Abstract
Purpose of review
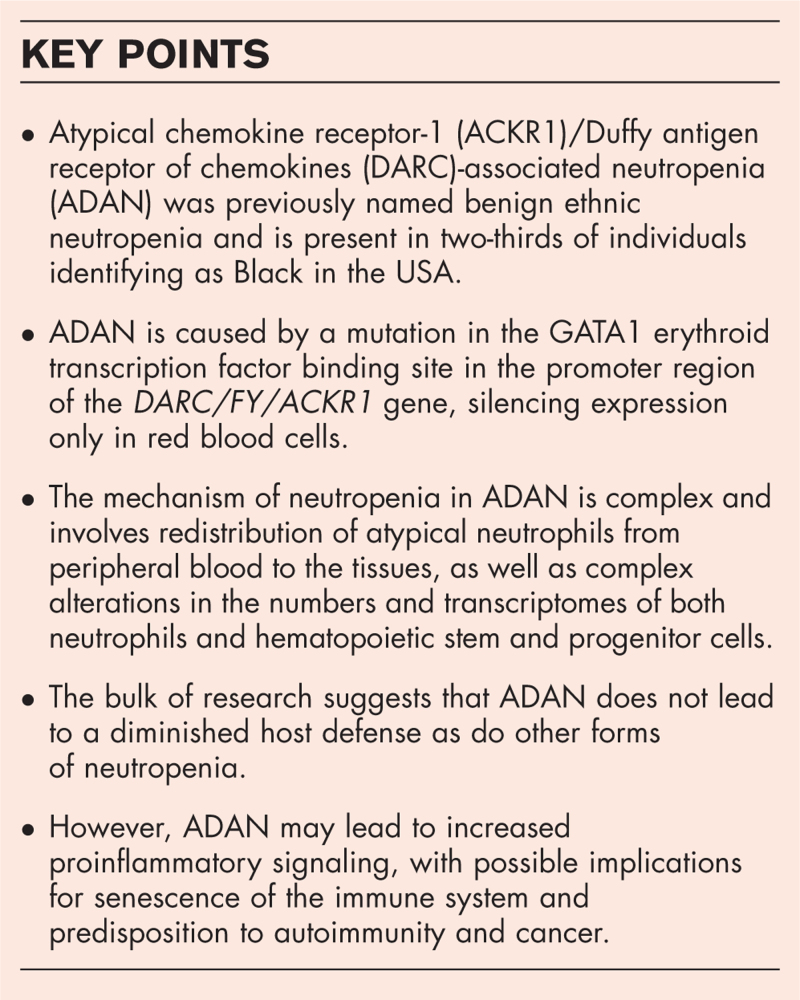
Atypical chemokine receptor-1 (ACKR1)/Duffy antigen receptor of chemokines (DARC)-associated neutropenia (ADAN; OMIM 611862), previously named benign ethnic neutropenia, and present in two-thirds of individuals identifying as Black in the USA, is associated with mild to moderate decreases in peripheral neutrophil counts that nevertheless do not lead to increased infections. Consequently, recent initiatives have sought to establish normal neutrophil count reference ranges for ADAN, considering it a normal variant rather than a clinical disorder requiring medical intervention.
Recent findings
A limited number of studies elucidating the mechanism of neutropenia in ADAN has suggested that neutrophils may redistribute from peripheral blood to the tissues including the spleen: this might explain why ADAN is not associated with increased risks of infection since the total number of neutrophils in the body remains normal. In this review, we critically examine the research underlying the molecular basis of ADAN.
Summary
Insights into the biology of neutrophils and their trafficking may inform the clinical interpretation of neutropenia in ADAN. The bulk of research suggests that ADAN does not lead to a diminished host defense as do other forms of neutropenia. However, ADAN may lead to increased proinflammatory signaling, with possible implications for senescence of the immune system and predisposition to autoimmunity and cancer.
