Abstract
We present a genome assembly from an individual female thistle gall fly, Urophora cardui (Arthropoda; Insecta; Diptera; Tephritidae). The genome sequence has a total length of 837.80 megabases. Most of the assembly is scaffolded into 6 chromosomal pseudomolecules. The mitochondrial genome has also been assembled and is 20.37 kilobases in length.
Keywords: Urophora cardui, thistle gall fly, genome sequence, chromosomal, Diptera
Species taxonomy
Eukaryota; Opisthokonta; Metazoa; Eumetazoa; Bilateria; Protostomia; Ecdysozoa; Panarthropoda; Arthropoda; Mandibulata; Pancrustacea; Hexapoda; Insecta; Dicondylia; Pterygota; Neoptera; Endopterygota; Diptera; Brachycera; Muscomorpha; Eremoneura; Cyclorrhapha; Schizophora; Acalyptratae; Tephritoidea; Tephritidae; Tephritinae; Myopitini; Urophora; Urophora cardui (Linnaeus 1758) (NCBI:txid503482).
Background
Urophora cardui (Linnaeus 1758), commonly known as the Canada Thistle Gall Fly or Thistle Stem Gall Fly, is a fruit fly in the family Tephritidae. The adult fly possesses a gloss black thorax, matte black abdomen, light cream to white scutellum, and a cream head dusted in rusty orange. Its wings are clear with distinct dark bands that, unlike others in the genus, fuse at the hind margin of the wing to form distinct a ‘M’ shape ( White, 1988).
Urophora cardui occurs throughout temperate Central Europe, with records spanning from the United Kingdom in the west to Southern Russia and the shores of Lake Baikal in the east. It has also been introduced to North America as a biological control agent to manage the population of its primary host plant, the Canada or Creeping Thistle ( Cirsium arvense), an invasive weed in that region ( Peschken & Harris, 1975). Its distribution is more latitudinally constrained with most records coming from between 45° and 65° North in its native Palearctic range between 35° and 50° North in its introduced Nearctic range ( GBIF.org, 2024). U. cardui is able to disperse relatively large distances ( Eber & Brandl, 1997; Schlumprecht, 1989) and the northern extent of its range may be increasing due to warmer summers, as demonstrated by observations in Finland ( Jansson, 1991).
Urophora cardui is a stem-gall-forming parasite of Cirsium thistles. In the central European and western parts of its range it is restricted to C. arvense. However, at the extremes of its range it switches host: to C. creticum in the eastern Mediterranean region ( White & Korneyev, 1989) and to C. setosum in the east, between Ukraine and northern Kazakhstan ( Korneyev & White, 1996). In both cases this host shift seems to reflect the relative rarity of the fly’s preferred host in these regions. In the wild females oviposit around 130 to 150 eggs in their lifetime divided into small clutches of between 1 and 12 offspring per multilocular gall ( Freese & Zwölfer, 1996). Eggs, laid in the vegetative shots, have an incubation period of 6.3 days at 24 °C ( Peschken & Harris, 1975). Galls develop around 15 days after oviposition and begin maturation at approximately 36 days, at which stage the larva enters 3rd instar and gains weight rapidly ( Lalonde & Shorthouse, 1985). At between 60 to 100 days post-oviposition, galls contain mature larvae which diapause overwinter and pupate within the gall during spring ( Peschken & Harris, 1975). Adults emerge in early summer with most UK records falling between June and September ( NBN Atlas Partnership, 2024).
Various aspects of this species ecology and life history have been studied due to its potential use as a classical biological control agent in North America ( Peschken & Derby, 1997; Peschken & Harris, 1975; Zwölfer et al., 1970). Since its first release in British Columbia in 1974 subsequent releases in Canada and the USA have established some seemingly stable populations which are thought to partially contribute to the ongoing control of C. arvense through stunting and reduced seed production ( De Clerck-Floate & Cárcamo, 2011; Peschken et al., 1982; Winston et al., 2016).
More recently this species, and its hymenopteran parasites, have been studied for a range of different topics: gall evolution ( Zwölfer, 1979), biogeographic dynamics ( Eber & Brandl, 1996), host-parasitoid interactions ( Johannesen & Seitz, 2003; Zwölfer et al., 2007; Zwölfer & Arnold-Rinehart, 1994), and population genetics ( Eber & Brandl, 1997; Johannesen et al., 2010; Seitz & Komma, 1984; Steinmetz et al., 2004). It is hoped that the high-quality reference genome presented here will be of great value to all these fields, especially the latter.
Genome sequence report
The genome of an adult female Urophora cardui ( Figure 1) was sequenced using Pacific Biosciences single-molecule HiFi long reads, generating a total of 27.86 Gb (gigabases) from 2.05 million reads, providing approximately 31-fold coverage. Primary assembly contigs were scaffolded with chromosome conformation Hi-C data, which produced 134.28 Gbp from 889.27 million reads, yielding an approximate coverage of 160-fold. Specimen and sequencing information is summarised in Table 1.
Figure 1. Photograph of the Urophora cardui (idUroCard1) specimen used for genome sequencing.
