Abstract
Partial deletion of the second hypervariable region from the envelope of the primary-like SF162 virus increases the exposure of certain neutralization epitopes and renders the virus, SF162ΔV2, highly susceptible to neutralization by clade B and non-clade B human immunodeficiency virus (HIV-positive) sera (L. Stamatatos and C. Cheng-Mayer, J. Virol. 78:7840–7845, 1998). This observation led us to propose that the modified, SF162ΔV2-derived envelope may elicit higher titers of cross-reactive neutralizing antibodies than the unmodified SF162-derived envelope. To test this hypothesis, we immunized rabbits and rhesus macaques with the gp140 form of these two envelopes. In rabbits, both immunogens elicited similar titers of binding antibodies but the modified immunogen was more effective in eliciting neutralizing antibodies, not only against the SF162ΔV2 and SF162 viruses but also against several heterologous primary HIV type 1 (HIV-1) isolates. In rhesus macaques both immunogens elicited potent binding antibodies, but again the modified immunogen was more effective in eliciting the generation of neutralizing antibodies against the SF162ΔV2 and SF162 viruses. Antibodies capable of neutralizing several, but not all, heterologous primary HIV-1 isolates tested were elicited only in macaques immunized with the modified immunogen. The efficiency of neutralization of these heterologous isolates was lower than that recorded against the SF162 isolate. Our results strongly suggest that although soluble oligomeric envelope subunit vaccines may elicit neutralizing antibody responses against heterologous primary HIV-1 isolates, these responses will not be broad and potent unless specific modifications are introduced to increase the exposure of conserved neutralization epitopes.
Analysis of the crystal structure of the gp120 human immunodeficiency virus (HIV) envelope subunit indicated that neutralization epitopes are primarily clustered in one face of this protein, which is naturally occluded within the oligomeric envelope form, i.e., that present on the surface of virions and infected cells (16, 37). These structural observations are supported by numerous immunochemical and virological studies (1, 24, 25, 27, 28, 31, 35, 38, 40).
Several reports have indicated that specific modifications (such as deglycosylations and loop deletions) introduced in the envelope glycoproteins of HIV and simian immunodeficiency virus (SIV) may increase the exposure of neutralization epitopes. Wyatt et al. demonstrated that on the background of the HXB2 virus, a laboratory-adapted CXCR4-using (X4-using) virus, deletions of the first, second, and third hypervariable regions (V1, V2, and V3 loops, respectively) of the gp120 envelope subunit increase the exposure of epitopes participating in HIV envelope-CD4 and -coreceptor binding (38, 40). Subsequently, it was demonstrated that the simultaneous deletion of the V1 and V2 loops from the envelope of this virus increases it susceptibility to neutralization by anti-V3 loop and certain CD4-induced monoclonal antibodies (MAbs) (3). Reitter et al. reported that elimination of specific asparagine-linked glycosylation sites located in the V1 loop of SIVmac239 results in the exposure of neutralization epitopes and, importantly, increases their immunogenicity (25). Infection of macaques with SIVmac239-derived viruses expressing such partially deglycosylated envelopes results in the generation of antienvelope antibodies capable of neutralizing the parental virus SIVmac239, which displays a fully glycosylated envelope, more efficiently than antibodies elicited during infection of macaques with SIVmac239 itself.
We previously reported that on the background of the SF162 virus, a primary-like CCR5-using (R5-using) isolate, deletion of the 30 amino acids from the central region of the V2 loop (SF162ΔV2) does not abrogate its infectivity but renders it highly susceptible to neutralization by sera collected from patients infected with heterologous HIV type 1 (HIV-1) isolates (30). We hypothesized that on the background of the SF162 envelope, partial elimination of the V2 loop increases the exposure of neutralization epitopes that are conserved among heterologous primary HIV-1 isolates.
In this study, we compared the immunogenic potentials of the unmodified SF162 and modified SF162ΔV2 (hereafter designated ΔV2) envelopes. Using the gene gun vaccination method, we immunized rabbits with the gp140 form of the SF162 and ΔV2 envelopes. We observed that both immunogens elicited the generation of similar antibody titers, but that the modified immunogen elicited higher titers of neutralizing antibodies against the parental SF162 virus than the unmodified immunogen. These results are in agreement with those previously reported in the case of SIVmac239 (25), because they suggest that specifically modified envelope immunogens are more effective than the corresponding unmodified envelope immunogens in eliciting neutralizing antibodies against the homologous parental virus. Additionally, the ΔV2-derived modified immunogen was more effective than the SF162-derived unmodified immunogen in generating antibodies capable of neutralizing heterologous primary HIV-1 isolates.
The immunogenicity of these two antigens was also evaluated in rhesus macaques, an animal model more closely related to humans and more suitable for HIV vaccine studies, using the DNA-prime–protein-boost vaccination method. Here too we recorded that the modified immunogen was more effective than the unmodified immunogen in generating potent neutralizing antibodies both against the homologous SF162ΔV2 and parental SF162 viruses. The antibodies elicited in macaques by the modified, but not unmodified, immunogen neutralized several, but not all, heterologous primary HIV-1 isolates. The neutralizing potential against the heterologous isolates tested was lower than that against the parental SF162 virus. Previous studies reported that cross-reactive neutralizing antibodies against primary HIV-1 isolates could be elicited in mice immunized with fusion-competent vaccines (18) or soluble oligomeric envelopes derived from a primary-like HIV envelope (41). Our studies indicate for the first time that potent cross-reactive neutralizing antibodies can be elicited in nonhuman primates immunized with soluble oligomeric subunit HIV envelope vaccines derived from an R5-using primary-like HIV-1 isolate. They strongly suggest, however, that specific envelope modifications can be introduced to increase the exposure of neutralization epitopes and increase the breadth and potency of these responses.
MATERIALS AND METHODS
Viruses.
The isolation and phenotypic characterization of the SF162 and SF162ΔV2 isolates were previously reported (5, 30). The primary clade B HIV-1 isolates 92US660, 92HT593, 92US657, 92US714, 92US727, 91US056, 91US054 and 93US073 were obtained from the NIH AIDS Research and Reference Reagent Program. All viral stocks were prepared and titrated in activated human peripheral blood mononuclear cells (PBMC).
Vaccines.
The DNA vector used to express our immunogens in rabbits is the pJW4303 (20). The DNA vector used to immunize rhesus macaques is derived from pCMVKm2 (4, 43). Both DNA plasmids contain the human cytomegalovirus enhancer/promoter elements, and the native leader peptide of the HIV envelope was replaced with that derived from the tissue-specific plasminogen activator gene. In the case of macaque immunizations, the DNA construct was codon optimized for high expression in mammalian cells. Both DNA vectors express the gp140 ectodomain form of the HIV envelope immunogen, with an intact gp120-gp41 cleavage site.
Protein-boosting immunizations were performed only in rhesus macaques to increase the titer of antibodies elicited following the DNA phase of immunization. For this purpose, the ΔV2 gp140 protein was produced in CHO cells and purified as stable soluble trimers (I. Srivastava et al., unpublished data). To increase the stability of these secreted oligomers, the gp120-gp41 cleavage site was eliminated by mutagenesis (9, 10, 32).
Immunizations (i) Rabbits.
Each animals received five DNA immunizations (each immunization consisting of 36 shots of 0.5 μg of DNA each) by the gene gun vaccination method (20) at weeks 0, 4, 8, 18, and 22. Blood was drawn 2 weeks following each immunization. Six animals (A1 to A6) were immunized with the unmodified SF162 gp140 immunogen, and six animals (A7 to A12) received the modified ΔV2 gp140 immunogen. Two animals (A13 and A14) served as controls and were immunized with the DNA vector alone.
(ii) Rhesus macaques.
