Abstract
The human microbiome emerges as a promising reservoir for diagnostic markers and therapeutics. Since host-associated microbiomes at various body sites differ and diseases do not occur in isolation, a comprehensive analysis strategy highlighting the full potential of microbiomes should include diverse specimen types and various diseases. To ensure robust data quality and comparability across specimen types and diseases, we employ standardized protocols to generate sequencing data from 1931 prospectively collected specimens, including from saliva, plaque, skin, throat, eye, and stool, with an average sequencing depth of 5.3 gigabases. Collected from 515 patients, these samples yield an average of 3.7 metagenomes per patient. Our results suggest significant microbial variations across diseases and specimen types, including unexpected anatomical sites. We identify 583 unexplored species-level genome bins (SGBs) of which 189 are significantly disease-associated. Of note, the existence of microbial resistance genes in one specimen was indicative of the same resistance genes in other specimens of the same patient. Annotated and previously undescribed SGBs collectively harbor 28,315 potential biosynthetic gene clusters (BGCs), with 1050 significant correlations to diseases. Our combinatorial approach identifies distinct SGBs and BGCs, emphasizing the value of pan-body pan-disease microbiomics as a source for diagnostic and therapeutic strategies.
Subject terms: Metagenomics, Clinical microbiology, Microbiome
In this large-scale metagenomics study encompassing 3,483 human host-derived samples from seven body sites, researchers identify 583 unreported single-genome bins and report 314 metagenome-disease as well as 814 biosynthetic gene-disease associations.
Introduction
Recent advancements in the field of microbiota research have spotlighted the complex interplay between non-communicable diseases and the human microbiome, offering novel avenues for understanding disease pathogenesis, identifying biomarkers, and developing new therapies1–8. Respective studies demonstrate site-specific changes in microbiota linked to disease development and progression. Large-scale metagenomic initiatives, such as the Human Microbiome Project, International Human Microbiome Consortium9, the American Gut Project, and other hallmark studies10–16, have compiled thousands of microbiota samples leading to results arguing all in the very same direction. Data on the impact of an organ-confined disease process in the microbiota of remote body sites remain, however, sparse. Understanding the dynamic relationship between health and disease, especially in the context of frequent co-morbidities, necessitates a holistic exploration of the human microbiome. One example is the discovery of the gut-brain axis, a refined communication between the intestinal microbiota, intestinal host cells, and the central nervous system via the vagal nerve17. Overall, the microbiome can be modified at sites remote from the primary disease process18,19. Thus, understanding the composition of metagenomes in health and disease in a systemic manner might be key to improving patient care.
Especially considering the growing health challenges spurred by demographic shifts and social influences, the imperative for enhanced treatment strategies becomes increasingly evident. For example, chronic inflammatory diseases encompass a diverse array of conditions such as periodontitis, chronic obstructive pulmonary disease (COPD), cystic fibrosis, cardiovascular diseases, heart failure, and ulcerative colitis, among others20–25. Respective ailments collectively pose a significant burden on individuals and healthcare systems alike. This calls for an in-depth exploration of underlying molecular mechanisms26,27. These and other non-communicable diseases are associated with chronic local and systemic inflammation and often occur as multimorbidities. Processes driving the development of multimorbidity are largely unknown but include a state of systemic hyperinflammation, metabolic changes, and senescence. While the underlying mechanisms leading to these (co)morbidities are not yet fully explored, emerging evidence suggests that chronic inflammatory diseases are intrinsically linked to perturbations in the composition and function of the human microbiota28. Imbalances in microbial communities residing at various body sites, including the oral cavity, respiratory tract, gastrointestinal tract, and skin, have been implicated in the initiation and perpetuation of inflammatory cascades29–32. This has led to the paradigm-shifting realization that the microbiota, once considered a bystander, plays a pivotal role in disease progression and resolution.
The pharmaceutical landscape has long drawn inspiration from nature, with a significant - but reducing - proportion (35% percent) of drugs on the market derived from natural products (NPs) and their producers. In 2021, 50 drugs were approved by the FDA33. Of those, 14 approvals represent biologics and 36 small molecules. Only four of the drugs are based on NPs which do not cover some of the greatest needs in human medicine, indicating the need to improve strategies to identify new NPs. These NPs are typically encoded by so-called biosynthetic gene Clusters (BGCs). Considering specific microbial interactions and their underlying chemical processes promises to identify new NPs that remain hidden in classical culture-based studies. Although most isolates are classified as commensals, the heightened prevalence of specific strains, notably methicillin-resistant S. aureus (MRSA), constitutes a substantial risk factor for severe and frequently fatal infections and finally led to epifadin as a new antimicrobial compound class. In a recent investigation, a novel dimension of microbial interactions was unveiled through the examination of the interplay between Staphylococcus epidermidis and S. aureus isolated from the human nasal region34.
Highly accurate, fast, and inexpensive next-generation sequencing paired with computational tools like antiSMASH35,36 are now used at scale in genome mining approaches to identify novel BGCs from microbial communities. As a consequence, important resources such as the BiG-FAM37 database are increasing in size and complexity, now hosting 1.2 million BGCs, and being potentially the dominating source for BGCs. However, the annotation of microbiota-derived BGCs originating from humans, especially in the context of BGCs present at different body sites, is largely absent, presenting a gap in evidence-based prioritization strategies aimed at identifying BGCs with the highest therapeutic potential. As an initial effort to address this gap, we developed ABC-HuMi38, a database featuring BGCs identified across various human body sites. Nevertheless, to provide a systematic disease context for diverse specimen types and diseases, data e.g. from meta-analyzes might not be sufficient. Here, metagenomes from deeply phenotyped cohorts must be analyzed in a standardized manner.
The aim of this study is to fill current gaps by comprehensively characterizing the pan-body alterations of the microbiome in single-organ disease and multimorbidity. The inclusion of different diseases is crucial in several aspects. The analysis of multiple disorders allows understanding the specificity of different abundances of species and BGCs for single diseases39. At the same time, it tremendously improves the definition of a “healthy” versus “normal” microbiome. Admittedly, the combination of multiple diseases affecting multiple organs and collecting multiple specimen types per patient bears significant challenges in including patients, measuring the sample with the least possible bias, and of course computational complexity and interpretation. Yet, our study provides steps forward in the ability to identify new diagnostic and therapeutic strategies as the basis for AI tools with increasing importance in NP development40.
Results
Standardized workflow considering multi-morbid patients
Our study results are derived from clinical, experimental, and computational components (Fig. 1a). Between 2021 and 2023 we collected a total of 3483 samples from 657 individuals spanning a broad spectrum of diseases (chronic inflammatory diseases of the lung, heart, eyes, intestine, skin, and oral cavity). Nine different departments at the Saarland University Hospital (dentistry, dermatology, cardiology, gastroenterology, pulmonology, ophthalmology, pediatry, periodontology, and sports medicine) recruited patients. The standardized sample collection and medical assessment across disciplines (e.g., each patient, independently of the enrolling clinics, medical assessment, and oral examination) was a key criterion within the clinical workflow. The enrolling sites transferred the specimens to the Department of Microbiology for biobanking and metagenomic sequencing and stored medical data in a database after manual curation. A respective approach specifically requires standardized protocols for metagenomic sequencing that we previously developed41. The computational analysis team obtained both, the raw sequencing data as well as the clinical information after blinded measurement of the metagenomes. We analyzed the microbial composition of multiple body sites, including the oral cavity (saliva, interdental plaque), the skin, the throat swabs, the gastro-intestinal tract (stool), and the eye (conjunctiva swabs), largely matching the affected organs (Fig. 1b). The standardized sequencing results combined with broad and well-curated medical annotations are the basis for the computational analyzes, linking known and not yet annotated bacterial species and their repertoire of BGCs to human pathologies, finally representing a unique resource for new NPs.
