Abstract
The E1B-55K and E4orf6 proteins of adenovirus type 5 are involved in viral mRNA export. Here we demonstrate that adenovirus infection does not inhibit the function of the E1B-55K nuclear export signal and that E1B-55K also shuttles in infected cells. Even during virus infection, E1B-55K was exported by the leptomycin B-sensitive CRM1 pathway, whereas E4orf6 transport appeared to be mediated by an alternative mechanism. Our results strengthen the potential role of E1B-55K as the “driving force” for adenoviral late mRNA export.
Late in adenovirus infection, the nuclear export of most cellular mRNAs is inhibited while viral mRNAs accumulate in the cytoplasm and are efficiently expressed. There is general agreement that human adenoviruses encode at least three early gene products, E1B-55K, E4orf6, and E4orf3, which promote viral replication by directly or indirectly modulating nucleocytoplasmic mRNA export (for reviews see references 14 and 29). Mutant viruses lacking one or more of these proteins are impaired in efficient transport of late viral transcripts and hence in virus replication (3, 4, 14). E1B-55K and E4orf6 were shown to interact with each other (15, 21, 26, 27), and this complex appears to play a critical role in late viral mRNA export (14). However, the detailed mechanism by which these adenoviral proteins promote viral but appear to inhibit cellular nucleocytoplasmic mRNA transport is still not well understood.
Previously, it was suggested that the shuttling of the E4orf6–E1B-55K complex in uninfected cells is mediated predominantly by the E4orf6 protein (6). In contrast, we recently demonstrated that E1B-55K alone is capable of nucleocytoplasmic shuttling via the CRM1 export pathway and contains a functional leucine-rich nuclear export signal (NES) (12). However, the issue of whether E1B-55K also shuttles in adenovirus-infected cells was not investigated. On the other hand, several groups reported shuttling of E4orf6 using heterokaryon assays (6, 8, 20, 23), and the postulated E4orf6 NES appeared to be important for virus viability (34). In contrast, E4orf6 transport in heterokaryon assays seemed to be independent of the postulated E4orf6 NES (20, 23) and the E4orf6 NES itself was reported to be inactive in a heterologous system (12).
In order to understand these observations and to broaden knowledge of the adenoviral nuclear mRNA export pathway, we addressed the nucleocytoplasmic trafficking of E1B-55K and E4orf6 in virus-infected cells.
The NES export pathway is active in adenovirus-infected cells.
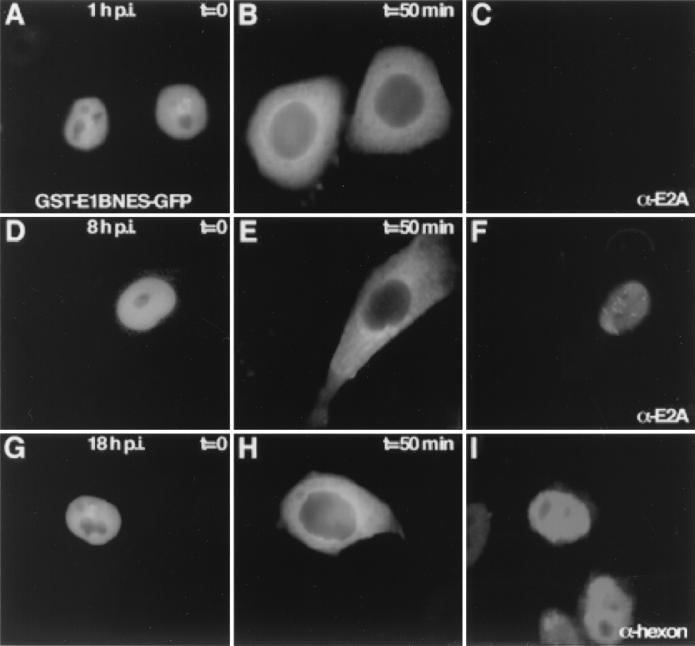
First, we investigated whether adenovirus infection interferes specifically or nonspecifically with export, mediated by the E1B-55K leucine-rich NES (12). Therefore, a hybrid protein composed of glutathione S-transferase (GST) linked to green fluorescent protein (GFP) and containing the E1B-55K NES (amino acids 83 to 93) (12) was microinjected into the nucleus of HeLa cells infected with human adenovirus type 5 (H5wt300). The recombinant GST-E1BNES-GFP export substrate was isolated from bacteria under nondenaturing conditions, and injection was carried out as described previously (25). To ensure infection of all cells, an inoculum of 30 PFU/cell was used as described previously (12). Following microinjection of GST-E1BNES-GFP (3 mg/ml) into the nucleus of HeLa cells at different times postinfection (p.i.), export was monitored directly by fluorescence microscopy using the appropriate GFP filters as described previously (12). Export occurred efficiently early (Fig. 1A and B) and later (Fig. 1D, E, G, and H) during infection. For each time point, about 30 cells were injected, and the kinetics of export were similar in all cells assayed. Comparable results were obtained in two independent experiments, and export was also active in infected Vero cells (data not shown). Microinjected GST-E4orf6NES-GFP was not exported in virus-infected HeLa cells, as already shown for semipermissive uninfected Vero cells (12) (data not shown). To verify infection, the cells were stained with the monoclonal antibody B6-8, specific for the 72-kDa E2A protein (24), at early time points (Fig. 1C and F). Staining with an antihexon antibody (Dako Diagnostics Ltd.) indicated adenovirus late protein expression at 18 h p.i and hence active late mRNA transport (Fig. 1I). Thus, the results strongly suggest that adenovirus replication does not interfere with the E1B-55K NES-mediated export pathway throughout infection.