Animals H445 and J408 were immunized with the modified ΔV2 gp140 immunogen, animals N472 and P655 were immunized with the unmodified SF162 gp140 immunogen, and animals M844 and H473 were immunized with the DNA vector alone. Before immunization, the animals were tested for antibodies to various simian viruses such as SIV, type D retroviruses, and simian T-lymphocytic virus type 1. Animals vaccinated with the modified envelope were immunized with DNA at weeks 0, 4, and 8, and animals vaccinated with the unmodified envelope were immunized with DNA at weeks 0, 4, and 9. The DNA (2 mg of DNA in 1 ml of endotoxin-free water each time per animal) was administered both intradermally at two sites (0.2 mg at each site) and intramuscularly (0.8 mg at two sites in the quadriceps muscles). Animals were immunized a fourth time with DNA and at the same time with the purified oligomeric ΔV2 or SF162 gp140 protein mixed with the adjuvant MF-59C. The proteins (0.1 mg of purified protein in 0.5 ml [total volume] per animal) were administered intramuscularly in the deltoids. The control animals received only adjuvant. This DNA-plus-protein booster immunization took place at week 27 for animals vaccinated with the modified immunogen and at week 48 for animals immunized with the unmodified immunogen. At week 38, the animals immunized with the modified, but not those immunized with the unmodified, immunogen were immunized one additional time with the adjuvanted protein alone (no DNA).
Antibody determination (i) Anti-gp140 antibodies.
Titers were determined throughout the immunization protocol by enzyme-linked immunosorbent assay (ELISA) as previously described (31, 33). Briefly, purified soluble oligomeric ΔV2 and SF162 gp140 proteins were used to coat ELISA plates (Immulon 2HB) (0.2 μg of protein in 0.1 ml of 100 mM NaHCO3 [pH 8.5]) by overnight incubation at 4°C. Nonadsorbed protein molecules were removed by washing with Tris-buffered saline (TBS), and the wells were blocked with SuperBlock (SB; Pierce). Heat-inactivated (56°C for 35 min) sera collected from the immunized animals were serially diluted in SB and added to the wells (0.1 ml per well) for 1 h at 37°C. In the case of rabbits, sera from control animals receiving the DNA vector alone were used as negative controls. In the case of macaques, preimmunization sera were used as negative controls. Unbound antibodies were removed by TBS washing, and the envelope-bound antibodies were detected with the use of goat anti-human (in the case of rhesus sera) or anti-rabbit (in the case of rabbit sera) immunoglobulin G coupled to alkaline phosphatase antibodies (Zymed Immunochemicals) as previously described (31). The optical density at 490 nm (OD490) of each well was recorded with a Bioluminometer (Molecular Dynamics). A plot of the OD490 signals versus serum dilution was generated, and endpoint antibody titers were determined as the highest postimmunization serum dilution that produces an OD490 value three times that of the OD490 produced by the preimmunization sera at their lowest dilution. Sera from various stages of immunization were tested at the same time.
(ii) Anti-V3 loop antibodies.
Titers of anti-V3 loop antibodies generated during immunization were determined by ELISA using the peptide CKSITIGPGRAFYATGDC, derived from the central region of the SF162-derived V3 loop. This peptide was diluted in 0.2 M sodium bicarbonate (pH 9.4) at a concentration of 1 μg/ml and then used to coat ELISA plates (0.1 ml per well) by overnight incubation at 37°C. The wells were washed with TBS and blocked with SB as described above. Sera collected before and after immunization were serially diluted in SB containing 0.3% (vol/vol), Tween and added (0.1 ml per well) for 2 h at room temperature. The plates were washed with TBS containing 0.3% (vol/vol) Tween, and V3 loop-bound antibodies were detected as described above with the use of immunoglobulin G coupled to alkaline phosphatase antibodies of the appropriate species. The antibody titers were determined as described above.
Neutralization assays.
Neutralization assays were performed using as target cells human PBMC activated for 3 days with phytohemagglutinin (3 μg/ml; Sigma) as previously described (21, 22, 30, 34). All HIV-1 isolates tested were grown and titrated in human PBMC, aliquoted, and kept frozen at −80°C until further use. Viruses (50 to 100 50% tissue culture infective doses in 50 μl of complete RPMI medium containing 20 U of interleukin-2 [Hoffmann-La Roche] per ml) were preincubated with an equal volume of serially diluted heat-inactivated (35 min at 56°C) sera for 1 h at 37°C in 96-well U-bottom plates (Corning). For each serum dilution, triplicate wells were used. Preimmunization sera from macaques and sera collected from rabbits immunized with the DNA vector alone were also incubated with the viruses and served as controls for nonspecific neutralization. To each well, 0.1 ml of complete medium containing 0.4 × 106 phytohemagglutinin-activated PBMC was added. Following overnight incubation at 37°C, half of the volume of each well was replaced with fresh, complete RPMI medium. Following centrifugation of the plates (5 min at 2,000 rpm), half of the volume of each well was again replaced with fresh medium. This procedure was repeated twice. The p24 antigen concentration in each well was evaluated at various points following infection (usually at days 4, 6, and 11), using an in-house ELISA p24- detection assay. The mean percent neutralization from triplicate wells and the standard deviation for each serum dilution were calculated based on p24 concentrations recorded in wells containing virus, cells, and no rabbit or macaque serum as previously described (34). However, we noticed that infection of some isolates was reduced in the presence of preimmunization sera (nonspecific neutralization). We decided therefore to present the results from our neutralization studies in two ways: (i) in the same figure, we present both the neutralization curve recorded with sera collected prior to vaccination (prebleeds) and that recorded with sera collected at various stages following vaccination; (ii) for each serum dilution, we calculate the difference between the percent neutralization recorded with postvaccination sera minus that recorded with prevaccination sera. In some figures, this difference (which we term specific neutralization) is plotted as a function of serum dilution. In parallel, we evaluated the susceptibilities of the various primary isolates to neutralization by MAbs 2F5 and 2G12.
During these neutralization experiments, we also evaluated the abilities of sera collected from macaques immunized with the recombinant SF2 gp120 envelope. This immunogen was previously tested as a potential vaccine against HIV and failed to raise cross-reactive neutralizing antibodies (22).
RESULTS
Generation of antibodies in rabbits.
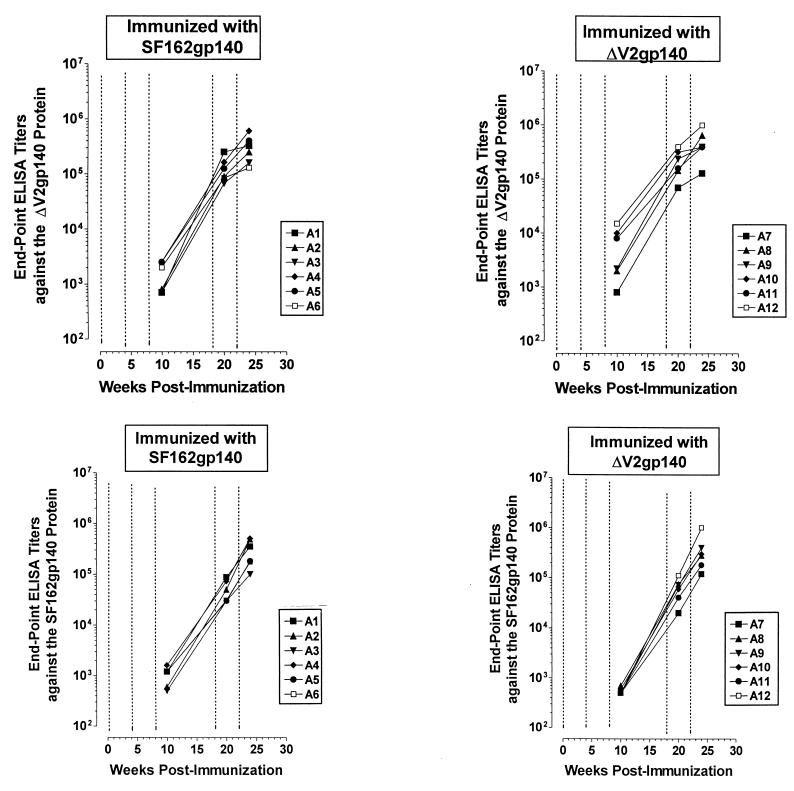
Both the SF162- and SF162ΔV2-derived immunogens elicited high titers of antibodies capable of binding to both oligomeric ΔV2 and SF162 gp140 (Fig. 1). As expected, variantions in the antibody titers were recorded throughout the vaccination schedule in animals belonging to either group. However, no statistically significant differences in antibody titers were recorded between the two animal groups throughout the immunization schedule. The antibody titers in each animal, regardless of whether it was immunized with the modified or the unmodified immunogen, were very weak during the first two immunizations (at 0 and 4 weeks). The fourth immunization (at 18 weeks) resulted in an increase in antibody titers, compared to the third immunization (8 weeks), between 2 and 3 log10 in both animal groups. The fifth immunization (22 weeks) increased the antibody titers, compared to the fourth immunization, against the SF162 gp140 antigen (by less than 1 log10) but not against the ΔV2 gp140 protein. At the end of the vaccination schedule, very potent endpoint ELISA binding antibody titers in the order of 105 to 106 were recorded in both animal groups against both antigens. Thus, it appears that in rabbits, based on the assay used here to determine antibody titers, the modified immunogen is as effective as the unmodified immunogen in eliciting the generation of antibodies even though the former immunogen lacks 30 amino acids from the V2 loop.
