Keywords: dog, high-throughput sequencing, Strongyloides stercoralis, Strongyloidiasis, zoonotic
Abstract
The rhabditid nematode Strongyloides stercoralis is known worldwide as the causative agent of strongyloidiasis in humans. In addition to public health concerns, S. stercoralis also infects dogs, which represent a possible reservoir for potentially zoonotic transmissions. We describe the first confirmed case of fatal disseminated infection in a dog in the Czech Republic. The microscopic and histological results were supported by a complex genotyping approach. Using high-throughput sequencing of the hypervariable region (HVR-IV) of 18S rDNA and Sanger sequencing of the partial cytochrome c oxidase subunit 1 gene (cox1), the potentially zoonotic haplotype/lineage A of S. stercoralis was confirmed, while the solely canine haplotype/lineage B was not found. The development of the disease is mainly associated with immunodeficiency, and in this case, it was triggered by inappropriate treatment, in particular the use of corticosteroids.
Introduction
Strongyloides stercoralis infects a range of hosts, including canids, felids, humans and non-human primates (NHPs). Resulting infection, usually termed strongyloidiasis, remains a major veterinary and public health challenge globally (Bradbury et al., 2021). The parasite has a unique life cycle in which it alternates between free-living and parasitic stages. The free-living stage is a single generation with a short life span. The parasitic generations include only parthenogenetic females living in the small intestine, that are ovoviviparous. The autoinfective filariform larvae may develop from rhabditiform larvae already in the intestine and can invade the intestinal mucosa or perianal area, causing autoinfection (Sandground, 1925; Streit, 2008; Page et al., 2018). In dogs, the infection may be asymptomatic. However, respiratory, dermatologic and gastrointestinal signs accompanied by vomiting and anorexia may occur in young animals, especially in puppies (Robertson and Thompson, 2002; Paradies et al., 2017), sometimes with fatal consequences (Dillard et al., 2007; Unterköfler et al., 2022). So far, none of the available anthelmintics are formally approved for the treatment of strongyloidiasis in dogs. In a few cases, successful off-label treatment has been demonstrated using moxidectin, milbemycin, praziquantel, fenbendazole and ivermectin, often in combination (Eydal and Skírnisson, 2016; Unterköfler et al., 2022; Deak et al., 2023). However, a single dose is not sufficient to eradicate the parasite, and successful therapy requires repeated applications (Itoh et al., 2009; Paradies et al., 2017; Basso et al., 2019). On the other hand, the administration of some drugs, such as glucocorticoids, can lead to a hyperinfection and worsen the health status (Basso et al., 2019), as they immunosuppress the host and act directly on the larvae, promoting accelerated moulting to the autoinfective stage (Ramanathan and Nutman, 2008).
Strongyloides stercoralis is being intensively studied in dogs because of its zoonotic potential (Gorgani-Firouzjaee et al., 2022). Infected free-roaming dogs could play a role in spreading the infection to other hosts including humans (Jaleta et al., 2017; Nagayasu et al., 2017) and reciprocally, humans could be a possible source of infection for dogs living in close contact with them, but this is yet to be proven (Bradbury and Streit, 2024). This extremely close coexistence between domestic carnivores and humans has created conditions for the mutual transmission of pathogenic and non-pathogenic organisms and has become part of the One Health concept, especially in Western society (Gray and Young, 2011; van Knapen and Overgaauw, 2014; Wetzels et al., 2021).
In the case of S. stercoralis two main haplotypes/lineages have been described: the potentially zoonotic haplotype/lineage A, which occurs in humans, dogs and mainly captive NHPs and the likely solely canine haplotype/lineage B (Bradbury et al., 2021). While haplotype/lineage A appears to be more widespread in dogs worldwide, haplotype/lineage B has been detected only in Australia, Cambodia, Myanmar and Grenada (Nosková et al., 2024). However, numerous cases of S. stercoralis infections in dogs are reported without genetic analysis (Schnyder et al., 2022), so the exact distribution of individual haplotypes/lineages worldwide is most likely inaccurate.
Basic microscopy, ideally following the Baermann larvoscopy (Schnyder et al., 2022), is essential for the detection of first-stage S. stercoralis larvae (L1; or occasionally infective L3 and parasitic females) from faecal samples (Basso et al., 2019; Schnyder et al., 2022), while oval eggs with U-shaped larvae are typical for some other Strongyloides species (Viney and Lok, 2015). Genetic-based methods are essential for the resolution of the individual haplotypes/lineages. Both the hypervariable region (HVR-IV) of 18S rDNA and cytochrome c oxidase subunit I (cox1) have the discriminatory power to differentiate S. stercoralis haplotypes/lineages (Beknazarova et al., 2019) and should be selected as the currently most appropriate markers, as there are no other discriminatory methods. Some haplotypes were recognized using the HVR-IV region of 18S, which correspond to the particular cox1 lineage, indicating that mixed infections with other Strongyloides species are not uncommon (Barratt et al., 2019) and this could be true also for the haplotypes/lineages of S. stercoralis in dogs. In recent years, increasingly complex genotyping methods have been used in Strongyloides research that enable the detection of multi-haplotypes infections in faecal samples (Barratt et al., 2019).
After receiving a sample from a dog with disseminated Strongyloides infection, we aimed to uncover which genetic haplotype and lineage is the causative agent of the disease. A high-throughput sequencing (HTS) approach was used to determine whether multiple haplotypes of HVR-IV overlap during this fatal infection. Sanger sequencing complemented the HTS results on the cox1 and HVR-I regions.
