Abstract
The widespread use of commercial feeds with high-fat content in aquaculture may lead to oxidative stress and inflammation in fish during culture, which may cause changes in fish muscle texture. Therefore, mitigating oxidative stress and inflammation during fish farming holds paramount importance in improving fish muscle quality. In this study, we investigated the effect of different dietary levels of chlorogenic acid (CGA; 0, 100, 200, 400, 800, 1600 mg/kg diet, P1–P6) in commercial diets on the muscle of blackspotted croaker (Protonibea diacanthus) through an 8-week feeding trial in open sea cages. The results showed that high dietary CGA levels (P5–P6) could significantly reduce muscle oxidative stress and inflammation (P < 0.05). Muscle toughness (hardness, chewiness, shear force, and gumminess) improved significantly as CGA levels increased (P < 0.05). It was also observed that the gap of muscle fiber was significantly reduced, while the muscle fiber density was significantly increased with the increase of CGA level (P < 0.05). Notably, dietary CGA also had a significant effect on collagen content in the muscle (P < 0.05), which may also be a crucial factor affecting muscle texture. Furthermore, it was found that the reduction of inflammation and increase of collagen deposition in muscle by dietary CGA may be related to the upregulation of transforming growth factor beta (TGF-β) signaling pathway. Finally, it can be concluded that a dietary CGA supplementation of 1173.11 mg/kg is suggested for the aquaculture of P. diacanthus, based on the muscle texture quality.
Keywords: antioxidant, blackspotted croaker, chlorogenic acid, pro-inflammatory response, texture
1. Introduction
The farming of aquatic organisms has experienced a surge in recent decades, supplying more than half of the global fish and seafood consumed by humans [1]. Aquafeeds for marine species have been a key factor in this development. However, for maximizing economic benefits and reducing protein intake and nitrogen emissions, aquaculture industries have increasingly utilized commercial diet with high-fat content [2]. Many studies have reported that excessive fat content in diets may lead to oxidative stress, inflammation, and decreased immune ability in fish, such as tilapia [3] and blunt snout bream [4, 5]. Oxidative stress and pro-inflammatory responses have been observed to decrease muscle quality in fish. The study revealed that a high-fat diet results in a reduction in muscle fiber diameter and a decrease in muscle hardness in grass carp, and these changes are closely associated with increased fat deposition and heightened pro-inflammatory responses within the muscle tissue [6]. Song et al. [7] reported that the increase of reactive oxygen species (ROS) in yellow river carp muscle led to a decrease in myofiber diameter and abnormal collagen metabolism, resulting in changes in muscle texture. The texture is a crucial quality attribute influencing consumers' purchasing behavior particularly for aquatic products due to their fragile properties [8]. In addition, the texture between muscle fibers and internal cross-linking of connective tissue are essential elements when assessing the quality of fresh aquatic products [9]. Thus, high levels of fat in commercial diets could lead to oxidative stress and pro-inflammatory responses, resulting in decreased fish meat quality and a subsequent reduction in aquaculture revenues.
Polyphenols are a major class of semi-water-soluble natural phytochemicals (from fruit and vegetable sources) with one or more benzene rings, which generally exist in nature in the form of glycosides [10]. The polyphenol holds significant potential as a dietary supplement for the protection against oxidative stress and pro-inflammatory responses in organisms [11–13]. Chlorogenic acid (CGA), a polyphenol derivative widely distributed in various natural plants such as honeysuckle, Eucommia, and chrysanthemum, [14], is widely used as additives in aquafeeds due to their multifaceted targeting effects, biodegradability, and minimal adverse effects [15]. Zhang et al. [16] found that CGA can partially counteract the negative effects of oxidized fish oil on channel catfish, alleviating hepatic oxidative stress and intestinal inflammation. Other studies have shown that dietary CGA can downregulate the gene expression of inflammation-related genes and upregulate the gene expression of antioxidant-related genes in juvenile largemouth bass under a high-fat diet [2]. It has also been shown that the addition of CGA to the diet can improve muscle quality in grass carp [17, 18]. However, it remains unclear whether the mechanism by which CGA improves muscle quality in fish is related to its anti-oxidative stress and anti-inflammatory response.
The blackspotted croaker (Protonibea diacanthus) is a benthic fish inhabiting warm coastal waters, with a wide distribution encompassing China, Korea, Japan, and Southeast Asian countries [19]. Recognized for its rapid growth, robust disease resistance, and significant economic benefits, this fish species has emerged as a promising candidate for offshore cage culture operations in China. However, the prolonged consumption of commercial diets has a potential trigger for oxidative stress and pro-inflammatory responses in fish, which can have detrimental effects on their health status and muscle quality, consequently resulting in a decline in market value. To address this concern, an investigation was conducted to evaluate the effects of different concentrations of CGA on muscle quality, antioxidant capacity, and pro-inflammatory responses of P. diacanthus in high-fat commercial diets.
2. Materials and Methods
2.1. Experimental Diets
The basal diet (P1) was used as a commercial puffed diet of China (5 mm diameter, 46.53% dry weight crude protein, and 16.15% crude lipid; Zhuhai Hailong Biotechnology Co., Zhuhai, China). CGA (Bide Pharmatech, Shanghai, China) was used as a supplement to the basal diet at the levels (P1–P6) of 0, 100, 200, 400, 800, and 1600 mg/kg diet, according to Sun et al. [17]. CGA powder (weight calculated according to added concentration) was dissolved in 1 L ddH2O to make the CGA solution. An electric spraying device was used to evenly spray different concentrations of the CGA solution into the base commercial puffed diet. After the diet completely absorbed the CGA solution, it was air-dried at room temperature for 48 h. The 2 g experimental diets was tested using peroxide value with reference to the detection method (GB 5009.227-2016). The general process is peroxide produced during the oxidation process of diets was reacted with iodide to generate iodine; then, the iodine was titrated by sodium thiosulfate standard titration solution; finally, the peroxide value of diets was calculated by iodine content. After the peroxide value of each experimental diet was detected to be less than 5 mmol/kg, the diet pellets were stored at −20°C until use. The proximate composition experimental diets were shown in Table 1. In our preliminary research, the optimal fat for P. diacanthus juveniles is about 11% [20], which is much lower than the fat content of this commercial diet.
Table 1.
Proximate composition (% dry matter) of experimental diets.
| Ingredients | Dietary CGA levels (mg/kg) | |||||
|---|---|---|---|---|---|---|
| 0 (P1) | 100 (P2) | 200 (P3) | 400 (P4) | 800 (P5) | 1600 (P6) | |
| Commercial diet (g/kg) | 1000.00 | 999.90 | 999.80 | 999.60 | 999.20 | 998.40 |
| CGA (mg/kg) | 0.00 | 100.00 | 200.00 | 400.00 | 800.00 | 1600.00 |
| Proximate composition (% dry weight) | ||||||
| Moisture | 7.56 | 7.45 | 7.48 | 7.51 | 7.58 | 7.42 |
| Crude protein | 46.53 | 46.12 | 46.88 | 45.89 | 46.24 | 46.07 |
| Crude lipid | 16.15 | 16.22 | 16.68 | 16.24 | 16.49 | 16.02 |
| Ash | 10.84 | 11.04 | 10.95 | 10.73 | 10.92 | 10.66 |
| Peroxide value (mmol/kg) | 3.35 | 3.62 | 3.77 | 3.22 | 3.40 | 3.64 |
Note: The basal diet was used as a commercial puffed diet of China (5 mm diameter, Zhuhai Hailong Biotechnology Co., Zhuhai, China).
