Abstract
Introduction
Gaining insight into patient characteristics to predict the success of procedures is crucial for improving outcomes and for preoperative counselling. We identified predictors of achieving a minimal clinically important difference (MCID) in lower urinary tract symptoms (LUTS) 3 months after Rezūm.
Material and methods
A retrospective study was conducted on patients treated with Rezūm. Patients with moderate or severe LUTS and a recorded International Prostate Symptom Score (IPSS) at 3 months were included and categorised into 2 cohorts based on experiencing a MCID at 3 months (≥ 25% improvement in IPSS). Predictors were identified through multivariate logistic regression analysis.
Results
Out of 174 patients, 134 (77%) achieved a MCID at 3 months, and those who did had a higher median baseline IPSS (20 [16–26] vs 15 [10–21], P <0.001) and were more likely to have severe LUTS at baseline (53.0% vs 35.0%, P = 0.046) when compared to those who did not experience a MCID at 3 months. Higher baseline IPSS (OR: 1.10, 95% CI 1.04–1.17) and larger baseline prostate volumes (OR: 1.03, 95% CI 1.0–1.05) were predictors of achieving a MCID at 3 months. More specifically, a significantly greater proportion of patients with severe LUTS (83.5 vs 70.8%, P = 0.046) and prostate volume ≥60 cc (94.6 vs 71.4%, P = 0.003) achieved MCID at 3 months when compared to patients with moderate LUTS and prostate volumes <60 cc, respectively.
Conclusions
More than three-quarters of patients treated with Rezūm achieved a MCID at 3 months. Patients with severe LUTS and prostate volumes ≥ 60 cc may be optimal candidates for experiencing early relief in LUTS following Rezūm.
Keywords: benign prostatic hyperplasia, minimal clinically important difference, lower urinary tract symptoms, minimally invasive surgical therapy, water vapor thermal therapy
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a complex pathologic process that often plays a significant role in causing lower urinary tract symptoms (LUTS) in men as they age [1]. LUTS is associated with significant morbidity due to both its high prevalence and its detrimental impact on quality of life (QoL). Prevalence rates measured both clinically and histologically range from 50% to 75% for men ages 50 years and older, increasing to approximately 80% for men over 70 years old [2, 3]. The International Prostate Symptom Score (IPSS) is a standardised 8-question screening tool used to evaluate LUTS severity, and it is frequently used to assess the QoL impact of LUTS and track changes over time. This makes it a valuable tool for quantifying the degree to which a patient’s symptoms change, particularly after initiating a change in management. Comparison of IPSS scores from before and after treatment can, therefore, also be used to determine whether a patient achieved clinically significant improvement [4].
Minimal clinically important difference (MCID) is often used in clinical research as a benchmark for the magnitude of improvement in patient-reported outcomes, which reflects clinical significance [5]. This concept was first applied to BPH therapy more than 20 years ago when Barry et al. showed that a 3-point improvement in IPSS is the minimum change required for a patient to perceive an improvement in LUTS after intervention with BPH pharmacological therapies [6]. Alternatively, subsequent studies analysing BPH therapies defined MCID as ≥25% improvement in IPSS [7, 8, 9, 10].
Treatment for LUTS ranges from lifestyle modifications to pharmacotherapy and surgery, including minimally invasive procedures such as the Rezūm system (Rezūm: Boston Scientific, Marlborough, MA, USA). Rezūm is a minimally invasive therapy that utilises a retractable needle to deliver high thermal energy from water vapor into excess prostatic tissue, leading to subsequent cell membrane disruption and ultimately tissue necrosis [11]. A multicentre randomised sham-controlled trial of Rezūm showed that it is capable of providing significant LUTS relief through 5 years [12]; however, not every patient is a good candidate for Rezūm therapy, nor do all patients have the same response to the procedure. Understanding the pertinent patient characteristics that may predict successful or failed procedures is key to improving efficacy and outcomes, as well as counselling patients preoperatively. Given the novelty of the Rezūm procedure, research into these specifics is still in its infancy and largely unknown. Therefore, the objective of this study is to identify predictors of achieving a MCID in LUTS following Rezūm therapy.
MATERIAL AND METHODS
After approval from the Western Institutional Review Board, a retrospective study was conducted on patients from a multi-ethnic population treated with Rezūm by 2 urologists in a single office between 1 December 2017 and 30 April 2019. Patients were included if they had a recorded baseline IPSS score between 8 and 35 (moderate [IPSS 8–19] and severe [IPSS ≥20] LUTS) and at least one follow-up within 12 months after treatment. Patients were excluded if they had no recorded IPSS score at 3 months, an active urinary tract infection within 7 days of the procedure, urinary retention requiring catheterisation on the day of the procedure, a prior Rezūm procedure, ongoing prostate cancer, or a prostate-specific antigen (PSA) ≥10 ng/ml, unless prostate cancer was ruled out by biopsy. Patients were included irrespective of prostate volume, and those with median lobes were not excluded.
All patients underwent pressure flow urodynamic studies to exclude other causes of LUTS such as detrusor overactivity or neurogenic bladder. Patients received antibiotics for one week prior to treatment and elected for either general anaesthesia or prostate block. During treatment, water vapor injections at 103°C for 9 seconds were utilised on the prostatic lateral and median lobes (if present). Standard procedure included one injection per centimetre of prostatic urethral length. All patients were catheterised following treatment. Patients were advised to use nonsteroidal anti-inflammatory drugs or acetaminophen as needed for pain control. Those who were taking BPH medications were counselled to continue their medications until their LUTS were reassessed at their 3-month follow-up.
Outcome measures included IPSS, QoL, maximum urinary flow rate (Qmax), prostate volume, postvoid residual (PVR), PSA, and usage of alpha blockers and/or 5-ARIs. Patient characteristics and outcome measures were collected through electronic medical records at baseline and postoperatively at 1, 3, 6, and/or 12 months. Qmax was collected through uroflowmetry with a minimum voided volume of ≥125 ml. Prostate volumes were measured through a transrectal ultrasound. Occurrence of adverse events (AE) within 3 months after treatment was recorded. Dysuria was defined as an AE in patients who had penile burning and penile pain.
Patients were categorised into 2 cohorts based on experiencing a MCID at 3 months postoperatively. MCID was defined as a ≥ 25% improvement in IPSS given that an evaluation of patient-reported, clinically meaningful differences may be highly influenced by varying baseline LUTS severity in our diverse patient population [5, 6, 10]. Demographic and clinical features were compared across relevant cohorts. Missing values were excluded from statistical analysis. Individual variables were tested for normality before analysis using the Shapiro-Wilk test. The independent 2-sample t-test was used for continuous, normally distributed variables, and the Mann–Whitney U test was used for continuous, non-normally distributed variables. The chi-square test was used for categorical variables when comparing characteristics between cohorts.
