Abstract
Background: Drug therapies have been widely applied for pain management, however, there are important side effects such as those related to corticosteroids and opioids. Recent studies demonstrated promising results using medical ozone as a safe, effective, and low-cost intervention for pain control. Objective: to review and critically analyze clinical studies that used ozone therapy for musculoskeletal pain. Methods: a literature search of various databases was performed to identify relevant studies. From a total of 249 records, 27 studies were included. Quality indicators, human and device factors that strongly influence the generation of evidence were considered, such as study design and device safety. We also mitigated biases, considering the safety and efficacy of the intervention itself. Results: Regarding safety, 77 (8%) of studies reported no adverse effects; concerning efficacy outcomes, medical ozone shows to be an effective intervention on musculoskeletal pain control. Important information about used devices were missing. Conclusions: medical ozone shows to be safe and effective; qualification of health professionals as well as the device safety are mandatory. However, there is a lack of requirements to identify the best therapeutic scheme; further longer, clinical and rigorous trials are needed.
Keywords: medical ozone, ozone therapy, pain, clinical trials, evidence
Introduction
Pain is a common condition that affects many people’s quality of life. Various treatments are used to manage pain, including medication, physical, and mechanical therapies. However, these methods are not always effective and can cause side effects. Medical ozone applied to ozone therapy is a complementary approach that is gaining increasing popularity in pain management.
Ozone therapy is a medical technique that uses ozone gas injection as a complementary treatment for a wide range of conditions, including chronic pain. This intervention has been used for decades as an option for many health conditions, like tissue repair, antimicrobial property, and others [1-4]. According to the literature, ozone therapy can relieve pain due to its analgesic and anti-inflammatory properties [5,6]. Furthermore, ozone therapy can be performed safely and effectively without causing serious side effects [7-11].
Generating evidence about new therapeutic options in pain control is a challenge. Many clinical studies for safety and efficacy of various interventions for musculoskeletal disorders have been performed in fields which are considered promising for chronic degenerative diseases, as regenerative medicine [12,13] and medical ozone added to other innovative therapies, such as stem cells and platelet-rich plasma (PRP) [1,14-18] have been hardly discussed. Unfortunately, most studies do not meet the necessary methodological rigor for scientific evidence generation, keeping alive studies with high risks of bias presenting unreliable results. In this review we performed a critical assessment of clinical studies and to achieve this goal we set specific requirements as follows: if study designs are appropriate; an adequate and representative sample size; long-term follow-ups, and others described throughout the text. These clinical qualitative indicators were identified in each study included in this scoping review and are highlighted and discussed. In addition, we included other indicators that are considered of great relevance to meet all the necessary criteria for identifying evidence in clinical trials outcomes having medical ozone as complementary intervention for pain control.
Materials and Methods
Study Design and Data Search Strategies
This study is a scope review of clinical trials using complementary ozone therapy for pain control. At first, literature search of the following databases was performed to identify relevant studies: PubMed, Medline, Web of Science, Elsevier, Trip Database Research Gate, and Cochrane Library. Keyword search terms included ozone therapy, medical ozone, safety, clinical trials. Studies with two decades of publication were included. Languages were restricted to Portuguese, Spanish, and English. Data search strategy is shown in Figure 1.
Figure 1.
Data search strategy.
Selection Criteria
Pilot studies, prospective, randomized, and non-randomized clinical trials were included in the review. Reviews, case controls, case reports or case series, and pre-clinical or in vitro studies were excluded. Only studies where clinical intervention was limited to administration of medical ozone to adult participants (minimum five participants per study) were eligible for inclusion in this review.
Data Extraction
We identified 249 records to include. A research team composed of PhDs and Master’s degrees read and analyzed the abstracts of screened papers (n=217), after removing duplicates, and those studies that did not meet the inclusion criteria (n=188) were excluded. The remaining studies (n=36) were read fully and included based on the eligibility criteria. At the end of search, five articles fully read were also excluded due to some divergences from the purpose of the study. In order to search for other studies that by chance were not found in the previous search, the references of each included study were searched again for additional findings. Twenty-seven (n=27) clinical studies were included in this review. A summary of the studies reviewed are presented in Figure 1.
Results and Discussion
Clinical Studies Worldwide Using Medical Ozone as Intervention
Various studies applied to humans with similar designs were reviewed. Considering that medical ozone currently is used on clinical practice for many health professionals in different conditions and countries, these studies are important to generate evidence about this therapeutic option. Even though many potential benefits of ozone to the musculoskeletal system are already known [19-21], it is necessary to the development of trials that may analyze administration techniques, with safe volume, concentration, and safe routes through the use of ozone generators with rigorous quality controls to reach the best results for good clinical practices.
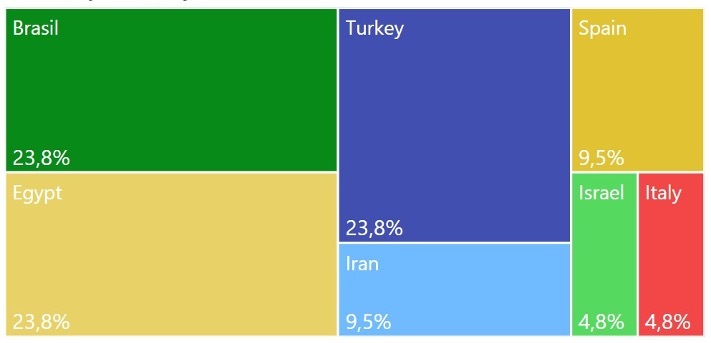
Figure 2 shows clinical trials in development around the world using medical ozone as intervention. To find these studies, we used the following strategies: data search in different platforms for registered trials, such as Cuban Public Registry of Clinical Trials (RPCEC), Peruvian Clinical Trial Registry (REPEC), Brazilian Registry of Clinical Trials (ReBEC), International Clinical Trials Registry Platform, and ClinicalTrials.Gov. Key words applied were pain; ozone; medical ozone; ozone therapy. We found in this search 58 clinical trials for pain disturbances. It was necessary to eliminate the duplicates identified (7), then we excluded the trials that did not meet our research criteria. At the end of search, we had a final number of 21 clinical trials, where five of them are from Brazil; five trials from Egypt; five from Turkey; two from Iran; two from Spain; one from Israel, and one last trial from Italy. For more detailed information, see Figure 2.
Figure 2.
Clinical studies worldwide using medical ozone as intervention.
