Abstract
Introduction
Robot-assisted laparoscopic surgery (RALS) and conventional laparoscopic surgery (LS) are the main options for ileal ureteral replacement (IUR). It is not clear which option is superior. The purpose of this study is to compare RALS and LS for IUR.
Material and methods
We searched MEDLINE, Embase, Web of Science, Scopus, Cochrane Central, and Google Scholar for studies comparing RALS and LS for IUR. The outcomes of interest are operative time, blood loss, postoperative stay, and Clavien-Dindo complications. Meta-analysis was performed with Rev Man version 5.4.
Results
We included 36 patients from 3 studies. The mean age was 44 years, with 53% male patients. Blood loss (MD -89.13 cc, CI -129.03 to -49.22, I2 = 0%) was significantly lower in patients undergoing RALS when comparing with LS. No differences were observed when comparing operative time (MD -10.99 minutes, CI -85.66 to 63.59, p = 0.77, I2 = 64%), postoperative stay (MD -2.56 days, CI -8.24 to 3.13, p = 0.38, I2 = 30%), and postoperative complications (OR 1.63, CI 0.27 to 10.02, p = 0.60, I2 = 0%).
Conclusions
Overall, we conclude that the robot-assisted technique showed less bleeding compared to the laparoscopic technique.
Keywords: robot-assisted, laparoscopic, ileal ureteral replacement, ileal ureter, ureteral injury
INTRODUCTION
Ileal ureteral replacement (IUR) is a ureteral reconstruction technique that replaces the damaged ureter by interposing a segment of ileum [1–6]. Initially, open IUR was primarily used for the management of ureteral strictures secondary to tuberculosis [1, 2]. This procedure ensures satisfactory urinary drainage and successful preservation of renal function, eliminating the need for permanent nephrostomy and nephrectomy. Nonetheless, the large incision and significant trauma associated with open bilateral IUR can limit its utilisation. Nowadays IUR is usually performed in cases of multiple stenosis, iatrogenic avulsion, and idiopathic fibrosis [1, 4, 6, 7].
With the advancements in the field, the first laparoscopic surgery (LS) was performed in 2005, and the first robot-assisted laparoscopic surgery (RALS) was performed in 2008 [6, 8]. Since then, robotic repair has become an increasingly viable option for complex ureteral defects [1, 4, 5, 9, 10]
Over the past decade, unilateral laparoscopic and robotic IUR procedures have been extensively performed [11], demonstrating successful outcomes characterised by reduced bleeding and accelerated postoperative recovery [12]. Additionally, several studies compared both minimally invasive techniques, concluding that robotic systems offer several benefits such as enhanced precision and dexterity when compared with laparoscopic surgery [2, 5–7, 9, 13, 14]
Multiple centres compared LS and RALS [1, 4, 5]; however, no previous meta-analysis was performed. Also, the absence of specific guidelines and the lack of information regarding the optimal approach underscore the need for further research. Thus, the purpose of this study is to conduct a meta-analysis of studies that assessed laparoscopic and robot-assisted ileal ureteral replacement.
MATERIAL AND METHODS
Eligibility
A search was conducted in the MEDLINE, Embase, Web of Science, Scopus, Cochrane, and Google Scholar databases from inception until June 2023 to identify randomised and non-randomised trials reporting the comparison of robot-assisted versus laparoscopic ileal ureteral replacement. We included adults (≥18 years old) who were patients undergoing ileal ureteral replacement due to any cause, such as stenosis, retroperitoneal fibrosis, radiation stricture, idiopathic fibrosis, and avulsions caused by tumours or previous surgery (e.g. ureteroscopic lithotripsy).
We excluded patients less than 18 years old and patients undergoing an open approach.
Search strategy
The search strategy included terms related to the intervention (robot-assisted laparoscopic surgery), the comparator branch (laparoscopic surgery), and terms related to ileal ureter replacement. This study is registered at PROSPERO (CRD42023431801).
Endpoints
Our outcomes of interest are the operative time, blood loss, postoperative hospital stay, and complications (Clavien-Dindo classification) [15].
Screening
The duplicates (n = 427) were removed using Endnote online™ 20 [16]. Two independent researchers (BP and MB) screened the studies by title and abstract, and disagreements were solved by a third (JA). Following this process, full text screening was performed.
Data extraction and risk of bias
Two independent researchers (BP and MB) extracted the data based on a predefined protocol, and disagreements were solved by a third (JA). Two authors independently extracted the data following predefined search criteria and quality assessment. The Cochrane ROBINS-I score was used to assess the quality of the studies [17].