2.2. Feeding Trial and Sampling
Juvenile P. diacanthus in good health were procured from a local marine fish hatchery (Raoping, Guangdong, China) and transported alive to the Nan'Ao Marine Biology Station (Shantou University, Shantou, China). Following a period of acclimation lasting 2 weeks, an 8-week feeding trial took place within floating cages in the offshore areas of the South China Sea. A total of 450 juvenile fish (initial weight of 20.04 ± 0.51 g, respectively) were randomly assigned into 18 cages (1 m × 1 m × 2 m, 25 fish per cage) with six experimental groups (P1–P6) in triplicate. Throughout the 8-week duration of the experiment, the fish were provided with the experimental diets until satiation twice daily, at 07:00 and 16:30. During the experiment, the water temperature ranged from 23°C to 28°C, the salinity was 30–34 g/L, the ammonia nitrogen level was less than 0.04 mg/L, and the dissolved oxygen level was higher than 6 mg/L.
At the end of the feeding trial, all fish were starved for 24 h, collected, and anesthetized with eugenol (1 : 10,000, Reagent, Shanghai, China) to reduce handling stress. All fish were counted and weighed at the beginning and end of the feeding trial. The muscle samples for histological analysis were kept in 4% paraformaldehyde at 4°C. Muscle samples for antioxidant and immune indices determination were assayed within 24 h of collection after storage at 4°C. Furthermore, muscle samples for RNA extraction and collagen content determination were collected from the sampled fish and stored at −80°C until analysis. For texture analysis, two pieces of muscle sample (2 cm × 2 cm) were collected for each fish and analyzed within 24 h. All animal procedures were carried out in accordance with the Guideline for the Care and Use of Laboratory Animals of Shantou University.
2.3. Antioxidant and Immune Indices in Muscle
Muscle samples were homogenized in ice-cold physiological saline 0.89% (w/v) buffer, and the homogenate centrifuged for 20 min at 800 g to collect the supernatant (assayed within 24 h), as described by Guo et al. [21]. The content of malondialdehyde (MDA) and activities of catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) were determined using assay kits (Nanjing Jiancheng Bioengineering Institute, China). The detected methods of these antioxidant enzyme assays were described as follows: MDA content, MDA in the degradation products of lipid peroxide can be condensed with thiobarbituric acid (TBA) to form a red product with a maximum absorption peak at 532 nm; CAT activity, CAT decomposition of H2O2 can be quickly halted by the addition of ammonium molybdate, and the remaining H2O2 reacts with ammonium molybdate to produce a yellowish complex; and GPx activity, GPx can promote the reaction of hydrogen peroxide (H2O2) with reduced glutathione (GSH) to produce H2O and oxidized glutathione (GSSG). Thus, the GPx activity can be determined by measuring the consumption of reduced GSH in this enzymatic reaction; in SOD activity, superoxide anion radical (O2−) is produced by the reaction system of xanthine and xanthine oxidase. The latter oxidizes hydroxylamine to form nitrite, which presents a purple red color under the action of a color developing agent. The light absorption is measured by a visible light spectrophotometer. When the sample contains SOD, the superoxide anion radical has a specific inhibition effect, so that the formation of nitrite is reduced, the absorbance value of the measurement tube is lower than that of the control.
The contents of interleukin 1β (IL1β) and interleukin 6 (IL6) were determined using Fish Interleukin 1β (IL-1β) ELISA Kit (Mlbio, Shanghai, China) and Fish Interleukin 6 (IL-6) ELISA Kit (Mlbio, Shanghai, China), respectively. The specific operation and calculation were performed according to the instructions. The principle of these determinations was the double antibody sandwich method. The samples were added to the coated micropores containing purified fish IL1β or L6 antibodies and then combined with horseradish peroxidase (HRP)-labeled IL1β or L6 antibodies to form a double antibody sandwich (antibody–antibody-enzyme-labeled antibody). After incubation and thorough washing, the substrate 3,3′,5,5′-Tetramethylbenzidine (TMB) was used for color development. The TMB was catalyzed by the HRP enzyme to appear blue and changed to the final yellow color under the action of acid, and the amount of IL1β or L6 in the sample was determined according to the depth of the color. The absorbance value (OD value) was measured at 450 nm, and the IL1β or L6 content was calculated according to the standard curve.
2.4. Measurement of Textural Quality Parameters in Muscle
For texture analysis, the muscle samples were examined using a Universal TA Texture Analyzer (Tengba Instrument Company, Shanghai, China) with a cylindrical probe (TA 25/1000) [22]. The measured parameters include hardness (g), chewiness (g), springiness, gumminess (g), and shear force (g). The type of measurement was determined using the following texture profile analysis (TPA) model parameters: before test speed 2.00 mm/s; test speed 1.00 mm/s; after test speed 2.00 mm/s; probe two times with a compression interval of 2 s; and deformation percentage was 20%. Each piece of sample was probed for five times, with an interval time of 30 s.
2.5. Hematoxylin and Eosin (H&E) Staining in Muscle
Muscle tissues were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) and routinely embedded in paraffin and sectioned. Paraffin sections (4 μm) were mounted on glass slides, dewaxed and dehydrated, and then stained with hematoxylin and eosin (H&E). The average diameter, gap, and density of muscle fiber were counted using Image J software (National Institutes of Health, USA). All sections were observed using an upright optical microscope (Nikon, Tokyo, Japan).
2.6. Determination of Microstructure of Muscle Using Transmission Electron Microscopy (TEM)
Microstructures of muscle were analyzed using a transmission electron microscope (HT7800/HT7700, HITACHI, Japan) at 160 kV. Muscle samples were fixed with 1% osmic acid for 2 h at room temperature and washed three times with PBS (pH 7.4). The samples were then dehydrated in a gradient series of ethanol solutions (70%, 80%, 90%, and 100%). The samples were embedded in Epone resin after removal of ethanol through two successive baths in propylene oxide and then polymerized at 70°C for 24 h. Finally, ultrathin sections were processed using an ultramicrotome (Leica UC7, Leica, Germany), deposited on copper grids, stained with 1% uranyl acetate, and photographed.
2.7. Proximate Composition and Collagen Content Analysis in Muscle
The proximate composition of diets and muscle was determined following the standard methods [23]. Moisture was determined by oven-drying at 105°C for 6 h (FUMA DGX-8053B, Shanghai, China). Crude protein (nitrogen × 6.25) was determined following the Kjeldahl method using the Kjeldahl Auto Sampler System 1035 Analyzer (Foss, Hoganas, Sweden). Crude lipid content was determined by ether extraction using Soxtec TM 8000 extraction system (Foss, Hoganas, Sweden). Ash content was measured using a muffle furnace (Carbolite CWF 11/5, Hope Valley, UK) at 550°C for 12 h. Collagen content was performed using Hydroxyproline Assay Kit following the user's manual (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Briefly, tissue samples were hydrolyzed with the hydrolyzation buffer containing NaOH at 95°C for 20 min. After cooling, the pH was adjusted to 6.0–6.8 by liquid A and liquid B. Then, the mixture was diluted by ddH2O, and the supernatant was collected after carbon adsorption and centrifugation according to the instruction. The Hyp concentration was determined by colorimetry at OD 560 nm, with standard Hyp (5 μg/ml) and ddH2O as control.
2.8. Gene Expression Analysis by qRT-PCR in Muscle
Total RNA from muscle tissues was isolated using the TRIzol reagent (Takara, Tokyo, Japan). The RNA quality was assessed by agarose gel electrophoresis, and the concentration of RNA was quantified by spectrophotometry (NanoDrop 20,480 thermocycler; Roche, Germany). The cDNA was then synthesized using the HiScript III RT SuperMix for qPCR (+gDNA wiper) Kit (Vazyme, Nanjing, China). The PCR reaction mix was set in a total volume of 10 μL with 5 μL SYBR Green I Master (Vazyme, Nanjin, China), 2 μL ddH2O, 1 μL of each primer (10 μM), and 1 μL of diluted cDNA (200 ng μL−1). All amplification reactions were run in triplicate following manufacture's instruction. The specificity and efficiency of the primers for each gene were determined by constructing a standard curve using serial dilutions of cDNA. Relative expression levels were calculated by 2−ΔΔCT method. 18S was used as the reference gene. All primers used were listed in Table 2.
