Abstract
Background:
Artificial urinary sphincter (AUS) placement remains the gold-standard treatment for post-prostatectomy urinary incontinence (PPUI), despite their need for periodic surgical revision.
Objective:
To understand the experiences of patients who undergo repeat AUS revisions.
Design:
Mixed design including quantitative surveys and qualitative interviews for thematic analysis.
Methods:
Men with ⩾2 revisions were collected from a single-institution, retrospective database of AUS patients. Participants were interviewed about their prostatectomy, incontinence, AUS placement, and revisions. A survey was administered utilizing validated tools (e.g., Decision Regret Scale (DRS), Incontinence Impact Questionnaire-7) for quantitative analysis. Interview transcripts were used for qualitative thematic analysis.
Results:
Of 26 respondents, 20 completed the interview. Twenty-three men completed the survey. The mean DRS score for prostatectomy was 24 (standard deviation (SD) = 27), indicating low regret. Median Incontinence Impact Questionnaire score was 54 (SD = 27), with 70% of participants describing their PPUI as “severe.” Participants experienced a significant decrease in daily pad usage with AUS placement (5.5 pre-AUS vs 1.4 post-AUS, p < 0.0001). Qualitative analysis revealed themes involving prostatectomy urgency, physician–patient relationships, expectation setting, and quality of follow-up. Most participants (96%) were satisfied with their initial AUS placement and endorsed a positive relationship with their urologist. However, 22% of participants were unaware of device limitations, including the need for revision. Some participants (26%) were uncertain of the status of their AUS, while some participants (35%) desired improved follow-up.
Conclusions:
Initial improvement and positive experiences with urologists motivate patients to undergo AUS repeat revision. Urologists should emphasize the limitations of the AUS before placement and follow up with patients to evaluate their needs for future care.
Keywords: AUS, post-prostatectomy urinary incontinence, qualitative, quality of life
Introduction
Prostatectomy, the dominant treatment option for prostate cancer, is a known cause of secondary post-prostatectomy urinary incontinence (PPUI). Although rates vary in the literature, PPUI affects an estimated 2%–65% of men long term.1,2 The detrimental symptoms of PPUI, characterized by involuntary urinary leakage upon exertion, significantly decrease the quality of life. 3 There are complex social, personal, and medical impacts of PPUI, which also renders decision-making for treatment equally as complicated.
The gold-standard treatment for post-prostatectomy stress urinary incontinence is the artificial urinary sphincter (AUS). Up to 80% of patients who undergo AUS placement will achieve continence. 4 Despite its safe and efficacious usage for the treatment of PPUI in men who have undergone a prostatectomy, several surgical and mechanical complications can occur, including infection, mechanical device failure, tissue erosion, and pump placement irritation. 5 Men who experience failure for these reasons can opt into undergoing one or more revision surgeries. These revision surgeries occur in approximately 8%–45% of AUS recipients.6,7
Though it is clear why patients may want to explore PPUI treatment options such as AUS placement, current literature does not yet characterize the complex experience of patients who seek repeated revision procedures. The emotional, physical, and financial implications of multiple revision procedures to treat male PPUI following prostatectomy demonstrate the need for further inquiry. The purpose of this study is to evaluate the longitudinal patient experience, from prostate cancer diagnosis and treatment through repeat AUS revision. In addition, we hope to identify the motivations for patients who elect to undergo 2+ AUS revision procedures. This will improve the understanding of patients’ decisions to undergo repeat AUS revision surgeries and promote patient-centered care for PPUI.
Methods
Participant collection
All participants were collected from a single-institution, retrospective database of patients who have undergone AUS placement or revision with seven surgeons between 1997 and 2023. The practice setting was a university-based academic institution. All living adult men who have undergone ⩾2 revisions were contacted via phone for interviews. Participants were offered a $150 gift card for participation in the long-form interview and a $10 gift card for participation in the short-form survey. This study and its design were IRB-approved (IRB00308729). Informed consent was obtained from all participants prior to the conduct of interviews.
Study design
This study consists of an open-ended, long-form interview in addition to a short-form quantitative survey. Interviews and surveys were recorded using the Rev call recording application (San Francisco, CA, USA). Long-form surveys were administered over the phone in an approximately 45-min conversational interview. The study team prepared topics that covered the major phases of urologic care that these participants experienced, including prostate cancer diagnosis and treatment, post-prostatectomy incontinence, initial AUS placement, and repeat AUS revision. However, participants were given the freedom to guide the conversation to whatever was most important to them. This allowed room for participants to open up about personal topics such as emotional and psychological well-being, personal relationships, values, self-confidence, struggles, successes, and how they might envision their future with their device.
