.2017.3826 [DOI] [PubMed] [Google Scholar]
- Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016. Oct;388(10053):1545–602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero M, Martín N. Depressive symptoms in neurodegenerative diseases. World J Clin Cases. 2015. Aug;3(8):682–93. 10.12998/wjcc.v3.i8.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galts CPC, Bettio LEB, Jewett DC, Yang CC, Brocardo PS, Rodrigues ALS, et al. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci Biobehav Rev. 2019;102:56-84. Epub 20190415. https://doi.org/ 10.1016/j.neubiorev.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Su KP. Inflammation in psychopathology of depression: Clinical, biological, and therapeutic implications. Biomedicine (Taipei). 2012;2(2):68–74. 10.1016/j.biomed.2012.03.002 [DOI] [Google Scholar]
- Das R, Emon MPZ, Shahriar M, Nahar Z, Islam SMA, Bhuiyan MA, et al. Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res. 2021;295:113568. Epub 20201110. https://doi.org/ 10.1016/j.psychres.2020.113568. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-41. Epub 20090115. https://doi.org/ 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su KP, Lai HC, Peng CY, Su WP, Chang JP, Pariante CM. Interferon-alpha-induced depression: Comparisons between early- and late-onset subgroups and with patients with major depressive disorder. Brain Behav Immun. 2019;80:512-8. Epub 20190504. https://doi.org/ 10.1016/j.bbi.2019.04.032. [DOI] [PubMed] [Google Scholar]
- Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017. Feb;60:1–12. 10.1016/j.bbi.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Archie SR, Al Shoyaib A, Cucullo L. Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics. 2021;13(11). Epub 20211026. https://doi.org/ 10.3390/pharmaceutics13111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Dong Z, Gan Q, Tang X, Xing J, Sheng X, et al. Matrix metalloproteinase 9 modulates immune response along with the formation of extracellular traps in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2023. Feb;133:108570. 10.1016/j.fsi.2023.108570 [DOI] [PubMed] [Google Scholar]
- Takata F, Nakagawa S, Matsumoto J, Dohgu S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front Cell Neurosci. 2021;15:661838. Epub 20210913. https://doi.org/ 10.3389/fncel.2021.661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:32. Epub 20130403. https://doi.org/ 10.3389/fneur.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009. Oct;65(4):702–8. 10.1227/01.NEU.0000351768.11363.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14(4):265-77. Epub 20130313. https://doi.org/ 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem Res. 2015;40(12):2583-99. Epub 20150507. https://doi.org/ 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerb R. Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006;234(1):4-33. Epub 20060228. https://doi.org/ 10.1016/j.canlet.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Amin ML. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights. 2013;7:27-34. Epub 20130819. https://doi.org/ 10.4137/DTI.S12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zailani H, Wu SK, Yang KJ, Malau IA, Liao HF, Chung YL, et al. Omega-3 polyunsaturated fatty acids in the prevention of relapse in patients with stable bipolar disorder: A 6-month pilot randomized controlled trial. Psychiatry Res. 2024. Jan;331:115633. 10.1016/j.psychres.2023.115633 [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003. Aug;13(4):267–71. 10.1016/S0924-977X(03)00032-4 [DOI] [PubMed] [Google Scholar]
- Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP, et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry. 2014. Oct;76(7):559–66. 10.1016/j.biopsych.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Iqbal AZ, Wu SK, Zailani H, Chiu WC, Liu WC, Su KP, et al. Effects of Omega-3 Polyunsaturated Fatty Acids Intake on Vasomotor Symptoms, Sleep Quality and Depression in Postmenopausal Women: A Systematic Review. Nutrients. 2023. Sep;15(19):4231. 10.3390/nu15194231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Tseng PT, Chen NY, Lin PC, Lin PY, Chang JP, et al. Safety and tolerability of prescription omega-3 fatty acids: A systematic review and meta-analysis of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2018;129:1-12. Epub 20180105. https://doi.org/ 10.1016/j.plefa.