Table 2.
Nucleotide sequences of the primers used in qRT-PCR.
| Primers | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | Efficient rate |
|---|---|---|---|
| COL1A1 | AACGGAGAGGATGGTGAGTC | AAGACCACGAGCACCCATAA | 107 |
| COL2A1 | ACCGCAAACACATCTGGTTC | TAGGCCACGCTGTTCTTACA | 99 |
| TGFβ1 | GGTGGGCAACGTAAGTGGTA | CGGCTCAGGCTCTTTTGGTA | 97 |
| TGFβ2 | AGCATCAGCATCACCTTGCT | ATTTTGGGGGTCTTGCCGAT | 107 |
| TGFβ3 | AGTCAAGTGCCGTTTCAGGT | TATCCTGTCCGCAACTCTGC | 102 |
| TGFβR2 | GGACAACGTGCTGAGAGACA | GCTTGTAGATGCGTTCTGCG | 91 |
| SMAD2 | CATCGACCGTCAGAGATGGC | CTGGACCTTCAGCCGGTTAC | 92 |
| IL1β | TGCTGAAGTCGTTCAGTCGTA | AGACGCCACCGAAACTTCAA | 99 |
| IL6 | TGCACACATGATTTGCCCCT | CCCAGGAGACTGACCAACAA | 96 |
| IL8 | CATCAGAGTCTTCGTCGCCT | GCTCGACTCCCAGACTTCTC | 93 |
| TNF-α | TCAGGCCAAACAGAAGCACT | TTCCAAATGGATGGCTGCCT | 92 |
| 18S | AGCTCGTAGTTGGACTTCGG | CGGCCTGCTTTGAACACTCT | 101 |
Abbreviations: COL1A1, collagen type I alpha 1 chain; COL2A1, collagen type II alpha 1 chain; IL1β, interleukin-1 beta; IL6, interleukin-6; IL8, interleukin-8; SMAD2, SMAD family member 2; TGFβ1, transforming growth factor beta 1; TGFβ2, transforming growth factor beta 2; TGFβ3, transforming growth factor beta 3; TGFβR2, transforming growth factor beta type 2 receptor; TNF-α, tumor necrosis factor-alpha.
2.9. Statistical Analysis
The growth indices of the fish fed diets with different CGA levels were calculated as follows:
Statistical analysis was performed using SPSS 21.0 (SPSS Inc., USA). After passing homogeneity and normal distribution tests, all data were subjected to one-way ANOVA analysis and Duncan's multiple test at the P < 0.05 level of significance. If the data have not passed, these data were subjected to heteroscedasticity test at the P < 0.05 level of significance. The trend analysis of CGA was used as orthogonal polynomial contrast analyses (linear and quadratic trend) at the P < 0.05 level of significance in this study.
3. Results
3.1. Effect of Dietary CGA on Growth Performance
The growth performance, feed utilization, and biometric parameters of P. diacanthus were shown in Table 3. After the 8-week feeding trial, no significant difference in survival rate (SR), weight gain (WG), specific growth rate (SGR), feed conversion ratio (FCR), condition factor (CF), viscerosomatic index (VSI), and hepatosomatic index (HIS) was observed among different dietary CGA supplementation groups (P1–P6).
Table 3.
Growth performance of P. diacanthus fed diets with different levels of CGA for 8 weeks.
| Growth performance | Dietary CGA levels (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 (P1) | 100 (P2) | 200 (P3) | 400 (P4) | 800 (P5) | 1600 (P6) | L. sig. | Q. sig. | |
| Initial weight (g) | 19.71 ± 2.87 | 20.35 ± 3.26 | 19.89 ± 2.42 | 20.88 ± 2.73 | 19.49 ± 1.85 | 19.92 ± 1.41 | 0.788 | 0.661 |
| Final weight (g) | 78.47 ± 11.65 | 82.35 ± 9.95 | 81.25 ± 14.81 | 85.23 ± 18.78 | 81.85 ± 11.90 | 81.41 ± 7.43 | 0.726 | 0.915 |
| SR (%) | 82.67 ± 2.31 | 82.67 ± 8.33 | 86.67 ± 4.62 | 81.33 ± 2.31 | 82.67 ± 2.31 | 85.33 ± 4.62 | 0.926 | 0.268 |
| WG (%) | 297.97 ± 15.54 | 306.78 ± 24.84 | 306.54 ± 31.74 | 305.84 ± 42.89 | 318.66 ± 26.27 | 308.63 ± 22.98 | 0.527 | 0.782 |
| SGR (% day−1) | 2.30 ± 0.23 | 2.34 ± 0.10 | 2.33 ± 0.13 | 2.33 ± 0.17 | 2.38 ± 0.11 | 2.34 ± 0.09 | 0.539 | 0.821 |
| CF (g/cm3) | 1.76 ± 0.11 | 1.72 ± 0.13 | 1.85 ± 0.12 | 1.85 ± 0.27 | 1.75 ± 0.18 | 1.74 ± 0.13 | 0.935 | 0.102 |
| VSI (%) | 6.51 ± 0.26 | 6.33 ± 0.23 | 6.23 ± 0.27 | 6.36 ± 0.33 | 6.41 ± 0.26 | 6.46 ± 0.34 | 0.926 | 0.268 |
| HIS (%) | 2.22 ± 0.21 | 2.27 ± 0.23 | 2.05 ± 0.26 | 2.14 ± 0.26 | 2.19 ± 0.18 | 2.18 ± 0.20 | 0.753 | 0.525 |
| FCR | 1.27 ± 0.11 | 1.24 ± 0.10 | 1.29 ± 0.11 | 1.31 ± 0.05 | 1.23 ± 0.05 | 1.22 ± 0.11 | 0.574 | 0.457 |
Note: Data were presented as mean ± SD (n = 3). Data in the same line with different superscripts were significantly different (P < 0.05).
Abbreviations: CF, condition factor; FCR, feed conversion ratio; HSI, hepatosomatic index; SGR, specific growth rate; SR, survival rate; VSI, viscerosomatic index; WG, weight gain.
3.2. Effect of Dietary CGA on Antioxidant and Immunity Index in Muscle
The antioxidant and immunity index in muscle of fish fed diet with different levels of dietary CGA were shown in Figure 1. The enzymatic activities of CAT, GPx, and SOD had a significant linear trend which increased with the increase of dietary CGA concentration in the diet. The enzymatic activities of CAT and SOD in the P5 group were significantly higher than the control group (P1). In contrast, the significant linear trend was observed in the content of MDA and interleukin 6 (IL6), which was decreased with the increase of dietary CGA concentration in the diet. Notably, the interleukin 1β (IL1β) content in the P5 group exhibited a statistically significant reduction when compared to the control group (P1). Similarly, the IL6 content in the 1600 mg/kg dietary CGA group (P6) displayed a significant reduction compared to the control group.
Figure 1.