Statistical analysis
This systematic review and meta-analysis were performed and reported in accordance with the Cochrane Collaboration Handbook for Systematic Review of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines [18].
Continuous outcomes are presented as the mean difference (MD) with 95% confidence interval (CI). Dichotomous data are presented as odds ratio (OR) with 95% CI. Pooled estimates were calculated with the random-effects model, considering that the patients came from different populations. Review Manager 5.4 (Cochrane Centre, The Cochrane Collaboration, Denmark) was used for statistical analysis.
RESULTS
Our search retrieved 1293 articles, of which 3 were included (Figure 1/PRISMA flow chart). Table 1 describes the baseline characteristics of included studies, which were Sim et al., Zhu et al., and Yuan et al. [1, 4, 6].
Figure 1.
Systematic review and study selection process.
Table 1.
Baseline characteristics of patients of included studies
| Study | Type of study | Language | Patients enrolled (N), RALS/LS | Mean age (range) – years, RALS/LS | Male (%), RALS/LS | Postoperative renal function, RALS/LS | Mean BMI (range) – kg/m2, RALS/LS | Side of disease | Ileum resection | Mean follow-up (range) – months, RALS/LS |
|---|---|---|---|---|---|---|---|---|---|---|
| Sim, A. 2014 | Retrospective cohort study | English | 1/4 | 65 ±11.7* (0)/58.5 ±12.7 (42–73) | 1 (100%)/1 (25%) | NS | NS/NS | NS | Intracorporeal in all surgery | 33 ±5.1* (0)/17.2 ±13.9 (2–35) |
| Zhu, W. 2021 | Retrospective cohort study | English | 10/15 | 41.3 ±8.7 (30–53)/38.9 ±13.7 (22–65) | 7 (70%)/9 (60%) | Normal in all patients | 25.6 ± 4 (20.2–32.2)/23.9 ±2.7 (18–27.7) | NS | Extracorporeal in all surgery | 12.9 ±5.4 (3–18)/15.4 ±5.3 (6–24) |
| Yuan, C. 2022 | Retrospective cohort study | English | 4/2 | 54.5 ±14.7 (39–74)/36 ±0 (0) | 2 (50%)/1 (50%) | Normal in all patients | 23.5 ±4.1 (18.9–27.9)/21.9 ±3.6 (19.4–24.5) | Unilateral only | Extracorporeal in all surgery | 11.2 ±4.8 (5–16)/20 ±15.5 (9–31) |
Note: The continuous variables were represented by mean ±SD (range)
BMI – body mass index; LS – laparoscopic surgery; RALS – robot-assisted laparoscopic surgery; NS – non specified; SD, standard deviation
According to the general principles of dealing with missing data from Cochrane, we imputed the standart deviation based on the mean from the others studies
We included 36 patients, 15 of whom underwent RALS and 21 LS. In the Sim et al. study, the mean age of all patients was 59.8 years. The causes for the indication of the IUR included ureteral avulsion, idiopathic and radiation stricture, and retroperitoneal fibrosis. However, the previous renal function of patients was not specified. In the Zhu et al. study, the patients had a mean age of 41.3 years, and the indication for intervention was extensive ureteral stenosis in all cases. Additionally, the preoperative renal function was normal in all patients, with a median creatinine level of 94 µmol/L. Finally, the Yuan et al. study involved a series of patients with an average age of 50 years. The primary aetiology leading to ureteral avulsion and subsequent intervention was related to the ureteroscopy during lithotripsy. Also, the renal function and serum electrolytes were normal in all patients.
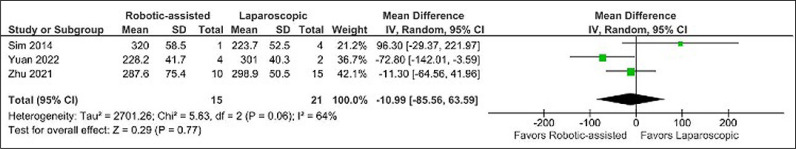
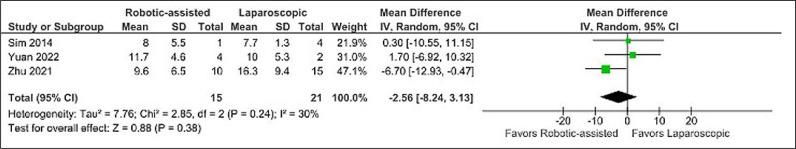
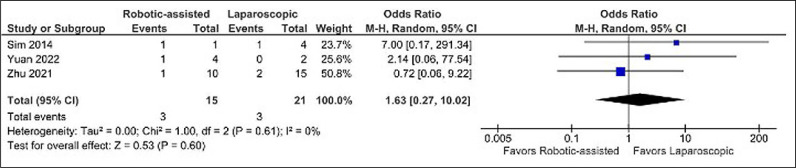
No difference was found when comparing operative time (MD -10.99 minutes, CI -85.66 to 63.59, p = 0.77, I2 = 64%) (Figure 2), postoperative hospital stay (MD -2.56 days, CI -8.24 to 3.13, p = 0.38, I2 = 30%) (Figure 4), and Clavien-Dindo complications (OR 1.63, CI 0.27 to 10.02, p = 0.60, I2 = 0%) (Figure 5).