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Chang JP, Tseng PT, Zeng BS, Chang CH, Su H, Chou PH, et al. Safety of Supplementation of Omega-3 Polyunsaturated Fatty Acids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2023. Nov;14(6):1326–36. 10.1016/j.advnut.2023.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zailani H, Satyanarayanan SK, Liao WC, Liao HF, Huang SY, Gałecki P, et al. Omega-3 Polyunsaturated Fatty Acids in Managing Comorbid Mood Disorders in Chronic Obstructive Pulmonary Disease (COPD): A Review. J Clin Med. 2023;12(7). Epub 20230402. https://doi.org/ 10.3390/jcm12072653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su KP. Nutrition, psychoneuroimmunology and depression: the therapeutic implications of omega-3 fatty acids in interferon-α-induced depression. Biomedicine (Taipei). 2015;5(4):21. Epub 20151128. https://doi.org/ 10.7603/s40681-015-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su KP, Matsuoka Y, Pae CU. Omega-3 Polyunsaturated Fatty Acids in Prevention of Mood and Anxiety Disorders. Clin Psychopharmacol Neurosci. 2015. Aug;13(2):129–37. 10.9758/cpn.2015.13.2.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Xiong JY, Chai YQ, Huang L, Tang ZY, Zhang XF, et al. Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system. Front Psychiatry. 2022;13:933704. Epub 20220831. https://doi.org/ 10.3389/fpsyt.2022.933704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010. Jan;2(1):a002907. 10.1101/cshperspect.a002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020. Nov;17(1):69. 10.1186/s12987-020-00230-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012. Nov;72(5):648–72. 10.1002/ana.23648 [DOI] [PubMed] [Google Scholar]
- Wu D, Chen Q, Chen X, Han F, Chen Z, Wang Y. The blood-brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther. 2023. May;8(1):217. 10.1038/s41392-023-01481-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999. Oct;147(1):185–94. 10.1083/jcb.147.1.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003. May;161(3):653–60. 10.1083/jcb.200302070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Yin Y, Du L. Blood-Brain Barrier Dysfunction in the Pathogenesis of Major Depressive Disorder. Cell Mol Neurobiol. 2022;42(8):2571-91. Epub 20211012. https://doi.org/ 10.1007/s10571-021-01153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchia GP, Nico B, Camassa LM, Mola MG, Loh N, Dermietzel R, et al. The role of aquaporin-4 in the blood-brain barrier development and integrity: studies in animal and cell culture models. Neuroscience. 2004;129(4):935–45. 10.1016/j.neuroscience.2004.07.055 [DOI] [PubMed] [Google Scholar]
- Salman MM, Kitchen P, Halsey A, Wang MX, Törnroth-Horsefield S, Conner AC, et al. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain. 2022. Mar;145(1):64–75. 10.1093/brain/awab311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JA, Hsu MS, Seldin MM, Binder DK. Expression of the Astrocyte Water Channel Aquaporin-4 in the Mouse Brain. ASN Neuro. 2015;7(5). Epub 20151021. https://doi.org/ 10.1177/1759091415605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997. Jan;17(1):171–80. 10.1523/JNEUROSCI.17-01-00171.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo C, Buccoliero C, Mola MG, Abbrescia P, Nicchia GP, Trojano M, et al. AQP4ex is crucial for the anchoring of AQP4 at the astrocyte end-feet and for neuromyelitis optica antibody binding. Acta Neuropathol Commun. 2019. Apr;7(1):51. 10.1186/s40478-019-0707-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva I, Silva J, Ferreira R, Trigo D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol Res Pract. 2021. Jan;3(1):5. 10.1186/s42466-021-00102-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JA, Hartz AMS, Bauer B. ABCB1 and ABCG2 Regulation at the Blood-Brain Barrier: Potential New Targets to Improve Brain Drug Delivery. Pharmacol Rev. 2023;75(5):815-53. Epub 20230327. https://doi.org/ 10.1124/pharmrev.120.000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora Lagares L, Pérez-Castillo Y, Novič M. Exploring the dynamics of the ABCB1 membrane transporter P-glycoprotein in the presence of ATP and active/non-active compounds through molecular dynamics simulations. Toxicology. 2024. Feb;502:153732. 10.1016/j.tox.2024.153732 [DOI] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31(6):246-54. Epub 20100424. https://doi.org/ 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elali A, Rivest S. The role of ABCB1 and ABCA1 in beta-amyloid clearance at the neurovascular unit in Alzheimer’s disease. Front Physiol. 2013;4:45. Epub 20130313. https://doi.org/ 10.3389/fphys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Rodriguez EM, Beurel E. Blood brain barrier and inflammation in depression. Neurobiol Dis. 2022. Dec;175:105926. 10.1016/j.nbd.2022.105926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie KL, Pelletier R, Arsenault A, Dupuis J, Bacon SL. Association between clinical depression and endothelial function measured by forearm hyperemic reactivity. Psychosom Med. 2010. Jan;72(1):20–6. 10.1097/PSY.0b013e3181c2d6b8 [DOI] [PubMed] [Google Scholar]