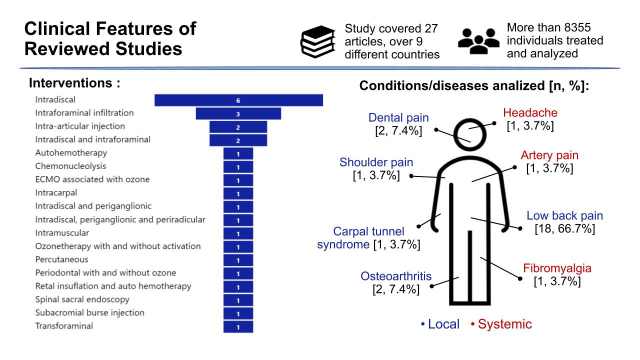
Clinical Features of Studies Reviewed
Figure 3 shows the main clinical features presented in the clinical studies reviewed. Considering the eligibility criteria of this review, the condition of the disease studied is related to acute or chronic musculoskeletal pain, where most of the studies are related to low back pain including lumbar disc herniation and/or lumbar degeneration disease. We considered studies eligible to include other pain from conditions that may have a relationship with the area of study interest. The registry in specifics platforms of Clinical trials was not applicable to all studies reviewed, as some of them are not fully characterized as studies of Phase I, II, or III. Furthermore, as we had concerns about quality indicators, we included studies that demonstrated reliability, traceability, and reproducibility in their results, even though some are not randomized, blinded (masked), controlled clinical trials.
Figure 3.
Clinical features of reviewed studies.
The outcome measure variables were based on quantitative tools for pain measurement. Among them, the visual analogic scale (VAS) is the most used in the studies, when we consider the primary outcomes. Other measurement methods were Oswestry Disability Index, Western Ontario and McMaster Universities (WOMAC) questionnaire, McNab classification, image, and dental exams.
The results presented in the reviewed studies were clinically and statistically significant, most of them showing differences in favor of groups of ozone intervention. We noticed a trend in pain relief from implementation and after 6 months of intervention. It reinforces the establishment of longer follow-ups in human studies, this issue we will discuss in the next section of this review.
Therapeutic Scheme
Table 1 presents the therapeutic scheme applied on subjects of the reviewed studies. We noticed a wide variability of concentrations/doses ranging from 20 mcg to 40 mcg, which is considered a moderate to high dose range [22,23]. The volume of ozone gas ranged from 3 ml to 20 ml except for two studies that used autohemotherapy which needs a larger volume. The active comparators chosen on the studies were mostly steroids, but magnesium and PRP were also mentioned.
Table 1. Therapeutic Scheme of Ozone Interventions from Reviewed Studies.
| Nº | Ref | Ozone intervention Arm | Pathway | Other intervention arm | Ozone Generator |
| 1 | [6] | intradiscal injection of ozone/oxygen mixture (10 ml, 25μg/ml) was performed under fluoroscopy guide | intradiscal | No | Not mentioned |
| 2 | [8] | 20 mL) with an O3 concentration of 20 μg/ml/mL | intramuscular (L4-L5) | Sham | Multiossigen PM95 generator (Multiossigen s.r.l., Gorle, Bergamo, Italy) |
| 3 | [9] | Ozone group received intradiscal oxygen-ozone therapy (4 to 7 mL of oxygen ozone mixture); ozone concentration of around 30 mcg/mL. | intradiscal | ozone-PIRFT group received a combination of oxygen-ozone therapy with PIRFT- percutaneous intradiscal radiofrequency thermocoagulation (radiofrequency lesioning at 80°C for 360 s). | Not mentioned |
| 4 | [14] | 20 mL oxygen–ozone mixture at a concentration of 30 mcg/mL | lumbar epidural space via spinal-sacral endoscopy | No | Medplus Philozon (Balneário Camboriú, Brazil) |
| 5 | [30] | 220 a 300 ml of blood is mixed with an equal volume of O3/O2 gas mixture at progressively in- creasing O3/O2 concentrations from 30 to 60 lg/mL p/mL of blood. | major autohemotherapy | No | Not mentioned |
| 6 | [34] | 25 ug in 3 ml | intraforaminal | periradicular infiltration of 2 mL (80 mg) of Methylprednisolone acetate; Depomedrol, Pfizer. | Not mentioned |
| 7 | [35] | 5 mL of ozone/oxygen mixture at 30 μg/mL concentration rate | percutaneous intradiscal | additional 5 mL of O2/O3 mix- ture at similar concentration rate was injected into the soft paravertebral tissues in the surroundings of the nerve root, together with a 2-mL solution of 1mL of Depo-Medrol 40mg/mL and 1 mL of Lidocaine Hydrochloride 20mg/mL. | Medical 99 IR, Multiossigen s.r.l., Gorle, Italy) |
| 8 | [36] | intradiscal and intraforaminal injections of O2/O3 plus an addition of a steroid and an anesthetic injections: 28 μg/ml /mI. Injected 5–7 mL of O2-O3 at intraforaminal level (mean, 6.5 mL) and 5–7 mL of O2-O3 inside the disk (mean, 5.8 mL) | Intradiscal, intraforaminal | intradiscal and intraforaminal injections of a steroid and an anesthetic only | OZO2 Futura; Alnitec, Cremosano, Italy |
| 9 | [37] | 5–10 mL of O2-O3 containing 40 mg/mL O3 intradiscal | intradiscal | 1 cc methylprednisolone and 1 cc 0.05% bupivacaine was injected into the foraminal region | Turkozone blue S |
| 10 | [38] | O2-O3 gas mixture was injected into the disc (3–4 ml) and the foramen (10 ml), concentration of 30–40 mcg/ml | intradiscal, periganglionic and periradicular oxygen-ozone (O2-O3) | No | Not mentioned |
| 11 | [39] | the gas mixture is injected into the disc (3-4ml) and into the epidural and intraforaminal spaces (10ml) at the concentrations of 30 micrograms/ml | percutaneous chemonucleolysis with periradicular and pariganglionic injection of oxygen-ozone mixture. | No | Not mentioned |
| 12 | [40] | (8 mL) injection of an oxygen-ozone mixture at an ozone concentration of 27 μg/ml | intradiscal and periganglionic | received identical oxygen-ozone injections, followed by a periganglionic injection of corticosteroid (1 mL of Depo-Medrone 40 mg | Multiossigen PM95 |
| 13 | [41] | 25 μg/mL in 20 ml | Intracarpal | methylprednisolone acetate 40mg, and 40 mg lidocaine in 20 mL | Not mentioned |
| 14 | [42] | 5 ml at a concentration of 27–30 μg/ml | intradiscal | periradicular injection of the same O2-O3 mixture (10 ml), steroid (1 ml), and local anesthetic (1 ml) | Ozo2 Futura, Alnitec, Italy |
| 15 | [43] | Not mentioned | autohemotransfusion in 55 patients and by ozone rectal insufflations in 10 patients | No | Not mentioned |
| 16 | [44] | 40 μg/mL, 3-5 ml | intervertebral disc for the intradiscal injection | PRP activated with ozone concentration of 80 μg/mL. | Evozone basic Plus, Reutlingen, Germany |
| 17 | [45] | 7 mg Betamethasone, preceded by a test dose of 1 milliliter 2% lidocaine with the addition of an O2-O3 mixture, with an ozone concentration of 25 microgram /mL. 3 mL of O2-O3 injected at transforaminal level | transforaminal | Magnesium sulphate with steroids, and steroids alone. Mg group= 7 mg Betamethasone, and 100 mg of magnesium sulphate diluted to 2 milliliters total volume with preservative-free normal saline, preceded by a test dose of 1 milliliter 2% lidocaine | Not mentioned |