Fig. 1. Study set up, metagenomics data and clinical information.
a Schematic Workflow describing the sample (upper arrow) and data flow (lower arrow) between clinicians, microbiology, and data science. The clinical data were kept separated from the measurement of microbiomes and only combined after measurement in the computational analysis. b Clinical sampling was focused on seven biospecimens (left blue part). We included patients from a wide range of clinical diseases that allows us analyzing the diagnostic potential of different specimen types across diseases. Created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. c Sankey plot for the number of samples included in the study at different intervals of the data generation process in relation to our quality control strategy. Specimen types are ordered vertically at each step in the pipeline by frequency of the respective specimen. d Number of reads for each sample colored by specimen. The horizontal line represents the 5 gigabase threshold at a paired-end read length of 150 bp. e Pruned upset plot displaying the most frequent co-occurrence of diseases within the dataset. The combinations are ordered with decreasing frequency, marking the combination of Hypertension and obesity as most common comorbidity in our study. f Ontology used throughout the study grouping diseases by biological systems and separating healthy control from diseased patients. Areas are proportional to the number of patients falling into each category. Patients may be represented multiple times if multiple diseases are diagnosed.
Metagenomic sequencing and clinical annotation yield 1931 high-quality samples
While the perfect world scenario encompasses a complete dataset where every patient’s sample is collected and each sample results in a high-quality (HQ) metagenome, in contrast, the real-world situation presents a more complex scenario. As not all patients consented to collecting all specimen types, and not all collected specimens yielded HQ metagenomes, we applied stringent quality control to avoid bias in the analysis of profiles. From the 3483 collected samples, we obtained 1931 HQ metagenomes after quality assessment. These stem from 515 individuals, marking an average of 3.7 microbiomes for each proband (Supplementary Data 1). We excluded samples for various reasons, the most important being an insufficient amount or quality of DNA (c.f. Methods, Fig. 1c). The sample removal process was unevenly distributed, with significantly lower losses of stool, saliva, plaque, and throat specimens, and conversely significantly increased loss of skin and eye specimens. For each metagenome passing the quality control assessment, regardless of the specimen type, we obtained an average of 5.3 gigabases (standard deviation +/− 2.3 gigabases) of metagenomic information after removing ambient human DNA (Fig. 1d). Together, the sequencing efforts generated 10.2 terabases of non-human sequencing information. We used this curated and annotated dataset of 1931 metagenomes and clinical data through the upcoming analyzes. This dataset excels in that over 90% of all samples belong to a patient with at least three different metagenomes available (Supplementary Fig. 1a). As a first analysis, we considered the observed comorbidity pattern of the enrolled individuals (Fig. 1e). The majority of 437 individuals were patients, i.e. probands diagnosed with at least one disease. We included further 46 participants as controls, encompassing individuals without known disease affection and an additional 36 competitive athletes. The five most frequent disease entities in the cohort were hypertension (32.4%), obesity (21.7%), periodontitis (18.8%), caries (16.7%), and diabetes (14.2%). We assessed comorbidities, with many patients suffering from two (23.7%) or three (17.4%) concurrent diseases, respectively. The most frequently observed association was diabetes and cardiac failure. Of note, covering a broad spectrum of diseases intrinsically and intentionally leads to a broad age spectrum (Supplementary Fig. 1b). Similarly, we observed an uneven gender distribution leaning towards more males than females, with a ratio of 58.1% to 41.9%. Together, these factors led to a slight yet statistically significant gender disparity persisting across different age groups (χ2 test p-value = 0.02). To reach sufficient statistical power we propose a three-tiered ontology framework for organizing our cohort analysis (Fig. 1f). By categorizing samples into hierarchical tiers, we ensured to enhance power while acknowledging the potential for increased heterogeneity and confounding factors within the data (Supplementary Data 2). We defined the associated cohort of a specimen as the second-level ontology category encompassing all diseases logically linked to the disease itself; for instance, all oral diseases are associated with the plaque specimen. This second ontology level gave the best overall balance between specificity and still having sufficient samples in the respective cohorts. We thus used this level throughout the manuscript and explicitly mention when another ontology level is the basis of a result.
Compositional analysis identifies a complex pattern of microbiome-disease associations
To get insights into the general distribution of metagenomes we computed an embedding based on the MinHash distances (Fig. 2a). This embedding highlighted the separation of metagenomes according to the specimen types, with samples from the oral cavity clustering together and being most different from the stool samples. Samples with lower bacterial and DNA abundance from the skin and the eye clustered between these two groups, however splitting up in two separate clusters. Those two clusters have no common confounding factors such as sequencing batches, age, gender, and others, with the only difference being the duplication rate of reads (Two-tailed unpaired Wilcoxon p-value < 10−41, Supplementary Fig. 2). To account for this effect in downstream analyzes, we adjusted our models for the duplication rate within samples. Taking the Shannon entropy as a measure for the diversity of the sequenced microbiota, we recognized a broader spread of the specimen types with lower DNA yield (Fig. 2b). Within the oral cavity samples throat-derived microbiomes are characterized by a decreased Shannon entropy as compared to saliva and plaque samples. In this regard, the throat samples followed a similar distribution as the stool samples. This observation posed the question of which bacterial species are driving the distances and whether those species are correlated to disease patterns. We thus computed the relative microbiome compositions at the genus level for each specimen type within each disease (Fig. 2c). The main driver for differences in microbial composition was the specimen types, prompting us to group the patterns within each type for the different diseases. Across the specimen types, our results suggested varying signatures for the most abundant bacterial genera with the competitive athletes frequently being the most deviating group. Of note, the patterns of the athletes only partially matched the standard control cohort. Especially for the skin deviating microbiome compositions are present in patients suffering from digestive tract disorders. Another obvious shift is a differential stool microbiome composition present in patients suffering from digestive tract disorders. To find significant changes in microbial compositions between disease cohorts and species, we carried out a differential abundance analysis at the species level. Splitting increased and decreased species in the different diseases confirms the complex patterns (Fig. 2d, e). For metabolic disorders and heart diseases, the largest number of significant species were recorded. Both were marked by a trend towards increased presence of species distributed across different specimen types. Among the specimen types, the oral cavity had overall the highest shares. Digestion disorders were characterized by a lower abundance of species in the stool samples at an increased frequency in the oral cavity samples. A similar pattern of higher abundance of species in the oral cavity is present in eye diseases. But these are also showing decreased species specifically in the samples taken from the eye. To get a broader overview, we grouped the patterns per specimen type and disease group (Supplementary Data 3). After adjusting p-values according to the number of species present in each specimen type, statistically significant differentially abundant hits emerged in nearly all cohort comparisons. Of note, the effects extended to specimen types not directly linked to the diseases; for example, 63 significant species were identified in the saliva in the case of neural disorders. Overall, for each disease group over 200 significant species were present, with saliva samples yielding the largest number of significant hits across the diseases. Remarkably, we detected a small number of significant results between controls and competitive athletes following adjustment for multiple testing. To assess the impact of comorbidity patterns on the results, we split the dataset into those patients with more than one diagnosed disease (Fig. 2f) and those with exactly one diagnosed disease (Fig. 2g). In the latter case, a reduced number of significant hits remained, suggesting that patients with comorbidities present higher alterations in the human microbiome, regardless of the body site. Because this might be partially due to the smaller number of patients with exactly one disease and the different number of samples per specimen type, we further analyzed the effect sizes as a robust measure in addition to the p-values. The results provided evidence for the validity of the reported patterns in general, however, also demonstrated substantial effects (absolute Cohens D > 0.5) in cases where the regression-based hypothesis test did not yield significance (Supplementary Fig. 3).