The short-form, quantitative survey was carried out via a 10-min phone call and begins with preliminary questions to better understand the participant’s journey with their AUS device. After verifying AUS revision history, each participant was surveyed on whether they felt that their decision to pursue revision was informed and confident. Participants are also asked about leakage levels and pad usage when their devices are functional/malfunctioning. The Decision Regret Scale (DRS), a validated research tool, was used to quantify participant distress or remorse about their prostate cancer treatment that caused their incontinence. 8 The Incontinence Impact Questionnaire-7 (IIQ-7) was used to quantify life quality and symptom distress in participants with incontinence. 9 Participants of the long-form interview also completed the short-form interview at the end of their encounter.
In addition to both the DRS and the IIQ-7, the study team generated a list of five possible motivations for pursuing repeat revisions and asked participants to identify which, if any, were motivations for them. These include (1) incontinence recurrence, (2) hope for incontinence improvement, (3) sunken cost (time, money, and resources already put into the treatment journey), (4) surgeon recommendation, and (5) previous successes. These were created internally and based on anecdotal experiences with previous patients. Participants were encouraged to provide other motivators for pursuing revision outside of these options.
Statistical analysis
Qualitative thematic analysis of long-form interviews was done using a combination of deductive and inductive coding of interview transcripts. Thematic analysis was done according to procedures previously published and widely used in qualitative research. 10 Recordings of participant interviews were transcribed using the Otter.ai transcription service (Mountain View, CA, USA). These transcriptions were then uploaded to Dedoose software (Los Angeles, CA, USA) for line-by-line coding. Codes were then organized by stage of treatment (e.g., prostatectomy, initial PPUI) as well as overarching concepts (e.g., related to physician-patient relationships). Codes with similar meanings were then grouped into themes. Themes were categorized by phase of AUS care, including prostate cancer treatment, PPUI, initial AUS placement, AUS Revision, and Future of AUS care. Certain themes about physician–patient relationships were also grouped. Finally, certain miscellaneous themes were included that did not fit these categories.
Reporting of the qualitative research is done using the consolidated criteria for reporting qualitative research (COREQ) guidelines. 11 A detailed explanation of these criteria is included in the Appendix.
Quantitative analysis was done for the short-form survey responses. Demographic data were assessed. Results from the DRS and IIQ-7 were analyzed using the tools’ respective scoring methods. Linear regression was done to assess the relationship between DRS and pad usage and the number of revisions. Self-reported pre- and post-AUS incontinence severity and pad usage were compared using paired t-tests. Data were analyzed using Microsoft Excel (Redmond, WA, USA). A p-value of 0.05 was considered significant.
Participant recruitment
Participants who had ⩾2 were included in this study to simultaneously evaluate participant experience with PPUI and initial AUS placement, as well as long-term care associated with device maintenance. Records of ⩾2 AUS revisions existed for 91 patients. Patients with device failure of any type (e.g., mechanical failure, erosion, infection) were included in participation. Certain patients were excluded due to death since the last visit (n = 4), their surgeon not participating in the study (n = 16), and cognitive impairment preventing recollection of their medical history (n = 2). In all, 43 participants were unable to be reached. Of the 26 respondents, 3 (12%) were not interested in participating, 3 (12%) completed the short-form questionnaire, and 20 (77%) completed both the long-form interview and short-form questionnaire. The mean long-form interview time was 38 min. Participant demographics are shown in Table 1. Table 2 shows a breakdown of the time between initial placement and revisions as well as the reason for revisions.
Table 1.
Participant demographics.
| Demographic | |
|---|---|
| Age, mean (SD) | 77 (7) |
| Number of AUS revisions, mean (SD) | 2.9 (.9) |
| Race | |
| White, n (%) | 18 (78) |
| Black, n (%) | 4 (18) |
| Asian, n (%) | 1 (4) |
| Education | |
| High school, n (%) | 3 (13) |
| Some college, n (%) | 5 (22) |
| Associate’s degree, n (%) | 1 (4) |
| Bachelor’s degree, n (%) | 3 (14) |
| Master’s degree, n (%) | 5 (22) |
| Doctorate degree, n (%) | 6 (26) |
| Marital status | |
| Single, n (%) | 2 (9) |
| Married, n (%) | 21 (91) |
| Able to complete activities of daily living independently, n (%) | 23 (100) |
AUS, artificial urinary sphincter; SD, standard deviation.
