Abstract
Metformin is widely used drugs in the treatment of type 2 diabetes mellitus. However, the mechanisms of action are complex and are still not fully understood yet. Metformin has a dose-dependent blood sugar-lowering effect. The most common adverse reactions of metformin are gastrointestinal symptoms, and women tend to be more experienced than men. A positive correlation between the administration of duration and the daily dose of metformin and the risk of vitamin B12 deficiency is confirmed. Novel glucose-lowering mechanism through the activation of AMP-activated protein kinase and alteration of gut microbiota composition is identified. In addition, metformin has immunomodulatory properties in various mechanisms, including anti-inflammatory actions, and so forth. Metformin improves insulin sensitivity, which may reduce the risk of tumor growth in certain cancers. The antiviral effects of metformin may occur through several mechanisms, including blocking angiotensin converting enzyme 2 receptor, and so forth. These potential mechanisms of metformin are promising in various clinical settings, such as inflammatory diseases, autoimmune diseases, cancer, and coronavirus disease 2019.
Keywords: Metformin, Diabetes Mellitus, Adverse Drug Reaction, Neoplasms, COVID-19
INTRODUCTION
Metformin is a widely used biguanide with a good safety profile that is the first-line treatment for type 2 diabetes mellitus (T2DM) [1,2]. However, the mechanisms of its therapeutic action are complex and are still not fully understood yet.
Metformin’s origins date back to 1772 when Galega officinalis was used to treat diabetic symptoms. In 1844–1861, Strecker synthesized guanidine, and Rathke synthesized biguanide in 1878–1879. In 1918, Watanabe confirmed the hypoglycemic effect of guanidine in animals, and Werner and Bell synthesized dimethylbiguanide in 1922. In 1957, Jean Sterne of France published the use of metformin for the treatment of diabetes, and metformin was introduced in the United Kingdom and other European countries in 1958 [3].
However, it took a long time for it to be approved by the US Food and Drug Administration (FDA), as it was first approved as a treatment for T2DM in 1994. FDA approval means that metformin is a safe and effective drug, and extensive clinical studies have proven its efficacy and safety. Additionally, metformin has a relatively low side effect profile compared to other hypoglycemic agents. Metformin has become one of the most widely used drugs in the treatment of T2DM. In 1998, the very important results of UK Prospective Diabetes Study were published, and it was reported that it slows the progression of prediabetes to T2DM in 2002. In 2011, metformin was included in the essential medicines lists of World Health Organization [3].
Recently, as the possibility of anti-inflammatory, cardiovascular protective, antibacterial, antiviral even anti-cancer effects beyond the anti-diabetic effect has been raised, understanding and various research on the mechanism of action of metformin are in progress [4].
PHARMACOLOGICAL TARGETS OF METFORMIN
Blood glucose levels are reduced both fasting and postprandial state, resulting in a decrease in hemoglobin A1c (HbA1c), as reported in numerous clinical trials [5,6]. Metformin has been shown to act via both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. At the molecular level, the findings vary depending on the doses of metformin used and duration of treatment, with clear differences between acute and chronic administration. Widely studied mechanisms of action, such as mitochondrial complex I of respiratory chain (mComplex-I) inhibition leading to AMPK activation, have only been observed in the context of suprapharmacological (>1 mM) metformin concentrations, which do not occur in the clinical setting. Recent studies have shown that clinically relevant (50–100 μM) concentrations of metformin inhibit hepatic gluconeogenesis in a substrate-selective manner, supporting a redox-dependent mechanism of metformin action [7,8].
Clinically relevant concentrations of metformin inhibit the lysosomal proton pump vacuolar ATPase (v-ATPase), which is a central node for AMPK activation following glucose starvation. Metformin-bound presenilin enhancer 2 (PEN-2) forms a complex with ATP6AP1 (accessory factor of v-ATPase), which leads to the inhibition of v-ATPase and the activation of AMPK without effects on cellular AMP levels. Metformin binds PEN2 and initiates a signaling route that intersects, through ATP6AP1, the lysosomal glucose-sensing pathway for AMPK activation [9,10].
The activation of AMPK by metformin could be consequent to mComplex-I inhibition and raised AMP through the canonical adenine nucleotide pathway or alternatively by activation of the lysosomal AMPK pool by other mechanisms involving the aldolase substrate fructose-1,6-bisphosphate or perturbations in the lysosomal membrane. In conditions of either glucose excess or gluconeogenic substrate excess, metformin lowers hexose monophosphates by mechanisms that are independent of AMPK-activation and most likely mediated by allosteric activation of phosphofructokinase 1 and/or inhibition of fructose-1,6-bisphosphatase 1 (FBP-1) [11].
Metformin is used to increase insulin sensitivity in insulin-resistant (IR) conditions such as diabetes, obesity, and polycystic ovary syndrome. There is a well-documented correlation between glucose transporter 4 (GLUT-4) expression and the level of IR. Therefore, the observed increase in peripheral glucose utilization after metformin administration most likely comes from the induction of GLUT-4 expression and its increased translocation to the plasma membrane. Overall, there are the complex interactions between metformin action, GLUT-4 expression, GLUT-4 translocation, and the observed increase in peripheral insulin sensitivity [12,13]. Metformin has a direct and AMPK-dependent effect on glucagon-like peptide 1 (GLP-1) secreting L cells and increases postprandial GLP-1 secretion, which seems to contribute to blood glucose-lowering effect of metformin [14].
PHARMACOKINETICS OF METFORMIN
Under fasting conditions, the bioavailability of the metformin hydrochloride 500 mg tablet is 50%–60%. The distribution volume for the 850 mg of metformin is approximately 654±358 L. Metformin is a hydrophilic base which exists at physiological pH as the cationic species, therefore, its passive diffusion through cell membranes should be very limited. It is absorbed predominately from the small intestine. Steady-state plasma concentration of the metformin is reached within 24 to 48 hours, and the elimination half-life is approximately 6.2 hours [15]. Metformin is excreted unchanged in the urine, with no significant hepatic metabolism or biliary excretion, and a fecal excretion of an unabsorbed fraction (20%–30%) is reported [16].
