Abstract
Urinary 8-hydroxydeoxyguanosine (8-OHdG) level is an oxidative stress marker in patients with cancer; however, little is currently known about the clinical relevance of urinary 8-OHdG levels in patients with prostate cancer at diagnosis. Voided urine samples were collected from patients at the time of prostate biopsy and stored at -80˚C after centrifugation. All of the patients were classified according to histology of the biopsy. Once the patients were diagnosed with prostate cancer, the standard of care and treatments were administered according to the standard guidelines. The association between clinicopathological parameters and urinary 8-OHdG and N-terminal telopeptide (NTx) levels were explored. A total of 409 patients received prostate biopsy, of which 190 were benign, 41 were diagnosed with prostatitis and 178 were diagnosed with prostate cancer. The urinary 8-OHdG/creatinine ratio was marginally associated with prostate size (P=0.052) but not with serum prostate-specific antigen levels (P=0.707). With correction for prostate size, the ratio of urinary 8-OHdG/creatinine was significantly higher in patients with prostate cancer than those without malignancy (P=0.004). Moreover, urinary 8-OHdG levels were weakly associated with urinary NTx levels (r2=0.04, P=0.009). In conclusion, urinary 8-OHdG levels normalized for prostate volume may reflect prostate cancer risk and could be used to predict prostate cancer aggressiveness.
Keywords: oxidative stress, 8-hydroxydeoxyguanosine, prostate cancer
Introduction
Prostate cancer is the most common solid malignancy in men in the Western world (1). In Taiwan, over the decade from 2010 to 2019, prostate cancer had the highest 10-year percent change rate of all types of cancer among men (2,3). Similar to other Asian populations, Taiwanese patients exhibit distinct characteristics compared with those in Western countries; these differences include variations in stage distribution, attitudes toward therapy modality and response to androgen deprivation therapy (4-6). Prostate cancer has a complex pathogenesis; while genetic factors, aging and ethnicity are non-modifiable risk factors, environmental factors, including infectious agents, dietary carcinogens or other environmental disruption compounds, such as phthalates (7), are modifiable. According to the cancer registry in Taiwan, the annual incidence of new prostate cancer diagnoses is rising, along with an increase in cancer treatment-related mortality and morbidities (8). Accumulating evidence has indicated that oxidative stress is associated with prostate cancer, suggesting that antioxidants may have the ability to protect men from this disease (9,10). Therefore, it is mandatory to investigate the relationship between environmental exposure-related oxidative stress and prostate cancer pathogenesis.
Oxidative stress can lead to DNA damage by causing modifications to the DNA bases. Guanine, one of the DNA bases, is particularly susceptible to oxidation, forming 8-hydroxydeoxyguanosine (8-OHdG). This modified DNA base is then released into the bloodstream and excreted in the urine. Notably, urinary 8-OHdG does not solely originate in the prostate, it reflects systemic oxidative stress, indicating the extent of DNA damage occurring throughout the body. Urinary 8-OHdG serves as a reliable and non-invasive biomarker of oxidative stress, and higher levels of urinary 8-OHdG indicate increased oxidative stress and potential DNA damage within cells (11). Elevated levels of urinary 8-OHdG have been associated with various pathogeneses linked to oxidative stress, including aging, metabolic diseases (12), cardiovascular diseases (13), neurodegenerative disorders (14) and cancer (15). In a previous immunohistochemical study, 8-OHdG was detected more in prostate cancer tissues than in benign prostate tissues (16). In addition, our previous study demonstrated that prostate volume was significantly positively associated with urinary 8-OHdG and serum inducible nitric oxide synthase (iNOS) (17). Furthermore, not only prostate enlargement, but also serum prostate-specific antigen (PSA) levels, were shown to be positively correlated with urinary 8-OHdG levels in a cohort of 207 men with a mean age of 62.5 years (17). Therefore, the present study aimed to investigate the relationship between urinary 8-OHdG levels and prostate carcinogenesis, in order to investigate the clinical relevance of urinary 8-OHdG levels in patients with prostate cancer at initial diagnosis.
Materials and methods
Patient population and study samples
The present single institution study obtained the approval and institutional oversight of the Institutional Review Board (IRB) for the Protection of Human Subjects at National Cheng Kung University Hospital (NCKUH; Tainan, Taiwan; IRB no. A-ER-101-181) on November 21, 2012. Patients were recruited from NCKUH between November 2012 and October 31, 2020. At the time of specimen collection, all patients provided written informed consent for study participation, subsequent retrospective review and publication. The patients who met the National Comprehensive Cancer Network criteria of prostate biopsy for prostate cancer diagnosis were invited to participate in this cohort study, and their data were collected into a transrectal ultrasound of prostate (TRUS) biopsy database created for this research (18). The indications for prostate biopsy were men aged >40 years old that exhibited any of the following criteria: i) Abnormal digital rectal examination with or without PSA elevation; ii) PSA >3.0 ng/ml in those with a suspicious family history of prostate cancer or a progressive increase in PSA in three consecutive measurements; iii) PSA >4.0 ng/ml. Patients with Foley catheterization, suprapubic cystostomy or an active urinary tract infection were excluded from the current study. Self-voided mid-stream urine was collected immediately before receiving a prostate biopsy, which was performed at an outpatient clinic of NCKUH as scheduled in the morning. All patients with newly diagnosed prostate cancer received subsequent staging and treatment according to the prostate cancer treatment guidelines at NCKUH and the d'Amico prostate cancer risk stratification (19). Those with a non-malignant biopsy were kept on for follow-up studies (18). A Consolidated Standards of Reporting Trials flowchart is shown in Fig. 1, which is a standardized diagram utilized to illustrate participant flow (20). The study was performed after obtaining the participants' informed consent in compliance with The Declaration of Helsinki.
Figure 1.