Figure 2.
Mean difference in operative time.
Figure 4.
Mean difference in postoperative hospital stay.
Figure 5.
Odds ratio of postoperative complications.
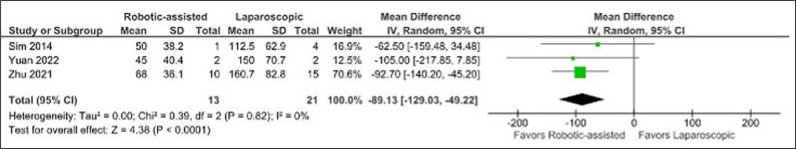
In terms of blood loss, the RALS approach showed superiority over LS (MD -89.13 cc, CI -129.03 to -49.22, p <0.0001, I2 = 0%) (Figure 3).
Figure 3.
Mean difference in blood loss.
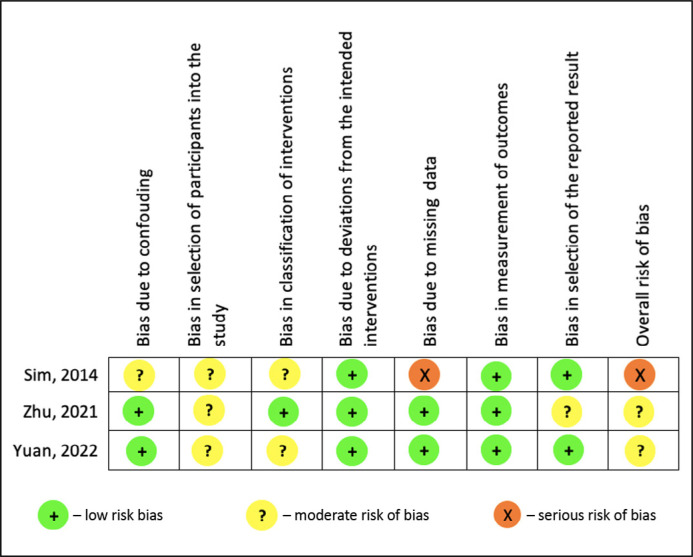
The articles exhibited a moderate bias, as assessed by the ROBINS-I score. Moreover, the study conducted by Sim et al. demonstrated a higher degree of bias when compared to others, due to the presence of missing data (see Figure 6).
Figure 6.
Risk of bias ROBINS-I of the included studies.
DISCUSSION
IUR is a treatment for long or multiple ureteral defects. Minimally invasive techniques (RALS or LS) have shown superiority when compared with open approach [1, 4, 14]. Nowadays, AUA and EAU do not report a preference for RALS or LS.
We included 3 studies in our meta-analysis (Table 1), and it was observed that the RALS group presented less blood loss, without differences in operative time, hospital stay, or complications. For the surgical data of the included studies, please refer to Table 2.
Table 2.