The oral absorption, hepatic uptake and renal excretion of metformin are mediated very largely by organic cation transporters (OCTs). Membrane transporters play a substantial role in its absorption, tissues distribution, and renal elimination. Several organic cation transporters are determinants of the pharmacokinetics of metformin, and many of them are important in its action, as entering mediators into target tissues [17].
Therefore, in order to find the individual differences in the therapeutic response to metformin, the effect of transporter polymorphisms on metformin is investigated. As a result, pharmacokinetic parameters of metformin were found to be affected by age, sex, ethnicity, and several OCT genes (e.g., SLC22A4) [18]. In meta-analysis, the associations between OCT genetic polymorphisms and metformin response and intolerance in patients with T2DM is revealed. The SLC22A1 rs622342 and rs628031 polymorphisms were potentially associated with glycemic response to metformin. This result may provide new insight into gene-based tailored medicine for diabetes [19].
The metformin has a dose-dependent blood sugar-lowering effect [14,20,21]. The pharmacokinetics of metformin have been reported to be similar in women and men, considering the weight differences between the sexes [22]. Food intake decreases the extent of absorption and delays the absorption of metformin. Specifically, when metformin is taken with food, the Cmax (maximum concentration) is 40% lower, the area under the curve is approximately 29% lower, and the Tmax (time to reach maximum concentration) is extended by 35 minutes compared to when the drug is taken in a fasted state. In particular, a diet rich in fat and high in calories can significantly decrease the amount and rate of absorption [23].
The metformin administration is not increased the risk of acidosis at an estimated glomerular filtration rate (eGFR) of 45 mL/min/1.73 m2 or higher, whereas may be increased at an eGFR of less than 30. Impaired renal function is followed by multiple complications because of the prolongation of the half-life and elevation of the plasma concentration of metformin. US FDA guidelines recommends that metformin should not be used at an eGFR less than 30 [24].
ADVERSE DRUG REACTIONS
Even though metformin is generally considered a safe drug, adverse reactions may occur in some patients. The most common adverse reactions are gastrointestinal symptoms, such as nausea and vomiting, which commonly appear in the early stages of taking the drug. Gastrointestinal symptoms can be alleviated when taken with a meal [25].
Other adverse effects commonly reported are abdominal pain or discomfort, and diarrhea. If the patients have reduced renal function, the risk of lactic acidosis, the most serious events, may be increased. Lactic acidosis causing symptoms such as myalgia, lethargy, dyspnea, abdominal pain, and confusion, is a rare event with an incidence of one in 30,000 patients, but can lead to fatal outcomes [26]. Generally, the observed cases occurred in patients who received high doses and/or had severe hepatic and renal impairment, old age, and alcoholism [27,28].
1. Sex-Related Difference and Intolerance
Adverse reactions of metformin may appear differently depending on sex. Some studies show that women tend to experience more gastrointestinal side effects than men [29,30]. This is because women’s gastrointestinal tract sensitivities (e.g., increase intestinal release of GLP-1) may be different from men’s [2].1 Because of differences in metabolism between women and men, interactions between metformin and other medications may occur differently. This may affect the frequency and severity of adverse reactions. Therefore, when taking metformin, it is necessary to monitor and manage adverse reactions depending on sex. Very few clinical studies have reported sex-related differences in efficacy and safety in the therapeutic use of metformin. The main differences are summarized (Table 1) [31].
Table 1.
Sex-related differences in diabetes mellitus complications and therapeutic use of metformin
| Variable | Female (vs. male) |
|---|---|
| Diabetes mellitus | - Higher all-cause mortality |
| - Higher risk PAD | |
| - Higher RR for oral, stomach, and kidney cancers, and leukemia | |
| Metformin treatment: | |
| Anti-hyperglycemic effect | No difference |
| Pharmacokinetics | No difference, considering weight difference and monitoring eGFR |
| ADRs | Higher |
| CRC incidence rate | Lower |
| CRC-specific mortality | Lower |
| COVID-19 | Lower severity and mortality |
Reprinted from Froldi G. Pharmaceuticals (Basel) 2024;17:478 [31].
PAD, peripheral artery disease; RR, relative risk; eGFR: estimated glomerular filtration rate; ADRs, adverse drug reactions; CRC, colorectal cancer; COVID-19, coronavirus disease 2019.
The associated factors with gastrointestinal intolerance of metformin are genotype variability, comorbidities, co-medications, and the history of abdominal surgery [32]. In euglycemic men, the metformin-induced microbial compositional changes were investigated whether the pre-treatment gut microbiota was related to gastrointestinal adverse events during metformin treatment. As a result, a reduced abundance of Intestinibacter spp. and Clostridium spp., as well as an increased abundance of Escherichia/Shigella spp. and Bilophila wadsworthia is found. Therefore, it is suggested that pre-treatment gut microbiota composition may be a determinant for development of gastrointestinal adverse effects following metformin intake [33]. Additionally, it was found that the mechanisms by microbial mediation of the therapeutic effects of metformin through short-chain fatty acid (SCFA) production, as well as potential microbiota-mediated mechanisms behind known intestinal adverse effects of a relative increase in abundance of Escherichia spp [34].
There are several strategies of gastrointestinal tolerance, such as an appropriate titration of immediate-release metformin, use of extended-release metformin, and the combination of metformin and gut microbiome modulators (GMMs), and so forth [32]. Modulating the composition of the gut microbiota was proposed as a method to manage chronic metabolic diseases (e.g., obesity, T2DM), and this is undertaken by using probiotics, prebiotics or synbiotics [35]. A pilot randomized trial was performed in patients with T2DM taking metformin with the GMMs vs placebo. The group taking with metformin/GMM combination were more tolerable than metformin/placebo. Also, patients taking the metformin/GMM combination had significantly lower fasting blood glucose levels [36].
2. Metformin-Induced Vitamin B12 Deficiency
Metformin exposure may be an iatrogenic cause for exacerbation of peripheral neuropathy in patients with T2DM [37]. Recently, in Chinese patients with T2DM, metformin treatment was associated with an increased risk of diabetic peripheral neuropathy (DPN) admission and this risk responds positively to the daily dose of metformin. In particular, the daily dose of metformin was positively associated with DPN risk. The risk of DPN was 1.5 and 4.3-fold higher in patients with doses of 1,000–2,000 mg/d and >2,000 mg/d, respectively. However, co-administration of vitamin B12 may avoid the risk of DPN associated with metformin use [38].
