Abstract
The purpose of this study was to examine the effects of far-infrared (FIR) heat on quality of life (QOL) in older adults. Participants were assigned to either a convective heat group (CON) or a convective and FIR group. Participants received six, 30-min heat sessions over the course of three weeks. Pre- and post-assessments included physical measures such as range of motion, gait speed, Timed Up and Go, and hand grip strength. Standardized questionnaires were used to determine pain severity and its interference with daily life, and the impact pain had on overall QOL. Pain severity was significantly reduced (from 3.31 to 2.5, p < .05) in the FIR group from pre-to-post, and pain interference was significantly reduced (from 1.26 to 0.43, p < .05) in the CON group from pre-to-post testing. Findings suggest that heat therapy was successful in reducing pain over time.
Keywords: ADAR, older adults, pain, quality of life, thermotherapy
Background
For a growing number of older adults, chronic health conditions and pain result in reduced quality of life (QoL) and significant health care costs (Centers for Disease Control and Prevention [CDC], 2019). The population of older adults in the United States has increased by 33% in a 10-year time frame, from 37.2 million in 2006 to 49.2 million in 2016 (Administration on Aging [AoA], 2017). By 2060, this number is expected to double to 98 million (AoA, 2017).
The prevalence of chronic pain typically increases with age and many older adults will face it in their lifetime (CDC, 2020; Nawai, 2019). As our nation faces an ongoing opioid epidemic and individuals struggle with addiction or side effects of pain medications, it is important to find alternative interventions for older adults to manage their pain (National Institutes on Health [NIH], 2020). The CDC recommends using nonopioid treatments for chronic pain whenever possible, supporting the use of interdisciplinary multimodal rehabilitation interventions (CDC, n.d.). Given the growing number of older adults, high rates of chronic health conditions, chronic pain challenges, and the significant costs associated with chronic pain management, interdisciplinary research is needed to identify interventions that improve pain-related QoL.
QoL for people living with chronic conditions and pain may be addressed through a variety of physiological interventions. The World Health Organization (WHO) defines QoL as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns” (WHO, n.d.) Correspondingly, the World Federation of Occupational Therapy has identified chronic health conditions as a research priority (WFOT, 2018). As chronic health conditions involving pain likely interfere with healthy ageing and may affect older adult’s perceptions about their position in life (Dagnino & Campos, 2022), treatment for such is increasingly removed from hospital-based settings, and identifying effective interventions that can take place within primary care and community-based settings is essential (Mackenzie et al., 2017). As the population of older adults increases throughout the world, research-based interventions that promote healthy ageing and disease prevention are also critical (Mackenzie et al., 2017). To this end, older adults with and without chronic conditions often identify pain as being a common problem and there is increasing evidence to suggest that some heat-related modalities may have a positive effect on pain (Hussain & Cohen, 2018; Sugie et al., 2020; Tsagkaris et al., 2022).
One recent method identified as a potential means of mitigating pain is the use of far-infrared (FIR) heating. Infrared radiation (IR) is a form of electromagnetic energy, with a wavelength longer than that of visible light, ranging between 700 nm and 1000 μm. IR is divided into three groups based upon wavelength: Near infrared (0.8–1.5 μm), middle infrared (1.5 to 5.6 μm), and FIR (5.6–1000 μm) (Toyokawa et al., 2003). FIR is able to penetrate up to 1.5 inches into the body, reaching deep skin, muscles, blood vessels, lymphatic glands, and nerves (Lee et al., 2019, Vatansever and Hamblin 2012). Previous research has shown that FIR may have an analgesic effect on the body via various mechanisms occurring at the cellular level, known as thermotherapy (Salm et al., 2019). Specifically, thermotherapy has been shown to promote muscle relaxation and increased circulation, thereby actively releasing tension and stiffness that may cause pain and other bodily discomfort. Conversely, in some cases, heat therapies may be contraindicated for conditions like edema (Brosseau et al., 2003).
Literature suggests that FIR sauna thermotherapy temperature is optimal for IR wavelengths between 45 and 60 °C. Considerable evidence suggests that heat therapy in the form of intense, short term, exposure can have substantial health benefits (Hussain and Cohen, 2018). The resulting elevated skin and body temperature initiates thermoregulatory pathways of the brain and CNS. This in turn causes vasodilation, increased heart rate, increased blood flow to the skin, increased cardiac output, and additional sweating. In essence, sauna thermotherapy expedites the human body’s capability to return to a homeostatic state by manipulating mechanisms and pathways in the body (Hussain and Cohen, 2018). Though several studies describe substantial benefits to IR therapy on healing, further research is required to specifically evaluate FIR effects on pain amelioration and the associated effects it has on increased QoL.