Material and methods
Clinical case
A one-year-old crossbred dog female was adopted from a Slovakian shelter in April 2022 with no medical records. From the beginning, the dog had repeated diarrhoea. Before the case was submitted to us, coproscopic examination was either not performed or included only faecal flotation with a negative result. In September 2022, the dog was presented to a veterinary clinic in Hradec Králové, Czech Republic as subfebrile, with profuse diarrhoea, apathy, cachexia (BCS 1/5). A fresh faecal sample was collected and examined using Sheather's flotation at the clinic. Baermann larvoscopy was not performed. Infrequent damaged unspecified larvae were microscopically detected after Sheather's flotation. The hematological examination was unremarkable. In the biochemical profile, liver enzymes and bilirubin were slightly elevated, amylase and pancreatic lipase were markedly elevated together with mild hyponatremia. The dog was dewormed with a single dose of moxidectin combined with imidacloprid (Advocate spot on). Furthermore, amoxicillin/clavulanate (22 mg kg−1, Noroclav inject.) and marbofloxacin (3 mg kg−1, Marbocyl FD inject.) were administrated. No larvae were detected after 6 days during the control Sheather's flotation. Dog condition did not improve; loss of appetite and vomiting persisted. Radiographic examination of the chest in latero-lateral projection showed a slightly accentuated bronchial pattern; endoscopy revealed abnormal findings in the duodenum, which was markedly hyperaemic throughout and had numerous miliary coagulum. The dog's condition deteriorated, with opisthotonus and bloody, profuse diarrhoea. Therefore, endoscopic examination, biopsy and subsequent histological examination were performed (LABOKLIN GmbH & Co. KG, see Histologic examination below). Based on the histological results, a faecal sample was sent to the University of Veterinary Sciences Brno for coproscopic examination. Strongyloides infection was diagnosed based on the microscopic and molecular identification and treated with Flubendazole (Flubenol KH paste) 22 mg kg−1 for 3 consecutive days. During the control flotation of the sample from the 3rd day after flubendazole administration (19th day after Advocate spot application), non-motile Stongyloides stages were observed. On the fourth day after initiation of anthelmintic therapy, the dog developed severe respiratory distress due to polypnea associated with complete anorexia. The respiratory distress was treated with corticosteroids, methylprednisolone (1 mg kg−1, SoluMedrol 40 mg inject.) and opiates (butorphanol 0.2 mg kg−1, Butomidor inject.), which led to relief of the polypnea. The dog, however, died the following day.
Histologic examination
A total of 27 samples, taken during the endoscopy, from the stomach, duodenum, ileum and colon were submitted to the LABOKLIN GmbH and Co. KG, for histological examination. The samples ranged from 1 mm in diameter up to 5 × 2 × 2 mm and included mostly mucosal surfaces. All samples were fixed in 10% phosphate-buffered formalin for 24–48 h to ensure correct fixation. They were then trimmed and processed according to laboratory standards (Fischer et al., 2008), embedded in paraffin wax, cut at 4–5 μm thickness and stained with Haematoxylin-Eosin (HE) and Periodic Acid-Schiff stain (PAS). The slides were scanned and analysed using image analysis software (NIS-elements software) (Nikon, Japan); Aperio ImageScope (Leica, Germany).
Faecal sample preparation, DNA extraction and molecular assays
A 200 ml suspension of faecal sample in water for molecular identification and the same amount of suspension fixed in formalin for coproscopic examination were sent to Department of Pathology and Parasitology, University of Veterinary Sciences Brno, Czech Republic in October 2022. Due to submission of a non-standard sample, the Baermann larvoscopy was not performed. The nematode stages in formalin-preserved sample were let to sediment, collected individually by a pipette and observed and measured using the light microscope with Nomarski differential contrast (Olympus BX41) at 100 × magnification and photographed (Olympus AX70).
The faecal–water suspension was centrifuged in 50-ml falcon tubes. The sediment was transferred into 1.5 ml tubes and dried overnight at 37°C before DNA isolation (Murphy et al., 2000). Total DNA from one sample was extracted using the DNeasy PowerSoil Kit (QIAGEN, Germany) according to the manufacturer's protocol. The most frequently used markers for Strongyloides spp. amplification were selected (Barratt et al., 2019), namely two hypervariable regions (HVRs) of 18S rDNA (HVR-I and HVR-IV) and the mitochondrial gene cox1. The HVR-I region (434 bp) was amplified using primers New_HVR-I_F and New_HVR-I_R, whereas cox1 (650 bp) was amplified with pair primers TJ5207 and TJ5208 (Jaleta et al., 2017). The volume and mixture of the PCR reaction of HVR-I region and cox1 were performed according to Nosková et al. (2023) (Table 1). Sanger sequencing was performed by Macrogen Europe (Netherlands). To reveal the potential occurrence of multiple Strongyloides haplotypes, the library for HTS was created using a 2-step-PCR approach, performed using primers New_HVR-IV_F and New_HVR-IV_R (Barratt et al., 2019), amplifying the HVR-IV region (255 bp). In the first step of PCR inner locus specific primers for the HVR-IV region amplification (New_HVR-IV_F and New_HVR-IV_R) were used, additionally containing nextera adaptor sequences at 5′ ends following design from a 16S Metagenomic Sequencing Library Preparation protocol. The first amplification was performed in a 10 μL reaction volume, including Kapa 2 G Robust Hot Star polymerase (Kapa Biosystems, United States), 0.25 μL of each primer and 2 μL of DNA template, before the second amplification was performed with the same polymerase, 4 μL of Nextera primers with adaptors and 2 μL diluted product in a 20 μL reaction volume (Table 1). The final library was sequenced using MiSeq Reagent Kit v3 (2 × 300 bp pair-end reads) by Illumina MiSeq platform (Illumina, USA).