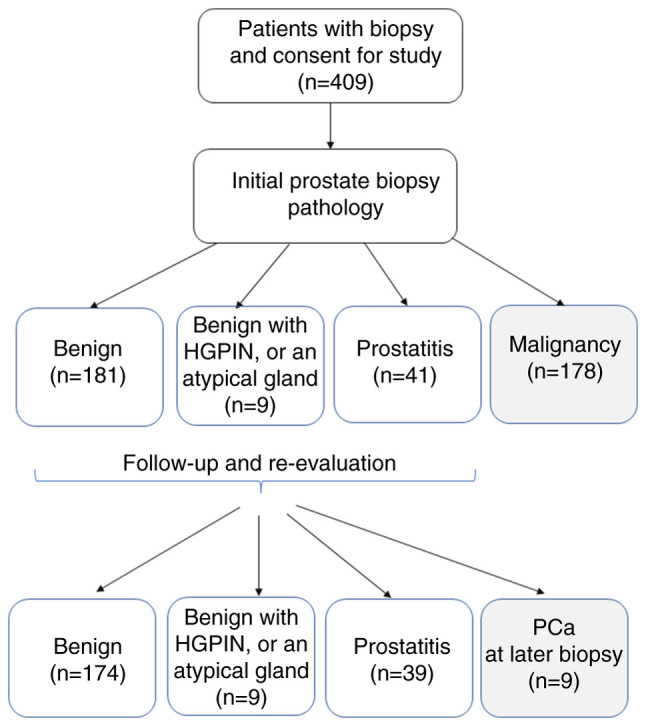
Consolidated Standards of Reporting Trials flow chart of the current study. HGPIN, high-grade prostatic intraepithelial neoplasia; PCa, prostate cancer.
Urinary 8-OHdG and N-terminal telopeptide (NTx) ELISA
Once the urine specimen was collected, it was centrifuged at room temperature for 10 min at 600 x g, and separated into two portions, supernatants and cell pellets. The cell pellet was collected after harvesting 1-2 ml of the supernatant without disturbing the pellet. Both portions were separately stored at -80˚C. The supernatant without cells, cell debris or other particulates (such as crystals) was investigated using a urinary 8-OHdG ELISA according to a previously described method (17). The results were normalized to urine creatinine levels. Urinary 8-OHdG (oxidatively damaged DNA adducts) levels were measured using a highly sensitive 8-OHdG Check ELISA kit (cat. no. KOG-200SE; Japan Institute for the Control of Aging); the sensitivity of this kit was reported as 0.125 ng/ml. Urinary NTx levels were measured with the human NTx I (Cross Linked N-telopeptide of type I collagen) ELISA kit (cat. no. E-EL-H0836; Elabscience; Elabscience Bionovation Inc.) according to the manufacturer's protocol; this kit recognizes human NTx Ⅰ in studied samples, and the sensitivity was reported as 1.88 ng/ml and the detection range as 3.13-200 ng/ml. There is no significant interference or cross-reactivity between human NTx Ⅰ and its analogs (standards or controls), and the coefficient of variation is <10%, according to the manufacturer's manual; thus indicating that this assay is highly specific and accurate.
Statistical analysis
The normal distribution of the data was assessed using descriptive statistics and frequency distribution, followed by the Shapiro-Wilk normality test. The comparison of urinary 8-OHdG levels among patients with benign prostatitis and malignancy, or localized, locally advanced and de novo metastatic diseases, was calculated using one-way ANOVA and Spearman's rank correlation coefficient. If the ANOVA test was significant, a post-hoc analysis with Tukey's multiple comparisons test was performed to compare the differences between each group. Unpaired Student's t-test was used to compare the differences between initial and later diagnosis of prostate cancer. χ2 test or Fisher's exact test (when the expected count was ≤5 in ≥20% of cells) was used to assess contingency tables. The association between urinary 8-OHdG and NTx levels was determined using a linear regression analysis. The association of urinary 8-OHdG/creatinine ratio with age, PSA, prostate volume and PSA density (PSAD; PSA level divided by prostate volume) was also assessed using linear regression analysis. Statistical analysis was performed using SPSS version 12.0 (SPSS, Inc.) and GraphPad Prism 6.00 for Windows (Dotmatics) software packages. P<0.05 (two-sided) was considered to indicate a statistically significant difference.
Results
Patient characteristics
From November 2012 to October 31, 2020, 409 patients were enrolled in the current study at an outpatient clinic before prostate biopsy, including 190 patients with a benign biopsy (including an atypical gland and prostate intraepithelial neoplasm), 41 patients with prostatitis and 178 with malignancy. The median age of the patients was 68 years (range, 43-91 years). As compared with non-malignant (benign or prostatitis) patients, those with a malignant histology were older (P<0.0001), and had higher PSA values (P=0.001), a lower prostate volume (P<0.0001) and higher PSAD (P=0.010) (Table I).
Table I.
Basic characteristics of the studied cohort at initial biopsy.
