In the published article, there was an error in Figure 1 as published. In the Figure 1 there are several errors in the panels, which are crucial for the selection of patients included in the study. “Uveitis registry” was changed to “AIDA registry”; “Behçet's disease” was removed from the middle right hand side panel; “Behçet's disease patients with ocular involvement treated with JAK inhibitors” was changed to “Patients treated with JAK inhibitors” in the bottom right hand side panel. The corrected Figure 1 and its caption appear below.
Figure 1.
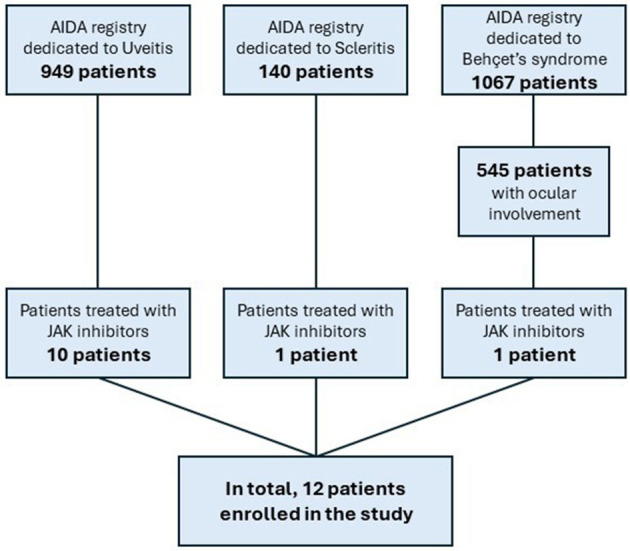
Flow-chart explaining the selection of patients included in this study starting from the total number of patients included in the AutoInflammatory Disease Alliance (AIDA) Network project.
In the published article, there was an error in Table 1 as published. In Table 1, there is a typographical error in the second to last row, “Extra ocular” was changed to “Extraocular”. The corrected Table 1 and its caption appear below.
Table 1.
Demographic, clinical and therapeutic features describing the twelve patients enrolled.
| Sex | Age at ocular disease onset, years | Age at diagnosis, years | Ocular diagnosis | Systemic or oculo-specific diagnosis | Treatment performed | Age at the start of treatment, years | Main reason for starting JAK inhibitors | Treatment duration, months | GCs at start, mg/day | GCs at last assessment, mg/day |
|---|---|---|---|---|---|---|---|---|---|---|
| Female | 33 | 35 | Posterior uveitis (multifocal choroiditis) | Punctate inner choroidopathy | Upadacitinib 15 mg/day plus leflunomide 20 mg/day | 38.5 | Ocular activity | 6 | 0 | 0 |
| Female | 51.2 | 51.4 | Pars planitis | Idiopathic | Upadacitinib 15 mg/day | 54.2 | Ocular activity | 20 | 0 | 0 |
| Female | 81.2 | 81.2 | Anterior uveitis (iridocyclitis) | Idiopathic | Upadacitinib 15 mg/day plus azathioprine 200 mg/day | 83.3 | Ocular activity | 3 | Sub-tenon corticosteroids | 0 |
| Male | 29.8 | 36.3 | Anterior uveitis (iridocyclitis) | Seronegative spondyloarthritis | Upadacitinib 15 mg/day | 57 | Extraocular activity | 3 | 0 | 0 |
| Female | 40.9 | 40.9 | Panuveitis | Seronegative spondyloarthritis | Upadacitinib 15 mg/day | 49.5 | Ocular activity | 6 | 0 | 0 |
| Female | 59.2 | 59.2 | Anterior sclerouveitis | Rheumatoid arthritis | Baricitinib 2 mg/day | 61 | Both ocular and extraocular activity | 17 | PDN 15 | 0 |
| Male | 12.8 | 13.8 | Panuveitis | Vogt-Koyanagi-Harada syndrome | Baricitinib 4 mg/day | 19.9 | Ocular activity | 7 | PDN 15 | 7.5 PDN |
| Female | 29 | 29 | Posterior uveitis (multifocal choroiditis) | Idiopathic | Baricitinib 4 mg/day | 45.2 | Ocular activity | 3 | PDN 30 | 12.5 PDN |
| Male | 32.5 | 39 | Anterior uveitis | Behçet's syndrome | Upadacitinib 15 mg/day plus azathioprine 200 mg/day | 57 | Ocular activity | 11 | Peribulbar corticosteroid injections | 0 |
| Male | 44 | 44 | Anterior scleritis, posterior scleritis | Psoriatic arthritis | Upadacitinib 15 mg/day plus sulfasalazine 1,000 mg/day | 50 | Both ocular and extraocular activity | 7 | 0 | 0 |
| Female | 22.6 | 22.6 | Anterior uveitis (iridocyclitis) | Seronegative spondyloarthritis | Tofacitinib 10 mg/die | 57.8 | Extraocular activity | 8 | PDN 50 | 0 |
| Male | 2.5 | 2.6 | Anterior uveitis | ANA positive juvenile idiopathic arthritis | Baricitinib 4 mg/day | 26.5 | Both ocular and extraocular activity | 12 | 0 | 0 |
Each row is referred to one patient.
ANA, anti-nuclear autoantibodies; GCs, glucocorticoids; JAK, Janus Kinase; PDN, prednisone.
In the published article, there was an error. There are four sentences in which there are either structural or typographical errors.
A correction has been made to Patients and methods, Paragraph 1. This sentence previously stated:
“international AutoInflammatory Disease Alliance (AIDA) Network registries dedicated to uveitis, scleritis and Behçet's disease”.
The corrected sentence appears below:
“International AutoInflammatory Disease Alliance (AIDA) Network registries dedicated to uveitis, scleritis and Behçet's syndrome”.
A correction has been made to Results, Paragraph 1. This sentence previously stated:
“and one from the International AIDA Network registry dedicated to Behçet's disease (10)”.
The corrected sentence appears below:
“and one from the International AIDA Network registry dedicated to Behçet's syndrome (10)”.
A correction has been made to Treatment details, Paragraph 8. This sentence previously stated:
“Four patients were receiving combination therapy with cDMARDs at the start of treatment with JAK inhibitors: two were on azathioprine, and one on sulfasalazine. The follow-up period while on combination therapy was three months and eleven months for the patients on azathioprine, and seven months for the patient treated with sulfasalazine”.
The corrected sentence appears below:
“Four patients were receiving combination therapy with cDMARDs at the start of treatment with JAK inhibitors: two were on azathioprine, one was on leflunomide, and one was on sulfasalazine. The follow-up period while on combination therapy was three months and eleven months for the patients on azathioprine, seven months for the patient in therapy with sulfasalazine, and six months for the patient treated with leflunomide”.
A correction has been made to Discussion, Paragraph 6. This sentence previously stated:
“The lack of Gas withdrawal in these two cases was either due to systemic disease activity or the short follow-up duration”.
The corrected sentence appears below:
“The lack of GCs withdrawal in these two cases was either due to systemic disease activity or the short follow-up duration”.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.