Table 1.
Primers and reaction conditions
| Primers | Sequence 5′→ 3′ | Reaction conditions | Region of gene | References |
|---|---|---|---|---|
| TJ5207 TJ5208 | TTTGATTGTTACCTGCTTCTATTTT TTTTACACCAGTAGGAACAGCAA | 2 min at 94°C; 35 cycles of 20 s at 94°C, 15 s at 50°C, 90 s at 72°C; and 7 min at 72°C | cox1 | Jaleta et al. (2017) |
| HVRI F HVRI R | GCTCATTATAACAGCTATAGACTACACGGTA CCACAACAATCATTTTATGCACTTGG | 3 min at 95°C; 35 cycles of 15 s at 95°C, 15 s at 60°C, 15 s at 72°Cs; and 1 min at 72°C | 18S rDNA (HVR-I) | Barratt et al. (2019) |
| HVRIV F HVRIV R | CGGGCCGGACACTATAAGG ATCTCTAAACAGGAACATAATGATCACTAC | 3 min at 95°C; 30 cycles of 15 s at 95°C, 15 s at 60°C, 15 s at 72°Cs; and 1 min at 72°C | 18S rDNA (HVR-IV) | |
| Nextera adaptor F | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG | 3 min at 95°C; 16 cycles of 15 s at 95°C, 30 s at 55°C, 30 s at 72°C; and 72°C for 3 min | - | 16S metagenomic sequencing library preparation protocol, Illumina |
| Nextera adaptor R | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG |
Data processing and phylogenetic analyses
Raw FASTQ sequences of the HVR-I of 18S rDNA and partial cox1 genes obtained after Sanger sequencing were checked manually and trimmed in Geneious 9.1.5 (www.geneious.com). Raw HVR-IV of 18S rDNA FASTQ sequences obtained using HTS were trimmed using Skewer (Jiang et al., 2014) and denoised using the dada2 package (Callahan et al., 2016) in Rstudio (https://www.rstudio.com). The taxonomic identification for all sequences (one from HVR-I, one from HVR-IV and one from cox1) were performed using BLAST software against the BLAST nt database (Altschul et al., 1990), available online at the National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/.
For phylogenetic analyses, 154 sequences of the partial cox1 gene of S. stercoralis from dogs were downloaded from Genbank and checked for duplicates. In addition, 12 sequences from humans and NHPs were included. Phylogenetic analysis of partial cox1 sequences was reconstructed by Bayesian inference (BI) in MrBayes 3.2.6 (Huelsenbeck and Ronquist, 2001). Only unique dog sequences from 11 localities were included in the phylogenetic analysis. The Muscle alignment (715 bp) of cox1 consisted of one sequence obtained during the present project and 61 sequences from Genbank, including Necator americanus (AJ417719) as an out-group. Nodal support was assessed to 106 replicates. Substitution GTR + G model was used as selected by Modelgenerator (Keane et al., 2006).
Results
Detection of adult parthenogenetic female and pathological findings
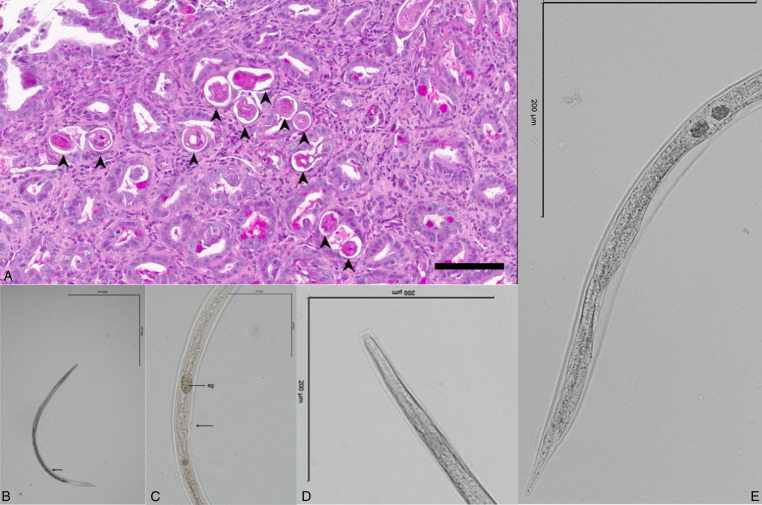
Within the intestinal samples of duodenum, ileum and colon, the mucosa, lamina propria and submucosa, when available, were diffusely infiltrated and expanded by moderate numbers of lymphocytes, cells, as well as scattered eosinophils. Embedded within the intestinal crypts there were numerous cross and tangential sections of degenerated adult nematodes and larvae (Fig. 1A). The villi were overall slightly blunted and occasionally fused. The intestinal crypts often contained an increased amount of mucinoid material, as well as a small number of cellular debris. The associated lymphoid tissue in these areas was often prominent and hyperplastic. The stomach samples were histologically unremarkable, with no evidence of parasites or overt inflammation, other than mild superficial erosion and apical haemorrhages. High number of adult parthenogenetic S. stercoralis females (average length 1300 μm) were observed in faecal suspension using microscopy. The typical morphological characters such as elongated (filariform) oesophagus (Fig. 1B), vulval region (Fig. 1C), buccal cavity (Fig. 1D) and tail (Fig. 1E) were observed.
Figure 1.
(A) Multiple cross sections of degenerated nematode parasites (arrows) embedded in the intestinal crypt, the remaining mucosal interstitial contains variable numbers of lymphocytes, plasma cells and scattered eosinophils, scale bar 80 μm; (B) general view of Strongyloides stercoralis parasitic female from dog, arrow indicates vulva, scale bar 500 μm; (C) vulval region of S. stercoralis parasitic female, arrow indicates vulva, scale bar 100 μm, abbreviation: eg – egg; (D) buccal cavity of S. stercoralis parasitic female, scale bar 200 μm and (E) tail of S. stercoralis parasitic female, scale bar 200 μm.