All tests were 2-sided with a significance threshold of P <0.05. Normally distributed data are presented as the mean ± standard deviation. Non-normally distributed data are presented as the median (interquartile range). Logistic regression was used for univariate and subsequent multivariate analysis of predictors for achieving MCID. Covariates that were significantly associated with achieving MCID on univariate analysis were subsequently included in a multivariate model. Before multivariate analysis, variables to be included were tested for collinearity using the Spearman correlation coefficient with a threshold of >0.6. Changes in outcomes after treatment were assessed using the Wilcoxon signed-rank test, stratified by those who achieved MCID and those who did not. All statistical analyses were performed using SPSS, version 28 (IBM Corp., Armonk, NY, USA).
RESULTS
Demographics and baseline characteristics
During the study period, a total of 218 patients were screened for eligibility, of whom 174 met inclusion criteria. Forty-four patients were excluded for the following reasons: no baseline IPSS at 3 months (31), prior treatment with Rezūm (8), or urinary retention at baseline (5).
A total of 134 patients (77.0%) experienced a MCID at 3 months while 40 (23.0%) did not. The overall cohort was racially diverse, with the majority being Asian (37.4%), Non-Hispanic Black (28.7%), and Hispanic (19.0%). Significant differences in baseline characteristics in those who experienced a MCID at 3 months and those who did not, respectively, were higher median IPSS (20 [16–26] vs 15 [10–21], p <0.001) and higher median IPSS symptom scores for Intermittency (P <0.001), urgency (P = 0.040), weak stream (P = 0.002), and straining (P = 0.004). Additionally, a significantly higher proportion of patients who experienced a MCID at 3 months had severe LUTS at baseline (53.0%) when compared to those who did not experience a MCID at 3 months (35.0%, P = 0.046) (Table 1).
Table 1.
Baseline demographics and characteristics
| Characteristic | All Patients | ≥25% IPSS improvement | <25% IPSS improvement | P |
|---|---|---|---|---|
| Sample Size | 174 | 134 | 40 | |
| Age, years, mean (SD) | 63.3 (8.57) | 63.0 (8.57) | 64.2 (8.59) | 0.43 |
| Race/Ethnicity, N (%) Non-Hispanic White Non-Hispanic Black Hispanic Asian |
26 (14.9) 50 (28.7) 33 (19.0) 65 (37.4) |
19 (14.2) 40 (29.9) 29 (21.6) 46 (34.3) |
7 (17.5) 10 (25.0) 4 (10.0) 19 (47.5) |
0.25 |
| Prostatic Urethral Length, cm, median (IQR) | 2.5 (2.0–3.0) | 2.5 (2.1–3.0) | 2.5 (2.0–2.5) | 0.22 |
| Presence of Median Lobe, N (%) | 123 (70.7) | 93 (69.4) | 30 (75.0) | 0.50 |
| Past Medical History, N (%) Urinary Retention Hypertension Erectile Dysfunction Dyslipidaemia Diabetes Nephrolithiasis |
11 (6.4) 102 (58.6) 75 (43.1) 67 (38.5) 54 (31.0) 9 (5.2) |
9 (6.8) 80 (59.7) 56 (41.8) 51 (38.1) 41 (30.6) 5 (3.7) |
2 (5.1) 22 (55.0) 19 (47.5) 16 (40.0) 13 (32.5) 4 (10.0) |
0.71 0.60 0.52 0.83 0.82 0.12 |
| Previous BPH Procedure, N (%) | 49 (29.3) | 41 (32.0) | 8 (20.5) | 0.17 |
| Alpha Blockers and/or 5-ARI Usage, N (%) | 155 (90.6) | 121 (92.4) | 34 (85.0) | 0.16 |
| Prostate Volume, cc, median (IQR) | 42.0 (35.0–56.0) | 44.0 (36.0–60.0) | 40.5 (30.8–51.8) | 0.064 |
| Prostate Volume breakdown, N (%) <30 cc 30–80 cc >80 cc |
23 (13.5) 135 (79.4) 12 (7.1) |
16 (12.3) 103 (79.2) 11 (8.5) |
7 (17.5) 32 (80.0) 1 (2.5) |
0.34 |
| PSA, ng/ml, median (IQR) | 1.4 (0.4–3.3) | 1.5 (0.7–3.6) | 1.4 (0.8–2.9) | 0.27 |
| Qmax, ml/s, median (IQR) | 10.0 (7.4–13.4) | 10.2 (7.4–13.8) | 8.6 (7.6–12.7) | 0.45 |
| PVR, ml, median (IQR) | 0 (0–31.7) | 0 (0–34.9) | 0 (0–50.3) | 0.98 |
| IPSS, median (IQR) Total Q1 (Incomplete Emptying) Q2 (Frequency) Q3 (Intermittency) Q4 (Urgency) Q5 (Weak Stream) Q6 (Straining) Q7 (Nocturia) |
19 (14–25) 3 (2, 4) 3 (2, 5) 3 (2, 4) 3 (1, 5) 3 (2, 4) 2 (0, 4) 3 (2, 4) |
20 (16–26) 3 (2, 4) 3 (2, 5) 3 (2, 4) 3 (2, 5) 3 (2, 5) 2 (1, 4) 3 (2, 4) |
15 (10–21) 2 (1, 4) 3 (1, 5) 2 (0, 3) 2 (0, 4) 2 (0, 4) 1 (0, 3) 3 (2, 5) |
<0.001 0.13 0.27 <0.001 0.040 0.002 0.004 0.12 |
| IPSS Severity breakdown, N (%) Moderate LUTS (IPSS 8–19) Severe LUTS (IPSS 20–35) |
89 (51.1) 85 (48.9) |
63 (47.0) 71 (53.0) |
26 (65.0) 14 (35.0) |
0.046 |
| QoL, median (IQR) | 5 (3–6) | 5 (4–6) | 5 (3–6) | 0.68 |
BPH – benign prostatic hyperplasia; 5-ARI – 5-alpha reductase inhibitor; PSA – prostate-specific antigen; Qmax – maximum flow rate; IPSS – International Prostate Symptom Score; QoL – quality of life; LUTS – lower urinary tract symptoms
Procedural characteristics
Most of the patients elected for general anaesthesia (85.1%). A median of 2 injections (1–2) were given per lateral prostatic lobe, and all patients were catheterised for a median of 5 days (4–5) following treatment. There were no significant differences in procedural characteristics between those who experienced a MCID at 3 months and those who did not (Table 2).