Fig. 2. Compositional analysis, and link of microbiota to diseases.
a Two-dimensional Uniform Manifold Approximation and Projection (UMAP) embedding of pairwise computed mash distances, colored by biospecimen of the sample. b Alpha-diversity of all samples, colored by specimen. As a measure of species richness, we selected the Shannon diversity. c Relative genus abundance for each cohort of the second ontology level, divided by biospecimen. Only labels for the 20 most abundant genera are displayed. d Sorted log-fold changes of differentially abundant species matching the visualized results of the next panel. Each panel is split vertically separating positive and negative log-fold changes. e–g Number of differentially abundant species after p-value adjustment of ANCOMBC results revealed during analysis across all cohorts and specimen combinations (q-val <0.05). Numbers in the circles represent the number of specimens included in the respective analysis. h Center-log ratio (CLR) normalized abundance counts of selected species-cohort-specimen combinations. The visualized diseased cohort is indicated by the text above each panel, whereas the selected biospecimen is indicated by the color of the writing. The first row of panels displays potential pathogen candidates with the highest statistical significance and a pathogen score of one. The second row of panels displays saliva samples of commensal bacteria candidates with a commensal score larger than eighteen (min(n) = 50). Boxplot follows Tukey’s style indicating the median as well as the second and third quantiles within boxes. Whiskers extend up to 1.5 times the interquartile range in the presence of outliers.
In order to highlight potential systematic differences between healthy and diseased cohorts, we analyzed the highest hierarchy level in our disease ontology: all patients versus all controls (Fig. 2h, Supplementary Data 4). Our results suggested that Staphylococcus epidermidis and Corynebacterium pseudogenitalium are significantly more frequent in skin swabs from patients suffering from coronary artery disease. Moreover, we detected a significant increase of Capnocytophaga gingivalis in saliva samples from aniridia patients, as well as Porphyromonas endodontalis in interdental plaque from obesity patients, and Lachnoanaerobaculum saburreum in interdental plaque from aniridia patients. Furthermore, Streptococcus australis. Lachnospiraceae bacterium oral taxon 096, Streptococcus sp. A12, Streptococcus sp. HMSC067H01, and Aggregatibacter aphrophilus appeared in decreased abundance in saliva in most analyzed diseases. These commensal bacteria are the five species, which across all analyzed diseases displayed a significant decrease. Only two species demonstrated differential abundance across the disease comparisons. Specifically, Bacteroides cellulosilyticus exhibited a significant decrease in stool samples from patients with digestive ailments, while Streptococcus vestibularis displayed a significant decrease in saliva samples from aniridia patients and an increase in saliva samples from Parkinson’s disease patients. Overall, the abundance analysis of known and annotated bacteria yielded significant disease annotations. However, the body still seems to harbor microbes that are not characterized and annotated in databases42. This motivated the following analysis of assembled metagenomic data, specifically in the context of existing antimicrobial resistance (AMR) genes.
AMR analyzes suggest pan-microbiome resistance within patients
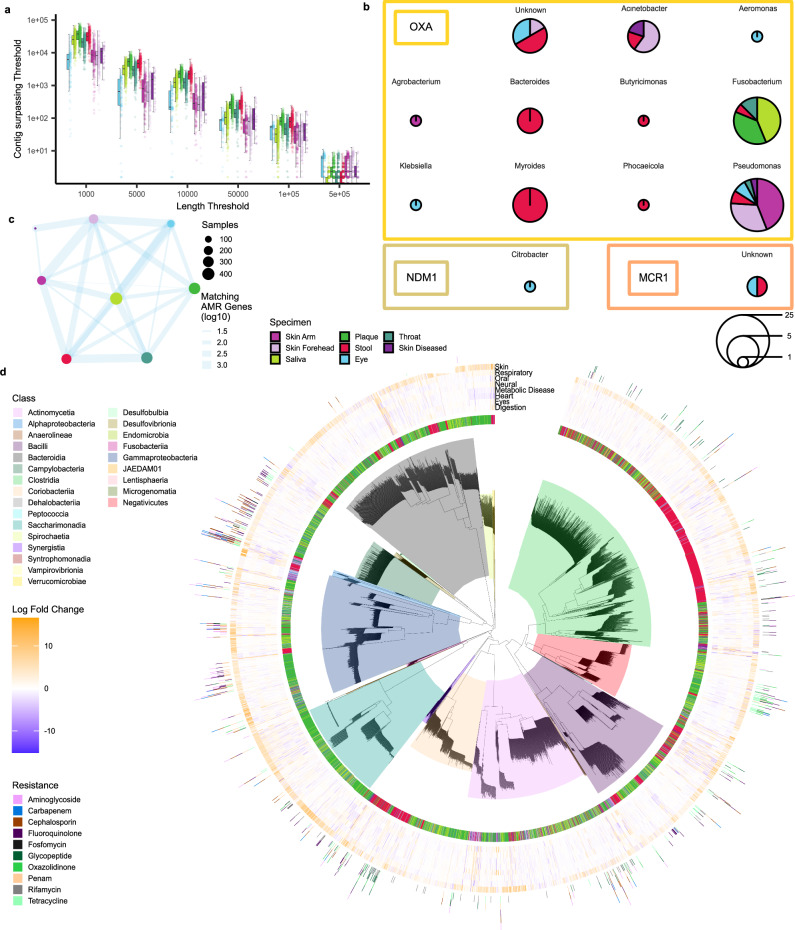
From the 10.2 terabases of sequencing information, we generated 450 million scaffolds by computing metagenomic assemblies for each sample separately. Following the nature of short-read metagenomic sequencing, metagenomics assemblies yield short contigs. Nonetheless, 19 million fragments exceeded 1 kilobase and 300,000 fragments surpassed the 50 kb mark (Fig. 3a, Supplementary Data 5). Intriguingly, long contigs were present in samples from all specimen types, including the skin and the eye. One of the main reasons for metagenomic assemblies is to search for the presence of antimicrobial resistance genes. We thus performed resistance gene profiling based on the assembled fragments (Fig. 3b), pinpointing a consistent prevalence of the mef(A) resistance gene. This gene was present in 1484 of 1931 samples (77%), spanning a set of 493 of 515 distinct individuals (96%).
Fig. 3. Assembly and resistance gene analysis.
a Distribution of the number of scaffolds in each sample at various length limits, colored by specimen as box-whisker plot (n = 1931). The boxplot follows a similar style to Fig. 2h. b Sequence of pie charts indicating the presence of emerging antimicrobial resistance genes. Panels are subdivided by genus that was assigned to the contig where resistance genes have been detected. Pie charts scale with the number of measurements in different samples and are colored by the relative frequency of the sample’s biospecimen. c Network visualization of counts of shared antimicrobial resistance (AMR) genes among different biospecimen samples derived from the same patient. Note, any resistance gene annotated by AMRFinderPlus was used for this plot. d Dereplicated SGBs defined from our data. Visualized information includes biospecimen of the initial sample where the SGB was derived from, selected resistance information taken from Pathofact, and effect size of differential coverage analysis for selected cohorts. Note, the visualized differential coverage focuses only on the biospecimen of the initial sample where the SGB has been defined from that is also visualized in the central ring.