- Shang B, Wang T, Zhao S, Yi S, Zhang T, Yang Y, et al. Higher Blood-brain barrier permeability in patients with major depressive disorder identified by DCE-MRI imaging. Psychiatry Res Neuroimaging. 2024;337:111761. Epub 20231123. https://doi.org/ 10.1016/j.pscychresns.2023.111761. [DOI] [PubMed] [Google Scholar]
- Lopez-Vilchez I, Diaz-Ricart M, Navarro V, Torramade S, Zamorano-Leon J, Lopez-Farre A, et al. Endothelial damage in major depression patients is modulated by SSRI treatment, as demonstrated by circulating biomarkers and an in vitro cell model. Transl Psychiatry. 2016;6(9):e886. Epub 20160906. https://doi.org/ 10.1038/tp.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraets AF, van Agtmaal MJ, Stehouwer CD, Sörensen BM, Berendschot TT, Webers CA, et al. Association of markers of microvascular dysfunction with prevalent and incident depressive symptoms: the Maastricht Study. Hypertension. 2020. Aug;76(2):342–9. 10.1161/HYPERTENSIONAHA.120.15260 [DOI] [PubMed] [Google Scholar]
- Tchalla AE, Wellenius GA, Sorond FA, Travison TG, Dantoine T, Lipsitz LA. Elevated circulating vascular cell Adhesion Molecule-1 (sVCAM-1) is associated with concurrent depressive symptoms and cerebral white matter Hyperintensities in older adults. BMC Geriatr. 2015. Jun;15(1):62. 10.1186/s12877-015-0063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi P, Brondino N, Emanuele E. Increased proapoptotic serum activity in patients with chronic mood disorders. Arch Med Res. 2008. Feb;39(2):242–5. 10.1016/j.arcmed.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017. Dec;20(12):1752–60. 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020. Nov;10(1):373. 10.1038/s41398-020-01054-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman E, Taler M, Flug R, Gur S, Dar S, Bormant G, et al. Serum claudin-5 levels among patients with unipolar and bipolar depression in relation to the pro-inflammatory cytokine tumor necrosis factor-alpha levels. Brain Behav Immun. 2023;109:162-7. Epub 20230124. https://doi.org/ 10.1016/j.bbi.2023.01.015. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang J, Teng T, Yin B, He Y, Jiang Y, et al. Biomarkers of intestinal permeability and blood-brain barrier permeability in adolescents with major depressive disorder. J Affect Disord. 2023. Feb;323:659–66. 10.1016/j.jad.2022.11.058 [DOI] [PubMed] [Google Scholar]
- Domenici E, Willé DR, Tozzi F, Prokopenko I, Miller S, McKeown A, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One. 2010;5(2):e9166. Epub 20100211. https://doi.org/ 10.1371/journal.pone.0009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobińska K, Szemraj J, Czarny P, Gałecki P. Role of MMP-2, MMP-7, MMP-9 and TIMP-2 in the development of recurrent depressive disorder. J Affect Disord. 2016;205:119-29. Epub 20160630. https://doi.org/ 10.1016/j.jad.2016.03.068. [DOI] [PubMed] [Google Scholar]
- Hamed R, Elmalt H, Salama A, Abozaid S, Elaziz SA. Role of matrix metalloproteinase-2, matrix metalloproteinase-9, and tissue inhibitors of metalloproteinase-1 in recurrent depression. Sci J Al-Azhar Med Fac Girls. 2020;4(2):295. 10.4103/sjamf.sjamf_51_20 [DOI] [Google Scholar]
- Garvin P, Nilsson L, Carstensen J, Jonasson L, Kristenson M. Plasma levels of matrix metalloproteinase-9 are independently associated with psychosocial factors in a middle-aged normal population. Psychosom Med. 2009;71(3):292-300. Epub 20090205. https://doi.org/ 10.1097/PSY.0b013e3181960e7f. [DOI] [PubMed] [Google Scholar]