FIG. 1.
Adenovirus infection does not affect NES-mediated export. Recombinant GST-E1BNES-GFP (3 mg/ml) was microinjected into the nuclei of adenovirus-infected HeLa cells at different times p.i. (A, D, and G). Export of the substrate was monitored by GFP fluorescence and occurred with similar kinetics at early (A and B) or late times (D, E, G, and H) of infection. Infection was verified by immunostaining against the 72-kDa E2A protein (C and F) or the hexon protein (I).
Adenovirus infection does not inhibit E1B-55K nucleocytoplasmic transport.
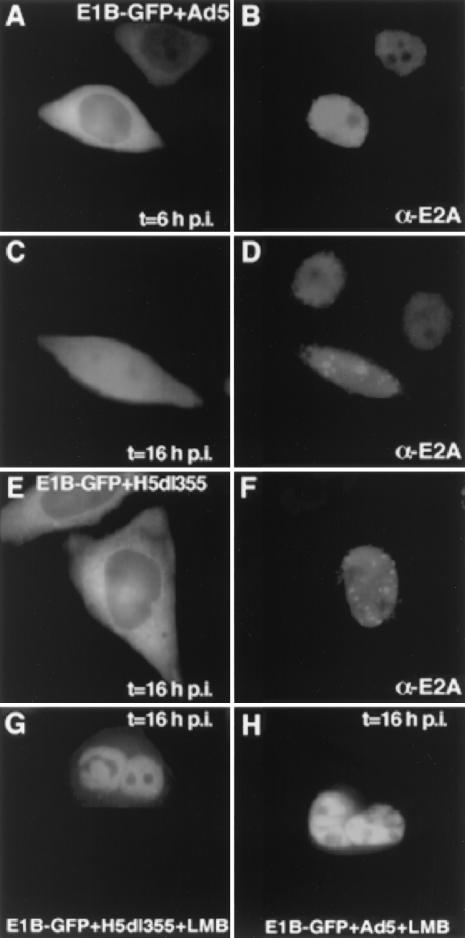
Although the E1B-55K NES itself was active in virus-infected cells, it was crucial to examine the nucleocytoplasmic shuttling of the complete protein in the presence of other viral proteins. Therefore, the E1B-55K–GFP hybrid (12) was transiently expressed in H5wt300-infected HeLa cells (30 PFU/cell) using calcium phosphate-DNA precipitates (30), and the cellular localization of E1B-55K–GFP was controlled at different times p.i. E1B-55K–GFP localized predominantly to the cytoplasm of infected cells, as reported for uninfected cells (12) (Fig. 2A). At late times p.i., E1B-55K–GFP became partially nuclear (Fig. 2C). This was most likely caused by the accumulation of the E4orf6 protein, known to direct E1B-55K to the nucleus in cotransfection experiments (12, 21, 27). Therefore, infection was performed using the E4orf6-deficient virus mutant H5dl355 (10). In the absence of E4orf6 E1B-55K–GFP was predominantly cytoplasmic even at late times p.i. (Fig. 2E), indicative of active E1B-55K shuttling in virus-infected cells. Productive infection was again verified by anti-72-kDa-E2A staining (Fig. 2B), and the characteristic pattern of viral factories indicated efficient adenovirus replication (Fig. 2D and F). E1B-55K–GFP localization did not significantly change at later times p.i. (20 and 24 h), although the cytopathic effect of adenovirus infection resulted in cell rounding (data not shown). Although E1B-55K has been reported to associate with the E4orf3 protein in nuclear structures during H5dl355 infection (15), the amount of expressed E4orf3 protein appeared to be insufficient to target E1B-55K–GFP efficiently to the nucleus.