There are no definite guidelines for screening, diagnosing, and treating vitamin B12 deficiency in patients with T2DM on metformin therapy. Therefore, in the literature, the prevalence of metformin-induced vitamin B12 deficiency is very widely ranged from 6% to 50%, because of the various deficiency cutoff values, the lack of clinical biomarkers in diagnosis, and the heterogeneous study populations concerning age, region, dietary habits, and the daily dose and use duration of metformin [39].
In a real-world evidence database study, a positive correlation between the duration of metformin use and the risk of vitamin B12 deficiency was revealed. Long-term (4 years or more) metformin use was associated with a significantly increased risk of vitamin B12 deficiency in patients with T2DM [40]. Current “metformin daily dose” is an accurate proxy of both cumulative metformin exposure and duration of T2DM. Therefore, metformin-induced vitamin B12 deficiency should be screened by annual laboratory monitoring regardless of the duration of T2DM—especially, 80 years old or more, vegetarian, daily 1,500 mg or more of metformin use, or no concomitant vitamin B12 supplementation [41].
NEW GLUCOSE-LOWERING MECHANISMS OF METFORMIN
1. AMPK-Dependent and AMPK-Independent Mechanism
Metformin inhibits hepatic glucose production through a mechanism linked to perturbation of intracellular ATP levels rather direct inhibition of gluconeogenic gene expression. No AMPK is essential for metformin inhibition of hepatic glucose production. Metformin has been shown to act via both AMPK-dependent and AMPK-independent mechanisms; by inhibition of mitochondrial respiration but also by inhibition of mitochondrial glycerophosphate dehydrogenase (mGPDH), and a mechanism involving the lysosome [7].
Metformin affects energy metabolism by activating AMPK. Activation of AMPK reduces the activity of enzymes (e.g., FBP-1, adenylate cyclase) in liver cells required for glucose production. AMPK senses the energy status of cells and regulates metabolic processes when energy is insufficient, suppressing glucose production and promoting fatty acid oxidation (Figure 1) [4].
Figure. 1.
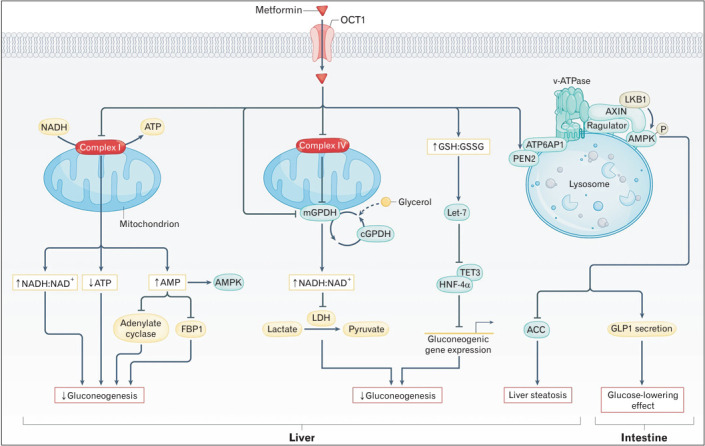
Proposed mechanisms for metformin-induced reductions in blood levels of glucose. Reprinted from Foretz M, et al. Nat Rev Endocrinol 2023;19:460-76.4) with permission from Springer Nature. NADH, nicotinamide adenine dinucleotide; ATP, adenosine triphosphate; AMPK, AMP-activated protein kinase; FBP1, fructose-1-6-bisphosphatase; mGPDH, mitochondrial glycerol-3-phosphate dehydrogenase; cGPDH, cytosolic glycerol-3-phosphate dehydrogenase; LDH, lactate dehydrogenase; GSH:GSSG, glutathione to oxidized glutathione ratio; TET3, Tet methylcytosine dioxygenase 3; HNF-4α, hepatocyte nuclear factor 4α; ATP6AP1, ATPase H+ transporting accessory protein 1; ACC, acetylCoA carboxylase; LKB1, liver kinase B1; GLP1, glucagon-like peptide 1; PEN2, presenilin enhancer 2.
It is accepted that mComplex-I as an opening point of metformin is therapeutic targets at the cellular level, and metformin causes inhibition of mitochondrial ATP production [42]. mComplex-I is the enzyme solely responsible for the oxidation of nicotinamide adenine dinucleotide (NADH) and therefore regeneration of NAD+ for catabolism. mComplex-I catalyzes the reduction of membrane ubiquinone (Q) to ubiquinol (QH2) by NADH provided from the upstream catabolic pathways (tricarboxylic acid cycle, fatty acid oxidation, malate-aspartate shuttle, and so forth). Even a slight inhibition of mComplex-I by metformin could result in the slowing down of oxidative phosphorylation (OXPHOS). These decreases adenylate energy charge and activates AMPK. The reduction in cellular energy charge could be sufficient to explain for the decrease in hepatic gluconeogenic flux [43].
There is a substantial data showing modulation of mitochondrial reactive oxygen species (ROS) production by metformin in preparations of cells, intact mitochondria, sub-mitochondrial particles, or isolated enzyme [42]. In addition to, it was suggested that metformin may bind differently to the active (A) and deactive (D) form, and is a weak effector of the A/D transition of mitochondrial complex I. At high concentrations, metformin increases the rate of spontaneous deactivation of complex I (A/D transition). This result implicates that it can either stimulate or inhibit mitochondrial ROS production depending on the preferential respiratory substrate [42].
Metformin non-competitively inhibits the redox shuttle enzyme mGPDH, resulting in an altered hepatocellular redox state, reduced conversion of lactate and glycerol to glucose, and decreased hepatic gluconeogenesis [44].
2. Modulation of Microbial Communities
Recent studies have shown that metformin interacts with gut microbes to influence metabolism. First, metformin is known to change the composition of the intestinal microbial community, with the effect of increasing the diversity of intestinal microorganisms. This can contribute to promoting the growth of beneficial microorganisms in the intestines and inhibiting the growth of certain harmful microorganisms.
Additionally, metformin improves intestinal permeability by strengthening barrier function and reduces intestinal inflammation, which plays an important role in maintaining the balance of intestinal microorganisms. Metformin reduces the absorption of glucose from the intestines and improves insulin sensitivity in muscle and fat cells, thereby contributing to blood sugar control by helping glucose to be absorbed more effectively into cells [33,34].