Table 2.
Characteristics of placement and revision.
| Placement | |
|---|---|
| Primary, n (%) | 17 (74) |
| Secondary, n (%) | 6 (26) |
| Revision 1 | |
| Mean time until revision, years (SD) | 3.6 (3.2) |
| Revision reason | |
| Mechanical, n (%) | 12 (60) |
| Erosion, n (%) | 5 (25) |
| Infection, n (%) | 0 (0) |
| Other, n (%) | 3 (15) |
| Revision component | |
| Partial, n (%) | 14 (78) |
| Total, n (%) | 4 (22) |
| Revision 2 | |
| Mean time until revision, years (SD) | 3.1 (3.0) |
| Revision reason | |
| Replacement, n (%) | 2 (9) |
| Mechanical, n (%) | 14 (64) |
| Erosion, n (%) | 2 (9) |
| Infection, n (%) | 2 (9) |
| Other, n (%) | 2 (9) |
| Revision component | |
| Partial, n (%) | 15 (71) |
| Total, n (%) | 6 (29) |
| Revision 3 | |
| Mean time until revision, years (SD) | 3.6 (3.23) |
| Revision reason | |
| Replacement, n (%) | 2 (22) |
| Mechanical, n (%) | 4 (44) |
| Erosion, n (%) | 2 (22) |
| Infection, n (%) | 1 (11) |
| Other, n (%) | 0 (0) |
| Revision component | |
| Partial, n (%) | 4 (44) |
| Total, n (%) | 5 (56) |
Not all surgical records were available for all patients, percentages and averages were based on available data for each variable. Primary: first procedure done for PPUI. Secondary: previous AUS placement, sling, or bulking done at an outside institution. Revision reason refers to mechanical failure of the device (e.g., cuff leak, tubing breakdown), urethral erosion under the cuff, infection of the device, or replacement of a previously explanted device. Revision component refers to total device replacement or of individual device components.
AUS, artificial urinary sphincter; SD, standard deviation.
Results
Quantitative survey results
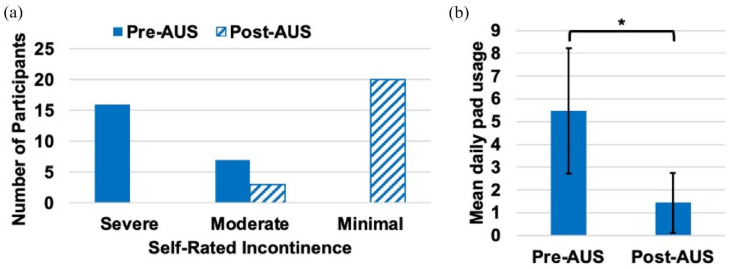
The mean DRS score was 24 (standard deviation (SD) = 27), indicating low regret for undergoing prostatectomy. There was no association between DRS score and pad usage (p = 0.2) or number of AUS revisions (p = 0.5) on linear regression. The mean IIQ-7 score was 54 (SD = 27), indicating the participants’ daily lives were moderately impacted by PPUI. Figure 1(a) shows participant’s self-rating of incontinence before and after implantation of their primary AUS. Change in daily pad usage is shown in Figure 1(b). In all, 22 (96%) participants found the AUS to be helpful and 18 (78%) participants had a functioning device at the time of study. Two participants have undergone subsequent permanent suprapubic cystostomy placement, eliminating the utility of any current or future AUS device. Of the remaining 21 participants, 17 (81%) currently use their device.
Figure 1.
Effect of primary AUS placement on incontinence. (a) Change in self-rated incontinence pre- and post-AUS. (b) Mean daily self-reported pad usage pre- and post-AUS. Error bars show SD, *p < 0.0001.
AUS, artificial urinary sphincter; SD, standard deviation.
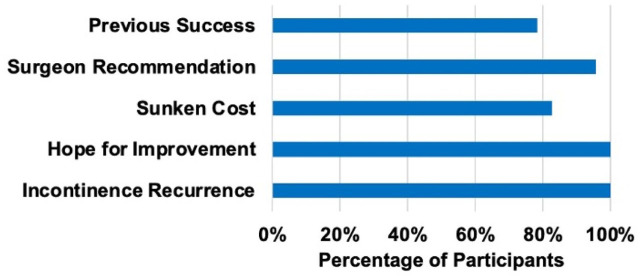
Of 23 participants, 22 (96%) felt they had all the information necessary to make an informed decision on whether to undergo their AUS revisions, and 23 (100%) were confident in that decision. Only 2 (9%) participants regretted the decision to undergo multiple revisions. Of 21 possible participants, all (100%) would consider undergoing AUS revision again in the future, if appropriate. Participants were read a list of potential motivators to pursue repeat revisions; the percentage of participants who agreed with each motivator is shown in Figure 2.