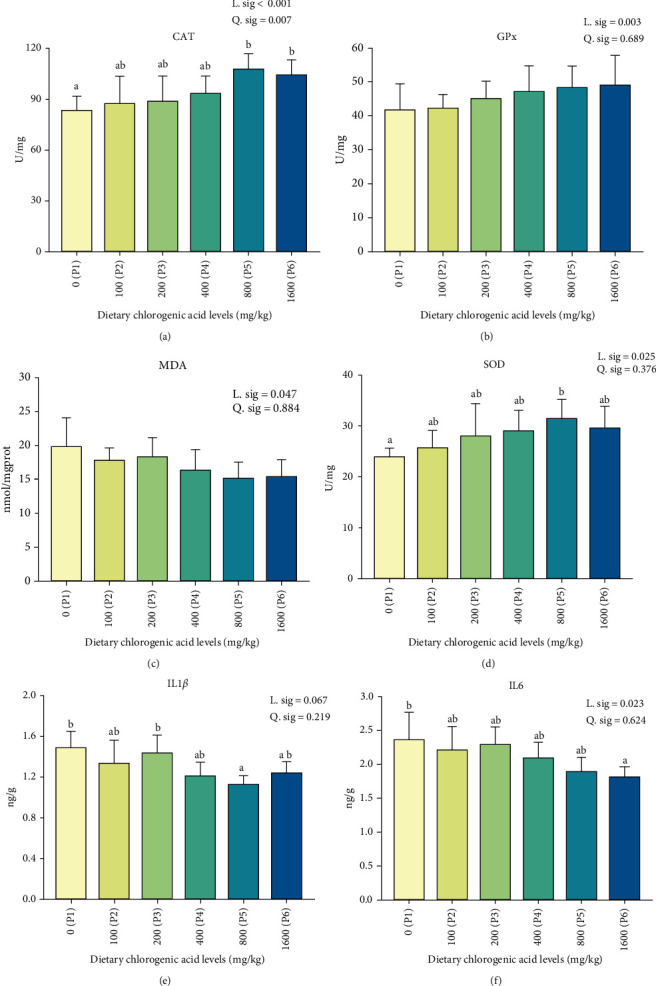
Effect of different levels of dietary CGA supplementation on antioxidation parameters (A) CAT, catalase activity; (B) GPx, glutathione peroxidase activity; (C) MDA, malondialdehyde content; (D) SOD, superoxide dismutase activity and inflammatory cytokines; (E) IL1β, interleukin 1β content; and (F) IL6, interleukin 6 content in the muscle of P. diacanthus. Data were shown as mean ± SD (n = 3). Columns with different letters indicated values with significant difference (P < 0.05).
3.3. Effect of Dietary CGA on Muscle Microstructure
The occurrence of muscle inflammation could lead to microstructural changes. The muscle microstructure of H&E staining in muscle was shown in Figure 2A. Dietary CGA had no significant effect on muscle fiber diameter (Figure 2B) but significantly linearly affected muscle fiber gap and muscle fiber density. With increasing dietary CGA levels, muscle fiber gap significantly linearly trended to decrease, with P5 and P6 groups significantly lower than the other groups (P1–P4; Figure 2C). On the contrary, muscle fiber density significantly linearly trended to increase with the increasing dietary CGA levels (Figure 2D). The muscle fiber density of the P5 group was significantly higher than that of the P1 and P3 groups. In the TEM observation of groups P1 and P5 (Figure 2E), we found that the sarcomeres of P5 were more complete, and the Z-line and M-line of the sarcomere were clear. However, the sarcomeres in the P1 were blurred, accompanied by the phenomenon of mitochondria swelling.
Figure 2.
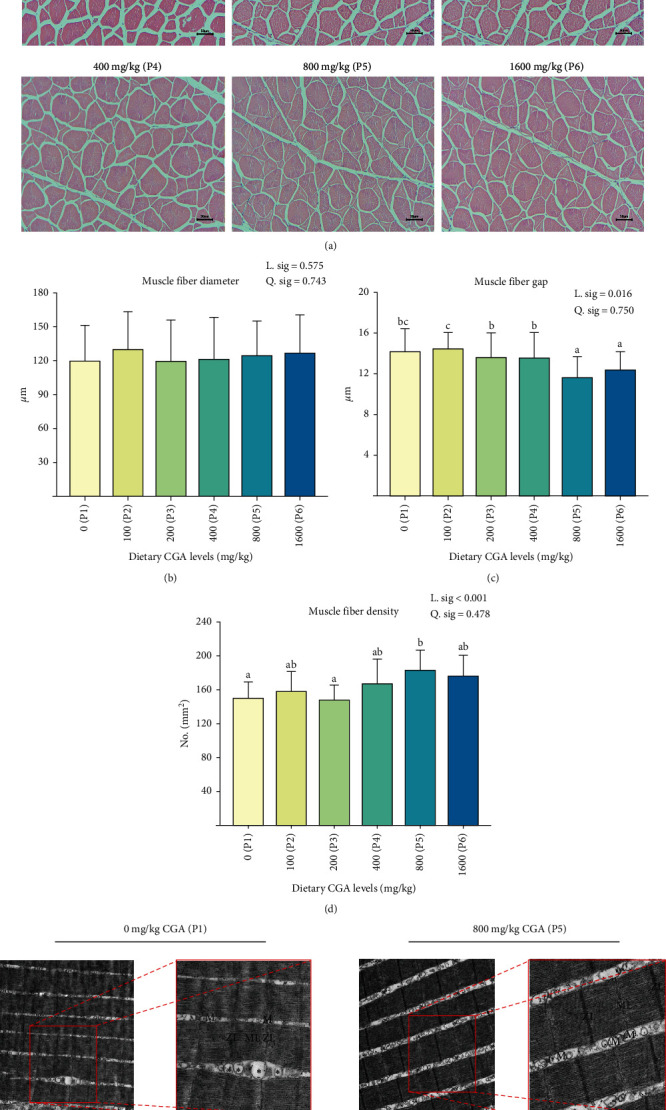
Effect of different levels of dietary CGA supplementation on muscle microstructure in P. diacanthus. (A) Microstructure observation of H&E staining in muscle (×200, scale bar is 50 μm); (B) analysis of muscle fiber diameter; (C) analysis of muscle fiber gap; (D) analysis of muscle fiber density; and (E) microstructure of muscle using transmission electron microscopy (before: ×5.0 k, scale bar is 1 μm; after focusing: ×10.0 k, scale bar is 500 nm). M, mitochondria; ML, M-line; ZL, Z-line; ∗swelling of mitochondria. Data were shown as mean ± SD (n = 3). Columns with different letters indicated values with significant difference (P < 0.05).
3.4. Effect of Dietary CGA on Texture Quality and Proximate Composition Muscle
The effects of dietary CGA on texture quality were shown in Table 4. Generally, the muscle texture quality (hardness, shear force, gumminess, and chewiness) had a significantly linear trend which improved with the increasing CGA levels in the diet. The muscle hardness, chewiness, shear force, and gumminess of P5 and P6 groups were significantly higher than other groups (P1–P4). The springiness of muscle had no significance on different dietary CGA levels. We conducted a quadratic regression analysis using muscle hardness as the dependent variable and calculated the optimal dietary chlorogenic acid level based on muscle texture quality for P. diacanthus to be 1173.11 mg/kg (Figure 3).
Table 4.
Texture of P. diacanthus fed diets with different levels of CGA for 8 weeks.
| Texture | Dietary CGA levels (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 (P1) | 100 (P2) | 200 (P3) | 400 (P4) | 800 (P5) | 1600 (P6) | L. sig. | Q. sig. | |
| Hardness (g) | 48.03 ± 3.46a | 54.70 ± 7.58a | 57.37 ± 11.03ab | 56.73 ± 9.46ab | 70.72 ± 1.15c | 67.38 ± 3.06bc | 0.001 | 0.901 |
| Shear force (g) | 497.66 ± 60.58a | 530.35 ± 93.61ab | 533.02 ± 100.07ab | 510.34 ± 31.46ab | 624.42 ± 24.26ab | 635.09 ± 56.05b | 0.013 | 0.365 |
| Gumminess (g) | 27.44 ± 3.01a | 31.07 ± 6.61a | 34.69 ± 8.59ab | 34.61 ± 6.83ab | 45.20 ± 3.78b | 43.65 ± 5.16b | 0.001 | 0.950 |
| Chewiness (g) | 14.22 ± 2.08a | 15.53 ± 5.60a | 19.04 ± 6.20ab | 18.09 ± 3.17ab | 25.05 ± 4.48b | 25.82 ± 5.05b | 0.002 | 0.660 |
| Springiness | 0.52 ± 0.02 | 0.49 ± 0.09 | 0.54 ± 0.06 | 0.52 ± 0.02 | 0.55 ± 0.06 | 0.59 ± 0.05 | 0.074 | 0.489 |
Note: Data were presented as mean ± SD (n = 3). Data in the same line with different superscripts were significantly different (P < 0.05).