Overall, our data suggested that when a resistant gene is identified in one specimen of a patient, there is an increased likelihood of detecting the same resistance gene in other specimens from the same patient. (Fig. 3c). We report 120 of such significantly associated biospecimen and gene combinations after p-value adjustment (Fisher exact test p-value < 0.05; Supplementary Data 6). Particularly noteworthy is the significant prevalence of resistance genes observed within skin samples, which maintain continual contact with the external environment. In this context, our results suggested that the detection of a resistance gene within either the arm or forehead microbiome corresponds to a probability of 16.1% for the arm microbiome and 13.1% for the forehead microbiome to encounter the same resistance gene within the patient’s stool. Furthermore, we screened for emerging resistance genes in Gram-negative bacteria against carbapenems and colistin, which constitute a global health threat. In our data, we detected several bla-Oxa genes, encoding various β-Lactamases in Acinetobacter sp., Klebsiella sp., Pseudomonas sp., and more (Fig. 3b). Of note, we did not detect the most prevalent bla-Oxa-48 across the study cohort. In conjunctiva swabs, we however observed the New Delhi metallo β-lactamase-1 (NDM-1) in Citrobacter sp. Moreover, the plasmid-mediated resistance to colistin, mcr-1, was found in one conjunctiva swab and one stool sample. When correlating the AMR genes to the different cohorts, we found surprisingly few statistically significant hits, suggesting that the presence of resistance genes is similar in cases and controls independent of the specimen type.
Unveiling 314 microbial genomes associated with diseases
In light of the observed disease associations of known bacteria and the limited association patterns of AMRs in health and disease states, it is reasonable to ask for disease annotations of the not yet annotated bacteria. Therefore, we generated species genome bins (SGBs) and probed their potential links to diseases. We reconstructed a total of 4380 dereplicated microbial genomes derived from 1448 high-quality samples. SGBs were further compared to multiple references to assess their novelty, highlighting 583 undescribed SGBs (Supplementary Data 7). Of known SGBs, 146 lack species representation in GTDB r214. Out of the 4380 genomes, 886 were marked as high quality ( > 90 completeness), of which 72 were displayed perfect completeness and 80 were not yet documented. Notably, oral microbiome specimens (plaque and saliva) exhibited a higher proportion of undescribed genomes (Supplementary Fig. 4), accounting for 72% of novelty and 75% of lack of species assignments in known SGBs.
Utilizing the available coverage data, we assessed SGB enrichment within cohort-specimen combinations, revealing 10170 statistically significant combinations with an absolute log fold change exceeding two, among which 1059 involved previously undescribed SGBs (Supplementary Data 8). The pattern of disease associations in known and unannotated species, and the limited number of significant AMR genes between patients and diseases suggest other factors that might impact physiological or pathophysiological conditions in the host caused by bacteria. Here, the potential of microbes as natural producers that carry BGCs with broad functional scope must be recognized.
Coverage-guided genome mining highlights 814 disease-related core biosynthetic genes
Among all metagenomic assembled scaffolds surpassing the 50 kb length threshold, we predicted a total of 28,315 BGCs. To identify pertinent candidates for further examination, we tailored the BigMAP43 workflow to harmonize with our data strategy (Fig. 4a). Employing coverage profiles for each sample-predicted core biosynthetic gene pair, we acquired an informed assessment of whether a BGC exhibited enrichment or depletion within specific disease cohorts. Notably, our observations reveal that 9% of core biosynthetic genes demonstrate remarkable specificity to the originating samples (Supplementary Fig. 5). Nonetheless, upon focusing exclusively on matching specimen-disease cohort pairings, we unveiled a total of 1050 statistically significant differentially altered coverages following p-value adjustment for the plethora of tested genes (Fig. 4b). With 119 contigs Rothia aeria contributed the most fragments that harbor significantly associated genes (Fig. 4c). Focusing on the functional aspect of these BGCs we observed significant differences in the distribution of BGC classes across tested cohorts, despite similar starting distributions (Supplementary Fig. 6a). In order to better understand the allegedly synthesized compounds that could be produced by the highlighted BGCs we compared them to the MIBiG database44 (Supplementary Fig. 6c). Here, the four BGCs containing significant genes with highest similarity to existing MIBiG entries encoded for streptine with at least 80% similarity (Supplementary Fig. 6d). Consistently, all four clusters were highlighted in the same cohort. Apart from michiganin A no other MIBiG similarities over 40% were observed. Further exploring these findings and specifically examining individual BGCs, we searched for potential pathogenic or protective attributes, including the coverage information (i.e. whether higher or lower coverage within the control cohort was observed). It is crucial to note, however, that this methodology offers insights into the genomic presence but does not encompass the transcriptional activity of BGC genes or the overall concentration of potentially bioactive compounds. Nevertheless, the presented data strongly advocates for prioritizing future investigations into the functionality of the identified BGCs concerning their association with diseases. A prioritized search should consider features like the similarity to documented BGCs, complexity of the BGC in terms of biotechnological suitability, and quality measures linked to the prediction of the BGC.
Fig. 4. Evidence-supported genome mining and disease association.
a Schematic representation of our proposed BGC prioritization strategy representing an adapted version of the BiGMAP workflow. Metagenomic assembly is performed for each sample, followed by BGC prediction. Next, all samples are aligned against all core biosynthetic genes of predicted BGCs. Coverage information is extracted, and downstream analysis is performed. b Volcano plot of the differential BGC coverage analysis results. In this visualization, only matching biospecimen – initial BGC contig combinations are visualized, constituting only a fraction of all results. The unadjusted two-tailed unpaired Wilcoxon test p-values are shown with two horizontal lines representing the 0.05 threshold, both before and after p-value adjustment. c Predicted host species distribution of the assembled DNA fragments where significantly associated core biosynthetic genes reside. Color reflects the number of significant BGCs. d Comparison of the highest correlating effect sizes, comparing differential BGC coverage results between alternative diets and diseases. The effect size of the vegetarian-omnivore comparison is visualized on the y-axis. On the x-axis, the cohort named above the panel is compared against the healthy cohort. For the fourth panel, the minimum effect size across all cohort comparisons is taken for each BGC and compared against the diet comparison.
Impact of confounders on the microbiota showcased by the diet of individuals
As the last aspect of our study, we emphasize the importance of considering confounding factors. Inherent to study designs, such as the one applied in this study, is a broad spectrum of confounders that might impact the results. Correlations between individuals’ early-life breastfeeding experience, gender, and educational attainment in relation to the microbial communities across various body sites exists45. Especially the sex has a large impact46 but also factors such as ethnicity and geography47–49. The regional proximity and a largely shared ethnology of individuals in our study account for these factors but other obvious and non-obvious confounders remain, potentially impacting our results. One of those is the diet that impacts microbial compositions50,51. Because the diet was one of the variables included in our questionnaire, we performed a specific analysis of the diet, testing the dietary information related to the disease context. To this end, we also added data from a longitudinal investigation of the planetary health diet on the gut microbiome52. In the vegetarian stool cohort, we identified eleven significantly diminished microbial species, including those previously linked with alternative diets. Notably, species like Bifidobacterium animals, Alistipes inops, and Phascolarctobacterium faecium, known for producing short-chain fatty acids through dietary fiber fermentation, were more abundant in omnivorous participants. In contrast, Dialister sp. CAG 357, associated with inflammation, exhibited higher levels in omnivores. With respect to the SGBs, only one hit with respect to the diet remained, derived from plaque: Saccharimonas sp013333645. The absence of statistically significant differences in our coverage analysis might be due to the limited number of vegetarians/vegans. Nonetheless, we asked for shared signatures concerning disease correlations. Accounting for limitations in using p-values, we again evaluated effect sizes for each BGC and correlated them with disease effect sizes. In this analysis, we identified negative Spearman coefficients such as −0.35 for coronary artery disease in the forehead skin microbiome, −0.30 for heart diseases in the eye, and −0.28 in the plaque of diabetes patients (Fig. 4d, Supplementary Data 9). In sum, our results provide evidence that confounding factors do have an influence on the metagenomic patterns, but despite these relevant factors, disease signals remain.