Trans-ethnic gut microbiota alterations of metformin have been established, with the increased abundance of two taxonomic units from Bacteroides and a reduced abundance of one taxonomic unit from Faecalibacterium [45]. The gut microbial changes after metformin administration is associated with increased levels of Escherichia spp. and decreased levels of Intestinibacter spp. in both euglycemic adults and patients with T2DM [33,46]. Also, in subjects with treatment-naïve type 2 diabetes, an association between metformin and the abundance of Akkermansia muciniphila is shown. However, no correlations between %HbA1c and A. munciniphila abundance [46].
Metformin may increase the concentration of SCFAs, which gut microbes produce by fermenting fiber. The most common SCFAs include acetic acid, propionic acid, and butyric acid. They are produced during the fermentation process of intestinal microorganisms, and each SCFA has a variety of physiological functions. SCFAs are used as an energy source for intestinal cells and play an important role in maintaining the health of the intestinal mucosa [34,35]. In particular, butyric acid is the main energy source for intestinal mucosal cells, promoting cell growth and regeneration. SCFAs contribute to alleviating intestinal inflammatory conditions by regulating the activation of immune cells and reducing the secretion of inflammatory cytokines. Additionally, metabolites of SCFAs help regulate blood sugar by improving insulin sensitivity in liver and muscle cells.
Metformin may affect the metabolic activity of gut microbiota by activating metabolic pathways such as AMPK. Modulation of these pathways can help control blood sugar by controlling the metabolic pathways mediated by microorganisms. Additionally, changes in the gut microbiota may also have an impact on regulating inflammatory responses. Metformin may contribute to reducing chronic inflammation by reducing the production of inflammatory cytokines through the composition of the gut microbiome.
Altering the bacterial composition of the gut can affect multiple aspects related to energy homeostasis, including nutrient absorption and the secretion of gut-derived hormones, such as GLP-1. In particular, the lipopolysaccharides (LPS) derived from gram-negative bacteria can be absorbed intestinally, leading to endotoxemia that triggers systemic inflammation and insulin resistance [47].
In mice high-fat diet (HFD), metformin restored the tight junction protein occluding-1 levels in gut, reversed the elevated gut permeability and serum LPS levels, and increased the abundance of beneficial bacteria Lactobacillus and A. muciniphila [47]. In addition, metformin not only decreased dysfunctional gut permeability (leaky gut) and inflammation, but also increased goblet cell mass and mucin production in HFD-fed mice [48]. As a result, metformin may attenuate endotoxemia and enhances insulin signaling in high-fat fed mice, which could be contributed to its anti-diabetic effects.
3. Microbial Metabolic Relationship
The gut microbiota influences host metabolism via the modulation of metabolites, including the LPS, bile acids, trimethylamine N-oxide, and SCFAs.
The primary bile acids, cholic acid and chenodeoxycholic acid in humans, are agonists of farnesoid X receptor (FXR), and activation of the FXR-small heterodimer partner axis in the liver inhibits the sterol 12 α-hydroxylase expression and bile acid synthesis. In metagenomics and metabolomics analysis from individuals with treatment-naïve T2DM, metformin treatment revealed that, via inhibition of intestinal FXR signaling, Bacteroides fragilis was decreased and the bile acid glycoursodeoxycholic acid was increased in the gut. In addition, HFD-fed mice colonized with B. fragilis were predisposed to more severe glucose intolerance, and the benefits of metformin on glucose intolerance were vanished [49].
Recent evidence indicates that intestinal gluconeogenesis (IGN) has beneficial effects on glucose and energy homeostasis. The SCFAs, propionate and butyrate, which are generated by fermentation of soluble fiber by the gut microbiota, activate IGN via complementary mechanisms. Butyrate, is a key energy substrate for both colonocytes and enterocytes, activates IGN gene expression through a cAMP-dependent mechanism, while propionate, is classically described as an efficient hepatic gluconeogenic substrate, and activates IGN gene expression via a gut-brain neural circuit involving the fatty acid receptor FFAR-3. Thus, the regulation of IGN is necessary for the metabolic benefits associated with SCFAs and soluble fiber [50].
Novel pharmacotherapy have been extensively studied to obtain actual effects for inflammatory bowel disease (IBD) patients. Among these drugs, metformin has been reported to exert benefits via its anti-inflammatory effect. Additionally, evidence from cellular to clinical models of IBD demonstrated significant positive effects of metformin on inflammatory pathways, oxidative stress, gut barrier integrity, and gut microbiota [51].
Administration of A. muciniphila, which was enriched in metformin-treated mice, alleviated the colonic inflammation and mucus barrier disruption. Metformin alleviated dextran sulfate sodium-induced ulcerative colitis in mice, and protected against cell damage via affecting the gut microbiota. Metformin was also associated with decreased pathogenic Escherichia-Shigella and increased the abundance of beneficial Lactobacillus and Akkermansia. Therefore, metformin treatment appears to induce anti-inflammatory effects, thus ameliorating symptoms, and the abundance of beneficial taxa and restored microbial diversity, suggesting a viable strategy against IBD [52,53].
IMMUNOMODULATORY MECHANISMS OF METFORMIN: DIMINISHING OF INFLAMMATION
Metformin has gained attention for its immunomodulatory effects beyond glucose regulation. Metformin exhibits a range of immunomodulatory effects through various mechanisms, including anti-inflammatory actions, modulation of immune cell activity, gut microbiota alterations, and AMPK activation.
Metformin has been shown to reduce inflammation by decreasing levels of pro-inflammatory cytokines and inhibiting the activation of the nuclear factor (NF)-κB pathway, which is crucial for the inflammatory response [54]. Metformin influences various immune cells. It enhances the M2 polarization of macrophages, promoting anti-inflammatory responses [55]. Metformin can modulate T cell activation and differentiation, leading to altered immune responses, particularly in autoimmune conditions.
The activation of AMPK and mammalian target of rapamycin complex 1 pathway may be involved in this process. Recent studies using Extracellular Flux Analyzer demonstrated that metformin alters the activities of glycolysis, OXPHOS, lipid oxidation, and glutaminolysis, which tightly link to the modulation of cytokine production in CD4+ and CD8+ T cells in various disease states, such as virus infection, autoimmune diseases, aging, and cancers [56].