Figure 2.
Percentage of participants who agreed with five possible motivators for pursuing repeat AUS revisions. Previous success refers to improvement following previous AUS placements/revisions. Sunken cost refers to the idea that the participant did not want to lose the progress that he had already made with his previous surgeries.
AUS, artificial urinary sphincter.
Thematic analysis
Table 3 shows major themes and exemplifying excerpts that were discussed in multiple interviews, separated by stage of medical care (i.e., prostate cancer treatment, PPUI, primary AUS placement, AUS revision, future of AUS care, and physician-patient relationships throughout care). Miscellaneous themes that do not fit in a particular stage of care are also shown. The number of interviews in which each theme was present is listed Table 3.
Table 3.
Major themes present in interview transcripts.
| Theme | Number of interviews (%) | Participant ID | Transcript excerpt |
|---|---|---|---|
| Prostate cancer treatment | |||
| 1. Urgency to physically remove the cancerous prostate after diagnosis | 10 (50) | 32 | I mean, I wanted it out the next day. . .I was told to come in Monday morning, after Thanksgiving, with my wife, and we went through a couple of different options. And I made the mistake by waiting five seconds to say take it out, which was one of the options. |
| 2. Prioritization of life-saving cancer treatment over the risk of subsequent incontinence | 5 (25) | 67 | I was more concerned with the cancer and just getting rid of it, at least my wife was. And I was aware of [the risks], but I’m willing to take my chances, you know? |
| Post-prostatectomy stress urinary incontinence | |||
| 3. Lack of understanding of the risk of incontinence following prostatectomy | 12 (60) | 91 30 |
No, I didn’t know any of [the risks]. I didn’t know, I didn’t know that I would have any problem. I thought everything would be fine until afterwards. But we talked about pads and the need for one or two pads a day, or three and how many pads that kind of thing. But we did have discussions about that. But at the time, I probably wasn’t hearing those discussions, because you know, I’ve never had any incontinence problems ever. |
| 4. PPUI impacted participants’ lives | 17 (85) | 36 | It ruins your life. Basically, they save your life, and then they ruin it in the same process |
| Initial AUS placement | |||
| 5. Delay in PPUI treatment | 4 (20) | 67 | I never got the information there that artificial sphincter was a possibility. And I probably would have gone to see [my urologist] sooner had I known that that possibility existed. |
| 6. Lack of awareness of the AUS limitations (e.g., finite lifespan, need for revision) | 5 (25) | 55 | I would not hold back on the cons, because, you know, for me, finding out the cons be, you know, on my own, totally pissed me off, because I felt I should have been told. |
| 7. Satisfaction with primary AUS results | 17 (85) | 66 | Hey, I loved it. I thought it was the greatest thing since sliced bread. |
| AUS revision | |||
| 8. Recurrence of incontinence drove revision, with continued leakage between revision | 9 (45) | 82 | It leaked. And then I went back to [my urologist] and he did something else. And when I got I’ve got out of the way after I healed back up. It worked pretty good. But never like it did in the beginning. And then and then a year and then gradually it went back to leaking again. And then I went back to [my urologist] and he fixed it again. And it lasted a month or two and it leaked and I went back the third time I think and when I got when he got done, it didn’t work at all. It was worse than when I started |
| 9. Lack of awareness that device failure and revision were possible | 6 (30) | 91 | Had I realized that it would go bad, and that I would, at some point, need to have it replaced? Had I known that I could have saved myself, I don’t know how many years. Maybe two or three years of agony if I just had known that. And I would have immediately had it taken care of and I wouldn’t have had to go through all of these infections and stuff. |
| 10. Mixed results following AUS revision. Six participants expressed disappointment, 11 expressed satisfaction, and 2 had mixed feelings | 19 (95) | 68 66 |
Life was good again afterwards. That one was a very disappointing surgery, because that was, I mean, that one didn’t work. |
| AUS future | |||
| 11. Uncertainty as to what options were for future AUS care | 6 (30) | 63 | I’ve been still having problems, and I, I don’t know if there’s anything else that can be done. . . I sort of got a sense after this last revision, that that was the last thing he thought he could do. But like I say, I haven’t really communicated with them. And maybe that’s not correct. |
| 12. Desire for increased follow-up with their surgeons | 8 (40) | 46 | I thought there might have been some follow up that happens over the years. Just to see if I was doing okay, or not, but I have no idea if, the thing is, is that if there’s anything else I should be doing. |