Discussion
In our extensive metagenomic sequencing investigation, we analyzed 1931 samples following rigorous quality control. We maintained a standardized data generation protocol across multiple biospecimen samples obtained from the same individuals. Having the different microbiomes measured from the same patient offers a fairer comparison of differentially abundant microbes between different sample types and disease entities. We deliberately included and categorized a diverse spectrum of dominantly chronic inflammatory diseases, as well as globally widespread diseases. While the standardized sampling strategy and the inclusion of multiple disease cohorts represent a core strength of our study, we acknowledge the challenges that remain. One of those is the impact of obvious and less obvious confounders. The broad spectrum of diseases with different ages of onset leads to a broad age distribution. With an additional gender distribution leaning towards more males than females, with a ratio of 58.1% to 41.9% we have a second confounding factor. Others include concomitant medications, ethnicity, geographic location, and the diet. Of note, respective confounding factors are correlated to each other (e.g. the nutrition is linked to the geographic origin), making the local sampling characteristics an advantage of our study. Further, standardized operating procedures add to the stability of the results. To investigate the impact of one confounder in detail, we compared the dietary association with microbiomes in the context of disease associations with the microbiomes. These results suggest that this confounder has an impact on the metagenomes, but that the disease trajectories remained despite this influence.
One major aim of our study was to identify diagnostic patterns. Indeed, our results suggest a complex pattern of disease-to-microbiome associations depending on the specimen types. We reached the highest diagnostic power from gut and oral cavity samples. Here, the low abundance of DNA in the eye or the skin and smaller cohort sizes might lead to lower overall diagnostic values. Still, several interesting hits remained in those specimen types, especially in the case of acne inversa and the skin. It is important to highlight that all associations discovered in this study need in-depth considerations and validation. Examples of associations include the increased presence of Otrichia sp. oral taxon 225 species in the saliva of patients suffering from Parkinson´s disease. Parkinson´s disease remains challenging to diagnose, for which additional testing for biomarkers in easily accessible body fluids, such as saliva, would provide great potential for improved diagnostic procedures53. However, the question of what comes first - the microbes or the disease, remains to be solved in functional studies.
Beyond diagnostic associations of microbiota to diseases, one aim of our study was the examination of antimicrobial resistances because we speculate that microbial dark matter carries resistance gene information that needs to be monitored. The most prevalent resistance gene identified across all specimens was mef(A), encoding a resistance against macrolide antibiotics. This macrolide efflux gene was first described in 1996 and has emerged rapidly in Streptococcus sp. worldwide54–57. Therefore, it is not surprising that we observed such a high prevalence in our study cohort. Furthermore, we identified emerging resistance genes against carbapenem and colistin, both used to treat infections with Gram-negative bacteria. These resistances display a global health threat as treatment options are limited. The most prevalent carbapenem resistance genes are related to Oxacillin-hydrolyzing (OXA) carbapenemases and New Delhi metallo beta-lactamases (NDM)58,59. Colistin had been abandoned for the treatment of Gram-negative infections for many decades but has been reintroduced as a last-resort antibiotic in the last decade. First described in 2011, the plasmid-mediated colistin resistance gene mcr-1 displays another challenging global health threat, as it spreads rapidly and decreases the options for last-resort antibiotics in case of multi-resistant Gram-negative infections60,61. In our study cohort, including 515 patients from southwest Germany, we identified two patients colonized by NDM-1 positive Citrobacter freundii, two patients colonized by mcr-1 carrying Gammaproteobacteria, and a variety of OXA mediated resistances against carbapenems. Interesting are blaOXA-50 carrying Pseudomonas aeruginosa, blaOXA-270 carrying Acinetobacter pittii, and blaOXA-58 carrying Acinetobacter baumannii. The carbapenem hydrolyzing activity of blaOXA-50 and blaOXA-270 has neither been confirmed nor denied. The carbapenemase blaOXA-58, however, was first described in 1995 and has spread globally ever since, posing one of the major carbapenem resistance genes in Acinetobacter baumannii62. We did not observe any bacterial blaOXA-48, which displays a now emerging resistance against carbapenems63. Our study setup also allows us to compare resistance genes across different specimens of the same patient. Here, resistance genes on the skin were indicative of carrying the same resistance genes in the gut.
Another important aim was to explore the functional capabilities of the measured microbiomes and to assess these abilities for potential associations to diseases. While there are many functional aspects of interest that may be explored in the future such as a vast diversity in genes, the pathways they are involved in, or the regulatory ncRNAs that may regulate them, we focused our attention on BGCs64. BGCs encode for molecular machineries, building natural products that are screened as a source of therapies. Our study setup was thought to enable a prioritization of BGCs with respect to therapeutic potential. By categorizing the data into distinct cohorts at various disease ontology levels, we identified BGCs that exhibited differential abundance and coverage. Beyond potential pathogenic species markers, we uncovered benign BGCs that displayed heightened coverage in healthy control groups. These BGCs warrant further exploration in vitro, offering promising avenues for medical discoveries, including the potential development of antibiotic compounds65. As a next step, we plan to thoroughly investigate these promising BGCs for their potential beneficial properties.
Methods
Clinical sampling
Clinical samples were obtained from study participants after having obtained written informed consent at Saarland University Medical Center in Homburg, Germany. Approval for the study was granted by the ethics committee of the local medical association (Ärztekammer des Saarlandes) with the identification number 131/20. Participants were not compensated. Per patient, an extensive medical examination was conducted by medical staff and diseases of interest for this study were identified. If such a disease was present, an in-depth medical history was obtained, including major factors that might influence microbial compositions in and on the human body, such as medication, lifestyle choices pertaining to diet, activity, smoking and for example alcohol uptake, as well as co-morbidities. Sex was derived from insurance documents. Subsequently, clinical samples were obtained: saliva, interdental plaque, conjunctiva swab, throat swab, stool, and skin swabs of the forehead and arm region, as well as in case of Acne inversa – affected skin areas. Concisely, fecal samples were procured from participants through utilization of a paper toilet-hat and a sterile collection tube complete with an integrated spoon, yielding an approximate range of 500 mg to 1 g of stool. Plaque samples were gathered through the use of twelve disposable micro applicators (Catalog No. MSF400, Microbrush International, Grafton, WI). Each quadrant involved brushing three interdental spaces, and subsequent transfer of all micro applicators to an ESwab transport tube (Copan Diagnostics, Brescia, Italy) along with ESwab Amies Medium (Copan Diagnostics). Saliva samples were obtained using 50-ml sterile, conic falcon tubes. Participants were instructed to deposit unstimulated saliva into the sterile falcon tube for a duration of 5 minutes. Conjunctiva specimens were acquired utilizing an ESwab. Eversion of the lower eyelid facilitated swabbing along the complete extent of the lower fornix thrice. Throat swabs were taken cautiously by medical staff, avoiding contact to saliva, tonsils, gums, and teeth, by streaking the throat-area 3–5 times. The forehead and arm were swabbed using an ESwab and wetting the nylon-flocked swab with the provided Amies Medium prior to contact with the skin. The areas were swabbed roughly to ensure removing bacterial mass not just from the surfaces, but also from hair follicles for example. Subsequent placement of the swabs into the designated transport medium was followed by freezing the tube at −80 °C.
DNA extraction
DNA was extracted from all native samples using the Qiagen QiAamp Microbiome Kit (Qiagen, Hilden, Germany). The DNA was extracted according to the manufacturer’s protocol. Briefly, swabs were vortexed in 1.5 ml Amies Medium for 2 minutes. The Amies medium containing the microbial mass from each sample was then used for DNA extraction according to the manufacturer’s recommendation. For fecal samples, 250 mg of stool was used and mixed with 500 µl of buffer AHL. Micro applicators used to collect interdental plaque were mixed with 1 ml 1x PBS (pH = 7.4) and vortexed rigorously for 2 minutes. 1 ml PBS containing bacterial cells was then used for DNA extraction. Saliva samples were vortexed briefly to allow homogenization of the sample. Then, 1 ml of saliva was used for DNA extraction according to the manufacturer’s recommendations. Utilizing the MP Biomedicals™ FastPrep-24™ 5 G Instrument (FisherScientific GmbH, Schwerte, Germany), mechanical disruption of bacterial cells was carried out. The operational parameters were set to a velocity of 6.5 m/s for 45 seconds, executed twice, with intervals of 5 minutes of ice storage separating each lysis cycle. After the lysis procedure, DNA was extracted into 50 µl elution buffer. To determine the DNA concentration, comprehensive microvolume UV-Vis measurements were performed using the NanoDrop 2000/2000c (ThermoFisher Scientific, Wilmington, DE)41.