FIG. 1.
Development of antibodies in rabbits. Six animals (A1 to A6) were immunized with DNA expressing the unmodified SF162 gp140 immunogen, and six (A7 to A12) were immunized with DNA expressing the modified ΔV2 gp140 immunogen. Titers were determined 2 weeks following each immunization, as described in Materials and Methods, against the oligomeric SF162 and ΔV2 gp140 proteins. Dashed lines indicate times of immunizations.
Neutralizing activity in rabbit sera against the SF162 and SF162ΔV2 isolates.
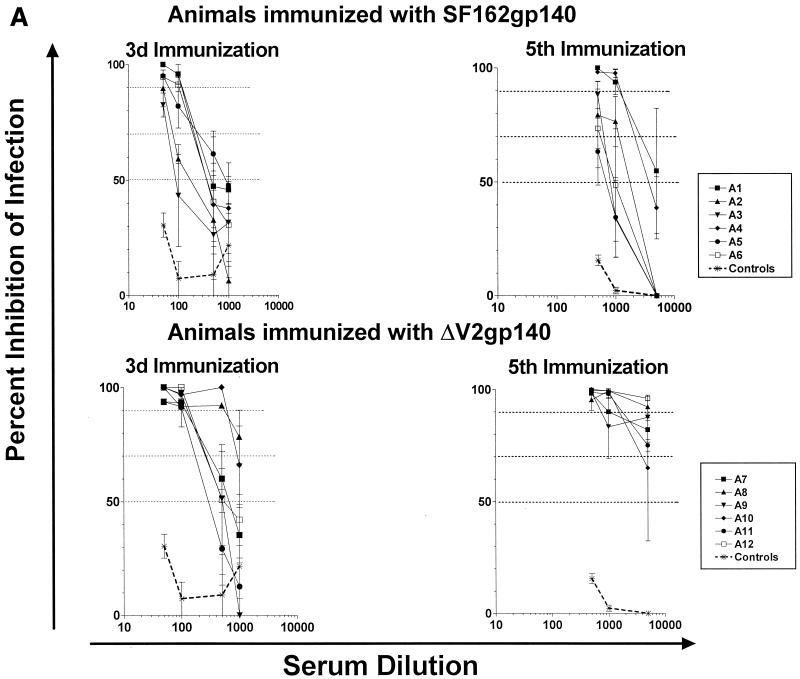
Both immunogens generated neutralizing antibodies against the SF162ΔV2 virus following the third DNA immunization (Fig. 2A). A trend toward higher neutralization titers in the modified immunogen-vaccinated group was recorded. Thus, the mean (± standard error) serum dilutions at which 70% inhibition of infection was recorded for SF162 gp140- and ΔV2 gp140-immunized animals were 179 (±34) and 483 (±148), respectively. At this stage of vaccination, while two (A8 and A9) out of six animals immunized with the modified immunogen elicited neutralizing antibodies against the parental SF162 isolate, none of the animals immunized with the unmodified immunogen elicited antibodies capable of doing so (Fig. 2B). However, the number of animals that generated neutralizing antibodies against the SF162 and SF162ΔV2 viruses increased with each subsequent immunization, so that at the end of the immunization schedule (i.e., after the fifth immunization) all animals had generated neutralizing antibodies against the SF162 virus. In addition, the neutralizing potency of each serum, regardless of whether the animal was vaccinated with the modified or unmodified immunogen, increased with each immunization.
FIG. 2.
Results of neutralization experiments using rabbit sera collected following the third and fifth immunizations against the SF162ΔV2 (A) and SF162 (B) viruses. Data are representative of at least three independent experiments. Symbols indicate the mean percent neutralization and standard deviation from triplicate wells. Dashed lines indicate 50, 70, and 90% inhibition of infection. Asterisks (controls) represent neutralization curves obtained with sera collected from animals that were immunized with the DNA vector alone and are indicative of nonspecific neutralization.
At the end of the immunization schedule, sera collected from rabbits immunized with the modified immunogen had higher neutralization potency against both SF162ΔV2 and SF162 viruses than sera collected from animals immunized with the unmodified immunogen. Six out of six animals immunized with the modified immunogen elicited antibodies capable of neutralizing the SF162ΔV2 virus between 70 and 100% at a 1:5,000 dilution (Fig. 2A). In contrast, at the same serum dilution only one (A1) of the six animals vaccinated with the unmodified envelope developed antibody responses able to neutralize SF162ΔV2 infection, and that by only 50%. The remaining five animals in this group failed to elicit antibody responses potent enough to neutralize SF162ΔV2 infection to any significant extent at this dilution. Differences in neutralizing potential between sera collected from animals immunized with the modified immunogen and those immunized with the unmodified immunogen were also evident when their abilities to neutralize the SF162 virus were compared (Fig. 2B). Sera collected from four (A8, A9, A10, and A12) out of six animals immunized with the modified antigen neutralized SF162 infection between 70 and 90% at 1:100 to 1:300 dilutions. In contrast, none of the sera collected from animals immunized with the unmodified antigen could inhibit SF162 infection by 70 to 90% at the same dilutions.
Generation of cross-reactive neutralizing antibodies in rabbits.
The fact that the SF162ΔV2-derived envelope immunogen was capable of eliciting higher titers of neutralizing antibodies against the parental SF162 isolate (which expresses the full envelope) than the immunogen derived from the SF162 isolate itself prompted us to examine whether the modified immunogen was also more effective in eliciting cross-reactive neutralizing antibodies, i.e., antibodies capable of neutralizing heterologous to the vaccine primary HIV-1 isolates. We tested several such isolates whose neutralization susceptibility to various MAbs was previously documented (8). Only two (92US714 and the 92HT593) out of the six isolates examined were neutralized by antibodies elicited by the unmodified immunogen (Table 1). All animals except animal A1 developed neutralizing antibodies against 92US714, while only animals A2 and A5 generated neutralizing antibodies against 92HT593. In contrast, four out of the six animals immunized with the modified ΔV2 gp120 immunogen generated cross-reactive neutralizing antibodies against most of the heterologous isolates tested. In addition, the neutralization potency of sera collected from animals immunized with the modified immunogen was higher than that of sera collected from animals immunized with the unmodified immunogen (Table 1). Thus, although 80% inhibition of infection was frequently recorded with the former sera, this level of inhibition was recorded in only two instances (sera from animal A5 versus the 92US714 and 92HT593 isolates).
TABLE 1.
Generation of cross-reactive neutralizing antibodies in rabbits
| gp140 | Animal | Specific neutralizationa
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 91US054
|
92US657
|
92US660
|
92HT593
|
91US056
|
92US714
|
||||||||
| 50% | 80% | 50% | 80% | 50% | 80% | 50% | 80% | 50% | 80% | 50% | 80% | ||
| Unmodified SF162 | A1 | − | − | − | − | − | − | − | − | − | − | − | − |
| A2 | − | − | − | − | − | − | + | − | − | − | + | − | |
| A3 | − | − | − | − | − | − | − | − | − | − | + | − | |
| A4 | − | − | − | − | − | − | − | − | − | − | + | − | |
| A5 | − | − | − | − | − | − | + | + | − | − | + | + | |
| A6 | − | − | − | − | − | − | − | − | − | − | + | − | |
| Modified ΔV2 | A7 | + | + | − | − | − | − | + | + | + | + | + | − |
| A8 | + | + | − | − | − | − | + | + | + | + | + | + | |
| A9 | + | − | + | − | − | − | + | + | + | + | + | + | |
| A10 | − | − | + | + | − | − | + | + | − | − | + | + | |
| A11 | − | − | − | − | − | − | − | − | − | − | − | − | |
| A12 | − | − | − | − | − | − | − | − | − | − | − | − | |
Neutralizing activity was evaluated at 1:10 dilution, taking into consideration the nonspecific neutralization recorded with sera collected from animals vaccinated with the DNA vector alone (see Materials and Methods for details). −, 50% specific neutralization was not recorded; +, 50 or 80% specific neutralization was recorded. Results are from three independent neutralization experiments.