| Parameter | Entire cohort | Benign (PIN, AG) | Prostatitis | Malignancy | P-valuea | P-valueb, Malignancy vs. Benign/Malignancy vs. Prostatitis |
|---|---|---|---|---|---|---|
| Number of patients | 409 | 190 | 41 | 178 | - | - |
| Age, years | <0.0001 | <0.0001/0.0002 | ||||
| Median (range) | 68 (43-91) | 66 (43-86) | 66 (50-80) | 71 (53-91) | ||
| 25-75% percentile | 62, 74 | 61, 72 | 60, 72 | 66, 76 | ||
| PSA, ng/ml | 0.001 | 0.0013/0.0868 | ||||
| Geometric mean | 13.91 | 7.7 | 8.9 | 28.8 | ||
| 95% CI | 12.2-15.8 | 7.1-8.5 | 7.2-10.1 | 22.7-36.6 | ||
| Range | 0.6-8332 | 0.6-41.9 | 1.4-49.7 | 1.8-8332 | ||
| Prostate volume, ml | <0.0001 | <0.0001/0.0234 | ||||
| Mean ± SD | 49.8±35.4 | 55.0±27.1 | 54.5±26.5 | 43.1±21.5 | ||
| PSAD, ng/ml/ml | 0.010 | 0.0107/0.1944 | ||||
| Geometric mean | 0.32 | 0.16 | 0.18 | 0.75 | ||
| 95% CI | 0.28-0.36 | 0.14-0.17 | 0.15-0.22 | 0.59-0.94 | ||
| Range | 0.03-307 | 0.03-1.55 | 0.06-0.97 | 0.06-307 | ||
| Urinary creatinine, mg/dl | 0.680 | |||||
| Geometric mean | 73.5 | 73.0 | 74.3 | 74.0 | ||
| 95% CI | 68.6-78.9 | 66.1-80.6 | 57.9-95.4 | 66.3-82.5 | ||
| 8-OHdG, ng/ml | 0.144 | |||||
| Geometric mean | 3.3 | 3.0 | 3.2 | 3.6 | ||
| 95% CI | 2.9-3.7 | 2.6-3.6 | 2.2-4.6 | 3.0-4.4 | ||
| 8-OHdG/creatinine, x10-6 | 0.377 | |||||
| Geometric mean | 4.5 | 4.17 | 4.29 | 4.77 | ||
| 95% CI | 4.1-4.9 | 3.72-4.67 | 3.39-5.43 | 4.26-5.34 | ||
| 8-OHdG/creatinine/prostate volume, x10-6/ml | 0.004 | 0.0065/0.070 | ||||
| Median | 0.10 | 0.09 | 0.08 | 0.12 | ||
| 95% CI | 0.03-0.31 | 0.03-0.27 | 0.03-0.25 | 0.04-0.38 |
aOne-way ANOVA;
bPost-hoc analysis with Tukey's multiple comparisons test. 8-OHdG, 8-hydroxydeoxyguanosine; AG, atypical gland CI, confidence interval; PIN, prostate intraepithelial neoplasm; PSA, prostate-specific antigen; PSAD, PSA density.
Urinary 8-OHdG levels and normalization with urine creatinine level and prostate volume
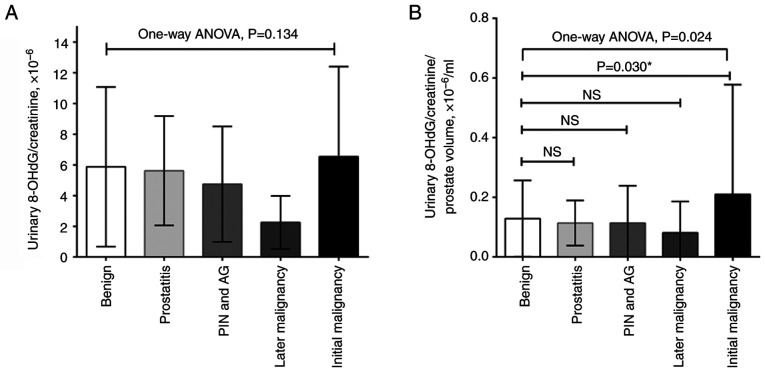
At the time of prostate biopsy, urinary 8-OHdG levels in patients with prostate cancer were higher than those without malignancy (3.6 vs. 3.2 or 3.0 ng/ml; P=0.144; Table I); however, this was not significant. During correction for urinary creatinine levels, malignant patients had higher ratios of urinary 8-OHdG/creatinine than the non-malignant patients (4.77x10-6 vs. 4.29x10-6 or 4.17x10-6; P=0.377; Table I); however, this was also not significant. Since prostate enlargement can be induced by oxidative stress, and there was a borderline association between prostate volume and 8-OHdG/creatinine ratio in the current study (Table SI), the present study further corrected the ratio with prostate volume (17). The results demonstrated that patients with malignancies exhibited a significantly higher ratio of urinary 8-OHdG/creatinine per prostate volume than the other subgroups (0.12x10-6/ml vs. 0.08x10-6/ml or 0.09x10-6/ml; P=0.004; Table I). A post-hoc analysis with Tukey's multiple comparisons test showed that the initial malignancy group exhibited a higher urine 8-OHdG/creatinine ratio per prostate volume than the benign group (P=0.030; Fig. 2).
Figure 2.
Ratio of urinary 8-OHdG/creatinine in the studied cohort. (A) Without or (B) with correction for prostate volume, the urinary 8-OHdG/creatinine ratio in patients with prostate cancer was significantly higher than that in patients with an initial benign biopsy. There were nine patients diagnosed with prostate cancer during follow-up. *Post hoc analysis with Tukey's multiple comparisons test. 8-OHdG, 8-hydroxydeoxyguanosine; AG, atypical gland; NS, not significant; PIN, prostate intraepithelial neoplasm.
During follow-up, another nine patients were diagnosed with prostate cancer, including two from the prostatitis subgroup and seven from the initial benign subgroup (Table SII). Among the four subgroups with an initially benign biopsy, there were no significant differences in age, PSA, prostate volume, urinary 8-OHdG/creatinine ratio or urinary 8-OHdG/creatinine ratio per prostate volume (P>0.05); however, the later malignancy group exhibited highest PSAD levels within all the groups (P=0.027).
Prostate cancer risk stratification and urinary 8-OHdG/creatinine ratio
During the study period, a total of 187 patients were diagnosed with prostate cancer, including 178 at the initial biopsy and nine at a later repeat biopsy. As compared with patients with prostate cancer diagnosed at the initial biopsy, those with later malignancy were younger and had lower urinary 8-OHdG/creatinine ratios (P=0.010 and P=0.031, respectively). Due to the small number of patients diagnosed with a later malignancy, there were no statistical differences in Gleason grading, clinical TNM stage (21) and cancer recurrent risk when comparing with patients initially diagnosed with prostate cancer (Fisher's exact test; P=0.578, P=0.404, and P=0.183, respectively) (Table II). A total of 50 patients received subsequent radical prostatectomy, and there was no difference between prostate cancer at initial diagnosis and later diagnosis in terms of pathological staging or grade (Table SIII).