Library Preparation, sequencing, and quality control
Extracted DNA from all native samples was sent to Novogene Company Limited (Cambridge, UK) for metagenomic library preparation and subsequent paired-end (PE150) Illumina sequencing (HiSeq). Before sequencing, potential genomic DNA degradation was measured with the fragment analyzer platform AATI (Agilent Technologies, CA, USA). The DNA concentration was measured using Qubit (Thermo Fisher, Wilmington, DE). Beads-based size selection of libraries selected for 500 bp fragments. The Novogene NGS DNA Library Prep Set (Cat No.PT004) was used for library preparation. From the study, we excluded sparsely collected biospecimens (n = 47), substandard (n = 1304), and anomalous samples (n = 201).
Next-generation sequencing data preprocessing
The first step of data analysis was host read removal with KneadData66 (version (v): 0.7.4; command line arguments (cla): “--trimmomatic-options = ’LEADING:3 TRAILING:3 MINLEN:50’ --bowtie2-options = ’--very-sensitive --no-discordant --reorder’“). Due to the high contamination load among skin and eye samples, we additionally ran the human sra-human-scrubber67 (v: 1.0.2021_05_05) after KneadData. Paired-end reads were only kept if none of the read pairs mapped to the human reference. After decontamination, we performed sequence overrepresentation analysis and quality assurance with fastp68 (v: 0.20.1; cla: “--overrepresentation_analysis”) and visualized results with MultiQC69 (v: 1.11).
Reference-free beta-diversity analysis
Mash70 (v: 2.3; cla: “sketch -S 23 -k 31 -s 5000 -r -m 2”) was used to compute and compare MinHash distances. A two-dimensional embedding was generated in R with the UMAP package71 (v: 0.2.8). After noticing the separation of the low-input biospecimen into two clusters, we split the low-input samples by their clustering behavior during outlier removal. During this outlier removal, we performed for each biospecimen a Grubb’s test on the mean of all pairwise MinHash distances and removed the most significant outlier. This procedure was repeated iteratively until no more significant outliers were left. In the case of low-input biospecimen, this algorithm was performed for each subcluster instead.
Reference-based compositional analysis
MetaPhlAn366 (v: 3.0.13; cla: “-t rel_ab_w_read_stats --unknown_estimation –add_viruses”) on the mpa_v30_CHOCOPhlAn_201901 database was used to profile quality controlled samples. Relative counts were rescaled to absolute counts based on the number of reads and virus counts were removed. Shannon diversity was used as an alpha-diversity measure. Reference-based beta-diversity was assessed with non-metric multidimensional scaling on Bray-Curtis distances. Differential abundance analysis was performed with ANCOM-BC72 (v: 1.6.2). P-values were adjusted via Benjamini-Hochberg adjustment. Note, that we only tested specimen-cohort combinations with more than ten samples in each cohort. While the athletic and sports cohorts were tested against healthy controls, all other diseases were tested against the union of healthy control and sports cohorts, i.e. the healthy cohort. Samples that were part of the control and disease cohort during testing, such as athletes with diseases, were removed. Based on the differential abundance analysis results, we searched for interesting pathogens and commensal bacteria to further investigate. To this end, we defined the pathogenicity score as the number of various diseases where a pathogen is predicted to be significantly more abundant in the diseased cohort in at least one biospecimen. Similarly, we define the commensal score as the number of disease cohorts where a bacterial species is significantly reduced in the diseased cohort for at least one biospecimen. In visualizations of abundances, absolute counts were center log ratio normalized.
Metagenomic assembly
We assembled each sample with SPAdes73 (v: 3.15.4; cla: “--meta”) and monitored the assembly quality with QUAST74 (v: 5.0.2; cla: “-s”). Scaffolds were binned with MetaBAT275 (v: 2.15; cla: “--seed 420”). MAGs across all samples were aggregated and dereplicated with dRep76 (v: 3.4.2; cla: “-comp 50 -con 5 --checkM_method lineage_wf --S_algorithm fastANI --S_ani 0.95 -nc 0.5”). Mid and high-quality genomes were selected for subsequent analysis using a similar MIMAG’s77: mid-quality genomes (MQ) with completeness >= 50% and contamination <5%, and completeness >90% and contamination <5% for high-quality (HQ) genomes. MAGS with average differences <5% ANI were clustered into 4,380 SGBs. To assess the novelty of the SGBs, we conducted a comparison against multiples references (99,376 genomes), including GTDB r21478, the Unified Human Gastrointestinal Genome collection79, the Singapore Platinum Metagenomes Project80, and Pasolli et al.81, utilizing Mash distances (<= 0.05) followed by validation through FastANI82. Based on the comparison, SGBs were classified into two categories: (i) known, if a match to a known source was found (>= 95% ANI), (ii) novel, if no matches were found. If multiple matches were found for a sequence, the match with the best ANI was retained. GTDB-TK83 (v: 2.3.0; cla: “classify_wf”) in combination with GTDB r21478 was used to annotate SGBs with taxonomic information. Differential coverage analysis on SGBs was performed by first, aggregating all bins creating one reference file, and then aligning all samples against the newly created reference file with Bowtie284 (v: 2.3.4.3; cla: “-a”). Afterward, coverage information was extracted from each alignment with SAMtools idxstats85 (v: “1.16.1”). Differential coverage analysis was organized identically to the differential abundance analysis, i.e. cohort-specimen multiplicity needed to be larger than ten and the control cohorts altered if sports or athletes were considered for testing. However, here, a Wilcoxon rank sum test was executed, performing Benjamini-Hochberg adjustment to adjust for the total number of tested dereplicated SGBs.
Virulence Analysis
On each dereplicated SGB, we ran PathoFact86 (commit v: 55d8240). To capture resistances that did not end up in any final bins, we ran AMRFinderPlus87 (v: 3.11.4) and complemented the information with Kraken88 (v:2.1.2; cla: “--use-mpa-style”; database version: k2-pluspf_20220908) taxonomic classifications for each contig.
Genome mining
After assembly, all contigs with a length > 50,000 bp were mined for BGCs with antiSMASH89 (v: 6.1.1; cla: “--genefinding-tool prodigal --cb-knownclusters --cb-subclusters –asf”). Next, all core biosynthetic genes that were annotated by antismash were extracted and aggregated into one file. Reads of all samples were then aligned against this reference and coverage information was extracted for each contig-sample combination, following the procedure described for the SGB analysis. Similarly, differential coverage analysis was repeated identically to the SGB analysis. P-value adjustment was performed using Benjamini-Hochberg adjustment, adjusting for the number of tested core biosynthetic genes. Taxonomic assignment of BGC fragments was performed using Kraken288 (v: 2.1.2; cla: “--use-mpa-style”) with the standard database (v:k2_pluspf_20220908).
