ABSTRACT
Anaemia is a common complication of chronic kidney disease (CKD) and is associated with poor long-term outcomes and quality of life. The use of supplemental iron, erythropoiesis-stimulating agents (ESAs) and blood transfusions has been the mainstay for treatment of anaemia in CKD for more than 3 decades. Despite available treatments, CKD patients with anaemia are undertreated and moderate–severe anaemia remains prevalent in the CKD population. Anaemia has consistently been associated with greater mortality, hospitalization, cardiovascular events and CKD progression in CKD patients, and the risk increases with anaemia severity.
Hypoxia-inducible factor (HIF) prolyl hydroxylase (PH) inhibitors have a novel mechanism of action by mimicking the body's response to hypoxia and have emerged as an alternative to ESAs for treatment of anaemia in CKD. Their efficacy in correcting and maintaining haemoglobin has been demonstrated in >30 phase 3 clinical trials. Additionally, HIF activation results in various pleiotropic effects beyond erythropoiesis, with cholesterol reduction and improved iron homeostasis and potential anti-inflammatory effects. The long-term safety of these agents, particularly with respect to cardiovascular and thromboembolic events, and their possible effect on tumour growth needs to be fully elucidated. This article presents in detail the effects of HIF-PH inhibitors, describes their mechanisms of action and pharmacologic properties and discusses their place in the treatment of anaemia in CKD according to the available evidence.
Keywords: anaemia, chronic kidney disease, erythropoiesis-stimulating agents, erythropoietin, hypoxia-inducible factor prolyl hydroxylase inhibitors
EPIDEMIOLOGY AND OUTCOMES OF ANAEMIA IN THE CHRONIC KIDNEY DISEASE (CKD) POPULATION
Anaemia is a clinical hallmark of CKD and its prevalence and severity increases with progression of CKD [1–3]. It is twice as prevalent in patients with CKD compared with the general population [1] and is a substantial healthcare burden associated with increased healthcare resource utilization [4–6].
Patients with CKD who develop anaemia have an increased risk of adverse health outcomes, including major cardiovascular events, hospitalization, progression to kidney failure and mortality [7–11]. Despite the prevalence of anaemia in CKD and its significant consequences for patient outcomes, it is often undertreated worldwide [1, 3] and typically less than half of patients with anaemia receive conventional anaemia medication within 1 year of nephrology follow-up [12].
Following the establishment of the role of the hypoxia-inducible factor (HIF) pathway in the physiological response to hypoxia, HIF prolyl hydroxylase (HIF-PH) enzyme inhibitors have been developed as an alternative to erythropoiesis-stimulating agents (ESAs) for the treatment of anaemia in CKD. Although they have been approved and utilized in many countries worldwide, some of these treatments have only recently been licensed by the European Medicines Agency (EMA), Medicines and Healthcare products Regulatory Agency (MHRA) and the US Food and Drug Administration (FDA).
This review outlines the mechanism of action of HIF-PH inhibitors (HIF-PHis) and considers their role in the management of anaemia in CKD by presenting the evidence from several phase 3 randomized clinical trials.
TREATMENT OF ANAEMIA IN CKD IN THE PRE-HIF-PHI ERA
The key facets of managing anaemia in CKD include iron supplementation, recombinant human erythropoietin (EPO) and its analogues, referred to as ESAs, and red blood cell (RBC) transfusion [13, 14]. An overview of the evolution of anaemia management in CKD is shown in Supplementary Fig. S1.
Iron
Iron is essential for erythropoiesis, intracellular oxygen transport and oxidative reactions needed for metabolic processes. Absolute and functional iron deficiency are common in CKD, driven by decreased dietary intake, decreased intestinal absorption, increased iron losses and altered iron homeostasis, including elevated levels of the regulator protein hepcidin due to chronic inflammation and poor kidney clearance [15]. Addressing iron deficiency with supplementation is the first-line therapy for anaemia in CKD [14] and it decreases the exposure to ESA therapy and RBC transfusion requirements [16, 17].
The Ferinject assessment in patients with Iron deficiency anaemia and Non-Dialysis-dependent Chronic Kidney Disease (FIND-CKD) study indicated that the use of intravenous (IV) ferric carboxymaltose to target higher ferritin levels (400–600 versus 100-200 µg/l) was more efficacious for increasing haemoglobin in non-dialysis CKD patients and delayed the time to initiation of other anaemia management (blood transfusion, ESA, other iron therapy), with no safety concerns in terms of cardiovascular events or infections [18]. The Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) trial demonstrated that proactive high-dose IV iron sucrose supplementation in incident haemodialysis (HD) patients [held if ferritin >700 µg/l or transferrin saturation (TSAT) ≥40%] lowered the composite risk of all-cause death, non-fatal myocardial infarction (MI), non-fatal stroke and heart failure (HF) hospitalization compared with a reactive low-dose regimen [19] without increasing the risk of infections.
ESAs
Erythropoietin derivatives have been studied since 1989 and transformed the treatment of anaemia in CKD by effectively increasing haemoglobin levels and avoiding regular blood transfusions [20].
Landmark clinical trials in anaemia showed no improvements in clinical outcomes with normalization of haemoglobin with ESAs in CKD patients [21–24]. The Normal Hematocrit Cardiac Trial (NHCT) in HD patients with cardiovascular disease (CVD) was stopped early for futility after results of the interim analysis were nearing the statistical boundary of a higher mortality rate in the normal-haematocrit group [21]. In patients with non-dialysis CKD, correction of haemoglobin to levels >13 g/dl was associated with an increased risk of cardiovascular events in the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial [22] and no cardiovascular benefit in the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta (CREATE) [23] and Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT) [24] trials. The increased cardiovascular events may be related to ESA dosing rather than the haemoglobin level per se [25–27] or to fluctuations in haemoglobin level [28].
MECHANISM OF ACTION OF HIF-PHI
The HIF pathway is an exquisite oxygen-sensing mechanism enabling adaptation according to the oxygen content by controlling the transcription of >1000 hypoxia-responsive genes [29]. Among its functions, HIF coordinates the response to hypoxia by stimulating erythropoietin production in the kidneys and liver and favouring intestinal iron absorption and availability[30].
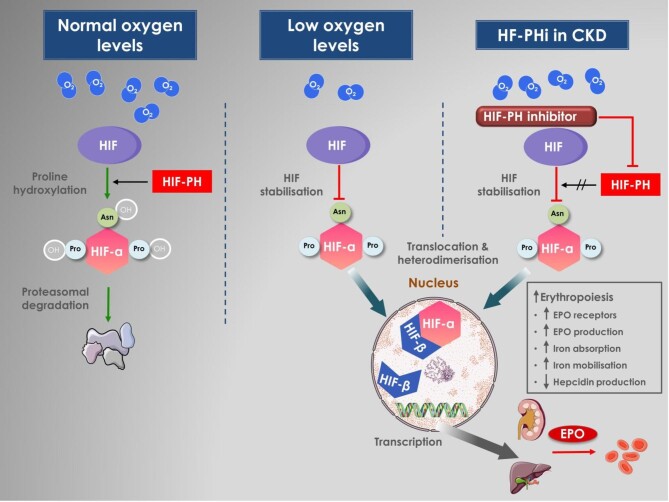
HIF is a heterodimeric DNA-binding complex composed of two basic helix–loop–helix proteins: one hypoxia-inducible α-subunit (HIF-1α, HIF-2α, HIF-3α) and the constitutive HIF-β [31]. While HIF-α subunits are highly inducible by hypoxia, the HIF-β subunit is a non-oxygen-responsive nuclear protein with other roles in transcription processes [32]. The HIF-2α subunit has been recognized as the primary mediator of erythropoiesis [33]. HIF is regulated by a family of prolyl hydroxylase domain (PHD) enzymes, of which there are three isoforms (PHD1, PHD2, and PHD3) that serve as cellular oxygen sensors. In the presence of oxygen and/or iron, PHD enzymes hydroxylate prolines in HIF-α, thereby targeting it for proteasomal degradation [30, 34]. Under hypoxic conditions or iron deficiency, PHD enzyme activity is supressed and the HIF-α escapes proteasomal degradation and accumulates [35]. It then translocates to the nucleus and dimerizes with HIF-β, forming the HIF-α/β heterodimer, which binds to the hypoxia response elements of target genes inducing, among other responses, erythropoiesis [36] (Fig. 1).
Figure 1:
HIF pathway in the presence of normal oxygen levels, under hypoxic conditions and after pharmacological inhibition of the prolyl hydroxylases. In conditions of normal oxygen tension, HIF-α is hydroxylated by the oxygen-sensitive HIF-prolyl hydroxylases (HIF-PHs) and undergoes rapid proteasomal degradation. Factor inhibiting HIF (FIH) is an asparaginyl (Asn) hydroxylase enzyme that regulates the transcriptional activity of HIF. Under hypoxic conditions, HIF-PH is inactive and cannot hydroxylate HIF-α, which then accumulates, translocates to the nucleus and forms heterodimers with the HIF-β, resulting in an active HIF complex. The HIF complex activates transcription of multiple genes promoting erythropoiesis via stimulation of endogenous erythropoietin production and regulators of iron metabolism.
HIFs are the main regulators of EPO production and iron availability via the following mechanisms: upregulation of EPO receptors and endogenous EPO production; an increase in intestinal iron absorption; an increase in iron uptake by proerythrocytes and promotion of erythrocyte maturation; and inhibition of hepcidin production in the liver [37, 38]. Prolyl hydroxylation can be pharmacologically inhibited by HIF-PHi—also referred to as HIF stabilizers—thereby stimulating these effects and enhancing erythropoiesis [39, 40]. The clinically available compounds have various degrees of inhibition of PHD isoforms in vitro, but to some degree this depends on the assay that is used [33, 41, 42]. They all appear to be potent inhibitors of PHD1–3, although PHD2 is considered the most important isoform from a physiological perspective [33, 42]. In a direct comparison of cellular assays, vadadustat was less potent at inhibiting PHD2 compared with roxadustat, daprodustat and molidustat. Differences in the effect on HIF-1α and HIF-2α are also apparent between the compounds, with vadadustat having a preference for HIF-2α over HIF-1α and roxadustat demonstrating the highest efficacy on HIF stabilization [42].
The peritubular interstitial EPO-producing cells are predominantly located in a zone of the kidney with relative hypoxia [43], where small decreases in blood oxygen stimulate upregulation of the HIF-2α leading to increased transcription of the EPO genes and increased levels of circulating EPO. There is evidence that HIF is activated spontaneously in HD patients living 1300–1400 m above sea level, as they have higher haemoglobin levels despite requiring lower ESA and iron doses [44]. Similarly, in patients with CKD, the risk of anaemia was lower at higher altitude [45].
A major class of genes moderated by HIFs include those involved in iron handling and metabolism. The impact of the HIF pathway on iron homeostasis is modulated by HIF-2α stimulation of iron absorption in the duodenum and by suppression of hepcidin [43]. Hepcidin reduces dietary iron absorption and blocks the release of stored iron from macrophages and the liver by decreasing the expression of ferroportin, an iron exporter, leading to reduced circulating iron levels [46].
HIF activation results in a broad physiologic response with various pleiotropic effects (beneficial, neutral or harmful). Many of these effects are context dependent and can go in opposite directions depending on the duration and severity of the hypoxic state. In animal studies, pharmacological inhibition of HIF-PH had a renoprotective effect from ischaemic injury caused by AKI [47, 48], although other experimental studies showed increased fibrosis following HIF activation [49]. Many of the genes involved in angiogenesis, such as vascular endothelial growth factors (VEGFs), are directly induced by HIF-1α [50]. The molecular mechanisms underlying cancer metabolism are significantly influenced by HIF-1α [36], however, studies on gene expression have so far failed to establish the impact of HIF-1α on tumour angiogenesis and growth [51, 52]. In contrast, under certain experimental conditions, PHD inhibition reconstituted tumour vessels and normalized the tumour microenvironment, which are essential for response to chemotherapy [53].
THERAPEUTIC USE OF HIF-PHI IN THE MANAGEMENT OF ANAEMIA IN NON-DIALYSIS-DEPENDENT CKD (NDD-CKD)
The search strategy used to identify phase 3 trials of HIF-PHi in adult CKD patients with anaemia is described in the supplementary material and Supplementary Table S1. All available phase 3 trials of HIF-PHi versus placebo in NDD-CKD are summarised in Table 1 and the trials of HIF-PHi versus ESAs are summarised in Table 2.