Substantial studies have indicated that metformin exerts its beneficial or deleterious effect by multiple mechanisms, apart from AMPK-dependent mechanism, also including several AMPK-independent mechanisms, such as restoring of redox balance, affecting mitochondrial function, modulating gut microbiome and regulating several other signals, such as FBP-1, protein phosphatase 2A, fibroblast growth factor 21, Sirtuin 1, and mammalian target of rapamycin (mTOR). On the basis of these multiple mechanisms, researchers tried to repurpose this old drug and further explored the possible indications and adverse effects of metformin [57].
The neutrophil extracellular traps (NETs) are composed of DNA associated with nuclear and cytosolic neutrophil proteins. NET markers, including myeloperoxidase, citrullinated histone H3, neutrophil elastase, and cell-free double-stranded DNA, can easily be measured in serum or tissue specimens. NETs propagate a pathologic inflammatory response with consequent tissue injury and thrombosis. Many diabetic complications—such as stroke, retinopathy, impaired wound healing, and coronary artery disease—involve these mechanisms [58]. Metformin, as compared to placebo, significantly reduced the concentrations of NET components in the plasma of patients with pre-diabetes who were randomized, independently from glucose control [59]. This finding suggests that metformin can dampen NETosis in activated neutrophils, which is important for host defense against pathogens and is involved in inflammatory-mediated tissue damage and thrombosis.
Overall, these properties suggest potential therapeutic applications for metformin in conditions characterized by immune dysregulation, such as autoimmune diseases and certain cancers. Further research is needed to fully elucidate these mechanisms and their clinical implications.
METFORMIN AND CANCER
1. Mechanism of Action
Metformin has attracted attention for its potential antitumor and anticancer effects. Metformin improves insulin sensitivity, potentially lowering insulin levels, which may reduce the risk of tumor growth since high insulin levels have been linked to certain cancers. At the molecular level, metformin triggers AMPK activation and signal transducer and activator of transcription 3 inactivation, leading to altered production of the effector cytokines tumor necrosis factor and interleukin-10 by immune cells [60]. Metformin activates AMPK, a cellular energy sensor that can inhibit cancer cell proliferation and induce apoptosis (programmed cell death). Repurposing an inexpensive anti-cancer drug was also invigorated by pre-existing results from anti-tumor effects and inhibition of tumor growth by metformin via inhibition of mitochondrial OXPHOS associated with AMPK-dependent/-independent mechanisms [61]. It may also slow tumor growth by inhibiting the mTOR pathway, which is important for cell growth and proliferation.
Metformin is being investigated for its potential to improve the efficacy of existing chemotherapy and targeted therapies. When used in combination with traditional anticancer drugs, it may help improve treatment outcomes. While metformin shows promise in cancer prevention and treatment, it is not approved as a primary anti-cancer medication.
2. Research Evidences
The first observational study identifying the effectiveness of metformin as a cancer prevention and treatment agent began in 2005. Since then, a significant number of basic, clinical, observational, and experimental studies have been conducted and are currently ongoing. In the past, several preclinical and clinical studies have suggested that metformin may reduce the incidence of certain cancers, including liver, pancreas, colon, and breast cancers [61]. Some studies have shown that diabetic patients taking metformin have a lower risk of developing cancer compared to those not taking the drug.
Even though the results of early observational studies were positive, recent randomized controlled trials (RCTs) are increasingly negative. For example, a recent large-scale RCT evaluating the effect of metformin as an adjuvant therapy for breast cancer patients did not find a clinical benefit in disease-free survival or overall survival [62].
These results may be due to the fact that most RCTs are conducted on subjects without diabetes, compared to observational studies targeting diabetic patients. In other words, the clinical results of metformin use may be different among subjects with or without diabetes. Additionally, the effects of metformin may vary depending on metabolic phenotype, and in particular, body mass index may modulate the clinical benefits associated with metformin use and cancer outcomes. The effect of metformin may also vary depending on the cancer stage when metformin is started. Metformin may not be effective against all types of cancer, as a certain cancer may be not responsive to insulin levels [63].
In meta-analysis of studies on the relationship between diabetes medications and cancer risk over the past 10 years, the most numerous papers were related to colorectal cancer, followed by pancreatic cancer, breast cancer, liver cancer, prostate cancer, and lung cancer. All RCTs were single except for pancreatic cancer (three cases), many were cohort studies, and some were case-control studies. In biguanide-related studies (44 cohorts, 20 case-controls), an inverse relationship was observed with colorectal cancers (risk ratio [RR], 0.85; 95% confidence interval [CI], 0.78–0.92) and liver cancers (RR, 0.55; 95% CI, 0.46–0.66) [64].
Metformin treatment for cancer should focus on specific promising phenotype or genotype subgroups. In other words, it will be necessary to select a precise treatment target according to the type of cancer. For example, in breast cancers, medication and dosage of metformin are determined on patient characteristics (e.g., obesity, IR, estrogen receptor-positive, etc.).
Therefore, research into the actual effects of metformin depending on the type of cancer treated, the cumulative dose of metformin, or whether or not other hypoglycemic agents are used in combination is still needed.
PLEIOTROPIC EFFECTS OF METFORMIN: OBESITY, OSTEOPOROSIS, AND OSTEOARTHRITIS
1. Obesity
Weight reduction effects are generally seen at doses aimed at controlling blood sugar levels and will vary depending on individual circumstances. The initial dose for weight reduction starts at 500 mg, taken 1–2 times a day. Thereafter, the dose can be increased up to a maximum of 2,000 mg (in 500 mg increments). A maximum daily dose of 2,000–2,500 mg is generally recommended. Metformin usually has the effect of reducing weight by an average of 1 to 3 kg over a period of 6 months to 1 year, and this effect varies depending on the individual’s dietary habits, amount of exercise, metabolic status, and so forth.
By its mechanism of action, metformin improves insulin sensitivity, facilitating glucose metabolism in the body and contributing to reducing excessive fat storage. In a recent systematic review and meta-analysis, it was concluded that the optimal dose in obesity is 1,000 mg/d for 3 months in adolescents and 3,000 mg/d for 6 months in adults [55].