Dietary alterations comparison
All day zero samples from the dataset of Rehner et al.52 were taken and processed according to the new data. The dataset was then extended by our dataset, however, only using our healthy controls. The dataset was from then on analyzed independently. If comparisons were performed, e.g. in statistical tests, the vegan/vegetarian cohort was always compared to the omnivore cohort.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Our appreciation extends to all individuals associated with Saarland University, Saarland University Medical Center, and Helmholtz Institute for Pharmaceutical Research Saarbrücken, whose contributions significantly enriched this study, albeit not reflected in authorship. Furthermore, we extend our gratitude to the participants of this study, whose consent allowed us to acquire and scrutinize clinical samples, thereby unraveling insights into their intricate microbial composition and functions. The compute infrastructure for this project was funded by the DFG [469073465], received by AK. This work was further supported financially by the Saarland University, the UdS-HIPS TANDEM initiative, and the TALENTS Marie Skłodowska-Curie COFUND-Action of the European Commission [101081463], received by LAGM. The views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union, which cannot be held responsible for them.
Author contributions
R.M., S.L.B., A.K., and R.B. supervised the research and obtained research funding. S.L.B., A.K., G.P.S., and J.R. designed the experiments. J.R. and N.L. performed the experiments, and together with GPS and LAGM analyzed the data. G.P.S., A.G., and L.A.G.M. designed the computational analysis. M.H., B.S., S.K., F.M., V.K., M.K., T.M., T.B., E.F., B.S., S.F., S.S.G., S.K., M.Z., F.G., J.ö.R., T.V., C.H., M.S., M.L.S., M.U., A.B., N.L.H., M.C.M., J.M.S., and R.B. supervised clinical sample collection and medical assessment of participants and contributed to the medical and experimental workflow. M.P.G., S.R., T.B., E.F., S.F., S.S.G., M.K., F.G., V.K., C.G., L.D., and A.H. collected clinical samples and performed medical assessment of participants health. J.R., G.P.S., and A.K. wrote the paper. All authors reviewed and edited the paper. D.K., J.H., K.B., T.A.M.G., C.F. C.B., and O.V.K. supported the annotation of the Biosynthetic Gene Clusters. J.M.S. supported the interpretation of the gut-related microbiota.
Peer review
Peer review information
Nature Communications thanks Marnix Medema and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The metagenomic sequencing data after removing ambient human DNA generated in this study has been deposited in the Sequencing Read Archive under the accession code PRJNA1057503.
Competing interests
G.P.S., R.M., and A.K. are co-founders of MooH GmbH, a company developing metagenomic based oral health tests. FM is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung. His institution (Saarland University) has received scientific support from Ablative Solutions, Medtronic, and ReCor Medical. He has received speaker honoraria/consulting fees from Ablative Solutions, Amgen, Astra-Zeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, ReCor Medical, Servier, and Terumo. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Georges P. Schmartz, Jacqueline Rehner, Madline P. Gund, Verena Keller, Sören L. Becker, Rolf Müller, Robert Bals, Andreas Keller.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-52598-7.
References
- 1.Potrykus, M., Czaja-Stolc, S., Stankiewicz, M., Kaska, L. & Malgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients13, 10.3390/nu13113839 (2021). [DOI] [PMC free article] [PubMed]
- 2.Kahrstrom, C. T., Pariente, N. & Weiss, U. Intestinal microbiota in health and disease. Nature535, 47 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Becker, A. et al. Effects of resistant starch on symptoms, fecal markers, and gut microbiota in parkinson’s disease - The RESISTA-PD Trial. Genomics Proteom. Bioinforma.20, 274–287 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puschhof, J. & Elinav, E. Human microbiome research: growing pains and future promises. PLoS Biol.21, e3002053 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsanos, A. H. et al. in Biomarkers for Endometriosis: State of the Art (ed Thomas D’Hooghe) 41-75 (Springer International Publishing, 2017).
- 6.Hajjo, R., Sabbah, D. A. & Al Bawab, A. Q. Unlocking the potential of the human microbiome for identifying disease diagnostic biomarkers. Diagnostics (Basel)12, 10.3390/diagnostics12071742 (2022). [DOI] [PMC free article] [PubMed]
- 7.Li, M. et al. Performance of gut microbiome as an independent diagnostic tool for 20 diseases: cross-cohort validation of machine-learning classifiers. Gut Microbes15, 2205386 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long, D. R. et al. Contribution of the patient microbiome to surgical site infection and antibiotic prophylaxis failure in spine surgery. Sci. Transl. Med16, eadk8222 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Integrative, H. M. P. R. N. C. The integrative human microbiome project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe16, 276-289, 10.1016/j.chom.2014.08.014 (2014). [DOI] [PMC free article] [PubMed]
- 10.Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature473, 174–180 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature464, 59–65 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature500, 541–546 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Zhou, X. et al. Longitudinal profiling of the microbiome at four body sites reveals core stability and individualized dynamics during health and disease. Cell Host Microbe32, 506–526 e509 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd-Price, J., Abu-Ali, G. & Huttenhower, C. The healthy human microbiome. Genome Med8, 51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costea, P. I. et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol3, 8–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature569, 655–662 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan, Y. et al. The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice. Nat. Microbiol.8, 787–802 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worby, C. J. et al. Longitudinal multi-omics analyses link gut microbiome dysbiosis with recurrent urinary tract infections in women. Nat. Microbiol.7, 630–639 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battaglia, T. W. et al. A pan-cancer analysis of the microbiome in metastatic cancer. Cell187, 2324–2335 e2319 (2024). [DOI] [PubMed] [Google Scholar]
- 20.Wang, R., Li, Z., Liu, S. & Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open13, e065186 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redondo, M., Keyt, H., Dhar, R. & Chalmers, J. D. Global impact of bronchiectasis and cystic fibrosis. Breathe (Sheff.)12, 222–235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharya, S., Heidler, P. & Varshney, S. Incorporating neglected non-communicable diseases into the national health program-A review. Front Public Health10, 1093170 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pakdin, M., Zarei, L., Bagheri Lankarani, K. & Ghahramani, S. The cost of illness analysis of inflammatory bowel disease. BMC Gastroenterol.23, 21 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zannetos, S., Zachariadou, T., Zachariades, A., Georgiou, A. & Talias, M. A. The economic burden of adult asthma in Cyprus; a prevalence-based cost of illness study. BMC Public Health17, 262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortaredona, S. & Ventelou, B. The extra cost of comorbidity: multiple illnesses and the economic burden of non-communicable diseases. BMC Med15, 216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramesh, S. & Kosalram, K. The burden of non-communicable diseases: A scoping review focus on the context of India. J. Educ. Health Promot12, 41 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloom, D. E. et al. The global economic burden of noncommunicable diseases. (Program on the Global Demography of Aging, 2012).