FIG. 2.
E1B-55K–GFP shuttles in adenovirus-infected cells. HeLa cells were transfected with the E1B-55K–GFP expression plasmid (3 μg of plasmid DNA) and 10 h later infected with the indicated adenoviruses (30 PFU/cell). The localization of E1B-55K–GFP was monitored by fluorescence microscopy, and infection was verified by immunostaining against the 72-kDa E2A protein (B, D, and F). In H5wt300-infected cells, E1B-55K–GFP localized predominantly to the cytoplasm at early times p.i. (A) and became partially nuclear 16 h p.i. (C). Infection with the E4orf6-deficient virus H5dl355 resulted in cytoplasmic E1B-55K–GFP localization throughout infection (E), indicative of active nuclear export. LMB treatment for 30 min caused nuclear accumulation of E1B-55K–GFP, demonstrating that nuclear import was not affected by adenovirus infection (G and H).
To ensure that the cytoplasmic localization of E1B-55K–GFP was not merely caused by blocking nuclear import, the cells were treated with the NES export inhibitor leptomycin B (LMB) (10 nM final concentration for 30 min) (12). If nuclear import was inhibited by adenovirus infection the cytoplasmic localization of E1B-55K–GFP should not change. However, LMB treatment resulted in complete nuclear accumulation of E1B-55K–GFP in all cells, demonstrating that nuclear import was not affected by H5wt300 or H5dl355 infection (Fig. 2G and H). At least 30 transfected or infected cells were studied, and similar results were obtained in two independent experiments.
E1B-55K and E4orf6 shuttle in heterokaryon assays.
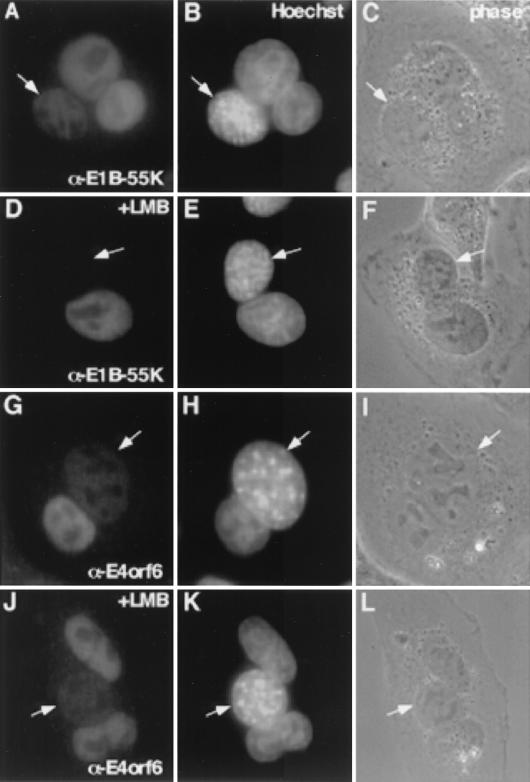
To address whether E1B-55K is capable of shuttling not only when expressed in trans but also when produced during adenovirus infection, we performed heterokaryon assays. HeLa cells were infected with H5wt300 at 30 PFU/cell, washed, and 8 h later seeded with uninfected mouse NIH 3T3 cells at a ratio of 1:3. Cells were cultured and fused 8 h later using polyethylene glycol (PEG 1500; Roche Diagnostica GmbH) as described previously (33). Briefly, cells were washed with phosphate-buffered saline, and 2 ml of PEG was added for 2 min. Subsequently, the cells were extensively washed with phosphate-buffered saline and further incubated at 37°C. Cycloheximide (50 μg/ml) was added 30 min prior to fusion and was present throughout the experiments to prevent new protein synthesis. At various time points, the cells were fixed and stained with the anti-E1B-55K monoclonal antibody 2A6 (28) (Fig. 3). To discriminate between infected human donor and uninfected mouse acceptor nuclei, staining was performed with Hoechst 33258 (Molecular Probes) (1 μg/ml) for 15 min. As already presented in various reports (1, 7, 17), Hoechst dye produces a more punctate staining of mouse nuclei (Fig. 3) than of HeLa nuclei. Figure 3A and B illustrate that virus-produced E1B-55K was exported from the donor and imported into the mouse acceptor nuclei as early as 30 min after fusion, indicative of active nucleocytoplasmic shuttling. Staining of heterokaryons with the E4orf6-specific monoclonal antibody RSA3 (19) also detected E4orf6 in the acceptor nuclei (Fig. 3E and F), as reported for uninfected cells (6, 8, 20). In contrast to the efficient export of E1B-55K (Fig. 3A), the cells had to be incubated for at least 1 h to detect E4orf6 in the acceptor nuclei. As a control, incubation of the fused cells at 4°C resulted in no detectable accumulation of E1B-55K or E4orf6 (data not shown). Expression of late viral proteins was verified by staining with a monoclonal antihexon antibody, indicative of active late mRNA transport and translation at 16 h p.i. (data not shown).