Metformin may have an appetite-suppressing effect and may alter the composition of the gut microbiome, promoting the growth of beneficial microorganisms, which may benefit metabolic health. Several studies have shown that metformin has a weight reduction effect even in obese patients. In particular, these effects may be more pronounced in insulin-resistant patients.
2. Osteoporosis
It has been suggested that its direct action on osteoblasts through AMPK activation resulting in osteoblasts differentiation, proliferation, and bone matrix synthesis. Some studies have shown that metformin may improve bone mineral density (BMD). In particular, there are cases where increased BMD has been observed in diabetic patients [65].
Metformin may help improve insulin resistance and promote metabolic health. This may also have a positive effect on bone health. The anti-inflammatory effects of metformin may contribute to reducing the inflammatory response associated with osteoporosis (OP). Since chronic inflammation is associated with bone loss, alleviating it may benefit bone health. However, even though metformin is being studied as a potential option for the treatment of OP, it is not currently approved as a first-line drug to treat OP.
3. Osteoarthritis
There are several mechanisms by which metformin contributes to the management of osteoarthritis (OA). There is increasing evidence from pre-clinical studies and clinical trials that metformin can slow OA progression by modulating inflammatory and metabolic factors [66].
First, metformin can help reduce the inflammatory response. This is achieved by inhibiting inflammatory pathways such as NF-κB and reducing the production of inflammatory cytokines. These actions may contribute to relieving joint inflammation and reducing pain. OA patients are often accompanied by obesity or metabolic syndrome. Metformin reduces the burden on joints by improving cellular energy metabolism and promoting fat metabolism by improving insulin sensitivity and activating AMPK.
In addition, metformin can help the survival of articular cartilage cells by reducing cellular stress and inhibiting the apoptosis pathway. This may help slow the progression of OA. Metformin may affect the regulation of pain signal in neuronal pathways.
METFORMIN AND COVID-19
Metformin has been shown to have potential antibacterial and antiviral effects. Some research suggests that metformin may contribute to inhibiting the growth of certain bacteria. In particular, there are reports that metformin may help reduce the risk of bacterial infections by controlling inflammation associated with obesity. Metformin also affects the gut microbiome, contributing to maintaining microbial balance, which may help prevent intestinal bacterial infections.
The antiviral effects of metformin may occur through several mechanisms, including activating AMPK, improving insulin resistance, modulating inflammatory responses, blocking viral receptors (e.g., angiotensin converting enzyme 2), and inducing apoptosis. Studies on the impact of metformin on the treatment of coronavirus disease 2019 (COVID-19) are ongoing.
Retrospective electronic health record data from T2DM subjects (n=25,326) treated with metformin, the odds ratio (OR) of contracting COVID-19 was high in subjects with obesity (OR, 1.93; 95% CI, 1.64–2.28), hypertension (OR, 2.46; 95% CI, 2.07–2.93), and diabetes (OR, 2.11; 95% CI, 1.78–2.48). Diabetes was associated with a dramatic increase in mortality (OR, 3.62; 95% CI, 2.11–6.2) and emerged as an independent risk factor in this diverse population. Interestingly, metformin treatment prior to diagnosis of COVID-19 was independently associated with a significant reduction in mortality in subjects with diabetes and COVID-19 (OR, 0.33; 95% CI, 0.13–0.84; P=0.021) [67]. A meta-analysis of 10,233 subjects (nine studies) showed than metformin is associated with lower mortality in pooled non-adjusted model (OR, 0.45; 95% CI, 0.25–0.81; I2=63.9%, P=0.026) and pooled adjusted model (OR, 0.64; 95% CI, 0.43–0.97; I2=52.1%, P=0.064) [68]. RCTs are needed to confirm these findings.
Even though early results may be positive, additional research is needed to conclude that metformin is effective in treating COVID-19. The following is related to the potential treatment of metformin for COVID-19. First, since severe patients with COVID-19 often have an excessive inflammatory response, it has been argued that metformin may contribute to alleviating inflammation. Second, people with diabetes are at high risk of developing severe illness if infected with COVID-19, and metformin may help manage blood sugar, which may contribute to reducing the risk of complications related to COVID-19. Third, some studies have raised the possibility that metformin may inhibit the replication of severe acute respiratory syndrome coronavirus 2. However, research on the mechanism is still in its early stages and more data and evidence are needed.
CONCLUSION
Metformin is one of the most common medications used worldwide for more than 60 years. However, the underlying mechanisms of action is not fully understood yet. Lately, there are substantial pre-clinical and clinical studies being developed to identify novel glucose-lowering mechanism and to verify the clinical benefits in various clinical settings, such as obesity, polycystic ovary syndrome, and cancer.
Novel glucose-lowering mechanisms of metformin through the AMPK signaling pathway is identified. Even though it has been regarded that the hepatic mechanism of action is occurred exclusively, there is now emerging evidence that extrahepatic mechanism of action, notably the gut microbiota, are involved. In addition, metformin has immunomodulatory properties in various diseases, such as cancer, inflammatory disease, autoimmune disease, and infectious diseases, involving direct or indirect regulation of the host innate and adaptive immune response.
The results of early observational studies have suggested that metformin may reduce the incidence of certain cancers. However, recent RCTs are a little disappointing. This difference of clinical outcomes may be due to the fact that most RCTs are conducted on subjects without T2DM, whereas observational studies targeting diabetic patients. In meta-analysis of studies on the relationship between biguanide and cancer risk over the past 10 years, an inverse relationship was observed with colorectal and liver cancers. Therefore, metformin treatment for cancer should focus on a precise target (e.g., specific phenotype or genotype) according to the type of cancer.
In treating COVID-19, even though early evidences may be positive, further research results is needed to conclude that metformin is effective.