Specific measures of pain can be assessed using a variety of tools, including validated measures in addition to those that are more subjective. Our study utilized the Canadian Occupational Performance Measure (COPM) to examine subjective pain and the corresponding impact it has on performance and satisfaction of activities of daily living, as well as the Brief Pain Inventory (BPI), which is an assessment of current and chronic pain. It is well-documented that pain can play a significant role in one’s QoL, which is a subjective measure of one’s satisfaction with their well being (Hadi et al., 2019; Henderson et al., 2021). One validated survey that examines QoL is the Short Form 36 (SF-36), which examines a variety of domains impacting one’s life over the course of several weeks. The SF-36 is a well-known clinically based tool that is effective in teasing out specific areas that are impacting one’s QoL.
In addition to pain, many physical factors are known to play a role in one’s ability to function adequately throughout the day, such as ambulation and functional mobility. These physical measures can be assessed using a variety of tools such as walking speed, Timed Up and Go (TUG), range of motion, and grip strength. Using a robust battery of assessments may allow for a more holistic perspective of one’s vitality, examining the myriad factors that impact one’s ability to complete those daily tasks that are important to the individual. Moreover, gait speed and the TUG test have been strong predictors of morbidity and mortality (Veronese et al., 2018) in older adults and thus are good correlaries for QoL when examining this population. Similarly, grip strength has long been used in occupational and clinical settings as an analog for whole-body strength, and is considered a relevant means of assessing physical function (Musalek & Kirchengast, 2017).
The current body of literature has robust data on the use of FIR in therapeutic settings, however, there is little data specific to the population of older adults. The purpose of this study was to examine the impact of FIR heating, delivered by means of a thermotherapy pod, on the pain, QoL, and physical function in older adults.
Methods
Participants:
Community-dwelling older adults (60 years of age and older) were recruited through flyers posted at local senior living communities and through University newsletters (n = 18 enrolled, n = 17 finished the study). The flyers invited potential participants to an information session where investigators shared details about the study and addressed questions. Additional follow-up information was provided by email and phone. Exclusion criteria included (a) being unable to provide informed consent; (b) being unable to get in and out of the thermotherapy pod used in the study; (c) being unable to fit in the thermotherapy pod; and (d) contraindications to heating/heat modalities. Participants were screened using a pre-screen questionnaire and decisional capacity questionnaire to ensure they met all eligibility criteria. Participants who met the eligibility criteria were taken through an informed consent process by the research team, which included information on the potential risks and benefits, and they were required to recite back expectations of the study and potential risks. Individuals involved in the study received free pain management education and six thermotherapy sessions with no financial compensation. All of the study processes were reviewed and approved by the Institutional Review Board at St Catherine University.
Study Design:
This study utilized a pretest-posttest, within-subjects design (see Figure 1 for the timing and sequencing of the study elements). The goal of the study was to determine whether or not the use of FIR has an impact on QoL over the course of three weeks. Participants were randomized into one of two groups: (a) Convection Heat Only (CON Only) received convection heat, at approximately 60 °C in the thermotherapy pod, as well as education on pain management and (b) FIR Heat plus Convection Heat (FIR + CON) received FIR heat along with convection heat, at approximately 60 °C, and pain management education. Participants were randomly placed into groups according to a computer-generated block randomization with a block size of four and an allocation ratio of 1:1. Following recruitment and consenting, participants were assessed using a battery of tests, followed by six heat treatment sessions with pain management education. Once completed, participants were asked to conduct the same battery of assessments that were initially tested.
Figure 1.
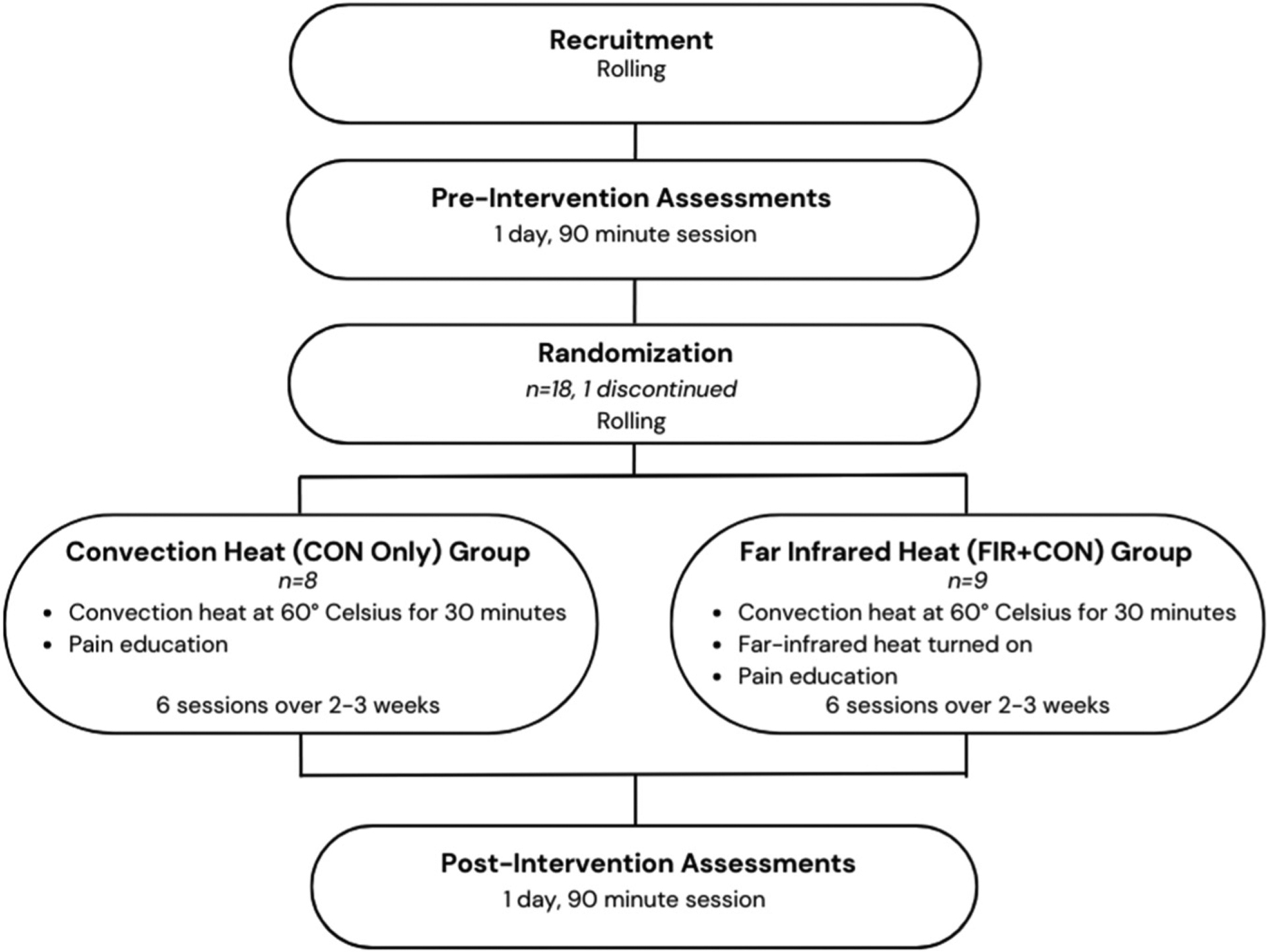
Study design.
Pain Management Education:
Pain management education included a 15-min narrated presentation on the science of pain and pain management strategies utilizing evidence-based practice (AOTA, 2021; Cameron, 2018; Louw et al., 2013; Nawai, 2019; Van Oosterwijck et al., 2013). This was supplemented with participant education handouts to support pain management entitled, “Better Sleep” (Deardorff, 2016; Tang et al., 2012; Teslow & Molitor, 2021), “Physical Activity” (Geneen et al., 2017; Oregon Pain Guidance, 2022; WHO, 2020), and “Activity Pacing” (Guy et al., 2019; Reeves, n.d.). A formative evaluation process was used to determine whether the material was effective for use in the study. Fifteen adults aged 60+ reviewed and provided feedback on the pain education materials, which was based on the Flesch-Kincade Grade Level, deemed accessible for just under the eighth-grade level. The narrated presentation included closed captioning and all print materials met the State of Minnesota’s accessibility standards for font style, size, and color contrast (Minnesota Information Technology Services, n.d.). One hundred percent of the respondents (N = 15) said they would recommend the materials to others and agreed the handouts explained pain management techniques in a way that was easy to understand.
Outcome Measures:
Height was measured in centimeters and weight was measured in pounds and converted to kilograms using a research-grade scale and stadiometer. Grip strength was measured using the best of three measurements using a hand-grip dynamometer (Jamar), where participants were asked to stand with elbows at 90 degrees and squeeze as hard as possible. Hip and ankle range of motion was measured in the sagittal plane using a digital inclinometer (Acumar). The TUG was done twice, with the fastest test being used for analysis. Participants were instructed to “go” from a seated position, and walk around the marker on the floor and return to their original seated position. As soon as the participant was fully seated, time was recorded to the nearest tenth of a second. Similarly, gait speed was measured over the course of four meters, beginning with the signal, “go” and ending when the participant crossed the 4 m line. QoL was measured using the Short Form 36 (SF-36), which is a 36-question survey that measures eight domains of health status using Likert-scale questions. Pain severity and the corresponding impact it had on daily activities, was measured using the BPI, which consisted of 9 questions read out loud and the participant identifying the answer that most closely fit their experience. Finally, the COPM was used to identify activities that were most meaningful for participants and the corresponding impact that pain had on those activities.