FIG. 3.
E1B-55K but not E4orf6 shuttles via the CRM1 export pathway during virus infection. HeLa cells infected with H5wt300 were fused with uninfected mouse NIH 3T3 cells using PEG and fixed at the indicated times postfusion. Alternatively, cells were incubated with LMB (10 nM final concentration) for 30 min prior to fusion, and LMB was present after fusion. Staining was performed with monoclonal antibodies against E1B-55K or E4orf6. E1B-55K (A and B) (30 min postfusion) as well as E4orf6 (G and H) (1 h postfusion) were detectable in the acceptor nuclei (arrows). Whereas E1B-55K export was blocked by LMB treatment (D and E) (1 h postfusion), E4orf6 was still detectable in the acceptor nuclei (J and K) (1 h postfusion). Human donor and mouse acceptor nuclei were discriminated by Hoechst staining (B, E, H, and K). To control for heterokaryon formation, phase-contrast images of the respective areas are shown (C, F, I, and L).
Shuttling of E1B-55K is mediated by active CRM1-mediated export, whereas E4orf6 transport appears to be nonspecific.
To discriminate between nonspecific transport and NES-mediated export, we performed heterokaryon assays in the presence of LMB. LMB specifically binds and inactivates the NES export receptor CRM1 (9, 13). Prior to fusions the cells were incubated with LMB (10 nM final concentration) and cycloheximide (50 μg/ml) for 30 min, and the drugs were present after fusion. One hour postfusion, the cells were fixed and stained for E1B-55K or E4orf6. Interestingly, whereas E1B-55K export was blocked by LMB treatment (Fig. 3C and D), E4orf6 was still detectable in the acceptor nuclei (Fig. 3G and H). This experimental approach allowed us to uncouple the transport of E1B-55K and E4orf6 and to demonstrate that E1B-55K was actively exported via the CRM1 pathway even in the course of an adenovirus infection. The smaller (34-kDa) E4orf6 protein appeared to be transported by a nonspecific mechanism, as already reported for other small proteins (2, 5, 33). Ten different syncytia were examined for each fusion, and similar results were obtained in two independent experiments.