Development of antibodies in rhesus macaques vaccinated with the modified ΔV2 gp140 immunogen.
The above results prompted us to evaluate the immunogenic potential of the unmodified SF162 gp140 and modified ΔV2 gp140 antigens in rhesus macaques, an animal model where the protective potential of vaccine-elicited antibodies can eventually be evaluated. Macaques were vaccinated with these two immunogens by the DNA-prime–protein-boost vaccination method.
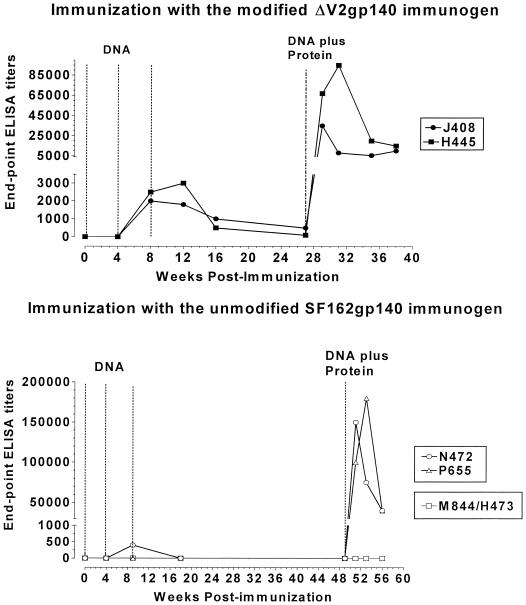
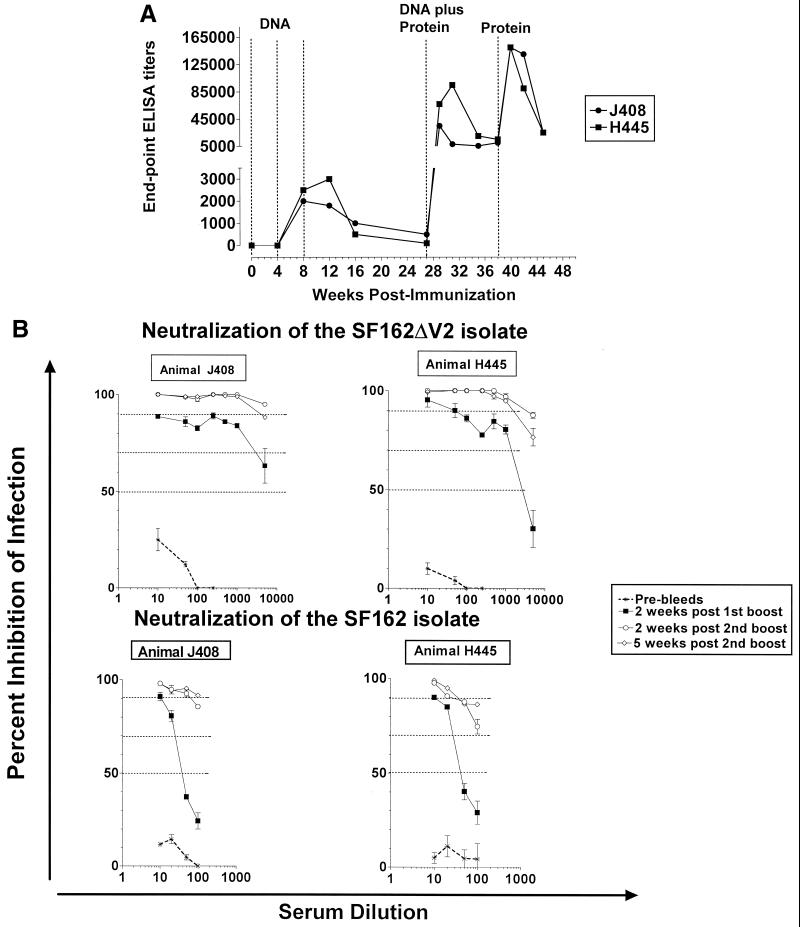
Envelope-specific antibodies became detectable following the second DNA immunization (Fig. 3). At this stage, endpoint ELISA titers in animals immunized with the modified antigen (animals J408 and H445) were in the order of 1:2,000. In contrast, in animals immunized with the unmodified envelope (animals N472 and P655), antibodies were detectable only in animal N472 (endpoint ELISA titers in the order of 1:500). With the exception of animal H445, the third DNA immunization did not further increase the antibody titers. Anti-gp120 and anti-gp41 antibodies were generated synchronously during DNA immunization (data not shown).
FIG. 3.
Generation of antibodies in rhesus macaques. The generation of antienvelope antibodies in two animals (J408 and H445) immunized with the modified ΔV2gp140 immunogen and two animals (P655 and N472) immunized with the unmodified SF162gp140 immunogen, as well as control animals (M844 and H473) immunized with the DNA vector alone, were determined by ELISA using the corresponding protein as described in Materials and Methods. Dashed lines indicate times of immunizations. DNA, animals received three monthly immunizations with DNA vectors expressing the gp140 form of each immunogen. Control animals received the DNA vector alone. DNA plus Protein, animals received a fourth DNA immunization and at the same time were immunized with the corresponding CHO cell-produced oligomeric gp140 proteins, mixed in the adjuvant MF-59C. Control animals received adjuvant alone.
During the subsequent 5 to 10 months of observation, antibodies were undetectable in animals immunized with the unmodified SF162 gp140 immunogen, while in animals immunized with the modified ΔV2 gp140 immunogen the antibodies were always detectable, but their titers declined over time.
Following the DNA-plus-protein booster immunization, the antibody titers increased significantly in all animals. At their peak values (reached within 2 to 4 weeks postboosting), endpoint ELISA antibody titers in animals immunized with the modified ΔV2 gp140 immunogen were 1:30,000 for animal J408 and 1:110,000 for animal H445. The titers decreased gradually over time and remained stable at approximately 1:8,000 for several weeks in both animals. Higher peak antibody titers were recorded in animals vaccinated with the unmodified SF162 gp140 immunogen (endpoint ELISA antibody titers of 1:150,000 in animal N472 and 175,000 in animal P655). During the following 7 weeks of observation, the antibody titers decreased more rapidly in both animals to approximately 1:35,000. Thus, in contrast to what we recorded in rabbits, in macaques the unmodified immunogen generated higher titers of binding antibodies than the modified immunogen.
As expected, anti-HIV envelope antibodies were not generated in control animals (M844 and H473) immunized with the DNA vector alone.
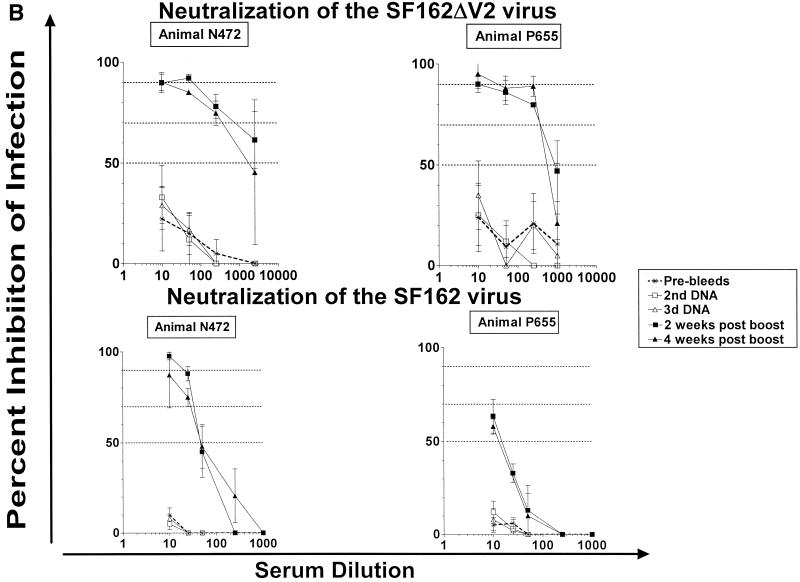
Neutralizing activity of macaque sera against the homologous SF162ΔV2 and parental SF162 isolates.