Statistical Analysis:
Comparisons of pretest and posttest means were analyzed for all variables using two-tailed paired t-tests; the mean pretest to posttest change for all variables was compared between heat treatment regimens using two-tailed independent t-tests. SPSS version 29 was used for all analyses, using an alpha level of 0.05.
Results
BPI:
We analyzed pain severity and pain interference measures from the BPI to examine potential changes in pain after participants experienced their assigned heating intervention. BPI scores (Figure 2) were significantly reduced from the pre-intervention assessment to the post-intervention assessment for both treatment groups (paired t-tests); this reduction did not differ significantly between the two treatment groups (independent t-test). Specifically, pain severity (Figure 2(a)) dropped 21% (p = .015) in the FIR + CON group and 35% in the CON Only group (p = .039). Additionally, we found that pain interference (Figure 2(b)) dropped 20% (p = .046) in the FIR + CON group and 64% (p = .002) in the CON Only group. However, the pretest to posttest drop in BPI pain measures did not differ between the FIR + CON and CON Only treatment groups: p-values were .976 for pain severity (Figure 3(a)) and 0.362 for pain interference (Figure 3(b)). Thus, we found no evidence that FIR heat, when added to convective heat, was any more effective at pain reduction than convection heat alone, but that participants from both heating groups experienced drops in pain measures after 6 30-minute heat treatments.
Figure 2.
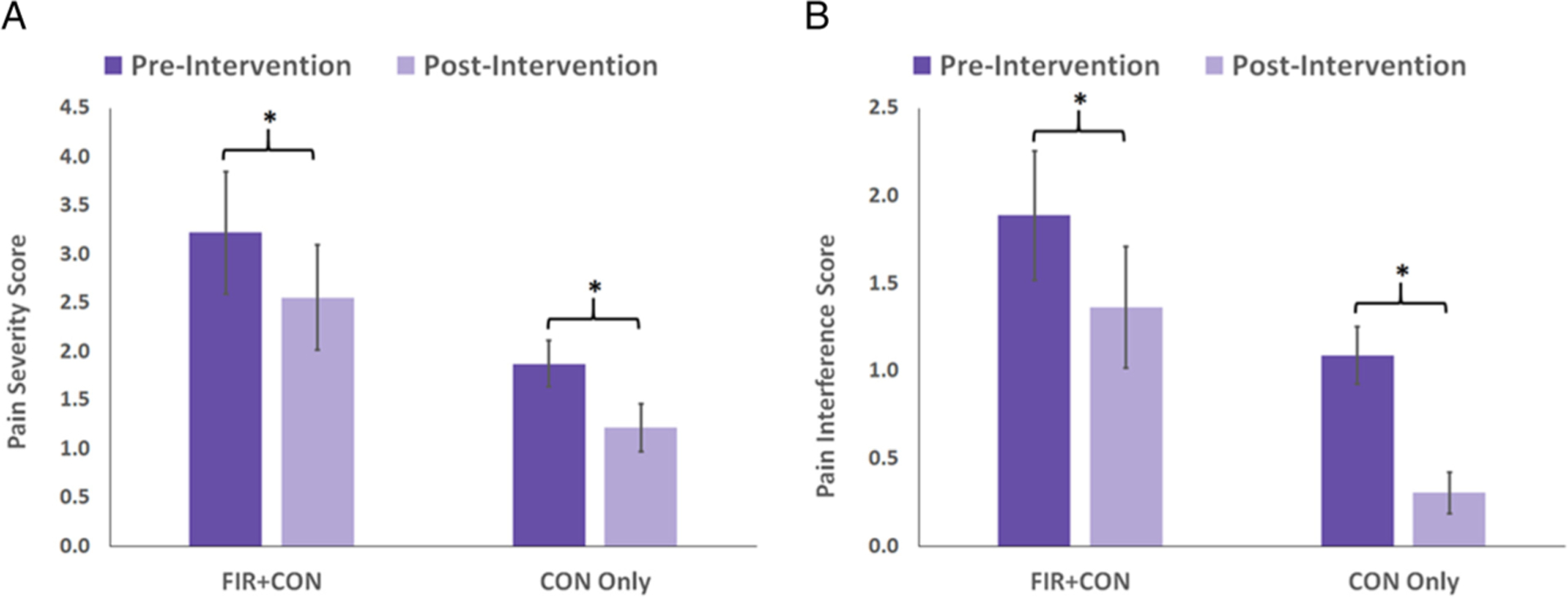
Pre-intervention versus post-intervention BPI measures for each heat treatment group. Pain severity (A) was statistically significantly lower after the heat intervention for both the FIR + CON (p = .015) and CON Only (p = .039) treatment groups. Likewise, pain interference (B) was also statistically significantly lower after the heat intervention for both the FIR + CON (p = .046) and CON Only (p = .002) treatment groups. FIR = far infrared; BPI: Brief Pain Inventory; CON = convective heat group.
Figure 3.