Keywords: atypical chemokine receptor-1, atypical chemokine receptor-1 Duffy antigen receptor of chemokines-associated neutropenia, Duffy antigen receptor of chemokines, Duffy-null, neutropenia
INTRODUCTION
Duffy-null associated neutrophil count
Neutropenia has historically been defined as an absolute neutrophil count (ANC) of <1500 cells/μl in adults and associated with an increased risk of infection that correlates with its severity. The prevalence of neutropenia varies by the population studied, being rare in Europe and North America but more common in Africa and the Arabian Peninsula, suggesting a genetic influence. Over time, the neutropenia seen in individuals of African or Middle Eastern descent was recognized as not increasing the risk of infection or hematologic malignancy and was thus termed benign ethnic neutropenia (BEN). Subsequently, the molecular basis for BEN was linked to a variation in the atypical chemokine receptor-1 [ACKR1 or Duffy antigen receptor of chemokines (DARC)] gene, and BEN was renamed ACKR1/DARC-associated neutropenia (ADAN). A limited number of studies elucidating the mechanism of neutropenia in ADAN has suggested that neutrophils may redistribute from peripheral blood to the tissues including the spleen: this would explain why ADAN is not associated with increased risks of infection since the total number of neutrophils in the body remains normal. In this review, we critically examine the research underlying the molecular basis of ADAN.
Box 1.
no caption available
MOLECULAR BASIS OF ATYPICAL CHEMOKINE RECEPTOR-1/DUFFY ANTIGEN RECEPTOR OF CHEMOKINES-ASSOCIATED NEUTROPENIA
The Duffy blood group system, also known as the FY antigen system, is comprised of DARC glycoprotein antigens on red blood cells (RBCs) [1]. The DARC protein is produced by the FY gene located on chromosome 1. There are two primary Duffy antigens, Fya and Fyb, determined by the alleles FYA and FYB, respectively. These antigens differ by a single amino acid due to a point mutation in the FY gene. The Duffy blood group is clinically significant in transfusion medicine, as mismatched blood transfusions can lead to hemolytic reactions [2].
The Duffy antigen DARC/FY, which later was re-designated ACKR1, is a nonsignaling chemokine receptor that is expressed on RBCs, endothelial cells, and Purkinje cells of the cerebellum. It has been found to act as a multispecific receptor, binding promiscuously to inflammatory chemokines of both the C–C and C–X–C families, including: CCL2/ MCP-1, CCL5/RANTES, CCL7, CCL11, CCL13, CCL14, CCL17, CXCL5/ ENA-78, CXCL6, IL8/CXCL8, CXCL11, CXCL1/ NAP-3/MSGA-α/KC/GRO-α, and CXCL7/NAP-2. ACKR1 structurally resembles a G-protein coupled receptor (GPCR) but does not couple to G proteins and therefore fails to induce the full spectrum of downstream intracellular signals that characterize GPCRs. It is often considered as an interceptor (internalizing receptor) or chemokine-scavenging receptor or chemokine decoy receptor. It can control chemokine levels and localization via high-affinity chemokine binding, resulting in chemokine sequestration, degradation, or transcytosis. ACKR1 may also affect the function of other receptors indirectly. For instance, ACKR1 has been shown to exist as a constitutive homo-oligomer but also hetero-oligomerizes with the CC chemokine receptor CCR5. The formation of this heterodimer impairs chemotaxis and calcium flux through CCR5, although CCR5 is not internalized in response [3,4]. ACKR1 also acts as the receptor for the human malarial parasites Plasmodium vivax (P. vivax) and Plasmodium knowlesi (P. knowlesi), and thus may modulate the process of RBC invasion in malaria: Duffy null individuals are resistant to infection by P. vivax.
The Duffy null phenotype or Fy(a-b-) leads to the absence of Duffy antigens on red blood cells. The genetic locus related to the Duffy null phenotype was mapped to a polymorphism (SNP rs2814778 at chromosome 1q23.2) [5]. This results in a mutation in the GATA1 erythroid transcription factor binding site in the promoter region of the DARC/FY/ACKR1 gene, silencing expression only in red blood cells. The erythrocyte silent phenotype, FyBES is prevalent in Africa, parts of the Arabian Peninsula, and descendants from these regions. In the United States, approximately two in three individuals identifying as Black have the Duffy-null phenotype. The median ANC in Duffy-null individuals is significantly lower than in Duffy nonnull individuals, with approximately one-fourth of Duffy-null individuals having an ANC of <2000 cells/μl [6]. This results in a clinically lower ANC compared to the commonly used reference population established from individuals of Asian or European descent, of whom nearly 100% are Duffy nonnull [7]. Individuals with the Duffy null phenotype have no increased risk of infection but are often incorrectly labeled as having neutropenia. This can result in unnecessary, expensive, and invasive testing; delayed or discontinued chemotherapy or other critical medications; exclusion from clinical trials, and other negative consequences. Because of this, it is now being proposed by the American Society of Clinical Pathology: ‘Don’t perform an extensive work-up in otherwise healthy neutropenic patients of African or Middle Eastern ancestry prior to Duffy-null phenotype testing.’ There are also ongoing efforts to establish normal ANC reference ranges for Duffy-null individuals. One recent single institution study of 120 Duffy-null adults indicated a normal ANC reference range between 1210 and 5390 cells/μl [8▪]. Although this and other studies have reported similar ANC ranges, a smaller Swedish study of 66 adults found 9% with very low ANC values between 100 and 490 cells/μl [9▪]. Also, children may have lower ANC values (less than 500 cells/μl) according to anecdotal reports [10].