Table 1:
NDD-CKD: HIF-PHi versus placebo
| Study | Comparator | Patients, n | CKD stage | Treatment period (weeks) | Target Hb (g/dl) | Efficacy endpoints | Safety endpoints |
|---|---|---|---|---|---|---|---|
| Roxadustat (vs placebo) | |||||||
| ALPS [56] (Europe) | Placebo | 594 | 3–5 | 52–104 | 10–12 | Mean Hb change at 28–52 weeks: 1.99 vs 0.30 g/dl Patients with Hb response at 24 weeks: 79.2% vs 9.9% |
Hypertension: 22.3% vs 13.8% Nausea: 9.5% vs 3.0% Diarrhoea: 8.4% vs 3.4% |
| ANDES [54] (global) | Placebo | 922 | 3–5 | 52–208 | 10–12 | Mean Hb change at 28–52 weeks: 2.00 vs 0.16 g/dl Patients with Hb response at 24 weeks: 86.0% vs 6.6% Rescue therapy at 52 weeks: 8.9% vs 28.9% RBC transfusion at 52 weeks: 5.6% vs 15.4% IV iron use at 52 weeks: 2.5% vs 4.9% ESA rescue at 52 weeks: 2.1% vs 6.7% |
Hypertension: 15.5% vs 8.9% Hyperkalaemia: 18.2% vs 13.4% Constipation: 17.2% vs 11.1% |
| OLYMPUS [55] (global) | Placebo | 2782 | 3–5 | 52–208 weeks | 10–12 | Mean Hb change at 28–52 weeks: 1.75 vs 0.40 g/dl Patients with Hb response at 24 weeks: 77.0% vs 8.5% Rescue therapy: 18.4% vs 41.7% RBC transfusion: 12.7% vs 23.3% IV iron use: 4.3% vs 7.9% ESA rescue: 4.7% vs 23.6% |
UTI: 12.8% vs 8.0% Hypertension: 11.5% vs 9.1% Hyperkalaemia: 8.5% vs 6.9% |
| Chen et al. [57] (China) | Placebo | 154 | 3–5 | 8 weeks (followed by an 18-week open-label period) | ≥10 | Mean Hb change at 7–9 weeks: 1.90 vs −0.40 g/dl Patients with mean Hb ≥10 g/dl at 7–9 weeks: 67% vs 6% Rescue therapy: 3% vs 12% Mean hepcidin change at 9 weeks: −56.1 vs −15.1 ng/ml |
Hyperkalaemia: 16% vs 8% Metabolic acidosis: 12% vs 2% |
| Daprodustat (vs placebo) | |||||||
| ASCEND-NHQ [58] (global) | Placebo | 614 | 3–5 | 28 weeks | 11–12 | Mean Hb change at 24–28 weeks: 1.58 vs 0.19 g/dl Patients with Hb increase ≥1 g/dl at 28 weeks: 77% vs 18% Rescue therapy: <1% vs 10% Change in the SF-36 vitality (fatigue) score at 28 weeks: 7.3 vs 1.9 points |
Hypertension: 7% vs 5% Retinal disorder: <1% vs 3.0% |
Table 2:
NDD-CKD: HIF-PHi versus ESAs
| Study | Comparator | Patients, n | CKD stage | Treatment period (weeks) | Target Hb (g/dl) | Efficacy endpoints | Safety endpoints |
|---|---|---|---|---|---|---|---|
| Roxadustat (vs ESAs) | |||||||
| DOLOMITES [60] (Europe) | Darbepoetin alfa | 616 | 3–5 | 104 | 10–12 | Patients with Hb response at 24 weeks: 89.5% vs 78.0% Mean monthly IV iron use at 1–36 weeks: 34.7 vs 69.6 mg Time to first use of IV iron at 1–36 weeks: HR 0.46 (95% CI 0.26–0.78) |
MACE: 11.8% vs 14.0% [HR 0.81 (95% CI 0.52–1.25)] MACE+: 16.7% vs 18.1% [HR 0.90 (95% CI 0.61–1.32)] VTE: 2.5% vs 0.7% [HR 3.63 (95% CI 0.76–17.20)] |
| Akizawa et al. [61] (Japan) | Darbepoetin alfa | 334 | 2–5 | 24 | 10–12 | Mean Hb change at 18–24 weeks: 0.15 vs 0.22 g/dl Patients with target Hb at 18–24 weeks: 77.1% vs 85.5% |
Hypertension: 2.3% vs 3.8% Hyperkalaemia: 3.8% vs 3.8% Nasopharyngitis: 19.1% vs 26.0% Retinal haemorrhage: 31.4% vs 39.8% |
| Daprodustat (vs ESAs) | |||||||
| ASCEND-ND [62] (Global) | Darbepoetin alfa | 3872 | 3–5 | 52 | 10–11 | Mean Hb change at 28–52 weeks: 0.74 vs 0.66 g/dl Use of rescue therapy: 2.0% vs 3.3% RBC transfusion: 12.8% vs 3.5% |
MACE (ITT analysis): 19.5% vs 19.2% [HR 1.03 (95% CI 0.89–1.19)] MACE (on-treatment analysis): 14.1% vs 10.5% [HR 1.40 (95% CI 1.17–1.68)] Cancer-related death or tumour progression or recurrence: 3.7% vs 2.5% [RR 1.47 (95% CI 1.03–2.10)] Oesophageal or gastric erosions: 3.6% vs 2.1% [RR 1.70 (95% CI 1.16–2.49)] |
| Nangaku et al. [63] (Japan) |
Epoetin beta pegol | 299 | 3–5 | 52 | 11–13 | Mean Hb level at 40–52 weeks (ITT): 12.0 vs 11.9 g/dl Patients with target Hb at 40–52 weeks: 92% vs 92% [OR 1.01 (95% CI 0.33–3.04)] |
Hypertension: 3.0% vs 5.0% Hyperkalaemia: 8.0% vs 5.0% Nasopharyngitis: 33.0% vs 37.0% |
| Vadadustat (vs ESAs) | |||||||
| PRO2TECT [64] (global) | Darbepoetin alfa | 3476 | 3–5 | 52 | 10–11 (USA) 10–12 (non-USA) |
Mean Hb change at 24–36 weeks: 0.74 vs 0.66 g/dl RBC transfusion 24–36 weeks: 2.7% vs 2.2% (ESA untreated), 1.6% vs 1.2% (ESA treated) ESA rescue 24–36 weeks% vs 12.8% (ESA untreated), 5.0% vs 13.4% (ESA treated) |
MACE: 22.0% vs 19.9% [HR 1.17 (95% CI 1.01–1.36)] MACE+: 25.9% vs 24.5% [HR 1.11 (95% CI 0.97–1.27)] Hypertension: 17.7% vs 22.1% (ESA untreated), 14.4% vs 14.8% (ESA treated) Hyperkalaemia: 12.3% vs 15.6% (ESA untreated), 9.4% vs 9.9% (ESA treated) |
| Nangaku et al. [65] (Japan) | Darbepoetin alfa | 304 | 3–5 | 52 | 11–13 | Mean Hb level at 20–24 weeks: 11.7 vs 11.9 g/dl Patients with target Hb at 52 weeks: 71.4% vs 84.5% (ESA non-users), 79.2% vs 76.6% (ESA users) |
Adverse drug reaction (≥1): 13.2% vs 4.6% Hypertension: 1.3% vs 7.2% Nasopharyngitis: 24.5% vs 28.1% |
| Enarodustat (vs ESAs) | |||||||
| SYMPHONY-ND [67] (Japan) | Darbepoetin alfa | 216 | 3–5 | 24 | 10–12 | Mean Hb level at 20–24 weeks: 10.96 vs 10.87 g/dl Patients with target Hb at 24 weeks: 88.6% vs 87.9% |
Retinal disorders: 3.7% vs 0.9% Upper respiratory tract infection: 17.8% vs 22.9% Hypertension: 4.7% vs 4.6% |
| Molidustat vs ESAs | |||||||
| MIYABI ND-C [66] (Japan) | Darbepoetin alfa | 162 | 3–5 | 52 | 11–13 | Mean Hb level at 30–36 weeks: 11.28 vs 11.70 g/dl Mean Hb change at 30–36 weeks: 1.32 vs 1.69 g/dl Patients with target Hb at 30–36 weeks: 68.3% vs 85.0% Mean IV iron use: 2.89 vs 11.22 mg/week |
Serious TEAE: 17.1% vs 7.6% MACE: 7.3% vs 0.0% Hyperkalaemia: 12.2% vs 11.4% Nasopharyngitis: 31.7% vs 26.6% |
| MIYABI ND-M [68] (Japan) | Darbepoetin alfa | 164 | 3–5 | 52 | 11–13 | Mean Hb level at 30–36 weeks: 11.67 vs 11.53 g/dl Mean Hb change at 30–36 weeks: 0.36 vs 0.24 g/dl Patients with target Hb at 30–36 weeks: 72.0% vs 76.8% |
Serious TEAE: 32.9% vs 26.8% MACE: 3.7% vs 1.2% Hyperkalaemia: 2.4% vs 8.5% Hypertension: 2.4% vs 6.1% Diabetic retinopathy: 3.7% vs 1.2% |
| Desidustat (vs ESAs) | |||||||
| DREAM-ND [69] (India and Sri Lanka) | Darbepoetin alfa | 588 | 3–5 | 24 | 10–12 | Mean Hb change at 16–24 weeks: 1.95 vs 1.83 g/dl Mean Hb level at 16–24 weeks: 10.90 vs 10.77 g/dl Patients with Hb response at 24 weeks: 77.8% vs 68.5% Mean hepcidin change at 24 weeks: −12.0 vs 7.8 ng/ml |
Serious TEAE: 8.2% vs 6.1% Hypertension: 1.7% vs 5.8% |
Table 2 outlines details of phase 3 comparator trials with ESA therapy, including two roxadustat (one from Europe [60] and one from Japan [61]), two daprodustat (one global [62] and one from Japan [63]), two vadadustat (one global [64] and one from Japan [65]), two molidustat (Japan [66, 68]), one enarodustat (Japan [67]) and one desidustat (South Asia [69]).
Hb: haemoglobin; ITT: intention to treat; OR: odds ratio; MACE: major adverse cardiac event (composite of death, non-fatal MI and/or stroke); MACE+: expanded major adverse cardiac event (MACE plus hospitalization for either heart failure or unstable angina or MACE plus hospitalization for either HF or a thromboembolic event); TEAE: treatment emergent adverse event; vs: versus.
Haemoglobin correction and maintenance
In the placebo-comparator trials, roxadustat and daprodustat were superior in achieving and maintaining target haemoglobin levels for up to 4 years and minimized the requirements for rescue RBC transfusion or ESA therapy [54–59]. A pooled analysis of the ANDES (NCT01750190) [54], OLYMPUS (NCT02174627) [55] and ALPS (NCT01887600) [56] trials of roxadustat versus placebo in 4277 patients showed a greater increase in haemoglobin (1.9 versus 0.2 g/dl), greater haemoglobin response (80 versus 9%) and less requirement for rescue therapy in the first 52 weeks of treatment (9% versus 31%) in the roxadustat arm [59].
HIF-PHis have consistently shown non-inferiority compared with ESAs in improving and maintaining haemoglobin levels [60–69]. The ASCEND-ND (NCT02876835) global trial of daprodustat versus darbepoetin alfa including 3872 patients demonstrated a non-inferior change in haemoglobin over 52 weeks with a between-group difference of 0.08 g/dl [95% confidence interval (CI) 0.03–0.13] [62]. The PRO2TECT trials (NCT02648347 and NCT02680574) showed non-inferiority of vadadustat versus darbepoetin in ESA-naïve [between-group difference in haemoglobin change 0.04 g/dl (95% CI −0.06–0.14)] and ESA-treated patients [difference 0.00 g/dl (95% CI −0.10–0.09)] [64]. A meta-analysis of ESA-comparator daprodustat clinical trials including 4406 patients showed a non-significant mean difference in haemoglobin change between the daprodustat and ESA groups [−0.01 g/dl (95% CI −0.38–0.35)] [70].
The potential for rapid haemoglobin increases with HIF-PHi therapy has been noted in some of the trials. In a Japanese trial of daprodustat at a starting dose of 4 mg/day versus epoetin beta pegol, 13% of ESA-naïve patients on daprodustat had a haemoglobin increase >2 g/dl in the first 4 weeks, requiring early daprodustat protocol adjustment [63]. In the ESA-comparator desidustat trial, 48% of patients on desidustat overshot the haemoglobin target compared with 40.8% on ESAs [69]. Although in the phase 3 clinical trials, larger increases in haemoglobin levels were observed for roxadustat compared with daprodustat and vadadustat [71], no difference in haemoglobin change from baseline among different HIF-PHis was observed in a recent meta-analysis [72]. The sharp increases in haemoglobin levels do not necessarily imply improved efficacy, but rather the choice of a relatively high starting dose.
Effects on iron homeostasis and hepcidin
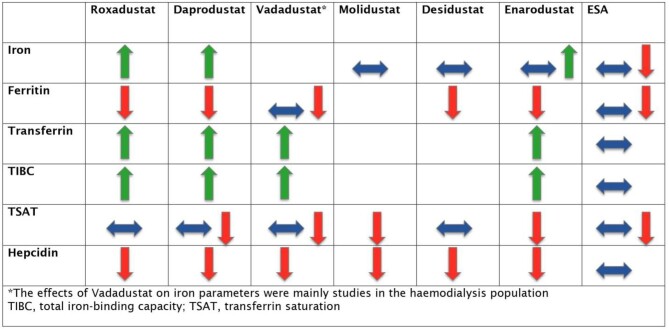
The main effects of HIF-PHi on iron homeostasis are summarized in Fig. 2.
Figure 2:
Effects of different HIF-PHis and ESAs in parameters of iron homeostasis and hepcidin.