Figure 3.
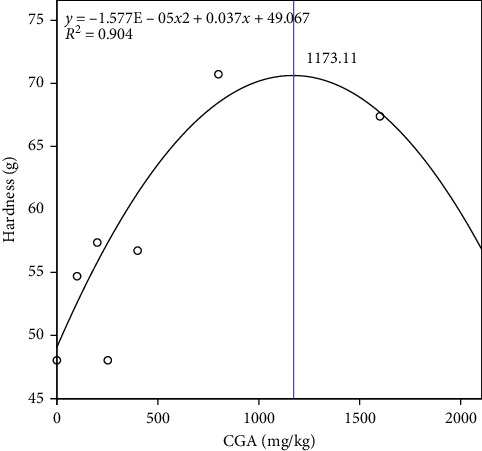
Optimal dietary CGA requirement for P. diacanthus muscle texture quality.
The dietary CGA level had no significant effect on the moisture, crude protein, crude fat, and crude ash of muscle but had a significant effect on the linear trend of the collagen content (Figure 4A). Muscle collagen content increased with increasing dietary CGA levels. The muscle collagen content of the P5 and P6 groups was significantly higher than that of P1 and P2 groups. Pearson correlation analysis showed that there was a positive correlation between muscle collagen content and muscle texture traits (Figure 4B).
Figure 4.
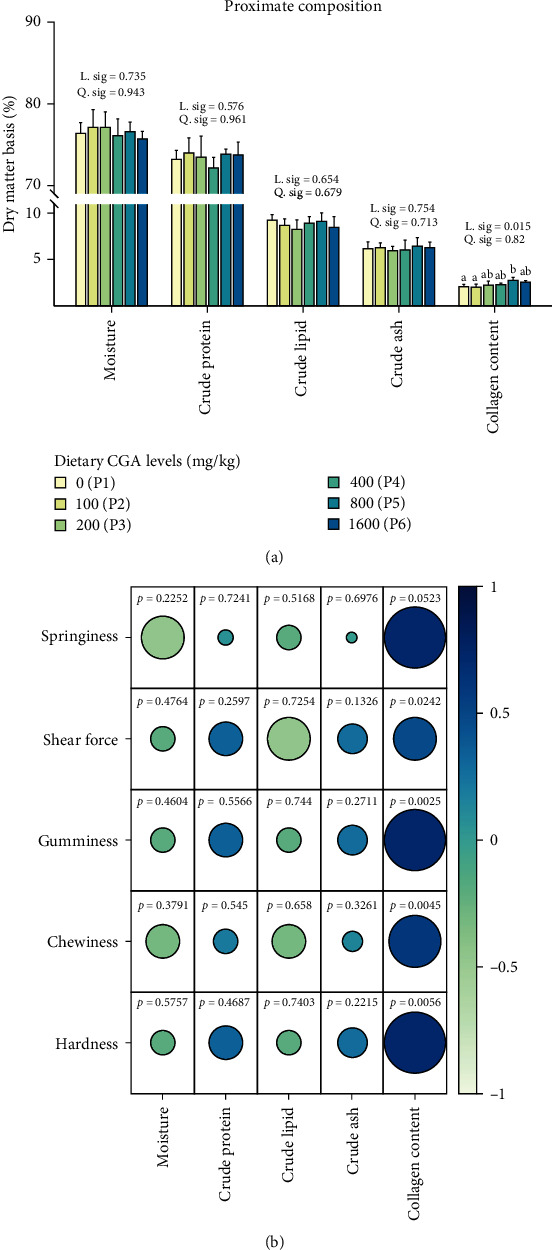
Effect of different levels of dietary CGA supplementation on muscle proximate composition in P. diacanthus. (A) Moisture, crude protein, crude fat, crude ash, and collagen content of muscle and (B) Pearson correlation analysis of muscle proximate composition and texture. Data were shown as mean ± SD (n = 3). Columns with different letters indicated values with significant difference (P < 0.05).
3.5. Effect of Dietary CGA on Gene Expression of Inflammation, Collagen, and TGF-β in Muscle
In order to further study the molecular mechanism of dietary CGA on regulation of muscular inflammation and quality, we detected the expression of TGF-β, inflammatory factors, and collagen. As shown in Figure 5A, the expression levels of two inflammatory factors, IL1β and TNF-α, were significantly linearly decreased with the increase of dietary CGA levels. The expressions of IL1β and TNF-α of P5 group were significantly lower than the control group (P1). Conversely, the gene expression of TGFβ1, TGFβ2, and TGFβR2 significantly linearly increased with the increasing dietary CGA levels (Figure 5B), accompanied by a trend of upregulation of COL1A1 (Figure 5C). Fish fed diet with 800 mg/kg CGA (P5) significantly promoted the expression of TGFβR2 and TGFβ2 than fish fed with the control diet (P1), and fish fed diet with 1000 mg/kg CGA (P6) significantly improved the expression of COL1A1 and TGFβ1 than fish fed with the control diet (P1).
Figure 5.
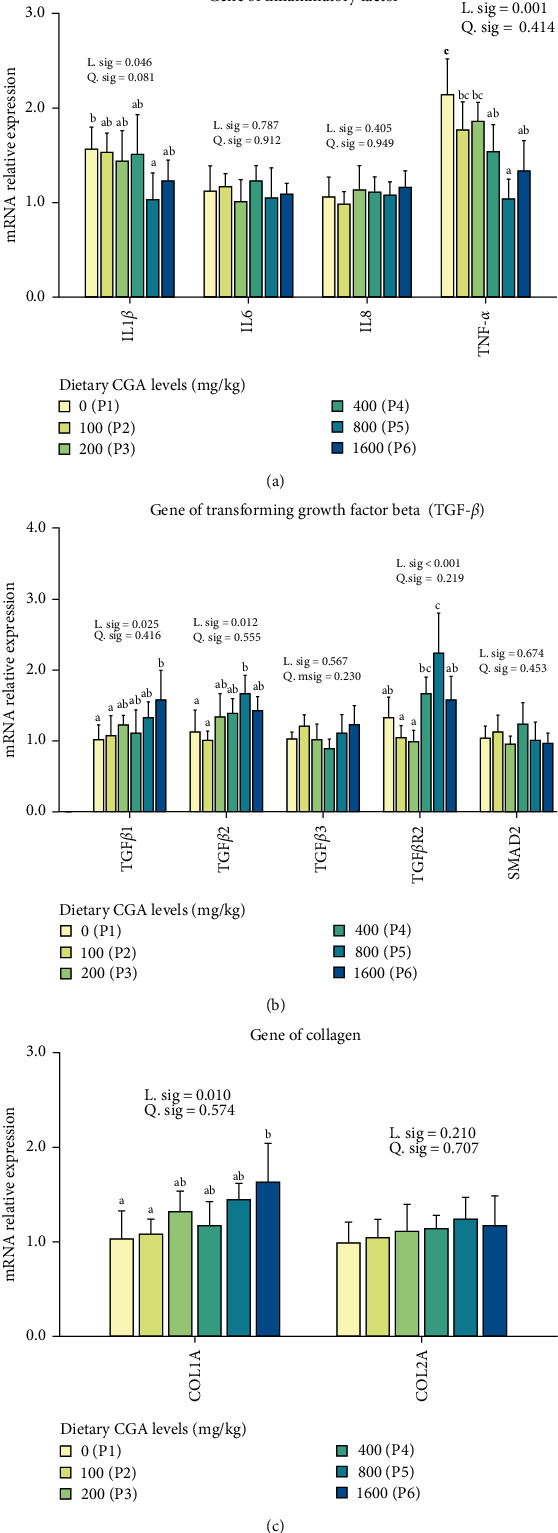
Effect of different levels of dietary CGA supplementation on gene expression of inflammatory factors (A), TGF-β (B), and collagen (C) in the muscle of P. diacanthus. Data were shown as mean ± SD (n = 3). Columns with different letters indicated values with significant difference (P < 0.05).