Surgical data of included studies
| Study | Patients enrolled (N), RALS/LS | Mean operative time (range) – min, RALS/LS | Mean blood loss (range) – ml, RALS/LS | Mean postoperative hospital stay (range) – days, RALS/LS | Postoperative complications (Clavien–Dindo), RALS/LS |
|---|---|---|---|---|---|
| Sim, A. 2014 | 1/4 | 320 ±58.5* (0)/223.7 ±52.5 (150–270) | 50 ±38.2* (0)/112.5 ±62.9 (50–200) | 8 ±5.5* (0)/7.7 ±1.3 (6–10) | 1 patient grade III (migrated stent) (100%)/1 patient grade I (fever) (25%) |
| Zhu, W. 2021 | 10/15 | 287.6 ±75.4 (227–450)/298.9 ±50.5 (183–360) | 68 ±36.1 (10–100)/160.7 ±82.8 (50–400) | 9.6 ±6.5 (5–27)/16.3 ±9.4 (9–49) | 1 patient grade II (10%)/2 patients grade II (13.3%) |
| Yuan, C. 2022 | 4/2 | 228.2 ±41.7 (191–283)/301 ±40.3 (273–330) | 45 ±40.4 (10–100)/150 ±70.7 (100–200) | 11.7 ±4.6 (7–18)/10 ±5.3* (0) | 1 patient grade II (incomplete intestinal obstruction) (25%)/none (0%) |
Note: The continuous variables were represented by mean ±SD (range)
BMI – body mass index; LS – laparoscopic surgery; RALS – robot-assisted laparoscopic surgery; NS – non specified; SD – standard deviation
According to the general principles of dealing with missing data from Cochrane, we imputed the standart deviation based on the mean from the others studies
Lower blood loss may be attributed to the enhanced precision, dexterity, more degrees of freedom, and improved visualisation of robotic surgery, which increases the safety in the surgical environment. While our study demonstrates statistical significance in the observed outcome, the initial 89-ml reduction in bleeding within the robotic group may not immediately seem substantial. It is essential to consider the specific surgical context of IUR — primarily one enteroanastomosis and 2 ureteral enteroanastomosis. This procedure typically involves minimal blood loss when compared to more invasive surgeries like radical prostatectomy with extensive bloody dissections. Also, given our small sample size, caution is warranted in drawing definitive conclusions. The observed blood loss reduction in the robotic group may become more pronounced with the inclusion of additional cohorts.
Initially, we expected longer operative time for RALS due to the additional time required for docking and undocking the robot. However, the mean surgical time favoured LS only in the Sim et al. cohort, possibly because the RALS group consisted of only one patient, which limited the comparison between the 2 groups. Interestingly, the shorter operating time observed in the RALS approach draws attention to the greater precision and agility during the surgical procedure.
The included studies reported similar lengths of hospital stay. These findings are similar to the studies that observed shorter convalescence periods and reduced narcotic requirements when comparing minimally invasive procedure and open surgery for IUR [14].
We also assessed postoperative complications using the Clavien-Dindo classification [15]. In the Sim et al. cohort, one patient who underwent RALS experienced a migrated stent (grade III), and another patient developed fever (grade II). Yuan et al. reported one patient who underwent RALS, who experienced an incomplete intestinal obstruction (grade II). Similarly, in the Zhu et al. study, 2 patients developed urinary tract infection (grade II), one had an incomplete ileus (grade II), and one experienced an incisional wound infection (grade III). Overall, all cohorts exhibited grade II or grade III complications, most of which required nonoperative management.
It is worth noting that the literature associates completely intracorporeal anastomosis with increased risk of long-term complications, such as anastomotic leaks, and fistulas, due to less meticulous and secure pyelo-ileal and ileo-vesical anastomosis [6, 19, 20].
The comparison of RALS and LS have been investigated in other procedures. Wang et al. [21] conducted a meta-analysis with 7952 patients comparing RALS and LS radical prostatectomy for localised prostate cancer. No differences were observed when comparing blood loss, however, postoperative complications were less frequent in the RALS group. These findings suggest that RALS may be the preferred approach in various surgical procedures, albeit for different reasons.
In terms of limitations, the small number of patients precluded a subgroup analysis of completely intra- or extracorporeal conduct of ileal anastomosis. As discussed previously, it has been hypothesised that these subgroups could have different surgical outcomes. Additionally, the potential influence of pre-existing renal function on the long-term outcomes of patients undergoing IUR was not explored in our work because Sim et al. did not specify the renal function of their patients, and the cohort of Yuan et al. simply classified it as normal for all participants.
Our study leaves certain questions unanswered. One issue is regarding the impact of preoperative renal function; another pertains to the effects of an anti-reflux papillary valve, as some studies consider it a reliable technique to prevent urine reflux [22], while others argue that ileal peristalsis would naturally suppress the reflux [23]. These areas are opportunities for further research and investigation, providing valuable insights into optimising patient selection and refining surgical techniques.
Given the slight superiority of the RALS over LS observed in our study, we believe it is pivotal to perform further studies in this area to provide stronger evidence of one technique over the other, or even to ascertain whether these techniques provide similar results. This is the first meta-analysis comparing these techniques. With additional studies, stronger evidence may be obtained, which may even provide data for guideline updates.