| 13. Interest in attempting further surgery if there were improvements made in the AUS device | 2 (10) | 1 | Yeah, I’d be the first one to go in line. I don’t mind the third operation. If there’s a new device, you know what I’m saying? I would gamble. Absolutely. Tell me we gotta put the same device and blah, blah, blah. |
| Physician–patient relationship | |||
| 14. Spending time on patient education during AUS consultations improved the physician–patient relationship | 10 (50) | 32 | He’s as nice as he can be. I’ve learned that the better the doctor is, the more humble they are. And when I had the consultation with [my doctor], he explained everything to me, took all the time in the world. And, you know, told me what to do, or what he was going to do. |
| 15. Distrust of the surgeon was an obstacle preventing further surgery | 5 (25) | 82 | I think they were all in a hurry. I think they all were ready to go home. And by the end the whole thing was botched. And [my urologist] didn’t say it but when I went back to have it fixed, he said “we’re gonna do it right this time.” Which led me to believe something was botched up. And I laid there all day waiting to be operated on. It was a disaster. . . But anyway, in fact, I’m mentally looking for another urologist. |
| Miscellaneous themes | |||
| 16. Hesitation for further surgery with age | 2 (10) | 1 | I’m in my 70s now. I don’t want the surgery to get into trouble and make it so my lifespan is shorter than this. So, there’s a kind of severe anxiety for that third surgery unless it’s new devices |
| 17. Desire for a community of patients who were experiencing similar medical issues | 7 (35) | 55 | It’s good to know that you’re not the only one out there that has this this problem, you know? |
| 18. Supplementation of physician-provided information with independent research was helpful | 10 (50) | 66 | I did a quite a bit of reading on my own too. I mean, I know I read [my doctor’s] book. And I mean, I did a lot of research. So, you know, in addition to what doctors tell me, I’m one that does a lot of independent research and like to verify things that I’m told. So I was well informed. |
| 19. AUS could complicate other medical procedures | 4 (20) | 46 | When you go in for other medical tests, be it MRI or CT scan or something? The explanation to the technician and to the medical team, you know, to be careful. Be careful with Foley operations. Don’t put a Foley in there. |
AUS, artificial urinary sphincter; CT, computed tomography; MRI, magnetic resonance imaging.
Discussion
Our study found that patients were glad to engage in life-saving prostate cancer treatment and found success in primary AUS placement, which motivated them to return for revision. Participants were eager to treat their PPUI but found that long-term AUS care had both advantages and disadvantages that required personalized consideration along with their urologist. To our knowledge, this study is the first to employ quantitative and qualitative methods to evaluate these complex experiences in this patient population.
Through exploration of patient experiences starting with prostate cancer treatment, we found that some patients wished they had found the AUS earlier (Theme 5). Their PPUI was significant enough to warrant intervention (Theme 4), but they may have been unaware that this intervention existed before meeting with a reconstructive urologist at this institution. Increasingly, it is recognized that prostate cancer patients deserve care long after the immediate postoperative period. 12 Indeed, AUA guidelines state that continued symptom management after prostatectomy, including PPUI, should be part of comprehensive prostate cancer treatment. 13 That said, Watson et al. found that only 31% of patients with moderate/severe leakage issues were referred to a specialist, and only 73% were able to discuss this issue with their oncologist postoperatively. 14 Our findings demonstrate that delay in PPUI treatment can lead to increased distress in this patient population. Incorporating reconstructive urologists into multidisciplinary prostate cancer teams may streamline care for patients suffering from PPUI.
This may also encourage shared decision-making (SDM), recently identified as a factor in reducing decisional regret for those with leakage. 15 The essence of SDM, in which healthcare choices are made by patients and their providers as a team, incorporates evidence on the risks and benefits of procedures with patient values. 16 Hampson et al. utilized a quantitative survey to quantify SDM in their participants’ healthcare experiences to demonstrate that SDM benefits patients in this population. 15 Our paper expands on Hampson et al. by qualitatively defining aspects of SDM that were important to these patients to determine clinical applications of that finding.