In placebo-comparator trials of roxadustat, most studies showed a reduction in ferritin levels with Roxadustat [54, 55, 57]. Serum iron and TSAT were unchanged or increased and total iron-binding capacity (TIBC) was increased in the roxadustat group [54–57]. These changes overall reflect an increase in erythropoiesis and iron mobilization with roxadustat.
In the ESA-comparator trials, HIF-PHi demonstrated a similar [60–63, 69] or greater [65–67] decrease in ferritin levels compared with ESA therapy. A single trial showed increased ferritin levels with HIF-Phi [73]. Iron levels were relatively unchanged in both groups in three trials [60–62], similarly increased in two trials [66, 67], increased with ESA therapy in two trials [63, 69] and increased with HIF-PHi in one trial [73]. Among the ESA randomized controlled trials (RCTs), TSAT was higher with ESA therapy in six trials [62, 63, 65–67, 69] and relatively unchanged with both HIF-PHi and ESA therapy in three trials [60, 61, 73]. HIF-PHi had a greater effect in increasing TIBC [61–63, 65, 67, 73] and transferrin levels [61, 63] compared with ESAs.
Phase 3 trials have noted a significant decrease in hepcidin levels in patients treated with HIF-PHis compared with placebo and ESAs [54–57, 60–63, 65–67, 69].
HIF-PHis have been shown to reduce the requirements for IV iron supplementation, however, this finding was not universally replicated in the trials. It should be noted that protocols for iron administration were not standardized in phase 3 trials, introducing inconsistency in prescription practices. Two studies found less need for IV iron utilization in patients on roxadustat compared with placebo [54, 55]. Patients in the roxadustat arm of the DOLOMITES study (NCT02021318) required less supplemental IV iron compared with patients on ESA therapy [60]. However, the iron protocol differed between treatment groups, with a preference for the oral route as first-line therapy in the roxadustat group, which likely introduced bias in favour of roxadustat. Lower dosing of IV iron was used in the molidustat group compared with darbepoetin alfa group in two studies [66, 68]. In contrast, the ASCEND-ND and SYMPHONY-ND (StudY to compare the efficacy and safety of enarodustat with darbepoetin alfa in aneMic Patients with cHrOnic kidNeY disease Not requiring Dialysis) studies of daprodustat and enarodustat versus darbepoetin alfa demonstrated similar IV iron requirements in both groups [62, 67]. Whether the potential advantage of a reduced requirement for IV iron therapy with HIF-PHi translates to a decreased number of IV iron infusions, which would be more relevant particularly in NDD-CKD patients, remains to be confirmed.
Efficacy in elevated inflammatory states
The efficacy of HIF-PHi therapy in achieving and maintaining a haemoglobin response was not impaired by the presence of elevated C-reactive protein (CRP) levels [54, 55, 61]. A pooled analysis of three RCTs in patients with CRP greater than the upper limit of normal comparing roxadustat with placebo showed a haemoglobin change of 2 g/dl in the roxadustat group versus 0.3 g/dl in the placebo group [59]. In a post hoc analysis of a study of roxadustat versus darbepoetin alfa, low-grade inflammation (CRP ≥3 mg/l) was associated with a requirement for higher doses of darbepoetin but not Roxadustat [74]. The MIYABI ND-C (MolIdustat once dailY improves renal Anaemia By Inducing erythropoietin Non-Dialysis Correction) trial showed similar haemoglobin levels in the subgroups of patients with high (>3 mg/l) and low (≤3 mg/l) CRP [66]. These findings should be interpreted with caution as trials excluded patients with chronic inflammatory states.
Effect on cholesterol
Roxadustat, daprodustat and desidustat significantly decreased total cholesterol and low-density lipoprotein (LDL) [54–57, 60, 63, 69] but also slightly decreased high-density lipoprotein (HDL) cholesterol [54, 56] compared with placebo and ESA therapy. This likely reflects the role of HIF in the activity of acetyl coenzyme-A and 3-hydroxy-3-methylglutaryl coenzyme-A reductase, which are essential in cholesterol synthesis [46].
In the pooled analysis of the placebo-comparator roxadustat trials, ANDES [54], OLYMPUS [55] and ALPS [56], the change in LDL cholesterol over 12–28 weeks was −17.3 mg/dl in the roxadustat group versus +2.6 mg/dl in the placebo group [59].
In the ESA-comparator trials, a Japanese study showed a decrease in LDL and HDL cholesterol in the daprodustat group compared with no change in the ESA group [63]. The DREAM-ND study (NCT04012957) found a significant decrease in LDL cholesterol with desidustat compared with darbepoetin alfa but no significant change in HDL, total cholesterol, triglyceride and apolipoproteins A1 and B [69]. The MIYABI ND-C trial showed no difference in the total cholesterol levels between patients treated with molidustat and darbepoetin [66].
It is unclear whether the decrease in serum cholesterol translates into a positive effect on atherosclerotic plaque stabilization, as is the case for statins.
Health-related quality of life (HRQoL)
Phase 3 trials comparing roxadustat with placebo demonstrated no significant change in HRQoL or functional health scoring [54–56]. In the DOLOMITES study, roxadustat was non-inferior to darbepoetin alfa for changes in patient-reported HRQoL measurements [60].
The ASCEND-NHQ trial (NCT03409107) reported a greater mean change in the 36-item Short Form Health Survey (SF-36) vitality score in the daprodustat than in the placebo group (7.3 versus 1.9 points), which translates to an improvement in fatigue with daprodustat [58]. However, patients receiving daprodustat achieved higher haemoglobin levels. The DREAM-ND trial demonstrated an increase in HRQoL scoring in patients taking desidustat, but this was not different from the ESA arm [69].
Kidney transplant recipients
The efficacy and safety of HIF-PHi in kidney transplant recipients is poorly investigated. Roxadustat has been reported to be effective in treating post-transplant anaemia in small case series from Japan [75, 76] and China [77, 78], however, studies with a long follow-up are required to investigate potential effects on the immune system and interactions with immunosuppressive drugs.
THERAPEUTIC USE OF HIF-PHI IN THE MANAGEMENT OF ANAEMIA IN DIALYSIS-DEPENDENT CKD (DD-CKD)
All available phase 3 trials of HIF-PHis versus ESAs in DD-CKD are summarized in Table 3.
Table 3:
DD-CKD: HIF-PHis versus ESAs
| Study | Comparator | Patients, n | Population | Treatment period (weeks) | Target Hb (g/dl) | Efficacy endpoints | Safety endpoints |
|---|---|---|---|---|---|---|---|
| Roxadustat (vs ESAs) | |||||||
| HIMALAYAS [79] (global) | Epoetin alfa | 1043 | Incident HD and PD (90%/10%) | 52 | ≥11 | Mean Hb change at 28–52 weeks: 2.57 vs 2.36 g/dl (USA), 2.62 vs 2.44 g/dl (Europe) Patients with Hb response: 84.3% vs 79.5% (USA), 88.2% vs 84.4% (Europe) RBC transfusion: 7.3% vs 6.4% Monthly IV iron use per PEM: 58.1 vs 88.7 mg |
Fatal TEAEs: 12.1% vs 11.4% Hypertension: 19% vs 17% Diarrhoea: 13.8% vs 7.4% AVF thrombosis: 11.3% vs 8.9% |
| SIERRAS [81] (USA) | Epoetin alfa | 741 | Incident and prevalent HD and PD (95%/5%) | 52 | ≈11 | Mean Hb change at 28–52 weeks: 0.39 vs −0.09 g/dl Patients with mean Hb ≥10 g/dl: 66.1% vs 58.6% RBC transfusion: 12.5% vs 21.1% Monthly IV iron use per PEM: 17.1 vs 37 mg |
Fatal TEAEs: 16.8% vs 15.7% Nausea: 17% vs 16.2% Hypertension: 16.8% vs 12.7% |
| ROCKIES [80] (global) | Epoetin alfa | 2133 | Incident and prevalent HD and PD (89%/11%) | 52 | ≈11 | Mean Hb change at 28–52 weeks: 0.77 vs 0.68 g/dl Patients with mean Hb ≥10 g/dl: 85.3% vs 89.2% RBC transfusion: 9.8% vs 13.2% Mean monthly IV iron use: 58.7 vs 91.4 mg |
Acute MI: 3.7% vs 3.9% Hypertension: 8.8% vs 8.9% Pneumonia: 8.7% vs 9.6% AVF thrombosis: 7.4% vs 5.4% |
| PYRENEES [84] (Europe) | Epoetin alfa or darbepoetin alfa | 836 | Prevalent HD and PD (94%/6%) | 52–104 | 10–12 | Mean Hb change at 28–36 weeks: 0.43 vs 0.19 g/dl Patients with target Hb at 28–36 weeks: 84.2% vs 82.4% Mean monthly IV iron use: 12 vs 44.8 mg |
Fatal TEAEs: 16.2% vs 13.1% Hypertension: 17.9 vs% 18.8% AVF thrombosis: 12.1% vs 7.4% All-cause death: 18.8% vs 14% |
| Chen et al. [85] (China) | Epoetin alfa | 305 | Prevalent HD and PD (89%/11%) | 26 | 10–12 | Mean Hb change: 0.7 vs 0.5 g/dl Patients with mean Hb ≥10 g/dL: 87% vs 88.5% Mean hepcidin change: −30.2 vs −2.3 ng/ml Mean TSAT change: −5.7% vs −7.6% |
Upper respiratory infection: 18.1% vs 11% Hyperkalaemia: 7.4% vs 1% AVF complication: 2.9% vs 3% |
| Akizawa et al. [91] (Japan) | Darbepoetin alfa | 303 | Prevalent HD | 24s | 10–12 | Mean Hb change: −0.04 vs −0.03 g/dl Patients with target Hb: 79.3% vs 83.4% IV iron use: 22.7% vs 20.4% |
Serious TEAEs: 20.7% vs 14.5% Nasopharyngitis: 34.7% vs 26.3% Vomiting: 6.7% vs 2% Shunt stenosis: 7.3% vs 8.6% |
| Hou et al. [92] (China) | ESAs | 129 | Prevalent PD | 24 | 10–12 | Mean Hb change: 2.5 vs 2.2 g/dl Patients with Hb response: 96% vs 92% Mean hepcidin change: −46.6 vs −5.9 ng/ml |
Hypertension: 6% vs 7% Hyperkalaemia: 8% vs 2% Insomnia: 6% vs 0% |
| Daprodustat (vs ESAs) | |||||||
| ASCEND-ID [82] (global) | Darbepoetin alfa | 312 | Incident HD and PD (81%/19%) | 52 | 10–11 | Mean Hb change at 28–52 weeks: 1.02 vs 1.12 g/dl Mean monthly IV iron use: 142 vs 128 mg Mean hepcidin change: −29.8 vs −11.4 ng/ml |
First occurrence of MACE: 12% vs 10% Hypertension: 18% vs 16% Diarrhoea: 9% vs 7% |
| ASCEND-D [86] (global) | Epoetin alfa or darbepoetin alfa | 2964 | Prevalent HD and PD (88.5%/11.5%) | 52 | 10–11 | Mean Hb change at 28–52 weeks: 0.28 vs 0.10 g/dl RBC transfusion: 15.7% vs 18.3% Mean monthly IV iron use: 90.8 vs 99.9 mg |
First occurrence of MACE: 25.2% vs 26.7% Rapid increase in Hb: 4.1% vs 1.6% Vascular access thrombosis: 10.4% vs 12.5% |
| ASCEND-TD [87] (global) | Epoetin alfa | 407 | Prevalent HD | 52 | 10–11 | Mean Hb change: −0.04 vs 0.02 g/dl Patients with mean Hb 10–11.5 g/dl: 80% vs 64% RBC transfusion: 8% vs 12% Mean monthly IV iron use: 98.1 vs 106.2 mg |
First occurrence of MACE: 12% vs 10% Hypertension: 9% vs 11% Non-fatal stroke: 3% vs 0% |
| Akizawa et al. [88] (Japan) | Darbepoetin alfa | 271 | Prevalent HD | 40–52 | 10–12 | Mean Hb change: 0 vs 0 g/dl Patients with target Hb: 88% vs 90% Mean monthly IV iron use: 14 vs 25 mg Mean hepcidin levels: 37.9 vs 51.5 ng/ml Mean TSAT change: 0.3% vs −2.1% |
Diarrhoea: 15% vs 9% Contusion: 13% vs 8% Nasopharyngitis: 42% vs 54% Pain in extremity: <1% vs 7% |
| Vadadustat (vs ESAs) | |||||||
| INNO2VATE [83] (global) | Darbepoetin alfa | 3923 | Incident and prevalent HD and PD | 52 | 10–11 (USA) 10–12 (non-USA) |
Mean Hb change at 24–36 weeks: 1.26 vs 1.58 g/dl (incident), 0.19 vs 0.36 g/dl (prevalent) Patients with target Hb at 24–36 weeks: 43.6% vs 56.9% (incident), 49.2% vs 53.2% (prevalent) RBC transfusion at 24–36 weeks: 1.3% vs 1.8% (incident), 2% vs 1.3% (prevalent) ESA rescue at 0–23 weeks: 20.4% vs 16% (incident), 27.6% vs 30.2% (prevalent) |
First occurrence of MACE: 18.2% vs 19.3% Serious AEs: 49.7% vs 56.5% (incident), 55% vs 58.3% (prevalent) Drug-related AEs: 3.9% vs 2.7% (incident), 9.6% vs 3.8% (prevalent) AVF thrombosis: 3.4% vs 5.4% (incident), 6% vs 4.4% (prevalent) |
| Nangaku et al. [93] (Japan) | Darbepoetin alfa | 323 | Prevalent HD | 20–24 | 10–12 | Mean Hb level: 10.61 vs 10.65 g/dl Patients with target Hb: 75.4% vs 75.7% IV iron use: 30.9% vs 33.3% |
Adverse drug reaction: 11.1% vs 3.7% Nasopharyngitis: 45.7% vs 45.3% Shunt stenosis: 14.2% vs 16.1% Retinal disorder: 13% vs 9.9% |
| Enarodustat (vs ESAs) | |||||||
| SYMPHONY-HD [73] (Japan) | Darbepoetin alfa | 173 | Prevalent HD | 20–24 | 10–12 | Mean Hb level: 10.73 vs 10.85 g/dl Patients with target Hb: 77.9% vs 88.4% Mean IV iron use: 74 vs 70.2 mg |
Vomiting: 10.3% vs 2.3% Retinal disorder: 6.9% vs 3.5% |
| Molidustat (vs ESAs) | |||||||
| MIYABI HD-M [89] (Japan) | Darbepoetin alfa | 229 | Prevalent HD | 52 | 10–12 | Mean Hb change at 33–36 weeks: −0.14 vs −0.07 g/dl Patients with target Hb at week 52: 74.6% vs 81.5% Patients with an increase in Hb of >0.5 g/dl/week: 49% vs 47.3% Use of rescue treatment: 11.1% vs 1.3% Mean weekly IV iron use: 18.2 vs 15.2 mg |
Serious TEAE: 24.2% vs 18.4% Neoplasms: 9.8% vs 5.3% Patients with MACE: 3.3% vs 2.6% Ocular TEAE: 30.1% vs 18.4% |
| Desidustat (vs ESAs) | |||||||
| DREAM-D [90] (India) | Epoetin alfa | 392 | Prevalent HD | 24 | 10–12 | Mean Hb change at 16–24 weeks: 0.95 vs 0.80 g/dl Patients with target Hb: 59.2% vs 48.4% Median time to achieve target Hb: 4 vs 8 weeks |
At least one TEAE: 48% vs 46.4% Nausea: 3.6% vs 1.5% Hyperkalaemia: 2.6% vs 0.5% Oedema: 2% vs 0.5% |
Table 3 details HIF-Phi- versus ESA-comparator phase 3 trials in the dialysis-dependent population. This includes six trials of roxadustat (two global [79, 80], one US [81], one Europe [84], one Japan [91] and one China [85]), four trials of daprodustat (three global [82, 86, 87], one Japan [88]), one trial of molidustat (Japan [89]), three trials of vadadustat (2 global [83], 1 Japan [93]), one enarodustat (Japan [73]) and one desidustat (South Asia [90]).