Table 2.
Rezūm procedural characteristics
| All Patients | ≥25% IPSS improvement | <25% IPSS improvement | P | |
|---|---|---|---|---|
| Anaesthesia method, n (%) General Prostate block |
148 (85.1) 26 (14.9) |
117 (87.3) 17 (12.7) |
31 (77.5) 9 (22.5) |
0.13 |
| Total prostatic injections, median (IQR) Injections per lateral lobe Injections per medial lobe |
2 (1–2) 1 (0–1) |
2 (1–2) 1 (0–1) |
2 (1–2) 1 (1–1) |
0.082 0.37 |
| Number of days catheterized postoperatively, median (IQR) | 5 (4–5) | 5 (4–5) | 4 (4–5) | 0.42 |
IPSS – International Prostate Symptom Score; n – number of patients; IQR – interquartile range
Safety
The most common AE was gross haematuria (68.6%) followed by penile burning (66.1%) and penile pain (36.3%). Eight patients (4.9%) were surgically retreated within 12 months following treatment; 5 with TURP and 3 with staged Rezūm. There were no significant differences in the occurrence and duration of all serious and non-serious AEs between those who experienced a MCID at 3 months and those who did not (Table 3).
Table 3.
Occurrence and duration of postoperative adverse events
| Characteristic | All Patients | ≥25% IPSS improvement | <25% IPSS improvement | P |
|---|---|---|---|---|
| UTI, N (%) | 13 (7.5) | 10 (7.5) | 3 (7.5) | 0.99 |
| Sepsis, N (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| Surgical Retreatment, N (%) | 8 (4.9) | 8 (6.3) | 0 (0.0) | 0.12 |
| Vasovagal, N (%) | 3 (1.7) | 2 (1.5) | 1 (2.5) | 0.67 |
| Gross Haematuria N (%) Duration, days, median (IQR) |
116 (68.6) 10 (0–30) |
93 (69.9) 10 (0–30) |
23 (63.9) 7 (0–19) |
0.49 0.37 |
| Penile Burning N (%) Duration, days, median (IQR) |
111 (66.1) 10 (0–60) |
85 (64.9) 7 (0–60) |
26 (70.3) 14 (0–60) |
0.54 0.41 |
| Penile Pain N (%) Duration, days, median (IQR) |
61 (36.3) 0 (0–13) |
44 (33.6) 0 (0–9) |
17 (45.9) 0 (0–40) |
0.17 0.12 |
| Dysuria N (%) Duration, days, median (IQR) |
45 (26.9) 0 (0–7) |
32 (24.6) 0 (0–1) |
13 (35.1) 0 (0–26) |
0.20 0.16 |
| Sloughing N (%) Duration, days, median (IQR) |
32 (24.8) 0 (0–1) |
25 (25.0) 0 (0–1) |
7 (24.1) 0 (0–1) |
0.93 0.84 |
UTI – urinary tract infection; IQR – interquartile range; N – number of patients; IPSS – International Prostate Symptom Score
Efficacy
Patients who experienced a MCID at 3 months saw significant improvements in IPSS and all IPSS symptom scores at all follow-ups. Patients who had not experienced a MCID at 3 months went on to have significant improvements in IPSS and IPSS symptom scores for incomplete emptying, and nocturia by 12 months. All patients, regardless of experiencing a MCID at 3 months, had significant reductions in prostate volume and BPH medication usage at follow-up (Table 4).
Table 4.
Changes in outcomes measures from baseline to 12 months. Change, percentage change, and p values are in reference to the baseline value.
| Baseline | 1 month | 3 months | 6 months | 12 months | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Measure | All patients | ≥25% IPSS improvement | <25% IPSS improvement | All patients | ≥25% IPSS improvement | <25% IPSS improvement | All patients | ≥25% IPSS improvement | <25% IPSS improvement | All patients | ≥25% IPSS improvement | <25% IPSS improvement | All patients | ≥25% IPSS improvement | <25% IPSS improvement | |||||||||||||||
| N | 174 | 134 | 40 | 142 | 113 | 29 | 174 | 134 | 40 | 139 | 108 | 31 | 136 | 105 | 31 | |||||||||||||||
| Median (IQR) | 19 (14, 25) | 20 (16, 26) | 15 (10, 21) | 13 (9, 17) | 12 (8, 16) | 16 (12, 20) | 9 (5, 14) | 7 (4, 11) | 18 (13, 21) | 8 (5, 13) | 7 (4, 11) | 12 (8, 18) | 8 (4, 13) | 7 (4, 11) | 12 (8, 20) | |||||||||||||||
| Change | -6 (-11, -1) | -7 (-12, -3) | 0 (-4, 4) | -9 (-15, -5) | -11 (-17, -8) | 0 (-4, 3) | -11 (-15, -5) | -12 (-16, -8) | -3 (-11, -2) | -9 (-15, -5) | -10 (-16, -6) | -5 (-9, 2) | ||||||||||||||||||
| % Change | -29.0 (-56.0, -8.5) | -37.5 (-61.1, -14.6) | 0.0 (-20.7, 25.4) | -50.0 (-71.6, -29.0) | -61.8 (-75.0, -45.1) | 0.0 (-16.7, 36.5) | -61.5 (-73.7, -33.3) | -65.1 (-77.6, -46.1) | -20.0 (-57.9, 15.4) | -55.7 (-75.0, -30.8) | -61.1 (-78.0, -39.4) | -33.3 (-57.9, 20.0) | ||||||||||||||||||