- Shibasaki C, Itagaki K, Abe H, Kajitani N, Okada-Tsuchioka M, Takebayashi M. Possible association between serum matrix metalloproteinase-9 (MMP-9) levels and relapse in depressed patients following electroconvulsive therapy (ECT). Int J Neuropsychopharmacol. 2018. Mar;21(3):236–41. 10.1093/ijnp/pyx086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, et al. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One. 2012;7(8):e42676. Epub 20120803. https://doi.org/ 10.1371/journal.pone.0042676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijata M, Bączyńska E, Müller FE, Bijata K, Masternak J, Krzystyniak A, et al. Activation of the 5-HT7 receptor and MMP-9 signaling module in the hippocampal CA1 region is necessary for the development of depressive-like behavior. Cell Rep. 2022. Mar;38(11):110532. 10.1016/j.celrep.2022.110532 [DOI] [PubMed] [Google Scholar]
- Breviario S, Senserrich J, Florensa-Zanuy E, Garro-Martínez E, Díaz Á, Castro E, et al. Brain matrix metalloproteinase-9 activity is altered in the corticosterone mouse model of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2023. Jan;120:110624. 10.1016/j.pnpbp.2022.110624 [DOI] [PubMed] [Google Scholar]
- van der Kooij MA, Fantin M, Rejmak E, Grosse J, Zanoletti O, Fournier C, et al. Role for MMP-9 in stress-induced downregulation of nectin-3 in hippocampal CA1 and associated behavioural alterations. Nat Commun. 2014. Sep;5(1):4995. 10.1038/ncomms5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Hernández D, Martínez M, Robledo-Montaña J, Muñoz-López M, Virto L, Ambrosio N, et al. Neuroinflammation related to the blood-brain barrier and sphingosine-1-phosphate in a pre-clinical model of periodontal diseases and depression in rats. J Clin Periodontol. 2023;50(5):642-56. Epub 20230127. https://doi.org/ 10.1111/jcpe.13780. [DOI] [PubMed] [Google Scholar]
- Su KP. Mind-body interface: the role of n-3 fatty acids in psychoneuroimmunology, somatic presentation, and medical illness comorbidity of depression. Asia Pac J Clin Nutr. 2008;17 Suppl 1:151–7. [PubMed] [Google Scholar]
- González-Arias C, Sánchez-Ruiz A, Esparza J, Sánchez-Puelles C, Arancibia L, Ramírez-Franco J, et al. Dysfunctional serotonergic neuron-astrocyte signaling in depressive-like states. Mol Psychiatry. 2023. Sep;28(9):3856–73. 10.1038/s41380-023-02269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb JA, O’Neill K, Milner J, Mahajan GJ, Lawrence TJ, May WL, et al. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience. 2016;316:209-20. Epub 20151230. https://doi.org/ 10.1016/j.neuroscience.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittins RA, Harrison PJ. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J Affect Disord. 2011;133(1-2):328-32. Epub 20110417. https://doi.org/ 10.1016/j.jad.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Xie XH, Lai WT, Xu SX, Di Forti M, Zhang JY, Chen MM, et al. Hyper-inflammation of astrocytes in patients of major depressive disorder: evidence from serum astrocyte-derived extracellular vesicles. Brain Behav Immun. 2023. Mar;109:51–62. 10.1016/j.bbi.2022.12.014 [DOI] [PubMed] [Google Scholar]
- Kong H, Sha LL, Fan Y, Xiao M, Ding JH, Wu J, et al. Requirement of AQP4 for antidepressive efficiency of fluoxetine: implication in adult hippocampal neurogenesis. Neuropsychopharmacology. 2009. Apr;34(5):1263–76. 10.1038/npp.2008.185 [DOI] [PubMed] [Google Scholar]
- Liu X, Gu XH, Zheng LL, Xu LJ, Yang YJ, Yang G, et al. Autophagy promotes membrane trafficking of NR2B to alleviate depression by inhibiting AQP4 expression in mice. Exp Cell Res. 2022. Oct;419(1):113298. 10.1016/j.yexcr.2022.113298 [DOI] [PubMed] [Google Scholar]
- Westermair AL, Munz M, Schaich A, Nitsche S, Willenborg B, Muñoz Venegas LM, et al. Association of Genetic Variation at AQP4 Locus with Vascular Depression. Biomolecules. 2018;8(4). Epub 20181205. https://doi.org/ 10.3390/biom8040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong H, Zeng XN, Fan Y, Yuan ST, Ge S, Xie WP, et al. Aquaporin-4 knockout exacerbates corticosterone-induced depression by inhibiting astrocyte function and hippocampal neurogenesis. CNS Neurosci Ther. 2014;20(5):391-402. Epub 20140115. https://doi.org/ 10.1111/cns.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Yang L, Sun G, Qi S, Li B. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: the function of AQP4 and the glymphatic system. Psychopharmacology (Berl). 2017;234(3):365-79. Epub 20161112. https://doi.org/ 10.1007/s00213-016-4473-9. [DOI] [PubMed] [Google Scholar]