The mechanism leading to neutropenia in Duffy null individuals is a topic of investigation. ACKR1's primary function is regulation of chemokine bioavailability. When expressed in erythrocytes, it serves as a blood reservoir of cognate chemokines but also as a chemokine sink, buffering potential surges in plasma chemokine levels. Therefore, ACKR1 effectively sustains homeostatic levels of circulating chemokines and modulates chemokine gradients between tissues and the blood to mediate the influx of neutrophils and monocytes from blood vessels into tissues during immune responses. Due to the known role of ACKR1 in chemokine regulation, investigators have proposed that the Duffy null phenotype may lead to neutropenia by altering the concentrations and distribution of chemokines that regulate neutrophil production or migration.
A 2017 study suggested that neutropenia in Duffy-null individuals was due to ACKR1 deficiency in nucleated erythroid cells (NECs) in the bone marrow. ACKR1 was found to be crucial for interaction between hematopoietic stem and progenitor cells (HSPCs) and NECs, which was essential for hematopoiesis and normal neutrophil maturation. Disruption of ACKR1 in NECs impaired this interaction, leading to the production of atypical neutrophils that redistributed to the spleen, thereby causing neutropenia [11]. This was associated with altered numbers and decreased proliferation of HSPCs, shifts in proportions of individual HSPC subpopulations, changes in HSPC transcriptomes and altered expression of their functional surface molecules. The exact mechanism of ACKR1-mediated interactions between NECs and HSPCs is yet to be fully understood [12], and several other studies have explored related mechanisms. A recent study revealed that dimeric CXCL12/SDF-1 bound to the extracellular N-terminus of ACKR1 with low nanomolar affinity and was internalized along with ACKR1 [13]. The binding of CXCL12 to ACKR1 was contingent on ACKR1's conformation, which underwent gradual alterations throughout erythroid development, leading to a lack of CXCL12 binding in mature erythrocytes. Notably, the binding of CXCL12 to erythrocytes could be triggered by pretreatment with interleukin (IL)-8 or antibodies that targeted specific epitopes on ACKR1 [14]. Given the key role of CXCL12 in hematopoiesis and granulopoiesis [15–18], the interaction between CXCL12 and ACKR1 adds another layer of regulation to neutrophil production.
Individuals with atypical chemokine receptor-1 disruption in all cell types
Most Duffy-null individuals carry the FyBES allele, and the gene is still transcribed in nonerythroid cells. However, individuals with ACKR1 disruption in all cell types have also been identified [1,19], although rare. The few documented cases of the Fy(a−b−) phenotype in Europeans and Asians arise from mutations in the coding region of the FY∗A or FY∗B allele, which often lead to a premature stop codon. These mutations, when present in the homozygous state, prevent Duffy antigen expression on any cell in the body and thus are true Duffy-null phenotypes. Consequently, these individuals are at risk of being allo-immunized when exposed to red blood cells expressing Fy antigens.
Significant neutropenia was not reported in individuals with ACKR1 disruption in all cell types. In fact, in the mouse model, neutropenia developed only when ACKR1 was absent in the BM but was expressed in the venular endothelial cells. Mechanistically, this was consistent with the known contribution of ACKR1 on endothelial cells to optimize chemokine-driven neutrophil egress into tissues. When ACKR1 is expressed in endothelial cells, like other silent receptors, it internalized chemokines but did not effectively scavenge them. Instead, ACKR1 mediated a process called chemokine transcytosis, which led to apical retention of intact chemokines and more extravasation across endothelial monolayers [20]. Recently, Girbl et al.[21] reported that CXCL2 produced by transmigrating neutrophils were retained at endothelial cell junctions by ACKR1, providing a paradigm of self-guided unidirectional luminal-to-abluminal migration. Thus, in individuals with ACKR1 disruption in all cell types, including endothelial cells, NECs and RBCs, ANC remained high since neutrophils failed to efficiently migrate to peripheral tissues when ACKR1 was disrupted in endothelial cells. This also explains why mice with a global ACKR1 deficiency, despite having altered neutrophils, were not neutropenic [11].