| p value | <0.001 | <0.001 | 0.74 | <0.001 | <0.001 | 0.73 | <0.001 | <0.001 | 0.024 | <0.001 | <0.001 | 0.047 | ||||||||||||||||||
| IPSS – Q1 (Incomplete Emptying) | ||||||||||||||||||||||||||||||
| N | 174 | 134 | 40 | 142 | 113 | 29 | 174 | 134 | 40 | 139 | 108 | 31 | 136 | 105 | 31 | |||||||||||||||
| Median (IQR) | 3 (2, 4) | 3 (2, 4) | 2 (1, 4) | 2 (0, 3) | 1 (0, 3) | 3 (1, 3) | 0 (0, 2) | 0 (0, 1) | 3 (0, 4) | 0 (0, 2) | 0 (0, 2) | 1 (0, 3) | 0 (0, 3) | 0 (0, 2) | 1 (0, 3) | |||||||||||||||
| Change | -1 (-3, 0) | -1 (-3, 0) | 0 (-2, 1) | -2 (-3, 0) | -2 (-4, -1) | 0 (-1, 1) | -2 (-3, 0) | -2 (-3, -1) | 0 (-2, 0) | -2 (-3, 0) | -2 (-4, 0) | 0 (-3, 0) | ||||||||||||||||||
| % Change | -33.3 (-100, 0.0) | -40.0 (-100, 0.0) | 0.0 (-50.0, 83.3) | -77.5 (-100, 0) | -100 (-100, -33.3) | 0.0 (-50.0, 100) | -66.7 (-100, 0.0) | -75.0 (-100, -20.0) | 0.0 (-100, 0.0) | -66.7 (-100, 0.0) | -75.0 (-100, 0.0) | 0.0 (-100, 0.0) | ||||||||||||||||||
| p value | <0.001 | <0.001 | 0.69 | <0.001 | <0.001 | 0.76 | <0.001 | <0.001 | 0.15 | <0.001 | <0.001 | 0.023 | ||||||||||||||||||
| IPSS – Q2 (Frequency) | ||||||||||||||||||||||||||||||
| N | 174 | 134 | 40 | 142 | 113 | 29 | 174 | 134 | 40 | 139 | 108 | 31 | 136 | 105 | 31 | |||||||||||||||
| Median (IQR) | 3 (2, 5) | 3 (2, 5) | 3 (1, 5) | 3 (1, 4) | 3 (1, 4) | 4 (3, 5) | 2 (1, 4) | 1 (0, 3) | 4 (2, 5) | 1 (0, 3) | 1 (0, 3) | 2 (0, 3) | 2 (0, 4) | 1 (0, 3) | 3 (1, 4) | |||||||||||||||
| Change | 0 (-2, 1) | 0 (-2, 1) | 0 (-1, 2) | -1 (-3, 0) | -2 (-3, 0) | 0 (-1, 2) | -2 (-3, 0) | -2 (-3, -1) | -1 (-2, 1) | -1 (-3, 0) | -2 (-3, 0) | 0 (-1, 1) | ||||||||||||||||||
| % Change | 0.0 (-50.0, 33.0) | 0.0 (-60.0, 25.0) | 0.0 (-20.0, 83.3) | -40.0 (-80.0, 0.0) | -50.0 (-100, 0.0) | 0.0 (-38.3, 66.7) | -60.0 (-100, 0.0) | -66.7 (-100, -20.0) | -40.0 (-100, 6.3) | -45.0 (-100, 0.0) | -50.0 (-100, 0.0) | 0.0 (-60.0, 25.0) | ||||||||||||||||||
| p value | 0.034 | 0.005 | 0.27 | <0.001 | <0.001 | 0.20 | <0.001 | <0.001 | 0.033 | <0.001 | <0.001 | 0.35 | ||||||||||||||||||
| IPSS – Q3 (Intermittency) | ||||||||||||||||||||||||||||||
| N (paired values) | 174 | 134 | 40 | 142 | 113 | 29 | 174 | 134 | 40 | 139 | 108 | 31 | 136 | 105 | 31 | |||||||||||||||
| Median (IQR) | 3 (2, 4) | 3 (2, 4) | 2 (0, 3) | 0 (0, 2) | 1 (0, 3) | 0 (0, 1) | 0 (0, 2) | 0 (0, 1) | 1 (0, 4) | 0 (0, 2) | 0 (0, 1) | 0 (0, 2) | 0 (0, 2) | 0 (0, 1) | 1 (0, 3) | |||||||||||||||
| Change | -2 (-3, 0) | -2 (-3, 0) | -2 (-3, 0) | -2 (-3, 0) | -2 (-3, -1) | 0 (-2, 2) | -2 (-3, 0) | -2 (-3, -1) | -1 (-2, 1) | -2 (-3, 0) | -2 (-4, 0) | 0 (-2, 1) | ||||||||||||||||||
| % Change | -66.7 (-100, 0.0) | -66.7 (-100, 0.0) | -66.7 (-100, 0.0) | -100 (-100, 0.0) | -100 (-100, -50.0) | 0.0 (-95.0, 100) | -100 (-100, 0.0) | -100 (-100, -28.8) | -40.0 (-100, 25.0) |
-100 (-100, 0.0) | -100 (-100, 0.0) | 0.0 (-100, 50.0) |
||||||||||||||||||
| p value | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | 0.82 | <0.001 | <0.001 | 0.12 | <0.001 | <0.001 | 0.21 | ||||||||||||||||||
| IPSS – Q4 (Urgency) | ||||||||||||||||||||||||||||||
| N (paired values) | 174 | 134 | 40 | 142 | 113 | 29 | 174 | 134 | 40 | 139 | 108 | 31 | 136 | 105 | 31 | |||||||||||||||
| Median (IQR) | 3 (1, 5) | 3 (2, 5) | 2 (0, 4) | 3 (1, 4) | 3 (1, 4) | 4 (3, 4) | 2 (0, 4) | 1 (0, 3) | 4 (2, 5) | 1 (0, 3) | 1 (0, 3) | 2 (0, 5) | 1 (0, 3) | 1 (0, 3) | 3 (0, 4) | |||||||||||||||
| Change | 0 (-2, 1) | -1 (-2, 1) | 1 (-1, 3) | -1 (-2, 0) | -1 (-3, 0) | 1 (0, 3) | -1 (-3, 0) | -2 (-3, 0) | 0 (-1, 1) | -1 (-3, 0) | -1 (-3, 0) | 0 (-1, 1) | ||||||||||||||||||
| % Change | 0.0 (-61.7, 50.0) | -20.0 (-75.0, 25.0) | 33.3 (-10.0, 100) | -20.0 (-100, 0.0) | -33.3 (-100, 0.0) | 25.0 (0.0, 100) | -50.0 (-100, 0.0) | -63.3 (-100, 0.0) | 0.0 (-66.7, 100) | -50.0 (-100, 0.0) | -66.7 (-100, 0.0) | 0.0 (-50.0, 25.0) | ||||||||||||||||||
| p value | 0.22 | 0.012 | 0.021 | <0.001 | <0.001 | 0.017 | <0.001 | <0.001 | 0.99 | <0.001 | <0.001 | 0.98 | ||||||||||||||||||