Table 1. Specimen and sequencing data for Urophora cardui.
| Project information | |||
|---|---|---|---|
| Study title | Urophora cardui (thistle gall fly) | ||
| Umbrella BioProject | PRJEB62614 | ||
| Species | Urophora cardui | ||
| BioSample | SAMEA112232592 | ||
| NCBI taxonomy ID | 503482 | ||
| Specimen information | |||
| Technology | ToLID | BioSample accession | Organism part |
| PacBio long read sequencing | idUroCard1 | SAMEA112233058 | Whole organism |
| Hi-C sequencing | idUroCard1 | SAMEA112233058 | Whole organism |
| Sequencing information | |||
| Platform | Run accession | Read count | Base count (Gb) |
| Hi-C Illumina NovaSeq 6000 | ERR11496088 | 8.89e+08 | 134.28 |
| PacBio Sequel IIe | ERR11483519 | 2.05e+06 | 27.86 |
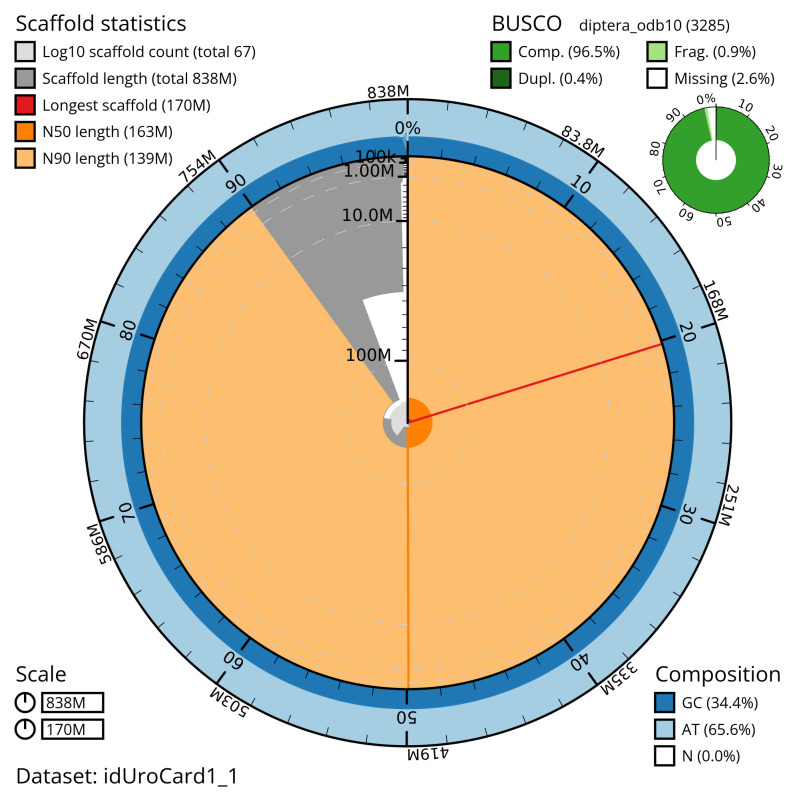
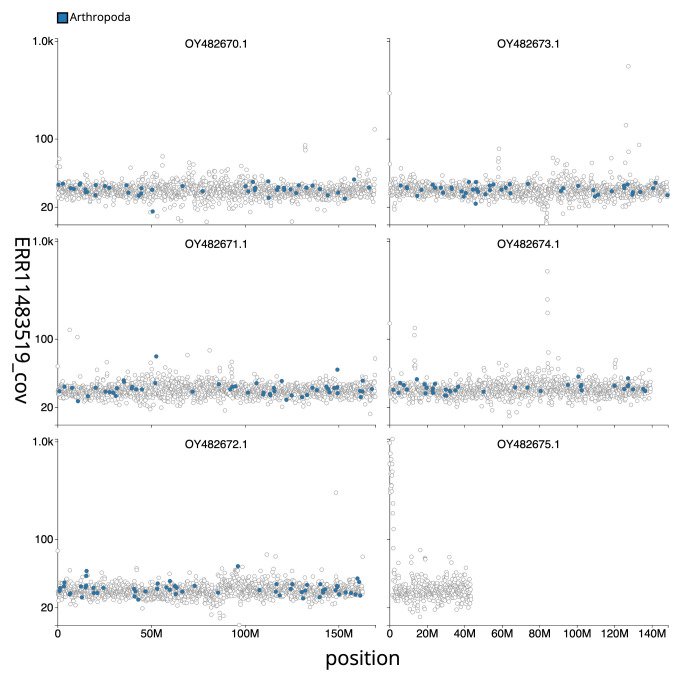
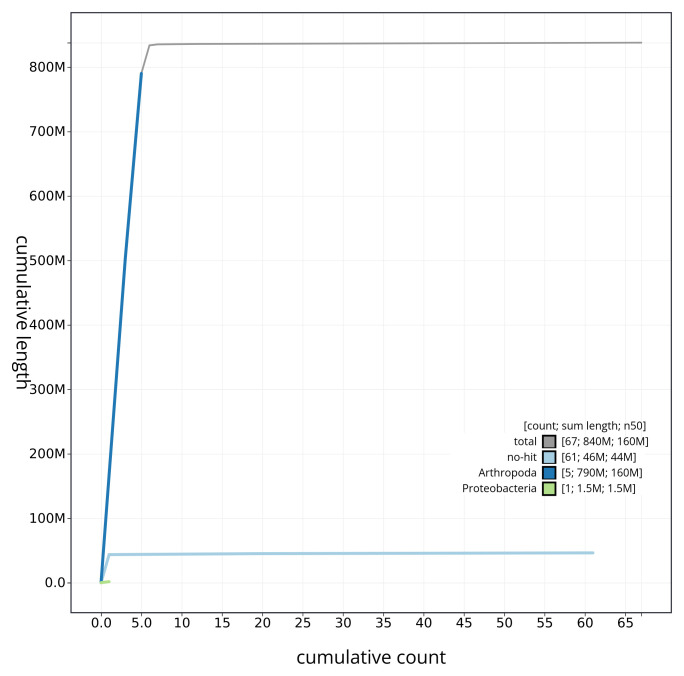
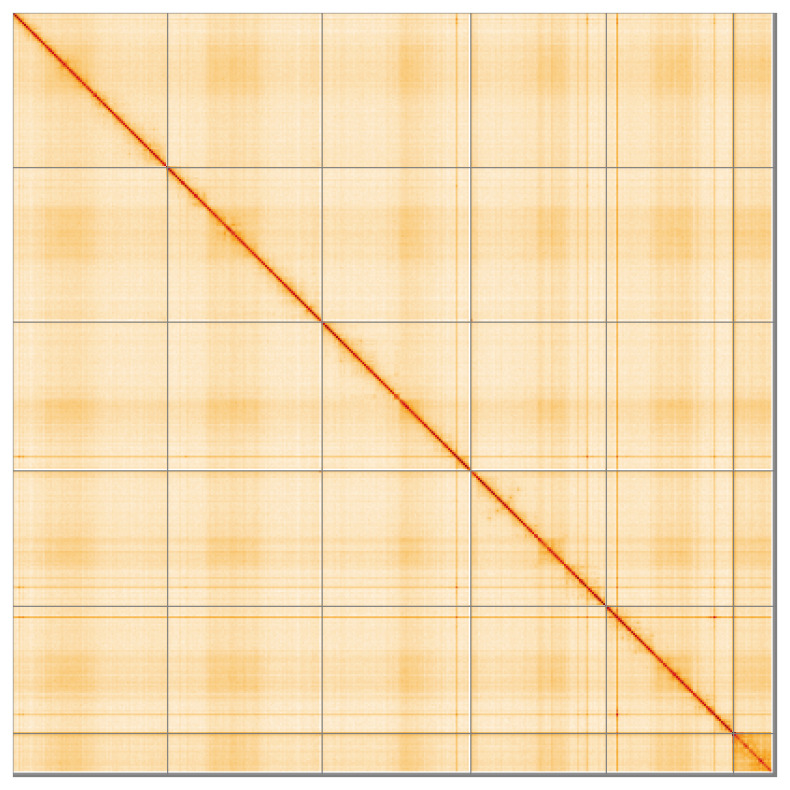
Manual assembly curation corrected 124 missing joins or mis-joins and five haplotypic duplications, reducing the scaffold number by 43.7%, and decreasing the scaffold N50 by 0.5%. The final assembly has a total length of 837.80 Mb in 66 sequence scaffolds with a scaffold N50 of 163.0 Mb ( Table 2). The total count of gaps in the scaffolds is 575. The snail plot in Figure 2 provides a summary of the assembly statistics, while Figure 3 shows the distribution of base coverage against position for sequences in each chromosome. The cumulative assembly plot in Figure 4 shows curves for subsets of scaffolds assigned to different phyla. Most (99.78%) of the assembly sequence was assigned to 6 chromosomal-level scaffolds. Chromosome-scale scaffolds confirmed by the Hi-C data are named in order of size ( Figure 5; Table 3). While not fully phased, the assembly deposited is of one haplotype. Contigs corresponding to the second haplotype have also been deposited. The mitochondrial genome was also assembled and can be found as a contig within the multifasta file of the genome submission.