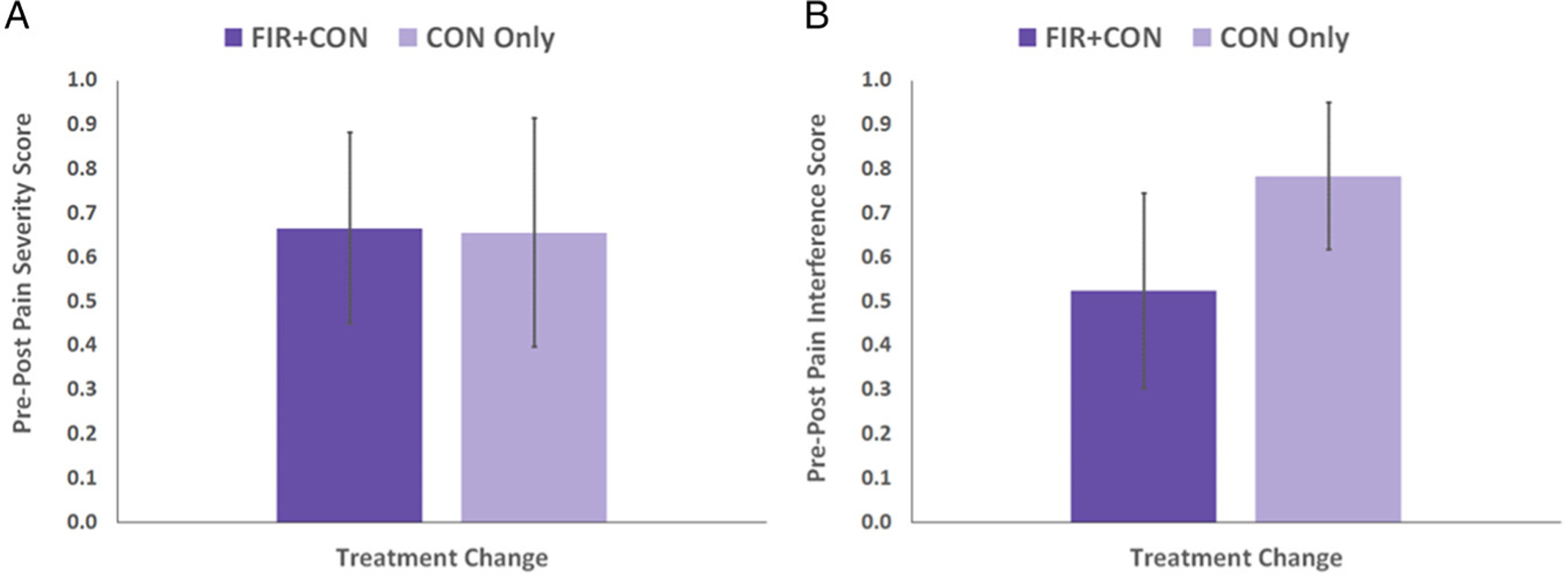
Change in Brief Pain Inventory (BPI) measures from pre- to post-intervention for each heat treatment group. The change from pre- to post-intervention was not statistically significantly different between the two heat treatment groups for either pain severity (A, p = .976) or pain interference (B, p = .362).
Other Outcome Measures:
For both treatment groups, there were no significant differences found between pretest and posttest means for all functional variables, which included L and R grip strength, L and R hip ROM, L and R ankle ROM, TUG, and gait speed. Additionally, there were no significant pretest to posttest differences in the COPM, as was the case with all but one domain of the Short Form 36 (SF-36); in the FIR + CON treatment group, there was a statistically significant reduction in the pain domain from pre- to post-treatment (p = .041).
Discussion
We found that heat therapy was successful in reducing pain in older adults over a short period of time, as both heat treatment groups had statistically significant decreases in pain measures from pre- to post-treatment (Figure 2). However, comparisons between treatment groups in the amount of pain reduction over the treatment period showed that the addition of FIR heat did not produce superior results to that of CON alone; there were no significant differences in pain relief experienced between the two heat groups (Figure 3). These findings suggest that heat, regardless of type, may be beneficial in improving pain severity. Given the high cost of managing chronic pain in older adults, the emphasis on shifting management of chronic pain away from hospital-based settings to community-based settings, and the goal of limiting the risk of opioid addiction, heat therapy may be an affordable and accessible modality to reduce pain.
While this study’s investigators found no changes if function and QOL for participants, previous authors have found pain reduction may correlate with such factors. Similarly, the reduction in pain noted by our participants is well documented in the literature and supports comparable findings in younger populations (Hannuksela & Ellahham, 2001). In their 2014 study, Dehghan & Farahbod found that thermotherapy was associated with a reduction in pain for those participants with low-back pain. While our study was not specifically looking at back pain, we did see a significant reduction in generalized pain in response to relatively acute heat application. Perhaps best aligned with our findings were those of Sugie et al. (2020), who found that low-temperature FIR heat had a positive impact on the Geriatric Depression Scale scores of older adults. Given we found no significant changes in QOL and satisfaction in activity performance, more research us needed to understand the type of amount of pain-related changes that would correspond to changes in QOL or support functional performance. Moreover, further research is needed, possibly with a larger sample size to better understand the necessary factors by which heat-therapy related pain reduction would result in functional and QOL changes.