| IPSS – Q5 (Weak Stream) | ||||||||||||||||||||||||||||||
| N | 174 | 134 | 40 | 142 | 113 | 29 | 174 | 134 | 40 | 139 | 108 | 31 | 136 | 105 | 31 | |||||||||||||||
| Median (IQR) | 3 (2, 4) | 3 (2, 5) | 2 (0, 4) | 1 (0, 3) | 1 (0, 3) | 2 (1, 3) | 0 (0, 2) | 0 (0, 1) | 2 (0, 3) | 0 (0, 2) | 0 (0, 2) | 1 (0, 3) | 1 (0, 2) | 0 (0, 2) | 1 (0, 4) | |||||||||||||||
| Change | -1 (-3, 0) | -2 (-3, 0) | 1 (-1, 2) | -2 (-3, 0) | -3 (-4, -1) | 0 (-1, 1) | -2 (-3, -1) | -2 (-4, -1) | -1 (-2, 1) | -2 (-3, 0) | -2 (-3, -1) | 0 (-2, 1) | ||||||||||||||||||
| % Change | -40.0 (-100, 0.0) | -60.0 (-100, 0.0) | 33.3 (-41.7, 100) | -100 (-100, -0.0) | -100 (-100, -50.0) | 0.0 (50.0, 45.8) | -66.7 (-100, -20.0) | -100 (-100, -33.3) | -33.3 (-75.0, 100) | -66.7 (-100, 0.0) | -75.0 (-100, -29.2) | 0.0 (-66.7, 100) | ||||||||||||||||||
| p value | <0.001 | <0.001 | 0.43 | <0.001 | <0.001 | 0.65 | <0.001 | <0.001 | 0.34 | <0.001 | <0.001 | 0.61 | ||||||||||||||||||
| IPSS – Q6 (Straining) | ||||||||||||||||||||||||||||||
| N | 174 | 134 | 40 | 142 | 113 | 29 | 174 | 134 | 40 | 139 | 108 | 31 | 136 | 105 | 31 | |||||||||||||||
| Median (IQR) | 2 (0, 4) | 2 (1, 4) | 1 (0, 3) | 0 (0, 1) | 0 (0-1) | 0 (0, 1) | 0 (0, 0) | 0 (0, 0) | 0 (0, 3) | 0 (0, 1) | 0 (0, 1) | 0 (0, 2) | 0 (0, 1) | 0 (0, 1) | 0 (0, 2) | |||||||||||||||
| Change | -1 (-2, 0) | -2 (-3, 0) | 0 (-2, 0) | -2 (-3, 0) | -2 (-3, -1) | 0 (-2, 2) | -1 (-3, 0) | -2 (-3, 0) | 0 (-2, 1) | -2 (-3, 0) | -2 (-3, 0) | 0 (-2, 0) | ||||||||||||||||||
| % Change | -60.0 (-100, 0.0) | -75.0 (-100, 0.0) | 0.0 (-77.5, 0.0) | -100 (-100, -0.0) | -100 (-100, -45.8) | 0.0 (-100, 100) | -66.7 (-100, 0.0) | -87.5 (-100, 0.0) | 0.0 (-100, 50.0) | -66.7 (-100, 0.0) | -75.0 (-100, 0.0) | 0.0 (-100, 0.0) | ||||||||||||||||||
| p value | <0.001 | <0.001 | 0.083 | <0.001 | <0.001 | 0.96 | <0.001 | <0.001 | 0.19 | <0.001 | <0.001 | 0.17 | ||||||||||||||||||
| IPSS – Q7 (Nocturia) | ||||||||||||||||||||||||||||||
| N | 174 | 134 | 40 | 142 | 113 | 29 | 174 | 134 | 40 | 139 | 108 | 31 | 136 | 105 | 31 | |||||||||||||||
| Median (IQR) | 3 (2, 4) | 3 (2, 4) | 3 (2, 5) | 2 (2, 3) | 2 (1, 3) | 2 (2, 4) | 2 (1, 3) | 2 (1, 3) | 3 (2, 4) | 2 (1, 3) | 2 (1, 3) | 2 (1, 4) | 2 (1, 3) | 2 (1, 2) | 2 (1, 3) | |||||||||||||||
| Change | 0 (-1, 0) | 0 (-1, 0) | 0 (-1, 1) | -1 (-1. 0) | -1 (-2, 0) | 0 (-1, 1) | -1 (-2, 0) | -1 (-2, 0) | -1 (-2, 0) | -1 (-2, 0) | -1 (-2, 0) | 0 (-1, 0) | ||||||||||||||||||
| % Change | 0.0 (0.0, -40.0) | 0.0 (-40.0, 0.0) | 0.0 (-45.0, 12.5) | -20.0 (-50.0, 0.0) | -33.3 (-50.0, 0.0) | 0.0 (-31.3, 33.3) | -25.0 (-50.0, 0.0) | -33.3 (-50.0, 0.0) | -20.0 (-50.0, 0.0) | -33.3 (-50.0, 0.0) | -33.3 (-57.5, 0.0) | 0.0 (-50.0, 0.0) | ||||||||||||||||||
| p value | <0.001 | <0.001 | 0.064 | <0.001 | <0.001 | 0.59 | <0.001 | <0.001 | 0.006 | <0.001 | <0.001 | 0.003 | ||||||||||||||||||
| QoL | ||||||||||||||||||||||||||||||
| N | 173 | 134 | 39 | 142 | 113 | 29 | 174 | 134 | 40 | 139 | 108 | 31 | 134 | 103 | 31 | |||||||||||||||
| Median (IQR) | 5 (3, 6) | 5 (4, 6) | 5 (3, 6) | 3 (2, 5) | 3 (2, 4) | 3 (2, 5) | 2 (1, 4) | 2 (1, 3) | 4 (3, 5) | 2 (1, 3) | 2 (1, 3) | 3 (2, 5) | 2 (1, 3) | 2 (1, 3) | 3 (2, 4) | |||||||||||||||
| Change | -1 (-3, 0) | -2 (-3, 0) | -1 (-3, 0) | -2 (-3, -1) | -3 (-3, -1) | 0 (-2, 1) | -2 (-4, -1) | -2 (-4, -1) | -1 (-3, 0) | -2 (-4, -1) | -2 (-4, -1) | -1 (-3, 0) | ||||||||||||||||||
| % Change | -33.3 (-50.0, 0.0) | -33.3 (-50.0, 0.0) | -16.7 (-50.0, 0.0) | -50.0 (-66.7, -18.3) | -60.0 (-75.0, -33.3) | 0.0 (-40.0, 33.3) | -50.0 (-75.0, -25.0) | -50.0 (-80.0, -33.3) | -33.3 (-60.0, 0.0) | -50.0 (-80.0, -20.0) | -66.7 (-80.0, -25.0) | -25.0 (-50.0, 0.0) | ||||||||||||||||||
| p value | <0.001 | <0.001 | 0.025 | <0.001 | <0.001 | 0.14 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.003 | ||||||||||||||||||
| Prostate volume (cc) | ||||||||||||||||||||||||||||||
| N | 170 | 130 | 40 | 133 | 105 | 28 | ||||||||||||||||||||||||
| Median (IQR) | 42.5 (35.0, 56.5) | 44.0 (36.0, 60.0) | 40.5 (30.8, 51.8) | 31.0 (23.0, 41.0) | 31.0 (23.0, 44.5) | 27.5 (21.5, 34.8) | ||||||||||||||||||||||||