Figure 2. Genome assembly of Urophora cardui, idUroCard1.1: metrics.
The BlobToolKit snail plot shows N50 metrics and BUSCO gene completeness. The main plot is divided into 1,000 size-ordered bins around the circumference with each bin representing 0.1% of the 837,836,207 bp assembly. The distribution of scaffold lengths is shown in dark grey with the plot radius scaled to the longest scaffold present in the assembly (169,694,088 bp, shown in red). . Orange and pale-orange arcs show the N50 and N90 scaffold lengths (162,960,411 and 139,447,831 bp), respectively. The pale grey spiral shows the cumulative scaffold count on a log scale with white scale lines showing successive orders of magnitude. The blue and pale-blue area around the outside of the plot shows the distribution of GC, AT and N percentages in the same bins as the inner plot. A summary of complete, fragmented, duplicated and missing BUSCO genes in the diptera_odb10 set is shown in the top right. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/idUroCard1_1/dataset/idUroCard1_1/snail.
Figure 3. Genome assembly of Urophora cardui, idUroCard1.1: Distribution plot of base coverage in ERR11483519 against position for sequences in the assembly.
Windows of 100 kb are coloured by phylum. The assembly has been filtered to exclude sequences with length < 2,550,000. An interactive version of this figure is available here.
Figure 4. Genome assembly of Urophora cardui idUroCard1.1: BlobToolKit cumulative sequence plot.
The grey line shows cumulative length for all sequences. Coloured lines show cumulative lengths of sequences assigned to each phylum using the buscogenes taxrule. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/idUroCard1_1/dataset/idUroCard1_1/cumulative.