CLINICAL CONSEQUENCES OF ATYPICAL CHEMOKINE RECEPTOR-1/DUFFY ANTIGEN RECEPTOR OF CHEMOKINES-ASSOCIATED NEUTROPENIA
The complex (and sometimes contradictory) effects of ADAN on host defense
ACKR1 is the receptor for the human malarial parasites P. vivax and P. knowlesi. Individuals with the Duffy-null genotype are resistant to malaria infection. This has led to the belief that the Duffy-null phenotype is a genetic adaptation to an environmental challenge (malaria). The low level of ACKR1 expression is associated with low susceptibility and resistance to infection. Red cells of heterozygotes for the silent allele bind substantially less P. vivax than those of individuals with two active FY alleles. Thus, even Duffy-negative heterozygosity offers significant protection, providing a selective advantage in regions where P. vivax is endemic. Of note, recent studies indicated that P. vivax infections can still occur in individuals with ADAN. It turns out that Fy protein is expressed in early erythropoietic stages in these individuals, enabling P. vivax invasion [22].
HIV-1 can also attach to RBCs via ACKR1, affecting trans-infection of target cells [23]. Moreover, ACKR1 expressed on RBCs influences plasma levels of HIV-1-suppressive and proinflammatory chemokines, such as CCL5/RANTES. ADAN has been implicated in higher rates of HIV-1 acquisition, but also associated with a survival advantage in leukopenic HIV-infected persons [24]. Low neutrophil counts in African mothers and newborns were associated with increased susceptibility to perinatal HIV infection [25]. However, neutrophil effector processes were not appreciably impaired in Duffy-null individuals regardless of HIV status [26].
In addition, neutrophils can sometimes also suppress the immune system (such as for myeloid-derived suppressor cells in cancer patients). Consequently, ADAN could theoretically impact other immune cell types, such as natural killer (NK) and CD8+ T cells, leading to altered phenotype, functionality, or homeostatic activity. However, the Duffy-null phenotype was not associated with significant dysfunction of either NK cells or cytotoxic T cells [27].
Recent studies also established a role for ACKR1 in SARS-CoV-2 pathogenesis. Exceptionally low COVID-19 mortality rates were observed in sub-Saharan Africa, a unique geographic region where ADAN is prevalent [28]. ACKR1 was identified as the most highly upregulated chemokine receptor in infected lungs, localizing to endothelial cells of veins and arterioles. Mice with complete absence of Ackr1 were shown to resist lethal SARS-CoV-2 challenges, although this preliminary study did not determine if the protection against SARS-CoV-2 was due to absence of Ackr1 on RBCs or (perhaps more likely) on endothelial cells [29].
Neutropenia and host defense in atypical chemokine receptor-1/Duffy antigen receptor of chemokines-associated neutropenia
Despite severe neutropenia typically impairing host defense, it has been reported that individuals with Duffy- null neutropenia do not exhibit compromised defense [30]. This may be attributed to several potential mechanisms. First, neutrophils in ACKR1-deficient mice were reported to be produced aberrantly, potentially having enhanced pathogen-killing abilities. This increased functionality might compensate for the reduced neutrophil count. Also, both Duffy-null individuals and ACKR1-deficient mice have been described to express a distinct profile of neutrophil effector molecules [4]. Second, ACKR1's cytokine modulation ability during infection can regulate the inflammatory state. The absence of ACKR1 on RBCs may alter cytokine concentrations/responses in the infected host, potentially leading to improved host defense. Finally, the Duffy-null genotype is known to confer an evolutionary advantage through natural selection by offering protection against malaria. Selective pressure exerted by a broad range of other infectious agents may have also contributed to the selection and fixation of polymorphisms in genes beyond ACKR1 within the Duffy-null population. In this scenario, selected pro-inflammatory mechanisms could emerge in any innate or adaptive immune component, so long as they enhanced host defense.