| Change | -13.0 (-18.5, -8.5) | -13.0 (-20.5, -8.5) | -13.0 (-16.0, -7.5) | |||||||||||||||||||||||||||
| % Change | -30.7 (-38.5, -20.3) | -29.8 (-37.8, -19.7) | -31.8 (-42.7, -21.9) | |||||||||||||||||||||||||||
| p value | <0.001 | <0.001 | <0.001 | |||||||||||||||||||||||||||
| Qmax (ml/s) | ||||||||||||||||||||||||||||||
| N | 127 | 96 | 31 | 28 | 24 | 4 | 25 | 21 | 4 | 33 | 27 | 6 | ||||||||||||||||||
| Median (IQR) | 10.0 (7.5, 13.4) | 10.2 (7.4, 13.8) | 8.6 (7.6, 12.7) | 17.3 (12.6, 22.4) | 18.0 (13.7, 22.6) | 11.0 (8.9, 14.6) | 12.5 (9.5, 18.6) | 12.5 (9.6, 19.8) | 11.6 (8.1, 16.4) | 15.9 (10.3, 22.2) | 16.5 (11.2, 23.1) | 11.4 (8.4, 17.3) | ||||||||||||||||||
| Change | 3.7 (1.8, 9.9) | 5.4 ( 1.8, 10.6) | 1.8 (1.0, 2.3) | 3.9 (-0.2, 7.3) | 4.5 (-1.2, 9.0) | 2.4 (0.1, 6.5) | 2.6 (-0.1, 9.1) | 5.2 (0.6, 10.3) | 0.4 (-1.2, 1.7) | |||||||||||||||||||||
| % Change | 38.3 (13.5, 131.2) | 53.8 (18.2, 177.2) | 16.5 (11.0, 26.2) | 28.9 (-2.5, 104.3 | 28.9 (-8.6, 134.7) | 19.8 (0.6, 85.6) | 27.1 (-1.1, 103.3) | 31.7 (6.0, 120.7) | 2.5 (-11.7, 16.9) | |||||||||||||||||||||
| p value | <0.001 | <0.001 | 0.068 | 0.006 | 0.013 | 0.14 | 0.003 | 0.004 | 0.60 | |||||||||||||||||||||
| PVR (ml) | ||||||||||||||||||||||||||||||
| N | 86 | 70 | 16 | 100 | 77 | 23 | 77 | 58 | 19 | 102 | 82 | 20 | ||||||||||||||||||
| Median (IQR) | 0.0 (0.0, 34.9) | 0.0 (0.0, 34.9) | 0.0 (0.0, 50.3) | 0.0 (0.0, 0.0) | 0.0 (0.0, 8.1) | 0.0 (0.0, 0.0) | 0.0 (0.0, 27.4) | 0.0 (0.0, 44.8) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 4.5) | 0.0 (0.0, 0.0) | ||||||||||||||||||
| Change | 0.0 (-31.7, 0.0) | 0.0 (-34.2, 8.1) | 0.0 (-26.1, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 10.5) | 0.0 (-78.4, 0.0) | 0.0 (-6.0, 0.0) | 0.0 (-47.6, 0.0) | 0.0 (0.0, 0.0) | |||||||||||||||||||||
| % Change | 0.0 (-41.2, 0.0) | 0.0 (-53.5, 23.4) | 0.0 (-12.5, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 25.0) | 0.0 (-63.9, 0.0) | 0.0 (-13.6, 0.0) | 0.0 (-58.9, 0.0) | 0.0 (0.0, 0.0) | |||||||||||||||||||||
| p value | 0.17 | 0.38 | 0.18 | 0.53 | 0.92 | 0.18 | 0.085 | 0.033 | 0.32 | |||||||||||||||||||||
| PSA (ng/ml) | ||||||||||||||||||||||||||||||
| N | 156 | 121 | 35 | 31 | 27 | 4 | 58 | 46 | 12 | |||||||||||||||||||||
| Median (IQR) | 1.4 (0.7, 3.4) | 1.5 (0.7, 3.6) | 1.4 (0.8, 2.9) | 2.5 (0.6, 4.5) | 3.0 (0.6, 4.8) | 1.0 (0.5, 3.7) | 2.5 (1.0, 4.5) | 2.8 (1.1, 5.1) | 1.4 (0.7, 2.7) | |||||||||||||||||||||
| Change | -0.4 (-1.3, 0.0) | -0.4 (-1.7, 0.0) | -0.4 (-1.1, 0.0) | -0.1 (-0.7, 0.8) | 0.0 (-0.4, 0.9) | -0.3 (-0.8, 0.1) | ||||||||||||||||||||||||
| % Change | -20.8 (-36.2, -1.7) | -20.8 (-36.2, -2.4) | -11.7 (-39.3, -0.4) | -5.3 (-24.7, 24.3) | -1.0 (-21.1, 36.9) | -11.3 (-37.0, 15.8) | ||||||||||||||||||||||||
| p value | 0.004 | 0.012 | 0.11 | 0.99 | 0.45 | 0.11 | ||||||||||||||||||||||||
| Alpha Blocker and/or 5-ARI usage | ||||||||||||||||||||||||||||||
| N | 171 | 131 | 40 | 140 | 109 | 31 | 112 | 87 | 25 | 113 | 90 | 23 | ||||||||||||||||||
| n, % | 155 (90.6) | 121 (92.4) | 34 (85.0) | 25 (17.9) | 19 (14.2) | 6 (19.4) | 19 (17.0) | 12 (9.0) | 7 (28.0) | 22 (19.5) | 17 (18.9) | 5 (21.7) | ||||||||||||||||||
| Change | -72.7 | -78.2 | -65.6 | -73.6 | -83.4 | -57.0 | -71.1 | -73.5 | -63.3 | |||||||||||||||||||||
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||||||||||||||
5-ARI – 5-alpha reductase inhibitor; PSA – prostate-specific antigen; Qmax – maximum flow rate; IPSS – International Prostate Symptom Score; QoL – quality of life; PVR – post-void residual
On multivariate analysis, higher IPSS scores and larger prostate volumes were the only independent predictors of achieving a MCID at 3 months. Each point increase in the baseline IPSS was associated with a 10% increase in the odds of achieving a MCID at 3 months (OR: 1.10, 95% CI 1.04, 1.17). Additionally, every 10-cc increase in baseline prostate volume was associated with a 30% increase in the odds of achieving a MCID at 3 months (OR: 1.03, 95% CI 1.00, 1.05) (Table 5).