Haplotypes and phylogenetic analysis of S. stercoralis
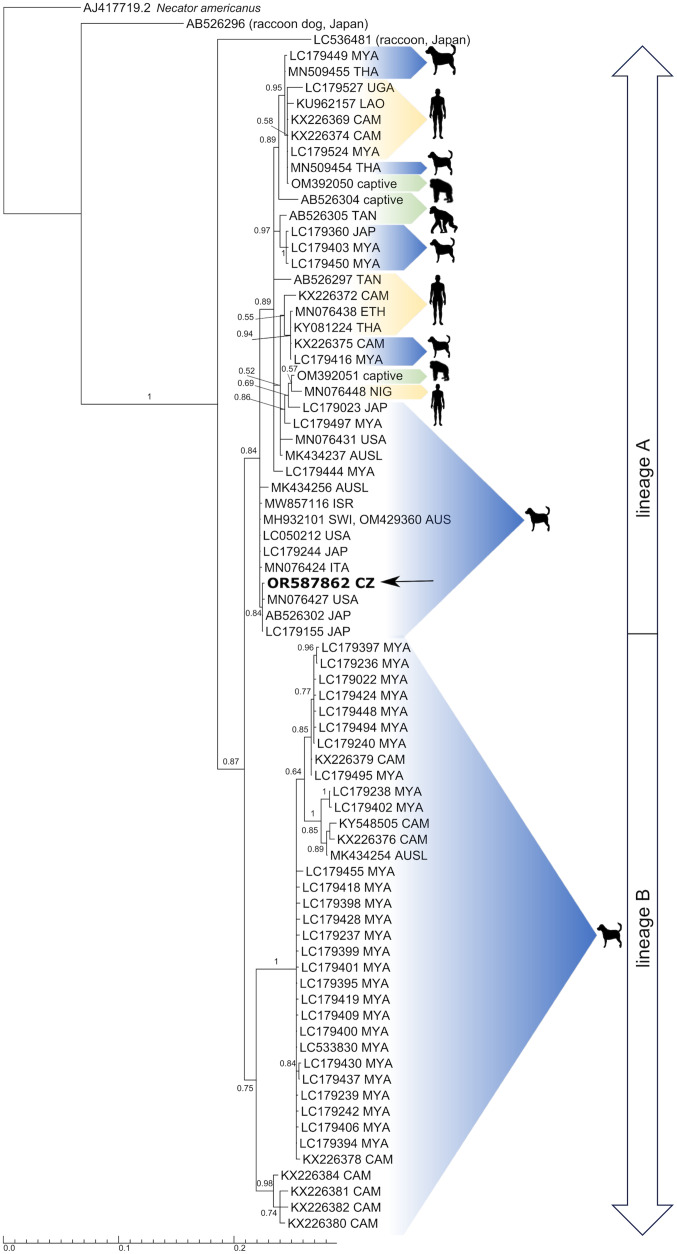
Strongyloides stercoralis sequences of the HVR-I (466 bp) and HVR-IV (290 bp) of 18S rDNA and partial cox1 (628 bp) were obtained from the examined dog faecal sample. BLAST search showed that sequence of the HVR-I region corresponded with haplotype VI while HVR-IV corresponded with haplotype A as marked in Barratt et al. (2019). Moreover, 97 046 high-quality reads were obtained using HTS and only potentially zoonotic HVR-IV haplotype A was detected, while the solely canine haplotype B was not recorded. The cox1 results also detected the potentially zoonotic lineage A. The resulting BI tree based on cox1 showed two separate S. stercoralis clades corresponding to lineages A and B, with our cox1 sequence (OR587862) placed in the A lineage (Fig. 2), clustering with high support (0.84 BI) into a sub-clade comprising two isolates from Japanese dogs and one isolate from a dog from USA. The pairwise sequence distance (PSD) within this sub-clade did not reach over 0.2% but differ from other sub-clades within lineage A from 1.8% to 4.2%. Two distinct subclades are shown also within lineage B and differed by 4 to 6.2%. Clades containing lineage A and B do not differ by more than 8.3%.
Figure 2.
Bayesian inference phylogenetic tree inferred from cox1 (715 bp) calculated from a muscle-constructed alignment using GTR + G model for nucleotide substitutions. Numbers at the branches indicate Bayesian posterior probability based on 106 replicates. Branch lengths indicate expected numbers of substitutions per nucleotide site. Sequence obtained during the current study is in bold.
Discussion
Surveillance of S. stercoralis in dogs, including molecular identification of positive cases, should be an important part of health management that can lead to a reduction in the risk of the parasite's impact on host health, as well as a reduction in fatal cases. Knowledge of particular S. stercoralis haplotypes/lineages helps evaluate the risk of zoonotic transmission to humans (Nagayasu et al., 2017). However, the combination of coproscopic and genetic analyses is not routinely performed in dogs. Here we present a case report of disseminated S. stercoralis infection in a dog evaluated by a combination of histology, microscopy and HTS approaches, with an appeal for the correct treatment approach.