Hb: haemoglobin; PEM: patient-exposure month; MACE: major adverse cardiac event (composite of death, non-fatal MI and/or stroke); AVF: arteriovenous fistula; TEAE: treatment emergent adverse event.
Haemoglobin correction and maintenance
The phase 3 trials demonstrated that all six HIF-PHis (roxadustat, daprodustat, vadadustat, molidustat, desidustat and enarodustat) were non-inferior to ESAs in haemoglobin correction and maintenance in studies of incident [79–83] or prevalent [73, 80, 81, 83–92] DD-CKD patients. The ROCKIES trial (NCT02174731) of 2133 dialysis patients showed non-inferiority of roxadustat to epoetin alfa (mean haemoglobin increase 0.77 versus 0.68 g/dl) and a similar proportion of time spent with a haemoglobin >10 g/dl [80]. In the ASCEND-D trial (NCT02879305) of 2964 dialysis patients, daprodustat was non-inferior to ESA therapy in correcting haemoglobin (mean haemoglobin increase 0.28 versus 0.10 g/dl) [86]. In the INNO2VATE trials (NCT02865850 and NCT02892149) including 3923 dialysis patients, vadadustat was non-inferior to darbepoetin alfa in haemoglobin efficacy (between-group difference in haemoglobin change −0.07 g/dl in the incident and −0.18 g/dl in the prevalent dialysis trial) [83].
Roxadustat compared with ESAs did not meet the non-inferiority criterion for the secondary endpoint of RBC transfusion in the HIMALAYAS trial (NCT03298451) [79] (4.3 versus 3.5 per 100 patient-exposure years), whereas it was non-inferior in the ROCKIES trial [80] (9.8 versus 13.2%) and superior in the SIERRAS (NCT02665065) [81] (12.5% versus 21.1%) and PYRENEES (NCT02278341) [84] (9.2% versus 12.9%) trials. Greater use of ESA rescue was shown in the roxadustat group in the ROCKIES [80] (3.7% versus 0.2%) and PYRENEES [84] (1.5% versus 0%) trials. A similar rate of rescue therapy was administered in both the daprodustat and ESA groups in the ASCEND-D [86], ASCEND-ID (NCT03029208) [82] and ASCEND-TD (NCT03400033) [87] trials. In the incident INNO2VATE trial, more patients in the vadadustat group required ESA rescue (20.4% versus 16%), whereas in the prevalent trial more patients in the ESA group required ESA rescue (27.6% versus 30.2%) [83].
Effects on iron homeostasis and hepcidin
The main effects of HIF-PHi on iron homeostasis are an increase in iron, transferrin and TIBC and a reduction in ferritin and hepcidin.
The roxadustat trials showed a reduction in ferritin in both the roxadustat and ESA groups, although the decrease was larger in the roxadustat group [79–81, 84, 91]. The SIERRAS trial noted greater reductions in patients with higher baseline ferritin levels [81]. The daprodustat trials showed a similar reduction in ferritin compared with ESAs [82, 86–88]. The INNO2VATE incident dialysis trial showed stable ferritin levels in both the vadadustat and ESA groups, but a greater reduction in ferritin was shown in the vadadustat group in the INNO2VATE prevalent dialysis trial [83].
HIF-PHi maintained or increased serum iron compared with ESAs in most clinical trials [73, 79–82, 85–88].
Overall, TSAT was stable or reduced at the same rate in both the HIF-PHi and ESA groups [79–88].
The TIBC and transferrin levels were elevated from baseline in the HIF-PHi compared with the ESA groups [73, 79, 80, 82, 85–88, 92, 93].
HIF-PHi was found to reduce hepcidin levels more than the comparator ESAs [73, 79–85, 87, 88, 91–93]. In the INNO2VATE trials, a greater decrease in hepcidin was noted in the vadadustat group of prevalent compared with incident dialysis patients [83].
Phase 3 trials demonstrated lower requirements for oral or IV iron supplementation with HIF-PHis compared with ESAs [79–82, 84–88, 93]. In the pooled analysis of the PYRENEES, SIERRAS, HIMALAYAS and ROCKIES trials, patients on roxadustat required a mean of 5.3 IV iron administrations per patient-year compared with 9.6 for ESA patients [94]. Two trials found similar iron requirements between the HIF-PHi and ESA groups [73, 91]. As for NDD-CKD, protocols for iron administration were not standardized in the trials, making it difficult to draw conclusions on IV iron needs.
Efficacy in elevated inflammatory states
The efficacy of haemoglobin response to HIF-PHi therapy was maintained in the context of elevated CRP in a number of trials of roxadustat, daprodustat, vadadustat and enarodustat.[73, 79, 81, 82, 84, 92, 93]. Three roxadustat trials demonstrated superior haemoglobin response in the context of an elevated CRP for the roxadustat versus the ESA group [80, 85, 92]. In other roxadustat trials, dose requirements were similar for both the high- and low-CRP roxadustat groups, but patients with a higher CRP treated with epoetin required increased doses and often achieved lower haemoglobin levels [79, 81, 85, 91]. A recent pooled analysis of four RCTs comparing roxadustat with ESA in patients stratified by quintiles of CRP at baseline showed a greater haemoglobin increase in the roxadustat group regardless of baseline CRP levels, without requirement for higher doses [95].
In the ASCEND-TD trial, patients classified as ESA hyporesponders did not respond better to daprodustat compared with their previous ESA treatment [87]. In other studies, patients with a higher baseline erythropoietin resistance index required higher doses of daprodustat to achieve haemoglobin targets [88] and enarodustat did not show a difference in dose needs compared with darbepoetin alfa in those with high CRP values (≥3 mg/l) [73]. However, vadadustat improved haemoglobin levels in patients who had not achieved targets with previous ESA therapy [93].
Effect on cholesterol
A consistent superior reduction in LDL cholesterol was reported in DD-CKD patients treated with HIF-PHis compared with ESAs [79–81, 84, 85, 90]. In the HIMALAYAS trial, roxadustat also decreased HDL cholesterol, non-HDL cholesterol and triglycerides [79]. In the ROCKIES [80] and Chen et al. [85] trials of roxadustat, a greater reduction in HDL and triglycerides was noted compared with epoetin alfa. In the DREAM-D trial, patients treated with desidustat had significantly lower apolipoprotein-B levels compared with those treated with ESAs [90].
HRQoL
The PYRENEES trial of roxadustat versus ESA therapy in prevalent dialysis patients found a greater improvement in the patient-reported HRQoL questionnaire in the roxadustat group [84]. However, in this trial, haemoglobin levels increased more rapidly and to higher levels in the roxadustat arm than in the ESA arm. The DREAM-D trial found no difference in HRQoL scoring between the desidustat and ESA groups [90].
SAFETY PROFILE OF HIF-PHI IN NDD-CKD AND DD-CKD
The broad spectrum of metabolic functions of the HIF pathway has raised safety concerns regarding its continuous activation from HIF-PHis [46]. Furthermore the effect of HIF-PHis on signalling pathways in metabolic processes other than the HIF pathway and their potential for epigenetic gene regulation is not fully understood [13, 46].
In the placebo-comparator trials in NDD-CKD patients for roxadustat and daprodustat, there were broadly comparable incidences of adverse events between placebo and HIF-PHi, and participants were more likely to withdraw due to adverse events in the placebo arms [54, 55, 57, 58]. Phase 3 active-comparator trials in NDD- and DD-CKD generally noted comparable adverse events to ESA therapy, although participants were more likely to withdraw due to adverse events in the HIF-PHi arms [60, 61, 64, 65, 68, 80, 81, 83–85, 91, 93].
Clinically important adverse events of different HIF-PHis from pooled analyses and meta-analyses are summarized in Table 4.