Table 5.
Univariate and multivariate regression of factors associated with achieving MCID at 3 months
| Characteristic | Unadjusted OR | Adjusted OR | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | 0.98 (0.94–1.03) | 0.43 | ||
| Race/Ethnicity Non-Hispanic White Non-Hispanic Black Hispanic Asian |
ref 1.47 (0.49–4.47) 2.67 (0.69–10.38) 0.89 (0.32–2.47) |
ref 0.49 0.16 0.83 |
||
| Prostate Volume, cc | 1.02 (1.00–1.05) | 0.037 | 1.03 (1.00–1.05) | 0.036 |
| PSA, ng/ml | 1.15 (0.96–1.39) | 0.13 | ||
| IPSS (Baseline) | 1.10 (1.04–1.17) | 0.001 | 1.10 (1.04–1.17) | 0.001 |
| QoL (Baseline) | 1.12 (0.85–1.49) | 0.42 | ||
| Past Medical History Urinary Retention Hypertension Erectile Dysfunction Dyslipidaemia Diabetes Nephrolithiasis |
1.35 (0.28–6.54) 1.21 (0.59–2.47) 0.79 (0.39–1.61) 0.92 (0.45–1.90) 0.92 (0.43–1.95) 0.35 (0.09–1.37) |
0.71 0.60 0.52 0.82 0.82 0.13 |
||
| Previous BPH Procedure | 1.83 (0.77–4.32) | 0.17 | ||
| Prostate Block | 0.50 (0.20–1.23) | 0.13 | ||
| Lateral Lobe Injections 2 3 4 |
1.35 (0.63–2.92) 3.15 (0.83–11.97) 2.46 (0.27–22.12) |
0.44 0.09 0.42 |
||
| Medial Lobe Injections 1 2 |
0.62 (0.27–1.43) 1.07 (0.11–10.31) |
0.26 0.95 |
||
OR – odds ratio; CI – confidence interval; BPH – benign prostatic hyperplasia; PSA – prostate-specific antigen; IPSS – International Prostate Symptom Score; QoL – quality of life; MCID – minimal clinically important difference
Sub-group analysis
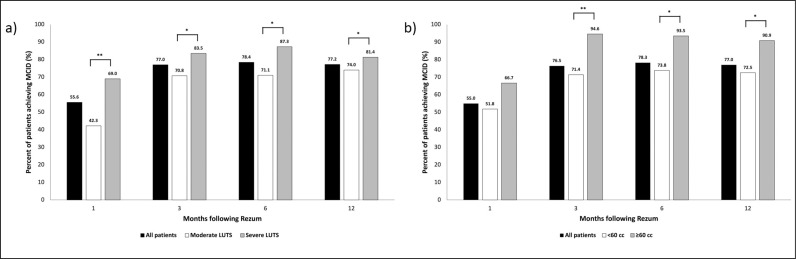
Patients were stratified by baseline LUTS severity (moderate [IPSS 8–19] and severe [IPSS ≥20] LUTS) and by prostate volume (small prostates [<60 cc] and large prostates [≥60 cc]) for further analysis. At all follow-up timepoints (up to 12 months postoperatively), a significantly greater proportion of patients with severe LUTS achieved MCID when compared to patients with moderate LUTS (Figure 1a). At 3, 6, and 12 months, a significantly greater proportion of patients with prostates ≥60 cc achieved MCID when compared to patients with prostates <60 cc (Figure 1b).
Figure 1.
Percentage of patients achieving minimal clinically important difference overtime stratified by baseline lower urinary tract symptoms severity (a); percentage of patients achieving MCID overtime stratified by baseline prostate volume (b).
MCID – minimal clinically important difference; LUTS – lower urinary tract symptoms
*P <0.05; **P <0.01
DISCUSSION
In our real-world, multi-ethnic population, we found that more than three-quarters of patients successfully achieved a MCID at 3 months. A higher baseline IPSS and larger prostate volumes were the only independent predictors of experiencing a MCID. More specifically, patients with severe LUTS (IPSS ≥20) or prostate volumes ≥60 cc were the optimal candidates of achieving MCID at 3 months.
Recently, Janakiraman et al. investigated risk factors of failing to achieve a MCID following Rezūm [10]. Similarly to our study, Janakiraman et al. defined MCID as ≥25% improvement in IPSS. The authors found that patients ultimately achieved MCID over time and that bladder outlet obstruction index and prior surgical BPH therapies were the independent predictors of failing to achieve a MCID. The results from Janakiraman et al. differ from our study in that we found that most patients achieved MCID by 3 months, without any significant changes in the percentage of patients achieving MCID in the subsequent follow-ups, and that lower baseline IPSS and smaller prostate volumes were risk factors of failing to achieve a MCID at 3 months. The differences in these results may be explained by the differences in baseline characteristics between the 2 study populations; our population was younger, more ethnically diverse, and had more patients with larger baseline prostate volumes and a greater number of prior BPH surgical therapies. However, further evaluation is warranted to elucidate the differences between our study and this previous study.
There has been one other study that has reported MCID rates of ≥25% improvement in IPSS at 3 months for Rezūm. Dixon et al. found that 86% of patients experienced a MCID at 3 months, which is slightly higher than our rate of 77% [13]. This discrepancy may be due to the effect of high variance of LUTS severity in their population compared to our study, which included a greater proportion of patients with moderate LUTS. As suggested by our findings, those with severe LUTS at baseline are more likely to achieve MCID at 3 months. Nevertheless, both studies highlighted significant improvements in IPSS and QoL in the total cohort from as early as one month and remaining throughout 12 months. This further supports the existing literature showing the rapid and durable improvement in LUTS following Rezūm therapy [13, 14].
We found that patients with moderate LUTS were less likely to achieve MCID. Therefore, those opting for Rezūm therapy for moderate symptoms may need to be counselled preoperatively on realistic expectations regarding LUTS improvements. On the other hand, more than two-thirds of patients with severe LUTS achieved MCID as early as one month, and more than 80% achieved MCID at later follow-ups. Thus, patients with severe LUTS may be optimal candidates to experience rapid and durable improvements in LUTS. That said, it is notable that none of the patients who failed to achieve a MCID at 3 months were surgically retreated by 12 months. This may be due to these patients being managed with BPH medications, opting against undergoing an additional BPH surgery, or seeking care elsewhere.