Table II.
Clinicopathological characteristics of patients with PCa according to the time of diagnosis.
| Parameter | Total | Initial PCa | Later PCa | P-value |
|---|---|---|---|---|
| Number of patients | 187 | 178 | 9 | |
| Median age, years (range) | 71 (53-91) | 71 (53-91) | 63 (55-73) | 0.010a |
| 25%-75% percentile | 65-76 | 66-76 | 61-70 | |
| Mean PSA, ng/ml (95% CI) | 27.5 (21.9-34.6) | 28.8 (22.7-36.6) | 10.9 (7.0-16.8) | 0.471a |
| Mean ±SD prostate volume, ml | 43.0±21.6 | 43.1±21.5 | 41.5±24.5 | 0.833a |
| Mean PSAD, ng/ml2 (95% CI) | 0.72 (0.57-0.89) | 0.75 (0.59-0.94) | 0.31 (0.19-0.50) | 0.560a |
| Mean 8-OHdG/crea., x10-6 (95% CI) | 4.6 (4.1-5.3) | 4.8 (4.2-5.6) | 1.7 (0.9-3.2) | 0.031a |
| Median 8-OHdG/crea./PV, x10-6/ml (95% CI) | 0.12 (0.10-0.14) | 0.12 (0.10-0.15) | 0.05 (0.02-0.09) | 0.294a |
| Gleason grade | 0.578b | |||
| 3+3 | 58 | 53 | 5 | |
| 3+4 | 21 | 20 | 1 | |
| 4+3 | 29 | 28 | 1 | |
| 4+4, 3+5, 5+3 | 33 | 32 | 1 | |
| 5+5, 5+4, 4+5 | 46 | 45 | 1 | |
| Clinical TNM staging | 0.404b | |||
| cT1c N0 | 53 | 48 | 5 | |
| cT2a,b,c N0 | 37 | 35 | 2 | |
| cT3aN0 | 20 | 19 | 1 | |
| cT3b-4N0 | 27 | 26 | 1 | |
| Any cT, cN+ | 11 | 11 | 0 | |
| Any cT, Any cN, M+ | 39 | 39 | 0 | |
| D'Amico prostate cancer risk stratification | 0.183b | |||
| Localized (low risk) | 25 | 24 | 1 | |
| Localized (intermediate risk) | 41 | 36 | 5 | |
| Localized (high risk) | 44 | 42 | 2 | |
| Locally advanced (very high risk) | 26 | 25 | 1 | |
| Nodal | 10 | 10 | 0 | |
| Metastatic | 41 | 41 | 0 | |
| Treatment options | 0.083b | |||
| Active surveillance/observation | 22 | 20 | 2 | |
| Radical prostatectomy | 50 | 45 | 5 | |
| Radiotherapy | 31 | 29 | 2 | |
| ADT | 51 | 51 | 0 | |
| Visit other hospitals or options | 33 | 33 | 0 |
aUnpaired t-test;
bFisher's exact test. 8-OHdG, 8-hydroxydeoxyguanosine; ADT, androgen deprivation therapy; CI, confidence interval; crea., creatinine; PCa, prostate cancer.
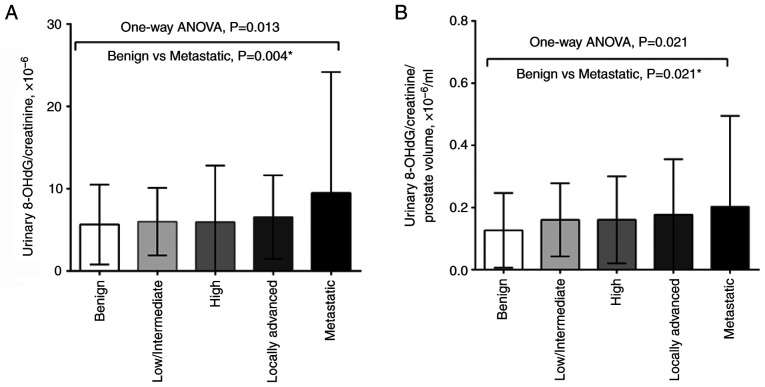
Significant differences in urinary 8-OHdG/creatinine ratio were detected between different prostate cancer risk groups using one-way ANOVA, and the Tukey's post hoc test indicated that the significant differences were between the benign and metastatic groups, regardless of whether the urinary 8-OHdG/creatinine ratio was normalized to prostate volume (P=0.004 and P=0.021, respectively) (Fig. 3). The Spearman's rank correlation coefficient revealed that was a negligible correlation between patient risk group and urinary 8-OHdG levels, with or without prostate volume correction (Fig. S1).
Figure 3.
One-way ANOVA were used to determine the association between urinary 8-OHdG levels and prostate cancer risk stratification (A) without or (B) with prostate volume correction. *Post hoc analysis with Tukey's multiple comparisons test. 8-OHdG, 8-hydroxydeoxyguanosine.
Association of 8-OHdG and NTx levels in the urine
Urinary NTx levels were measured in a total of 188 studied patients, including 178 patients diagnosed with prostate cancer at the initial biopsy and 10 random patients with an initial benign biopsy. The geometric mean value of urinary NTx level was 30.8 ng/ml with a range of 0.5-386 ng/ml (95% CI, 47.5-65.6 ng/ml). The association between 8-OHdG and NTx levels in the urine was analyzed, and the results showed a weakly positive association between both factors in the urine (linear regression, r2=0.04; P=0.009) (Fig. 4).
Figure 4.