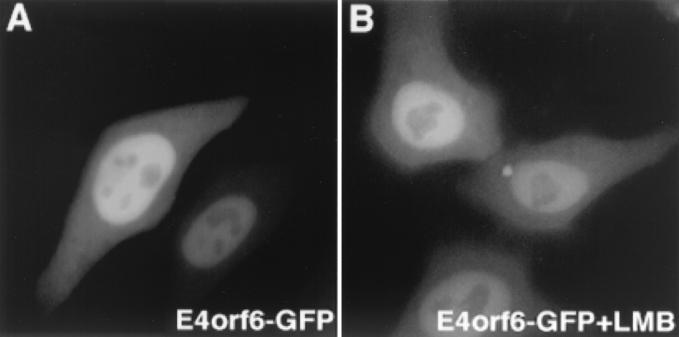
After completion of this work, Rabino and colleagues reported a only partial inhibition of E4orf6 shuttling by LMB in heterokaryon assays (23). Although we did not observe this inhibition in our assay 1 h after fusion, this report supports our observations on E4orf6 export. Since LMB binds and inactivates CRM1 irreversibly (13), one would expect a complete inhibition of E4orf6 export if transport is mediated by a CRM1-dependent NES. One could speculate that the prolonged LMB-PEG treatment (2.5 h, used by Rabino et al.) may have nonspecifically affected the cells in terms of the cytotoxic side effects of LMB, explaining the partial inhibition. On the other hand, we found no rationale why E4orf6 transport should be affected by LMB, since the suggested NES seemed to be inactive (12) and an alternative active leucine-rich NES has not yet been identified. To further clarify the LMB-sensitive trafficking of E4orf6, we expressed E4orf6 as a GFP hybrid, which allows highly sensitive detection in living cells (12). In transient transfections, E4orf6-GFP localized primarily in the nuclei of HeLa cells, although the protein was also detectable in the cytoplasm (Fig. 4A). Assuming that E4orf6-GFP is constantly shuttling via the NES-mediated pathway, LMB treatment should result in complete nuclear accumulation by blocking export, as already demonstrated for a variety of other shuttle proteins (11, 12, 16, 31, 32). However, even treatment with LMB (20 nM final concentration) for over 2 h did not change the steady-state localization of E4orf6-GFP, indicating that the localization of E4orf6 is LMB insensitive and thus not mediated by a leucine-rich NES (Fig. 4B). To avoid artifacts caused by overexpression, we studied especially low-level-expressing cells. In addition, E4orf6-GFP transport could not be blocked by LMB in cell fusion assays. Of note, a E4orf6-GFP hybrid lacking the postulated NES displayed a similar intracellular localization and transport behavior, and incubation of the transfected cells for 6 h at 39°C did not significantly change the localization of the E4orf6-GFP or the E1B-55K–GFP hybrid protein (data not shown). The mechanism by which relatively small proteins can be transported between the nucleus and the cytoplasm in general and its biological significance for protein function awaits certainly further investigation.
FIG. 4.
E4orf6-GFP does not respond to LMB treatment. HeLa cells were transfected with the E4orf6-GFP expression plasmid (3 μg of plasmid DNA) and 16 h later observed by fluorescence microscopy. In living cells, E4orf6-GFP localized predominantly to the nucleus but was also detectable in the cytoplasm (A). LMB treatment for 2 h did not affect the intracellular distribution of E4orf6-GFP (B).
In order to understand the adenoviral mRNA transport machinery it is first essential to identify and characterize potential adenoviral shuttle proteins in the absence of other viral components. Subsequently, transport has to be further analyzed in combination with other viral proteins, especially during viral replication. Therefore, this study was undertaken to investigate the shuttling capability of the E1B-55K and E4orf6 proteins in the context of adenovirus infection. We found that not only the previously identified E1B-55K NES (12) but also the complete E1B-55K protein was actively exported by the CRM1-dependent pathway in adenovirus-infected cells. E4orf6, on the other hand, appeared to be transported via a nonspecific mechanism. Clearly, E4orf6 mediates essential functions during adenovirus infection, and various mutations in the E4orf6 protein have been shown to inactivate or alter these biological activities (12, 18, 20, 34). However, so far the observed effects could not be directly linked to nucleocytoplasmic transport. Our study also underlines the necessity of carefully characterizing a potential active shuttle protein. Cell fusion assays certainly provide important information but additional experimental approaches are required. A shuttle protein exported by the CRM1-dependent export pathway should meet the following criteria. First, export should be inhibited by LMB treatment. Second, mutations of essential leucine residues in the NES should abolish nuclear export. Third, the NES should be transferable to a heterologous protein and export should be assayed in the absence of import (e.g., by microinjection of the respective NES-containing recombinant substrate into the nucleus). So far, E1B-55K but not E4orf6 meets all these requirements even in adenovirus-infected cells (reference 12 and this report), suggesting that it is more likely to be the potential “driving force” for adenoviral late mRNA transport.