Infections of dogs with S. stercoralis are considered rare in the Czech Republic. Only one case has been documented in the literature, reporting unidentified Strongyloides eggs found in dog faeces (Valkounová, 1982). Since only S. stercoralis L1 are typically detected in faeces of infected dogs, evaluation of this case is speculative. Recently, 9-week-old Chihuahuas imported to Switzerland from a Czech breeding station, were tested positive for S. stercoralis, suggesting that the infection had already been introduced from the Czech Republic (Schnyder et al., 2022). Overall, cases of canine strongyloidiasis have emerged throughout Europe in the last decade (e.g. Dillard et al., 2007; Riggio et al., 2013; Eydal and Skírnisson, 2016; Paradies et al., 2017; Bourgoin et al., 2018; Basso et al., 2019; Hall et al., 2020; Schnyder et al., 2022; Unterköfler et al., 2022; Deak et al., 2023) suggesting an increasing occurrence or recent spread across Europe, likely due to the importation of dogs (Schnyder et al., 2022), including from Strongyloides-endemic countries (Unterköfler et al., 2022). However, improved awareness, diagnosis and immunosuppression in dogs may also play a role in the increased number of cases (Cervone et al., 2016). The dog in this study was adopted from Slovakia, which is considered one of the endemic countries for Strongyloides in both dogs and humans (Štrkolcová et al., 2017). In addition, transmission of S. stercoralis between humans and dogs was suspected as IgG antibodies against S. stercoralis were detected in serum samples from children and rhabditiform larvae of S. stercoralis were detected in dogs in a Roma settlement in Slovakia (Štrkolcová et al., 2017), although without molecular data. Further reports of strongyloidiasis in humans, but without molecular data, have been reported mainly from Eastern and Southern Europe (Ottino et al., 2020), possibly influenced by increasing global migration (Asundi et al., 2019; Marrone et al., 2023).
Although most case reports and prevalence studies of infected dogs with S. stercoralis were based on microscopic diagnostics, some studies also included the genotyping of Strongyloides (Basso et al., 2019; Salant et al., 2021; Unterköfler et al., 2022). Unfortunately, the haplotypes/lineages are not always reported, as in the case of Chihuahuas imported into Switzerland from the Czech Republic (Schnyder et al., 2022). So far, only haplotype/lineage A has been detected in dogs in Europe, including the presented case (Nosková et al., 2024). In the Czech Republic, the potentially zoonotic S. stercoralis haplotype A was also molecularly confirmed in captive orangutans kept in zoological gardens (Nosková et al., 2023). In humans in Europe, haplotype A has only been confirmed in Italy (Barratt et al., 2019).
Until now, studies on Strongyloides infection in dogs carried out in Europe have been conducted based on classical sequencing, which has its limitations when it comes to detecting multiple haplotypes. Conventional PCR analyses followed by Sanger sequencing provide only a single sequence and are therefore suitable for detection of Strongyloides from a single larva, for example, but the molecular identification of Strongyloides from total DNA extracted from faeces, which may contain multiple Strongyloides haplotypes, requires more complex genotyping approaches (Nosková et al., 2024). Hypothetically, the almost complete absence of haplotype B could be due to the dominance of haplotype A in potentially mixed infections, which is preferentially detected by Sanger sequencing. Though, only the potentially zoonotic haplotype A was detected in our study when HTS was applied, and the previous fatal cases of disseminated strongyloidiasis analysed by molecular tools also revealed only the potentially zoonotic haplotype A so far. (Basso et al., 2019; Unterköfler et al., 2022). It appears that haplotype A is pathogenic or at least pathogenic under certain conditions, while the pathogenicity of haplotype B infecting dogs is so far unknown. However, in many studies, the haplotype was not identified, and it should be considered in the future. Perhaps if veterinary clinics, together with specialized institutions (e.g. universities, genomic or research centres), have the opportunity to perform HTS identification, the valuable results would fill the gaps regarding the genetic diversity and distribution of Strongyloides infection and clarify the possible pathogenicity of haplotype B.
In general, S. stercoralis disease progression in dogs varies from a latent course, especially in adult immunocompetent dogs, to fatal cases in young or immunocompromised dogs (Cervone et al., 2016). Clinical signs include diarrhoea, erythema, respiratory problems, epaxial atrophy, nervous signs such as paresis, tremor, comatose states, recurrent epileptiform seizures, torticollis (Dillard et al., 2007; Basso et al., 2019; Schnyder et al., 2022; Unterköfler et al., 2022) or opisthotonus as in our study. Respiratory symptoms are attributed with Strongyloides infection as L3 enter the bloodstream and migrate through the trachea into the intestine (Page et al., 2018). Disseminated strongyloidiasis develops when the host is exposed to long-term autoinfection. Strongyloides larvae have previously been found post-mortem not only in the intestinal mucosa and respiratory tract, but also in the CNS, spleen, kidneys and muscles (Thamsborg et al., 2016; Paradies et al., 2017). Strongyloidiasis in humans has a similar course. Most infections are mild, but under certain conditions (e.g. if the patient is immunosuppressed) dissemination may occur. While the mild course of the disease is characterised by gastrointestinal, pulmonary and dermatological symptoms, the infection can lead to severe systemic disease with fatal consequences. In a disseminated form, the larvae can occur in any organ and lead to systemic bacterial sepsis (Hagelskjaer, 1994; Qu et al., 2016; Karanam et al., 2021). In the case of the dog described in this study, the necropsy was not authorised by the dog's owner. However, we detected typical pathologies in the duodenum, ileum and colon by endoscopy. In addition, we observed parasitic females in the faecal sample, which is rare and suggests disseminated disease (Basso et al., 2019).