| 18 | [46] | 8 ml of ozone (O2-O3) with a concentration of 12 μg/ml and 2 ml of lidocaine 1% | subacromial bursa | 1 ml of triamcinolone 40 mg/ml with 2 ml of lidocaine 1% , physical therapy, including shoulder ROM exercises, posterior capsule stretching, and isometric exercises | Not mentioned |
| 19 | [47] | OAHT, 14 treatments in the seven-week period | endovenous | intravenously with 0.5 ng/kg/m’ prostacyclin (trometamol salt, Endoprost 50, Italfarmaco) for 28 days using 0.1 mg Endoprost solution diluted in 500 mL saline solution. Infusion lasted about 6 hours | Ozonline International (Medica, Bologna) |
| 20 | [48] | group 1 (n = 21), ozone treatment with no activation (NA); group 2 (n = 22), ozone treatment with manual dynamic activation (MDA); group 3, (n = 21), ozone treatment with passive ultrasonic activation (PUA); group 4 (n = 23), ozone treatment with sonic activation (SA) | subcutaneous | no ozone treatment (control group) | Dentozone, Analytical & Medical Technology, Mumbai, Maharashtra, India |
| 21 | [49] | 5-mL mixture of O2–O3, containing 40 mg/mL O3 | intradiscal | dexamethasone (8 mg) and 0.05% bupivacaine (1 mL) intraforaminal | Turkozone Blue S |
| 22 | [50] | Group 4 Ozone (3 doses weekly) | intra articular injections (IAIs) | Group 1 HA (3 doses weekly), Group 2 PRP (2 doses with 3 weeks interval), Group 3 PRGF (2 doses with 3 weeks interval) | Ozonibaric P (made by Sedecal, Spain) |
| 23 | [51] | 16 applications in an 8-week period (standard therapy) | intraforaminal | intraforaminal steroid injections | Not mentioned |
| 24 | [52] | 10 ml oxygen-ozone mixture (25–30 μg/ml) | intraforaminal | Same treatment with additional injection of 1ml of compound betamethasone (2 mg/ml, Schering- Plough Labo, N.V. Belgium) | Ozoneline E80, Italy |
| 25 | [53] | non-surgical periodontal therapy (SRP) + ozone therapy (GROUP A) | local injection | standard treatment (GROUP B) | Ozone DTA, Sweden & Martina Company; Carrara San Giorgio, Veneto, Italy) |
| 26 | [54] | 20 μg/ml of ozone (OZ) once a week for 8 consecutive weeks | intra-articular | Placebo | Ozone & Life O&L 3.0 (Sao Jose dos Campos —Brazil) |
| 27 | [55] | Not described | percutaneous application (ozonolysis) | percutaneous steroid treatment | Not mentioned |
The routes of administration presented by the authors were local injections minimally invasive, except for two studies that used major autohemotherapy and endovenous. All local active comparators were also minimally invasive, as ozone intervention.
The ozone generators have an important paper on the safety and efficacy of the gas as a therapeutic option. Efficiency and rigorous quality control of these devices allow researchers and health professionals to use a concentration and a volume strictly defined for each treatment plan, establishing standards and treatment methods, and controlling variables not foreseen in the study protocols or patients’ records. In the reviewed studies, 10 did not mentioned the generator used in the trials.
There is a concern about using ozone therapy as a complementary option because of its various options of administration. The success of an intervention is influenced by different factors: the experience and legal qualification of the professional who is practicing, the severity of illness or condition, the therapeutic scheme chosen, considering all the points we discussed before, and the quality of ozone gas generator. Therefore, we understand that reproducibility and traceability are essentials for protecting subjects and patients from damage and iatrogenic conditions resulting from this therapy.
Guidelines of Quality Requirements for Ozone Devices
The ozone generator is a device that converts oxygen into ozone in a controlled manner. The main technologies used for ozone generation in medical application generators are electrical discharge and UV light, the former being more efficient and reliable.
The quality parameters that must be taken into account in the generation of ozone are quality of the oxygen used, constructive characteristics of the equipment, and application conditions, as commented upon below.
Oxygen quality: According to ISCO3 [24], the equipment must produce ozone from medical oxygen, with a minimum purity of 99.5%. In this way, it is also guaranteed that the oxygen is free of moisture, a factor that impairs the quality of the ozone generated.
Constructive characteristics of the generator: The constructive characteristics of the generator must meet the following requirements: external and internal piping, rigid or flexible, as well as all connections, made of ozone resistant material; ability to measure real-time ozone concentration using a photometric method, algorithmic calculation methods, or both; fully reliable internal ventilation or ventilation system, which must stop ozone generation when there is not enough of it; high quality ozone generating cell material, the most recommended material being quartz glass. In addition, solenoid valves must be used to open or close the oxygen intake and/or the O2/O3 mixture outlet; an internal or external cleaning pump must be connected to the generator, connected to a catalytic destroyer; and finally, an internal catalytic destroyer or directly connected to the generator to eliminate the possibility of ozone escaping into the ambient air [24].
Conditions for application: For the use of ozone gas, the ozone generator equipment must present technical characteristics and functionalities that guarantee the effectiveness in the delivery of the gas, safety for the user and patient, and efficiency in the consumption of resources.
Device effectiveness: Efficacy is the ability of the device to produce the expected result in terms of quality and effectiveness. In the application of the ozone generator for medicinal purposes, factors that must be considered to achieve effectiveness in the use of the gas are [24]: the user must be able to easily identify and adjust the desired ozone concentration between 1 µg/mL and 80 µg/mL, with increments of 1 µg/mL. The margin of error must not exceed ±10%; the outlet nozzle must be protected against the flow into the generator of any unwanted solid or liquid contaminants when not in use; it must allow easy fitting and detaching of a syringe to be filled; it must not allow any ozone to escape from the generator into the ambient air, allowing for easy cleaning and disinfection. In addition, it is recommended that the ozone output flow be adjustable, to better suit different applications. The concentration and flow must be tested in the laboratory with the issuance of the respective report or certificate and the ozone generator must be registered with the competent health authority of each country where the device is used. As for maintenance and calibration, these must be carried out at intervals established by the manufacturer or by local legislation.