Figure 5. Genome assembly of Urophora cardui idUroCard1.1: Hi-C contact map of the idUroCard1.1 assembly, visualised using HiGlass.
Chromosomes are shown in order of size from left to right and top to bottom. An interactive version of this figure may be viewed at https://genome-note-higlass.tol.sanger.ac.uk/l/?d=L-VARUgZTPCe69o4zIhIHA.
Table 2. Genome assembly data for Urophora cardui, idUroCard1.1.
| Genome assembly | ||
|---|---|---|
| Assembly name | idUroCard1.1 | |
| Assembly accession | GCA_960531455.1 | |
| Accession of alternate haplotype | GCA_960531475.1 | |
| Span (Mb) | 837.80 | |
| Number of contigs | 642 | |
| Contig N50 length (Mb) | 3.0 | |
| Number of scaffolds | 66 | |
| Scaffold N50 length (Mb) | 163.0 | |
| Longest scaffold (Mb) | 171.21 | |
| Assembly metrics * | Benchmark | |
| Consensus quality (QV) | 60.8 | ≥ 50 |
| k-mer completeness | 100.0% | ≥ 95% |
| BUSCO ** | C:96.5%[S:96.1%,D:0.4%],F:0.9%,M:2.6%,n:3,285 | C ≥ 95% |
| Percentage of assembly mapped
to chromosomes |
99.78% | ≥ 95% |
| Sex chromosomes | Not identified | localised homologous pairs |
| Organelles | Mitochondrial genome: 20.37 kb | complete single alleles |
* Assembly metric benchmarks are adapted from column VGP-2020 of “Table 1: Proposed standards and metrics for defining genome assembly quality” from Rhie et al. (2021).
** BUSCO scores based on the diptera_odb10 BUSCO set using version 5.3.2. C = complete [S = single copy, D = duplicated], F = fragmented, M = missing, n = number of orthologues in comparison. A full set of BUSCO scores is available at https://blobtoolkit.genomehubs.org/view/idUroCard1_1/dataset/idUroCard1_1/busco.
Table 3. Chromosomal pseudomolecules in the genome assembly of Urophora cardui, idUroCard1.
| INSDC
accession |
Name | Length (Mb) | GC% |
|---|---|---|---|
| OY482670.1 | 1 | 169.38 | 34.5 |
| OY482671.1 | 2 | 169.69 | 34.5 |
| OY482672.1 | 3 | 162.96 | 34.5 |
| OY482673.1 | 4 | 148.57 | 34.5 |
| OY482674.1 | 5 | 139.45 | 34.5 |
| OY482675.1 | 6 | 43.56 | 34.5 |
| OY482676.1 | MT | 0.02 | 17.0 |
The estimated Quality Value (QV) of the final assembly is 60.8 with k-mer completeness of 100.0%, and the assembly has a BUSCO v5.3.2 completeness of 96.5% (single = 96.1%, duplicated = 0.4%), using the diptera_odb10 reference set ( n = 3,285).
Metadata for specimens, BOLD barcode results, spectra estimates, sequencing runs, contaminants and pre-curation assembly statistics are given at https://links.tol.sanger.ac.uk/species/503482.
Methods
Sample acquisition
An adult female Urophora cardui (specimen ID Ox002373, ToLID idUroCard1) was netted in Wytham Woods, Oxfordshire, UK (latitude 51.77, longitude –1.34) on 2022-05-28. The specimen was collected by Leila Franzen and Liam Crowley (University of Oxford), identified by Liam Crowley (University of Oxford) and preserved on dry ice.
The initial species identification was verified by an additional DNA barcoding process according to the framework developed by Twyford et al. (2024). A small sample was dissected from the specimens and stored in ethanol, while the remaining parts of the specimen were shipped on dry ice to the Wellcome Sanger Institute (WSI). The tissue was lysed, the COI marker region was amplified by PCR, and amplicons were sequenced and compared to the BOLD database, confirming the species identification ( Crowley et al., 2023). Following whole genome sequence generation, the relevant DNA barcode region was also used alongside the initial barcoding data for sample tracking at the WSI ( Twyford et al., 2024). The standard operating procedures for Darwin Tree of Life barcoding have been deposited on protocols.io ( Beasley et al., 2023).
Nucleic acid extraction
The workflow for high molecular weight (HMW) DNA extraction at the Wellcome Sanger Institute (WSI) Tree of Life Core Laboratory includes a sequence of core procedures: sample preparation and homogenisation, DNA extraction, fragmentation and purification. Detailed protocols developed by the WSI Tree of Life laboratory are publicly available on protocols.io ( Denton et al., 2023b).
In sample preparation, the idUroCard1 sample was weighed and dissected on dry ice ( Jay et al., 2023). Tissue from the whole organism was homogenised using a PowerMasher II tissue disruptor ( Denton et al., 2023a). HMW DNA was extracted using the Automated MagAttract v1 protocol ( Sheerin et al., 2023). DNA was sheared into an average fragment size of 12–20 kb in a Megaruptor 3 system with speed setting 30 ( Todorovic et al., 2023). Sheared DNA was purified by solid-phase reversible immobilisation, using AMPure PB beads to eliminate shorter fragments and concentrate the DNA ( Strickland et al., 2023). The concentration of the sheared and purified DNA was assessed using a Nanodrop spectrophotometer and Qubit Fluorometer using the Qubit dsDNA High Sensitivity Assay kit. Fragment size distribution was evaluated by running the sample on the FemtoPulse system.