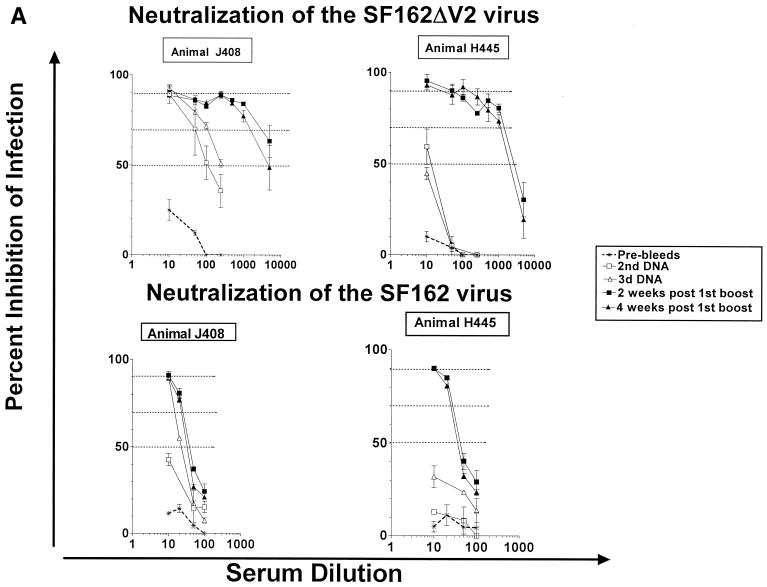
During the DNA phase of immunization, only animals immunized with the modified ΔV2 gp140 immunogen elicited neutralizing antibodies against the SF162 and SF162ΔV2 viruses (Fig. 4). Following the second DNA immunization, animal J408 developed neutralizing antibodies against the homologous SF162ΔV2, but not the parental SF162, isolate (Fig. 4A). The titer of neutralizing antibodies in animal J408 increased following the third DNA immunization, at which point neutralization of both isolates was recorded, although the titers of binding antibodies did not increase in parallel (Fig. 3). In contrast, much weaker neutralizing antibody responses against the SF162ΔV2 and no neutralizing responses against the SF162 virus were elicited in animal H445, even though this animal generated titers of binding antibodies similar to those generated in animal J408 (Fig. 3).
FIG. 4.
Neutralizing activities of rhesus macaque sera. Neutralization activities against the SF162 and SF162ΔV2 viruses of sera collected from animals immunized with the modified ΔV2 gp140 (A) and the unmodified (B) SF162 gp140 immunogens were determined as described in Materials and Methods. Dashed lines indicate 50, 70, and 90% inhibition of infection. Results are representative of three to five independent experiments. Data indicate the mean and standard deviation from triplicate wells. Pre-bleeds, sera collected before vaccination; 2nd DNA and 3rd DNA, sera collected 1 month following the second and the third, respectively, DNA administrations; 2 and 4 weeks post 1st boost, sera collected 2 and 4 weeks, respectively, following the DNA-plus-protein booster immunization.
Two weeks following the DNA-plus-protein booster immunization, sera collected from animals immunized with either immunogen inhibited SF162ΔV2 infection. The neutralization potency of sera collected from animals immunized with the modified immunogen was higher than that of sera collected from animals immunized with the unmodified immunogen. For example, 50% inhibition of SF162ΔV2 infection was recorded at dilutions of 1:2,000 to 1:5,000 from the former sera, but this level of inhibition was not recorded at this dilutions with the latter sera. Both ΔV2 gp140-immunized animals generated strong neutralizing antibodies against the parental SF162 virus, while only one (N472) of the two animals immunized with the SF162 gp140 immunogen generated neutralizing antibodies against this virus. Changes in the neutralizing potency of these sera were not recorded during the subsequent 2 weeks, even tough changes in the antibody titer levels were detectable during this period (Fig. 3). Control animals (M844 and H473) vaccinated with the vector alone did not develop neutralizing antibodies (data not shown).
Neutralization of heterologous primary HIV-1 isolates by macaques sera.
The breath of the neutralizing antibody responses elicited in macaques immunized with the modified and unmodified immunogens was evaluated by comparing the abilities of sera collected from macaques immunized with these two immunogens to block infection of heterologous primary clade B HIV-1 isolates. During our serum neutralization experiments, we evaluated in parallel the susceptibility of these isolates to neutralization by two of the most commonly used primary-isolate-neutralizing MAbs (2F5 and 2G12) (Table 2).
TABLE 2.
Neutralization of heterologous primary HIV-1 isolates by macaque sera
| Isolate (coreceptor usage) | MAb
|
% Neutralizationa with immunogenb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔV2 gp140
|
SF162 gp140
|
SF2 gp120
|
||||||||
| 2F5 | 2G12 | J408
|
H445
|
P655 (A) | N472 (A) | L714 | L814 | |||
| A | B | A | B | |||||||
| 91US056(R5) | 60 | 70 | 90 | — | 65 | — | — | — | — | — |
| 92US714(R5) | 70 | 20 | 85 | — | 85 | — | — | — | — | — |
| 92US660(R5) | 75 | 70 | 50 | — | 80 | — | — | — | — | — |
| 92HT593(R5X4) | 75 | 80 | — | — | — | — | — | — | — | — |
| 92US657(R5) | NT | NT | — | — | — | 65 | — | — | — | — |
| BZ167(R5X4) | 90 | 75 | NT | — | NT | 80 | NT | NT | NT | NT |
| ADA(R5) | NT | NT | 90 | 50 | 90 | 80 | NT | NT | NT | NT |
Percent neutralization of a given HIV-1 isolate by sera (1:10 dilution) collected from animals immunized with the modified ΔV2 gp140 (J408 and H445), unmodified SF162 gp140 (P655 and N472), and recombinant SF2 gp120 (L714 and L814), calculated as described in Materials and Methods, taking into consideration the nonspecific neutralization recorded with sera collected from the same animals before immunization.
A, sera collected 2 weeks following the DNA-plus-protein booster immunization; B, sera collected 2 weeks following the final protein booster immunization of animals J408 and H445. Values represent averages from two to three independent experiments. The susceptibility of these isolates to neutralization by 2F5 and 2G12 at 25 μg of MAb per ml is also presented. —, not detected; NT, not tested.
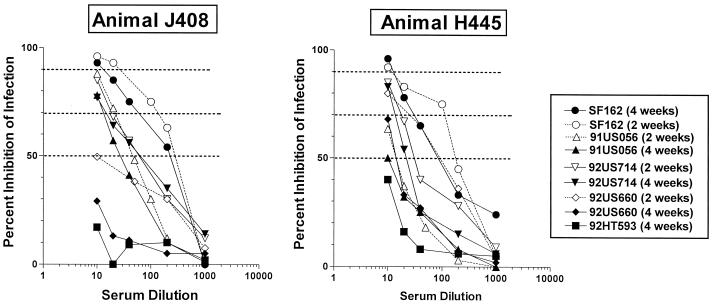
Heterologous isolate neutralization was not recorded (less than 50% inhibition of infection at 1:10 serum dilution) during the DNA phase of immunization in macaques (data not shown). Two weeks following the DNA-plus-protein booster immunization, sera collected from the two animals vaccinated with the modified ΔV2 gp140 protein neutralized some of the heterologous primary HIV-1 isolates tested (Fig. 5). At the lowest serum dilution tested (1:10), and when nonspecific neutralization recorded with preimmunization sera was taken into consideration (see Materials and Methods for details), 80 to 90% inhibition of infection was recorded only with the ADA, 91US056, and 92US714 isolates by J408 sera and with the ADA, 92US714, and 92US660 isolates with the H445 sera (Fig. 5 and Table 2). The cross-neutralizing activity of the sera collected from these two animals differed. For example, 92US660 infection was inhibited by 80 and 50% by H445 and J408 sera, respectively. The serum cross-neutralizing activity decreased during the subsequent weeks of observation (Fig. 5). Sera collected 5 weeks following this DNA-plus-protein booster immunization had no cross-reactive neutralizing activity, even though potent neutralization of the SF162 and SF162ΔV2 isolates was still recorded.