- 28.Campbell, C. et al. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines11, 10.3390/biomedicines11020294 (2023). [DOI] [PMC free article] [PubMed]
- 29.Giordano-Kelhoffer, B. et al. Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review. Biomedicines10, 10.3390/biomedicines10081803 (2022). [DOI] [PMC free article] [PubMed]
- 30.Bjerre, R. D. et al. Skin dysbiosis in the microbiome in atopic dermatitis is site-specific and involves bacteria, fungus and virus. BMC Microbiol21, 256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santana, P. T., Rosas, S. L. B., Ribeiro, B. E., Marinho, Y. & de Souza, H. S. P. Dysbiosis in inflammatory bowel disease: pathogenic role and potential therapeutic targets. Int. J. Mol. Sci.23, 10.3390/ijms23073464 (2022). [DOI] [PMC free article] [PubMed]
- 32.Yang, D., Xing, Y., Song, X. & Qian, Y. The impact of lung microbiota dysbiosis on inflammation. Immunology159, 156–166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de la Torre, B. G. & Albericio, F. The pharmaceutical industry in 2021. An analysis of FDA drug approvals from the perspective of molecules. Molecules27, 10.3390/molecules27031075 (2022). [DOI] [PMC free article] [PubMed]
- 34.Torres Salazar, B. O. et al. Commensal production of a broad-spectrum and short-lived antimicrobial peptide polyene eliminates nasal Staphylococcus aureus. Nat. Microbiol9, 200–213 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blin, K. et al. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res51, W46–W50 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blin, K., Shaw, S., Medema, M. H. & Weber, T. The antiSMASH database version 4: additional genomes and BGCs, new sequence-based searches and more. Nucleic Acids Res.10.1093/nar/gkad984 (2023). [DOI] [PMC free article] [PubMed]
- 37.Kautsar, S. A., Blin, K., Shaw, S., Weber, T. & Medema, M. H. BiG-FAM: the biosynthetic gene cluster families database. Nucleic Acids Res49, D490–D497 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch, P. et al. ABC-HuMi: the atlas of biosynthetic gene clusters in the human microbiome. Nucleic Acids Res.10.1093/nar/gkad1086 (2023). [DOI] [PMC free article] [PubMed]
- 39.Keller, A. et al. Toward the blood-borne miRNome of human diseases. Nat. Methods8, 841–843 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Mullowney, M. W. et al. Artificial intelligence for natural product drug discovery. Nat. Rev. Drug Discov.22, 895–916 (2023). [DOI] [PubMed] [Google Scholar]
- 41.Rehner, J. et al. Systematic cross-biospecimen evaluation of dna extraction kits for long- and short-read multi-metagenomic sequencing studies. Genomics Proteom. Bioinforma.20, 405–417 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowarsky, M. et al. Numerous uncharacterized and highly divergent microbes which colonize humans are revealed by circulating cell-free DNA. Proc. Natl Acad. Sci. USA114, 9623–9628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascal Andreu, V. et al. BiG-MAP: an automated pipeline to profile metabolic gene cluster abundance and expression in microbiomes. mSystems6, e0093721 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terlouw, B. R. et al. MIBiG 3.0: a community-driven effort to annotate experimentally validated biosynthetic gene clusters. Nucleic Acids Res51, D603–D610 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding, T. & Schloss, P. D. Dynamics and associations of microbial community types across the human body. Nature509, 357–360 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim, Y. S., Unno, T., Kim, B. Y. & Park, M. S. Sex differences in gut microbiota. World J. Mens. Health38, 48–60, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deschasaux, M. et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med.24, 1526–1531 (2018). [DOI] [PubMed] [Google Scholar]
- 48.He, Y. et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med.24, 1532–1535 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Gaulke, C. A. & Sharpton, T. J. The influence of ethnicity and geography on human gut microbiome composition. Nat. Med.24, 1495–1496 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Singh, R. K. et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med15, 73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morais, S. et al. Cryptic diversity of cellulose-degrading gut bacteria in industrialized humans. Science383, eadj9223 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rehner, J. et al. The effect of a planetary health diet on the human gut microbiome: a descriptive analysis. Nutrients15, 10.3390/nu15081924 (2023). [DOI] [PMC free article] [PubMed]
- 53.Tolosa, E., Garrido, A., Scholz, S. W. & Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol.20, 385–397 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clancy, J. et al. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol. Microbiol22, 867–879 (1996). [DOI] [PubMed] [Google Scholar]
- 55.Daly, M. M., Doktor, S., Flamm, R. & Shortridge, D. Characterization and prevalence of MefA, MefE, and the associated msr(D) gene in Streptococcus pneumoniae clinical isolates. J. Clin. Microbiol42, 3570–3574 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ardanuy, C. et al. Distribution of subclasses mefA and mefE of the mefA gene among clinical isolates of macrolide-resistant (M-phenotype) Streptococcus pneumoniae, viridans group streptococci, and Streptococcus pyogenes. Antimicrob. Agents Chemother.49, 827–829 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harimaya, A. et al. High prevalence of erythromycin resistance and macrolide-resistance genes, mefA and ermB, in Streptococcus pneumoniae isolates from the upper respiratory tracts of children in the Sapporo district, Japan. J. Infect. Chemother.13, 219–223 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Codjoe, F. S. & Donkor, E. S. Carbapenem resistance: a review. Med Sci (Basel)6, 10.3390/medsci6010001 (2017). [DOI] [PMC free article] [PubMed]
- 59.Khan, A. U., Maryam, L. & Zarrilli, R. Structure, genetics and worldwide spread of new delhi metallo-beta-lactamase (NDM): a threat to public health. BMC Microbiol17, 101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, R. et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun.9, 1179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gregoire, N., Aranzana-Climent, V., Magreault, S., Marchand, S. & Couet, W. Clinical Pharmacokinetics and Pharmacodynamics of Colistin. Clin. Pharmacokinet.56, 1441–1460 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Coelho, J., Woodford, N., Afzal-Shah, M. & Livermore, D. Occurrence of OXA-58-like carbapenemases in Acinetobacter spp. collected over 10 years in three continents. Antimicrob. Agents Chemother.50, 756–758 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fursova, N. K. et al. The spread of bla OXA-48 and bla OXA-244 carbapenemase genes among Klebsiella pneumoniae, Proteus mirabilis and Enterobacter spp. isolated in Moscow, Russia. Ann. Clin. Microbiol Antimicrob.14, 46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavlopoulos, G. A. et al. Unraveling the functional dark matter through global metagenomics. Nature622, 594–602 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donia, M. S. et al. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell158, 1402–1414 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beghini, F. et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife10, 10.7554/eLife.65088 (2021). [DOI] [PMC free article] [PubMed]
- 67.Katz, K. S. et al. STAT: a fast, scalable, MinHash-based k-mer tool to assess Sequence Read Archive next-generation sequence submissions. Genome Biol.22, 270 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ewels, P., Magnusson, M., Lundin, S. & Kaller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics32, 3047–3048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ondov, B. D. et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol.17, 132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McInnes et al. UMAP: Uniform manifold approximation and projection. J Open Source Softw.3, 861 (2018).
- 72.Lin, H. & Peddada, S. D. Analysis of compositions of microbiomes with bias correction. Nat. Commun.11, 3514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nurk, S., Meleshko, D., Korobeynikov, A. & Pevzner, P. A. metaSPAdes: a new versatile metagenomic assembler. Genome Res27, 824–834 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mikheenko, A., Saveliev, V. & Gurevich, A. MetaQUAST: evaluation of metagenome assemblies. Bioinformatics32, 1088–1090 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Kang, D. D. et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ7, e7359 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sieber, C. M. K. et al. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat. Microbiol3, 836–843 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bowers, R. M. et al. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat. Biotechnol.35, 725–731 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parks, D. H. et al. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res.50, D785–D794 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Almeida, A. et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol.39, 105–114 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gounot, J. S. et al. Genome-centric analysis of short and long read metagenomes reveals uncharacterized microbiome diversity in Southeast Asians. Nat. Commun.13, 6044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasolli, E. et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell176, 649–662 e620 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jain, C., Rodriguez, R. L., Phillippy, A. M., Konstantinidis, K. T. & Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun.9, 5114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaumeil, P. A., Mussig, A. J., Hugenholtz, P. & Parks, D. H. GTDB-Tk v2: memory friendly classification with the genome taxonomy database. Bioinformatics38, 5315–5316 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Danecek, P. et al. Twelve years of SAMtools and BCFtools. Gigascience10, 10.1093/gigascience/giab008 (2021). [DOI] [PMC free article] [PubMed]
- 86.de Nies, L. et al. PathoFact: a pipeline for the prediction of virulence factors and antimicrobial resistance genes in metagenomic data. Microbiome9, 49 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feldgarden, M. et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep.11, 12728 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wood, D. E., Lu, J. & Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol.20, 257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blin, K. et al. antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res49, W29–W35 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The metagenomic sequencing data after removing ambient human DNA generated in this study has been deposited in the Sequencing Read Archive under the accession code PRJNA1057503.