Sequencing
Pacific Biosciences HiFi circular consensus DNA sequencing libraries were constructed according to the manufacturers’ instructions. DNA sequencing was performed by the Scientific Operations core at the WSI on a Pacific Biosciences Sequel IIe instrument. Hi-C data were also generated from whole organism tissue of idUroCard1 using the Arima-HiC v2 kit. The Hi-C sequencing was performed using paired-end sequencing with a read length of 150 bp on the Illumina NovaSeq 6000 instrument.
Genome assembly, curation and evaluation
Assembly
The HiFi reads were first assembled using Hifiasm ( Cheng et al., 2021) with the --primary option. Haplotypic duplications were identified and removed using purge_dups ( Guan et al., 2020). The Hi-C reads were mapped to the primary contigs using bwa-mem2 ( Vasimuddin et al., 2019). The contigs were further scaffolded using the provided Hi-C data ( Rao et al., 2014) in YaHS ( Zhou et al., 2023) using the --break option. The scaffolded assemblies were evaluated using Gfastats ( Formenti et al., 2022), BUSCO ( Manni et al., 2021) and MERQURY.FK ( Rhie et al., 2020).
The mitochondrial genome was assembled using MitoHiFi ( Uliano-Silva et al., 2023), which runs MitoFinder ( Allio et al., 2020) and uses these annotations to select the final mitochondrial contig and to ensure the general quality of the sequence.
Assembly curation
The assembly was decontaminated using the Assembly Screen for Cobionts and Contaminants (ASCC) pipeline (article in preparation). Flat files and maps used in curation were generated in TreeVal ( Pointon et al., 2023). Manual curation was primarily conducted using PretextView ( Harry, 2022), with additional insights provided by JBrowse2 ( Diesh et al., 2023) and HiGlass ( Kerpedjiev et al., 2018). Scaffolds were visually inspected and corrected as described by Howe et al. (2021). Any identified contamination, missed joins, and mis-joins were corrected, and duplicate sequences were tagged and removed. The curation process is documented at https://gitlab.com/wtsi-grit/rapid-curation (article in preparation).
Evaluation of the final assembly
A Hi-C map for the final assembly was produced using bwa-mem2 ( Vasimuddin et al., 2019) in the Cooler file format ( Abdennur & Mirny, 2020). To assess the assembly metrics, the k-mer completeness and QV consensus quality values were calculated in Merqury ( Rhie et al., 2020). This work was done using the “sanger-tol/readmapping” ( Surana et al., 2023a) and “sanger-tol/genomenote” ( Surana et al., 2023b) pipelines. The genome readmapping pipelines were developed using the nf-core tooling ( Ewels et al., 2020), use MultiQC ( Ewels et al., 2016), and make extensive use of the Conda package manager, the Bioconda initiative ( Grüning et al., 2018), the Biocontainers infrastructure ( da Veiga Leprevost et al., 2017), and the Docker ( Merkel, 2014) and Singularity ( Kurtzer et al., 2017) containerisation solutions. The genome was also analysed within the BlobToolKit environment ( Challis et al., 2020) and BUSCO scores ( Manni et al., 2021; Simão et al., 2015) were calculated.
Table 4 contains a list of relevant software tool versions and sources.
Table 4. Software tools: versions and sources.
| Software tool | Version | Source |
|---|---|---|
| BlobToolKit | 4.2.1 | https://github.com/blobtoolkit/blobtoolkit |
| BUSCO | 5.3.2 | https://gitlab.com/ezlab/busco |
| bwa-mem2 | 2.2.1 | https://github.com/bwa-mem2/bwa-mem2 |
| Gfastats | 1.3.6 | https://github.com/vgl-hub/gfastats |
| Hifiasm | 0.16.1-r375 | https://github.com/chhylp123/hifiasm |
| HiGlass | 1.11.6 | https://github.com/higlass/higlass |
| Merqury.FK | d00d98157618f4e8d1a9190026b19
b471055b22e |
https://github.com/thegenemyers/MERQURY.FK |
| MitoHiFi | 2 | https://github.com/marcelauliano/MitoHiFi |
| PretextView | 0.2 | https://github.com/wtsi-hpag/PretextView |
| purge_dups | 1.2.3 | https://github.com/dfguan/purge_dups |
| sanger-tol/genomenote | v1.0 | https://github.com/sanger-tol/genomenote |
| sanger-tol/readmapping | 1.1.0 | https://github.com/sanger-tol/readmapping/tree/1.1.0 |
| YaHS | yahs-1.1.91eebc2 | https://github.com/c-zhou/yahs |
Wellcome Sanger Institute – Legal and Governance
The materials that have contributed to this genome note have been supplied by a Darwin Tree of Life Partner. The submission of materials by a Darwin Tree of Life Partner is subject to the ‘Darwin Tree of Life Project Sampling Code of Practice’, which can be found in full on the Darwin Tree of Life website here. By agreeing with and signing up to the Sampling Code of Practice, the Darwin Tree of Life Partner agrees they will meet the legal and ethical requirements and standards set out within this document in respect of all samples acquired for, and supplied to, the Darwin Tree of Life Project.