Device Efficiency: Another important factor to be considered is the efficiency in the application of ozone gas, considering the entire life cycle of the equipment, from design, through construction, use and final disposal.
Efficiency is the ability of the device to produce the desired result using the least possible resources. It focuses on optimizing resources, such as producing the concentration of ozone with the lowest consumption of electricity.
Some impact factors are relevant to efficiency, such as: initial investment of the equipment, where attention must be paid in the development of the project, to the cost ratio of the component x energy consumption for operation, considering the useful life of the device. Software is another impact factor, where programs must be developed to manage the operation of the equipment in order to consume less energy and oxygen.
Device Safety: The equipment can have its operation monitored through sensors and intelligent software, improving the control of its operation and management of its resources. As a result, better levels of effectiveness, safety, and efficiency can be achieved.
Another way to ensure user safety is the presence of alarms and alerts (visual, digital, and/or audible) that inform about irregular functioning or failures during its use.
Quality Indicators Related to Evidence in Clinical Trials Using Ozone Therapy for Pain Control
In this section, information gathered from the selected trials are described in Appendix A and included the following variables: study design, interventions, sample size, age range, blinding (masking), allocation methods, pairing, follow-up, clinical trial registry, and ethical considerations. We consider that all the issues here analyzed work as quality indicators of human studies and will help us to understand the limitations and vantages on generating evidence of the clinical trials included in this review.
Sample size: Clinical studies are essential for determining the efficacy and safety of new treatments, drugs, and medical devices. The results of these studies play a critical role in determining whether a new intervention will be approved for widespread use and ultimately benefit patients. One of the most crucial aspects of a clinical study is the sample size, which can greatly impact the validity and reliability of the results.
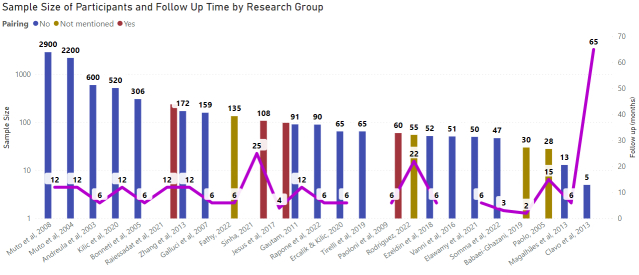
A sample is a subset of the population that is selected for study, and the sample size refers to the number of individuals included in the sample. The choice of sample size is important because it affects the statistical power of the study. Statistical power refers to the ability of a study to detect an effect if it exists. A study with a small sample size may have low statistical power, making it difficult to detect an effect even if it is present in the population. Conversely, a study with a large sample size may have high statistical power, increasing the chances of detecting an effect if it exists. The larger the sample, the greater the power and confidence of the study. In addition, an adequate sample size allows for the occurrence of randomization. Different designs need specific sample sizes. For instance, pilot studies are designed with short samples, while phase 2/3 clinical studies need a larger number of subjects to validate the findings and assure, they have causality with the intervention. Figure 4 shows the sample sizes in 27 reviewed studies, varying from minimum of five subjects to maximum of 2900 subjects.
Figure 4.
Sample size of participants and follow-up of reviewed studies.
The sample size also affects the precision of the study results. Precision refers to the degree of variability in the results. A study with a large sample size will generally have lower variability, resulting in more precise results. This increased precision can lead to more reliable conclusions about the efficacy and safety of a new intervention.
The choice of sample size also affects the generalizability of the results. Generalizability refers to the ability to apply the results of a study to other populations. A study with a large sample size is more likely to be representative of the population, increasing the chances that the results can be generalized to other populations.
A study with a large sample size increases the chances of detecting an effect if it exists, increases precision, and increases generalizability. It is essential for researchers to carefully consider the sample size in their studies to ensure that the results are valid, reliable, and applicable to other populations.
Matching and Age Range: Matching is a statistical technique used in clinical studies to match or balance groups of participants regarding important variables such as age, sex/gender, medical history, among others. The objective of pairing is to reduce the influence of these variables in the analysis of study results, to guarantee that the differences between groups are attributed to the treatment or to the factor of interest in assessment, and not to other variables.
Age is an important variable in many clinical studies, as it can affect response to treatment or the factor of interest. In addition, it is common to have a large age variation among participants in clinical trials.
In these situations, it is important to consider age when pairing participants. This is done to ensure that age variation does not affect study results and that differences between groups are attributable to treatment or the factor of interest rather than age. Pairing can be done in several ways, such as pairs, blocks, or layering.
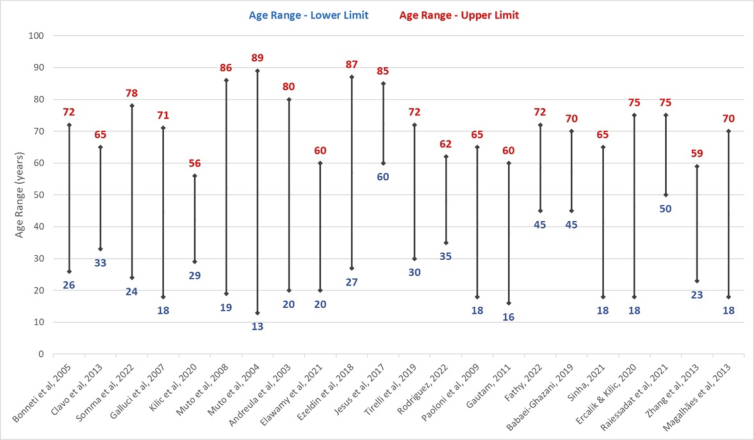
Age range in clinical studies must be divided into subgroups, to create pairings as homogeneous as possible. It will allow realistic comparisons among the subjects enrolled in the study. If age range is very large, distinct standards of comparison will prevail regarding human physiological dimension.
In our review, we identified in most of the 27 studies a huge discrepancy among minimal and maximum age of subjects, hindering an assertive analysis of the results (Figure 5).
Figure 5.
Age range of subjects enrolled in clinical studies using ozone therapy.
Randomization: Randomization is considered one of the main strategies to minimize the influence of extrinsic factors on the results of clinical studies [25]. It helps to avoid systematic selection of participants who may have a different response to treatment or who may be more predisposed to developing adverse effects, which could compromise the validity of the results.
Randomization is carried out at random, which means that each participant has an equal chance of being included in any one of the treatment groups. This helps to ensure that participant characteristics are similar across groups, which is important for assessing the efficacy and safety of the treatment or medication in question.
Furthermore, randomization allows differences between groups to be attributed to treatment or medication rather than to other factors such as age, sex/gender, or medical history of participants [25]. This is crucial so that study results are reliable and can be used to make clinical decisions. In clinical trials it is recommended the possibility of subjects to be assigned by chance. Randomization provides a way of determining the effects of interventions [26], avoiding allocation bias and inconsistences in the results. Fourteen studies in this review had randomization in their design.