- Iorio R. Treatment-Resistant Depression and Aquaporin-4 Autoantibodies: Is There a Link? Biol Psychiatry. 2015. Jul;78(1):e1–2. 10.1016/j.biopsych.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Gur S, Taler M, Bormant G, Blattberg D, Nitzan U, Vaknin-Dembinsky A, et al. Lack of association between unipolar or bipolar depression and serum aquaporin-4 autoantibodies. Brain Behav Immun. 2020;88:930-4. Epub 20200505. https://doi.org/ 10.1016/j.bbi.2020.05.001. [DOI] [PubMed] [Google Scholar]
- Brückl TM, Uhr M. ABCB1 genotyping in the treatment of depression. Pharmacogenomics. 2016;17(18):2039-69. Epub 20161205. https://doi.org/ 10.2217/pgs.16.18. [DOI] [PubMed] [Google Scholar]
- Chang HH, Chou CH, Yang YK, Lee IH, Chen PS. Association between ABCB1 Polymorphisms and Antidepressant Treatment Response in Taiwanese Major Depressive Patients. Clin Psychopharmacol Neurosci. 2015. Dec;13(3):250–5. 10.9758/cpn.2015.13.3.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Fukuda T, Serretti A, Wakeno M, Okugawa G, Ikenaga Y, et al. ABCB1 (MDR1) gene polymorphisms are associated with the clinical response to paroxetine in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):398-404. Epub 20070915. https://doi.org/ 10.1016/j.pnpbp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Ray A, Tennakoon L, Keller J, Sarginson JE, Ryan HS, Murphy GM, et al. ABCB1 (MDR1) predicts remission on P-gp substrates in chronic depression. Pharmacogenomics J. 2015. Aug;15(4):332–9. 10.1038/tpj.2014.72 [DOI] [PubMed] [Google Scholar]
- Blázquez A, Gassó P, Mas S, Plana MT, Lafuente A, Lázaro L. One-Year Follow-up of Children and Adolescents with Major Depressive Disorder: Relationship between Clinical Variables and Abcb1 Gene Polymorphisms. Pharmacopsychiatry. 2016;49(6):248-53. Epub 20160616. https://doi.org/ 10.1055/s-0042-108202. [DOI] [PubMed] [Google Scholar]
- Ma G, Huang X, Bi Y, Ren D, Xu F, Sun Q, et al. Association study between ABCB1, ABCB6 and ABCG1 polymorphisms and major depressive disorder in the Chinese Han population. Psychiatry Res. 2018;270:1170-1. Epub 20180523. https://doi.org/ 10.1016/j.psychres.2018.05.045. [DOI] [PubMed] [Google Scholar]
- Shan XX, Qiu Y, Xie WW, Wu RR, Yu Y, Wu HS, et al. ABCB1 Gene Is Associated With Clinical Response to SNRIs in a Local Chinese Han Population. Front Pharmacol. 2019;10:761. Epub 20190704. https://doi.org/ 10.3389/fphar.2019.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Lima L, Carvalho S, Brandão A, Barroso F, Cruz A, et al. ABCB1 C1236T, G2677TA and C3435T Genetic Polymorphisms and Antidepressant Response Phenotypes: Results from a Portuguese Major Depressive Disorder Cohort. Int J Mol Sci. 2024;25(10). Epub 20240508. https://doi.org/ 10.3390/ijms25105112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AB, Bousman CA, Ng CH, Byron K, Berk M. ABCB1 polymorphism predicts escitalopram dose needed for remission in major depression. Transl Psychiatry. 2012;2(11):e198. Epub 20121127. https://doi.org/ 10.1038/tp.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeleń A, Świechowski R, Żebrowska-Nawrocka M, Sałagacka-Kubiak A, Szmajda-Krygier D, Gałecki P, et al. Importance of selected ABCB1 SNPs for the level of severity of depressive symptoms and effectiveness of recurrent depressive disorder therapy. Gene. 2023. Jan;851:147021. 10.1016/j.gene.2022.147021 [DOI] [PubMed] [Google Scholar]
- Zailani H, Satyanarayanan SK, Liao WC, Hsu YT, Huang SY, Gałecki P, et al. Roles of Omega-3 Polyunsaturated Fatty Acids in Managing Cognitive Impairment in Chronic Obstructive Pulmonary Disease: A Review. Nutrients. 2023. Oct;15(20):4363. 10.3390/nu15204363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JP, Su KP, Mondelli V, Pariante CM. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: a Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology. 2018;43(3):534-45. Epub 20170725. https://doi.org/ 10.1038/npp.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HF, Liu WC, Zailani H, Yang CC, Chen TB, Chang CM, et al. A 12-week randomized double-blind clinical trial of eicosapentaenoic acid intervention in episodic migraine. Brain Behav Immun. 2024;118:459-67. Epub 20240316. https://doi.org/ 10.1016/j.bbi.2024.03.019. [DOI] [PubMed] [Google Scholar]
- Chang JP, Lin CY, Lin PY, Shih YH, Chiu TH, Ho M, et al. Polyunsaturated fatty acids and inflammatory markers in major depressive episodes during pregnancy. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80(Pt C):273-8. Epub 20170520. https://doi.org/ 10.1016/j.pnpbp.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007. Jul;68(7):1056–61. 10.4088/JCP.v68n0712 [DOI] [PubMed] [Google Scholar]