CONCLUSIONS
In our work, the robot-assisted technique showed less bleeding than the laparoscopic technique. The other surgical outcomes were similar. Because this meta-analysis had a reduced number of evaluated patients, further studies and comparisons are needed to ratify our work.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Yuan C, Li Z, Wang J, et al. Ileal ureteral replacement for the management of ureteral avulsion during ureteroscopic lithotripsy: a case series. BMC Surg. 2022; 22: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams HF, Buchbinder MI. Experience with ileal ureters. Bull N Y Acad Med. 1977; 53: 329-337. [PMC free article] [PubMed] [Google Scholar]
- 3.Vecchio R, MacFayden BV, Palazzo F. History of laparoscopic surgery. Panminerva Med. 2000; 42: 87-90. [PubMed] [Google Scholar]
- 4.Sim A, Todenhöfer T, Mischinger J, et al. Intracorporeal ileal ureter replacement using laparoscopy and robotics. Cent European J Urol. 2014; 67: 420-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosso AA, Di Maida F, Mari A, et al. Totally intracorporeal robotic ileal ureter replacement: Focus on surgical technique and outcomes. Minerva Urol Nephrol. 2021; 73: 532-539. [DOI] [PubMed] [Google Scholar]
- 6.Zhu W, Xiong S, Fang D, et al. Minimally invasive ileal ureter replacement: Comparative analysis of robot-assisted laparoscopic versus conventional laparoscopic surgery. Int J Med Robot. 2021; 17: e2230. [DOI] [PubMed] [Google Scholar]
- 7.Kocot A, Kalogirou C, Vergho D, Riedmiller H. Long-term results of ileal ureteric replacement: a 25-year single-centre experience. BJU Int. 2017; 120: 273-279. [DOI] [PubMed] [Google Scholar]
- 8.Wagner JR, Schimpf MO, Cohen JL. Robot-assisted laparoscopic ileal ureter. JSLS. 2008; 12: 310-313. [PMC free article] [PubMed] [Google Scholar]
- 9.George EI, Brand TC, LaPorta A, Marescaux J, Satava RM. Origins of Robotic Surgery: From Skepticism to Standard of Care. JSLS. 2018; 22: e2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morey AF, Brandes S, Dugi DD 3rd, et al. Urotrauma: AUA guideline. J Urol. 2014; 192: 327-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill IS, Savage SJ, Senagore AJ, Sung GT. Laparoscopic ileal ureter. J Urol. 2000; 163: 1199-1202. [PubMed] [Google Scholar]
- 12.Kochkin AD, Knutov AV, Sevryukov FA. Laparoscopic ureteral substitution: Ileocalicostomy combined with psoas hitch. Urologiia. 2020; 2020: 78-81. [PubMed] [Google Scholar]
- 13.Brandao LF, Autorino R, Zargar H, et al. Robotic ileal ureter: a completely intracorporeal technique. Urology. 2014; 83: 951-954. [DOI] [PubMed] [Google Scholar]
- 14.Varkarakis I. Laparoscopic and Robotic Reconstructive Surgery of the Ureter. Smith's Textbook of Endourology: 3rd Edition. Volume 2, 2012: 1036-1059. [Google Scholar]
- 15.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250: 187-196. [DOI] [PubMed] [Google Scholar]
- 16.Team TE . EndNote. EndNote 20 ed. Philadelphia, PA: Clarivate, 2013. [Google Scholar]
- 17.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MA-O, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armatys SA, Mellon MJ, Beck SD, Koch MO, Foster RS, Bihrle R. Use of ileum as ureteral replacement in urological reconstruction. J Urol. 2009; 181: 177-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung BI, Hamawy KJ, Zinman LN, Libertino JA. The use of bowel for ureteral replacement for complex ureteral reconstruction: long-term results. J Urol. 2006; 175: 179-183. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Wang Q, Wang S. A Meta-analysis of Robot Assisted Laparoscopic Radical Prostatectomy Versus Laparoscopic Radical Prostatectomy. Open Med (Wars). 2019; 14: 485-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu YM, Feng C, Kato H, Xie H, Zhang XR. Long-term Outcome of Ileal Ureteric Replacement With an Iliopsoas Muscle Tunnel Antirefluxing Technique for the Treatment of Long-segment Ureteric Strictures. Urology. 2016; 88: 201-206. [DOI] [PubMed] [Google Scholar]
- 23.Waldner M, Hertle L, Roth S. Ileal ureteral substitution in reconstructive urological surgery: is an antireflux procedure necessary? J Urol. 1999; 162: 323-326. [PubMed] [Google Scholar]