FIG. 5.
Neutralization of heterologous clade B primary HIV-1 isolates by macaque sera. Neutralization activities of sera collected 2 and 4 weeks following the DNA-plus-protein booster immunization against isolates heterologous to the vaccine primary HIV-1 isolates were determined as described in Materials and Methods. Dashed lines indicate 50, 70, and 90% inhibition of infection. The values represent specific neutralization, which is defined as the difference between the percent virus neutralization recorded with sera collected following vaccination and that recorded with sera collected prior to the initiation of vaccination. Data points indicate the mean percent specific neutralization from two independent experiments.
Despite the fact that following this DNA-plus-protein booster immunization, the binding antibody titers in animals vaccinated with the unmodified immunogen were higher than those in animals vaccinated with the modified immunogen (Fig. 3), the former sera failed to neutralize any of the heterologous isolates tested (Table 2) (i.e., less than 50% specific neutralization was recorded). Thus, although in rabbits the unmodified immunogen was able to elicit (albeit much less efficiently than the modified immunogen) neutralizing antibodies against some heterologous primary HIV-1 isolates (Table 1), it failed to do so in rhesus macaques.
In parallel, we evaluated the susceptibility of the heterologous isolates to neutralization by sera collected from macaques that had been immunized with the recombinant SF2-derived gp120 protein. This protein was previously evaluated as a vaccine candidate and was ineffective in eliciting cross-reactive neutralizing antibodies; i.e., less than 50% neutralization at serum dilutions of 1:10 was recorded (22). All of the isolates tested here were not susceptible to neutralization by antibodies elicited by the SF2 gp120 protein (Table 2).
Second booster immunization with the modified ΔV2 gp140 protein.
Although the above results indicated that the modified ΔV2 gp140 immunogen was indeed more effective in eliciting cross-reactive neutralizing antibody responses than the unmodified immunogen, these responses were weaker than those recorded against the parental SF162 isolate (Fig. 5). In an effort to further increase the potency and breath of these responses, we attempted to further boost the antibody titers in animals H445 and J408 by immunizing them one additional time with the purified oligomeric ΔV2 gp140 protein (this time in the absence of DNA immunization).
An increase in antibody titers was indeed recorded following this protein boost, so that at their peak values (1:145,000 endpoint ELISA titers) the titers were approximately threefold higher than those recorded during the first booster immunization with DNA plus protein (Fig. 6A). In parallel, we recorded a significant increase in the titer of neutralizing antibodies against the homologous SF162ΔV2 and parental SF162 isolates (Fig. 6B). No differences in the neutralizing potential of the sera collected 2 and 5 weeks following this last boost were recorded, even though the binding antibody titers decreased significantly during the same period. Unexpectedly, however, the neutralizing potential of the same sera against most of the heterologous primary isolates tested generally decreased (Table 2). Thus, with the exception of the BZ167, 92US657, and ADA isolates, all of the heterologous isolates tested were resistant to neutralization by sera collected 2 weeks following the second boost. Interestingly, although isolate 92US657 was resistant to neutralization by sera collected following the first boost, it became susceptible to neutralization by sera collected from animal H445 following the second boost.
FIG. 6.
Generation of binding and neutralizing antibodies following the second booster immunization with the modified ΔV2 gp140 protein. (A) The generation of antienvelope antibodies in two rhesus macaques (J408 and H445) vaccinated with the modified ΔV2 gp140 immunogen was determined by ELISA as described in Materials and Methods. Dashed lines indicate times of immunizations. DNA, animals received three monthly immunizations with DNA vectors expressing the gp140 form of this immunogen; DNA plus Protein, animals received a fourth DNA immunization and purified oligomeric ΔV2 gp140 protein; Protein, the animals were immunized with the purified oligomeric ΔV2 gp140 protein alone. (B) Neutralization activities against the SF162ΔV2 and SF162 isolates of sera following the second boost were compared to those of sera collected following the first boost (see also Fig. 4). Nonspecific neutralization recorded with preimmunization sera (Pre-bleeds) is also shown.
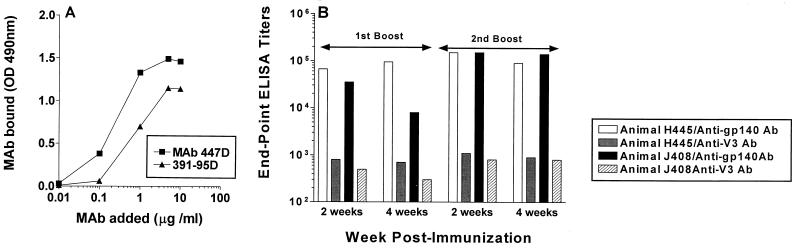
Generation of anti-V3 loop antibodies in rhesus macaques vaccinated with the modified ΔV2 gp140 immunogen.
One explanation for the increase in neutralizing activity against the parental SF162 and homologous SF162ΔV2 viruses and the decrease in neutralizing activity against the heterologous isolates following the second booster immunization is that multiple immunizations with the modified ΔV2 gp140 protein increased the titer of antibodies directed against epitopes that are uniquely (or predominantly) expressed on the SF162 and SF162ΔV2 envelopes. It is conceivable that multiple immunizations with the ΔV2 gp140 protein result in the generation of high titers of anti-V3 loop antibodies. To determine the titers of such antibodies, we used V3 loop peptide-based ELISAs using the SF162/SF162ΔV2-derived V3 loop (Fig. 7). This peptide was recognized by antibodies binding to both linear (447D) (7, 12) and conformational (391-95D) (29) epitopes (Fig. 7A). Although anti-V3 loop antibodies were generated upon immunization of macaques with the modified ΔV2 gp140 immunogen, their titers were much lower than those against the entire envelope (Fig. 7B). In addition, the second booster immunization did not increase the titer of anti-V3 loop antibodies. It should be noted, however, that certain anti-V3 loop antibodies present in the sera of these animals may not interact efficiently with the V3 loop peptide in an ELISA format, while they may bind to their epitopes on the native envelope (23). Additionally, the V3 loop peptide used here does not span the carboxy and amino termini of the V3 loop, and our assay does not detect antibodies targeting these two regions. Thus, a more detailed examination of the epitope specificity of the antibodies elicited by the modified ΔV2 gp140 immunogen is required.
FIG. 7.
Presence of anti-V3 loop antibodies in sera collected from macaques immunized with the modified ΔV2 gp140 immunogen. The development of anti-V3 loop antibodies was determined by ELISA using the V3 loop peptide derived from the SF162/SF162ΔV2 envelope. (A) We first examined whether the captured V3 loop peptide interacts with specific anti-V3 loop MAbs recognizing linear (447D) and conformational (391-95D) V3 loop epitopes. (B) We next determined the titer of anti-V3 loop antibodies present in sera collected 2 and 4 weeks following the 1st and 2nd boosts from the two vaccinated animals. As a comparison, we also include the titers of total antienvelope antibodies present in the same sera.
DISCUSSION
In this study we compared the immunogenicities of soluble oligomeric gp140 envelope proteins derived from related neutralization-resistant (SF162) and neutralization-susceptible (SF162ΔV2) viruses (30). The only difference between the two immunogens is the absence of 30 amino acids from the V2 loop of the SF162ΔV2-derived immunogen (30).
We first performed immunization studies in rabbits, where we observed that although both proteins elicited similar titers of binding antibodies (Fig. 1), the modified immunogen elicited higher titers of neutralizing antibodies against isolates expressing not only the modified SF162ΔV2 envelope but also the unmodified parental SF162 envelope (Fig. 2). Our results are in agreement with those made on the background of the SIVmac239 virus (25) because they suggest that specifically modified envelope immunogens may elicit antibodies capable of neutralizing isolates expressing the parental unmodified envelope. However, our results contrast with those obtained during the immunization of rabbits with a more extensively modified HIV-1 envelope-derived immunogen, which lacked not only the V2 loop but also the V1 and V3 loops (20). The reasons for this discrepancy are not known, but it would be interesting to determine whether the triple-loop-deleted SF162 envelope also fails to elicit neutralizing antibodies.