Recently, Rabino and colleagues (23) reported that adenoviral late mRNA transport and protein expression were not inhibited by excessive LMB treatment. This is surprising, since we observed a general cytotoxic effect of LMB after 5 to 6 h of treatment, whereas Rabino et al. found the expression of late viral proteins almost unaffected by LMB treatment for 12 h. Since the activity of different LMB preparations appears to vary and LMB is also a cytotoxic drug, we routinely test the LMB preparations prior to use. Treatment of cells transiently expressing the human T-cell leukemia virus type 1 Rex-GFP or the E1B-55K–GFP protein for 30 min resulted in complete nuclear accumulation of the GFP hybrid proteins by blocking nuclear export as reported elsewhere (11, 12). Therefore, the influence of LMB treatment on adenovirus late mRNA export may require reevaluation. On the other hand, the observation of Rabino and colleagues (23) emphasizes the fact that it is still unclear if and how adenovirus late mRNAs are actively export by adenoviral shuttle proteins and what the corresponding cis-acting RNA elements are. Certainly, the mRNA transport mechanism during adenovirus replication is less well understood than that in complex retroviruses, in which a single transport protein interacts specifically with a defined cis-acting RNA element (e.g., the Rev-RRE axis for human immunodeficiency virus type 1 [22]). Given the multifunctionality of the E1B-55K and E4orf6 proteins, there are also other essential steps in the adenoviral replication cycle and in the processes of cellular transformation where shuttling may be essential. There is therefore an urgent need for a recombinant type 5 adenovirus expressing a NES-deficient E1B-55K and/or E4orf6 protein, to investigate the impact of NES-mediated shuttling on mRNA transport and the adenovirus life cycle.
Acknowledgments
We thank Bernhard Fleckenstein for continuous support. Purified adenovirus type 5 and LMB were kindly provided by Walter Dörfler and the Novartis Research Institute Vienna, respectively.
This work was supported by the Deutsche Forschungsgemeinschaft, Johannes und Frieda Marohn-Stiftung, and the Wilhelm-Sander Stiftung.
REFERENCES
- 1.Amirand C, Viari A, Ballini J P, Rezaei H, Beaujean N, Jullien D, Kas E, Debey P. Three distinct sub-nuclear populations of HMG-I protein of different properties revealed by co-localization image analysis. J Cell Sci. 1998;111:3551–3561. doi: 10.1242/jcs.111.23.3551. [DOI] [PubMed] [Google Scholar]
- 2.Borer R A, Lehner C F, Eppenberger H M, Nigg E A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 3.Bridge E, Ketner G. Interaction of adenoviral E4 and E1b products in late gene expression. Virology. 1990;174:345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- 4.Bridge E, Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989;63:631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee S, Stochaj U. Diffusion of proteins across the nuclear envelope of HeLa cells. BioTechniques. 1998;24:668–674. doi: 10.2144/98244rr04. [DOI] [PubMed] [Google Scholar]
- 6.Dobbelstein M, Roth J, Kimberly W T, Levine A J, Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan X C, Steitz J A. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc Natl Acad Sci USA. 1998;95:15293–15298. doi: 10.1073/pnas.95.26.15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer N, Voss M D, Mueller-Lantzsch N, Grasser F A. A potential NES of the Epstein-Barr virus nuclear antigen 1 (EBNA1) does not confer shuttling. FEBS Lett. 1999;447:311–314. doi: 10.1016/s0014-5793(99)00313-0. [DOI] [PubMed] [Google Scholar]
- 9.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 10.Halbert D N, Cutt J R, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heger P, Rosorius O, Hauber J, Stauber R H. Titration of cellular export factors, but not heteromultimerization, is the molecular mechanism of trans-dominant HTLV-1 Rex mutants. Oncogene. 1999;18:4080–4090. doi: 10.1038/sj.onc.1202762. [DOI] [PubMed] [Google Scholar]
- 12.Krätzer F, Rosorius O, Heger P, Hirschmann N, Dobner T, Hauber J, Stauber R H. The adenovirus type 5 E1B–55K oncoprotein is a highly active shuttle protein and shuttling is independent of E4orf6, p53 and Mdm2. Oncogene. 2000;19:850–857. doi: 10.1038/sj.onc.1203395. [DOI] [PubMed] [Google Scholar]
- 13.Kudo N, Wolff B, Sekimoto T, Schreiner E P, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 14.Leppard K N. Regulated RNA processing and RNA transport during adenovirus infection. Semin Virol. 1998;8:301–307. [Google Scholar]