Specifically, our participants highlighted the importance of thorough patient education during consultations is paramount to treatment satisfaction. Physician-driven patient education, an important part of SDM, improved the physician–patient relationship (Theme 14) and served as a major motivator for patients to trust surgeon recommendations for repeat revisions. Furthermore, our participants expressed disappointment when tenets of SDM were not properly incorporated into consultations, such as expectation setting and explanation of potential treatment disadvantages (Themes 3 and 6). Urologists should intentionally discuss the limitations of the AUS in treating PPUI, including the need for revision, so patients can properly engage in SDM and minimize regret. Studies have shown that incorporating the use of written, audiovisual, interactive digital, verbal feedback, and multicomponent interventions may improve risk comprehension and recall.17–21 Future work could focus on the development of a decision-making tool to aid urologists in properly preparing their patients for postoperative events that make imminent revision less shocking.
In addition, the findings of this study support close and continued follow-up of repeat AUS patients, which some patients expressed as an unmet need in their care (Theme 12). This would be helpful in promptly addressing patients in which incontinence reoccurs (Theme 8), as well as those who are uncertain about their device status and/or whether there is anything that can be done to improve their recurrence symptoms (Theme 11). Urologists may be responsible for patients who have these devices implanted and revised, especially as revised devices are more likely to fail. 22 Uncertainty of device status due to loss of follow-up could prove dangerous to patients who require catheterization, cystoscopy, or salvage radiation by other providers who are less familiar with the device (Theme 19). 23 One possible strategy to improve follow-up procedures without overburdening patients, physicians, and hospital systems would be to incorporate asynchronous, telehealth questionnaires, which have proven useful to surgeons in other specialties. 24 Providing AUS patients with scheduled, physician-driven contact could help them remain cognizant of their devices and the possibility of long-term care.
We acknowledge the limitations of our study. First, the sample size was limited by the number of patients at our institution. The study team attempted to contact each potential participant multiple times to maximize participation, ultimately completing a satisfactory number of long-form interviews to complete a content analysis. Still, these findings may certainly be impacted by failure cause, cultural, racial, ethnic, educational, or socioeconomic factors, but given low participant numbers no stratification was appropriate. Volunteer bias, in which participants with uniquely positive or negative experiences were more likely to participate, was considered. Likewise, we did not elect to contact individuals who did not require multiple revisions, nor made other choices for treatment of their PPUI. Future studies including these patients could help provide further insight into the diverse PPUI patient experience. Using neutral language in scripted questions and being careful to collect information on positive and negative aspects of care were done to help minimize this bias. The patients’ responses were subject to recall bias, and no objective check was performed to corroborate their reported level of leakage. Finally, because multiple team members conducted interviews, a questionnaire script and interview outline were developed to help maintain uniformity throughout. We believe that all major limitations were properly addressed to maximize the generalizability of our findings.
Conclusion
Initial improvement in leakage and positive experiences with urologists motivate patients to undergo AUS repeat revision. Urologists should emphasize the limitations and lifespan of the AUS before initial placement and follow up with patients to evaluate the needs for future care.
Acknowledgments
None.
Appendix
Appendix Table 1.
COREQ guidelines.