- Lin PY, Chang CH, Chong MF, Chen H, Su KP. Polyunsaturated Fatty Acids in Perinatal Depression: A Systematic Review and Meta-analysis. Biol Psychiatry. 2017;82(8):560-9. Epub 20170309. https://doi.org/ 10.1016/j.biopsych.2017.02.1182. [DOI] [PubMed] [Google Scholar]
- Liao Y, Xie B, Zhang H, He Q, Guo L, Subramanieapillai M, et al. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl Psychiatry. 2019. Aug;9(1):190. 10.1038/s41398-019-0515-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JP, Tseng PT, Zeng BS, Chang CH, Su H, Chou PH, et al. Safety of Supplementation of Omega-3 Polyunsaturated Fatty Acids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2023;14(6):1326-36. Epub 20230809. https://doi.org/ 10.1016/j.advnut.2023.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DY, Tsao YY, Leung YM, Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for ω-3 fatty acids. Neuropsychopharmacology. 2010;35(11):2238-48. Epub 20100728. https://doi.org/ 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgórzyńska E, Dziedzic B, Gorzkiewicz A, Stulczewski D, Bielawska K, Su KP, et al. Omega-3 polyunsaturated fatty acids improve the antioxidative defense in rat astrocytes via an Nrf2-dependent mechanism. Pharmacol Rep. 2017;69(5):935-42. Epub 20170420. https://doi.org/ 10.1016/j.pharep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Borsini A, Nicolaou A, Camacho-Muñoz D, Kendall AC, Di Benedetto MG, Giacobbe J, et al. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: relevance for major depression and for human hippocampal neurogenesis. Mol Psychiatry. 2021;26(11):6773-88. Epub 20210616. https://doi.org/ 10.1038/s41380-021-01160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Lin L, Bazinet RP, Chien YC, Chang JP, Satyanarayanan SK, et al. Clinical Efficacy and Biological Regulations of ω-3 PUFA-Derived Endocannabinoids in Major Depressive Disorder. Psychother Psychosom. 2019;88(4):215-24. Epub 20190703. https://doi.org/ 10.1159/000501158. [DOI] [PubMed] [Google Scholar]
- Lamon-Fava S, So J, Mischoulon D, Ziegler TR, Dunlop BW, Kinkead B, et al. Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation. Prostaglandins Leukot Essent Fatty Acids. 2021;164:102219. Epub 20201205. https://doi.org/ 10.1016/j.plefa.2020.102219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamon-Fava S, Liu M, Dunlop BW, Kinkead B, Schettler PJ, Felger JC, et al. Clinical response to EPA supplementation in patients with major depressive disorder is associated with higher plasma concentrations of pro-resolving lipid mediators. Neuropsychopharmacology. 2023. May;48(6):929–35. 10.1038/s41386-022-01527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Satyanarayanan SK, Li A, Yan L, Zhao Z, Yuan Q, et al. Unraveling the impact of Omega-3 polyunsaturated fatty acids on blood-brain barrier (BBB) integrity and glymphatic function. Brain Behav Immun. 2024. Jan;115:335–55. 10.1016/j.bbi.2023.10.018 [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000. Oct;2(10):737–44. 10.1038/35036374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Henning SM, Guan J, Grogan T, Elashoff D, Cohen P, et al. Effect of dietary omega-3 fatty acids on castrate-resistant prostate cancer and tumor-associated macrophages. Prostate Cancer Prostatic Dis. 2020;23(1):127-35. Epub 20190822. https://doi.org/ 10.1038/s41391-019-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Sui C, Meng F, Ma P, Jiang Y. The omega-3 polyunsaturated fatty acid docosahexaenoic acid inhibits proliferation and progression of non-small cell lung cancer cells through the reactive oxygen species-mediated inactivation of the PI3K /Akt pathway. Lipids Health Dis. 2017;16(1):87. Epub 20170503. https://doi.org/ 10.1186/s12944-017-0474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Kawana K, Tomio K, Yamashita A, Isobe Y, Nagasaka K, et al. Matrix metalloproteinase (MMP)-9 in cancer-associated fibroblasts (CAFs) is suppressed by omega-3 polyunsaturated fatty acids in vitro and in vivo. PLoS One. 2014;9(2):e89605. Epub 20140227. https://doi.org/ 10.1371/journal.pone.0089605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun EJ, Song KS, Shin S, Kim S, Heo JY, Kweon GR, et al. Docosahexaenoic acid suppresses breast cancer cell metastasis by targeting matrix-metalloproteinases. Oncotarget. 2016. Aug;7(31):49961–71. 