Pain education has been identified as an important component of Occupational Therapists’ multimodal approach to chronic pain management which supports this study’s delivery of education on sleep, physical activity, and activity pacing to both treatment groups. This education may have impacted the reduction in pain reported and therefore supports the ongoing use of occupational therapy-based education interventions for pain management in older adults. Additionally, this study supports the continued use of heat as a preparatory method for individuals to engage in meaningful occupations (AOTA, 2021). Furthermore, our findings support the AOTA’s assertion that interdisciplinary and biopsychosocial approaches that include multimodal interventions for pain management are the current best practice (AOTA, 2014).
The research environment may also have impacted the study results. Participants reported a positive experience with the study, which provided regular engagement and conversation with undergraduate and graduate student researchers on a college campus. The study parameters of meeting with students for eight sessions over the course of three weeks increased their social participation and community engagement. When asked to describe the experience during post-test assessments, participants reported “‘This has been great fun!’, ‘It was fun and something to do!’, ‘I really enjoyed our conversations … it was a nice feeling’ and ‘I love learning new things!’” These intergenerational connections and social participation may have positively impacted participant QoL and their subsequent report of pain.
Limitations:
Due to the small sample size, this study did not include a group of individuals who received pain education with no heat intervention. However, the lack of a true control group was purposeful, as participants would have known which arm of the study they were on (they would realize there was no heat in the pod); this could have caused a bias in the self-reported pain and other QoL scores that we collected. This purposeful exclusion may limit the conclusions that can be drawn about the role of education and lifestyle pain management strategies, as well as the role of heat therapy in reducing pain. It is also worth noting that our intervention was conducted over a short period of time, whereas some of the previously conducted research examining FIR utilized longer periods of time, ranging from 1–3 months. Other notable considerations include limited knowledge and/or control of participants’ daily activity, which may have contributed to pain amelioration or exacerbation. Convenience sampling was utilized during recruitment, with recruitment targeted towards older adults living in close proximity to the University, as well as older adults already connected to the University. This contributed to participants not being representative of the general population regarding race, ethnicity, and gender.
Future Research:
Based on the results of this study, future research is warranted. Future studies could include an additional group who receive pain education and no heat treatments, to discriminate between the effects of standard pain education and the effects of the thermotherapy. This study provided short-term exposure to heat therapy for three weeks. Future studies could explore the impact of long-term, regular exposure to heat therapy. Additionally, future studies could gather longitudinal data from study participants to collect data on pain to see if and how it changes over time. Lastly, recruitment of participants in the future should better reflect the diversity and demographics of the older adult population in the United States.
Conclusion:
Findings suggest that heat therapy was successful in reducing pain over time, but that the combination of FIR and convective heat was not superior to the effect of convective heat alone. Our results did not discriminate between the effects of the FIR + CON heat combination and the independent effect of FIR heat on QoL. A combination of pain management education and heat therapy may benefit older adults who are living with pain. However, further research is required to properly identify the relationship between QoL and the use of FIR.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIA MSTEM: Advancing Diversity in Aging Research through Undergraduate Education (R25) (grant number 1R25AG060892-01).
Biographies
Melanie Homan, OTD, OTR/L is an adjunct instructor at St. Catherine University Occupational Therapy Department. Her work focuses on older adult quality of life and home modifications through rebuilding together to support safety and independence for community-dwelling older adults.
Shavonnye U. L. Rath is currently a 4th-year student at St. Catherine University. She will graduate in May 2024 with a Bachelor of Science in Exercise and Sports Science with minors in Biology and Longevity & Aging. She is a part of the Katies for Aging Research and Equity program funded by the National Institute on Aging.
Virginia L. S. Green, OTD, OTR/L, is an assistant professor in the occupational therapy department at St. Catherine University. She serves as the doctoral capstone coordinator for the occupational therapy entry-level Doctor of Occupational Therapy (OTD EL) Program, in which she oversees each phase of the student doctoral capstone from development to completion and dissemination. She also serves as the level I fieldwork coordinator for the occupational therapy program in which she coordinates level I student clinical experiences with community partners. Ginny teaches entering the profession, psychological and social factors impacting performance, fieldwork practice reflection, and doctoral capstone experience.
Jennifer Hutson, PhD, OTR/L, ATP made substantial contributions to the conception and design of the research study and participated in critical review of the manuscript to evaluate and provide edits on the accuracy of the work.