| Domain 1: Research team and reflexivity | |
|---|---|
| Personal characteristics | |
| 1. Interviewer/facilitator | (a) Phillip Huffman, (b) Gabriella Ewachiw, (c) Ryan Johnson |
| 2. Credentials | (a) BA, (b) n/a, (c) BA |
| 3. Occupation | (a) Medical student, (b) undergraduate student and research assistant, (c) post-baccalaureate student and research assistant |
| 4. Gender | (a) Male, (b) female, (c) male |
| 5. Experience and training | (a) Medical school, (b) n/a, (c) n/a. All interviewers were educated on the purpose of the AUS, the surgical procedure of implantation and revision, and perioperative complication risks. This was done through a literature review and didactic education. |
| Relationship with participants | |
| 6. Relationship established | No previous relationships were established with the participants. |
| 7. Participant knowledge of the interviewer | Interviewers introduced themselves and their role on the study team to all participants. Study goals were detailed in the recruitment phase. |
| 8. Interviewer characteristics | Interviewers’ interests in urology were provided to the participants. |
| Domain 2: study design | |
| Theoretical framework | |
| 9. Methodological orientation and Theory | Thematic analysis |
| Participant selection | |
| 10. Sampling | All possible participants were contacted (i.e., consecutive sampling) |
| 11. Method of approach | Telephone with 2 attempts to contact |
| 12. Sample size | 23 |
| 13. Non-participation | 3 potential participants declined to participate |
| 14. Setting of data collection | Variable (home, clinic, workplace). |
| 15. Presence of non-participants | No |
| 16. Description of sample | See Table 2 for participant demographics. Interviews were collected in two rounds: one in the spring of 2022 and one in the spring of 2023. |
| Data collection | |
| 17. Interview guide | Questions included in the short-form survey, as well as major themes included in the long-form survey, are provided by the authors. The survey was not pilot-tested. |
| 18. Repeat interviews | No |
| 19. Audio/visual recording | Use of audio recording (Rev iOS software) |
| 20. Field notes | Field notes made during the interview |
| 21. Duration | 30–60 min |
| 22. Data saturation | Not discussed |
| 23. Transcripts returned | No |
| Domain 3: analysis and findings | |
| Data analysis | |
| 24. Number of data coders | One |
| 25. Description of coding tree | Description of the coding process provided |
| 26. Derivation of themes | Themes derived from data collected |
| 27. Software | Dedoose |
| 28. Participant checking | Participants were not provided a copy of their transcript |
| Reporting | |
| 29. Quotations presented | Yes, with the participant number provided |
| 30. Data and findings consistent | Yes |
| 31. Clarity of major themes | Major themes clearly presented in the findings |
| 32. Clarity of minor themes | Minor themes described and discussed |
AUS, artificial urinary sphincter; COREQ, consolidated criteria for reporting qualitative research.
Footnotes
ORCID iD: Phillip J. Huffman  https://orcid.org/0000-0003-4736-9430
https://orcid.org/0000-0003-4736-9430
Contributor Information
Phillip J. Huffman, The Brady Urological Institute at JHBMC, 4940 Eastern Avenue, 301 Building, Suite 3100, Baltimore, MD 21224, USA.
Gabriella Ewachiw, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Ryan Johnson, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Mitchell M. Huang, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA
Hasan Dani, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Pedro G. Knijnik, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA
Arthur F. da Silva, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA
Arthur L. Burnett, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA
Jacek L. Mostwin, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA
Edward J. Wright, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA
Andrew J. Cohen, James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA
Declarations
Ethics approval and consent to participate: All participants were read a verbal informed consent agreement and verbally consented to participate. This study, including the informed consent, was approved by the Johns Hopkins IRB (IRB00308729).
Consent for publication: All participants consented for their responses to be published during the verbal consent process.
Author contributions: Phillip J. Huffman: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Gabriella Ewachiw: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Ryan Johnson: Data curation; Methodology; Writing – original draft; Writing – review & editing.
Mitchell Huang: Data curation; Formal analysis; Writing – review & editing.
Hasan Dani: Data curation; Resources; Writing – review & editing.
Pedro Knijnik: Data curation; Writing – review & editing.
Arthur da Silva: Data curation; Writing – review & editing.
Arthur L. Burnett: Data curation; Supervision; Writing – review & editing.
Jacek L. Mostwin: Data curation; Supervision; Writing – review & editing.
Edward Wright: Data curation; Supervision; Writing – review & editing.
Andrew J. Cohen: Conceptualization; Formal analysis; Funding acquisition; Methodology; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the incentive provided for participation was generously gifted by the Oliver and Harriet Sockwell Foundation.
The authors declare that there is no conflict of interest.
Availability of data and material: Data from quantitative surveys were provided. Direct quotes from interview transcripts are provided in the manuscript according to COREQ guidelines.