4. Discussion
In this study, dietary CGA had no significant effect on the growth performance of P. diacanthus. This observation aligns with previous reports, as demonstrated by Koi carp [24], grass carp [17], and shrimp (Litopenaeus vannamei) [25]. These findings suggest that CGA could have no obvious impact on growth of aquatic organisms. However, some studies have shown that dietary CGA has positive effects on farmed animals. In crucian carp, dietary CGA significantly increased the weight, WG rate, and SGR and tended to significantly reduce the feed coefficient [26]. Ghafarifarsani et al. [27] found that CGA-supplemented diets significantly improved growth performance parameters (FW, WG, SGR, and FCR) of rainbow trout. The same phenomenon was observed in experiments of adding CGA to the high-fat diet of largemouth bass [2]. Overall, due to differences in species, environment, and other factors, the impact of CGA on animal growth performance may vary, and further research may be needed.
Our analyses on MDA, a recognized indicator of lipid peroxidation in tissues that indirectly suggest cellular damage [28], were found to inversely correlate with concentrations of dietary CGA. Rather, the activities of SOD, CAT, and GPx in muscle, pivotal antioxidant enzymes documented for their role in counteracting free radicals and mitigating oxidative damage in fish [29], increased with increasing dietary CGA concentrations. It is worth noting that oxidative stress has been implicated in influencing immune responses and apoptosis of animals [30]. In this study, we found that the fish fed appropriate dietary CGA could significantly reduce the contents of IL1β and IL6, which reflects inflammation [31], compared to fish fed with the control diet in muscle. Furthermore, histological examinations (TEM), a reliable diagnostic tool for myopathies [32], were shown that fish fed with the control diet had more inflammation than fish fed diet with appropriate dietary CGA (P5), suggesting dietary CGA is a promising feed additive with commendable antioxidant and anti-inflammatory properties.
Prolonged inflammation damages the structure of fish muscles, leading to changes in muscle texture [33]. To mitigate the immune damage induced by oxidative stress, the utilization of feed additives has shown promise in enhancing muscle characteristics, subsequently improving muscle quality and economic value. Our study indicated that appropriate CGA supplementation in the diet could significantly enhance muscle hardness, chewiness, and shear force of P. diacanthus, thereby augmenting muscle toughness, as similarly observed in pigs [34] and grass carp [18]. These changes in muscle texture, which are closely linked to the palatability of fish meat, are often attributed to alterations in the structure of muscle fibers. Our results demonstrate that dietary CGA supplementation could increase the density of muscle fibers and decrease the gap size of the muscle fiber, consistent with what's observed in crisp grass carp [35]. Similar changes in muscle fiber morphology were also observed when dietary CGA was incorporated into the diet of channel catfish (Ictalurus punctatus) [16]. Also, moisture, protein content, fat content, and collagen content are important determinants for muscle texture [36]. In our study, while CGA did not significantly influence crude protein, crude fat, or moisture content, it significantly elevated collagen content. Further correlation analysis revealed a significant positive relationship between muscle texture properties and collagen content, with higher toughness observed as collagen content increased. Collagen is an essential structural protein that maintains tissue tensile strength [37], and it is a major factor in determining muscle texture [38, 39]. Sun et al. [17] found that dietary CGA can significantly increase the content of collagen and alkaline-insoluble collagen in grass carp muscle. In grass carp muscle cell, Yang et al. [40] also found that CGA could significantly increase the hydroxyproline concentration of the culture medium and significantly increase the mRNA expression of genes related collagen metabolism in muscle cell. Based on the foregoing, we hypothesize that the improvement in the texture of P. diacanthus muscle by CGA may be related to alterations in the muscle fiber structure and an increase in collagen content.
Transforming growth factor beta (TGF-β) is a pivotal molecule in the regulation of various cellular processes, including cell proliferation, differentiation, survival, migration, immune cell function, activation, and deactivation [41]. TGF-β is a family of pluripotent cytokines that comprises three isoforms: TGFβ1, 2, and 3. The interplay of TGF-β and other cytokine signals, such as Smad, interleukin, and Foxp3, is crucial in maintaining the balance between immunity and tolerance [42]. TGFβ1 knockout mice have been shown to develop autoimmune disorders and multifocal inflammation in internal organs, indicating its immune suppressive role in these organs [43, 44]. In this investigation, we observed that dietary CGA significantly reduced the expression of the inflammatory cytokines IL1β and TNF-α and increased the expression of TGFβ1 and TGFβ2 in the muscle of P. diacanthus, which is similar to the results of a dietary CGA supplementation experiment in koi carp [24]. This suggests that dietary CGA may upregulate TGF-β expression to exert its anti-inflammatory effects and further affect the muscle texture.
For another aspect, a large body of evidence has suggested that TGF-β cytokines play a key role in regulating collagen deposition [45, 46]. Collagen is the most important structural protein in the extracellular matrix (ECM) [37], and many studies have revealed that ECM is essential for maintaining the tensile strength and structural integrity of tissues [47]. TGF-β can activate ECM deposition by inducing collagen types I, III, IV, VII, and X; fibronectin; and proteoglycans. This effect is further enhanced through its inhibitory effect on matrix degradation, decreased synthesis of proteases, and increased levels of protease inhibitors [48]. In this study, the collagen gene (COL1A1) was also significantly upregulated by dietary CGA, in concert with the TGF-β, similar to a recent study in mice showing that dietary CGA upregulated collagen and TGF-β in wound mice [49]. Our result indicated that the collagen deposition may be promoted by dietary CGA through upregulation of TGF-β, which also contributed to the improved muscle texture. However, the molecular mechanisms of CGA for immune response, collagen deposition, and texture still need to be further explored, such as protein expression and protein interaction.
5. Conclusions
In summary, our results demonstrated that appropriate dietary CGA could reduce the oxidative stress and immune response in P. diacanthus muscle and improve the collagen content and texture quality of muscle, possibly by suppression of inflammation and enhancement of collagen deposition in muscle by upregulation of TGF-β. Based on the regression curve analysis of muscle hardness, the optimal dietary CGA requirement for P. diacanthus was 1173.11 mg/kg. Our data could provide theoretical basis for developing high-efficiency feed additives for improving muscle quality of fish products during aquaculture.
Contributor Information
Fan Lin, Email: linfan@stu.edu.cn.
Xiaobo Wen, Email: wenxbo@scau.edu.cn.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Haoran Zhang and Haoji Guo contributed equally to the work.
Funding
This work was financially supported by National Natural Science Foundation of China (no. 31972806), Natural Science Foundation of Guangdong Province (2022A1515010778), Special Fund Project for Science and Technology Innovation Strategy of Guangdong Province (STKJ2021099, STKJ202209036), Guangdong Province Basic and Applied Basic Research Fund (2021A1515110179) and Department of Education of Guangdong Province (2022ZDZX4006).