Marcella J. Myers, PhD is professor of biology at St. Catherine University. As founding director of the Women’s Health Integrative Research Center and co-PI for the National Institute on Aging-funded Katies for Aging Research and Equity program, she focuses her investigative time and energy on equitably increasing healthspan, mobility, and vitality across the lifespan.
Joshua D. Guggenheimer is an associate professor and program director of the Exercise & Sport Science program at St. Catherine University. He teaches exercise physiology and conducts research in the areas of exercise and activity as it pertains to longevity and healthy aging.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Administration on Aging. (2017). Profile of older Americans. https://www.acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2017OlderAmericansProfile.pdf
- American Occupational Therapy Association. (2014). Occupational therapy’s role with pain rehabilitation [Fact sheet]. https://www.aota.org/~/media/Corporate/Files/AboutOT/Professionals/WhatIsOT/HW/Facts/Pain%20Rehabilitation%20fact%20sheet.pdf
- American Occupational Therapy Association (2021). Role of occupational therapy in pain management. The American Journal of Occupational Therapy, 75(Supplement_3). 10.5014/ajot.2021.75S3001 [DOI] [PubMed] [Google Scholar]
- Brosseau L, Yonge KA, Robinson V, Marchand S, Judd M, Wells G, & Tugwell P (2003). Thermotherapy for treatment of osteoarthritis. Cochrane Database of Systematic Reviews, 4. 10.1002/14651858.CD004522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MH (2018). Physical agents in rehabilitation: From research to practice (5th ed.). Elsevier/Saunders. [Google Scholar]
- Centers for Disease Control and Prevention. (n.d.). Nonopioid treatments for chronic pain. https://www.cdc.gov/drugoverdose/pdf/nonopioid_treatments-a.pdf
- Centers for Disease Control and Prevention. (2019). Health and economic costs of chronic diseases. https://www.cdc.gov/chronicdisease/about/costs/index.htm
- Centers for Disease Control and Prevention. (2020). Chronic pain and high-impact chronic pain among U.S. adults, 2019. https://www.cdc.gov/nchs/products/databriefs/db390.htm
- Dagnino APA, & Campos MM (2022). Chronic pain in the elderly: Mechanisms and perspectives. Frontiers in Human Neuroscience, 16. 10.3389/fnhum.2022.736688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff W (2016). Practicing good sleep hygiene. https://www.spine-health.com/wellness/sleep/practicing-good-sleep-hygiene
- Dehghan M, & Farahbod F (2014). The efficacy of thermotherapy and cryotherapy on pain relief in patients with acute low back pain, a clinical trial study. Journal of Clinical and Diagnostic Research, 8(9), LC01–LC04. 10.7860/JCDR/2014/7404.4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, & Smith BH (2017). Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database of Systematic Reviews, 4(4). 10.1002/14651858.CD011279.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy L, McKinstry C, & Bruce C (2019). Effectiveness of pacing as a learned strategy for people with chronic pain: A systematic review. The American Journal of Occupational Therapy, 73(3), 7303205060p1–7303205060p10. 10.5014/ajot.2019.028555 [DOI] [PubMed] [Google Scholar]
- Hadi MA, McHugh GA, & Closs SJ (2019). Impact of chronic pain on patients’ quality of life: A comparative mixed-methods study. Journal of Patient Experience, 6(2), 133–141. 10.1177/2374373518786013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannuksela ML, & Ellahham S (2001). Benefits and risks of sauna bathing. The American Journal of Medicine, 110(2), 118–126. 10.1016/S0002-9343(00)00671-9 [DOI] [PubMed] [Google Scholar]
- Henderson KN, Killen LG, O’Neal EK, & Waldman HS (2021). The cardiometabolic health benefits of sauna exposure in individuals with high-stress occupations. A mechanistic review. International Journal of Environmental Research and Public Health, 18(3), 1105. 10.3390/ijerph18031105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain J, & Cohen M (2018). Clinical effects of regular dry sauna bathing: A systematic review. Evidence-Based Complementary and Alternative Medicine, 2018, 1–30. https://www.hindawi.com/journals/ecam/2018/1857413/ 10.1155/2018/1857413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Seo Y, Kim Y-W, Kim S, Bae H, Choi J, Lim I, Bang H, Kim J-H, & Ko J-H (2019). Far-infrared radiation stimulates platelet-derived growth factor mediated skeletal muscle cell migration through extracellular matrix-integrin signaling. The Korean Journal of Physiology & Pharmacology, 23(2), 141–150. 10.4196/kjpp.2019.23.2.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw A, & Puentedura E, & International Spine and Pain Institute. (2013). Therapeutic neuroscience education: Teaching patients about pain: A guide for clinicians. International Spine and Pain Institute. [Google Scholar]