The treatment of strongyloidiasis has not yet been clarified, as efficacy has only been surveyed in a small number of dogs. Ivermectin and fenbendazole appears to be the most effective anthelminthics, although S. stercoralis infection in dogs is not listed in indications of any canine anthelminthics containing these substances (Paradies et al., 2017; Schnyder et al., 2022). Nevertheless, a single administration is not considered sufficient, as the excretion of the larvae in the faeces persists (e.g. Paradies et al., 2017; Schnyder et al., 2022) and a repetition of the therapy at different intervals is usually necessary (Thamsborg et al., 2016). Single dose of ivermectin is particularly insufficient for disseminated infection (Buonfrate et al., 2022). Although the examined dog was treated against intestinal parasites, the effect on Strongyloides infection was insufficient, although the dog was classified as negative after the first treatment. Control of S. stercoralis infection can only be considered successful if the faecal examination repeatedly shows a negative larvoscopy result (Schnyder et al., 2022). Unfortunately, in most cases of disseminated strongyloidiasis, prolonged therapy is not possible due to the deteriorating condition of the patient (Basso et al., 2019). Patients with the disseminated form often suffer from secondary bacterial infections, respiratory failure, requiring appropriate intensive care (Cervone et al., 2016). The development of a disseminated form can be promoted by the use of glucocorticoids (Mansfield et al., 1996). Even short courses (6–17 days) of corticosteroids in immunocompetent patients without underlying immunosuppressive conditions have even been associated with death (Krolewiecki and Nutman, 2019). If a Strongyloides infection is detected in a dog taking corticosteroids, their use must be ceased immediately as there is a risk of development of the disseminated form (Graham et al., 2019). The fatal case of the disseminated form in the examined dog was probably triggered by the prescription of corticosteroids. However, the disseminated infection in the studied dog developed before the administration of corticosteroids, when the dog showed opisthotomus, as the central nervous system can also be affected in a disseminated infection (Concha et al., 2005).
Conclusion
The increasing occurrence of S. stercoralis in dogs in various European countries shows that this parasite appears to pose an increasing risk in Europe. The cross-border transportation of dogs from endemic countries and the accumulation of dogs in animal shelters could contribute to the spread of the infection. So far, only the potentially zoonotic haplotype/lineage A is known to be circulating in dogs in Europe, which in turn could pose a risk to human health. Due to the potential for cross-transmission between humans and dogs, S. stercoralis is a classic candidate for One Health approaches, where attention must be paid to accurate control and diagnosis in both humans and dogs. The correct treatment approaches should include: (1) Appropriate methods for the detection of Strongyloides infection, such as the Baermann larvoscopy or PCR, should be included in routine diagnostics in dogs. If faecal flotation is the only diagnostic method, the diagnostic reports should clearly state that the method performed cannot reliably detect Strongyloides so the veterinarians who commission the test are aware. (2) The repeated therapy and the confirmation of the treatment success should be stated only after several negative larvoscopy results. (3) If the Strongyloides infection is detected in a dog taking corticosteroids, their use must be ceased immediately as there is a risk of development of disseminated form. (4) Subsequently, appropriate molecular tools, ideally using HTS approaches, are highly recommended for Strongyloides-positive samples to raise awareness of the spread of individual S. stercoralis haplotypes/lineages. (5) General awareness of this spreading parasite should be raised among physicians, veterinarians and clinicians, together with caution in the use of glucocorticoids.
Acknowledgements
We acknowledge the CF Genomics CEITEC MU supported by the NCMG research infrastructure (LM2023067 funded by MEYS CR) for their support with obtaining scientific data presented in this paper. Computational resources were provided by the e-INFRA CZ project (ID:90254), supported by the Ministry of Education, Youth and Sports of the Czech Republic. The authors would like to thank Bethan Mason for language correction and improvement.
Data availability statement
Sequence based cox1 of Strongyloides stercoralis is available and uploaded to GenBank under the accession numbers OR587862.
Author contributions
EN conceived the study, wrote the manuscript, performed the coproscopic examination, lab work, and phylogenetic analysis, VS wrote the article, performed the coproscopic examination, VH performed the veterinary examination, AC-E performed the histological examination, TK performed the lab work, VI performed statistical analyses, DM reviewed and edited the manuscript, BP supervised the project and wrote the manuscript.
Financial support
This project was supported from Masaryk University (MUNI/A/1602/2023).
Competing interests
The authors declare that no competing interests exist.
Ethical standards
Not applicable.