Further, the Wellcome Sanger Institute employs a process whereby due diligence is carried out proportionate to the nature of the materials themselves, and the circumstances under which they have been/are to be collected and provided for use. The purpose of this is to address and mitigate any potential legal and/or ethical implications of receipt and use of the materials as part of the research project, and to ensure that in doing so we align with best practice wherever possible. The overarching areas of consideration are:
• Ethical review of provenance and sourcing of the material
• Legality of collection, transfer and use (national and international)
Each transfer of samples is further undertaken according to a Research Collaboration Agreement or Material Transfer Agreement entered into by the Darwin Tree of Life Partner, Genome Research Limited (operating as the Wellcome Sanger Institute), and in some circumstances other Darwin Tree of Life collaborators.
Funding Statement
This work was supported by Wellcome through core funding to the Wellcome Sanger Institute [206194, <a href=https://doi.org/10.35802/206194>https://doi.org/10.35802/206194</a>] and the Darwin Tree of Life Discretionary Award [218328, <a href=https://doi.org/10.35802/218328>https://doi.org/10.35802/218328 </a>].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 4 approved]
Data availability
European Nucleotide Archive: Urophora cardui (thistle gall fly). Accession number PRJEB62614; https://identifiers.org/ena.embl/PRJEB62614 ( Wellcome Sanger Institute, 2024). The genome sequence is released openly for reuse. The Urophora cardui genome sequencing initiative is part of the Darwin Tree of Life (DToL) project. All raw sequence data and the assembly have been deposited in INSDC databases. The genome will be annotated using available RNA-Seq data and presented through the Ensembl pipeline at the European Bioinformatics Institute. Raw data and assembly accession identifiers are reported in Table 1 and Table 2.
Author information
Members of the University of Oxford and Wytham Woods Genome Acquisition Lab are listed here: https://doi.org/10.5281/zenodo.12157525.
Members of the Darwin Tree of Life Barcoding collective are listed here: https://doi.org/10.5281/zenodo.12158331
Members of the Wellcome Sanger Institute Tree of Life Management, Samples and Laboratory team are listed here: https://doi.org/10.5281/zenodo.12162482.
Members of Wellcome Sanger Institute Scientific Operations: Sequencing Operations are listed here: https://doi.org/10.5281/zenodo.12165051.
Members of the Wellcome Sanger Institute Tree of Life Core Informatics team are listed here: https://doi.org/10.5281/zenodo.12160324.
Members of the Tree of Life Core Informatics collective are listed here: https://doi.org/10.5281/zenodo.12205391.
Members of the Darwin Tree of Life Consortium are listed here: https://doi.org/10.5281/zenodo.4783558.
References
- Abdennur N, Mirny LA: Cooler: scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics. 2020;36(1):311–316. 10.1093/bioinformatics/btz540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allio R, Schomaker-Bastos A, Romiguier J, et al. : MitoFinder: efficient automated large-scale extraction of mitogenomic data in target enrichment phylogenomics. Mol Ecol Resour. 2020;20(4):892–905. 10.1111/1755-0998.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley J, Uhl R, Forrest LL, et al. : DNA barcoding SOPs for the Darwin Tree of Life project. protocols.io. 2023; [Accessed 25 June 2024]. 10.17504/protocols.io.261ged91jv47/v1 [DOI] [Google Scholar]
- Challis R, Richards E, Rajan J, et al. : BlobToolKit – interactive quality assessment of genome assemblies. G3 (Bethesda). 2020;10(4):1361–1374. 10.1534/g3.119.400908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Concepcion GT, Feng X, et al. : Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18(2):170–175. 10.1038/s41592-020-01056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley L, Allen H, Barnes I, et al. : A sampling strategy for genome sequencing the British terrestrial arthropod fauna [version 1; peer review: 2 approved]. Wellcome Open Res. 2023;8:123. 10.12688/wellcomeopenres.18925.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Veiga Leprevost F, Grüning BA, Alves Aflitos S, et al. : BioContainers: an open-source and community-driven framework for software standardization. Bioinformatics. 2017;33(16):2580–2582. 10.1093/bioinformatics/btx192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clerck-Floate R, Cárcamo H: Biocontrol arthropods: new denizens of Canada’s grassland agroecosystems. Arthropods Can Grassl. 2011;2:291–321. Reference Source [Google Scholar]
- Denton A, Oatley G, Cornwell C, et al. : Sanger Tree of Life sample homogenisation: PowerMash. protocols.io. 2023a. 10.17504/protocols.io.5qpvo3r19v4o/v1 [DOI] [Google Scholar]
- Denton A, Yatsenko H, Jay J, et al. : Sanger Tree of Life wet laboratory protocol collection V.1. protocols.io. 2023b. 10.17504/protocols.io.8epv5xxy6g1b/v1 [DOI] [Google Scholar]