Allocation methods: For the results of clinical trial studies to be valid, it is crucial that participants are allocated appropriately between treatment groups. We identified 11 studies that described their allocation methods.
Various allocation methods are used to ensure that the allocation is fair and balanced. One of the most common methods is simple randomization, which consists of randomly allocating participants to one of the treatment groups [25]. This method is easy to implement and allows the characteristics of the participants to be similar between the groups, which is important to assess the efficacy and safety of the treatment or medication in question.
Another method is stratified randomization, which consists of subdividing participants into homogeneous groups based on relevant characteristics, such as age or medical history, and then randomly allocating them to one of the treatment groups [26]. This method allows for a more even distribution of participants across groups, which can increase the accuracy of results.
In addition, the block allocation method is used when it is desired to avoid imbalances between treatment groups [25]. In this method, participants are allocated into groups of fixed size, which are called blocks. Randomization is performed within each block, which ensures that the distribution between treatment groups is more balanced.
Masking, a key element for reliable results: The findings from clinical studies play a critical role in guiding clinical decision-making and shaping health policy. Therefore, it is crucial to ensure that the results of clinical studies are accurate, reliable, and free from bias. One important strategy for achieving this goal is to use a technique known as masking.
Masking is the process of concealing the treatment allocation of study participants from the individuals who are evaluating the outcomes of the study. This can be done in several ways, including single-masking, where only the participant is unaware of their treatment allocation, or double-masking, where both the participant and the evaluator are masked to the treatment allocation.
The importance of masking in clinical studies is widely recognized and has been demonstrated in numerous studies and meta-analyses. A systematic review of randomized controlled trials (RCTs) [27] showed that masking reduced the risk of performance bias, which occurs when the knowledge of treatment allocation influences the assessment of outcomes. Furthermore, masking also reduced the risk of detection bias, which occurs when the knowledge of treatment allocation influences the measurement of outcomes.
Another systematic review [28] found that masking was associated with a reduction in the risk of bias in RCTs. They also found that studies that were single-masked had a higher risk of bias than studies that were double-masked.
Masking can also help to reduce the influence of placebo effects, which can have a significant impact on the outcomes of clinical studies. Placebo effects occur when the mere expectation of receiving a treatment has an impact on the observed outcome, regardless of the actual treatment received. In double-masked studies, the placebo effect is equalized between the treatment and control groups, reducing the risk of bias in the results.
In addition, masked studies are more likely to produce accurate and reliable results, which are essential for guiding clinical decision-making and shaping health policy. In our search, there were 11 studies non-masked, 12 fully masked, and four single-masked.
Follow-up: Clinical studies play a critical role in evaluating the safety and efficacy of new medical interventions, such as drugs, devices, or procedures. However, the results of a clinical study are not always representative of the long-term outcomes of the intervention. Therefore, follow-up is an essential component of clinical research, as it provides valuable information about the long-term safety and efficacy of the intervention being studied.
Follow-up refers to the ongoing monitoring of participants in a clinical study after they have received the intervention. This can include periodic assessments of health status, ongoing monitoring of side effects, and the collection of data on the long-term outcomes of the intervention. The length of follow-up can vary depending on the nature of the intervention and the outcome being studied, but it can last for many years after the completion of the study.
One of the primary benefits of follow-up in clinical studies is that it allows researchers to assess the long-term safety and efficacy of the intervention. For example, a study may show that an intervention is effective in the short-term, but follow-up can reveal that the intervention is associated with significant side effects or long-term complications identifying adverse events that may not be noticed in a short period of time. In these cases, follow-up can inform clinical decision-making and help to ensure that the intervention is used safely and effectively. It is strongly recommended for new therapeutic approaches in clinical trials the follow-ups must be designed to have a long-term duration [26,29]. In this review as shown in Figure 4, we identified by a red line across the studies the follow-up period, where it has a high variability. Of all 27 studies, nine presented follow-ups equal or over 12 months; 11 studies had 6 months follow-up; and seven studies were defined in this review with a short follow-up for their participants, covering a period of less than 6 months.
Regarding the benefits of establishing a long-term follow-up, it certainly helps to identify unexpected side effects or complications of the intervention. For instance, a study may show that an intervention is effective in the short-term, but follow-up may reveal that the intervention is associated with serious side effects or long-term complications that were not initially recognized. In these cases, follow-up can help to ensure that the intervention is used safely and effectively and can inform clinical decision-making.
In addition, follow-up can also provide valuable information about the natural course of the condition: a study follow-up of several years after the completion of the study which intends to assess the long-term outcomes of the condition will possibly identify the factors that may influence the progression of the condition. This information can be used to improve the care of individuals with the condition and to develop more effective interventions in the future.
Clinical trial registration and ethics information: Ethics is of huge importance concerning human trials, to guarantee the subjects safety and the quality of study conduction according to good clinical practices. Trials registry is also an important issue because it shows transparency regarding the design of the study, in addition to being a form of scientific dissemination of information with other researchers. We collected data about clinical trials registry and ethics, but some studies did not mention this information.
Adverse events: Although ozone therapy has been widely used and has been reported to be a generally well-tolerated technique, there are concerns about potential adverse events associated with it. Several clinical studies have examined adverse events associated with ozone therapy. A randomized, double-blind, placebo-controlled RCT of patients undergoing ozone therapy for the treatment of chronic low back pain found that the technique was well tolerated, and no serious side effects were reported [6]. However, another study with patients undergoing ozone therapy for the treatment of chronic headache found that some patients experienced acute pain and ecchymosis at the site of administration of the therapy [30].
Furthermore, there is evidence that ozone therapy may increase the risk of adverse reactions in patients with a history of allergy to ozone or other medicinal gases [31-33]. Therefore, it is important that patients with a history of allergy inform their physician before undergoing ozone therapy.
The intensity of adverse events may vary from patient to patient, but it is important that they be evaluated in terms of their relationship with ozone therapy. A causality analysis helps determine whether the adverse event was caused by ozone therapy or by other factors. Some adverse events probably are related to ozone therapy if they occur shortly after administering the therapy and disappear quickly. Other adverse events are considered unrelated to ozone therapy if there is no clear temporal relationship between therapy administration and the adverse event.
The assessment of the causality and intensity of adverse events is important to ensure patient safety and to better understand the potential risks of ozone therapy. In addition, this assessment can also help to improve recommendations for the use of ozone therapy in specific patients.
Considering the severity rating of adverse events associated with ozone therapy, the literature shows it varies widely, from mild reactions such as local pain, to severe reactions such as systemic allergic reactions. It is important that clinicians and patients are aware of the possibility of adverse events and carefully weigh the risks and benefits of ozone therapy.