References
- Altschul SF, Gish W, Miller W, Myers EW and Lipman DJ (1990) Basic local alignment search tool. Journal of Molecular Biology 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Asundi A, Beliavsky A, Liu XJ, Akaberi A, Schwarzer G, Bisoffi Z, Requena-Méndez A, Shrier I and Greenaway C (2019) Prevalence of strongyloidiasis and schistosomiasis among migrants: a systematic review and meta-analysis. The Lancet Global Health 7, e236–e248. [DOI] [PubMed] [Google Scholar]
- Barratt JLN, Lane M, Talundzic E, Richins T, Robertson G, Formenti F, Pritt B, Verocai G, de Souza JN, Soares NM, Traub R, Buonfrate D and Bradbury RS (2019) Correction: a global genotyping survey of Strongyloides stercoralis and Strongyloides fuelleborni using deep amplicon sequencing. PLoS Neglected Tropical Diseases 15, e0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso W, Grandt LM, Magnenat AL, Gottstein B and Campos M (2019) Strongyloides stercoralis infection in imported and local dogs in Switzerland: from clinics to molecular genetics. Parasitology Research 118, 255–266. [DOI] [PubMed] [Google Scholar]
- Beknazarova M, Barratt JLN, Bradbury RS, Lane M, Whiley H and Ross K (2019) Detection of classic and cryptic Strongyloides genotypes by deep amplicon sequencing: a preliminary survey of dog and human specimens collected from remote Australian communities. PLoS Neglected Tropical Diseases 13, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgoin G, Jacquet-Viallet P and Zenner L (2018) Fatal strongyloidiasis in a puppy from France. Veterinary Record Case Reports 6, 6–10. [Google Scholar]
- Bradbury RS and Streit A (2024) Is strongyloidiasis a zoonosis from dogs? Philosophical Transactions of the Royal Society B: Biological Sciences 379, 1–13. doi: 10.1098/rstb.2022.0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury RS, Pafčo B, Nosková E and Hasegawa H (2021) Strongyloides genotyping: a review of methods and application in public health and population genetics. International Journal for Parasitology 51, 1153–1166. [DOI] [PubMed] [Google Scholar]
- Buonfrate D, Rodari P, Barda B, Page W, Einsiedel L and Watts MR (2022) Current pharmacotherapeutic strategies for Strongyloidiasis and the complications in its treatment. Expert Opinion on Pharmacotherapy 23, 1617–1628. [DOI] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA and Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nature Methods 13, 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervone M, Giannelli A, Otranto D and Perrucci S (2016) Strongyloides stercoralis hyperinfection in an immunosuppressed dog from France. Revue Veterinaire Clinique 51, 55–59. [Google Scholar]
- Concha R, Harrington W and Rogers AI (2005) Intestinal strongyloidiasis: recognition, management, and determinants of outcome. Journal of Clinical Gastroenterology 39, 203–211. doi: 10.1097/01.mcg.0000152779.68900.33 [DOI] [PubMed] [Google Scholar]
- Deak G, Ionică AM, Taulescu M, Negoescu A, Ifteme C, Roșoiu M and Mihalca AD (2023) A severe case of hyperinfection by Strongyloides stercoralis in a pet dog from Romania. Parasitology International 100, 102849. [DOI] [PubMed] [Google Scholar]
- Dillard KJ, Saari SAM and Anttila M (2007) Strongyloides stercoralis infection in a Finnish kennel. Acta Veterinaria Scandinavica 49, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eydal M and Skírnisson K (2016) Strongyloides stercoralis found in imported dogs, household dogs and kennel dogs in Iceland. Icelandic Agricultural Sciences 29, 39–51. [Google Scholar]
- Fischer AH, Jacobson KA, Rose J and Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols 3, 4986–4988. [DOI] [PubMed] [Google Scholar]
- Gorgani-Firouzjaee T, Kalantari N, Chehrazi M, Ghaffari S and Shahdin S (2022) Global prevalence of Strongyloides stercoralis in dogs: a systematic review and meta-analysis. Journal of Helminthology 96, e11. [DOI] [PubMed] [Google Scholar]
- Graham JA, Sato M, Moore AR, McGrew AK, Ballweber LR, Byas AD and Dowers KL (2019) Disseminated Strongyloides stercoralis infection in a dog following long-term treatment with budesonide. Journal of the American Veterinary Medical Association 254, 974–978. [DOI] [PubMed] [Google Scholar]
- Gray PB and Young SM (2011) Human – pet dynamics in cross-cultural perspective. Anthrozoös 24, 17–30. [Google Scholar]
- Hagelskjaer L (1994) A fatal case of systemic strongyloidiasis and review of the literature. European Journal of Clinical Microbiology and Infectious Diseases 13, 1102–1103. [DOI] [PubMed] [Google Scholar]
- Hall E, Lyriaki M, Whitworth F and Black V (2020) Severe strongyloidosis in dogs. Veterinary Record 186, 354–355. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP and Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics (Oxford, England) 17, 754–755. [DOI] [PubMed] [Google Scholar]
- Itoh N, Kanai K, Hori Y, Nakao R, Hoshi F and Higuchi S (2009) Fenbendazole treatment of dogs with naturally acquired Strongyloides stercoralis infection. Veterinary Record 164, 559–560. [DOI] [PubMed] [Google Scholar]
- Jaleta TG, Zhou S, Bemm FM, Schär F, Khieu V, Muth S, Odermatt P, Lok JB and Streit A (2017) Different but overlapping populations of Strongyloides stercoralis in dogs and humans—dogs as a possible source for zoonotic strongyloidiasis. PLoS Neglected Tropical Diseases 11, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lei R, Ding SW and Zhu S (2014) Skewer: a fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinformatics 15, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanam L SK, Basavraj GK and Papireddy CKR (2021) Strongyloides stercoralis hyper infection syndrome. Indian Journal of Surgery 83, 582–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Creevey CJ, Pentony MM, Naughton TJ and McInerney JO (2006) Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evolutionary Biology 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewiecki A and Nutman TB (2019) Strongyloidiasis: a neglected tropical disease. Infectious Disease Clinics of North America 33, 135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield LS, Niamatali S, Bhopale V, Volk S, Smith G, Lok JB, Genta RM and Schad GA (1996) Strongyloides stercoralis: maintenance of exceedingly chronic infections. The American Journal of Tropical Medicine and Hygiene 55, 617–624. [DOI] [PubMed] [Google Scholar]