References
- 1.Sprague M., Betancor M. B., Tocher D. R. Microbial and Genetically Engineered Oils as Replacements for Fish Oil in Aquaculture Feeds. Biotechnology Letters . 2017;39(11):1599–1609. doi: 10.1007/s10529-017-2402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin P., Xie S., Zhuang Z., et al. Chlorogenic Acid Improves Health in Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diets: Involvement of Lipid Metabolism, Antioxidant Ability, Inflammatory Response, and Intestinal Integrity. Aquaculture . 2021;545 doi: 10.1016/j.aquaculture.2021.737169.737169 [DOI] [Google Scholar]
- 3.Jia R., Cao L.-P., Du J.-L., et al. Effects of High-Fat Diet on Antioxidative Status, Apoptosis and Inflammation in Liver of Tilapia (Oreochromis niloticus) via Nrf2, TLRs and JNK Pathways. Fish & Shellfish Immunology . 2020;104:391–401. doi: 10.1016/j.fsi.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Lu K.-L., Xu W.-N., Wang L.-N., Zhang D.-D., Zhang C.-N., Liu W.-B. Hepatic β-Oxidation and Regulation of Carnitine Palmitoyltransferase (CPT) I in Blunt Snout Bream Megalobrama amblycephala Fed a High Fat Diet. PLoS ONE . 2014;9(3) doi: 10.1371/journal.pone.0093135.e93135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu K.-L., Xu W.-N., Liu W.-B., Wang L.-N., Zhang C.-N., Li X.-F. Association of Mitochondrial Dysfunction with Oxidative Stress and Immune Suppression in Blunt Snout Bream Megalobrama amblycephala Fed a High-Fat Diet. Journal of Aquatic Animal Health . 2014;26(2):100–112. doi: 10.1080/08997659.2014.893460. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y., Zhong L., Liu Y., et al. Dietary Sanguinarine Supplementation Recovers the Decrease in Muscle Quality and Nutrient Composition Induced by High-Fat Diets of Grass Carp (Ctenopharyngodon idella) Animal Nutrition . 2024;17:208–219. doi: 10.1016/j.aninu.2024.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song D., Yun Y., He Z., et al. Effects of Faba Bean (Vicia faba L.) on Fillet Quality of Yellow River Carp (Cyprinus carpio) via the Oxidative Stress Response. Food Chemistry . 2022;388 doi: 10.1016/j.foodchem.2022.132953.132953 [DOI] [PubMed] [Google Scholar]
- 8.Hwang Y. J., Hwang S. A., Lee J. W. Sensory Property Improvement of Jokbal (Korean pettitoes) Made from Frozen Pig Feet by Addition of Herbal Mixture. Korean Journal for Food Science of Animal Resources . 2016;36(1):19–22. doi: 10.5851/kosfa.2016.36.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J.-H., Sun D.-W., Han Z., Zeng X.-A. Texture and Structure Measurements and Analyses for Evaluation of Fish and Fillet Freshness Quality: A Review. Comprehensive Reviews in Food Science and Food Safety . 2014;13(1):52–61. doi: 10.1111/1541-4337.12043. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadifar E., Yousefi M., Karimi M., et al. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Reviews in Fisheries Science & Aquaculture . 2021;29(4):478–511. doi: 10.1080/23308249.2020.1818689. [DOI] [Google Scholar]
- 11.Ahmadifar E., Moghadam M. S., Dawood M. A. O., Hoseinifar S. H. Lactobacillus fermentum and/or Ferulic Acid Improved the Immune Responses, Antioxidative Defence and Resistance against Aeromonas hydrophila in Common Carp (Cyprinus carpio) Fingerlings. Fish & Shellfish Immunology . 2019;94:916–923. doi: 10.1016/j.fsi.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Armobin K., Ahmadifar E., Adineh H., et al. Quercetin Application for Common Carp (Cyprinus carpio): I. Effects on Growth Performance, Humoral Immunity, Antioxidant Status, Immune-Related Genes, and Resistance against Heat Stress. Aquaculture Nutrition . 2023;2023:10. doi: 10.1155/2023/1168262.1168262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yılmaz S., Ergün S. Trans-Cinnamic Acid Application for Rainbow Trout (Oncorhynchus mykiss): I. Effects on Haematological, Serum Biochemical, Non-Specific Immune and Head Kidney Gene Expression Responses. Fish & Shellfish Immunology . 2018;78:140–157. doi: 10.1016/j.fsi.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Liang N., Kitts D. D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients . 2016;8(1) doi: 10.3390/nu8010016.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H., Tian Z., Cui Y., Liu Z., Ma X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Comprehensive Reviews in Food Science and Food Safety . 2020;19(6):3130–3158. doi: 10.1111/1541-4337.12620. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Wang Z., Shi Y., Xia L., Hu Y., Zhong L. Protective Effects of Chlorogenic Acid on Growth, Intestinal Inflammation, Hepatic Antioxidant Capacity, Muscle Development and Skin Color in Channel Catfish Ictalurus punctatus Fed an Oxidized Fish Oil Diet. Fish & Shellfish Immunology . 2023;134 doi: 10.1016/j.fsi.2022.108511.108511 [DOI] [PubMed] [Google Scholar]
- 17.Sun W., Li X., Xu H., Chen J., Xu X., Leng X. Effects of Dietary Chlorogenic Acid on Growth, Flesh Quality and Serum Biochemical Indices of Grass Carp (Ctenopharyngodon idella) Aquaculture Nutrition . 2017;23(6):1254–1263. doi: 10.1111/anu.12500. [DOI] [Google Scholar]
- 18.Yang H., Li X.-Q., Xu Z., Cheng Z., Leng X.-J. Effects of Three Active Components in Eucommia ulmoides on Growth and Flesh Quality of Grass Carp (Ctenopharyngodon Idellus) Based on Transcriptomics. Aquaculture Nutrition . 2020;26(6):1895–1907. doi: 10.1111/anu.13109. [DOI] [Google Scholar]
- 19.Dutta S., Giri S., Dutta J., Hazra S. Blackspotted Croaker, Protonibea diacanthus (Lacepède, 1802): A New Dimension to the Fishing Pattern in West Bengal, india. Croatian Journal of Fisheries . 2014;72(1):41–44. doi: 10.14798/72.1.684. [DOI] [Google Scholar]
- 20.Li W., Wei F., Xu B., Wang S., Li S., Xuan X. Dietary Lysine Requirement of Juvenile Protonibea diacanthus. Aquaculture Nutrition . 2020;26(4):1289–1294. doi: 10.1111/anu.13084. [DOI] [Google Scholar]
- 21.Guo H., Chen C., Yan X., et al. Effects of Different Dietary Oil Sources on Growth Performance, Antioxidant Capacity and Lipid Deposition of Juvenile Golden Pompano Trachinotus ovatus. Aquaculture . 2021;530 doi: 10.1016/j.aquaculture.2020.735923.735923 [DOI] [Google Scholar]
- 22.Ma L. L., Kaneko G., Wang X. J., et al. Effects of Four Faba Bean Extracts on Growth Parameters, Textural Quality, Oxidative Responses, and Gut Characteristics in Grass Carp. Aquaculture . 2020;516 doi: 10.1016/j.aquaculture.2019.734620.734620 [DOI] [Google Scholar]
- 23.AOAC. Association of Official Analytical Chemists . 18th. Arlington: VA; 2006. Official Methods of Analysis. [Google Scholar]
- 24.Xu G., Xing W., Yu H., et al. Evaluation of Chlorogenic Acid Supplementation in Koi (Cyprinus carpio) Diet: Growth Performance, Body Color, Antioxidant Activity, Serum Biochemical Parameters, and Immune Response. Aquaculture Nutrition . 2022;2022:10. doi: 10.1155/2022/2717003.e2717003 [DOI] [Google Scholar]