The assessment of the severity of adverse events can be influenced by factors such as the dosage and method of administration of ozone therapy. Therefore, it is important for clinicians to follow strict safety and dosing protocols to minimize the risk of adverse events development and either carefully consider the potential benefits and risks before undergoing ozone therapy.
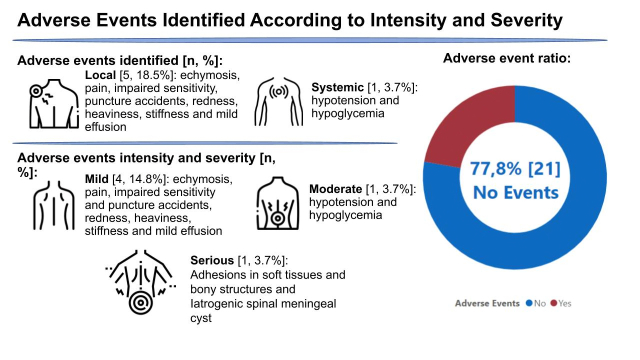
Figure 6 shows the adverse events reported in the 27 reviewed studies, where according to intensity and severity, 77.8% had no adverse events; 14.8% were mild adverse events; 3.7% were considered moderate adverse events, and 3.7% serious adverse events. Among the severe adverse events, none was related to death or disability. Furthermore, these events were also classified as local or systemic, as follows: 18.5% had local events and 3.7% of studies had systemic adverse events.
Figure 6.
Adverse events according to location, intensity, and severity.
Conclusion
We performed a deep review of clinical studies using medical ozone for pain control. Among these conditions, low back pain was the most studied over the last few decades. Based on the identified scientific data, where low risks were identified for the use of medicinal ozone as a complementary therapy for treating musculoskeletal pain, we consider this intervention safe, which is confirmed by the report of few adverse events in the 27 clinical studies reviewed, most of which are classified as mild, followed by moderate adverse events. Furthermore, we identified that these events were mostly developed in the site of infusion. We also believe that ozone therapy, performed with concentrations and volumes with a lowest possible dose, with gas release and production performed by a reliable generator, is certainly a new approach for many conditions related to human motricity. Additionally, we assert that medical ozone therapy is both a safe and effective treatment option for managing musculoskeletal pain.
Although there is growing evidence that ozone therapy is effective in pain management, there is still a need for more research to fully understand the variables of this approach and to assess its effectiveness compared to other pain management methods, considering criterion standard or new ones. However, current evidence highlighted in this review suggest that ozone therapy may be a viable option for those seeking safe and effective pain relief.
We believe this study contributes valuable insights into the potential of ozone therapy yet underscores the importance of continued research to fully establish its comparative effectiveness in pain management. We also reinforce the need for the development of new clinical trials with long-term follow-ups, with the inclusion of more homogeneous samples, having reliable controls of quality for both study design and ozone devices.
Further and finally, we understand that the well-being and protection of study participants is a high priority in the development of clinical trials, therefore we strongly recommend that the application of medicinal ozone must be carried out by trained professionals, to further minimize the possible risks related to the intervention.
Glossary
- RPCEC
Cuban Public Registry of Clinical Trials
- REPEC
Peruvian Clinical Trial Registry
- ReBEC
Brazilian Registry of Clinical Trials
- VAS
visual analogic scale
- WOMAC
Ontario and McMaster Universities
- PRP
Platelet-rich plasma
- RCTs
randomized controlled trials
Appendix A.
Author Contributions
LTA performed the data search and data extraction; wrote the manuscript; PCS performed the tables and data extraction; MM designed the study and reviewed the manuscript.
Conflicts of Interest
We declare there are no conflict of interests.
Source of Funding
The company where the author PCS works paid for a computer technology professional to create the illustrations. The authors declared no source of funding.
References
- Gurger M, Once G, Yilmaz E, Demir S, Calik I, Say Y, et al. The effect of the platelet-rich plasma and ozone therapy on tendon-to-bone healing in the rabbit rotator cuff repair model. J Orthop Surg Res. 2021. Mar;16(1):202. 10.1186/s13018-021-02320-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci VA. Ozone: A New Medical Drug. Italy: Springer; 2011. p. 295. 10.1007/978-90-481-9234-2 [DOI] [Google Scholar]
- Sánchez GM. Challenges of ozone therapy and access to information sources. Rev Cuba Farm. 2014;48:347–9. [Google Scholar]
- Elvis AM, Ekta JS. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011. Jan;2(1):66–70. 10.4103/0976-9668.82319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Fei W, Zhang L, et al. Intrathecal injection of ozone alleviates CCI-induced neuropathic pain via GluR6-NF-kB/p65 signalling pathway in rats. Mol Med Rep. 2020;•••:231. [DOI] [PubMed] [Google Scholar]
- Ozcan S, Muz A, Yildiz Altun A, Onal SA. Intradiscal ozone therapy for lumbar disc herniation. Cell Mol Biol (Noisy-le-grand). 2018. Apr;64(5):52–5. 10.14715/cmb/2018.64.5.8 [DOI] [PubMed] [Google Scholar]
- Rodríguez D, Felipe J, León R, Baspineiro TA, Knoll S. Pain Relief and Functional Improvements after Spinal Ozone and PRP Injections for Symptomatic Lumbar Herniated Disc. J Regen Med. 2022;53:1–8.