We are needed more data and precise evidences about the effects of metformin for a range of diseases beyond T2DM.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Wang Y, Hussain SM, Wluka AE, Lim YZ, Abram F, Pelletier JP, et al. Association between metformin use and disease progression in obese people with knee osteoarthritis: data from the Osteoarthritis Initiative: a prospective cohort study. Arthritis Res Ther. 2019;21:127. doi: 10.1186/s13075-019-1915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cicero AF, Tartagni E, Ertek S. Metformin and its clinical use: new insights for an old drug in clinical practice. Arch Med Sci. 2012;8:907–17. doi: 10.5114/aoms.2012.31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60:1566–76. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 4.Foretz M, Guigas B, Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol. 2023;19:460–76. doi: 10.1038/s41574-023-00833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 6.Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35:446–54. doi: 10.2337/dc11-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–85. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev. 2021;42:77–96. doi: 10.1210/endrev/bnaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma T, Tian X, Zhang B, Li M, Wang Y, Yang C, et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603:159–65. doi: 10.1038/s41586-022-04431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Patnana PK, Nimmagadda SC. Low-dose metformin and PEN2-dependent lysosomal AMPK activation: benefits outnumber side effects. Signal Transduct Target Ther. 2022;7:178. doi: 10.1038/s41392-022-01040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agius L, Ford BE, Chachra SS. The metformin mechanism on gluconeogenesis and AMPK activation: the metabolite perspective. Int J Mol Sci. 2020;21:3240. doi: 10.3390/ijms21093240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman R, Kravos NA, Jensterle M, Janez A, Dolzan V. Metformin and insulin resistance: a review of the underlying mechanisms behind changes in GLUT4-mediated glucose transport. Int J Mol Sci. 2022;23:1264. doi: 10.3390/ijms23031264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020;472:1273–98. doi: 10.1007/s00424-020-02417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahne E, Sun EW, Young RL, Hansen M, Sonne DP, Hansen JS, et al. Metformin-induced glucagon-like peptide-1 secretion contributes to the actions of metformin in type 2 diabetes. JCI Insight. 2018;3:e93936. doi: 10.1172/jci.insight.93936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12:235–46. doi: 10.1111/j.1365-2125.1981.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang X, Giacomini KM. Transporters involved in metformin pharmacokinetics and treatment response. J Pharm Sci. 2017;106:2245–50. doi: 10.1016/j.xphs.2017.04.078. [DOI] [PubMed] [Google Scholar]
- 18.Saiz-Rodriguez M, Ochoa D, Zubiaur P, Navares-Gomez M, Roman M, Camargo-Mamani P, et al. Identification of transporter polymorphisms influencing metformin pharmacokinetics in healthy volunteers. J Pers Med. 2023;13:489. doi: 10.3390/jpm13030489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng A, Gong C, Xu Y, Liang X, Chen X, Hong W, et al. Association between organic cation transporter genetic polymorphisms and metformin response and intolerance in T2DM individuals: a systematic review and meta-analysis. Front Public Health. 2023;11:1183879. doi: 10.3389/fpubh.2023.1183879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai Y, Kazumori K, Takeshima T, Iwasaki K, Tanaka Y. Effects of Increasing metformin dose vs adding/switching to dipeptidyl peptidase-4 inhibitors on glycemic control in patients with type 2 diabetes. Diabetes Ther. 2021;12:897–911. doi: 10.1007/s13300-021-01017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, et al. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care. 2001;24:489–94. doi: 10.2337/diacare.24.3.489. [DOI] [PubMed] [Google Scholar]
- 22.Ilias I, Rizzo M, Zabuliene L. Metformin: sex/gender differences in its uses and effects-narrative review. Medicina (Kaunas) 2022;58:430. doi: 10.3390/medicina58030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun ML, Liu F, Yan P, Chen W, Wang XH. Effects of food on pharmacokinetics and safety of metformin hydrochloride tablets: a meta-analysis of pharmacokinetic, bioavailability, or bioequivalence studies. Heliyon. 2023;9:e17906. doi: 10.1016/j.heliyon.2023.e17906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orloff J, Min JY, Mushlin A, Flory J. Safety and effectiveness of metformin in patients with reduced renal function: a systematic review. Diabetes Obes Metab. 2021;23:2035–47. doi: 10.1111/dom.14440. [DOI] [PubMed] [Google Scholar]
- 25.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetes Prevention Program Research Group Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–7. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–75. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung SC, Chang YK, Liu JS, Kuo KL, Chen YH, Hsu CC, et al. Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. Lancet Diabetes Endocrinol. 2015;3:605–14. doi: 10.1016/S2213-8587(15)00123-0. [DOI] [PubMed] [Google Scholar]
- 29.de Vries ST, Denig P, Ekhart C, Mol PG, van Puijenbroek EP. Sex differences in adverse drug reactions of metformin: a longitudinal survey study. Drug Saf. 2020;43:489–95. doi: 10.1007/s40264-020-00913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Jong L, Harmark L, van Puijenbroek E. Time course, outcome and management of adverse drug reactions associated with metformin from patient’s perspective: a prospective, observational cohort study in the Netherlands. Eur J Clin Pharmacol. 2016;72:615–22. doi: 10.1007/s00228-016-2019-z. [DOI] [PubMed] [Google Scholar]
- 31.Froldi G. View on metformin: antidiabetic and pleiotropic effects, pharmacokinetics, side effects, and sex-related differences. Pharmaceuticals (Basel) 2024;17:478. doi: 10.3390/ph17040478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes Metab. 2017;19:473–81. doi: 10.1111/dom.12854. [DOI] [PubMed] [Google Scholar]
- 33.Bryrup T, Thomsen CW, Kern T, Allin KH, Brandslund I, Jorgensen NR, et al. Metformin-induced changes of the gut microbiota in healthy young men: results of a non-blinded, one-armed intervention study. Diabetologia. 2019;62:1024–35. doi: 10.1007/s00125-019-4848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–6. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seicaru EM, Popa Ilie IR, Catinean A, Craciun AM, Ghervan C. Enhancing metformin effects by adding gut microbiota modulators to ameliorate the metabolic status of obese, insulin-resistant hosts. J Gastrointestin Liver Dis. 2022;31:344–54. doi: 10.15403/jgld-4248. [DOI] [PubMed] [Google Scholar]