CONCLUSION
In conclusion, the infection response in Duffy-null individuals can be complex and context-dependent. The evidence supporting the notion that Duffy-null neutropenic individuals have normal responses to infection needs careful evaluation and confirmation across various infection and disease conditions. This remains a highly relevant question, as the decision to lower diagnostic standards for Duffy-null neutropenic individuals hinges on a significant assumption. This assumption, thus far backed by current clinical observations, is that neutropenia in these individuals does not increase their risk of infection.
Proinflammatory signaling and hematopoietic stem cell dynamics in atypical chemokine receptor-1/Duffy antigen receptor of chemokines-associated neutropenia
Despite the lack of association with increased infections, ADAN may not be benign in all contexts. In addition to having complex effects on HIV acquisition rate, as described above, ADAN individuals have been reported to have increased immunoglobulin IgE levels and susceptibility to asthma [31], higher rates of morbidity and mortality after acute lung injury [32], and a higher incidence of triple-negative breast cancer (so-called because of low expression levels of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2) [33]. ACKR1 is a key regulator that binds chemokines involved in inflammatory responses, and its absence in erythrocytes and NECs was shown to provoke a proinflammatory profile that may favor autoimmune reactions or alterations in the tumor microenvironment [34]. Palmblad et al. found that 28 of 64 ADAN individuals tested had autoantibodies to neutrophils (as compared with less than 1% of control individuals) [9▪]. Excessive proinflammatory chemokine signaling in ADAN may also contribute to the senescence-associated secretory phenotype or SASP, believed to play a role in senescence of the immune system during aging [34]. Redistribution of peripheral blood neutrophils to tissues such as the spleen may also have complex effects on hematopoiesis or stem cell clonal dynamics, which are incompletely understood at present.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Hoher G, Fiegenbaum M, Almeida S. Molecular basis of the Duffy blood group system. Blood Transfus 2018; 16:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutbush M, Mollison PL, Parkin DM. A new human blood group. Nature 1950; 165:188–189. [Google Scholar]
- 3.Chakera A, Seeber RM, John AE, et al. The Duffy antigen/receptor for chemokines exists in an oligomeric form in living cells and functionally antagonizes CCR5 signaling through hetero-oligomerization. Mol Pharmacol 2008; 73:1362–1370. [DOI] [PubMed] [Google Scholar]
- 4.Crawford KS, Volkman BF. Prospects for targeting ACKR1 in cancer and other diseases. Front Immunol 2023; 14:1111960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reich D, Nalls MA, Kao WHL, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet 2009; 5:e1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merz LE, Story CM, Osei MA, et al. Absolute neutrophil count by Duffy status among healthy Black and African American adults. Blood Adv 2023; 7:317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagheri M, Chung CP, Dickson AL, et al. White blood cell ranges and frequency of neutropenia by Duffy genotype status. Blood Adv 2023; 7:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪.Merz LE, Osei MA, Story CM, et al. Development of Duffy null-specific absolute neutrophil count reference ranges. JAMA 2023; 329:2088–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]; First development of a hospital system-wide reference range for absolute neutrophil count in ADAN individuals.
- 9▪.Palmblad J, Sohlberg E, Nilsson CC, et al. Clinical and immunological features in ACKR1/DARC-associated neutropenia. Blood Adv 2024; 8:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent study suggesting that the absolute neutrophil count in ADAN can be below 500 cells/μL and that many ADAN individuals have autoantibodies against neutrophils.