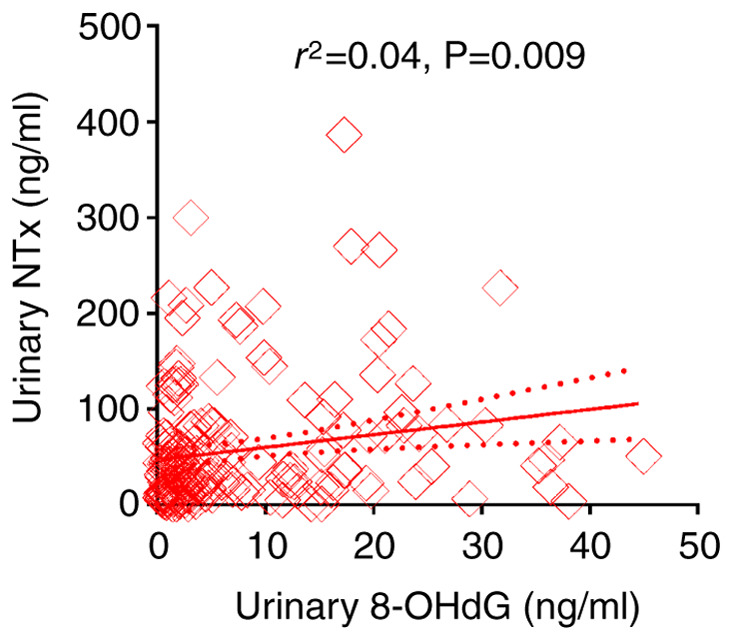
Association between NTx and 8-OHdG levels in the urine. The solid line represents the linear regression and the dotted lines represent the standard error. 8-OHdG, 8-hydroxydeoxyguanosine; NTx, N-terminal telopeptide.
Discussion
The present study demonstrated that urinary 8-OHdG levels with prostate volume correction were elevated in patients with malignancy at the time of prostate biopsy compared with those with non-malignant histology, although there was an absence of a direct association between urinary 8-OHdG and cancer in this analysis. Significant differences in urinary 8-OHdG/creatinine ratio were also determined between the different prostate cancer risk groups. Moreover, urinary 8-OHdG levels were significantly associated with urinary NTx levels. Taken together, urinary 8-OHdG levels may reflect the involvement of oxidative stress in the initiation and progression of prostate carcinogenesis.
Several studies have reported on the association between oxidative stress and prostate cancer (22-24), with several studies noting changes in pro-oxidant/antioxidant balance in clinical prostate cancer samples, rodent models and prostate cell lines (9,25,26). In a previous study, malignant prostate samples demonstrated elevated levels of thiobarbituric acid reactive substances and reduced levels of glutathione peroxidase and CuZn-superoxide dismutase (SOD) in comparison with the benign group (27). Upregulated oxidative stress profiles have also been detected in human prostate cancer and it has been suggested that antioxidant defense systems might be impaired (28). Miyake et al (29) reported that the urinary 8-OHdG/creatinine ratio in patients with prostate cancer was significantly elevated as compared with that in age-matched healthy controls, which indicates the importance of oxidative stress in the early events of prostate cancer, including cell proliferation, differentiation, apoptosis and carcinogenesis. Ohtake et al (16) reported that 8-OHdG was more highly expressed in prostate cancer tissues in comparison to benign prostate tissues using immunohistochemical analysis. In addition, our recent publication identified a positive association between serum PSA levels (or PSAD) and urinary 8-OHdG levels in men with a mean age of 62.5 years, which was associated with prostate enlargement (17). Since urinary 8-OHdG is not solely of prostate origin, it reflects systemic oxidative stress, indicating the extent of DNA damage occurring throughout the body. According to our previous study, prostate volume was significantly positively associated with urinary 8-OHdG and serum iNOS levels (17), thus the present study normalized urinary 8-OHdG levels to prostate volume. The results provide further evidence that oxidative stress may partly contribute to the initiation of prostate cancer and prostate enlargement. To the best of our knowledge, the present study is the first to demonstrate a significant association between urinary 8-OHdG levels and d'Amico risk stratification, thereby aiding in predicting patient risk during initial diagnosis.
Our previous study showed that urinary di(2-ethylhexyl)phthalate (DEHP) metabolites, which serve as evidence of phthalate exposure, were associated with urinary 8-OHdG levels, serum PSA and prostate volume (17). Phthalates are well-known inducers of oxidative stress associated with several diseases (30,31). Numerous studies have proposed that exposure to phthalates, both in vivo and in vitro, leads to oxidative stress in patients, primarily by decreasing the levels of SOD and glutathione (17); however, their role in prostate carcinogenesis remains unclear. Some in vitro studies have shown that prostate cancer cells exhibit various inherent responses to oxidative stress, including reactive oxygen species generation, and activation of matrix metalloproteinase-9 (MMP-9) by causing changes in the MMP-9 structure, which is involved in the breakdown of extracellular matrix components that is required for aggressive phenotypes (32). In addition, some phthalates (such as DEHP, benzyl butyl phthalate and diisobutyl phthalate) can upregulate cyclin D1 and proliferating cell nuclear antigen, downregulate P21 and stimulate LNCaP proliferation (33). Phthalates may also promote prostate cancer progression through the Hedgehog pathway in LNCaP cells (34). Although there is a lack of causal mechanistic experiments regarding oxidative stress and prostate cancer progression, the present results demonstrated significant differences in urinary 8-OHdG/creatinine ratio between the different prostate cancer risk groups, and identified a positive relationship between urinary 8-OHdG levels and NTx levels. Such evidence provides strong clinical evidence that oxidative stress serves an important role in the process of prostate cancer progression. However, Spearman's rank correlation coefficient showed no significant correlation between patient risk group and urinary 8-OHdG levels; this lack of correlation indicates the need for further research to explore potential non-linear relationships or other influencing factors.