- 15.Leppard K N, Everett R D. The adenovirus type 5 E1b 55K and E4 Orf3 proteins associate in infected cells and affect ND10 components. J Gen Virol. 1999;80:997–1008. doi: 10.1099/0022-1317-80-4-997. [DOI] [PubMed] [Google Scholar]
- 16.McBride K M, McDonald C, Reich N C. Nuclear export signal located within the DNA-binding domain of the STAT1 transcription factor. EMBO J. 2000;19:6196–6206. doi: 10.1093/emboj/19.22.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser F G, Dorman B P, Ruddle F H. Mouse-human heterokaryon analysis with a 33258 Hoechst-Giemsa technique. J Cell Biol. 1975;66:676–680. doi: 10.1083/jcb.66.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevels M, Rubenwolf S, Spruss T, Wolf H, Dobner T. Two distinct activities contribute to the oncogenic potential of the adenovirus type 5 E4orf6 protein. J Virol. 2000;74:5168–5181. doi: 10.1128/jvi.74.11.5168-5181.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nevels M, Tauber B, Kremmer E, Spruss T, Wolf H, Dobner T. Transforming potential of the adenovirus type 5 E4orf3 protein. J Virol. 1999;73:1591–1600. doi: 10.1128/jvi.73.2.1591-1600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orlando J S, Ornelles D A. An arginine-faced amphipathic alpha helix is required for adenovirus type 5 E4orf6 function. J Virol. 1999;73:4600–4610. doi: 10.1128/jvi.73.6.4600-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ornelles D A, Shenk T. Localization of the adenovirus early region 1B 55-kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J Virol. 1991;65:424–429. doi: 10.1128/jvi.65.1.424-429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlakis G N, Stauber R H. Regulatory proteins of HIV-1. In: Saksena N, editor. Human immunodeficiency viruses: biology, immunology and molecular biology. Genoa, Italy: Medical Systems SpA; 1998. pp. 103–122. [Google Scholar]
- 23.Rabino C, Aspegren A, Corbin-Lickfett K, Bridge E. Adenovirus late gene expression does not require a Rev-like nuclear RNA export pathway. J Virol. 2000;74:6684–6688. doi: 10.1128/jvi.74.14.6684-6688.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reich N, Sarnow P, Duprey E, Levine A J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA binding protein. Virology. 1983;128:480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- 25.Rosorius O, Heger P, Stelz G, Hirschmann N, Hauber J, Stauber R H. Direct observation of nucleo-cytoplasmic transport by microinjection of GFP-tagged proteins in living cells. BioTechniques. 1999;27:350–355. doi: 10.2144/99272rr02. [DOI] [PubMed] [Google Scholar]
- 26.Rubenwolf S, Schutt H, Nevels M, Wolf H, Dobner T. Structural analysis of the adenovirus type 5 E1B 55-kilodalton-E4orf6 protein complex. J Virol. 1997;71:1115–1123. doi: 10.1128/jvi.71.2.1115-1123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarnow P, Hearing P, Anderson C W, Halbert D N, Shenk T, Levine A J. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J Virol. 1984;49:692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarnow P, Ho Y S, Williams J, Levine A J. Adenovirus E1B 58 kD tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kD cellular protein in transformed cells. Cell. 1982;28:387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- 29.Shenk T. Adenoviridae and their replication. In: Fields B, Knipe D, Howley P, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2111–2148. [Google Scholar]
- 30.Stauber R, Gaitanaris G A, Pavlakis G N. Analysis of trafficking of Rev and transdominant Rev proteins in living cells using green fluorescent protein fusions: transdominant Rev blocks the export of Rev from the nucleus to the cytoplasm. Virology. 1995;213:439–449. doi: 10.1006/viro.1995.0016. [DOI] [PubMed] [Google Scholar]
- 31.Stauber R H, Afonina E, Gulnik S, Erickson J, Pavlakis G N. Analysis of intracellular trafficking and interactions of cytoplasmic HIV-1 Rev mutants in living cells. Virology. 1998;251:38–48. doi: 10.1006/viro.1998.9295. [DOI] [PubMed] [Google Scholar]
- 32.Stauber R H, Krätzer F, Schneider G, Hirschmann N, Hauber J, Rosorius O. Investigation of nucleo-cytoplasmic transport using UV-guided microinjection. J Cell Biochem. 2001;80:388–396. doi: 10.1002/1097-4644(20010301)80:3<388::aid-jcb130>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Stauber R H, Pavlakis G N. Intracellular trafficking and interactions of the HIV-1 Tat protein. Virology. 1998;252:126–132. doi: 10.1006/viro.1998.9400. [DOI] [PubMed] [Google Scholar]
- 34.Weigel S, Dobbelstein M. The nuclear export signal within the E4orf6 protein of adenovirus type 5 supports virus replication and cytoplasmic accumulation of viral mRNA. J Virol. 2000;74:764–772. doi: 10.1128/jvi.74.2.764-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]