- 10.Parisi X, Bledsoe JR. Discerning clinicopathological features of congenital neutropenia syndromes: an approach to diagnostically challenging differential diagnoses. J Clin Pathol 2024; 0:1–19. [DOI] [PubMed] [Google Scholar]
- 11.Duchene J, Novitzky-Basso I, Thiriot A, et al. Atypical chemokine receptor 1 on nucleated erythroid cells regulates hematopoiesis. Nature Immunol 2017; 18:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Permanyer M, Bošnjak B, Förster R. Dual role for atypical chemokine receptor 1 in myeloid cell hematopoiesis and distribution. Cell Mol Immunol 2018; 15:399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutjahr JC, Crawford KS, Jensen DR, et al. The dimeric form of CXCL12 binds to atypical chemokine receptor 1. Sci Signal 2021; 14:eabc9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klei TRL, Aglialoro F, Mul FPJ, et al. Differential interaction between DARC and SDF-1 on erythrocytes and their precursors. Sci Rep 2019; 9:16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehrpouri M. The contributory roles of the CXCL12/CXCR4/CXCR7 axis in normal and malignant hematopoiesis: a possible therapeutic target in hematologic malignancies. Eur J Pharmacol 2022; 920:174831. [DOI] [PubMed] [Google Scholar]
- 16.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25:977–988. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Dépond M, He L, et al. CXCR4/CXCL12 axis counteracts hematopoietic stem cell exhaustion through selective protection against oxidative stress. Sci Rep 2016; 6:37827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karpova D, Bonig H. Concise review: CXCR4/CXCL12 signaling in immature hematopoiesis--lessons from pharmacological and genetic models. Stem Cells 2015; 33:2391–2399. [DOI] [PubMed] [Google Scholar]
- 19.Rios M, Chaudhuri A, Mallinson G, et al. New genotypes in Fy(a-b-) individuals: nonsense mutations (Trp to stop) in the coding sequence of either FY A or FY B. Br J Haematol 2000; 108:448–454. [DOI] [PubMed] [Google Scholar]
- 20.Pruenster M, Mudde L, Bombosi P, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol 2009; 10:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girbl T, Lenn T, Perez L, et al. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity 2018; 49:1062–1076. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dechavanne C, Dechavanne S, Bosch J, et al. Duffy antigen is expressed during erythropoiesis in Duffy-negative individuals. Cell Host Microbe 2023; 31:2093–2106. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He W, Neil S, Kulkarni H, et al. Duffy antigen receptor for chemokines mediates trans-infection of HIV-1 from red blood cells to target cells and affects HIV-AIDS susceptibility. Cell Host Microbe 2008; 4:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni H, Marconi VC, He W, et al. The Duffy-null state is associated with a survival advantage in leukopenic HIV-infected persons of African ancestry. Blood 2009; 114:2783–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kourtis AP, Hudgens MG, Kayira D. Neutrophil count in African mothers and newborns and HIV transmission risk. N Engl J Med 2012; 367:2260–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naidoo KK, Ngubane A, Gaza P, et al. Neutrophil effector functions are not impaired in Duffy antigen receptor for chemokines (DARC)-null Black South Africans. Front Immunol 2019; 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naidoo KK, Shangase ZB, Rashid T, et al. The DARC-null trait is associated with moderate modulation of NK cell profiles and unaltered cytolytic T cell profiles in black South Africans. PLoS One 2020; 15:e0242448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabore JW, Karamagi HC, Kipruto HK, et al. COVID-19 in the 47 countries of the WHO African region: a modelling analysis of past trends and future patterns. Lancet Global Health 2022; 10:e1099–e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majumdar S, Weaver JD, Pontejo SM, et al. <em>Atypical Chemokine Receptor 1 (Ackr1)</em>-deficient mice resist lethal SARS-CoV-2 challenge. bioRxiv 2023; doi:10.1101/2023.06.05.543759. [Google Scholar]
- 30.Legge SE, Christensen RH, Petersen L, et al. The Duffy-null genotype and risk of infection. Hum Mol Genet 2020; 29:3341–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vergara C, Tsai YJ, Grant AV, et al. Gene encoding Duffy antigen/receptor for chemokines is associated with asthma and IgE in three populations. Am J Respir Crit Care Med 2008; 178:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kangelaris KN, Sapru A, Calfee CS, et al. The association between a Darc gene polymorphism and clinical outcomes in African American patients with acute lung injury. Chest 2012; 141:1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman LA, Jenkins B, Chen Y, et al. Hereditary susceptibility for triple negative breast cancer associated with western Sub-Saharan African ancestry: results from an International Surgical Breast Cancer COLLABORATIVE. Ann Surg 2019; 270:484–492. [DOI] [PubMed] [Google Scholar]
- 34.Jinna N, Rida P, Su T, et al. The DARC side of inflamm-aging: Duffy antigen receptor for chemokines (DARC/ACKR1) as a potential biomarker of aging, immunosenescence, and breast oncogenesis among high-risk subpopulations. Cells 2022; 11:3818. [DOI] [PMC free article] [PubMed] [Google Scholar]