In rabbits, both the unmodified SF162 and modified ΔV2 gp140 immunogens elicited neutralizing antibodies against several heterologous primary HIV-1 isolates, but the potential of the modified immunogen to do so was greater (Table 1). Thus, not only did more animals vaccinated with the modified immunogen elicit cross-reactive neutralizing antibodies, but also the breadth and potency of the cross-neutralizing responses were higher in sera collected from these animals than from animals immunized with the unmodified immunogen. We believe that this is a consequence of the greater immunogenicity of conserved neutralizing epitopes on the modified immunogen tested here. Consequently, the modified immunogen more effectively elicits antibodies recognizing these epitopes than the unmodified immunogen. If V2 loop deletion increases the exposure of conserved neutralization epitopes that are poorly exposed on the SF162 envelope, then these two immunogens will elicit different titers of the same antibodies. If V2 loop deletion results in exposure of neutralization epitopes that are not exposed on the SF162 envelope, then the two immunogens will elicit different types of antibodies. An alternative explanation for the increased ability of the modified ΔV2gp140 immunogen to elicit neutralizing antibodies was previously proposed by our group (30). It is possible that deletion of the V2 loop from the SF162 envelope results in an alteration of the ratio of neutralization and nonneutralization epitopes on this envelope, so that more of the former epitopes are present on the SF162ΔV2 than on the SF162 envelope. If this hypothesis is correct, immunization with the modified ΔV2 gp140 envelope would be expected to elicit a proportionally higher number of neutralizing antibodies than immunization with the unmodified SF162 gp140 envelope. The above possibilities are not mutually exclusive.
Our vaccination studies conducted in rhesus macaques confirm the observations made in rabbits, that the modified ΔV2 gp140 immunogen is more effective than the unmodified SF162 gp140 in eliciting neutralizing antibodies against isolates expressing the parental SF162 envelope and heterologous envelopes. Neutralization of heterologous primary HIV-1 isolates was less efficient than neutralization of the parental SF162 virus, which in turn was less efficiently neutralized than the homologous SF162ΔV2 virus. This suggests that the epitopes recognized by these cross-reactive antibodies are less accessible on the surface of the heterologous isolates than on the surface of the SF162 virus and much less accessible than they are on the surface of the SF162ΔV2 virus. Based on our previous neutralization studies with MAbs, we believe that deletion of the V2 loop from the SF162 envelope increases the exposure of epitopes participating in envelope-CD4 and -coreceptor binding (30). Such epitopes become only transiently exposed during the displacement of the V1 and V2 loops that take place upon HIV envelope-CD4 binding (37–39). It is possible that these epitopes are normally masked within the oligomeric envelope structure by the V2 loop and deletion of this loop renders them permanently exposed. The difference in neutralization susceptibility between the SF162 and heterologous isolates may be the result of different envelope glycosylation patterns and/or different positioning of the V2 loop on the SF162 and heterologous isolates tested here. Additionally, we expect that although cross-reactive antibodies were elicited by this specific envelope modification, a large fraction of the antibodies elicited by the ΔV2 gp140 immunogen are targeting epitopes unique to the ΔV2 and SF162 envelopes. Our current results suggest that these unique epitopes must be located in envelope regions other than the V3 loop. This is also supported by our observations that the heterologous primary HIV-1 isolates tested here were resistant to neutralization by sera collected from macaques immunized with the recombinant monomeric SF2 gp120 protein, which primarily elicits anti-V3 loop antibodies (36). The lack of generation of high anti-V3 loop antibodies by our soluble oligomeric immunogens may not be due to the specific antigenic structure of these immunogens, because a recent report indicated that immunization with the oligomeric envelope derived from the HXB2 isolate also failed to elicit high titers of V3 loop-directed antibodies (11). Several heterologous primary isolates tested here were, however, completely resistant to neutralization by the antibodies elicited by the modified immunogen. Either these isolates may lack the epitopes recognized by the antibodies elicited by this immunogen or, as mentioned above, these epitopes may be more efficiently masked on these particular isolates than on the isolates susceptible to neutralization. By identifying the epitopes recognized by the neutralizing antibodies elicited by the ΔV2 gp140 immunogen, we may be able to determine whether these epitopes are absent from the heterologous isolates that are resistant to neutralization or whether they are more efficiently occluded on the surface of these isolates.
The envelopes of the SF162 and SF162ΔV2 viruses use the CCR5 cellular protein to mediate virus-cell fusion in the presence of CD4 (30). The heterologous primary isolates tested here used either exclusively CCR5 or both CCR5 and CXCR4 (Table 2). We did not examine whether the antibodies generated by the immunogens tested here, especially the modified one, would have neutralization potential against isolates that exclusively utilize CXCR4 to infect CD4+ T cells. Failure of our immunogens to do so would be an indication that the antibodies elicited may recognize epitopes participating in gp120-CCR5 interaction. Although, we believe that an effective vaccine against HIV should elicit a broad spectrum of neutralizing antibodies, such a vaccine should primarily elicit neutralizing antibodies against R5-using HIV-1 isolates because such isolates are more effective than X4-using HIV-1 isolates in establishing a primary HIV-1 infection in exposed humans (15, 19, 26, 42).
Some differences were recorded during the immunization of rabbits and macaques. For example, in rabbits both immunogens elicited similar binding antibody titers, while the unmodified immunogen elicited higher titers of binding antibodies in macaques. Also, although in rabbits the unmodified immunogen elicited neutralizing antibodies (albeit infrequently and at low titers) against a few heterologous HIV-1 isolates, it failed to do so in macaques. Finally, some isolates, such as 92HT593 was neutralized by rabbit but not by macaque sera. Whether these differences are due to differences in the way the immune systems of these two animal species reacts to the same immunogen, or whether they are due to the different methods of immunization (gene gun in the case of rabbits and intramuscular plus intradermal needle DNA injections followed by protein immunization in the case of macaques) merits further investigation.
It is important to note that in the case of the heterologous primary HIV-1 isolates tested here, we rarely recorded 90% inhibition of the infection with the neutralization assay that we used at the lowest serum dilution evaluated (1:10) and that the cross-neutralizing activity was lost within 5 weeks following the DNA-plus-protein booster immunization. We corrected our neutralization data for nonspecific neutralization recorded with autologous sera collected before vaccination, because it was recently reported that preimmunization sera (1:4 dilution) collected from immunized human volunteers could inhibit by up to 80% the infection of several primary HIV-1 isolates (2). Rabbit or macaque preimmunization sera (1:10 dilution) used here generally did not inhibit by over 30 to 35% the infection of the isolates tested. Although it is possible that 90% inhibition of infection may be recorded more frequently at lower serum dilutions, we believe that to increase the potency and breadth of neutralization, additional envelope modifications must be introduced. These modifications should increase the exposure and/or the number of conserved neutralization epitopes on the immunogen. However, because an increase in epitope exposure may not automatically result in an increase of epitope immunogenicity (20), the effect of a particular envelope modification on envelope immunogenicity can be determined empirically only by in vivo immunogenicity studies in relevant animal models. Nevertheless, the achievement of the observed level of cross-reactive neutralizing activity in a nonhuman primate warrants further evaluation of this particular envelope modification approach. Neutralization epitopes may also become exposed during the propagation of HIV in cells lacking receptor molecules (14, 17) or by thermal or chemical treatment of virions (13). Envelope molecules derived from such viruses, when used as immunogens, may also elicit high titers of neutralizing antibodies.
It would also be important to compare the protective potentials of antibodies elicited by our modified and unmodified immunogens. In this regard, we recently reported that the antibodies elicited by the modified ΔV2gp140 immunogen offered partial protection from challenged with the related pathogenic SIV-HIV himeric virus SHIVSF162P4 (6). However, the ability of the antibodies elicited by our modified immunogen to protect macaques from heterologous viral challenge is not yet known. Importantly, vaccination methodologies that generate and sustain significant titers of cross-reactive neutralizing antibodies must be developed.