Although these cellular and clinical studies support that oxidative stress is crucial in the etiology or progression of prostate cancer, several large clinical trials have failed to support the ability of supplemental dietary antioxidants, such as vitamin E, to reduce prostate cancer risk. These studies include the Prostate, Lung, Colorectal and Ovarian; Alpha-Tocopherol, Beta-Carotene Prevention; and Selenium and Vitamin E Cancer Prevention Trial studies (35-37). However, vitamin E has been reported to exhibit some benefit in a particular subgroup of patients; smokers supplemented with 50 mg vitamin E daily exhibited a significantly lower prostate cancer incidence (32%) and a significantly lower prostate cancer mortality rate (41%) than those assigned to receive a placebo (38). Despite the absence of clinical trials supporting the efficacy of supplemental dietary antioxidants, it may still be worthwhile exploring therapies that target the antioxidant response.
Notably, 8-OHdG is an oxidized nucleoside of DNA that is the most frequently studied and detected biomarker of oxidative DNA damage. Upon DNA repair, 8-OHdG is soon excreted in the urine, and can be measured using ELISA and HPLC-MS methods. Evidence has demonstrated that urinary 8-OHdG is a well-known biomarker of generalized, cellular oxidative stress, and a risk factor for atherosclerosis, diabetes and cancer (13). By contrast, blood 8-OHdG may represent systemic oxidative stress from the tissues and circulating lymphocytes. Few studies have focused on blood 8-OHdG because several challenges exist for detecting 8-OHdG in the blood; notably, techniques such as ELISA may not be sensitive enough, as their detection limits can be higher than the normal range found in healthy individuals (39,40). Our previous study demonstrated that urinary 8-OHdG levels were positively associated with prostate enlargement (17). Therefore, the urinary 8-OHdG levels in the current study theoretically reflect oxidative stress both in the prostate and the whole body. The prostate is one of the most vulnerable organs in the body. While the absence of a direct association between urinary 8-OHdG and cancer is noted in the present analysis, it does not preclude its potential value as an indicator of oxidative stress or DNA damage, both of which are linked to cancer initiation and progression. Notably, compared with prostate volume, the normalized urinary 8-OHdG levels were a worse marker for predicting prostate cancer. However, urinary 8-OHdG level reflects systemic oxidative stress, which not only promotes prostate enlargement (17), but might induce the initiation and progression of prostate cancer, as evidenced by the association between urinary 8-OHdG and NTx levels. Further research into the biological mechanisms underlying how environmental exposures induce oxidative stress, as evidenced by urinary 8OH-dG levels, and their connection to prostate cancer could illuminate their potential clinical relevance for diagnosing and treating the disease.
There were several limitations in the current study. First, there are several factors associated with oxidative stress, such as aging, prostate volume, infection/inflammation, cancer and environmental exposure. In the current study, it was revealed that prostate volume and cancer were the two most notable contributing factors to oxidative stress. In contrast with large-scale clinical trials, the number of study patients in the current study was relatively small to explore the effect of other factors. Second, urinary 8-OHdG levels might be influenced by cancer volume. Although the impact of tumor percentage was not investigated in the current study, the results revealed that urinary 8-OHdG levels were associated with risk stratification, which might partly reflect tumor burden. A future study may be conducted with more patients receiving radical prostatectomy. Third, the research objective was to utilize the existing TRUS biopsy database to observe potential clinically significant phenomenal; therefore, the required sample size in the current study was not explicitly calculated, which may influence the statistical power. Since the present study enrolled 409 patients, it may be considered a reasonable size. The present data also showed that patient age may be a better marker than the urinary 8-OHdG/creatinine ratio regarding prostate cancer detection (P=0.01 vs. P=0.031). Notably, as the present study utilized an existing TRUS biopsy database, disparities in both population size and age distribution were present during group comparisons; therefore, it is difficult to make comparisons to determine which factor is better. Additionally, some patient information was not collected and analyzed in the current study, including smoking, drug history, occupation history, comorbidities and medication history. These factors may interfere with urinary 8-OHdG levels. In future studies, increasing participant enrollment with detailed medical and social history records may help mitigate these potential biases. Fourth, the present study did not perform immunohistochemical analysis to clarify whether 8-OHdG was expressed more highly in prostate cancer tissues than in benign prostate tissues, and 8-OHdG was not measured in the blood for comparison with urinary levels. Although both the blood test and tissue assay for the existence of 8-OHdG might theoretically reflect the action of oxidative stress on prostate carcinogenesis, blood 8-OHdG cannot accurately reflect the oxidative stress status of the prostate or malignant tissue. Our previous study showed that oxidative status and urinary 8-OHdG levels were positively correlated with prostate enlargement in patients with lower urinary tract symptoms that exhibited relatively normal serum PSA levels (17). Therefore, we focused on the influence of oxidative stress on prostate carcinogenesis in the present study. It was hypothesized that the action of phthalate exposure is not specific for prostate enlargement but also for prostate cancer. The non-specific effect of phthalate exposure on the prostate is similar with the non-specific characteristic of the origin of PSA. Both benign and malignant prostate tissue can produce and secrete PSA into the lumen of the prostate gland, which can leak into the blood stream, and benign prostate cells can produce more PSA mRNA than malignant prostate cancer cells (41). The present study demonstrated a substantial association between prostate volume-normalized urinary 8-OHdG/creatinine ratio and the aggressiveness of prostate cancer. This finding suggested its potential as a non-invasive and easily accessible predictor for assessing the influence of oxidative stress on prostate cancer aggressiveness at initial diagnosis. Nevertheless, larger studies are necessary to yield more robust results. At present, we are dedicated to investigating the influence and molecular mechanism of phthalate exposure on prostate carcinogenesis in order to validate and reinforce our findings, thereby improving their clinical applicability and upholding the highest standards of patient care through rigorous scientific research.