10.18632/oncotarget.10266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JK, Yu HN, Noh EM, Kim JM, Hong OY, Youn HJ, et al. DHA blocks TPA-induced cell invasion by inhibiting MMP-9 expression via suppression of the PPAR-γ/NF-κB pathway in MCF-7 cells. Oncol Lett. 2017;13(1):243-9. Epub 20161111. https://doi.org/ 10.3892/ol.2016.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio C, Piazzi G, Vitaglione P, Fogliano V, Munarini A, Prossomariti A, et al. Inflammation increases NOTCH1 activity via MMP9 and is counteracted by Eicosapentaenoic Acid-free fatty acid in colon cancer cells. Sci Rep. 2016;6:20670. Epub 20160211. https://doi.org/ 10.1038/srep20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Wu YN, Wang SL, Lin QH, He MF, Liu QL, et al. Docosahexaenoic Acid Modulates Invasion and Metastasis of Human Ovarian Cancer via Multiple Molecular Pathways. Int J Gynecol Cancer. 2016. Jul;26(6):994–1003. 10.1097/IGC.0000000000000746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadian M, Shekari N, Soltani-Zangbar MS, Mohammadi A, Mansoori B, Maralbashi S, et al. Docosahexaenoic acid suppresses migration of triple-negative breast cancer cell through targeting metastasis-related genes and microRNA under normoxic and hypoxic conditions. J Cell Biochem. 2020;121(3):2416-27. Epub 20191112. https://doi.org/ 10.1002/jcb.29464. [DOI] [PubMed] [Google Scholar]
- Luchian I, Goriuc A, Sandu D, Covasa M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int J Mol Sci. 2022;23(3). Epub 20220204. https://doi.org/ 10.3390/ijms23031806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alva P, Solís-Suárez DL, Cifuentes-Mendiola SE, García-Hernández AL. A diet rich in omega-3 fatty acid improves periodontitis and tissue destruction by MMP2- and MMP9-linked inflammation in a murine model. Odontology. 2024;112(1):185-99. Epub 20230628. https://doi.org/ 10.1007/s10266-023-00831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt SB, Hakki SS, Kantarci A. Differential effects of resolvin D1 and resolvin E1 on cementoblast function. J Periodontol. 2023;94(11):1351-62. Epub 20230615. https://doi.org/ 10.1002/JPER.22-0510. [DOI] [PubMed] [Google Scholar]
- Nicolai E, Sinibaldi F, Sannino G, Laganà G, Basoli F, Licoccia S, et al. Omega-3 and Omega-6 Fatty Acids Act as Inhibitors of the Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Activity. Protein J. 2017. Aug;36(4):278–85. 10.1007/s10930-017-9727-9 [DOI] [PubMed] [Google Scholar]
- Liuzzi GM, Latronico T, Rossano R, Viggiani S, Fasano A, Riccio P. Inhibitory effect of polyunsaturated fatty acids on MMP-9 release from microglial cells--implications for complementary multiple sclerosis treatment. Neurochem Res. 2007;32(12):2184-93. Epub 20070711. https://doi.org/ 10.1007/s11064-007-9415-9. [DOI] [PubMed] [Google Scholar]
- Chitranjali T, Anoop Chandran P, Muraleedhara Kurup G. Omega-3 fatty acid concentrate from Dunaliella salina possesses anti-inflammatory properties including blockade of NF-κB nuclear translocation. Immunopharmacol Immunotoxicol. 2015;37(1):81-9. Epub 20141113. https://doi.org/ 10.3109/08923973.2014.981639. [DOI] [PubMed] [Google Scholar]
- Melo CP, Saito P, Martinez RM, Staurengo-Ferrari L, Pinto IC, Rodrigues CC, et al. Aspirin-Triggered Resolvin D1 (AT-RvD1) Protects Mouse Skin against UVB-Induced Inflammation and Oxidative Stress. Molecules. 2023. Mar;28(5):2417. 10.3390/molecules28052417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho SC, Hindi SM, Kumar A, Marques MJ. Effects of omega-3 on matrix metalloproteinase-9, myoblast transplantation and satellite cell activation in dystrophin-deficient muscle fibers. Cell Tissue Res. 2017. Sep;369(3):591–602. 10.1007/s00441-017-2640-x [DOI] [PubMed] [Google Scholar]
- Kamata R, Bumdelger B, Kokubo H, Fujii M, Yoshimura K, Ishida T, et al. EPA Prevents the Development of Abdominal Aortic Aneurysms through Gpr-120/Ffar-4. PLoS One. 2016;11(10):e0165132. Epub 20161020. https://doi.org/ 10.1371/journal.pone.0165132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugo H, Zaima N, Mouri Y, Tanaka H, Yanagimoto K, Urano T, et al. The preventive effect of fish oil on abdominal aortic aneurysm development. Biosci Biotechnol Biochem. 2016;80(6):1186-91. Epub 20160329. https://doi.org/ 10.1080/09168451.2016.1146073. [DOI] [PubMed] [Google Scholar]