Table 4:
Safety outcomes of different HIF-PHis (data from pooled analyses and meta-analyses)
| Outcome | Roxadustat | Daprodustat | Vadadustat | |||
|---|---|---|---|---|---|---|
| NDD-CKD | DD-CKD | NDD-CKD | DD-CKD | NDD-CKD | DD-CKD | |
| All-cause mortality | Roxadustat vs placebo HR 1.08 (95% CI 0.93–1.26) [59] RR 0.40 (95% CI 0.06–2.84) [111] |
Roxadustat vs ESAs RR 1.13 (95% CI 0.95–1.34) [94] |
Daprodustat vs placebo RR 0.54 (95% CI 0.09–3.31) [111] Daprodustat vs ESAs RR 1.01 (95% CI 0.87–1.17) [70] |
Daprodustat vs ESAs RR 0.99 (95% CI 0.86–1.14) [70] |
Vadadustat vs placebo RR 1.43 (95% CI 0.15–13.27)[111] |
|
| CV event | Roxadustat vs ESAs RR 1.00 (95% CI 0.88–1.14) [112] |
Daprodustat vs ESAs RR 0.96 (95% CI 0.85–1.08) [112] |
Vadadustat vs ESAs RR 0.94 (95% CI 0.83–1.07) [112] |
|||
| MACE | Roxadustat vs placebo HR 1.10 (95% CI 0.96–1.27) [59] |
Roxadustat vs ESAs RR 1.09 (95% CI 0.95–1.26) [94] |
Daprodustat vs ESAs RR 1.05 (95% CI 0.94–1.18) [70] |
Daprodustat vs ESAs RR 0.89 (95% CI 0.89–0.98) [70] |
||
| MACE+ | Roxadustat vs placebo HR 1.07 (95% CI 0.94–1.21) [59] |
Roxadustat vs ESAs RR 0.98 (95% CI 0.86–1.11) [94] |
||||
| MI | Roxadustat vs placebo HR 1.29 (95% CI 0.90–1.85) [59] |
Roxadustat vs ESAs RR 0.59 (95% CI 0.29–1.21) [113] RR 1.05 (95% CI 0.81–1.35) [94] |
Daprodustat vs ESAs RR 1.08 (95% CI 0.84–1.38) [70] |
Daprodustat vs ESAs RR 0.74 (95% CI 0.59–0.92) [70] |
||
| Stroke | Roxadustat vs placebo HR 1.25 (95% CI 0.82–1.90) [59] |
Roxadustat vs ESAs RR 1.01 (95% CI 0.69–1.50) [94] |
Daprodustat vs ESAs RR 1.41 (95% CI 0.86–2.29) [70] |
Daprodustat vs ESAs RR 0.78 (95% CI 0.50–1.20 )[70] |
||
| Hospitalization for heart failure | Roxadustat vs placebo HR 0.93 (95% CI 0.75–1.16) [59] |
Roxadustat vs ESAs RR 0.39 (95% CI 0.17–0.89) [113] RR 0.91 (95% CI 0.73–1.14)[94] |
Daprodustat vs ESAs RR 1.02 (95% CI 0.36–2.87) [70] |
Daprodustat vs ESAs RR 1.01 (95% CI 0.82–1.25) [70] |
||
| Cancer-related death or tumour progression or recurrence | Roxadustat vs ESAs RR 0.25 (95% CI 0.03–2.24) [112] |
Daprodustat vs ESAs RR 0.86 (95% CI 0.60–1.24) [112] |
Vadadustat vs ESAs RR 0.77 (95% CI 0.29–2.03) [112] |
|||
| AVF thrombosis | Roxadustat vs placebo 0.7 vs 0.2 per 100 PY [59] |
Roxadustat vs ESAs RR 1.43 (95% CI 1.09–1.87) [113] RR 1.15 (95% CI 1.04–1.27) [112] 5.7 vs 3.9 per 100 PY [94] |
Daprodustat vs ESAs RR 0.78 (95% CI 0.66–0.92) [112] |
Vadadustat vs ESAs RR 0.98 (95% CI 0.83–1.16) [112] |
||
| Hypertension | Roxadustat vs placebo RR 1.45 (95% CI 1.12–1.87) [113] 9.0 vs 6.6 per 100 PY [59] |
Roxadustat vs ESAs RR 1.13 (95% CI 0.93–1.37) [113] RR 1.00 (95% CI 0.88–1.13) [112] MD 1.00 (95% CI 0.81–1.24) [114] 8.3 vs 6.9 per 100 PY [94] |
Daprodustat vs ESAs RR 1.00 (95% CI 0.85–1.16) [112] MD 0.95 (95% CI 0.82–1.10) [114] |
Vadadustat vs ESAs RR 0.81 (95% CI 0.69–0.96) [112] MD 0.74 (95% CI 0.60–0.91) [114] |
||
| Hyperkalaemia | Roxadustat vs placebo RR 1.41 (95% CI 1.08–1.85) [113] 7.0 vs 5.7 per 100 PY [59] |
Roxadustat vs ESAs RR 1.03 (95% CI 0.80–1.33) [113] RR 1.03 (95% CI 0.78–1.37) [112] |
Daprodustat vs ESAs RR 0.91 (95% CI 0.63–1.33) [112] |
Vadadustat vs ESAs RR 0.84 (95% CI 0.60–1.17) [112] |
||
MD: mean difference; MACE: major adverse cardiac event (composite of death, non-fatal MI and/or stroke); MACE+: expanded major adverse cardiac event (MACE plus hospitalization for either HF or unstable angina or MACE plus hospitalization for either HF or a thromboembolic event)
All-cause mortality
A meta-analysis of 46 studies including 27 338 patients across all the currently available HIF-PHis found no significant differences in mortality compared with placebo or ESAs in both the DD-CKD and NDD-CKD subgroups [96]. A meta-analysis of eight studies comparing daprodustat with ESAs showed no difference in mortality in the DD-CKD and NDD-CKD groups [70].
A pooled analysis of 4277 patients in the ANDES [54], OLYMPUS [55] and ALPS [56] trials for roxadustat versus placebo in NDD-CKD patients showed non-inferiority for all-cause mortality [59].
Similarly, a pooled analysis of the HIMALAYAS [79], ROCKIES [80], SIERRAS [81] and PYRENEES [84] roxadustat ESA-comparator trials in DD-CKD patients demonstrated non-inferiority of roxadustat for all-cause mortality [94]. There was a numerically higher risk of all-cause mortality in the subgroup of stable dialysis patients converted from ESA to roxadustat compared with incident dialysis patients treated with roxadustat [hazard ratio (HR) 1.23 (95% CI 1.02–1.49) versus HR 0.83 (95% CI 0.57–1.19)]. This is confounded by the change of ESA to a new therapy and the possible impact this may have had on haemoglobin levels.
Cardiovascular safety
The majority of phase 3 trials demonstrated non-inferiority of HIF-PHis to placebo and ESA therapy for major cardiac events in NDD-CKD and DD-CKD patients [55, 60, 62, 79–81, 84, 86]. A Cochrane meta-analysis of 51 studies including 30 994 NDD-CKD and DD-CKD patients showed little or no difference between HIF-PHis and ESAs for cardiovascular death [relative risk (RR) 1.05 (95% CI 0.88–1.26)], non-fatal MI [RR 0.91 (95% CI 0.76–1.10)] and non-fatal stroke [RR 1.06 (95% CI 0.71–1.56)] [97].
A pooled analysis of the ANDES [54], OLYMPUS [55] and ALPS [56] trials of roxadustat compared with placebo in NDD-CKD patients found roxadustat to be non-inferior for major adverse cardiovascular events (MACE; composite of death, non-fatal MI and/or stroke) [HR 1.10 (95% CI 0.96–1.27)] and expanded MACE (MACE plus hospitalization for either HF or unstable angina or MACE plus hospitalization for either HF or a thromboembolic event) [HR 1.07 (95% CI 0.94–1.21)] [59]. In the dialysis population, a pooled analysis of four roxadustat ESA-comparator clinical trials (HIMALAYAS [79] ROCKIES [80], SIERRAS [81] and PYRENEES [84]) revealed non-inferiority for MI, unstable angina, stroke and HF requiring hospitalization [94].
In a meta-analysis of eight clinical trials including 3839 DD-CKD and 4406 NDD-CKD patients, daprodustat compared with ESAs was associated with a significantly reduced incidence of MACE [RR 0.89 (95% CI 0.89–0.98)] in DD-CKD patients but not in the NDD-CKD cohort [RR 1.05 (95% CI 0.94–1.18)] [70]. The reduced incidence of MACE in the DD-CKD group was driven by a decrease in the incidence of MI [RR 0.74 (95% CI 0.59–0.92)]. A post hoc analysis of three Japanese phase 3 trials in NDD-CKD and DD-CKD patients comparing daprodustat with ESAs found no difference in the incidence of MACE [RR 0.86 (95% CI 0.29–2.52)] [98].
A meta-analysis comparing vadadustat with placebo or darbepoetin alfa including NDD-CKD and DD-CKD patients found no difference in the incidence of cardiac events [RR 1.03 (95% CI 0.88–1.20)] or non-fatal stroke [RR 0.92 (95% CI 0.55–1.57)] [99]. In contrast, a pooled analysis of the PRO2TECT NDD-CKD trials of 3471 patients comparing vadadustat with darbepoetin alfa showed a higher risk for MACE [HR 1.17 (95% CI 1.01–1.36)] in the vadadustat group [64]. This appeared to be driven by the subset of patients enrolled outside of the US randomized to a higher haemoglobin target (10–12 g/dl versus 10–11 g/dl).
Thrombotic events
In a pooled analysis of trials in NDD-CKD patients, roxadustat was associated with an increased incidence of arteriovenous (AV) access thrombosis (1.5 versus 0.9 per 100 patient-years), deep vein thrombosis (DVT) (0.7 versus 0.2 per 100 patient-years) and pulmonary thromboembolism (0.3 versus 0.1 per 100 patient-years) compared with placebo [59]. A meta-analysis of roxadustat in NDD-CKD trials noted an increased risk of DVT compared with placebo [RR 3.80 (95% CI 1.50–9.64)] [100]. In the ASCEND-ND trial, more patients treated with daprodustat developed vascular access thrombosis compared with ESAs (2.1% versus 1.5%) [62].
In patients on dialysis, phase 3 trials have noted higher rates of AV dialysis access thrombosis in patients treated with HIF-PHis compared with ESAs [79–81, 83, 84]. However, other studies have found similar or fewer AV access thrombosis episodes with HIF-Phis [73, 85, 87]. A pooled analysis of Japanese phase 3 trials of daprodustat found a similar incidence of thromboembolic events between the daprodustat and ESA groups [98].
Malignancy
In a recent meta-analysis of 26 studies with 24 387 NDD and DD-CKD patients, the risk of cancer was similar between HIF-PHis and ESAs [RR 0.93 (95% CI 0.76–1.13)] [72]. A post hoc analysis from three phase 3 Japanese studies in NDD-CKD and DD-CKD patients noted similar cancer-related adverse events in the daprodustat and ESA groups (1.28 versus 1.53 per 100 patient-years, respectively) [98].
In the ASCEND-ND trial, cancer-related outcomes (death or tumour progression or recurrence) were more frequent with daprodustat compared with ESAs [3.7% versus 2.5%; RR 1.47 (95% CI 1.03–2.10)] [62]; the imbalance for cancer-related events between the two treatment groups was attenuated in post hoc analyses taking into account the longer darbepoetin dosing intervals [101]. A pooled analysis of studies on roxadustat compared with placebo in patients with NDD-CKD showed no increased risk of malignancy with Roxadustat [59].
In dialysis patents, the MIYABI HD-M trial (NCT03543657) demonstrated an increased incidence of neoplasm episodes (9.8% versus 5.3%) in the molidustat arm compared with darbepoetin [89]. However, the trial sample size was rather small and the follow-up short for reliably assessing the risk of malignancy.
Retinopathy
The neo-vascularization effect of HIF-PHis has been postulated to worsen ocular pathology, such as diabetic retinopathy [13, 46]. For this reason, most of the phase 3 clinical trials excluded patients with severe retinopathy.
The pooled Japanese daprodustat analysis of trials in NDD- and DD-CKD patients found no increased risk for retinal events or aggravation of underlying retinal disease [98].
The SYMPHONY-ND study demonstrated increased VEGF levels and increased retinal adverse events of enarodustat compared with ESAs (3.7% versus 0.9%) [67]. All the remaining Japanese NDD-CKD trials showed no increased risk of ocular disorders related to HIF-PHi therapy [61, 63, 65, 91].
The SYMPHONY-HD trial reported an increased risk of retinal adverse events with vadadustat (6.9% versus 3.5%), although the VEGF levels were lower in the vadadustat compared with the darbepoetin group [73]. The ASCEND-ID trial also reported an increased incidence of ocular adverse events with daprodustat compared with ESAs (3.4 versus 0.79 per 100 patient-years) [82].
Hypertension
Although hypertension is an established complication of ESA therapy, comparator trials of HIF-PHis versus ESAs in NDD-CKD patients have not shown significant differences in the development of hypertension [61, 64, 69]. A meta-analysis of NDD-CKD roxadustat trials noted a higher incidence of hypertension in the roxadustat group compared with placebo [RR 1.37 (95% CI 1.13–1.65)] [59]. However, another meta-analysis of NDD-CKD patients has reported a lower risk of hypertension with HIF-PHis compared with ESAs [RR 0.89 (95% CI 0.81–0.98)] [102].
In comparator trials of DD-CKD patients there were no significant differences in the development of hypertension between the HIF-PHi and ESA groups [73, 79, 81, 84, 86, 90].
Other studies in both NDD- and DD-CKD patients suggest a beneficial effect of HIF-PHis on blood pressure compared with ESAs, such as fewer requirements for titration of anti-hypertensives [63, 65, 85, 87, 88, 93].
Other potential adverse effects
Other less commonly reported potential adverse effects are described in the supplementary material.
APPROVED HIF-PHIS
Currently approved HIF-PHis are summarized in Supplementary Table S2.
Roxadustat was the first-in-class HIF-PHi approved for treatment of anaemia in patients with DD-CKD and NDD-CKD and is the most studied globally. Roxadustat was granted marketing authorization by the EMA in August 2021 for patients with anaemia associated with CKD, whether they are on dialysis or not (EMA/453588/2021). It was rejected by the FDA in July 2021 because of safety concerns. More specifically, the efficacy and safety of roxadustat was assessed by the FDA and EMA in a phase 3 program of eight multicentre randomized studies involving 9600 patients worldwide with CKD anaemia. Although both agencies considered the evidence provided for efficacy of roxadustat was substantial, the FDA raised significant safety concerns. The safety of roxadustat was assessed by the FDA using pooled analyses of studies of roxadustat versus placebo [54–56] or darbepoetin alfa [60] in NDD-CKD and roxadustat versus ESA [79–81, 84] in DD-CKD. Using on-treatment analyses (as opposed to intention-to-treat analyses) that were requested by the FDA to minimize the effect of including unexposed person-times or events, the risk of MACE was higher for roxadustat compared with placebo in the NDD-CKD population [HR 1.38 (95% CI 1.11–1.70)] and similar to ESA in the DD-CKD population [HR 1.02 (95% CI 0.88–1.20)] [103]. Of note, in the USA, ESAs are not used as frequently as in Europe for the treatment of anaemia in NDD-CKD (28% in the USA compared with 57% in Germany) [104]. Roxadustat was also associated with a higher risk for thrombotic events, vascular access thrombosis and seizures compared with placebo in the NDD-CKD population and ESA in the DD-CKD population. The FDA did not approve roxadustat and called for an additional clinical trial on the safety of roxadustat in both the NDD-CKD and DD-CKD populations. In contrast, the EMA concluded that the cardiovascular and mortality risks appeared to be similar to ESA based on data from the ‘haemoglobin correction studies’ in NDD-CKD and DD-CKD and considered that evaluation in other data pools (including comparison with placebo and in stable dialysis patients) are associated with methodological and study design issues complicating interpretation. Thus the risk of MACE, MACE+ and all-cause mortality in the ‘haemoglobin correction studies’ was similar compared with ESAs [HR 0.79 (95% CI 0.61–1.02), HR 0.78 (95% CI 0.62–0.98) and HR 0.78 (95% CI 0.57–1.05), respectively] and the benefits were considered greater than its risks [105].