- Mackenzie L, Coppola S, Alvarez L, Cibule L, Maltsev S, Loh SY, Mlambo T, Ikiugu MN, Pihlar Z, Sriphetcharawut S, Baptiste S, & Ledgerd R (2017). International occupational therapy research priorities: A Delphi study. Occupational Therapy Journal of Research, 37(2), 72–81. 10.1177/1539449216687528 [DOI] [PubMed] [Google Scholar]
- Minnesota Information Technology Services. (n.d.). Accessibility matters. https://mn.gov/mnit/assets/CompleteSetAccessibilityQuickCards2017_tcm38-294078.pdf
- Musalek C, & Kirchengast S (2017). Grip strength as an indicator of health-related quality of life in old age-A pilot study. International Journal of Environmental Research and Public Health, 14(12), 1447. 10.3390/ijerph14121447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. (2020). Opioid crisis and pain management. https://www.drugabuse.gov/nidamed-medical-health-professionals/opioid-crisis-pain-management
- Nawai A (2019). Chronic pain management among older adults: A scoping review. Sage Open Nursing, 5, 237796081987425. 10.1177/2377960819874259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oregon Pain Guidance. (2022). Pain education toolkit. https://www.oregonpainguidance.org/paineducationtoolkit/
- Reeves L (n.d.). Nonpharmacological pain management strategies: A lifestyle redesign® approach. https://www.occupationaltherapy.com/ot-ceus/all/%23/term:4982/
- Salm DC, Belmonte LAO, Emer AA, dos Santos Leonel L, de Brito RN, da Rocha CC, Martins TC, dos Reis DC, Moro ARP, Mazzardo-Martins L, Kviecinski MR, Bobinski F, Salgado ASI, Cidral-Filho FJ, & Martins DF (2019). Aquatic exercise and far infrared (FIR) modulates pain and blood cytokines in fibromyalgia patients: A double-blind, randomized, placebo-controlled pilot study. Journal of Neuroimmunology, 337, 577077–577077. 10.1016/j.jneuroim.2019.577077 [DOI] [PubMed] [Google Scholar]
- Sugie M, Harada K, Takahashi T, Nara M, Fujimoto H, Kyo S, & Ito H (2020). Effectiveness of a far-infrared low-temperature sauna program on geriatric syndrome and frailty in community-dwelling older people. Geriatrics & Gerontology International, 20(10), 892–898. 10.1111/ggi.14003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NK, Goodchild CE, Sanborn AN, Howard J, & Salkovskis PM (2012). Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: A multilevel daily process study. Sleep, 35(5), 675–87A. 10.5665/sleep.1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslow R, & Molitor WL (2021). Environmental factors impacting sleep among older adults across practice settings. SIS Quarterly Practice Connections, 6(4), 22–25. [Google Scholar]
- Toyokawa H, Matsui Y, Uhara J, Tsuchiya H, Teshima S, Nakanishi H, Kwon AH, Azuma Y, Nagaoka T, Ogawa T, & Kamiyama Y (2003). Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Experimental Biology and Medicine, 228(6), 724–729. 10.1177/153537020322800612 [DOI] [PubMed] [Google Scholar]
- Tsagkaris C, Papazoglou AS, Eleftheriades A, Tsakopoulos S, Alexiou A, Gaman MA, & Moysidis DV (2022). Infrared radiation in the management of musculoskeletal conditions and chronic pain: A systematic review. European Journal of Investigation in Health, Psychology and Education, 12(3), 334–343. 10.3390/ejihpe12030024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oosterwijck J, Meeus M, Paul L, DeSchryber M, Pascal A, Lambrecht L, & Nijs J (2013). Pain physiology education improves health status and endogenous pain inhibition in fibromyalgia: A double-blind randomized controlled trial. Clinical Journal of Pain, 29, 873–882. 10.1097/AJP.0b013e31827c7a7d [DOI] [PubMed] [Google Scholar]
- Vatansever F, & Hamblin MR (2012). Far infrared radiation (FIR): Its biological effects and medical applications. Photonics & Lasers in Medicine, 1(4), 255–266. 10.1515/plm-2012-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese N, Stubbs B, Volpato S, Zuliani G, Maggi S, Cesari M, Lipnicki DM, Smith L, Schofield P, Firth J, Vancampfort D, Koyanagi A, Pilotto A, & Cereda E (2018). Association between gait speed with mortality, cardiovascular disease and cancer: A systematic review and meta-analysis of prospective cohort studies. Journal of the American Medical Directors Association, 19(11), 981–988.e7. 10.1016/j.jamda.2018.06.007 [DOI] [PubMed] [Google Scholar]
- World Federation of Occupational Therapy. (2018). WFOT international research priorities. https://wfot.org/resources/wfot-research-priorities
- World Health Organization. (n.d.). WHOQOL: Measuring quality of life. https://www.who.int/tools/whoqol
- World Health Organization. (2020). Physical activity. Fact sheet 385. www.who.int/mediacentre/factsheets/fs385/en/