During the second booster immunization (protein alone) of macaques with the modified ΔV2 gp140 immunogen, we observed that although an increase in the potency of neutralizing antibody responses was recorded in the case of the homologous and parental viruses (Fig. 6), a parallel increase against heterologous primary HIV-1 isolates was not recorded (Table 2). Thus, multiple immunizations with the purified modified ΔV2 gp140 protein may preferentially increase the titer of antibodies recognizing epitopes that are unique to the SF162 and ΔV2 envelopes. Whether this is related to the fact that the DNA-expressed protein has an intact gp120-gp41 cleavage site, while this site is absent from the CHO cell-produced protein is not known. It is possible that conserved neutralization epitopes are more efficiently exposed on soluble oligomeric gp140 proteins with an intact gp120-gp41 cleavage site. In that respect, differences in the structures of gp140 proteins derived from the SF162 and ΔV2 envelopes with intact or absent gp120-gp41 cleavage sites were reported by our group (32).
In summary, our current data strongly suggest that unless specific modifications are introduced in soluble oligomeric envelope constructs derived from R5-using, primary HIV-1 isolates, the breadth and potency of the neutralizing responses elicited by such vaccines will be weak. The challenge is to identify additional envelope modifications that would further increase the immunogenicity of neutralization epitopes whose structures are conserved among primary HIV-1 isolates.
ACKNOWLEDGMENTS
This study was supported by NIH grants AI47708-01 (L.S.), AI44309-01 (L.S.), AI44309 (S.L.), and AI40337 (S.L.).
We acknowledge Cecilia Cheng-Mayer and James Bradac for many helpful discussions throughout this study and John Donnelly for critical reading of the manuscript.
REFERENCES
- 1.Bou-Habib D C, Roderiquez G, Oravesz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bures R, Gaitan A, Zhu T, Graziosi C, McGrath K M, Tartaglia J, Caudrelier P, El Habib R, Klein M, Lazzarin A, Stablein D M, Deers M, Corey L, Greenberg M L, Schwartz D H, Montefiori D C. Immunization with recombinant canarypox vectors expressing membrane- anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2000;16:2019–2035. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- 3.Cao J, Sullivan N, Desjardin E, Parolin C, Robinson J, Wyatt R, Sodroski J. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J Virol. 1997;71:9808–9812. doi: 10.1128/jvi.71.12.9808-9812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman B S, Thayer R M, Vincent K A, Haigwood N L. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 1991;19:3979–3986. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng-Mayer C, Quiroga M, Tung J W, Dina D, Levy J A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990;64:4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherpelis S, Shrivastava I, Gettie A, Jin X, D H D, Barnett S W, Stamatatos L. DNA vaccination with the human immunodeficiency virus type 1 SF162ΔV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CD8+ T-cell-depleted rhesus macaques. J Virol. 2001;75:1547–1550. doi: 10.1128/JVI.75.3.1547-1550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conley A J, Gorny M K, Kessler J A, II, Boots L J, Ossorio-Castro M, Koenig S, Lineberger D W, Emini E A, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Souza M P, Livnat D, Bradac J A, Bridges S H. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. AIDS Clinical Trials Group Antibody Selection Working Group. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 9.Earl P L, Koenig S, Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. I Virol, 1991;65:31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earl P L, Moss B. Mutational analysis of the assembly domain of the HIV-1 envelope glycoprotein. AIDS Res Hum Retroviruses. 1993;9:589–594. doi: 10.1089/aid.1993.9.589. [DOI] [PubMed] [Google Scholar]
- 11.Earl P L, Sugiura W, Montefiori D C, Broder C C, Lee S A, Wild C, Lifson J, Moss B. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J Virol. 2001;75:645–653. doi: 10.1128/JVI.75.2.645-653.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorny M K, Conley A J, Karwowska S, Buchbinder A, Xu J-Y, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grovit-Ferbas K, Hsu J F, Ferbas J, Gudeman V, Chen I S. Enhanced binding of antibodies to neutralization epitopes following thermal and chemical inactivation of human immunodeficiency virus type 1. J Virol. 2000;74:5802–5809. doi: 10.1128/jvi.74.13.5802-5809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman T L, LaBranche C C, Zhang W, Canziani G, Robinson J, Chaiken I, Hoxie J A, Doms R W. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc Natl Acad Sci USA. 1999;96:6359–6364. doi: 10.1073/pnas.96.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 16.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaBranche C C, Hoffman T L, Romano J, Haggarty B S, Edwards T G, Matthews T J, Doms R W, Hoxie J A. Determinants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions required for coreceptor specificity. J Virol. 1999;73:10310–10319. doi: 10.1128/jvi.73.12.10310-10319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaCasse R A, Follis K E, Trahey M, Scarborough J D, Littman D R, Nunberg J H. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283:357–362. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 20.Lu S, Wyatt R, Richmond J F L, Mustafa F, Wang S, Weng J, Montefiori D C, Sodroski J, Robinson H L. Immunogenicity of DNA vaccines expressing human immunodeficiency virus type 1 envelope glycoprotein with and without deletions in the V1/V2 and V3 regions. AIDS Res Hum Retroviruses. 1998;14:151–155. doi: 10.1089/aid.1998.14.151. [DOI] [PubMed] [Google Scholar]
- 21.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S the NIAID AIDS Vaccine Evaluation Group. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 23.Moore J P. The reactivities of HIV-1+ human sera with solid-phase V3 loop peptides can be poor predictors of their reactivities with V3 loops on native gp120 molecules. AIDS Res Hum Retroviruses. 1993;9:209–219. doi: 10.1089/aid.1993.9.209. [DOI] [PubMed] [Google Scholar]
- 24.Moore J P, McKeating J A, Huang Y, Askenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 26.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 27.Sattentau Q J, Moore J P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sattentau Q J, Moore J P, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seligman S J, Binley J M, Gorny M K, Burton D R, Zolla-Pazner S, Sokolowski K A. Characterization by serial competition ELISAs of HIV-1 V3 loop epitopes recognized by monoclonal antibodies. Mol Immunol. 1996;33:737–745. doi: 10.1016/0161-5890(96)00044-2. [DOI] [PubMed] [Google Scholar]
- 30.Stamatatos L, Cheng-Mayer C. An envelope modification that renders a primary, neutralization resistant, clade B HIV-1 isolate highly susceptible to neutralization by sera from other clades. J Virol. 1998;72:7840–7845. doi: 10.1128/jvi.72.10.7840-7845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamatatos L, Cheng-Mayer C. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J Virol. 1995;69:6191–6198. doi: 10.1128/jvi.69.10.6191-6198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatatos L, Lim M, Cheng-Mayer C. Generation and structural analysis of soluble oligomeric envelope proteins derived from neutralization-resistant and neutralization-susceptible primary HIV-1 isolates. AIDS Res Hum Retroviruses. 2000;16:981–994. doi: 10.1089/08892220050058407. [DOI] [PubMed] [Google Scholar]
- 33.Stamatatos L, Wiskerchen M, Cheng-Mayer C. Effect of major deletions in the V1 and V2 loops of a macrophage-tropic HIV-1 isolate on viral envelope structure, cell-entry and replication. AIDS Res Hum Retroviruses. 1998;14:1129–1139. doi: 10.1089/aid.1998.14.1129. [DOI] [PubMed] [Google Scholar]
- 34.Stamatatos L, Zolla-Pazner S, Gorny M, Cheng-Mayer C. Binding of antibodies to virion-associated gp120 molecules of primary-like human immunodeficiency virus type 1 (HIV-1) isolates: effect on HIV-1 infection of macrophages and peripheral blood mononuclear cells. Virology. 1997;229:360–369. doi: 10.1006/viro.1997.8443. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanCott T C, Mascola J R, Loomis-Price L D, Sinangil F, Zitomersky N, McNeil J, Robb M L, Birx D L, Barnett S. Cross-subtype neutralizing antibodies induced in baboons by a subtype E gp120 immunogen based on an R5 primary human immunodeficiency virus type 1 envelope. J Virol. 1999;73:4640–4650. doi: 10.1128/jvi.73.6.4640-4650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 38.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt R, Sullivan N, Thali M, Repke H, Ho D, Robinson J, Posner M, Sodroski J. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J Virol. 1993;67:4557–4565. doi: 10.1128/jvi.67.8.4557-4565.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Wyatt R, Sodroski J. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J Virol. 2001;75:1165–1171. doi: 10.1128/JVI.75.3.1165-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu T, Mo H, Wang N, Nam D S, Coa Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 43.zur Megede J, Chen M C, Doe B, Schaefer M, Greer C E, Selby M, Otten G R, Barnett S W. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J Virol. 2000;74:2628–2635. doi: 10.1128/jvi.74.6.2628-2635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]