- Paoloni M, Di Sante L, Cacchio A, Apuzzo D, Marotta S, Razzano M, et al. Intramuscular oxygen-ozone therapy in the treatment of acute back pain with lumbar disc herniation: a multicenter, randomized, double-blind, clinical trial of active and simulated lumbar paravertebral injection. Spine. 2009. Jun;34(13):1337–44. 10.1097/BRS.0b013e3181a3c18d [DOI] [PubMed] [Google Scholar]
- Gautam S, Rastogi V, Jain A, Singh AP. Comparative evaluation of oxygen-ozone therapy and combined use of oxygen-ozone therapy with percutaneous intradiscal radiofrequency thermocoagulation for the treatment of lumbar disc herniation. Pain Pract. 2011;11(2):160–6. 10.1111/j.1533-2500.2010.00409.x [DOI] [PubMed] [Google Scholar]
- Babaei-Ghazani A, Fadavi HR, Eftekharsadat B, Ebadi S, Ahadi T, Ghazaei F, et al. A Randomized Control Trial of Comparing Ultrasound-Guided Ozone (O2-O3) vs Corticosteroid Injection in Patients With Shoulder Impingement. Am J Phys Med Rehabil. 2019. Nov;98(11):1018–25. 10.1097/PHM.0000000000001240 [DOI] [PubMed] [Google Scholar]
- Talebi HM, Javaheri J, Fakhari MS. Successful treatment of a high-risk diabetic foot ulcer by ozone therapy and collagen powder: A case report. Clin Case Rep. 2024. Apr;12(4):e8728. 10.1002/ccr3.8728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Chung PK, Suh DS, Heo DB, Tak DH, Koh YG. Implantation of mesenchymal stem cells in combination with allogenic cartilage improves cartilage regeneration and clinical outcomes in patients with concomitant high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2020. Feb;28(2):544–54. 10.1007/s00167-019-05729-3 [DOI] [PubMed] [Google Scholar]
- Saw KY, Anz AW, Ng RC, Jee CS, Low SF, Dorvault C, et al. Arthroscopic Subchondral Drilling Followed by Injection of Peripheral Blood Stem Cells and Hyaluronic Acid Showed Improved Outcome Compared to Hyaluronic Acid and Physiotherapy for Massive Knee Chondral Defects: A Randomized Controlled Trial. Arthroscopy. 2021. Aug;37(8):2502–17. 10.1016/j.arthro.2021.01.067 [DOI] [PubMed] [Google Scholar]
- Magalhães FN, Soares SC, Torres JM, Ungaretti A, Cacciacarro MF, Teixeira MJ, et al. Effects of ozone applied by spinal endoscopy in patients with chronic pain related to failed back surgery syndrome: a pilot study. Neuropsychiatr Dis Treat. 2013;9:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico G, Isakovic J, Song MS, Rustichelli F, Travagli V, Mitrecic D. Ozone influences migration and proliferation of neural stem cells in vitro. Neurosci Lett. 2020. Nov;739:135390. 10.1016/j.neulet.2020.135390 [DOI] [PubMed] [Google Scholar]
- Huang P, Wang R, Pang X, Yang Y, Guan Y, Zhang D. Platelet-rich plasma combined with ozone prevents cartilage destruction and improves weight-bearing asymmetry in a surgery-induced osteoarthritis rabbit model. Ann Palliat Med. 2022. Feb;11(2):442–51. 10.21037/apm-21-1510 [DOI] [PubMed] [Google Scholar]
- Patel P. Ozone and stem cell a possible cure for Rheumatoid Arthritis. J Stem Cell Res Regenerative Med. 2020;3:2.
- de Araújo LT, Macêdo CT, Damasceno PK, das Neves ÍG, de Lima CS, Santos GC, et al. Clinical Trials Using Mesenchymal Stem Cells for Spinal Cord Injury: Challenges in Generating Evidence. Cells. 2022. Mar;11(6):1–26. 10.3390/cells11061019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu M, Lin X, et al. Low-Concentration Oxygen/Ozone Treatment Attenuated Radiculitis and Mechanical Allodynia via PDE2A-cAMP/cGMP-NF-B/p65 Signaling in Chronic Radiculitis Rats. Pain Res Manag. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek EK, Sahinturk F, Gul E, Tepeoglu M, Araz C, Haberal B. Effect of Ozone Therapy on Epidural Fibrosis in Rats. World Neurosurg. 2023. Jul;175:e296–302. 10.1016/j.wneu.2023.03.075 [DOI] [PubMed] [Google Scholar]
- Spassim MR, Dos Santos RT, Rossato-Grando LG, Cardoso L, da Silva JS, de Souza SO, et al. Intra-articular ozone slows down the process of degeneration of articular cartilage in the knees of rats with osteoarthritis [Internet]. Knee. 2022. Mar;35:114–23. 10.1016/j.knee.2022.03.003 [DOI] [PubMed] [Google Scholar]
- Viebahn-Hänsler R, León Fernández OS, Fahmy Z. Olga Sonia León Fernández & Ziad Fahmy. Ozone in Medicine: The Low-Dose Ozone Concept—Guidelines and Treatment Strategies. Ozone Sci Eng. 2012;34(6):408–24. 10.1080/01919512.2012.717847 [DOI] [Google Scholar]
- Bocci V, Zanardi I, Travagli V. Oxygen/ozone as a medical gas mixture. A critical evaluation of the various methods clarifies positive and negative aspects. Med Gas Res. 2011. Apr;1(1):6. 10.1186/2045-9912-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISCO3 . (2019). International Scientific Committee of Ozone Therapy: Scientific Report 2019. [Internet]. cited 2023 Nov 27. Available from: [http://isco3.org/2019- scientific-report.] [Google Scholar]
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. CONSORT GROUP (Consolidated Standards of Reporting Trials). The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001. Apr;134(8):663–94. 10.7326/0003-4819-134-8-200104170-00012 [DOI] [PubMed] [Google Scholar]
- Herbert RD, Kasza J, Bø K. Analysis of randomised trials with long-term follow-up. BMC Med Res Methodol. 2018. May;18(1):48. 10.1186/s12874-018-0499-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001. May;344(21):1594–602. 10.1056/NEJM200105243442106 [DOI] [PubMed] [Google Scholar]
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004. May;291(20):2457–65. 10.1001/jama.291.20.2457 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick T, Perrier L, Shakik S, Cairncross Z, Tricco AC, Lix L, et al. Assessment of Long-term Follow-up of Randomized Trial Participants by Linkage to Routinely Collected Data: A Scoping Review and Analysis. JAMA Netw Open. 2018. Dec;1(8):e186019. 10.1001/jamanetworkopen.2018.6019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavo B, Santana-Rodriguez N, Gutierrez D, Lopez JC, Suarez G, Lopez L, et al. Long-term improvement in refractory headache following ozone therapy. J Altern Complement Med. 2013. May;19(5):453–8. 10.1089/acm.2012.0273 [DOI] [PubMed] [Google Scholar]
- Zhou PE, Qian ZM, McMillin SE, Vaughn MG, Xie ZY, Xu YJ, et al. Relationships between long-term ozone exposure and allergic rhinitis and bronchitic symptoms in Chinese children. Toxics. 2021. Sep;9(9):1–12. 10.3390/toxics9090221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Lee PH, Lee SH, Park CS, Jang AS. Impact of ozone on claudins and tight junctions in the lungs. Environ Toxicol. 2018. Jul;33(7):798–806. 10.1002/tox.22566 [DOI] [PubMed] [Google Scholar]