- Marrone R, Mazzi C, Ouattara H, Cammilli M, Pontillo D, Perandin F and Bisoffi Z (2023) Screening for neglected tropical diseases and other infections in African refugees and asylum seekers in Rome and Lazio region, Italy. Travel Medicine and Infectious Disease 56, 102649. [DOI] [PubMed] [Google Scholar]
- Murphy MA, Waits LP and Kendall KC (2000) Quantitative evaluation of fecal drying methods for brown bear DNA analysis. Wildlife Society Bulletin 28, 951–957. [Google Scholar]
- Nagayasu E, Aung MPPTHH, Hortiwakul T, Hino A, Tanaka T, Higashiarakawa M, Olia A, Taniguchi T, Win SMT, Ohashi I, Odongo-Aginya EI, Aye KM, Mon M, Win KK, Ota K, Torisu Y, Panthuwong S, Kimura E, Palacpac NMQ, Kikuchi T, Hirata T, Torisu S, Hisaeda H, Horii T, Fujita J, Htike WW and Maruyama H (2017) A possible origin population of pathogenic intestinal nematodes, Strongyloides stercoralis, unveiled by molecular phylogeny. Scientific Reports 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosková E, Modrý D, Baláž V, Červená B, Jirků-Pomajbíková K, Zechmeisterová K, Leowski C, Petrželková KJ, Pšenková I, Roman V, Kessler SE, Ngoubangoye B and Setchell JM (2023) Identification of potentially zoonotic parasites in captive orangutans and semi-captive mandrills: phylogeny and morphological comparison. American Journal of Primatology 85, e23475. [DOI] [PubMed] [Google Scholar]
- Nosková E, Sambucci KM, Petželková KJ, Červená B, Modrý D and Pafčo B (2024) Strongyloides in non-human primates: significance for public health control. Philosophical Transactions of the Royal Society B: Biological Sciences 379, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottino L, Buonfrate D, Paradies P, Bisoffi Z, Antonelli A, Rossolini GM, Gabrielli S, Bartoloni A and Zammarchi L (2020) Autochthonous human and canine Strongyloides stercoralis infection in Europe: report of a human case in an Italian teen and systematic review of the literature. Pathogens (Basel, Switzerland) 9, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W, Judd JA and Bradbury RS (2018) The unique life cycle of Strongyloides stercoralis and implications for public health action. Tropical Medicine and Infectious Disease 3, 1–11. doi: 10.3390/tropicalmed3020053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies P, Iarussi F, Sasanelli M, Capogna A, Lia RP, Zucca D, Greco B, Cantacessi C and Otranto D (2017) Occurrence of strongyloidiasis in privately owned and sheltered dogs: clinical presentation and treatment outcome. Parasites & Vectors 10, 1–9. doi: 10.1186/s13071-017-2275-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu T, Yang Q, Yu M and Wand J (2016) Fatal Strongyloides stercoralis hyperinfection syndrome in a patient with chronic kidney disease. Medicine 95, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R and Nutman TB (2008) Strongyloides stercoralis infection in the immunocompromised host. Current Infectious Disease Reports 10, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggio F, Mannella R, Ariti G and Perrucci S (2013) Intestinal and lung parasites in owned dogs and cats from central Italy. Veterinary Parasitology 193, 78–84. [DOI] [PubMed] [Google Scholar]
- Robertson ID and Thompson RC (2002) Enteric parasitic zoonoses of domesticated dogs and cats. Microbes and Infection 4, 867–873. [DOI] [PubMed] [Google Scholar]
- Salant H, Harel M, Moreshet A, Baneth G, Mazuz ML and Yasur-Landau D (2021) Strongyloides stercoralis in a pomeranian dog in Israel. Israel Journal of Veterinary Medicine 76, 81–85. [Google Scholar]
- Sandground J (1925) Speciation and specificity in the nematode genus Strongyloides. Journal of Parasitology 12, 59–80. [Google Scholar]
- Schnyder M, Reichler I, Eichenberger RM, Hofer-Inteeworn N, Kümmerle-Fraune C and Grimm F (2022) Strongyloides stercoralis in Swiss dogs – a retrospective study suggests an increasing occurrence of this potentially zoonotic parasite as a consequence of dog imports. Schweizer Archiv fur Tierheilkunde 164, 89–104. [DOI] [PubMed] [Google Scholar]
- Streit A (2008) Reproduction in Strongyloides (Nematoda): a life between sex and parthenogenesis. Parasitology 153, 285–294. [DOI] [PubMed] [Google Scholar]
- Štrkolcová G, Goldová M, Bocková E and Mojžišová J (2017) The roundworm Strongyloides stercoralis in children, dogs, and soil inside and outside a segregated settlement in Eastern Slovakia: frequent but hardly detectable parasite. Parasitology Research 116, 891–900. [DOI] [PubMed] [Google Scholar]
- Thamsborg SM, Ketzis J, Horii Y and Matthews JB (2016) Strongyloides spp. infections of veterinary importance. Parasitology 144, 274–284. doi: 10.1017/S0031182016001116 [DOI] [PubMed] [Google Scholar]
- Unterköfler MS, Eipeldauer I, Merz S, Pantchev N, Hermann J, Brunthaler R, Basso W and Hinney B (2022) Strongyloides stercoralis infection in dogs in Austria: two case reports. Parasites and Vectors 15, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkounová J (1982) Parasitological investigation of children's sandboxes and dog faeces from public areas of housing development in Prague. Folia Parasitologica 29, 133–138. [PubMed] [Google Scholar]
- van Knapen F and Overgaauw P (2014) Dogs and transmission of infection to Man, “respected member of the family?”. Zoonoses-Infections Affecting Humans and Animals: Focus on Public Health Aspects, 575–587. doi: 10.1007/978-94-017-9457-2 [DOI] [Google Scholar]
- Viney ME and Lok JB (2015) The biology of Strongyloides spp. WormBook: the Online Review of C. elegans Biology, 1–17. doi: 10.1895/wormbook.1.141.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzels SU, Strachan CR, Conrady B, Wagner M, Burgener IA, Virányi Z and Selberherr E (2021) Wolves, dogs and humans in regular contact can mutually impact each other's skin microbiota. Scientific Reports 11, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence based cox1 of Strongyloides stercoralis is available and uploaded to GenBank under the accession numbers OR587862.