- 36.Burton JH, Johnson M, Johnson J, Hsia DS, Greenway FL, Heiman ML. Addition of a gastrointestinal microbiome modulator to metformin improves metformin tolerance and fasting glucose levels. J Diabetes Sci Technol. 2015;9:808–14. doi: 10.1177/1932296815577425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wile DJ, Toth C. Association of metformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathy. Diabetes Care. 2010;33:156–61. doi: 10.2337/dc09-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang R, Yu H, Wu J, Chen H, Wang M, Wang S, et al. Metformin treatment and risk of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus in Beijing, China. Front Endocrinol (Lausanne) 2023;14:1082720. doi: 10.3389/fendo.2023.1082720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yazidi M, Kammoun E, Oueslati I, Chihaoui M. Metformin-induced vitamin B12 deficiency in patients with type 2 diabetes: a narrative review with a practical approach for screening, diagnosing, and managing vitamin B12 deficiency. Korean J Fam Med. 2024;45:189–98. doi: 10.4082/kjfm.24.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurley-Kim K, Vu CH, Dao NM, Tran LC, McBane S, Lee J, et al. Effect of metformin use on vitamin B12 deficiency over time (EMBER): a real-world evidence database study. Endocr Pract. 2023;29:862–7. doi: 10.1016/j.eprac.2023.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Wee AKH, Sultana R. Determinants of vitamin B12 deficiency in patients with type-2 diabetes mellitus: a primary-care retrospective cohort study. BMC Prim Care. 2023;24:102. doi: 10.1186/s12875-023-02057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoval-Sanchez B, Ansari F, Lange D, Galkin A. Effect of metformin on intact mitochondria from liver and brain: concept revisited. Eur J Pharmacol. 2022;931:175177. doi: 10.1016/j.ejphar.2022.175177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–6. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez-Silva C, Kashani A, Hansen TH, Pinna NK, Anjana RM, Dutta A, et al. Trans-ethnic gut microbiota signatures of type 2 diabetes in Denmark and India. Genome Med. 2021;13:37. doi: 10.1186/s13073-021-00856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–8. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 47.Zhou ZY, Ren LW, Zhan P, Yang HY, Chai DD, Yu ZW. Metformin exerts glucose-lowering action in high-fat fed mice via attenuating endotoxemia and enhancing insulin signaling. Acta Pharmacol Sin. 2016;37:1063–75. doi: 10.1038/aps.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmadi S, Razazan A, Nagpal R, Jain S, Wang B, Mishra SP, et al. Metformin reduces aging-related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. J Gerontol A Biol Sci Med Sci. 2020;75:e9–21. doi: 10.1093/gerona/glaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24:1919–29. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Wanchaitanawong W, Thinrungroj N, Chattipakorn SC, Chattipakorn N, Shinlapawittayatorn K. Repurposing metformin as a potential treatment for inflammatory bowel disease: evidence from cell to the clinic. Int Immunopharmacol. 2022;112:109230. doi: 10.1016/j.intimp.2022.109230. [DOI] [PubMed] [Google Scholar]
- 52.Ke H, Li F, Deng W, Li Z, Wang S, Lv P, et al. Metformin exerts anti-inflammatory and mucus barrier protective effects by enriching Akkermansia muciniphila in mice with ulcerative colitis. Front Pharmacol. 2021;12:726707. doi: 10.3389/fphar.2021.726707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z, Liao W, Zhang Z, Sun R, Luo Y, Chen Q, et al. Metformin affects gut microbiota composition and diversity associated with amelioration of dextran sulfate sodium-induced colitis in mice. Front Pharmacol. 2021;12:640347. doi: 10.3389/fphar.2021.640347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Misirkic Marjanovic MS, Vucicevic LM, Despotovic AR, Stamenkovic MM, Janjetovic KD. Dual anticancer role of metformin: an old drug regulating AMPK dependent/independent pathways in metabolic, oncogenic/tumorsuppresing and immunity context. Am J Cancer Res. 2021;11:5625–43. [PMC free article] [PubMed] [Google Scholar]
- 55.Hui F, Zhang Y, Ren T, Li X, Zhao M, Zhao Q. Role of metformin in overweight and obese people without diabetes: a systematic review and network meta-analysis. Eur J Clin Pharmacol. 2019;75:437–50. doi: 10.1007/s00228-018-2593-3. [DOI] [PubMed] [Google Scholar]
- 56.Nojima I, Wada J. Metformin and its immune-mediated effects in various diseases. Int J Mol Sci. 2023;24:755. doi: 10.3390/ijms24010755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du Y, Zhu YJ, Zhou YX, Ding J, Liu JY. Metformin in therapeutic applications in human diseases: its mechanism of action and clinical study. Mol Biomed. 2022;3:41. doi: 10.1186/s43556-022-00108-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shafqat A, Abdul Rab S, Ammar O, Al Salameh S, Alkhudairi A, Kashir J, et al. Emerging role of neutrophil extracellular traps in the complications of diabetes mellitus. Front Med (Lausanne) 2022;9:995993. doi: 10.3389/fmed.2022.995993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menegazzo L, Scattolini V, Cappellari R, Bonora BM, Albiero M, Bortolozzi M, et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018;55:593–601. doi: 10.1007/s00592-018-1129-8. [DOI] [PubMed] [Google Scholar]
- 60.Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37:207–18. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ, Ennis M, Lemieux J, et al. Effect of metformin vs placebo on invasive disease-free survival in patients with breast cancer: the MA.32 randomized clinical trial. JAMA. 2022;327:1963–73. doi: 10.1001/jama.2022.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HS, Kim JH, Jang HJ, Lee J. The addition of metformin to systemic anticancer therapy in advanced or metastatic cancers: a meta-analysis of randomized controlled trials. Int J Med Sci. 2020;17:2551–60. doi: 10.7150/ijms.50338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, Mushashi F, Son S, Bhatti P, Dummer T, Murphy RA. Diabetes medications and cancer risk associations: a systematic review and meta-analysis of evidence over the past 10 years. Sci Rep. 2023;13:11844. doi: 10.1038/s41598-023-38431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melton LJ, 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res. 2008;23:1334–42. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lambova SN. Pleiotropic effects of metformin in osteoarthritis. Life (Basel) 2023;13:437. doi: 10.3390/life13020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crouse AB, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front Endocrinol (Lausanne) 2021;11:600439. doi: 10.3389/fendo.2020.600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lukito AA, Pranata R, Henrina J, Lim MA, Lawrensia S, Suastika K. The effect of metformin consumption on mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:2177–83. doi: 10.1016/j.dsx.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