References
- 1. Alivizatos G, Skolarikos A. Incontinence and erectile dysfunction following radical prostatectomy: a review. ScientificWorldJournal 2005; 5: 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen Y-C, Lin P-H, Jou Y-Y, et al. Surgical treatment for urinary incontinence after prostatectomy: a meta-analysis and systematic review. PLoS One 2017; 12: e0130867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silva LA, Andriolo RB, Atallah ÁN, et al. Surgery for urinary incontinence due to presumed sphincter deficiency after prostate surgery. Cochrane Database Syst Rev 2014; 2014(9): CD008306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van der Aa F, Drake MJ, Kasyan GR, et al. The artificial urinary sphincter after a quarter of a century: a critical systematic review of its use in male non-neurogenic incontinence. Eur Urol 2013; 63: 681–689. [DOI] [PubMed] [Google Scholar]
- 5. Srivastava A, Joice GA, Patel HD, et al. Causes of artificial urinary sphincter failure and strategies for surgical revision: implications of device component survival. Eur Urol Focus 2019; 5: 887–893. [DOI] [PubMed] [Google Scholar]
- 6. Averbeck MA, Woodhouse C, Comiter C, et al. Surgical treatment of post-prostatectomy stress urinary incontinence in adult men: report from the 6th International Consultation on Incontinence. Neurourol Urodyn 2019; 38: 398–406. [DOI] [PubMed] [Google Scholar]
- 7. Fuller TW, Ballon-Landa E, Gallo K, et al. Outcomes and risk factors of revision and replacement artificial urinary sphincter implantation in radiated and nonradiated cases. J Urol 2020; 204: 110–114. [DOI] [PubMed] [Google Scholar]
- 8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Mak Int J Soc Med Decis Mak 2003; 23: 281–292. [DOI] [PubMed] [Google Scholar]
- 9. Moore KN, Jensen L. Testing of the Incontinence Impact Questionnaire (IIQ-7) with men after radical prostatectomy. J Wound Ostomy Continence Nurs 2000; 27: 304–312. [DOI] [PubMed] [Google Scholar]
- 10. Moser A, Korstjens I. Series: practical guidance to qualitative research. Part 3: sampling, data collection and analysis. Eur J Gen Pract 2018; 24: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007; 19: 349–357. [DOI] [PubMed] [Google Scholar]
- 12. Dunn J, Heathcote P, Chambers SK. Prostate cancer survivorship care: if not now, when? BJU Int 2021; 127(Suppl 1): 30–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eastham JA, Auffenberg GB, Barocas DA, et al. Clinically localized prostate cancer: AUA/ASTRO guideline, Part II: principles of active surveillance, principles of surgery, and follow-up. J Urol 2022; 208: 19–25. [DOI] [PubMed] [Google Scholar]
- 14. Watson E, Shinkins B, Frith E, et al. Symptoms, unmet needs, psychological well-being and health status in survivors of prostate cancer: implications for redesigning follow-up. BJU Int 2016; 117: E10–E19. [DOI] [PubMed] [Google Scholar]
- 15. Hampson LA, Suskind AM, Breyer BN, et al. Predictors of regret among older men after stress urinary incontinence treatment decisions. J Urol 2022; 207: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (Or it takes at least two to tango). Soc Sci Med 1997; 44: 681–692. [DOI] [PubMed] [Google Scholar]
- 17. Karan A, Somasundaram P, Michael H, et al. The effect of multimedia interventions on the informed consent process for cataract surgery in rural South India. Indian J Ophthalmol 2014; 62: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gyomber D, Lawrentschuk N, Wong P, et al. Improving informed consent for patients undergoing radical prostatectomy using multimedia techniques: a prospective randomized crossover study: computer-based consent for radical prostatectomy. BJU Int 2010; 106: 1152–1156. [DOI] [PubMed] [Google Scholar]
- 19. Fink AS, Prochazka AV, Henderson WG, et al. Enhancement of surgical informed consent by addition of repeat back: a multicenter, randomized controlled clinical trial. Ann Surg 2010; 252: 27–36. [DOI] [PubMed] [Google Scholar]
- 20. Smith HK, Manjaly JG, Yousri T, et al. Informed consent in trauma: does written information improve patient recall of risks? A prospective randomised study. Injury 2012; 43: 1534–1538. [DOI] [PubMed] [Google Scholar]
- 21. Mishra PK. A randomized controlled trial to assess the effect of audiotaped consultations on the quality of informed consent in cardiac surgery. Arch Surg 2010; 145: 383. [DOI] [PubMed] [Google Scholar]
- 22. Hebert KJ, Linder BJ, Morrisson GT, et al. A comparison of artificial urinary sphincter outcomes after primary implantation and first revision surgery. Asian J Urol 2021; 8: 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Otis-Chapados S, Kim J, Radomski SB. Artificial urinary sphincter cuffs and safe instrument/catheter passage guidelines. Neurourol Urodyn 2022; 41: 1764–1769. [DOI] [PubMed] [Google Scholar]
- 24. Beauharnais CC, Hill SS, Sturrock PR, et al. Efficacy and satisfaction of asynchronous TeleHealth care compared to in-person visits following colorectal surgical resection. Surg Endosc 2022; 36: 9106–9112. [DOI] [PMC free article] [PubMed] [Google Scholar]