- Diesh C, Stevens GJ, Xie P, et al. : JBrowse 2: a modular genome browser with views of synteny and structural variation. Genome Biol. 2023;24(1):74. 10.1186/s13059-023-02914-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eber S, Brandl R: Metapopulation dynamics of the tephritid fly Urophora cardui: an evaluation of incidence-function model assumptions with field data. J Anim Ecol. 1996;65(5):621–630. 10.2307/5741 [DOI] [Google Scholar]
- Eber S, Brandl R: Genetic differentiation of the tephritid fly Urophora cardui in Europe as evidence for its biogeographical history. Mol Ecol. 1997;6(7):651–660. 10.1046/j.1365-294X.1997.00236.x [DOI] [Google Scholar]
- Ewels P, Magnusson M, Lundin S, et al. : MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32(19):3047–3048. 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewels PA, Peltzer A, Fillinger S, et al. : The nf-core framework for community-curated bioinformatics pipelines. Nat Biotechnol. 2020;38(3):276–278. 10.1038/s41587-020-0439-x [DOI] [PubMed] [Google Scholar]
- Formenti G, Abueg L, Brajuka A, et al. : Gfastats: conversion, evaluation and manipulation of genome sequences using assembly graphs. Bioinformatics. 2022;38(17):4214–4216. 10.1093/bioinformatics/btac460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese G, Zwölfer H: The problem of optimal clutch size in a tritrophic system: the oviposition strategy of the thistle gallfly Urophora cardui (Diptera, Tephritidae). Oecologia. 1996;108(2):293–302. 10.1007/BF00334654 [DOI] [PubMed] [Google Scholar]
- GBIF.org: GBIF occurrence download. GBIF,2024; [Accessed 16 July 2024]. 10.15468/dl.qpm7th [DOI] [Google Scholar]
- Grüning B, Dale R, Sjödin A, et al. : Bioconda: sustainable and comprehensive software distribution for the life sciences. Nat Methods. 2018;15(7):475–476. 10.1038/s41592-018-0046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, McCarthy SA, Wood J, et al. : Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 2020;36(9):2896–2898. 10.1093/bioinformatics/btaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry E: PretextView (Paired REad TEXTure Viewer): a desktop application for viewing pretext contact maps.2022; [Accessed 19 October 2022]. Reference Source
- Howe K, Chow W, Collins J, et al. : Significantly improving the quality of genome assemblies through curation. GigaScience. 2021;10(1): giaa153. 10.1093/gigascience/giaa153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson A: Distribution and dispersal of Urophora cardui (Diptera, Tephritidae) in Finland in 1985-1991. Entomol Fenn. 1991;2(4):211–216. 10.33338/ef.83553 [DOI] [Google Scholar]
- Jay J, Yatsenko H, Narváez-Gómez JP, et al. : Sanger Tree of Life sample preparation: triage and dissection. protocols.io. 2023. 10.17504/protocols.io.x54v9prmqg3e/v1 [DOI] [Google Scholar]
- Johannesen J, Drüeke U, Seitz A: Parapatric diversification after post-glacial range expansion in the gall fly Urophora cardui (Tephritidae). J Biogeogr. 2010;37(4):635–646. 10.1111/j.1365-2699.2009.02253.x [DOI] [Google Scholar]
- Johannesen J, Seitz A: Larval distributions of the ectoparasitoid wasp Eurytoma robusta relative to the host tephritid gall fly Urophora cardui. Entomol Exp Appl. 2003;107(1):47–55. 10.1046/j.1570-7458.2003.00040.x [DOI] [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, et al. : HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018;19(1): 125. 10.1186/s13059-018-1486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneyev V, White I: Fruit flies of the genus Urophora R.-D. (Diptera, Tephritidae) of Eastern Palaearctics. II. Review of species of the subgenus Urophora s. str. Communication 3. Entomol Rev. 1996;76:499–513. [Google Scholar]
- Kurtzer GM, Sochat V, Bauer MW: Singularity: scientific containers for mobility of compute. PLoS One. 2017;12(5): e0177459. 10.1371/journal.pone.0177459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde RG, Shorthouse JD: Growth and development of larvae and galls of Urophora cardui (Diptera, Tephritidae) on Cirsium arvense (Compositae). Oecologia. 1985;65(2):161–165. 10.1007/BF00379213 [DOI] [PubMed] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, et al. : BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol. 2021;38(10):4647–4654. 10.1093/molbev/msab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel D: Docker: lightweight linux containers for consistent development and deployment. Linux J. 2014;2014(239):2, [Accessed 2 April 2024]. Reference Source [Google Scholar]
- NBN Atlas Partnership: Urophora cardui (Linnaeus, 1758). NBN Atlas,2024; [Accessed 16 July 2024]. Reference Source
- Peschken D, Derby J: Establishment of Urophora cardui (Diptera: Tephritidae) on Canada thistle, Cirsium arvense (Asteraceae), and colony development in relation to habitat and parasitoids in Canada.In: Vertical Food Web Interactions: Evolutionary Patterns and Driving Forces.Springer,1997;53–66. 10.1007/978-3-642-60725-7_4 [DOI] [Google Scholar]
- Peschken DP, Finnamore DB, Watson AK: Biocontrol of the weed Canada thistle ( Cirsium arvense): releases and development of the gall fly Urophora cardui (Diptera: Tephritidae) in Canada. Can Entomol. 1982;114(4):349–357. 10.4039/Ent114349-4 [DOI] [Google Scholar]