- Bayram H, Rusznak C, Khair OA, Sapsford RJ, Abdelaziz MM. Effect of ozone and nitrogen dioxide on the permeability of bronchial epithelial cell cultures of non-asthmatic and asthmatic subjects. Clin Exp Allergy. 2002. Sep;32(9):1285–92. 10.1046/j.1365-2745.2002.01435.x [DOI] [PubMed] [Google Scholar]
- Bonetti M, Fontana A, Cotticelli B, Volta GD, Guindani M, Leonardi M. Intraforaminal O2-O3 versus periradicular steroidal infiltrations in lower back pain: randomized controlled study. Riv Ital di Ossigeno-Ozonoterapia. 2006;5:55. [PMC free article] [PubMed] [Google Scholar]
- Somma F, Negro A, D’Agostino V, Piscitelli V, Pace G, Tortora M, et al. COVID-19 and low back pain: previous infections lengthen recovery time after intradiscal ozone therapy in patients with herniated lumbar disc. Radiol Med. 2022. Jun;127(6):673–80. 10.1007/s11547-022-01500-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci M, Limbucci N, Zugaro L, Barile A, Stavroulis E, Ricci A, et al. Sciatica: treatment with intradiscal and intraforaminal injections of steroid and oxygen-ozone versus steroid only. Radiology. 2007. Mar;242(3):907–13. 10.1148/radiol.2423051934 [DOI] [PubMed] [Google Scholar]
- Kılıç M Lumbar Intradiscal Ozone Chemonucleolysis applied together with the epidural steroid treatment. SiSli Etfal Hastan Tip Bul / Med Bull Sisli Hosp. 2020;55(2):203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto M, Ambrosanio G, Guarnieri G, Capobianco E, Piccolo G, Annunziata G, et al. “Low back pain” e sciatalgia: trattamento con iniezione intradiscale-intraforaminale di O2-O3. La nostra esperienza. Radiol Med. 2008;113(5):695–706. 10.1007/s11547-008-0302-5 [DOI] [PubMed] [Google Scholar]
- Muto M, Andreula C, Leonardi M. Treatment of herniated lumbar disc by intradiscal and intraforaminal oxygen-ozone (O2-O3) injection. J Neuroradiol. 2004. Jun;31(3):183–9. 10.1016/S0150-9861(04)96989-1 [DOI] [PubMed] [Google Scholar]
- Andreula CF, Simonetti L, De Santis F, Agati R, Ricci R, Leonardi M. Minimally invasive oxygen-ozone therapy for lumbar disk herniation. AJNR Am J Neuroradiol. 2003. May;24(5):996–1000. [PMC free article] [PubMed] [Google Scholar]
- Elawamy A, Hassanien M, Talaat EA, Ali AM, Roushdy AS, Kamel EZ. Intra-carpal injection of ozone versus methylprednisolone in carpal tunnel syndrome of systemic sclerosis patients: A randomized single-blind clinical trial. Pain Physician. 2021. Jul;24(4):E453–8. [PubMed] [Google Scholar]
- Ezeldin M, Leonardi M, Princiotta C, Dall’olio M, Tharwat M, Zaki M, et al. Percutaneous ozone nucleolysis for lumbar disc herniation. Neuroradiology. 2018. Nov;60(11):1231–41. 10.1007/s00234-018-2083-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli U, Cirrito C, Pavanello M, Piasentin C, Lleshi A, Taibi R. Ozone therapy in 65 patients with fibromyalgia: an effective therapy. Eur Rev Med Pharmacol Sci. 2019. Feb;23(4):1786–8. [DOI] [PubMed] [Google Scholar]
- Rodriguez LM, Ramírez JF, Arancibia T, Knoll S, Lewandrowski K. Pain Relief and Functional Improvements after Spinal Ozone and PRP Injections for Symptomatic Lumbar Herniated Disc. J Regen Med. 2022;2022(11):5. [Google Scholar]
- Fathy W, Hussein M, Ibrahim RE, Abdel-Aziz MM, Adel S, Soliman SH, et al. Comparative effect of transforaminal injection of Magnesium sulphate versus Ozone on oxidative stress biomarkers in lumbar disc related radicular pain. BMC Anesthesiol. 2022. Aug;22(1):254. 10.1186/s12871-022-01789-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babael-Ghazani Arash MD, Hamid Reza Fadavi DO, Bina Eftekharsadat MD. Safoora Ebadi, PhD, Tannaz Ahadi, MD, Fatemeh Ghazaei, MD, and Mohamad Sadegh Khabbaz, MD. A Randomized Control Trial of Comparing Ultrasound-Guided Ozone (O2-O3) vs Corticosteroid Injection in Patients With Shoulder Impingement. Am J Phys Med Rehabil. 2019;11:98–9. [DOI] [PubMed] [Google Scholar]
- Di Paolo N, Bocci V, Salvo DP, Palasciano G, Biagioli M, Meini S, et al. Extracorporeal blood oxygenation and ozonation (EBOO): a controlled trial in patients with peripheral artery disease. Int J Artif Organs. 2005. Oct;28(10):1039–50. 10.1177/039139880502801012 [DOI] [PubMed] [Google Scholar]
- Sinha N, Asthana G, Parmar G, Langaliya A, Shah J, Kumbhar A, et al. Evaluation of Ozone Therapy in Endodontic Treatment of Teeth with Necrotic Pulp and Apical Periodontitis: A Randomized Clinical Trial [Internet]. J Endod. 2021. Dec;47(12):1820–8. 10.1016/j.joen.2021.09.006 [DOI] [PubMed] [Google Scholar]
- Ercalik T, Kilic M. Efficacy of intradiscal ozone therapy with or without periforaminal steroid injection on lumbar disc herniation: A double-blinded controlled study. Pain Physician. 2020. Sep;23(5):477–84. 10.36076/ppj.2020/23/477 [DOI] [PubMed] [Google Scholar]
- Raeissadat, et al. The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskelet Disorders. 2021; 22:134.-021-04017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni D, Galzio R, Kazakova A, Pantalone A, Sparvieri A, Salini V, et al. Intraforaminal ozone therapy and particular side effects: preliminary results and early warning. Acta Neurochir (Wien). 2016. Mar;158(3):491–6. 10.1007/s00701-015-2545-y [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ma Y, Jiang J, Ding T, Wang J. Treatment of the lumbar disc herniation with intradiscal and intraforaminal injection of oxygen-ozone. J Back Musculoskelet Rehabil. 2013;26(3):317–22. 10.3233/BMR-130386 [DOI] [PubMed] [Google Scholar]
- Rapone B, Ferrara E, Santacroce L, Topi S, Gnoni A, Dipalma G, et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int J Environ Res Public Health. 2022. Jan;19(2):2. 10.3390/ijerph19020985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes de Jesus CC, Dos Santos FC, de Jesus LM, Monteiro I, Sant’Ana MS, Trevisani VF. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: A randomized, double-blinded, placebo-controlled study. PLoS One. 2017. Jul;12(7):e0179185. 10.1371/journal.pone.0179185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri M, Grattacaso G, Di Tunno V, Marsecano C, Di Cesare E, Splendiani A, et al. MRI DWI/ADC signal predicts shrinkage of lumbar disc herniation after O2-O3 discolysis. Neuroradiol J. 2015. Apr;28(2):198–204. 10.1177/1971400915576658 [DOI] [PMC free article] [PubMed] [Google Scholar]