In conclusion, the present study demonstrated that patients with prostate cancer had higher urinary levels of the oxidative stress marker 8-OHdG normalized to prostate volume than non-malignant patients at the time of prostate biopsy. Furthermore, urinary 8-OHdG levels normalized to prostate volume were higher in more advanced disease and were positively associated with urinary NTx levels. These data highlight the evidence of oxidative stress in the etiology and progression of prostate cancer; however, further studies are required to identify the etiology of oxidative stress in prostate cancer patients.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by Taiwan Ministry of Science and Technology (grant nos. 111-2314-B-006-108- and 110-2314-B-006-065-MY3), the NCKUH (grant nos. NCKUH-11204018, NCKUH-11104011, NCKUH-11004022, NCKUH-10902045 and NCKUH-10802035) and the Ditmanson Medical Foundation Chia-Yi Christian Hospital (grant no. NCKUCYCH-P-11101-1).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
TST, YCJ and YST designed the study. HTT and YST were primarily responsible for conducting the experiments and confirm the authenticity of all the raw data. YCH and ISC acquired and analyzed the data, and wrote the manuscript. LNH and HTT assisted in data acquisition and interpretation, and edited the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study obtained the approval and institutional oversight of the Institutional Review Board (IRB) for the Protection of Human Subjects at National Cheng Kung University Hospital (IRB no.: A-ER-101-181). Written informed consent was obtained from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2. Health Promotion Administration (HPA): 2022 Health Promotion Administration Annual Report. HPA, Taipei City, 2022. https://www.hpa.gov.tw/EngPages/Detail.aspx?nodeid=1070&pid=16384. Accessed 18 May, 2024. [Google Scholar]
- 3.Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ, Lai MS. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J Formos Med Assoc. 2016;115:1076–1088. doi: 10.1016/j.jfma.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Lin YH, Chen KK, Chiu JH. Use of Chinese medicine among prostate cancer patients in Taiwan: A retrospective longitudinal cohort study. Int J Urol. 2011;18:383–386. doi: 10.1111/j.1442-2042.2011.02738.x. [DOI] [PubMed] [Google Scholar]
- 5.Inamoto T, Azuma H, Hinotsu S, Tsukamoto T, Oya M, Ogawa O, Kitamura T, Kazuhiro S, Naito S, Namiki M, et al. Age at diagnosis on prostate cancer survival undergoing androgen deprivation therapy as primary treatment in daily practice: Results from Japanese observational cohort. J Cancer Res Clin Oncol. 2014;140:1197–1204. doi: 10.1007/s00432-014-1638-y. [DOI] [PubMed] [Google Scholar]
- 6.Chen PM, Chen SC, Liu CJ, Hung MH, Tsai CF, Hu YW, Chen MH, Shen CC, Su TP, Yeh CM, et al. The association between prostate cancer and mood disorders: A nationwide population-based study in Taiwan. Int Psychogeriatr. 2015;27:481–490. doi: 10.1017/S104161021400218X. [DOI] [PubMed] [Google Scholar]
- 7.Balistreri CR, Candore G, Lio D, Carruba G. Prostate cancer: From the pathophysiologic implications of some genetic risk factors to translation in personalized cancer treatments. Cancer Gene Ther. 2014;21:2–11. doi: 10.1038/cgt.2013.77. [DOI] [PubMed] [Google Scholar]
- 8.Huang WK, Liu CH, Pang ST, Liu JR, Chang JW, Liaw CC, Hsu CL, Lin YC, See LC. Type of androgen deprivation therapy and risk of dementia among patients with prostate cancer in Taiwan. JAMA Netw Open. 2020;3(e2015189) doi: 10.1001/jamanetworkopen.2020.15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shukla S, Srivastava JK, Shankar E, Kanwal R, Nawab A, Sharma H, Bhaskaran N, Ponsky LE, Fu P, MacLennan GT, Gupta S. Oxidative stress and antioxidant status in high-risk prostate cancer subjects. Diagnostics (Basel) 2020;10(126) doi: 10.3390/diagnostics10030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drozdz-Afelt JM, Koim-Puchowska BB, Kaminski P. Analysis of oxidative stress indicators in Polish patients with prostate cancer. Environ Sci Pollut Res Int. 2022;29:4632–4640. doi: 10.1007/s11356-021-15922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Taie A, Sancar M, Izzettin FV. Chapter 17-8-Hydroxydeoxyguanosine: A valuable predictor of oxidative DNA damage in cancer and diabetes mellitus. In: Cancer (Second Edition). Preedy VR and Patel VB (eds). Academic Press, San Diego, pp179-187, 2021. [Google Scholar]
- 12.Li YS, Song MF, Kasai H, Kawai K. 8-hydroxyguanine in urine and serum as an oxidative stress marker: Effects of diabetes and aging. J Uoeh. 2013;35:119–127. doi: 10.7888/juoeh.35.119. [DOI] [PubMed] [Google Scholar]
- 13.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Miyaoka T, Ieda M, Hashioka S, Wake R, Furuya M, Liaury K, Hayashida M, Tsuchie K, Arauchi R, Araki T, et al. Analysis of oxidative stress expressed by urinary level of biopyrrins and 8-hydroxydeoxyguanosine in patients with chronic schizophrenia. Psychiatry Clin Neurosci. 2015;69:693–698. doi: 10.1111/pcn.12319. [DOI] [PubMed] [Google Scholar]
- 15.Yano T, Shoji F, Baba H, Koga T, Shiraishi T, Orita H, Kohno H. Significance of the urinary 8-OHdG level as an oxidative stress marker in lung cancer patients. Lung Cancer. 2009;63:111–114. doi: 10.1016/j.lungcan.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Ohtake S, Kawahara T, Ishiguro Y, Takeshima T, Kuroda S, Izumi K, Miyamoto H, Uemura H. Oxidative stress marker 8-hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. Mol Clin Oncol. 2018;9:302–304. doi: 10.3892/mco.2018.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang WH, Tsai YS, Wang JY, Chen HL, Yang WH, Lee CC. Sex hormones and oxidative stress mediated phthalate-induced effects in prostatic enlargement. Environ Int. 2019;126:184–192. doi: 10.1016/j.envint.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Moses KA, Sprenkle PC, Bahler C, Box G, Carlsson SV, Catalona WJ, Dahl DM, Dall'Era M, Davis JW, Drake BF, et al. NCCN Guidelines® Insights: Prostate cancer early detection, version 1.2023. J Natl Compr Canc Netw. 2023;21:236–246. doi: 10.6004/jnccn.2023.0014. [DOI] [PubMed] [Google Scholar]