- Peschken DP, Harris P: Host specificity and biology of Urophora cardui (Diptera: Tephritidae). A biocontrol agent for Canada thistle ( Cirsium arvense). Can Entomol. 1975;107(10):1101–1110. 10.4039/Ent1071101-10 [DOI] [Google Scholar]
- Pointon DL, Eagles W, Sims Y, et al. : sanger-tol/treeval v1.0.0 – Ancient Atlantis.2023. 10.5281/zenodo.10047654 [DOI] [Google Scholar]
- Rao SSP, Huntley MH, Durand NC, et al. : A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–1680. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, McCarthy SA, Fedrigo O, et al. : Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021;592(7856):737–746. 10.1038/s41586-021-03451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, et al. : Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21(1): 245. 10.1186/s13059-020-02134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumprecht H: Dispersal of the thistle gallfly Urophora cardui and its endoparasitoid Eurytoma serraiulae (Hymenoptera: Eurytomidae). Ecol Entomol. 1989;14(3):341–348. 10.1111/j.1365-2311.1989.tb00963.x [DOI] [Google Scholar]
- Seitz A, Komma M: Genetic polymorphism and its ecological background in Tephritid populations (Diptera: Tephritidae).In: Population Biology and Evolution.Springer,1984;143–158. 10.1007/978-3-642-69646-6_12 [DOI] [Google Scholar]
- Sheerin E, Sampaio F, Oatley G, et al. : Sanger Tree of Life HMW DNA extraction: automated MagAttract v.1. protocols.io. 2023; [Accessed 21 November 2023]. 10.17504/protocols.io.x54v9p2z1g3e/v1 [DOI] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, et al. : BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Johannesen J, Seitz A: Clinal genetic variation and the ‘rare allele phenomenon’ in random mating populations of Urophora cardui (Diptera: Tephritidae). Genetica. 2004;122(3):277–290. 10.1007/s10709-004-1419-7 [DOI] [PubMed] [Google Scholar]
- Strickland M, Cornwell C, Howard C: Sanger Tree of Life fragmented DNA clean up: manual SPRI. protocols.io. 2023. 10.17504/protocols.io.kxygx3y1dg8j/v1 [DOI] [Google Scholar]
- Surana P, Muffato M, Qi G: sanger-tol/readmapping: sanger-tol/readmapping v1.1.0 - Hebridean Black (1.1.0). Zenodo. 2023a. 10.5281/zenodo.7755669 [DOI] [Google Scholar]
- Surana P, Muffato M, Sadasivan Baby C: sanger-tol/genomenote (v1.0.dev). Zenodo. 2023b. 10.5281/zenodo.6785935 [DOI] [Google Scholar]
- Todorovic M, Sampaio F, Howard C: Sanger Tree of Life HMW DNA fragmentation: diagenode Megaruptor ®3 for PacBio HiFi. protocols.io. 2023. 10.17504/protocols.io.8epv5x2zjg1b/v1 [DOI] [Google Scholar]
- Twyford AD, Beasley J, Barnes I, et al. : A DNA barcoding framework for taxonomic verification in the Darwin Tree of Life Project [version 1; peer review: awaiting peer review]. Wellcome Open Res. 2024;9:339. 10.12688/wellcomeopenres.21143.1 [DOI] [Google Scholar]
- Uliano-Silva M, Ferreira JGRN, Krasheninnikova K, et al. : MitoHiFi: a python pipeline for mitochondrial genome assembly from PacBio high fidelity reads. BMC Bioinformatics. 2023;24(1): 288. 10.1186/s12859-023-05385-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasimuddin M, Misra S, Li H, et al. : Efficient architecture-aware acceleration of BWA-MEM for multicore systems.In: 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS).IEEE,2019;314–324. 10.1109/IPDPS.2019.00041 [DOI] [Google Scholar]
- Wellcome Sanger Institute: The genome sequence of the thistle gall fly, Urophora cardui (Linnaeus 1758). European Nucleotide Archive.[dataset], accession number PRJEB62614,2024.
- White IM: Tephritid flies: Diptera: Tephritidae, 1.In: Handbooks for the Identification of British Insects.London: Royal Entomological Society of London,1988. Reference Source [Google Scholar]
- White IM, Korneyev VA: A revision of the western Palaearctic species of Urophora Robineau-Desvoidy (Diptera: Tephritidae). Syst Entomol. 1989;14(3):327–374. 10.1111/j.1365-3113.1989.tb00289.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston R, Bell C, De Clerck-Floate R, et al. : Biological control of weeds in the northwest. The Forest Health Technology Enterprise Team,2016. [Google Scholar]
- Zhou C, McCarthy SA, Durbin R: YaHS: yet another Hi-C scaffolding tool. Bioinformatics. 2023;39(1): btac808. 10.1093/bioinformatics/btac808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwölfer H: Strategies and counterstrategies in insect population systems competing for space and food in flower heads and plant galls. Fortschr D Zool. 1979;25(2–3):331–353. Reference Source [Google Scholar]
- Zwölfer H, Arnold-Rinehart J: The evolution of interactions and diversity in plant-insect systems: the Urophora-Eurytoma food web in galls on palearctic cardueae.In: Biodiversity and Ecosystem Function.Berlin, Heidelberg: Springer Berlin Heidelberg,1994;211–233. 10.1007/978-3-642-58001-7_10 [DOI] [Google Scholar]
- Zwölfer H, Böheim M, Beck E: Eurytoma robusta (Hymenoptera: Eurytomidae), a local key factor in the population dynamics of Urophora cardui (Diptera: Tephritidae): a comparative analysis. Eur J Entomol. 2007;104(2):217–224. 10.14411/eje.2007.034 [DOI] [Google Scholar]
- Zwölfer H, Englert W, Pattullo W: Investigations on the biology, population ecology and distribution of Urophora cardui (L). Weed Projects for Canada. Prog Rep. 1970. [Google Scholar]