Vadadustat was granted marketing authorization by the EMA in April 2023 for treatment of anaemia in patients with DD-CKD (EMA/100938/2023), with a warning on the risk of thromboembolic events. In the NDD-CKD population, the non-inferiority of vadadustat compared with darbepoetin alfa for MACE was not demonstrated. Vadadustat has been rejected by the FDA on safety concerns regarding thromboembolic events and a case of severe drug-induced liver injury reported in phase 2 trial data.
Daprodustat was approved for use by the FDA in February 2023 for patients with DD-CKD, with a boxed warning for an increased risk of thrombotic events. It was not approved for NDD-CKD patients due to insufficient safety data in this population. On 22 June 2023, the EMA Committee for Medicinal Products for Human Use recommended granting of a marketing authorization for daprodustat only for DD-CKD (EMEA/H/C/005746). On 12 July 2023, the pharmaceutical company withdrew its application.
Roxadustat, vadadustat and daprodustat are commercialized in other countries outside the European Union and the USA; of note, the local regulatory authorities did not require extensive phase 3 data for the approvals.
Molidustat was submitted for EMA approval but was withdrawn in August 2019, with the company indicating its strategic plans to focus on the Japanese market.
The remaining two HIF-PHis have not been submitted to the EMA. Desidustat was approved for use in India in March 2022 and Enarodustat in Japan in September 2020 for the treatment of anaemia in NDD-CKD and DD-CKD patients.
ONGOING STUDIES WITH HIF-PHIS IN CKD
Although HIF-PHis have shown promise in haemoglobin correction and maintenance efficacy, long-term safety data are required to establish their role in the management of anaemia of CKD. At the moment, only a limited number of new clinical trials have been designed.
A study focusing on the long-term safety outcomes of molidustat in Japanese patients is currently recruiting (NCT04899661), with an estimated completion date of June 2027. A post-marketing phase 4 study will evaluate the long-term safety of desidustat in dialysis and non-dialysis patients but is not yet recruiting (NCT05515367).
Specific groups of patients were excluded from phase 3 clinical trials, including patients with a kidney transplant, those with significant CVD including HF, and those with active inflammatory disease. A study comparing roxadustat combined with sacubitril/valsartan versus recombinant human erythropoietin combined with angiotensin-converting enzyme inhibitor or angiotensin receptor blocker in Chinese patients with cardiorenal syndrome and anaemia is currently recruiting (NCT05053893). Another study examining the safety and efficacy of roxadustat in the treatment of HF in patients with CKD and anaemia (NCT05691257) is expected to start recruiting this year in China and Japan. A meta-analysis of studies evaluating the efficacy and safety of HIF-PHis compared with ESAs in patients with CKD and HF [106] is expected to publish its results later this year.
SUGGESTIONS FOR CLINICAL PRACTICE
The Asian Pacific Society of Nephrology has published recommendations for the use of HIF-PHis [107]. The Kidney Disease: Improving Global Outcomes (KDIGO) group recently published its conclusions from the 2021 controversies conference on novel anaemia therapies in CKD; however, the scope of the KDIGO report was not to provide specific recommendations on their use [108].
Major clinical trials have failed to conclusively demonstrate that, as a class, HIF-PHis are non-inferior to placebo or ESAs for cardiovascular, thrombotic or cancer complications. Given the mechanism of action for HIF-PHis, patients with known malignancy occurring in the 5 years preceding enrolment were excluded from clinical trials and the median follow-up of phase 3 studies was too short to reliably assess a pro-oncogenic effect. The same holds true for patients with polycystic kidney disease, as the rate of cyst growth was not assessed systematically in the trials.
Potential explanations for the different effects of different trials and agents include imbalances in patients’ characteristics and ethnicity, haemoglobin at baseline, prior ESA exposure and the types of analyses performed (intention-to-treat versus on-treatment analyses). Although a class effect is plausible, drug-specific effects may also contribute to differences in efficacy and safety outcomes.
Collectively, given the degree of uncertainty about the benefits and harms of HIF-PHis, the principle of shared decision making should be applied to ensure that the values of patients with diverse needs and perspectives are respected. Definitive answers on whether there is a specific population in which HIF-PHis should be preferred or avoided will evolve from comprehensive assessment of post-marketing surveillance data and a mandate for a registry has been proposed [109].
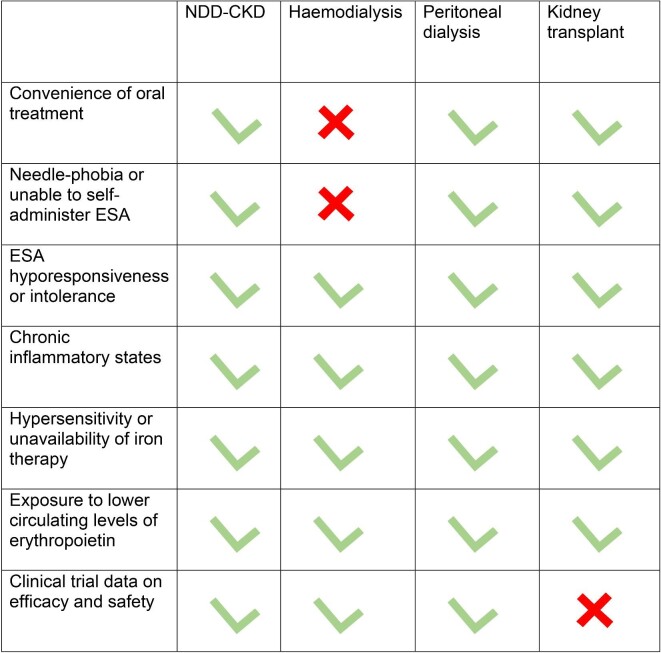
Based on existing evidence, our summary of suggestions for clinical practice is shown in Table 5. Potential advantages of the use of HIF-PHi compared with ESA therapy in different CKD populations are shown in Fig. 3.
Table 5:
Suggestions for clinical practice.
| Consider use of HIF-PHi |
| NDD-CKD or PD patients • Patient preference for oral treatment (accessibility, convenience, ease of administration, no storage requirements) • Challenges to starting or receiving ESAs (needle-phobia, unable to self-administer ESAs) • Challenges to administering iron therapy or when increased iron availability is desired • ESA hyporesponsiveness or intolerance • Chronic inflammatory states (CRP ≥3 mg/l) |
| HD patients • Patient preference for oral treatment • Home HD • Hypersensitivity or unavailability of IV iron • ESA hyporesponsiveness or intolerance • Chronic inflammatory states (CRP ≥3 mg/l) |
| Use with caution |
| • Vascular access with a high risk of thrombotic complication • Retinal disordersa • Autoimmune diseasesb • History of cured malignancy or without recurrence for at least 5 years • Kidney transplant recipientsc |
| Avoid or use with extreme caution |
| • Patient with a cardiovascular or thrombotic event in the previous 3 months • History of malignancy in the last 5 years • Polycystic kidney disease • Untreated proliferative diabetic retinopathy, macular degeneration and retinal vein occlusion • Idiopathic pulmonary arterial hypertension |
| Administration key points |
| • Ensure adequate iron stores prior to initiating treatment (ferritin >100 µg/l, TSAT >20%)d • Individualize dose to achieve and maintain target haemoglobin levels of 10–12 g/dl |
| Monitoring key points |
| • Avoid rapid rises in haemoglobin, e.g. >2 g/dl over 4 weeks, or very high haemoglobin levels (>12 g/dl)e; in the case of haemoglobin overcorrection, consider treatment discontinuation for haemoglobin levels >13 g/dl and dose decreases for haemoglobin levels of 12–13 g/dl • Monitor haemoglobin levels at least monthly until the target haemoglobin level of 10–12 g/dl is achieved and stabilized, thereafter as clinically indicated • Monitor potassium and liver function testsf |
Consider close ophthalmology follow-up.
Patients with a known chronic inflammatory disease that could impact erythropoiesis (e.g. systemic lupus erythematosus, rheumatoid arthritis, celiac disease), even if it was in remission were excluded in some of the trials.
Not enrolled in clinical trials, no information on potential interaction with immunosuppressive drugs, unknown effects on the immune system.
For HD patients, the PIVOTAL regime if ferritin <700 µg/l and TSAT ≤40% can be used (at least in patients with a relatively short dialysis duration and no signs of severe inflammation).
These can be associated with an increased risk of thrombotic complications.
Reports of hyperkalaemia and liver injury (uncommon) in clinical trials.
Figure 3:
Potential advantages of HIF-PHi compared to ESA therapy in different CKD populations.
CONCLUSIONS AND FUTURE DIRECTIONS
HIF-PHis offer an alternative pharmacological approach for anaemia correction in CKD but also mediate a number of metabolic pathways beyond anaemia correction that could improve patients’ outcomes and prognosis. However, HIF stabilization is not highly specific and may lead to metabolic cascades and gene expression with unfavourable effects. The phase 3 program of HIF-PHis is one of the largest global investigative programs ever conducted in CKD, with a plethora of data generated, which require careful analyses and vigilance as these agents are approved for use in clinical practice.
Although several large phase 3 trials have been published, they evaluate adverse events over a relatively short treatment period (52–104 weeks). Evidence for MACE non-inferiority compared with ESAs has been demonstrated in patients receiving dialysis [59, 83, 85, 88, 91], but questions remain in patients with NDD-CKD for some HIF-PHis [64, 110]. In fact, the EMA has approved only roxadustat for use in NDD-CKD. The reported data of thromboembolic events from phase 3 trials raise concerns of increased thrombotic risk with HIF‐PHis [59, 62, 79–81, 83, 84, 100]. There is a theoretical potential for oncogenesis based on the putative mechanisms of action of HIF-PHis, with conflicting evidence in patients with NDD-CKD [54, 62]. Regarding diabetic retinopathy, the available data from some of the phase 3 trials [88] are reassuring. Hyperkalaemia is an unexpected but relatively rare adverse effect of HIF-PHi therapy [59, 85, 110] and requires further evaluation. Finally, elucidation of potential benefits of HIF-PHis in ESA-hyporesponsive patients, inflammation, iron metabolism, alleviation of hypoxic kidney injury, rate of kidney function loss and quality of life is needed.
Supplementary Material
ACKNOWLEDGEMENTS
This work was planned as part of the activity of the European Renal Best Practice advisory board of the ERA.
Contributor Information
Sokratis Stoumpos, Renal and Transplant Unit, Queen Elizabeth University Hospital, Glasgow, UK; School of Cardiovascular and Metabolic Health, University of Glasgow, Glasgow, UK.
Kirsty Crowe, Renal and Transplant Unit, Queen Elizabeth University Hospital, Glasgow, UK.
Pantelis Sarafidis, 1st Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessalonki, Greece.
Jonathan Barratt, Department of Cardiovascular Sciences, University of Leicester, Leicester, UK.
Davide Bolignano, Department of Medical and Surgical Sciences, Nephrology Unit, “Magna-Graecia” University, Catanzaro, Italy.
Lucia Del Vecchio, Department of Nephrology and Dialysis, Sant’ Anna Hospital, ASST Lariana, Como, Italy.
Jolanta Małyszko, Department of Nephrology, Dialysis and Internal Medicine, Medical University of Warsaw, Warsaw, Poland.
Andrzej Więcek, Department of Nephrology, Transplantation and Internal Medicine, Medical University of Silesia, Katowice, Poland.
Alberto Ortiz, Division of Nephrology and Hypertension, IIS-Fundación Jiménez Díaz-Universidad Autónoma Madrid, Spain, RICORS2040, Spain.
Mario Cozzolino, Renal Division, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Milan, Italy.
FUNDING
SS research is funded by the Chief Scientific Officer (CSO), Scotland. AO research is supported by FIS/Fondos FEDER (PI22/00469, PI22/00050, PI21/00251, ERA-PerMed-JTC2022 (SPAREKID AC22/00027), FRIAT, Comunidad de Madrid en Biomedicina P2022/BMD-7223, CIFRA_COR-CM. Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0001) funded by European Union NextGenerationEU, Mecanismo para la Recuperación y la Resiliencia (MRR) and SPACKDc PMP21/00109, FEDER funds, COST Action PERMEDIK CA21165, supported by COST (European Cooperation in Science and Technology) 2023-2027 and PREVENTCKD Consortium Project ID: 101101220 Programme: EU4H DG/Agency: HADEA.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article.