- 25.Wang Y., Li Z., Li J., et al. Effects of Dietary Chlorogenic Acid on Growth Performance, Antioxidant Capacity of White Shrimp Litopenaeus vannamei under Normal Condition and Combined Stress of Low-Salinity and Nitrite. Fish & Shellfish Immunology . 2015;43(2):337–345. doi: 10.1016/j.fsi.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Jin X., Su M., Liang Y., Li Y. Effects of Chlorogenic Acid on Growth, Metabolism, Antioxidation, Immunity, and Intestinal Flora of Crucian Carp (Carassius auratus) Frontiers in Microbiology . 2023;13 doi: 10.3389/fmicb.2022.1084500.1084500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghafarifarsani H., Nedaei S., Hoseinifar S. H., Van Doan H. Effect of Different Levels of Chlorogenic Acid on Growth Performance, Immunological Responses, Antioxidant Defense, and Disease Resistance of Rainbow Trout (Oncorhynchus mykiss) Juveniles. Aquaculture Nutrition . 2023;2023:13. doi: 10.1155/2023/3679002.3679002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng S., Chen L., Qin J. G., et al. Effects of Replacement of Dietary Fish Oil by Soybean Oil on Growth Performance and Liver Biochemical Composition in Juvenile Black Seabream, Acanthopagrus schlegeli. Aquaculture . 2008;276(1–4):154–161. doi: 10.1016/j.aquaculture.2008.01.035. [DOI] [Google Scholar]
- 29.Sun S., Ye J., Chen J., Wang Y., Chen L. Effect of Dietary Fish Oil Replacement by Rapeseed Oil on the Growth, Fatty Acid Composition and Serum Non-Specific Immunity Response of Fingerling Black Carp, Mylopharyngodon piceus. Aquaculture Nutrition . 2011;17(4):441–450. doi: 10.1111/j.1365-2095.2010.00822.x. [DOI] [Google Scholar]
- 30.Marri V., Richner H. Immune Response, Oxidative Stress and Dietary Antioxidants in Great Tit Nestlings. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology . 2015;179:192–196. doi: 10.1016/j.cbpa.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Ng A., Tam W. W., Zhang M. W., et al. IL-1β, IL-6, TNF-α and CRP in Elderly Patients with Depression or Alzheimer’s Disease: Systematic Review and Meta-Analysis. Scientific Reports . 2018;8(1) doi: 10.1038/s41598-018-30487-6.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albayda J., van Alfen N. Diagnostic Value of Muscle Ultrasound for Myopathies and Myositis. Current Rheumatology Reports . 2020;22(11) doi: 10.1007/s11926-020-00947-y.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao S., Liu X., Han B., et al. Salmonid Alphavirus Non-Structural Protein 2 is a Key Protein that Activates the NF-κB Signaling Pathway to Mediate Inflammatory Responses. Fish & Shellfish Immunology . 2022;129:182–190. doi: 10.1016/j.fsi.2022.08.059. [DOI] [PubMed] [Google Scholar]
- 34.Wang W., Wen C., Guo Q., Li J., He S., Yin Y. Dietary Supplementation with Chlorogenic Acid Derived from Lonicera Macranthoides Hand-Mazz Improves Meat Quality and Muscle Fiber Characteristics of Finishing Pigs via Enhancement of Antioxidant Capacity. Frontiers in Physiology . 2021;12 doi: 10.3389/fphys.2021.650084.650084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu E., Fu B., Wang G., et al. Proteomic and Metabolomic Basis for Improved Textural Quality in Crisp Grass Carp (Ctenopharyngodon idellus C.et V) Fed with a Natural Dietary pro-Oxidant. Food Chemistry . 2020;325 doi: 10.1016/j.foodchem.2020.126906.126906 [DOI] [PubMed] [Google Scholar]
- 36.Pearce K. L., Rosenvold K., Andersen H. J., Hopkins D. L. Water Distribution and Mobility in Meat during the Conversion of Muscle to Meat and Ageing and the Impacts on Fresh Meat Quality Attributes—A Review. Meat Science . 2011;89(2):111–124. doi: 10.1016/j.meatsci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Andersen U. B., Thomassen M. S., Rørå A. M. B. Texture Properties of Farmed Rainbow Trout (Oncorhynchus mykiss): Effects of Diet, Muscle Fat Content and Time of Storage on Ice. Journal of the Science of Food and Agriculture . 1997;74:347–353. doi: 10.1002/(ISSN)1097-0010. [DOI] [Google Scholar]
- 38.Dransfield E. Intramuscular Composition and Texture of Beef Muscles. Journal of the Science of Food and Agriculture . 1977;28(9):833–842. doi: 10.1002/jsfa.2740280910. [DOI] [Google Scholar]
- 39.Light N., Champion A. E., Voyle C., Bailey A. J. The rôle of Epimysial, Perimysial and Endomysial Collagen in Determining Texture in Six Bovine Muscles. Meat Science . 1985;13(3):137–149. doi: 10.1016/0309-1740(85)90054-3. [DOI] [PubMed] [Google Scholar]
- 40.Yang H., Xu Z., Tan S., Zhang C., Li X., Leng X. In Vitro Effects of Eucommia ulmoides and Its Active Components on the Growth, Lipid Metabolism and Collagen Metabolism of Grass Carp (Ctenopharyngodon idellus) Hepatocyte and Intramuscular Fibroblast. Journal of Fish Biology . 2022;101(3):597–612. doi: 10.1111/jfb.15130. [DOI] [PubMed] [Google Scholar]
- 41.Li M. O., Wan Y. Y., Sanjabi S., Robertson A.-K. L., Flavell R. A. Transforming Growth Factor-β Regulation of Immune Responses. Annual Review of Immunology . 2006;24(1):99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura A., Wakabayashi Y., Mori T. Cellular and Molecular Basis for the Regulation of Inflammation by TGF-β. Journal of Biochemistry . 2010;147(6):781–792. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulkarni A. B., Karlsson S. Inflammation and TGFβ1: Lessons from the TGFβ1 Null Mouse. Research in Immunology . 1997;148(7):453–456. doi: 10.1016/S0923-2494(97)82669-7. [DOI] [PubMed] [Google Scholar]
- 44.Shull M. M., Ormsby I., Kier A. B., et al. Targeted Disruption of the Mouse Transforming Growth Factor-β1 Gene Results in Multifocal Inflammatory Disease. Nature . 1992;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cowin A. J., Hatzirodos N., Holding C. A., et al. Effect of Healing on the Expression of Transforming Growth Factor βs and their Receptors in Chronic Venous Leg Ulcers. Journal of Investigative Dermatology . 2001;117(5):1282–1289. doi: 10.1046/j.0022-202x.2001.01501.x. [DOI] [PubMed] [Google Scholar]
- 46.Jude E. B., Blakytny R., Bulmer J., Boulton A. J. M., Ferguson M. W. J. Transforming Growth Factor-Beta 1, 2, 3 and Receptor Type I and II in Diabetic Foot Ulcers. Diabetic Medicine . 2002;19(6):440–447. doi: 10.1046/j.1464-5491.2002.00692.x. [DOI] [PubMed] [Google Scholar]
- 47.Theocharis A. D., Skandalis S. S., Gialeli C., Karamanos N. K. Extracellular Matrix Structure. Advanced Drug Delivery Reviews . 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Ong V. H., Carulli M. T., Xu S., et al. Cross-Talk between MCP-3 and TGFβ Promotes Fibroblast Collagen Biosynthesis. Experimental Cell Research . 2009;315(2):151–161. doi: 10.1016/j.yexcr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Song L., Yang H., Liang D., et al. A Chlorogenic Acid-Loaded Hyaluronic Acid-Based Hydrogel Facilitates Anti-Inflammatory and pro-Healing Effects for Diabetic Wounds. Journal of Drug Delivery Science and Technology . 2022;70 doi: 10.1016/j.jddst.2022.103232.103232 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