In our study, more than 90% of patients with larger prostates (≥ 60 cc) achieved MCID at follow-ups after one month when compared to patients with smaller prostates (<60 cc). Multiple studies have shown Rezūm to be effective in men with large prostate glands [15–18]. Regarding our study, it is unclear if this observation is due to post-operative anatomical changes in the prostate or an association between prostate volume and improvement in LUTS. Moreover, the number of injections utilised was not associated with the likelihood of achieving MCID. A few recent studies have suggested a “less is more” Rezūm treatment approach after observing no relationship between the number of injections employed during Rezūm and improvements in LUTS [19–21]. Instead, they found that utilising more injections was associated with higher rates of AEs [20].
This study is not without limitations. First, the sample size of patients who did not achieve a MCID at 3 months was small, which limits further sub-analysis of these patients. Second, analysis of long-term outcomes, including retreatment rates, is warranted to assess the impact of failure to achieve MCID on the durability of Rezūm. Despite these limitations, our study includes patients from an ethnically diverse patient population, who are typically underrepresented in clinical research, and it offers important insights into preoperative counselling regarding expectations of clinical improvement in LUTS.
CONCLUSIONS
We found that more than three-quarters of patients treated with Rezūm therapy achieved a MCID at 3 months. Furthermore, more severe urinary symptoms at baseline and larger prostate volumes were independent predictors of experiencing MCID at 3 months. Given the paucity of data on patient characteristics associated with achieving MCID following Rezūm, our real-world study involving a diverse patient population offers novel insight that urologists should consider when counselling and managing patients who may be candidates for Rezūm therapy. Ultimately, Rezūm offers short-onset clinical benefit, with patients continuing to show clinical improvement for up to 12 months following the procedure. Our findings are particularly notable for the clinical management of patients with severe LUTS and large prostate volumes, who may have previously been offered treatment with a transurethral resection of the prostate and now may consider Rezūm therapy as a more appropriate first-line surgical procedure.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
We thank Dr. David S. Schnapp for helping to create our Rezūm database and the staff at DSS Urology for assisting with data collection.
ETHICS APPROVAL STATEMENT
The Western Institutional Review Board approved this study under protocol #1-1181739-1.
References
- 1.Roehrborn CG, Strand DW. Benign Prostatic Hyperplasia. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, editors. Campbell-Walsh-Wein Urology. 3. Twelfth ed. Canada: Elsevier; 2021. [Google Scholar]
- 2.Egan KB. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol Clin North Am. 2016; 43: 289-297. [DOI] [PubMed] [Google Scholar]
- 3.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984; 132: 474-479. [DOI] [PubMed] [Google Scholar]
- 4.Rees J. Patients not P values. BJU Int. 2015; 115: 678-679. [DOI] [PubMed] [Google Scholar]
- 5.Sedaghat AR. Understanding the Minimal Clinically Important Difference (MCID) of Patient-Reported Outcome Measures. Otolaryngol Head Neck Surg. 2019; 161: 551-560. [DOI] [PubMed] [Google Scholar]
- 6.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995; 154: 1770-1774. [DOI] [PubMed] [Google Scholar]
- 7.Chapple CR, Montorsi F, Tammela TL, Wirth M, Koldewijn E, Fernández Fernández E. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: results of an international, randomized, double-blind, placebo- and active-controlled clinical trial performed in Europe. Eur Urol. 2011; 59: 342-352. [DOI] [PubMed] [Google Scholar]
- 8.Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology. 1998; 51: 892-900. [DOI] [PubMed] [Google Scholar]
- 9.Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol. 1998; 160: 1701-1706. [PubMed] [Google Scholar]
- 10.Janakiraman S, Felice M, Pahouja G, et al. Risk Factors for Persistent Lower Urinary Tract Symptoms 1 Month Following Convective Water Vapor Thermal Therapy (CWVTT-Rezum). Urology. 2023; 179: 112-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McVary KT, Gange SN, Gittelman MC, et al. Minimally Invasive Prostate Convective Water Vapor Energy Ablation: A Multicenter, Randomized, Controlled Study for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. J Urol. 2016; 195: 1529-1538. [DOI] [PubMed] [Google Scholar]
- 12.McVary KT, Gittelman MC, Goldberg KA, et al. Final 5-Year Outcomes of the Multicenter Randomized Sham-Controlled Trial of a Water Vapor Thermal Therapy for Treatment of Moderate to Severe Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. J Urol. 2021; 206: 715-724. [DOI] [PubMed] [Google Scholar]
- 13.Dixon CM, Cedano ER, Pacik D, et al. Two-year results after convective radiofrequency water vapor thermal therapy of symptomatic benign prostatic hyperplasia. Res Rep Urol. 2016; 8: 207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babar M, Loloi J, Tang K, Syed U, Ciatto M. Emerging outcomes of water vapor thermal therapy (Rezum) in a broad range of patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia: A systematic review. Low Urin Tract Symptoms. 2022; 14: 140-154. [DOI] [PubMed] [Google Scholar]
- 15.Elterman D, Bhojani N, Vannabouathong C, Chughtai B, Zorn KC. Rezūm therapy for ≥80-ml benign prostatic enlargement: a large, multicentre cohort study. BJU Int. 2022; 130: 522-527. [DOI] [PubMed] [Google Scholar]
- 16.Garden EB, Shukla D, Ravivarapu KT, et al. Rezum therapy for patients with large prostates (≥ 80 g): initial clinical experience and postoperative outcomes. World J Urol. 2021; 39: 3041-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bole R, Gopalakrishna A, Kuang R, et al. Comparative Postoperative Outcomes of Rezūm Prostate Ablation in Patients with Large Versus Small Glands. J Endourol. 2020; 34: 778-781. [DOI] [PubMed] [Google Scholar]
- 18.Ines M, Babar M, Singh S, Iqbal N, Ciatto M. Real-world evidence with The Rezūm System: A retrospective study and comparative analysis on the efficacy and safety of 12 month outcomes across a broad range of prostate volumes. Prostate. 2021; 81: 956-970. [DOI] [PubMed] [Google Scholar]
- 19.Aladesuru O, Amankwah K, Elterman D, et al. Pilot Study of “Less is More” Rezum for Treatment of BPH. Urology. 2022; 165: 256-60. [DOI] [PubMed] [Google Scholar]
- 20.Babar M, Loloi J, Azhar U, et al. Rezum Outcomes in Relationship to Number of Injections: Is Less More? J Endourol. 2023; 37: 157-164. [DOI] [PubMed] [Google Scholar]
- 21.Babar M, Azhar U, Loloi J, et al. Water vapour thermal therapy (Rezum) outcomes at 4 years in relationship to the number of injections: is the 'less is more' treatment approach durable? BJU Int. 2023; 132: 266-267. [DOI] [PubMed] [Google Scholar]