CONFLICT OF INTEREST STATEMENT
SS has received consultancy or speaker fees or travel support from Astrazeneca, Astellas, and Vifor. AO has received grants from Sanofi and consultancy or speaker fees or travel support from Adviccene, Alexion, Astellas, Astrazeneca, Amicus, Amgen, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Lilly, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma and Spafarma and is Director of the Catedra UAM-Astrazeneca of chronic kidney disease and electrolytes. He has stock in Telara Farma.
REFERENCES
- 1. Wong MMY, Tu C, Li Y et al. Anemia and iron deficiency among chronic kidney disease stages 3–5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: often unmeasured, variably treated. Clin Kidney J 2020;13:613–24. 10.1093/ckj/sfz091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Astor BC, Muntner P, Levin A et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2002;162:1401–8. 10.1001/archinte.162.12.1401 [DOI] [PubMed] [Google Scholar]
- 3. Evans M, Bower H, Cockburn E et al. Contemporary management of anaemia, erythropoietin resistance and cardiovascular risk in patients with advanced chronic kidney disease: a nationwide analysis. Clin Kidney J 2020;13:821–7. 10.1093/ckj/sfaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. St. Peter WL, Guo H, Kabadi S et al. Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non-dialysis-dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol 2018;19:67. 10.1186/s12882-018-0861-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ershler WB, Chen K, Reyes EB et al. Economic burden of patients with anemia in selected diseases. Value Health 2005;8:629–38. 10.1111/j.1524-4733.2005.00058.x [DOI] [PubMed] [Google Scholar]
- 6. Baumeister SE, Böger CA, Krämer BK et al. Effect of chronic kidney disease and comorbid conditions on health care costs: a 10-year observational study in a general population. Am J Nephrol 2010;31:222–9. 10.1159/000272937 [DOI] [PubMed] [Google Scholar]
- 7. Locatelli F, Pisoni RL, Combe C et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2004;19:121–32. 10.1093/ndt/gfg458 [DOI] [PubMed] [Google Scholar]
- 8. Pereira AA, Sarnak MJ. Anemia as a risk factor for cardiovascular disease. Kidney Int Suppl 2003;87:S32–9. 10.1046/j.1523-1755.64.s87.6.x [DOI] [PubMed] [Google Scholar]
- 9. Hoshino J, Muenz D, Zee J et al. Associations of hemoglobin levels with health-related quality of life, physical activity, and clinical outcomes in persons with stage 3-5 nondialysis CKD. J Ren Nutr 2020;30:404–14. 10.1053/j.jrn.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 10. Toft G, Heide-Jørgensen U, Van Haalen H et al. Anemia and clinical outcomes in patients with non-dialysis dependent or dialysis dependent severe chronic kidney disease: a Danish population-based study. J Nephrol 2020;33:147–56. 10.1007/s40620-019-00652-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Astor BC, Coresh J, Heiss G et al. Kidney function and anemia as risk factors for coronary heart disease and mortality: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2006;151:492–500. 10.1016/j.ahj.2005.03.055 [DOI] [PubMed] [Google Scholar]
- 12. Lopes MB, Tu C, Zee J et al. A real-world longitudinal study of anemia management in non-dialysis-dependent chronic kidney disease patients: a multinational analysis of CKDopps. Sci Rep 2021;11:1784. 10.1038/s41598-020-79254-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Portolés J, Martín L, Broseta JJ et al. Anemia in chronic kidney disease: from pathophysiology and current treatments, to future agents. Front Med (Lausanne) 2021;8:642296. 10.3389/fmed.2021.642296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kidney Disease: Improving Global Outcomes Anemia Work Group . KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012;2:279–335. [Google Scholar]
- 15. Babitt JL, Eisenga MF, Haase VH et al. Controversies in optimal anemia management: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int 2021;99:1280–95. 10.1016/j.kint.2021.03.020 [DOI] [PubMed] [Google Scholar]
- 16. Macdougall IC, Bhandari S, White C et al. Intravenous iron dosing and infection risk in patients on hemodialysis: a prespecified secondary analysis of the PIVOTAL trial. J Am Soc Nephrol 2020;31:1118–27. 10.1681/ASN.2019090972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Macdougall IC, Bock AH, Carrera F et al. Renal function in patients with non-dialysis chronic kidney disease receiving intravenous ferric carboxymaltose: an analysis of the randomized FIND-CKD trial. BMC Nephrol 2017;18:24. 10.1186/s12882-017-0444-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macdougall IC, Bock AH, Carrera F et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 2014;29:2075–84. 10.1093/ndt/gfu201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Macdougall IC, White C, Anker SD et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 2019;380:447–58. 10.1056/NEJMoa1810742 [DOI] [PubMed] [Google Scholar]
- 20. Eschbach JW. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989;111:992–1000. 10.7326/0003-4819-111-12-992 [DOI] [PubMed] [Google Scholar]
- 21. Besarab A, Bolton WK, Browne JK et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998;339:584–90. 10.1056/NEJM199808273390903 [DOI] [PubMed] [Google Scholar]
- 22. Singh AK, Szczech L, Tang KL et al. Correction of anemia with epoetin Alfa in chronic kidney disease. N Engl J Med 2006;355:2085–98. 10.1056/NEJMoa065485 [DOI] [PubMed] [Google Scholar]
- 23. Drüeke TB, Locatelli F, Clyne N et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006;355:2071–84. 10.1056/NEJMoa062276 [DOI] [PubMed] [Google Scholar]
- 24. Pfeffer MA, Burdmann EA, Chen C-Y et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361:2019–32. 10.1056/NEJMoa0907845 [DOI] [PubMed] [Google Scholar]
- 25. Solomon SD, Uno H, Lewis EF et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 2010;363:1146–55. 10.1056/NEJMoa1005109 [DOI] [PubMed] [Google Scholar]
- 26. Szczech LA, Barnhart HX, Inrig JK et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008;74:791–8. 10.1038/ki.2008.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kilpatrick RD, Critchlow CW, Fishbane S et al. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clin J Am Soc Nephrol 2008;3:1077–83. 10.2215/CJN.04601007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drüeke TB. Lessons from clinical trials with erythropoiesis-stimulating agents (ESAs). Ren Replace Ther 2018;4:46. 10.1186/s41100-018-0187-2 [DOI] [Google Scholar]
- 29. Semenza GL. The genomics and genetics of oxygen homeostasis. Annu Rev Genomics Hum Genet 2020;21:183–204. 10.1146/annurev-genom-111119-073356 [DOI] [PubMed] [Google Scholar]
- 30. Gupta N, Wish JB. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis 2017;69:815–26. 10.1053/j.ajkd.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 31. Wang GL, Jiang BH, Rue EA et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995;92:5510–4. 10.1073/pnas.92.12.5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gu Y-Z, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol 2000;40:519–61. 10.1146/annurev.pharmtox.40.1.519 [DOI] [PubMed] [Google Scholar]
- 33. Haase VH. Hypoxia-inducible factor-prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int Suppl (2011) 2021;11:8–25. 10.1016/j.kisu.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol 2012;23:1631–4. 10.1681/ASN.2011111078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang BH, Semenza GL, Bauer C et al. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 1996;271:C1172–80. 10.1152/ajpcell.1996.271.4.C1172 [DOI] [PubMed] [Google Scholar]
- 36. Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ 2008;15:621–7. 10.1038/cdd.2008.12 [DOI] [PubMed] [Google Scholar]
- 37. Del Balzo U, Signore PE, Walkinshaw G et al. Nonclinical characterization of the hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat, a novel treatment of anemia of chronic kidney disease. J Pharmacol Exp Ther 2020;374:342–53. 10.1124/jpet.120.265181 [DOI] [PubMed] [Google Scholar]
- 38. Hanudel MR, Wong S, Jung G et al. Amelioration of chronic kidney disease-associated anemia by vadadustat in mice is not dependent on erythroferrone. Kidney Int 2021;100:79–89. 10.1016/j.kint.2021.03.019 [DOI] [PubMed] [Google Scholar]
- 39. Kurata Y, Tanaka T, Nangaku M. Hypoxia-inducible factor prolyl hydroxylase inhibitor in the treatment of anemia in chronic kidney disease. Curr Opin Nephrol Hypertens 2020;29:414–22. 10.1097/MNH.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 40. Sugahara M, Tanaka T, Nangaku M. Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int 2017;92:306–12. 10.1016/j.kint.2017.02.035 [DOI] [PubMed] [Google Scholar]
- 41. Tcholakov I, Grimshaw CE, Shi L et al. Time-dependent inhibition of PHD2. Biosci Rep 2017;37:BSR20170275. 10.1042/BSR20170275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yeh T-L, Leissing TM, Abboud MI et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem Sci 2017;8:7651–68. 10.1039/C7SC02103H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haase VH. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol 2010;299:F1–13. 10.1152/ajprenal.00174.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sibbel S, Maroni BJ, Brunelli SM. The effect of altitude on erythropoiesis-stimulating agent dose, hemoglobin level, and mortality in hemodialysis patients. J Nephrol 2017;30:821–9. 10.1007/s40620-016-0350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ng Y-H, Myers O, Shore X et al. The association of altitude and the prevalence of anemia among people with CKD. Am J Kidney Dis 2019;74:715–8. 10.1053/j.ajkd.2019.04.015 [DOI] [PubMed] [Google Scholar]
- 46. Haase VH. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int 2017;21(Suppl 1):S110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hill P, Shukla D, Tran MGB et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 2008;19:39–46. 10.1681/ASN.2006090998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kapitsinou PP, Jaffe J, Michael M et al. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol Renal Physiol 2012;302:F1172–9. 10.1152/ajprenal.00667.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stanigut AM, Pana C, Enciu M et al. Hypoxia-inducible factors and diabetic kidney disease-how deep can we go? Int J Mol Sci 2022;23:10413. 10.3390/ijms231810413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Forsythe JA, Jiang B-H, Iyer NV et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996;16:4604–13. 10.1128/MCB.16.9.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carmeliet P, Dor Y, Herbert J-M et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998;394:485–90. 10.1038/28867 [DOI] [PubMed] [Google Scholar]
- 52. Ryan HE, Poloni M, McNulty W et al. Hypoxia-inducible factor-1α is a positive factor in solid tumor growth. Cancer Res 2000;60:4010–5. [PubMed] [Google Scholar]
- 53. Nishide S, Uchida J, Matsunaga S et al. Prolyl-hydroxylase inhibitors reconstitute tumor blood vessels in mice. J Pharmacol Sci 2020;143:122–6. 10.1016/j.jphs.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 54. Coyne DW, Roger SD, Shin SK et al. Roxadustat for CKD-related anemia in non-dialysis patients. Kidney Int Rep 2021;6:624–35. 10.1016/j.ekir.2020.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fishbane S, El-Shahawy MA, Pecoits-Filho R et al. Roxadustat for treating anemia in patients with CKD not on dialysis: results from a randomized phase 3 study. J Am Soc Nephrol 2021;32:737–55. 10.1681/ASN.2020081150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shutov E, Sułowicz W, Esposito C et al. Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, double-blind, placebo-controlled study (ALPS). Nephrol Dial Transplant 2021;36:1629–39. 10.1093/ndt/gfab057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen N, Hao C, Peng X et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med 2019;381:1001–10. 10.1056/NEJMoa1813599 [DOI] [PubMed] [Google Scholar]
- 58. Johansen KL, Cobitz AR, Singh AK et al. The ASCEND-NHQ randomized trial found positive effects of daprodustat on hemoglobin and quality of life in patients with non-dialysis chronic kidney disease. Kidney Int 2022;103:1180–92. [DOI] [PubMed] [Google Scholar]
- 59. Provenzano R, Szczech L, Leong R et al. Efficacy and cardiovascular safety of roxadustat for treatment of anemia in patients with non–dialysis-dependent CKD: pooled results of three randomized clinical trials. Clin J Am Soc Nephrol 2021;16:1190–200. 10.2215/CJN.16191020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barratt J, Andric B, Tataradze A et al. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, open-label, active-controlled study (DOLOMITES). Nephrol Dial Transplant 2021;36:1616–28. 10.1093/ndt/gfab191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Akizawa T, Iwasaki M, Otsuka T et al. Phase 3 study of roxadustat to treat anemia in non-dialysis-dependant CKD. Kidney Int Rep 2021;6:1810–28. 10.1016/j.ekir.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Singh AK, Carroll K, Mcmurray JJV et al. Daprodustat for the treatment of anemia in patients not undergoing dialysis. N Engl J Med 2021;385:2313–24. 10.1056/NEJMoa2113380 [DOI] [PubMed] [Google Scholar]