- 19.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 20.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(c332) doi: 10.4103/0976-500X.72352. CONSORT Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer (Version 4.2024). National Comprehensive Cancer Network; 2024. Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459. Accessed 24 July, 2024. [Google Scholar]
- 22.Paschos A, Pandya R, Duivenvoorden WC, Pinthus JH. Oxidative stress in prostate cancer: Changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis. 2013;16:217–225. doi: 10.1038/pcan.2013.13. [DOI] [PubMed] [Google Scholar]
- 23.Liou GY, C'Lay-Pettis R, Kavuri S. Involvement of reactive oxygen species in prostate cancer and its disparity in african descendants. Int J Mol Sci. 2024;25(6665) doi: 10.3390/ijms25126665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biesiadecki M, Mołoń M, Balawender K, Kobylińska Z, Galiniak S. Shedding light on the shadows: Oxidative stress and its pivotal role in prostate cancer progression. Front Oncol. 2024;14(1393078) doi: 10.3389/fonc.2024.1393078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battisti V, Maders LDK, Bagatini MD, Reetz LG, Chiesa J, Battisti IE, Gonçalves JF, Duarte MM, Schetinger MR, Morsch VM. Oxidative stress and antioxidant status in prostate cancer patients: Relation to Gleason score, treatment and bone metastasis. Biomed Pharmacother. 2011;65:516–524. doi: 10.1016/j.biopha.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Rossetto IMU, Santos FR, da Silva HM, Minatel E, Mesquitta M, Salvador MJ, Montico F, Cagnon VHA. Tempol effect on oxidative and mitochondrial markers in preclinical models for prostate cancer. Toxicol Res (Camb) 2024;13(tfae056) doi: 10.1093/toxres/tfae056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, Erten K, Ozgök Y, Dimovski A. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 2006;39:176–179. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Oh B, Figtree G, Costa D, Eade T, Hruby G, Lim S, Elfiky A, Martine N, Rosenthal D, Clarke S, Back M. Oxidative stress in prostate cancer patients: A systematic review of case control studies. Prostate Int. 2016;4:71–87. doi: 10.1016/j.prnil.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyake H, Hara I, Kamidono S, Eto H. Oxidative DNA damage in patients with prostate cancer and its response to treatment. J Urol. 2004;171:1533–1536. doi: 10.1097/01.ju.0000116617.32728.ca. [DOI] [PubMed] [Google Scholar]
- 30.Han Q, Gao X, Wang S, Wei Z, Wang Y, Xu K, Chen M. Co-exposure to polystyrene microplastics and di-(2-ethylhexyl) phthalate aggravates allergic asthma through the TRPA1-p38 MAPK pathway. Toxicol Lett. 2023;384:73–85. doi: 10.1016/j.toxlet.2023.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Yang Y, Tao Y, Guo X, Cui Y, Li Z. Phthalates (PAEs) and reproductive toxicity: Hypothalamic-pituitary-gonadal (HPG) axis aspects. J Hazard Mater. 2023;459(132182) doi: 10.1016/j.jhazmat.2023.132182. [DOI] [PubMed] [Google Scholar]
- 32.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 33.Zhu M, Huang C, Ma X, Wu R, Zhu W, Li X, Liang Z, Deng F, Wu J, Geng S, et al. Phthalates promote prostate cancer cell proliferation through activation of ERK5 and p38. Environ Toxicol Pharmacol. 2018;63:29–33. doi: 10.1016/j.etap.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Yong W, Jiao C, Jianhui W, Yan Z, Qi P, Xiu W, Zuyue S, Yunhui Z. Mono-2-ethyhexyl phthalate advancing the progression of prostate cancer through activating the hedgehog pathway in LNCaP cells. Toxicol In Vitro. 2016;32:86–91. doi: 10.1016/j.tiv.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Virtamo J, Edwards BK, Virtanen M, Taylor PR, Malila N, Albanes D, Huttunen JK, Hartman AM, Hietanen P, Mäenpää H, et al. Effects of supplemental alpha-tocopherol and beta-carotene on urinary tract cancer: Incidence and mortality in a controlled trial (Finland) Cancer Causes Control. 2000;11:933–939. doi: 10.1023/a:1026546803917. [DOI] [PubMed] [Google Scholar]
- 36.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 37.Kirsh VA, Hayes RB, Mayne ST, Chatterjee N, Subar AF, Dixon LB, Albanes D, Andriole GL, Urban DA, Peters U. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. J Natl Cancer Inst. 2006;98:245–254. doi: 10.1093/jnci/djj050. PLCO Trial. [DOI] [PubMed] [Google Scholar]
- 38.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, Haapakoski J, Malila N, Rautalahti M, Ripatti S, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: Incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 39.Koide S, Kinoshita Y, Ito N, Kimura J, Yokoyama K, Karube I. Determination of human serum 8-hydroxy-2'-deoxyguanosine (8-OHdG) by HPLC-ECD combined with solid phase extraction (SPE) J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2163–2167. doi: 10.1016/j.jchromb.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Korkmaz KS, Butuner BD, Roggenbuck D. Detection of 8-OHdG as a diagnostic biomarker. J Lab Precis Med. 2018;3(95) [Google Scholar]
- 41.Qiu SD, Young CY, Bilhartz DL, Prescott JL, Farrow GM, He WW, Tindall DJ. In situ hybridization of prostate-specific antigen mRNA in human prostate. J Urol. 1990;144:1550–1556. doi: 10.1016/s0022-5347(17)39797-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.