- Wang JH, Eguchi K, Matsumoto S, Fujiu K, Komuro I, Nagai R, et al. The ω-3 polyunsaturated fatty acid, eicosapentaenoic acid, attenuates abdominal aortic aneurysm development via suppression of tissue remodeling. PLoS One. 2014;9(5):e96286. Epub 20140505. https://doi.org/ 10.1371/journal.pone.0096286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara T, Shimada K, Fukao K, Sai E, Sato-Okabayashi Y, Matsumori R, et al. Omega 3 Polyunsaturated Fatty Acids Suppress the Development of Aortic Aneurysms Through the Inhibition of Macrophage-Mediated Inflammation. Circ J. 2015;79(7):1470-8. Epub 20150430. https://doi.org/ 10.1253/circj.CJ-14-0471. [DOI] [PubMed] [Google Scholar]
- Shinto L, Marracci G, Baldauf-Wagner S, Strehlow A, Yadav V, Stuber L, et al. Omega-3 fatty acid supplementation decreases matrix metalloproteinase-9 production in relapsing-remitting multiple sclerosis. Prostaglandins Leukot Essent Fatty Acids. 2009;80(2-3):131-6. Epub 20090125. https://doi.org/ 10.1016/j.plefa.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinto L, Marracci G, Bumgarner L, Yadav V. The effects of omega-3 Fatty acids on matrix metalloproteinase-9 production and cell migration in human immune cells: implications for multiple sclerosis. Autoimmune Dis. 2011;2011:134592. Epub 20110720. https://doi.org/ 10.4061/2011/134592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew L, Sugiarto NU, Rajagopal SP, He J, Leask R, Norman JE, et al. The effect of omega-3 polyunsaturated fatty acids on the inflammatory response of the amnion. Prostaglandins Leukot Essent Fatty Acids. 2013. Sep;89(4):221–5. 10.1016/j.plefa.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Epitropoulos AT, Donnenfeld ED, Shah ZA, Holland EJ, Gross M, Faulkner WJ, et al. Effect of Oral Re-esterified Omega-3 Nutritional Supplementation on Dry Eyes. Cornea. 2016. Sep;35(9):1185–91. 10.1097/ICO.0000000000000940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre Y, Van Themsche C, Estève PO. Emerging features in the regulation of MMP-9 gene expression for the development of novel molecular targets and therapeutic strategies. Curr Drug Targets Inflamm Allergy. 2003. Sep;2(3):206–15. 10.2174/1568010033484133 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chen LH. Eicosapentaenoic acid prevents lipopolysaccharide-stimulated DNA binding of activator protein-1 and c-Jun N-terminal kinase activity. J Nutr Biochem. 2005. Feb;16(2):78–84. 10.1016/j.jnutbio.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Ramirez-Ramirez V, Macias-Islas MA, Ortiz GG, Pacheco-Moises F, Torres-Sanchez ED, Sorto-Gomez TE, et al. Efficacy of fish oil on serum of TNF α, IL-1β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid Med Cell Longev. 2013;2013:709493. Epub 20130618. https://doi.org/ 10.1155/2013/709493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Luo C, Feng Y, Yao X, Shi Z, Liang F, et al. Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. Faseb j. 2017;31(1):282-93. Epub 20161007. https://doi.org/ 10.1096/fj.201600896. [DOI] [PubMed] [Google Scholar]
- Zhang E, Wan X, Yang L, Wang D, Chen Z, Chen Y, et al. Omega-3 Polyunsaturated Fatty Acids Alleviate Traumatic Brain Injury by Regulating the Glymphatic Pathway in Mice. Front Neurol. 2020. Jul;11:707. 10.3389/fneur.2020.00707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Xie Y, Satyanarayanan SK, Zeng H, Liu Q, Huang M, et al. Omega-3 polyunsaturated fatty acids promote brain-to-blood clearance of β-Amyloid in a mouse model with Alzheimer’s disease. Brain Behav Immun. 2020. Mar;85:35–45. 10.1016/j.bbi.2019.05.033 [DOI] [PubMed] [Google Scholar]
- Kuan CY, Walker TH, Luo PG, Chen CF. Long-chain polyunsaturated fatty acids promote paclitaxel cytotoxicity via inhibition of the MDR1 gene in the human colon cancer Caco-2 cell line. J Am Coll Nutr. 2011. Aug;30(4):265–73. 10.1080/07315724.2011.10719969 [DOI] [PubMed] [Google Scholar]
- Gelsomino G, Corsetto PA, Campia I, Montorfano G, Kopecka J, Castella B, et al. Omega 3 fatty acids chemosensitize multidrug resistant colon cancer cells by down-regulating cholesterol synthesis and altering detergent resistant membranes composition. Mol Cancer. 2013;12:137. Epub 20131113. https://doi.org/ 10.1186/1476-4598-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012. Mar;142(3):592S–9S. 10.3945/jn.111.155259 [DOI] [PubMed] [Google Scholar]
- Chang J, Su KP. The lipid raft hypothesis: the relation among omega-3 fatty acids, depression and cardiovascular diseases [Review]. Taiwanese J Psychiatry. 2010;24:168–80. [Google Scholar]