- 63. Nangaku M, Hamano T, Akizawa T et al. Daprodustat compared with epoetin beta pegol for anemia in Japanese patients not on dialysis: a 52-week randomized open-label phase 3 trial. Am J Nephrol 2021;52:26–35. 10.1159/000513103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chertow GM, Pergola PE, Farag YMK et al. Vadadustat in patients with anemia and non–Dialysis-dependent CKD. N Engl J Med 2021;384:1589–600. 10.1056/NEJMoa2035938 [DOI] [PubMed] [Google Scholar]
- 65. Nangaku M, Kondo K, Kokado Y et al. Phase 3 randomized study comparing vadadustat with darbepoetin alfa for anemia in Japanese patients with nondialysis-dependent CKD. J Am Soc Nephrol 2021;32:1779–90. 10.1681/ASN.2020091311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yamamoto H, Nobori K, Matsuda Y et al. Efficacy and safety of molidustat for anemia in ESA-naive nondialysis patients: a randomized, phase 3 trial. Am J Nephrol 2021;52:871–83. 10.1159/000518071 [DOI] [PubMed] [Google Scholar]
- 67. Akizawa T, Nangaku M, Yamaguchi T et al. A phase 3 study of enarodustat in anemic patients with CKD not requiring dialysis: the SYMPHONY ND study. Kidney Int Rep 2021;6:1840–9. 10.1016/j.ekir.2021.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yamamoto H, Nobori K, Matsuda Y et al. Molidustat for renal anemia in nondialysis patients previously treated with erythropoiesis-stimulating agents: a randomized, open-label, phase 3 study. Am J Nephrol 2021;52:884–93. 10.1159/000518072 [DOI] [PubMed] [Google Scholar]
- 69. Agrawal D, Varade D, Shah H et al. Desidustat in anemia due to non-dialysis-dependent chronic kidney disease: a phase 3 study (DREAM-ND). Am J Nephrol 2022;53:352–60. 10.1159/000523961 [DOI] [PubMed] [Google Scholar]
- 70. Fatima K, Ahmed W, Fatimi AS et al. Evaluating the safety and efficacy of daprodustat for anemia of chronic kidney disease: a meta-analysis of randomized clinical trials. Eur J Clin Pharmacol 2022;78:1867–75. 10.1007/s00228-022-03395-y [DOI] [PubMed] [Google Scholar]
- 71. Locatelli F, Del Vecchio L. Hypoxia-inducible factor-prolyl hydroxyl domain inhibitors: from theoretical superiority to clinical noninferiority compared with current ESAs? J Am Soc Nephrol 2022;33:1966–79. 10.1681/ASN.2022040413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Minutolo R, Liberti ME, Simeaon V et al. Efficacy and safety of hypoxia-inducible factor prolyl hydroxylase inhibitors in patients with chronic kidney disease: meta-analysis of phase 3 randomized controlled trials. Clin Kidney J 2023;17:sfad143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Akizawa T, Nangaku M, Yamaguchi T et al. A phase 3 study of enarodustat (JTZ-951) in Japanese hemodialysis patients for treatment of anemia in chronic kidney disease: SYMPHONY HD study. Kidney Dis 2021;7:494–502. 10.1159/000517053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Akizawa T, Tanaka-Amino K, Otsuka T et al. Factors affecting doses of roxadustat versus darbepoetin alfa for anemia in nondialysis patients. Am J Nephrol 2021;52:702–13. 10.1159/000519043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Miki K, Nakamura Y, Yokoyama T et al. Therapeutic effect of roxadustat on patients with posttransplant anemia. Transplant Proc 2022;54:671–7. 10.1016/j.transproceed.2022.02.004 [DOI] [PubMed] [Google Scholar]
- 76. Nakamura N, Aso S, Nakagawa C et al. Efficacy and safety of hypoxia-inducible factor prolyl hydroxylase inhibitor therapy for anemia in renal transplantation patients by prior erythropoiesis stimulating agent use. Transplant Proc 2023;55:829–31. 10.1016/j.transproceed.2023.03.062 [DOI] [PubMed] [Google Scholar]
- 77. Li J, Ma K, Wang L et al. Efficacy and safety of roxadustat in the treatment of renal allograft anemia patients: a case series. Ann Palliat Med 2021;10:11859–67. 10.21037/apm-21-2916 [DOI] [PubMed] [Google Scholar]
- 78. Li H, Hu S-M, Li Y-M et al. Beneficial effect of roxadustat on early posttransplant anemia and iron utilization in kidney transplant recipients: a retrospective comparative cohort study. Ann Transl Med 2022;10:1360. 10.21037/atm-22-5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Provenzano R, Shutov E, Eremeeva L et al. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant 2021;36:1717–30. 10.1093/ndt/gfab051 [DOI] [PubMed] [Google Scholar]
- 80. Fishbane S, Pollock CA, El-Shahawy M et al. Roxadustat versus epoetin alfa for treating anemia in patients with chronic kidney disease on dialysis: results from the randomized phase 3 ROCKIES study. J Am Soc Nephrol 2022;33:850–66. 10.1681/ASN.2020111638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Charytan C, Manllo-Karim R, Martin ER et al. A randomized trial of roxadustat in anemia of kidney failure: SIERRAS study. Kidney Int Rep 2021;6:1829–39. 10.1016/j.ekir.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Singh AK, Cizman B, Carroll K et al. Efficacy and safety of daprodustat for treatment of anemia of chronic kidney disease in incident dialysis patients: a randomized clinical trial. JAMA Intern Med 2022;182:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eckardt K-U, Agarwal R, Aswad A et al. Safety and efficacy of vadadustat for anemia in patients undergoing dialysis. N Engl J Med 2021;384:1601–12. 10.1056/NEJMoa2025956 [DOI] [PubMed] [Google Scholar]
- 84. Csiky B, Schömig M, Esposito C et al. Roxadustat for the maintenance treatment of anemia in patients with end-stage kidney disease on stable dialysis: a European phase 3, randomized, open-label, active-controlled study (PYRENEES). Adv Ther 2021;38:5361–80. 10.1007/s12325-021-01904-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen N, Hao C, Liu B-C et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med 2019;381:1011–22. 10.1056/NEJMoa1901713 [DOI] [PubMed] [Google Scholar]
- 86. Singh AK, Carroll K, Perkovic V et al. Daprodustat for the treatment of anemia in patients undergoing dialysis. N Engl J Med 2021;385:2325–35. 10.1056/NEJMoa2113379 [DOI] [PubMed] [Google Scholar]
- 87. Coyne DW, Singh AK, Lopes RD et al. Three times weekly dosing of daprodustat versus conventional epoetin for treatment of anemia in hemodialysis patients: ASCEND-TD: a phase 3 randomized, double-blind, noninferiority trial. Clin J Am Soc Nephrol 2022;17:1325–36. 10.2215/CJN.00550122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Akizawa T, Nangaku M, Yonekawa T et al. Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: a randomized, double-blind, phase 3 trial. Clin J Am Soc Nephrol 2020;15:1155–65. 10.2215/CJN.16011219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Akizawa T, Yamada T, Nobori K et al. Molidustat for Japanese patients with renal anemia receiving dialysis. Kidney Int Rep 2021;6:2604–16. 10.1016/j.ekir.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gang S, Khetan P, Varade D et al. Desidustat in anemia due to dialysis-dependent chronic kidney disease: a phase 3 study (DREAM-D). Am J Nephrol 2022;53:343–51. 10.1159/000523949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Akizawa T, Iwasaki M, Yamaguchi Y et al. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol 2020;31:1628–39. 10.1681/ASN.2019060623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hou Y-P, Mao X-Y, Wang C et al. Roxadustat treatment for anemia in peritoneal dialysis patients: a randomized controlled trial. J Formos Med Assoc 2022;121:529–38. 10.1016/j.jfma.2021.06.004 [DOI] [PubMed] [Google Scholar]
- 93. Nangaku M, Kondo K, Ueta K et al. Efficacy and safety of vadadustat compared with darbepoetin alfa in Japanese anemic patients on hemodialysis: a phase 3, multicenter, randomized, double-blind study. Nephrol Dial Transplant 2021;36:1731–41. 10.1093/ndt/gfab055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barratt J, Sulowicz W, Schömig M et al. Efficacy and cardiovascular safety of roxadustat in dialysis-dependent chronic kidney disease: pooled analysis of four phase 3 studies. Adv Ther 2021;38:5345–60. 10.1007/s12325-021-01903-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Choukroun G, Harkavyi A, Santos V et al. #2959 Efficacy and safety of roxadustat in patients with anemia of dialysis-dependent ckd with or without inflammation: a pooled analysis of 4 phase 3 studies. Nephrol Dial Transplant 2023;38(Suppl 1):gfad063c_2959. [Google Scholar]
- 96. Takkavatakarn K, Thammathiwat T, Phannajit J et al. The impacts of hypoxia-inducible factor stabilizers on laboratory parameters and clinical outcomes in chronic kidney disease patients with renal anemia: a systematic review and meta-analysis. Clin Kidney J 2023;16:845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Natale P, Palmer SC, Jaure A et al. Hypoxia-inducible factor stabilisers for the anaemia of chronic kidney disease. Cochrane Database Syst Rev 2022;8:CD013751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nangaku M, Akizawa T, Nagakubo T et al. Safety of daprodustat in patients with anemia of chronic kidney disease: a pooled analysis of phase 3 studies in Japan. Ther Apher Dial 2022;26:1065–78. 10.1111/1744-9987.13839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xiong L, Zhang H, Guo Y et al. Efficacy and safety of vadadustat for anemia in patients with chronic kidney disease: a systematic review and meta-analysis. Front Pharmacol 2022;12:795214. 10.3389/fphar.2021.795214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Abdelazeem B, Shehata J, Abbas KS et al. The efficacy and safety of roxadustat for the treatment of anemia in non-dialysis dependent chronic kidney disease patients: an updated systematic review and meta-analysis of randomized clinical trials. PLoS One 2022;17:e0266243. 10.1371/journal.pone.0266243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Singh AK, McCausland FR, Claggett BL et al. Analysis of on-treatment cancer safety events with daprodustat versus conventional erythropoiesis-stimulating agents-post hoc analyses of the ASCEND-ND and ASCEND-D trials. Nephrol Dial Transplant 2023;38:1890–7. 10.1093/ndt/gfac342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zheng Q, Wang Y, Yang H et al. Cardiac and kidney adverse effects of HIF prolyl-hydroxylase inhibitors for anemia in patients with CKD not receiving dialysis: a systematic review and meta-analysis. Am J Kidney Dis 2023;81:434–445.e1. 10.1053/j.ajkd.2022.09.014 [DOI] [PubMed] [Google Scholar]
- 103. Winkelmayer WC, Walther CP. Cardiovascular safety of roxadustat in CKD anemia: a fig leaf named noninferiority. Clin J Am Soc Nephrol 2021;16:1155–7. 10.2215/CJN.08280621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wong MMY, Tu C, Li Y et al. Anemia and iron deficiency among chronic kidney disease stages 3-5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: often unmeasured, variably treated. Clin Kidney J 2020;13:613–24. 10.1093/ckj/sfz091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. European Medicines Agency . Assessment report. Evrenzo. 2021. https://www.ema.europa.eu/en/documents/assessment-report/evrenzo-epar-public-assessment-report_en.pdf
- 106. Fukuta H, Hagiwara H, Kamiya T. Hypoxia-inducible factor prolyl hydroxylase inhibitors for anemia in heart failure patients: a protocol for systematic review and meta-analysis. PLoS One 2022;17:e0275311. 10.1371/journal.pone.0275311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yap DYH, McMahon LP, Hao C‐M et al. Recommendations by the Asian Pacific Society of Nephrology (APSN) on the appropriate use of HIF-PH inhibitors. Nephrology (Carlton) 2021;26:105–18. 10.1111/nep.13835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ku E, Del Vecchio L, Eckardt KU et al. Novel anemia therapies in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2023;104:655–80. [DOI] [PubMed] [Google Scholar]
- 109. Wish JB. Treatment of anemia in kidney disease: beyond erythropoietin. Kidney Int Rep 2021;6:2540–53. 10.1016/j.ekir.2021.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chen N, Hao C, Peng X et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med 2019;381:1001–10. 10.1056/NEJMoa1813599 [DOI] [PubMed] [Google Scholar]
- 111. Zheng Q, Yang H, Sun L et al. Efficacy and safety of HIF prolyl-hydroxylase inhibitor vs epoetin and darbepoetin for anemia in chronic kidney disease patients not undergoing dialysis: a network meta-analysis. Pharmacol Res 2020;159:105020. 10.1016/j.phrs.2020.105020 [DOI] [PubMed] [Google Scholar]
- 112. Chen D, Niu Y, Liu F et al. Safety of HIF prolyl hydroxylase inhibitors for anemia in dialysis patients: a systematic review and network meta-analysis. Front Pharmacol 2023;14:1163908. 10.3389/fphar.2023.1163908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lei J, Li H, Wang S. Efficacy and safety of roxadustat in patients with chronic kidney disease: an updated meta-analysis of randomized controlled trials including 6,518 patients. Biomed Res Int 2022;2022:2413176. 10.1155/2022/2413176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen J, Shou X, Xu Y et al. A network meta-analysis of the efficacy of hypoxia-inducible factor prolyl-hydroxylase inhibitors in dialysis chronic kidney disease. Aging (Albany NY) 2023;15:2237–74. 10.18632/aging.204611 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article.